Abstract
Adult neurogenesis, defined here as progenitor cell division generating functionally integrated neurons in the adult brain, occurs within the hippocampus of numerous mammalian species including humans. The present review details various endogenous (e.g., neurotransmitters) and environmental (e.g., physical exercise) factors that have been shown to influence hippocampal adult neurogenesis. In addition, the potential involvement of adult-generated neurons in naturally-occurring spatial learning behavior is discussed by summarizing the literature focusing on traditional animal models (e.g., rats and mice), non-traditional animal models (e.g., tree shrews), as well as natural populations (e.g., chickadees and Siberian chipmunk).
Keywords: adult neurogenesis, hippocampus, spatial learning, memory, Morris water maze, food hoarding
1. Introduction
1.1. Brief history of adult neurogenesis
In the field of neuroscience, it was traditionally believed that neurogenesis, defined here as progenitor cell division generating functionally integrated neurons, only occurs in the developing brain (Ramon y Cajal, 1928). The first evidence of the formation of new neurons in adult rats came from Altman's pioneering studies using thymidine autoradiography (Ming and Song, 2005; Sidman et al., 1959)—a technique labeling dividing cells with [3H]-thymidine, which incorporates into the replicating DNA (Altman and Das, 1965; Altman, 1966; Altman and Das, 1967). Unfortunately, Altman's findings received little attention at that time. The topic of adult neurogenesis was revisited years later by Kaplan and his colleagues, who provided additional evidence for adult-generated neurons using electron microscopy. In particular, they demonstrated that [3H]-thymidine labeled cells in adult rats display ultra-structural characteristics of neurons (such as dendrites and synapses) (Kaplan and Hinds, 1977; Kaplan and Bell, 1984). However, these findings were also largely ignored. Methodological advancements provided evidence of adult neurogenesis in a variety of mammalian species, which, in turn, led to the acceptance that the adult mammalian brain displays neurogenesis (Gross, 2000; Kempermann and Gage, 1999; Lie et al., 2004). These methodological advancements included the introduction of novel methods to label new cells (e.g., 5-bromo-3’-deoxyuridine, BrdU, labeling) (Gratzner, 1982) and the distinction of neurons from glial cells (e.g., double-labeling of BrdU with neuronal markers such as TuJ1 and NeuN) (e.g., Ming and Song, 2005).
1.2. Common techniques to study adult neurogenesis
The numerous technical developments, which have facilitated the advancement of the field of adult neurogenesis, can be grouped into the following three distinct approaches: (1) using nucleotide analogs, (2) using genetic tools, and (3) using specific markers. First, exogenous nucleotide analogs, such as [3H]-thymidine, BrdU, iododeoxyuridine (IdU), and chlorodeoxyuridine (CldU), are usually administered via intraperitoneal injections and incorporate into the cellular DNA in place of endogenous thymidine during the DNA synthesis phase of the cell cycle (S phase) (Leuner and Gould, 2010; Miller and Nowakowski, 1988). Labeled cells can be identified using autoradiography (for [3H]-thymidine) and immunohistochemistry (for BrdU, IdU, and CldU). [3H]-thymidine and BrdU (but not IdU and CldU) are similar in their efficiency to label dividing cells (Leuner et al., 2009). However, BrdU is more commonly used as it allows phenotypic analysis (see review Lieberwirth and Wang, 2012) and stereological quantification of the newly-generated cells (Scharfman et al., 2005). Varying the pulsing paradigm and the elapsed examination time after pulsing allows the quantitative analysis of distinct stages of adult neurogenesis, namely cell proliferation, neuronal differentiation, and cell survival (Dayer et al., 2003; Paizanis et al., 2007). Furthermore, BrdU immunohistochemistry can be combined with labeling for cell type-specific markers. Fluorescent double- or triple-labeling combined with confocal microscopy can be used to determine whether BrdU-labeled cells express a neuronal or glial phenotype (for reviews see Lieberwirth and Wang, 2012; von Bohlen Und Halbach, 2007). However, BrdU immunostaining has limitations in that it requires tissue fixation and DNA denaturation—making this type of analysis unsuitable for living cells. Secondly, adult neurogenesis can be studied using genetic labeling with viral vectors. Any viral vector, such as the frequently used non-replicative retrovirus, requires invasive stereotaxic surgery to be injected into a specific brain region. These markers are dependent on nuclear membrane breakdown during mitosis, thus their expression is a good indicator of cell division (Christie and Cameron, 2006; Lewis and Emerman, 1994; Ming and Song, 2005). An advantage of viral vectors includes that they usually carry a reporter gene, such as green fluorescent protein, which allows easy identification of cells expressing the retrovirus even in living cells (Ming & Song 2005). As the green fluorescent protein fills the entire structure of the neuron (including the soma and processes), viral vectors allow electrophysiological and morphological examination of these neurons. However, the use of viral vectors is limited by low infection rates and infection variability between animals (Christie and Cameron, 2006). Lastly, adult neurogenesis can also be studied using endogenous cell cycle markers. These markers include nuclear antigens (such as Ki67, proliferating cell nuclear antigen [PCNA], and minichromosome marker-2 [MCM-2]), which are only expressed in actively diving cells (Galand and Degraef, 1989; Kee et al., 2002; Scholzen and Gerdes, 2000; Stoeber et al., 2001). The major limitation of these endogenous markers is that they can only be used to analyze cell proliferation, but not the other stages of adult neurogenesis (such as survival or neuronal differentiation) (Lieberwirth and Wang, 2012).
1.3. Evidence of adult neurogenesis in various brain regions
Using these common techniques to study adult-generated neurons, numerous studies have shown that adult neurogenesis continuously occurs in the subventricular zone (SVZ) of the rostral lateral ventricles as well as the subgranular zone of the dentate gyrus (DG) of the hippocampus. Newly-generated cells migrate from the SVZ along the rostral migratory stream to the main olfactory bulb (MOB). Most SVZ-generated cells differentiate into neurons and integrate into the existing olfactory circuitry—where they likely play a role in short-term olfactory memory, olfactory fear-conditioning, and long-term associative olfactory memory (Lledo and Saghatelyan, 2005; Ming and Song, 2005; Ming and Song, 2011). Similarly, most cells born in the subgranular zone migrate within the DG, differentiate into neurons (Figure 1), and integrate into the existing DG circuitry (Christie and Cameron, 2006; Lledo and Saghatelyan, 2005; Ming and Song, 2005). Various studies suggest that these adult-generated DG neurons may play a role in spatial learning, long-term spatial memory retention, trace conditioning, and contextual fear conditioning (Ming and Song, 2011). Although the majority of studies assessing adult neurogenesis has focused on the SVZ/MOB and DG, evidence has accumulated suggesting that adult neurogenesis also occurs in other non-traditional neurogenic brain regions (Fowler et al., 2008; see reviews Gould, 2007; Migaud et al., 2010). In particular, adult-generated neurons have been reported in the neocortex (Altman, 1962; Bernier et al., 2002; Cameron and Dayer, 2008; Dayer et al., 2005; Gould et al., 1999c; Gould et al., 2001), piriform cortex (Bernier et al., 2002), and striatum (Bedard et al., 2002; Bedard et al., 2006). Furthermore, numerous limbic structures such as the amygdala (Figure 2), hypothalamus, and medial preoptic area—brain regions essential to mediating social behaviors—have been reported to display adult-generated neurons (Akbari et al., 2007; Bernier et al., 2002; Fowler et al., 2002; Huang et al., 1998; Kokoeva et al., 2005; Lieberwirth et al., 2012; Okuda et al., 2009). Although various researchers have observed new neurons in these non-traditional brain regions, it should be noted that other researchers did not (Bhardwaj et al., 2006; Koketsu et al., 2003; Kornack and Rakic, 2001). Interestingly, correlative studies have shown that the social environment can influence the rate of adult neurogenesis within the limbic system. For example, positive social interactions (such as mating) increase, whereas negative interactions (such as social isolation; Figure 2) decrease the number of adult-generated limbic system neurons (for review see Lieberwirth and Wang, 2012). As these studies are correlational, future studies are needed to assess the causal link between adult-generated neurons in the limbic system and social behavior.
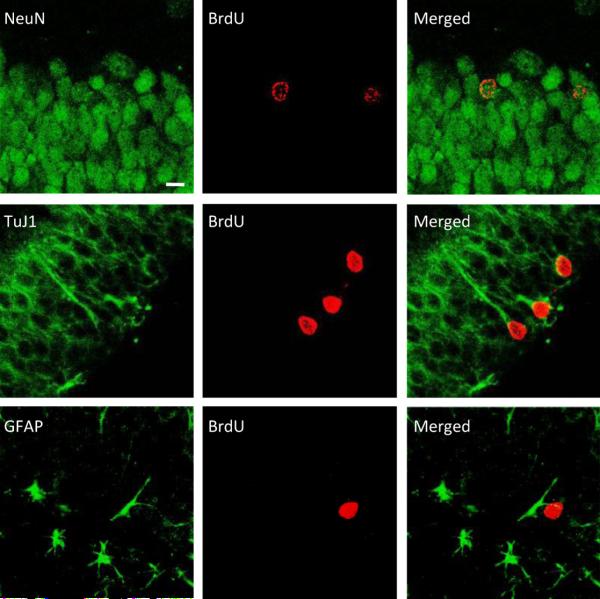
Figure 1.
Confocal microscopy images illustrating adult-generated cells (labeled with BrdU, red) in the dentate gyrus of the hippocampus in male rats. The majority of BrdU-labeled cells also co-expressed neuronal markers (NeuN and TuJ1), but not a glial marker (GFAP). Scale bar=10 μm. (Adapted from Leuner et al., 2010).
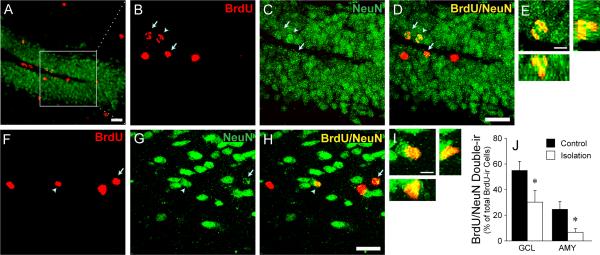
Figure 2.
Confocal microscopy images illustrating adult-generated cells (labeled with BrdU, red) which express a neuronal phenotype (labeled with NeuN, green) in the dentate gyrus (DG) of the hippocampus (A, B, C, D, and E) and the amygdala (AMY) (F, G, H, and I) in female prairie voles. The white box in Panel A indicates the area of the DG with high magnification (B–D). Arrows indicate cells double-labeled for BrdU/NeuN. Arrowheads indicate the double-labeled cell shown in the magnified image in the DG (E) and AMY (I). 3D co-localization of BrdU and NeuN is demonstrated using views along the y–z axis (right) and x–z axis (below). Scale bar=20 μm (A–D and F–H) or 5 μm (E and I). (J) Female prairie voles that were socially isolated for 6 weeks had significantly lower percentages of BrdU-labeled cells that co-expressed NeuN in the granular cell layer (GCL) of the DG and AMY, compared to control females that were continuously housed with the female cage mates. *p < 0.05. Error bars represent SEM. (Adapted from Lieberwirth et al., 2012).
Although numerous studies have focused on various aspects of the functional significance of adult-generated cells, the present review will only focus on adult neurogenesis in the hippocampus and its associated learning-memory functions. We will discuss various external (such as exercise) and internal (such as steroid hormones) factors influencing the distinct stages—cell proliferation, cell survival, and neuronal differentiation—of adult neurogenesis in the DG. In addition, we will highlight the different mammalian animal models (including laboratory as well as field animal models) that have been used to study hippocampal neurogenesis. Finally, as the DG is involved in spatial learning and memory functions (Broadbent et al., 2004; Galani et al., 1998; Riedel et al., 1999), we will also summarize the evidence indicating the potential involvement of adult-generated hippocampal neurons in behaviors displayed in the laboratory as well as naturally-occurring behaviors that require special learning and memory.
2. Adult neurogenesis in the hippocampus
2.1. Evidence of hippocampal neurogenesis across diverse species including humans
The occurrence of adult neurogenesis has been documented in a large variety of species covering a large range of animal classes, including fish (Pisces) (Byrd and Brunjes, 2001; Fernández et al., 2011), amphibians (Amphibia) (Raucci et al., 2006), reptiles (Reptila) (Font et al., 2001; Pérez-Cañellas and García-Verdugo, 1996), birds (Aves) (Alvarez-Buylla and Nottebohm, 1990; Alvarez-Buylla and Kirn, 1997; Ling et al., 1997), and mammals (Mammalia) (Fowler et al., 2008; Gould et al., 2000). Specifically, hippocampal adult neurogenesis has been observed in all mammalian species studied to date [hedgehogs (Bartkowska et al., 2009); marsupials (Harman et al., 2002); moles (Bartkowska et al., 2009); rodents including guinea pigs (Altman and Das, 1967), mice (Carlen et al., 2002; Kempermann et al., 1997; Kempermann et al., 2003), rats (Altman and Das, 1965; Cameron et al., 1993; Kaplan and Hinds, 1977), squirrels (Barker et al., 2005), and voles (Fowler et al., 2002; Galea and McEwen, 1999; Lieberwirth et al., 2012); rabbits (Gueneau et al., 1982); sheep (Brus et al., 2010; Hawken et al., 2009); tree shrews (Gould et al., 1997); several bat species (Amrein et al., 2007; Gatome et al., 2009); non-human primates (Gould et al., 1999b; Kornack and Rakic, 1999); and even humans (Eriksson et al., 1998; Knoth et al., 2010; Kukekov et al., 1999)]. Although substantial numbers of new cells are generated, only a small subset of these cells survives, matures into neurons, and integrates into the existing neuronal circuit (Cameron and McKay, 2001; Kempermann et al., 2003; von Bohlen Und Halbach, 2007). Interestingly, numerous studies have identified environmental and endogenous factors that influence hippocampal adult neurogenesis—suggesting that hippocampal adult neurogenesis may be an adaptive process to respond to environmental and/or internal challenges (Lledo et al., 2006).
2.2. A variety of factors influence hippocampal neurogenesis
2.2.1. Environmental factors
Previous research has indicated that various environmental factors influence the distinct stages of hippocampal neurogenesis including cell proliferation, neuronal differentiation, and/or survival. In particular, increased environmental complexity (relative to standard housing)—enhancing the opportunities for social, cognitive, sensory, and motor stimulation (Nithianantharajah and Hannan, 2006; van Praag et al., 2000)—has been shown to increase hippocampal cell survival in mice and rats, without affecting hippocampal cell proliferation (Bruel-Jungerman et al., 2005; Kempermann et al., 1997; Kempermann et al., 1998; Nilsson et al., 1999; Olson et al., 2006; van Praag et al., 1999b). Increased environmental complexity also significantly enhances neuronal differentiation in the murine DG (Brown et al., 2003; Kempermann et al., 1998; Kempermann et al., 2002; Tashiro et al., 2007). Voluntary physical activity (via wheel running) also heightens hippocampal adult neurogenesis by increasing cell proliferation, neuronal differentiation, as well as cell survival in the adult mouse (Brown et al., 2003; Stranahan et al., 2006; van Praag et al., 1999a; van Praag et al., 1999b; van Praag et al., 2005; Zhao et al., 2006). As increased environmental complexity consists of various components (including social, cognitive, sensory, and motor stimulation), van Praag and colleagues (1999a) separately investigated the components of environmental complexity and found that only one component—physical activity—is associated with enhanced hippocampal neurogenesis. There is evidence that voluntary physical activity and a complex environment increase vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF), which, in turn, positively influence hippocampal neurogenesis (Adlard et al., 2004; Cao et al., 2004; During and Cao, 2006; Fabel et al., 2003; Farmer et al., 2004).
Furthermore, increased environmental complexity enhances the number of learning opportunities as compared to standard housing (Leuner and Gould, 2010). Interestingly, learning also has beneficial effects on hippocampal neurogenesis (Gould et al., 1999d). In particular, hippocampal-dependent learning tasks, such as eye-blink conditioning, spatial navigation learning, and conditioned food preference, alter adult neurogenesis in the DG. Eye-blink conditioning and spatial navigation learning in the Morris water maze increase hippocampal cell proliferation (Dupret et al., 2007; Van der Borght et al., 2005) and survival (Ambrogini et al., 2000; Döbrössy et al., 2003; Epp et al., 2011; Gould et al., 1999a; Leuner et al., 2004; Leuner et al., 2006). Similarly, acquiring conditioned food preference, a hippocampus-dependent learning task, enhances cell survival in the rat DG (Olariu et al., 2005). In contrast, hippocampal-independent learning tasks (such as short-delay eye-blink conditioning, cued water maze training, and active shock avoidance) do not alter hippocampal neurogenesis (Gould et al., 1999a). However, the classification of learning tasks as hippocampus-dependent or hippocampus-independent does not completely account for the learning-induced effects on hippocampal adult neurogenesis (reviewed by Shors, 2008). Data from previous studies suggest that the stimulatory effect of learning on hippocampal adult neurogenesis depends on task difficulty (Leuner et al., 2006; Waddell and Shors, 2008) and depth of learning (Dalla et al., 2007; Leuner et al., 2004).
In contrast to the enhancement of neurogenesis due to exposure to a complex environment, voluntary physical activity, and learning, stress (due to both physical and psychosocial stressors) negatively affects adult neurogenesis in the DG (for review see Mirescu and Gould, 2006). For example, a physical stressor such as forced swimming results in the reduction of hippocampal cell proliferation in rats (Heine et al., 2004; Veenema et al., 2007). Acute foot shock reduces cell proliferation in male, but not female, rats (Malberg and Duman, 2003; Shors et al., 2007). Chronic, but not acute, restraint stress reduces both hippocampal cell proliferation and survival (Pham et al., 2003). Cell proliferation is also reduced in response to chronic unpredictable stress (Heine et al., 2004). Similarly to physical stressors, psychosocial stressors, such as the exposure to an aggressive conspecific, predator odor, or social isolation, reduce hippocampal neurogenesis (reviewed by Lieberwirth and Wang, 2012). In particular, acute as well as chronic social defeat stress reduces hippocampal cell proliferation and survival in marmoset monkeys (Callithrix jacchus), mice, rats, and tree shrews (Tupaia belangeri) (Czeh et al., 2001; Czeh et al., 2002; Czeh et al., 2007; Gould et al., 1997; Gould et al., 1998; Mitra et al., 2006; Simon et al., 2005; Thomas et al., 2007; Van Bokhoven et al., 2011; van der Hart et al., 2002; Yap et al., 2006). Hippocampal cell proliferation and survival are also reduced in response to predator odor exposure in male rats (Falconer and Galea, 2003; Tanapat et al., 2001). Furthermore, social isolation reduces hippocampal cell proliferation and cell survival in prairie voles (Microtus ochrogaster) and rats (Fowler et al., 2002; Lieberwirth et al., 2012; Spritzer et al., 2011). It should be noted that contrary to aversive social experiences (e.g., exposure to social defeat, resident-intruder, and social isolation), non-stressful social interactions including exposure to male pheromones, maternal experience, and interactions with conspecific pups increase hippocampal neurogenesis in mice, prairie voles, and rats (Furuta and Bridges, 2009; Mak et al., 2007; Ruscio et al., 2008) (for reviews see Gheusi et al., 2009; Lieberwirth and Wang, 2012).
Researchers have also focused on the effects of the administration of psychotropic drugs on hippocampal neurogenesis. As such, the chronic use of drugs of abuse including alcohol, cocaine, opioids, ecstasy, and nicotine negatively affect hippocampal neurogenesis by reducing cell proliferation and survival (Abrous et al., 2002; Dominguez-Escriba et al., 2006; Duman et al., 2001; Eisch et al., 2000; He et al., 2005; Hernandez-Rabaza et al., 2006; Herrera et al., 2003; Nixon and Crews, 2002; Yamaguchi et al., 2005). On the other hand, the exposure to antidepressants increase hippocampal cell proliferation and neuronal differentiation (Malberg et al., 2000).
2.2.2. Endogenous factors
In addition to environmental factors, various intrinsic factors (such as developmental morphogens, neurotrophic factors, neurotransmitters, and steroids) have been reported to influence hippocampal neurogenesis (Grote and Hannan, 2007; Jang et al., 2008; Lledo et al., 2006; Schinder and Gage, 2004; Vaidya et al., 2007; Zhao et al., 2008). In particular, data from previous studies have shown that morphogens including Notch, Shh, Wnts, and BMPs play a regulatory role in the maintenance, activation, and neural differentiation of adult neural precursors (for review see Ming and Song, 2011). By experimentally manipulating the levels of neurotrophic factors, such as BDNF, ciliary neurotrophic factor (CNTF), insulin-like growth factor-1 (IGF-1) and VEGF, it has been shown that such neurotrophic factors increase hippocampal cell proliferation. For example, the site-specific chronic infusion of BDNF into the DG increases adult neurogenesis in rats (Scharfman et al., 2005). Similarly, central CNTF administration increases the cell proliferation and neuronal differentiation in the murine DG (Emsley and Hagg, 2003). Additionally, the peripheral as well as central administration of IGF-1 enhances the proliferation and neuronal differentiation in the DG of mice and rats (Åberg et al., 2000; Lichtenwalner et al., 2001). Hippocampal cell proliferation is also upregulated in response to central administration or viral-vector-induced expression of VEGF (Cao et al., 2004; Jin et al., 2002). On the contrary, the genetic knockdown of TrkB—the high affinity receptor for BDNF—or the knockdown of CNTF cause a significant reduction in hippocampal neurogenesis (Bergami et al., 2008; Müller et al., 2009).
In addition to morphogens and neurotrophic factors, various types of neurotransmitters have been shown to influence hippocampal neurogenesis. For example, the activation of the gamma amino butyric acid (GABA) neurotransmitter system, the main inhibitory neurotransmitter, regulates adult hippocampal neurogenesis. On one hand, GABA system activation downregulates cell proliferation, on the other hand GABA system activation seems to accelerate the synaptic integration of adult-generated hippocampal neurons (Ge et al., 2007). Interestingly, the activation of the glutamate neurotransmitter system, the main excitatory neurotransmitter, negatively affects hippocampal neurogenesis. For example, the administration of an agonist to the NMDA subtype of the glutamate receptors reduces hippocampal neurogenesis by inhibiting cell proliferation (Cameron et al., 1995). Administering a NMDA receptor antagonist or lesioning the perforant path, the main glutamatergic input to the DG, results in an increase in hippocampal cell proliferation in rats, tree shrews (Tupaia belangeri), and gerbils (Bernabeu and Sharp, 2000; Cameron et al., 1995; Cameron et al., 1998; Gould et al., 1997). Another neurotransmitter that has been shown to influence hippocampal neurogenesis is serotonin. Serotonin upregulates hippocampal neurogenesis, whereas the inhibition of serotonin synthesis and the lesioning of the raphe nuclei, site of serotonin neurons, downregulate hippocampal neurogenesis (Brezun and Daszuta, 1999; Gould, 1999; Radley and Jacobs, 2002).
Lastly, hippocampal neurogenesis is also modulated by steroids, including both corticosteroids and gonadal steroids. Corticosteroids, such as glucocorticoids that are naturally released during the stress response, reduce hippocampal neurogenesis (Cameron and Gould, 1994; Gould and Tanapat, 1999; Wong and Herbert, 2005). Similarly, the administration of glucocorticoids decreases hippocampal cell proliferation in adult rats (Ambrogini et al., 2002; Cameron and Gould, 1994; Gould et al., 1992). On the contrary, reducing corticosteroid levels (via adrenalectomy) increases hippocampal cell proliferation and can even restore the age-related decline of hippocampal neurogenesis, which is observed due to high levels of corticosteroids (Cameron and Gould, 1994; Cameron and McKay, 1999; Gould et al., 1992; Rodriguez et al., 1998). Adrenalectomy accompanied by corticosterone replacement does not lead to an increase in hippocampal cell proliferation (Gould et al., 1992; Rodriguez et al., 1998). Similarly to adrenalectomy, the blockade of glucocorticoid receptors can normalize a stress-induced reduction of hippocampal adult neurogenesis (Oomen et al., 2007). In addition, naturally occurring differences in the stress response have been shown to influence hippocampal cell proliferation. Specifically, highly reactive rats show significantly reduced hippocampal cell proliferation compared to low reactive rats (Lemaire et al., 1999).
Similarly to the modulating effects of corticosteroids, gonadal steroids have been shown to affect hippocampal cell proliferation. In particular, hippocampal proliferation in female rats and meadow voles (Microtus pennsylvanicus) fluctuates corresponding to ovarian steroid changes across the course of the estrous and breeding cycle (Galea and McEwen, 1999; Ormerod and Galea, 2001; Pawluski et al., 2009; Tanapat et al., 1999). Acute estrogen administration (within 4 hours) upregulates cell proliferation in the DG of female meadow voles and rats, whereas acute estrogen administration suppresses cell proliferation after 48 hours (Ormerod and Galea, 2001; Ormerod et al., 2003; Tanapat et al., 1999). Gonadal steroids have also been shown to affect hippocampal cell survival. In particular, neuronal survival in the male rat DG is enhanced by chronic androgen exposure (Spritzer and Galea, 2007). Hippocampal cell survival in the male meadow vole also seems to fluctuate corresponding to the seasonal changes of androgens (Ormerod and Galea, 2003).
3. Hippocampal adult neurogenesis and its behavioral significance
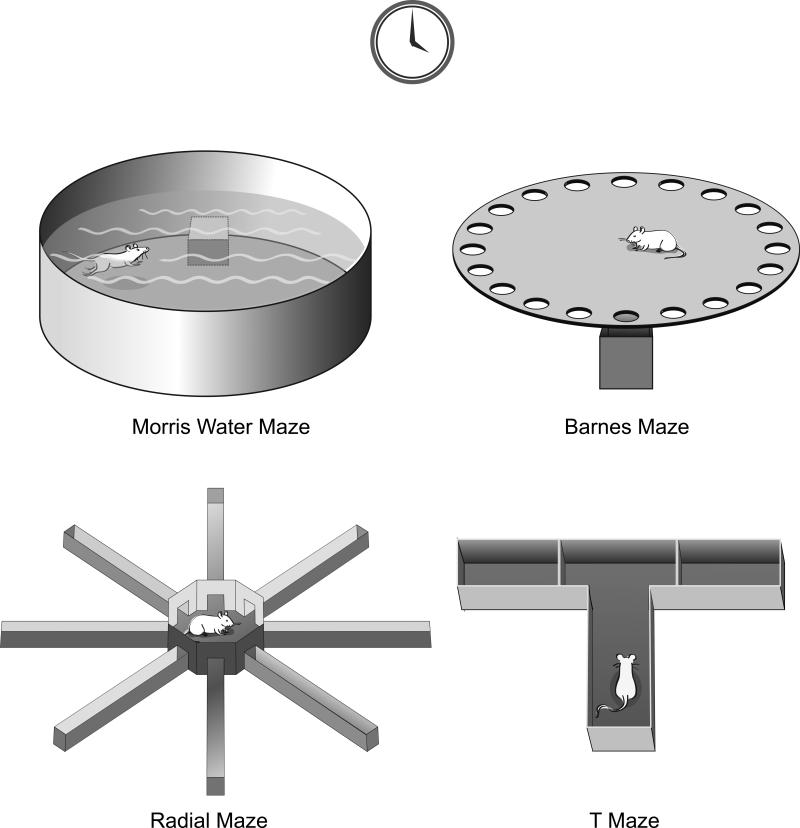
As the DG plays an essential role in learning and memory functions (such as converting short-term into long-term memories as well as spatial memory) (Broadbent et al., 2004; Burgess et al., 2002; Deacon et al., 2002a; Galani et al., 1998; Riedel et al., 1999) and hippocampal adult neurogenesis seems to be regulated by environmental factors and experience (Lledo et al., 2006; Zhao et al., 2008), researchers have investigated the functional significance of hippocampal adult-generated neurons. In the following section, we will review studies that focused on spatial learning and memory behaviors displayed by laboratory animals in the laboratory (using behavioral tests such as the Morris water maze or Barnes maze; Figure 3) or displayed by field animal species that naturally display spatial learning and memory behaviors (such as food hoarding).
Figure 3.
Schematic drawings of commonly used behavioral tasks in rodent research for hippocampus-dependent spatial learning and memory. These tasks include Morris water maze, Barnes maze, Radial arm maze, and T-maze.
3.1. Studying hippocampal adult neurogenesis using laboratory animals
3.1.1. Rats
One traditional animal model that has been used to study the functional implications of adult neurogenesis and its link to spatial learning has been the rat model. Hippocampus-dependent spatial learning (e.g., Morris water maze) has been reported to increase hippocampal neurogenesis (Epp et al., 2007; Gould et al., 1999a). Interestingly, the time and the proficiency of spatial learning seem to play a specific role. In particular, spatial learning increases the survival of 1-week old neurons, whereas younger and older neurons at the time of spatial learning are not more likely to survive (Ambrogini et al., 2000; Ambrogini et al., 2004; Dupret et al., 2007; Epp et al., 2007; Gould et al., 2000). Hippocampal cell proliferation seems to be upregulated only during the late, but not early, phase of spatial learning (Döbrössy et al., 2003; Dupret et al., 2007). Furthermore, high, but not low, proficiency spatial learning enhances hippocampal adult neurogenesis (Sisti et al., 2007). Such time- and proficiency-related enhancement of hippocampal neurogenesis suggests that the surviving adult-generated hippocampal neurons might play a role in these newly acquired spatial memories.
Further support of the involvement of newly generated cells in spatial learning comes from the observation that the reduction of adult neurogenesis causes impairments in spatial learning. In particular, the age-dependent reduction of hippocampal neurogenesis has been linked to impairments in learning the Morris water maze task (Drapeau et al., 2003; Driscoll et al., 2006). It is of interest to note that aged-impaired rats, which exhibit a lower level of adult neurogenesis than aged-unimpaired rats, show spatial memory impairments, whereas the aged-unimpaired rats do not show such impairments. Abolishing hippocampal adult neurogenesis by using low dose irradiation—an effective and rapid, but irreversible, method to reduce the number of young hippocampal neurons (Mizumatsu et al., 2003; Tada et al., 2000; Wojtowicz, 2006)—causes deficits in long-term retention, but not acquisition, in the Morris water maze (Snyder et al., 2005; Wojtowicz et al., 2008) and spatial working memory in the T-maze (Madsen et al., 2003). However, the ablation of adult neurogenesis using an anti-mitotic agent (such as methylazoxymethanol acetate, MAM) does not seem to affect spatial learning (Shors et al., 2002). One possible explanation for these apparently contradictory results is that spatial learning may only require a very small percentage of newly-generated cells (Moser et al., 1995; Snyder and Cameron, 2012). MAM-treatment, which does not fully ablate adult neurogenesis, may allow for a greater number of adult-generated hippocampal neurons than exist during senescence (Dupret et al., 2005; Shors et al., 2002). Another possible explanation is that the test of spatial memory must be sufficiently difficult to detect small changes in performance. For example, ablation of adult-generated hippocampal neurons leads to a deficit in the Morris water maze only if a long-delay time is used (Snyder et al., 2005; Zhang et al., 2008).
Whereas factors that reduce adult hippocampal neurogenesis impair spatial learning, factors that enhance adult neurogenesis seem to increase spatial learning. For example, increased environmental complexity enhances hippocampal adult neurogenesis as well as spatial learning in the Morris water maze (Nilsson et al., 1999). Increased environmental complexity can even ameliorate the stress-induced reduction in hippocampal cell proliferation as well as the spatial learning deficits (Veena et al., 2009). In sum, research in rats provides evidence that newly-generated hippocampal neurons play a role in spatial learning and memory.
3.1.2. Mice
In addition to rats, another traditional animal model to study the functional implications of adult neurogenesis is the mouse model. Similarly to findings in rats, hippocampus-dependent spatial learning enhances hippocampal adult neurogenesis in mice (Nilsson et al., 1999; Trouche et al., 2009). Furthermore, these adult-generated hippocampal neurons tend to incorporate into neuronal networks supporting spatial memory (Kee et al., 2007; Tashiro et al., 2007; Trouche et al., 2009). The reduction of adult neurogenesis causes impairments in spatial learning. Specifically, the age-dependent reduction of hippocampal neurogenesis has been linked to impairments in learning the Morris water maze task (van Praag et al., 2005). Experimentally abolishing hippocampal adult neurogenesis by using low dose irradiation also causes spatial memory deficits in the Barnes maze (Raber et al., 2004). Similarly, irradiation impairs the performance of mice in the Morris water maze, the radial arm maze, and a two-choice spatial discrimination task (Ben Abdallah et al., 2013; Clelland et al., 2009) (but see Raber et al., 2004). Correspondingly, the ablation of adult neurogenesis using temozolomide (TMZ), a DNA-alkylating agent, results in impaired performance in the Morris water maze task (Garthe et al., 2009; Garthe et al., 2013).
Whereas factors that reduce adult hippocampal neurogenesis impair spatial learning, factors that enhance adult neurogenesis seem to increase spatial learning. For example, running (via voluntary wheel running) enhances hippocampal adult neurogenesis as well as spatial learning in the Morris water maze (van Praag et al., 1999a; van Praag et al., 2005). Voluntary wheel running can even ameliorate the age-dependent reduction in hippocampal cell proliferation as well as the spatial learning deficits (van Praag et al., 2005). It should be noted that ablation studies in rats have been limited to the use of low dose irradiation and anti-mitotic treatment—techniques that lack specificity as well as have unwanted side effects (Bruel-Jungerman et al., 2007; Dupret et al., 2005; Schinder and Gage, 2004; Tada et al., 2000; Winocur et al., 2006). In mice, however, ablation of adult neurogenesis can also be achieved by using genetic tools, which allow for the reversible ablation of specific neurons. Several transgenic mouse lines have been developed, which allow the precise induction of death of neuronal precursors in the hippocampus. Using a transgenic mouse model to precisely induce the ablation of nestin-positive hippocampal neural precursors causes spatial deficits in the Morris water maze (Deng et al., 2009; Dupret et al., 2008). Similarly, the removal of TLX-positive neural precursors and the removal of NSE-positive cells also leads to spatial deficits in the Morris water maze and the Barnes maze, respectively (Imayoshi et al., 2008; Zhang et al., 2008). Interestingly, the ablation of GFAP-positive progenitor cells does not result in spatial deficits in the Morris water maze or the Y maze (Saxe et al., 2006). Although it could be argued that these GFAP-progenitor cells may not play a role in spatial learning and memory, it should be noted that the specific training protocol for the Morris water maze may be important to observe spatial learning and memory deficits induced by ablating adult hippocampal neurogenesis (Zhang et al., 2008). Using a transgenic technique that allows the ablation of previously-tagged adult-generated neurons, Arruda-Carvalho et al. (2011) ablated time-specifically neurons before or after Morris water maze learning. The authors observed that ablating adult-generated hippocampal neurons after memory formation, but not before, causes significant deficits in the Morris water maze task. In sum, various studies have shown a potential link between adult-generated hippocampal neurons in mice and spatial learning and memory behavior.
3.1.3. Other species
In addition to the traditional rat and mouse models to assess the functional implications of hippocampal neurons in spatial learning and memory, researchers have been assessing the presence of hippocampal adult neurogenesis in various non-traditional laboratory species, including guinea pigs (Altman and Das, 1967); rabbits (Gueneau et al., 1982); rodents such as California mice (Peromyscus californicus) (Glasper et al., 2011), meadow voles (Galea and McEwen, 1999; Ormerod and Galea, 2003), prairie voles (Fowler et al., 2002; Lieberwirth et al., 2012; Ruscio et al., 2008), and squirrels (Barker et al., 2005); sheep (Brus et al., 2010; Hawken et al., 2009); tree shrews (Tupaia belangeri) (Gould et al., 1997); and non-human primates (Gould et al., 1999b; Kornack and Rakic, 1999). Comparative research of the long-tailed wood mouse (Apodemus sylvaticus) and the bank vole (Clethrionomys glareolus) shows that wood mice have higher levels of hippocampal neurogenesis (Amrein et al., 2004b) and superior spatial learning capabilities (Galsworthy et al., 2005) than bank voles—providing correlational evidence that newly generated hippocampal neurons may play a functional role in spatial learning and memory . However, the causal relationship between adult-generated hippocampal neurons and spatial learning using non-traditional animal models has not yet been systematically investigated.
3.2. Studying hippocampal adult neurogenesis using field animal models
Numerous researchers have studied the potential link of hippocampal adult neurogenesis and its functional implications in spatial learning and memory using traditional laboratory animals such as rats and mice. However, using such animal models has various limitations and this approach cannot determine whether adult-generated neurons promote survival and reproductive fitness in natural populations (Barnea, 2010; Boonstra et al., 2001). Laboratory animals live in a highly controlled environment and are often exposed to inappropriate environmental stimuli—conditions that are not reflective of the natural environment (Barker et al., 2005). Standard laboratory housing is characterized by the surplus of food and water, unusually close proximity between conspecifics, environmental simplicity, and physical inactivity (Nottebohm, 2002). Further, naturally-occurring environmental stimuli such as predators, competition, and seasonal cues are usually absent. In turn, such impoverished and unnatural housing conditions may negatively impact the brain, in particular the DG (Gould and Gross, 2002). Indeed animals living in an enriched environment, either in the wild or in a relatively complex laboratory environment, display higher rates of hippocampal neurogenesis than laboratory animals living in standard laboratory housing (Amrein et al., 2004a; Barnea and Nottebohm, 1994; Epp et al., 2009; Kempermann et al., 1997; Kempermann et al., 1998; Kempermann et al., 2002; Nilsson et al., 1999; Nottebohm, 2002; Tarr et al., 2009).
In addition to the unnatural housing conditions, spatial learning/memory in laboratory animals is often assessed using tests such as the Morris water maze and Barnes maze (Paul et al., 2009). Although these tests measure spatial learning reliably, they have various limitations. One of the limitations is that these tests require extensive training. For example, rodents must be trained in the Morris water maze for 5-10 days with 4-6 trials per day (Morgan, 2009; Paul et al., 2009). Furthermore, these tests are rather stressful due to the requirement of negative reinforcers (such as water immersion, intense light, or loud noise) (Van Sluyters and Obernier, 2004) as well as food or water deprivation (Hyde et al., 1998). As the exposure to stressful stimuli reduces hippocampal neurogenesis (for review see Mirescu and Gould, 2006), such stressors might act as a confound in assessing the link between spatial learning and hippocampal neurogenesis.
For the above-mentioned reasons, various researchers have focused on establishing field animal models to assess the functional implications of hippocampal adult neurogenesis in spatial learning (Boonstra et al., 2001; Nottebohm, 2002). Such field models focus on studying animals in their natural habitat and rely on naturally displayed behaviors. Indeed, this approach eliminates various confounds associated with laboratory studies, namely the unnatural, controlled laboratory environment and the use of stress-inducing spatial tasks that often require extensive training. To this date, field studies have mainly focused on assessing hippocampal adult neurogenesis in animal species that display food hoarding behaviors, including bird species such as the black-capped chickadee (Parus atricapillus) (Barnea and Nottebohm, 1994; Barnea and Nottebohm, 1996; Chancellor et al., 2011; Hoshooley and Sherry, 2004; Hoshooley et al., 2006; Hoshooley and Sherry, 2007) and mountain chickadee (Poecile gambeli) (Freas et al., 2012; LaDage et al., 2010), as well as rodent species such as the eastern grey squirrel (Sciurus carolinensis) (Barker et al., 2005; Lavenex et al., 2000a; Lavenex et al., 2000b), red squirrel (Tamiasciurus hudsonicus) (Johnson et al., 2010), Siberian chipmunk (Tamias sibiricus) (Pan et al., 2013), and yellow-pine chipmunk (Tamias amoenus) (Barker et al., 2005). It is of importance to note that both avian and mammalian hippocampal formations originate from the reptilian dorsomedial cortex. Through evolution, the mammalian hippocampus developed structures such as the dentate gyrus and Ammon's horn—structures that seem to be lacking in the avian hippocampus. Instead the avian hippocampus has a V-shaped layer of densely packed neurons and a dorsomedial zone surrounding the V shape that can be seen as the mammalian equivalent to the hippocampus proper and dentate gyrus, respectively (reviewed in Lee, et al. 1998). Furthermore, both avian and mammalian hippocampi share cell types such as pyramidal and granule cells and these cells display similarities in connectivity to brain regions such as the septum and brain stem (but note the direct projections to the hypothalamus is absent in birds) It should be noted that although there are differences in the avian and mammalian hippocampus, there is homology in terms of anatomy, physiology (e.g., existence of adult neurogenesis), neurochemistry (e.g., similarity in occurrence of neuropeptides and neurotransmitters), function (specifically the involvement in spatial learning and memory), and postnatal plasticity (e.g., display of adult neurogenesis) (Colombo and Broadbent, 2000; Lee et al., 1998; Shiflett et al., 2004). In regards to differences of avian and mammalian adult hippocampal neurogenesis specifically, one main difference is that avian neurogenesis appears to be seasonally regulated (Barnea and Nottebohm, 1994; Smulders et al., 2000), whereas neurogenesis in most mammals lacks such seasonal fluctuations (but see Galea and McEwen, 1999; Lavenex et al., 2000a).
Food hoarding (caching) is a behavior that is characterized by storing food in locations concealed from other conspecifics and members of other species. Food is either larder or scatter hoarded. Larder hoarding refers to the food storage in one or few large stores (larders), whereas scatter hoarding refers to the formation of a large number of food storage sites (small hoards) (Vander Wall, 1990). Creating larder or small hoard food caches is an important adaptation to seasonal and unpredictable changes in food availability (Pravosudov and Clayton, 2002; Pravosudov and Smulders, 2010; Vander Wall, 1990). Relocating such caches in times of low food availability seems to be a hippocampus-dependent learning task as indicated by lesion studies. For example, lesions of the hippocampus disrupt the ability of Eurasian nutcrackers (Nucifraga caryocatactes), black-capped chickadees (Poecile atricapillus), and dark-eyed juncos (Junco hyemalis) to relocate their caches (Hampton and Shettleworth, 1996; Krushinskaya, 1966; Sherry and Vaccarino, 1989). Similarly, cytotoxic lesions of the hippocampus cause deficits in food hoarding in mice (Deacon et al., 2002b). Furthermore, researchers have reported a link between hoarding behavior and hippocampal size (for review see Clayton and Lee, 1998; Krebs et al., 1989; Krebs et al., 1996; Lucas et al., 2004; Sherry and Hoshooley, 2010). Comparing the hippocampal sizes of 23 bird species in 13 passerine families and subfamilies that differ in their food-storing behavior shows that food-storing birds have larger hippocampi than non-food-storing species (Sherry et al., 1989). In addition, food-storing bird species, which rely more extensively on stored food—such as the Clark's nutcracker (Nucifraga columbiana), have larger hippocampi than food-storing species that rely less on stored food—such as the gray-breasted jay (Aphelocoma ultramarina), the scrub jay (Aphelocoma coerulescens), and the pinyon jay (Gymnorhinus cyanocephalus) (Basil et al., 1996). In addition, bird species that store more food, store food for longer, or both have larger hippocampi than species that store less food or store food for a lesser duration (Hampton et al., 1995; Healy and Krebs, 1992; Healy and Krebs, 1996). Similarly to the findings in birds, the DG size of the Merriam's kangaroo rat (Dipodomys merriami), which relies extensively on stored food, is larger than the DG of the bannertail kangaroo rat (Dipodomys spectabilis), which relies less on stored food (Jacobs and Spencer, 1994). Due to the link between the DG and hoarding behavior, it is not surprising that researchers have started to investigate the potential involvement of adult-generated DG neurons in hoarding behavior.
The first study to investigate the potential link of hippocampal adult neurogenesis and spatial learning in natural populations was conducted in bird species that display food hoarding behavior. Specifically, black-capped chickadees (Parus atricapillus) showed a peak of adult-generated hippocampal neurons in fall—the season, in which hoarding behavior is highest (Barnea and Nottebohm, 1994). Such seasonal recruitment suggests that the adult-generated hippocampal neurons may play a role in the acquisition of new spatial memories. Interestingly, there were no seasonal differences in cell proliferation—suggesting that the seasonal difference in neuronal recruitment observed by Barnea and Nottebohm (1994) is likely due to an enhancement in survival (Hoshooley and Sherry, 2004). Comparing neuronal recruitment in black-capped chickadees (Parus atricapillus; food storing bird species) to the neuronal recruitment in the house sparrow (Passer domesticus; non-food storing bird species) allows the assessment about whether the increased neuronal recruitment in fall plays a role in food storing behavior. Indeed, black-capped chickadees (Parus atricapillus) show significantly greater hippocampal neuronal recruitment than house sparrows (Passer domesticus)—suggesting that the degree of hippocampal neurogenesis may be associated with spatial memory requirements of food-caching behavior (Hoshooley and Sherry, 2007). It should be noted that the authors did not observe seasonally fluctuating recruitment of adult-generated hippocampal neurons in chickadees (Parus atricapillus) as seen in Barnea and Nottebohm's study (1994). This discrepancy between studies may be due to differences in methodology (e.g., examination of wild vs. captive chickadees).
Additional support for the role of adult-generated hippocampal neurons in spatial memory comes from a study assessing a possible link between environmental harshness and adult neurogenesis (Chancellor et al., 2011). Environmental harshness is believed to cause a greater reliance on cached food, which, in turn might influence the rate of hippocampal neurogenesis (Pravosudov et al., 2015). The comparison of the number of adult-generated neurons in black-capped chickadees (Parus atricapillus) along an environmental gradient revealed that chickadees from harsh environments with more reliance on cached food have higher rates of hippocampal neurogenesis than birds from milder environments with less reliance on cached food (Chancellor et al., 2011). Therefore, this study provides evidence of the role of adult-generated hippocampal neurons in spatial memory. Further support of this link comes from an experiment conducted by LaDage et al. (2010)—assessing the effect of restricting food caching behavior in captivity on adult hippocampal neurogenesis in mountain chickadees (Poecile gambeli). The results showed that food caching behavior influenced adult hippocampal neurogenesis in captivity. Specifically, the mountain chickadees (Poecile gambeli), which were allowed to display food caching behavior, had significantly more new hippocampal neurons than the ones, which were denied to display food caching behavior.
Besides food hoarding avian species, food caching mammalian species, in particular rodents, have also been studied to assess the possible functional link of hippocampal adult neurogenesis and spatial memory. Contrary to the seasonal fluctuations observed in black-capped chickadees (Parus atricapillus) (Barnea and Nottebohm, 1994), the adult scatter-hoarding eastern gray squirrel (Sciurus carolinensis) does not display seasonal changes in cell proliferation (Lavenex et al., 2000a). Due to the absence of seasonal fluctuations of cell proliferation, Barker et al. (2005) assessed the potential link between adult-generated neurons and spatial memory by comparing levels of adult neurogenesis between scatter and larder hoarders. It was predicted that scatter hoarders, which rely more heavily on spatial memory than larder hoarders, should have a higher rate of hippocampal adult neurogenesis. Indeed, the eastern gray squirrel (Sciurus carolinensis), a scatter hoarder, displays a higher number of newly-generated cells, but not a higher number of young neurons than does the yellow pine chipmunk (Tamias amoenus), a larder hoarder. Interestingly, such differences are not observed between scatter hoarding (in eastern North America) and larder hoarding (in western North America) subpopulations of red squirrels (Tamiasciurus hudsonicus) (Johnson et al., 2010). Despite these seemingly contradictory findings, which may be in part due to methodological shortcomings (such as not assessing cell turnover), these studies provide evidence for significant differences of adult neurogenesis across species with different food storing behaviors.
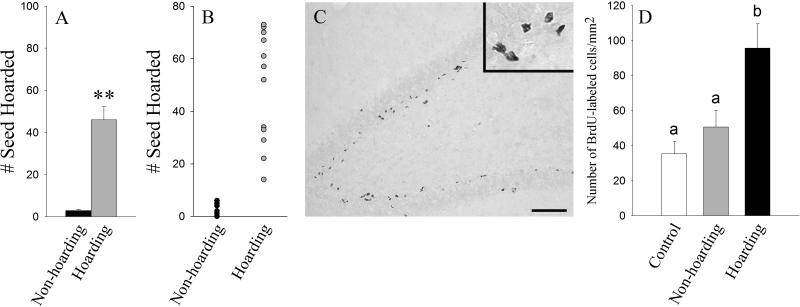
In addition to assessing the link between hippocampal adult neurogenesis and spatial memory using natural populations in the field, this link has also been investigated using natural populations in semi-natural enclosures. For example, the display of hoarding behavior, which shows large individual variations in these semi-natural enclosures in Siberian chipmunk (Tamias sibiricus), is associated with enhanced hippocampal cell proliferation (Pan et al., 2013) (Figure 4). It is of interest to note that only males, but not females, display this association between food hoarding and cell proliferation. Such sex differences may be due to less use of spatial memory via less cached seed retrieval by females or sex differences in spatial use patterns. However, there are no significant correlations between the level of hoarding behavior and cell survival. It is possible that the seed distribution and hoarding paradigm in this experiment was not vigorous enough to increase hippocampal cell survival. Although research has shown that food-caching animals may represent an excellent model to investigate the relationship between adult hippocampal neurogenesis and spatial memory, it should be noted that the studies mentioned above only show a correlational link between hoarding and hippocampal adult neurogenesis. Future research needs to be conducted to further elucidate the complex functional link between adult-generated hippocampal neurons and spatial memory as well as study the cause and effect relationship between hoarding behavior and hippocampal adult neurogenesis.
Figure 4.
Seed hoarding and newly-generated cells in the dentate gyrus (DG) of the hippocampus in Siberian chipmunks in semi-natural enclosures. Hoarding animals hoarded significantly more seeds than their non-hoarding counterparts (A) and showed large individual differences in seed-hoarding behavior (B). (C) Photomicrographs illustrating BrdU-labeled cells in the DG of hoarding animals. Scale bar=50 μm. The insert in Panel C shows BrdU-labeled cells in the DG with scale bar=10μm. (D) Hoarding animals had a higher density of BrdU-labeled cells in the DG than the control and non-hoarding animals (p < 0.01). Alphabetic letters indicate the results of the post-hoc test. Error bars represent SEM. (Adapted from Pan et al., 2013).
4. Conclusion
The well-documented evidence reviewed here suggests that adult neurogenesis in the hippocampus can be influenced by both environmental and endogenous factors. In addition, data also indicate a possible link between hippocampal adult neurogenesis and spatial learning and memory. Various environmental factors related to spatial learning and memory have been shown to enhance adult neurogenesis in the DG, whereas the reduction of hippocampal neurogenesis causes deficits in spatial learning and memory tasks. Although data from laboratory studies have contributed significantly to our understanding of adult neurogenesis and its functions, it will also be important to study adult neurogenesis and its functions in free-living animals. Studies utilizing natural populations displaying natural spatial learning and memory behaviors (such as hoarding) will provide a better understanding about whether adult-generated DG neurons promote survival and reproductive fitness in natural populations (Barnea, 2010; Boonstra et al., 2001).
Acknowledgments
We thank Charles Badland for his assistance with the figures and Hans Jorgensen for his critical reading of the manuscript. This research was supported by NIH grant NIMH-R01-089852 to ZXW and China National Natural Science Foundation grant (#31272321) to YLP.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- BrdU
5-bromo-3’-deoxyuridine
- CldU
chlorodeoxyuridine
- CNTF
ciliary neurotrophic factor
- DG
dentate gyrus of the hippocampus
- GABA
gamma amino butyric acid
- IdU
iododeoxyuridine
- IGF-1
insulin-like growth factor-1
- MAM
methylazoxymethanol acetate
- MCM-2
minichromosome marker-2
- MOB
main olfactory bulb
- PCNA
proliferating cell nuclear antigen
- SVZ
subventricular zone
- TMZ
temozolomide
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of interest: The authors have no financial disclosures and have no potential conflicts of interest.
References
- Åberg MAI, Åberg ND, Hedbäcker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrous DN, Adriani W, Montaron M-F, Aurousseau C, Rougon G, Le Moal M, Piazza PV. Nicotine self-administration impairs hippocampal plasticity. J Neurosci. 2002;22:3656–3662. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Akbari EM, Chatterjee D, Levy F, Fleming AS. Experience-dependent cell survival in the maternal rat brain. Behav Neurosci. 2007;121:1001–1011. doi: 10.1037/0735-7044.121.5.1001. [DOI] [PubMed] [Google Scholar]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- Altman J. Proliferation and migration of undifferentiated precursor cells in the rat during postnatal gliogenesis. Exp Neurol. 1966;16:263–278. doi: 10.1016/0014-4886(66)90063-x. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214:1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Ambrogini P, Cuppini R, Cuppini C, Ciaroni S, Cecchini T, Ferri P, Sartini S, Del Grande P. Spatial learning affects immature granule cell survival in adult rat dentate gyrus. Neurosci Lett. 2000;286:21–24. doi: 10.1016/s0304-3940(00)01074-0. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Barbanti I, Cuppini R. Persistently high corticosterone levels but not normal circadian fluctuations of the hormone affect cell proliferation in the adult rat dentate gyrus. Neuroendocrinology. 2002;76:366–372. doi: 10.1159/000067581. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Ciaroni S, Cuppini R. Learning may reduce neurogenesis in adult rat dentate gyrus. Neurosci Lett. 2004;359:13–16. doi: 10.1016/j.neulet.2003.12.123. [DOI] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Lipp HP. Granule cell number, cell death and cell proliferation in the dentate gyrus of wild-living rodents. Eur J Neurosci. 2004a;20:3342–3350. doi: 10.1111/j.1460-9568.2004.03795.x. [DOI] [PubMed] [Google Scholar]
- Amrein I, Slomianka L, Poletaeva II, Bologova NV, Lipp HP. Marked species and age-dependent differences in cell proliferation and neurogenesis in the hippocampus of wild-living rodents. Hippocampus. 2004b;14:1000–1010. doi: 10.1002/hipo.20018. [DOI] [PubMed] [Google Scholar]
- Amrein I, Dechmann DKN, Winter Y, Lipp H-P. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera). PloS One. 2007;2:e455. doi: 10.1371/journal.pone.0000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31:15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Wojtowicz JM, Boonstra R. Where's my dinner? Adult neurogenesis in free-living food-storing rodents. Genes Brain Behav. 2005;4:89–98. doi: 10.1111/j.1601-183X.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci U S A. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Recruitment and replacement of hippocampal neurons in young and adult chickadees: An addition to the theory of hippocampal learning. Proc Natl Acad Sci U S A. 1996;93:714–718. doi: 10.1073/pnas.93.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A. Wild neurogenesis. Brain, behavior and evolution. 2010;75:86–87. doi: 10.1159/000306483. [DOI] [PubMed] [Google Scholar]
- Bartkowska K, Turlejski K, Grabiec M, Ghazaryan A, Yavruoyan E, Djavadian RL. Adult neurogenesis in the hedgehog (Erinaceus concolor) and mole (Talpa europaea). Brain Behav Evol. 2009;76:128–143. doi: 10.1159/000320944. [DOI] [PubMed] [Google Scholar]
- Basil JA, Kamil AC, Balda R, Fite KV. Differences in hippocampal volume among food storing corvids. Brain Behav Evol. 1996;47:156–164. doi: 10.1159/000113235. [DOI] [PubMed] [Google Scholar]
- Bedard A, Cossette M, Levesque M, Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett. 2002;328:213–216. doi: 10.1016/s0304-3940(02)00530-x. [DOI] [PubMed] [Google Scholar]
- Bedard A, Gravel C, Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res. 2006;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- Ben Abdallah NMB, Filipkowski RK, Pruschy M, Jaholkowski P, Winkler J, Kaczmarek L, Lipp H-P. Impaired long-term memory retention: Common denominator for acutely or genetically reduced hippocampal neurogenesis in adult mice. Behav Brain Res. 2013;252:275–286. doi: 10.1016/j.bbr.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu R, Sharp FR. NMDA and AMPA/kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cereb Blood Flow Metab. 2000;20:1669–1680. doi: 10.1097/00004647-200012000-00006. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Bucholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Druid H, Eriksson PS, Frisen J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra R, Galea L, Matthews S, Wojtowicz JM. Adult neurogenesis in natural populations. Can J Physiol Pharmacol. 2001;79:297–302. [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: Facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Franceschini I, Keller M, Levy F. Evidence for cell proliferation in the sheep brain and its down-regulation by parturition and interactions with the young. Horm Behav. 2010;58:737–746. doi: 10.1016/j.yhbeh.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Byrd CA, Brunjes PC. Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience. 2001;105:793–801. doi: 10.1016/s0306-4522(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998;82:349–54. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Dayer AG. New interneurons in the adult neocortex: Small, sparse, but significant? Biol Psychiatry. 2008;63:650–655. doi: 10.1016/j.biopsych.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carlen M, Cassidy RM, Brismar H, Smith GA, Enquist LW, Frisen J. Functional integration of adult-born neurons. Curr Biol. 2002;12:606–608. doi: 10.1016/s0960-9822(02)00771-6. [DOI] [PubMed] [Google Scholar]
- Chancellor LV, Roth TC, LaDage LD, Pravosudov VV. The effect of environmental harshness on neurogenesis: A large-scale comparison. Dev Neurobiol. 2011;71:246–252. doi: 10.1002/dneu.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Lee DW. Memory and the hippocampus in food-storing birds. In: Balda RP, Pepperberg IM, Kamil AC, editors. Animal Cognition in Nature. Academic Press; New York: 1998. pp. 99–118. [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Broadbent N. Is the avian hippocampus a functional homologue of the mammalian hippocampus? Neurosci Biobehav Rev. 2000;24:465–484. doi: 10.1016/s0149-7634(00)00016-6. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, Toschi N, Fuchs E, Keck ME. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52:1057–1065. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: Hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and learning: Acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiol Learn Mem. 2007;88:143–148. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RMJ, Bannerman DM, Kirby BP, Croucher A, Rawlins JNP. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002a;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Croucher A, Rawlins JNP. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav Brain Res. 2002b;132:203–213. doi: 10.1016/s0166-4328(01)00401-6. [DOI] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döbrössy MD, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Dominguez-Escriba L, Hernandez-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, Garcia-Verdugo JM, Canales JJ. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci. 2006;24:586–594. doi: 10.1111/j.1460-9568.2006.04924.x. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Stone JC, Monfils MH, Tomanek B, Brooks WM, Sutherland RJ. The aging hippocampus: A multi-level analysis in the rat. Neuroscience. 2006;139:1173–1185. doi: 10.1016/j.neuroscience.2006.01.040. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- Dupret D, Montaron MF, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Methylazoxymethanol acetate does not fully block cell genesis in the young and aged dentate gyrus. Eur J Neurosci. 2005;22:778–783. doi: 10.1111/j.1460-9568.2005.04262.x. [DOI] [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During MJ, Cao L. VEGF, a mediator of the effect of experience on hippocampal neurogenesis. Curr Alzheimer Res. 2006;3:29–33. doi: 10.2174/156720506775697133. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neurol. 2003;183:298–310. doi: 10.1016/s0014-4886(03)00129-8. [DOI] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LA. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Epp JR, Barker JM, Galea LAM. Running wild: Neurogenesis in the hippocampus across the lifespan in wild and laboratory-bred Norway rats. Hippocampus. 2009;19:1040–1049. doi: 10.1002/hipo.20546. [DOI] [PubMed] [Google Scholar]
- Epp JR, Haack AK, Galea LA. Activation and survival of immature neurons in the dentate gyrus with spatial memory is dependent on time of exposure to spatial learning and age of cells at examination. Neurobiol Learn Mem. 2011;95:316–325. doi: 10.1016/j.nlm.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Falconer EM, Galea LA. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975:22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague–Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fernández AS, Rosillo JC, Casanova G, Olivera-Bravo S. Proliferation zones in the brain of adult fish Austrolebias (Cyprinodontiform: Rivulidae): A comparative study. Neuroscience. 2011;189:12–24. doi: 10.1016/j.neuroscience.2011.05.063. [DOI] [PubMed] [Google Scholar]
- Font E, Desfilis E, Pérez-Cañellas MM, García-Verdugo JM. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav Evol. 2001;58:276–295. doi: 10.1159/000057570. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Wang Z. Estrogen and adult neurogenesis in the amygdala and hypothalamus. Brain Res Rev. 2008;57:342–351. doi: 10.1016/j.brainresrev.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freas CA, LaDage LD, Roth TC, Pravosudov VV. Elevation-related differences in memory and the hippocampus in mountain chickadees, Poecile gambeli. Anim Behav. 2012;84:121–127. [Google Scholar]
- Furuta M, Bridges RS. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res Bull. 2009;80:408–13. doi: 10.1016/j.brainresbull.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galand P, Degraef C. Cyclin/PCNA immunostaining as an alternative to tritiated thymidine pulse labelling for marking S phase cells in paraffin sections from animal and human tissues. Cell Tissue Kinet. 1989;22:383–392. doi: 10.1111/j.1365-2184.1989.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Galani R, Weiss I, Cassel JC, Kelche C. Spatial memory, habituation, and reactions to spatial and nonspatial changes in rats with selective lesions of the hippocampus, the entorhinal cortex or the subiculum. Behav Brain Res. 1998;96:1–12. doi: 10.1016/s0166-4328(97)00197-6. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Amrein I, Kuptsov PA, Poletaeva II, Zinn P, Rau A, Vyssotski A, Lipp H-P. A comparison of wild-caught wood mice and bank voles in the Intellicage: assessing exploration, daily activity patterns and place learning paradigms. Behav Brain Res. 2005;157:211–217. doi: 10.1016/j.bbr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PloS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PloS One. 2013;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatome CW, Mwangi DK, Lipp H-P, Amrein I. Hippocampal neurogenesis and cortical cellular plasticity in Wahlberg's epauletted fruit bat: A qualitative and quantitative study. Brain Behav Evol. 2009;76:116–127. doi: 10.1159/000320210. [DOI] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming G.-l., Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo PM. A niche for adult neurogenesis in social behavior. Behav Brain Res. 2009;200:315–322. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Glasper ER, Kozorovitskiy Y, Pavlic A, Gould E. Paternal experience suppresses adult neurogenesis without altering hippocampal function in Peromyscus californicus. J Comp Neurol. 2011;519:2271–2281. doi: 10.1002/cne.22628. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology. 1999;21:46S–51S. doi: 10.1016/S0893-133X(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999a;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci U S A. 1999b;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999c;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: A possible role in learning. Trends Cogn Sci. 1999d;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci U S A. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Gross CG. Neurogenesis in adult mammals: Some progress and problems. J Neurosci. 2002;22:619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gratzner HG. Monoclonal antibody to 5-bromo-and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Gross CG. Neurogenesis in the adult brain: Death of a dogma. Nat Rev Neurosci. 2000;1:67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- Grote HE, Hannan AJ. Regulators of adult neurogenesis in the healthy and diseased brain. Clin Exp Pharmacol Physiol. 2007;34:533–545. doi: 10.1111/j.1440-1681.2007.04610.x. [DOI] [PubMed] [Google Scholar]
- Gueneau G, Privat A, Drouet J. Subgranular zone of the dentate gyrus of young rabbits as a secondary matrix. Dev Neurosci. 1982;5:345–358. doi: 10.1159/000112694. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Sherry DF, Shettleworth SJ, Khurgel M, Ivy G. Hippocampal volume and food-storing behavior are related in parids. Brain Behav Evol. 1995;45:54–61. doi: 10.1159/000113385. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Shettleworth SJ. Hippocampal lesions impair memory for location but not color in passerine birds. Behav Neurosci. 1996;110:831–835. doi: 10.1037//0735-7044.110.4.831. [DOI] [PubMed] [Google Scholar]
- Harman A, Meyer P, Ahmat A. Neurogenesis in the hippocampus of an adult marsupial. Brain Behav Evol. 2002;62:1–12. doi: 10.1159/000071955. [DOI] [PubMed] [Google Scholar]
- Hawken PA, Jorre TJ, Rodger J, Esmaili T, Blache D, Martin GB. Rapid induction of cell proliferation in the adult female ungulate brain (Ovis aries) associated with activation of the reproductive axis by exposure to unfamiliar males. Biol Reprod. 2009;80:1146–1151. doi: 10.1095/biolreprod.108.075341. [DOI] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. European Journal of Neuroscience. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Healy SD, Krebs JR. Food storing and the hippocampus in Corvids amount and volume are correlated. Proc R Soc Lond B Biol Sci. 1992;248:241–245. [Google Scholar]
- Healy SD, Krebs JR. Food storing and the hippocampus in Paridae. Brain Behav Evol. 1996;47:195–199. doi: 10.1159/000113239. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joëls M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–144. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Dominguez-Escriba L, Barcia JA, Rosel JF, Romero FJ, Garcia-Verdugo JM, Canales JJ. Binge administration of 3, 4-methylenedioxymethamphetamine (“ecstasy”) impairs the survival of neural precursors in adult rat dentate gyrus. Neuropharmacology. 2006;51:967–973. doi: 10.1016/j.neuropharm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yagüe AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, García-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci U S A. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshooley JS, Sherry DF. Neuron production, neuron number, and structure size are seasonally stable in the hippocampus of the food-storing black-capped chickadee (Poecile atricapillus). Behav Neurosci. 2004;118:345–355. doi: 10.1037/0735-7044.118.2.345. [DOI] [PubMed] [Google Scholar]
- Hoshooley JS, Phillmore LS, Sherry DF, Macdougall-Shackleton SA. Annual cycle of the black-capped chickadee: seasonality of food-storing and the hippocampus. Brain Behav Evol. 2006;69:161–168. doi: 10.1159/000096984. [DOI] [PubMed] [Google Scholar]
- Hoshooley JS, Sherry DF. Greater hippocampal neuronal recruitment in food-storing than in non-food-storing birds. Dev Neurobiol. 2007;67:406–414. doi: 10.1002/dneu.20316. [DOI] [PubMed] [Google Scholar]
- Huang L, DeVries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: Learning in three inbred strains of mice. Brain Research. 1998;785:236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Spencer WD. Natural space-use patterns and hippocampal size in kangaroo rats. Brain Behav Evol. 1994;44:125–132. doi: 10.1159/000113584. [DOI] [PubMed] [Google Scholar]
- Jang M-H, Song H, Ming G-L. Regulation of adult neurogenesis by neurotransmitters. In: Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Cold Spring Harbor Monograph Archive; Cold Spring Harbor, NY: 2008. pp. 397–423. [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Boonstra R, Wojtowicz JM. Hippocampal neurogenesis in food-storing red squirrels: the impact of age and spatial behavior. Genes Brain Behav. 2010;9:583–591. doi: 10.1111/j.1601-183X.2010.00589.x. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: Electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4:1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Meth. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. New nerve cells for the adult brain. Sci Am-American Edition. 1999;280:48–67. doi: 10.1038/scientificamerican0599-48. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: Sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu D, Mikami A, Miyamoto Y, Hisatsune T. Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J Neurosci. 2003;23:937–942. doi: 10.1523/JNEUROSCI.23-03-00937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Krebs JR, Sherry DF, Healy SD, Perry VH, Vaccarino AL. Hippocampal specialization of food-storing birds. Proc Natl Acad Sci U S A. 1989;86:1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs JR, Clayton NS, Healy SD, Cristol DA, Patel SN, Jolliffe AR. The ecology of the avian brain: food-storing memory and the hippocampus. Ibis. 1996;138:34–46. [Google Scholar]
- Krushinskaya N. Some complex forms of feeding behaviour of nutcracker Nucifraga caryocatactes, after removal of old cortex. Zh. Evol. Biokhim. Fiziol. 1966;11:563–568. [Google Scholar]
- Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O'brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- LaDage LD, Roth TC, 2nd, Fox RA, Pravosudov VV. Ecologically relevant spatial memory use modulates hippocampal neurogenesis. Proc Biol Sci. 2010;277:1071–1079. doi: 10.1098/rspb.2009.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Steele MA, Jacobs LF. The seasonal pattern of cell proliferation and neuron number in the dentate gyrus of wild adult eastern grey squirrels. Eur J Neurosci. 2000a;12:643–648. doi: 10.1046/j.1460-9568.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Steele MA, Jacobs LF. Sex differences, but no seasonal variations in the hippocampus of food-caching squirrels: A stereological study. J Comp Neurol. 2000b;425:152–166. [PubMed] [Google Scholar]
- Lee DW, Miyasato LE, Clayton NS. Neurobiological bases of spatial learning in the natural environment: Neurogenesis and growth in the avian and mammalian hippocampus. Neuroreport. 1998;9:R15–R27. [PubMed] [Google Scholar]
- Lemaire V, Aurousseau C, Le Moal M, Abrous DN. Behavioural trait of reactivity to novelty is related to hippocampal neurogenesis. Eur J Neurosci. 1999;11:4006–4014. doi: 10.1046/j.1460-9568.1999.00833.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. J Neurosci. 2006;26:13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J Comp Neurol. 2009;517:123–133. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–140. C1–3. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming G.-l., Gage FH. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Ann Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Liu Y, Jia X, Wang Z. Social isolation impairs adult neurogenesis in the limbic system and alters behaviors in female prairie voles. Horm Behav. 2012;62:357–366. doi: 10.1016/j.yhbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. The social environment and neurogenesis in the adult mammalian brain. Front Hum Neurosci. 2012;6:1–19. doi: 10.3389/fnhum.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zuo M, Alvarez-Buylla A, Cheng MF. Neurogenesis in juvenile and adult ring doves. J Comp Neurol. 1997;379:300–312. [PubMed] [Google Scholar]
- Lledo P-M, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Saghatelyan A. Integrating new neurons into the adult olfactory bulb: Joining the network, life-death decisions, and the effects of sensory experience. Trends Neurosci. 2005;28:248–254. doi: 10.1016/j.tins.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Brodin A, de Kort SR, Clayton NS. Does hippocampal size correlate with the degree of caching specialization? Proc Biol Sci. 2004;271:2423–2429. doi: 10.1098/rspb.2004.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PEG, Bolwig TG, Wörtwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: Possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: Reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: A comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 2010;32:2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sundlass K, Parker KJ, Schatzberg AF, Lyons DM. Social stress-related behavior affects hippocampal cell proliferation in mice. Physiol Behav. 2006;89:123–127. doi: 10.1016/j.physbeh.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Mizumatsu S, Monje ML, Morhardt DR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- Morgan D. Water maze tasks in mice: Special reference to Alzheimer's transgenic mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. CRC Press; Boca Raton, FL: 2009. pp. 281–292. [Google Scholar]
- Moser M-B, Moser EI, Forrest E, Andersen P, Morris RGM. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Chakrapani BPS, Schwegler H, Hofmann H-D, Kirsch M. Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells. 2009;27:431–441. doi: 10.1634/stemcells.2008-0234. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Why are some neurons replaced in adult brain? J Neurosci. 2002;22:624–628. doi: 10.1523/JNEUROSCI.22-03-00624.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda H, Tatsumi K, Makinodan M, Yamauchi T, Kishimoto T, Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J Neurosci Res. 2009;87:3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Shore LE, Brewer MD, Cameron HA. A natural form of learning can increase and decrease the survival of new neurons in the dentate gyrus. Hippocampus. 2005;15:750–762. doi: 10.1002/hipo.20097. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci. 2007;26:3395–3401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: A possible regulatory role for estradiol. Neuroscience. 2001;102:369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LAM. Reproductive status influences the survival of new cells in the dentate gyrus of adult male meadow voles. Neurosci Lett. 2003;346:25–28. doi: 10.1016/s0304-3940(03)00546-9. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- Paizanis E, Kelai S, Renoir T, Hamon M, Lanfumey L. Life-long hippocampal neurogenesis: Environmental, pharmacological and neurochemical modulations. Neurochem Res. 2007;32:1762–1771. doi: 10.1007/s11064-007-9330-0. [DOI] [PubMed] [Google Scholar]
- Pan Y, Li M, Yi X, Zhao Q, Lieberwirth C, Wang Z, Zhang Z. Scatter hoarding and hippocampal cell proliferation in Siberian chipmunks. Neuroscience. 2013;255:76–85. doi: 10.1016/j.neuroscience.2013.09.065. [DOI] [PubMed] [Google Scholar]
- Paul C-M, Magda G, Abel S. Spatial memory: Theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res. 2009;203:151–164. doi: 10.1016/j.bbr.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Pérez-Cañellas MM, García-Verdugo JM. Adult neurogenesis in the telencephalon of a lizard: A [3 H] thymidine autoradiographic and bromodeoxyuridine immunocytochemical study. Dev Brain Res. 1996;93:49–61. doi: 10.1016/0165-3806(96)00014-4. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Pravosudov V, Clayton NS. A test of the adaptive specialization hypothesis: population differences in caching, memory, and the hippocampus in black-capped chickadees (Poecile atricapilla). Behav Neurosci. 2002;116:515–522. [PubMed] [Google Scholar]
- Pravosudov VV, Smulders TV. Integrating ecology, psychology and neurobiology within a food-hoarding paradigm. Philos Trans R Soc B Biol Sci. 2010;365:859–867. doi: 10.1098/rstb.2009.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov VV, Roth TC, II, LaDage LD, Freas CA. Environmental Influences on Spatial Memory and the Hippocampus in Food-Caching Chickadees. Comp Cogn Behav Rev. 2015;10:25–43. doi: 10.1093/icb/icv029. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Res. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Degeneration and regeneration of the nervous system. Oxford University Press; London: 1928. [Google Scholar]
- Raucci F, Di Fiore MM, Pinelli C, D'Aniello B, Luongo L, Polese G, Rastogi RK. Proliferative activity in the frog brain: A PCNA-immunohistochemistry analysis. J Chem Neuroanat. 2006;32:127–142. doi: 10.1016/j.jchemneu.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Riedel G, Micheau J, Lam AGM, Roloff E.v.L., Martin SJ, Bridge H, De Hoz L, Poeschel B, McCulloch J, Morris RGM. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Montaron MF, Petry KG, Aurousseau C, Marinelli M, Premier S, Rougon G, Moal ML, Abrous DN. Complex regulation of the expression of the polysialylated form of the neuronal cell adhesion molecule by glucocorticoids in the rat hippocampus. Eur J Neurosci. 1998;10:2994–3006. doi: 10.1046/j.1460-9568.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav Brain Res. 2008;187:9–16. doi: 10.1016/j.bbr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Gage FH. A hypothesis about the role of adult neurogenesis in hippocampal function. Physiology. 2004;19:253–261. doi: 10.1152/physiol.00012.2004. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Vaccarino AL. Hippocampus and memory for food caches in black-capped chickadees. Behav Neurosci. 1989;103:308–318. [Google Scholar]
- Sherry DF, Vaccarino AL, Buckenham K, Herz RS. The hippocampal complex of food-storing birds. Brain Behav Evol. 1989;34:308–317. doi: 10.1159/000116516. [DOI] [PubMed] [Google Scholar]
- Sherry DF, Hoshooley JS. Seasonal hippocampal plasticity in food-storing birds. Philos Trans R Soc Lond B Biol Sci. 2010;365:933–943. doi: 10.1098/rstb.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Tomaszycki ML, Rankin AZ, DeVoogd TJ. Long-term memory for spatial locations in a food-storing bird (Poecile atricapilla) requires activation of NMDA receptors in the hippocampal formation during learning. Behav Neurosci. 2004;118:121–130. doi: 10.1037/0735-7044.118.1.121. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. From stem cells to grandmother cells: How neurogenesis relates to learning and memory. Cell Stem Cell. 2008;3:253–258. doi: 10.1016/j.stem.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Miale IL, Feder N. Cell proliferation and migration in the primitive ependymal zone; an autoradiographic study of histogenesis in the nervous system. Exp Neurol. 1959;1:322–333. doi: 10.1016/0014-4886(59)90024-x. [DOI] [PubMed] [Google Scholar]
- Simon M, Czeh B, Fuchs E. Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Res. 2005;1049:244–248. doi: 10.1016/j.brainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: Learning over time enhances memory and the survival of new neurons. Learn Mem. 2007;14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders TV, Shiflett MW, Sperling AJ, DeVoogd TJ. Seasonal changes in neuron numbers in the hippocampal formation of a food-hoarding bird: The black-capped chickadee. Dev Neurobiol. 2000;44:414–422. doi: 10.1002/1097-4695(20000915)44:4<414::aid-neu4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Cameron HA. Could adult hippocampal neurogenesis be relevant for human behavior? Behav Brain Res. 2012;227:384–390. doi: 10.1016/j.bbr.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Ibler E, Inglis W, Curtis MG. Testosterone and social isolation influence adult neurogenesis in the dentate gyrus of male rats. Neuroscience. 2011;195:180–190. doi: 10.1016/j.neuroscience.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber K, Tlsty TD, Happerfield L, Thomas GA, Romanov S, Bobrow L, Williams ED, Williams GH. DNA replication licensing and human cell proliferation. J Cell Sci. 2001;114:2027–2041. doi: 10.1242/jcs.114.11.2027. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Tarr BA, Rabinowitz JS, Imtiaz MA, DeVoogd TJ. Captivity reduces hippocampal volume but not survival of new cells in a food-storing bird. Dev Neurobiol. 2009;69:972–981. doi: 10.1002/dneu.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci U S A. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Vadodaria KC, Jha S. Neurotransmitter regulation of adult neurogenesis: putative therapeutic targets. CNS Neurol Disord Drug Targets. 2007;6:358–374. doi: 10.2174/187152707783220910. [DOI] [PubMed] [Google Scholar]
- Van Bokhoven P, Oomen CA, Hoogendijk WJ, Smit AB, Lucassen PJ, Spijker S. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci. 2011;33:1833–1840. doi: 10.1111/j.1460-9568.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Wallinga AE, Luiten PGM, Eggen BJL, Van der Zee EA. Morris water maze learning in two rat strains increases the expression of the polysialylated form of the neural cell adhesion molecule in the dentate gyrus but has no effect on hippocampal neurogenesis. Behav Neurosci. 2005;119:926–932. doi: 10.1037/0735-7044.119.4.926. [DOI] [PubMed] [Google Scholar]
- van der Hart MG, Czeh B, de Biurrun G, Michaelis T, Watanabe T, Natt O, Frahm J, Fuchs E. Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry. 2002;7:933–941. doi: 10.1038/sj.mp.4001130. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sluyters RC, Obernier JA. Guidelines for the care and use of mammals in neuroscience and behavioral research. Contemporary Topics in Laboratory Animal Science. 2004;43:48–52. [Google Scholar]
- Vander Wall SB. Food Hoarding in Animals. University of Chicago Press; Chicago, IL: 1990. [Google Scholar]
- Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Shankaranarayana Rao BS. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009;87:831–843. doi: 10.1002/jnr.21907. [DOI] [PubMed] [Google Scholar]
- Veenema AH, de Kloet ER, de Wilde MC, Roelofs AJ, Kawata M, Buwalda B, Neumann ID, Koolhaas JM, Lucassen PJ. Differential effects of stress on adult hippocampal cell proliferation in low and high aggressive mice. J Neuroendocrinol. 2007;19:489–498. doi: 10.1111/j.1365-2826.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- von Bohlen Und Halbach O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007;329:409–420. doi: 10.1007/s00441-007-0432-4. [DOI] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. Eur J Neurosci. 2008;27:3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM. Irradiation as an experimental tool in studies of adult neurogenesis. Hippocampus. 2006;16:261–266. doi: 10.1002/hipo.20158. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Wong EYH, Herbert J. Roles of mineralocorticoid and glucocorticoid receptors in the regulation of progenitor proliferation in the adult hippocampus. Eur J Neurosci. 2005;22:785–792. doi: 10.1111/j.1460-9568.2005.04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Arai H, Hori T, Shiga T. Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse. 2005;58:63–71. doi: 10.1002/syn.20182. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Takase LF, Kochman LJ, Fornal CA, Miczek KA, Jacobs BL. Repeated brief social defeat episodes in mice: Effects on cell proliferation in the dentate gyrus. Behav Brain Res. 2006;172:344–350. doi: 10.1016/j.bbr.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Zhang C-L, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]