Abstract
Today’s world population has an unprecedented risk of dying from the consequences of being overweight and obese. Chronic diseases such as cardiovascular disease, type 2 diabetes, and cancer are often accelerated because of excessive adiposity. Various biological mechanisms are implicated in the obesity-cancer link, particularly local and systemic inflammation as well as altered growth factor signaling pathways. In order to combat obesity-induced inflammation and the resulting increases in cancer risk and progression, the identification of safe and effective mechanism-based interventions is imperative. Notably, long chain omega-3 polyunsaturated fatty acids (PUFAs) modulate the secretion of pro-inflammatory cytokines, prostaglandins and other inflammatory mediators, restore insulin sensitivity, and can prevent or delay tumorigenesis. Delineating the precise mechanisms by which omega-3 PUFAs suppress obesity-induced inflammation will help identify promising key mechanistic targets and intervention strategies to break the obesity-cancer link.
Keywords: Obesity, Cancer, Omega-3 PUFAs
1. Introduction
The prevalence of obesity has risen dramatically within the past 30 years. Today nearly 40% of adults in the United States (U.S.) are considered obese [1]. The World Health Organization (WHO) estimates that 1.9 billion of the world’s population is overweight, including nearly 700 million obese, and these numbers continue to increase [2]. Overweight and obesity are characterized by excessive fat accumulation and classified by a weight-for-height index, commonly known as body mass index (BMI), with obesity defined as a BMI ≥ 30 kg/m2. Must et al. [3] demonstrated a strong correlation between obesity and mortality risk that increases with advancing age. Given that life expectancy in the U.S. and other industrialized countries is on the rise, the now heavier and older population has a greater chance of experiencing the adverse health consequences of being overweight and obese [4].
Obesity engenders a state of chronic, low-grade inflammation characterized by excessive secretion of inflammatory mediators by adipocytes, macrophages, and other cells, including the gut microbiota. These pro-inflammatory factors disrupt metabolic homeostasis and thereby promote insulin resistance, type 2 diabetes, cardiovascular disease, genome instability and cancer [5]. The disparity in mortality between obese individuals and their lean counterparts is attributed, at least in part, to this aberrant pro-inflammatory signaling and the resulting metabolic dysfunction [6]. Unfortunately, significant and sustained weight loss is difficult to achieve in obese individuals. Thus, anti-inflammatory interventions may be needed to reduce the inflammatory burden imposed with morbid adiposity levels. Numerous clinical and epidemiological studies have shown beneficial health effects with increased long chain omega-3 polyunsaturated fatty acids (PUFAs) consumption, including reductions in inflammation, hyperlipidemia and improved insulin signaling (Table 1) [7–9]. The anti-inflammatory and metabolic reprogramming properties of omega-3 PUFAs have been shown to delay the onset of cancer in several animal models, negating the pro-tumorigenic effects of obesity. This review will discuss how omega-3 PUFAs suppress obesity-associated pro-inflammatory adipokine secretion and growth factor signaling, as well as consider issues related to translating these mechanistic insights to decrease cancer development and progression.
Table 1.
Summary of various clinical research studies implicating the role of omega-3 PUFAs on obesity-induced cancer risk and progression.
| Author | Study Title | Sample Size/Target | Omega-3 PUFAs dose(s) | Effect |
|---|---|---|---|---|
| Hidaka et al. (2015) | Omega-3 and omega-6 Fatty acids in blood and breast tissue of high-risk women and association with atypical cytomorphology |
n = 70; women at high risk of breast cancer |
80 mg–1.1 g/day | Decreased breast epithelial proliferation and cytologic atypia; normalized serum omega-3:omega-6 |
| Fabian et al. (2015) | Modulation of breast cancer risk biomarkers by high-dose omega-3 fatty acids: phase II pilot study in postmenopausal women |
Postmenopausal women with cytologic evidence of hyperplasia |
1,860 mg EPA + 1500 mg DHA/day for 6 months |
Serum adiponecton normalization; Reduced levels of serum TNF-α and MCP-1 breast tissue levels |
| Sandhu et al. (2015) | Influence of obesity on breast density reduction by omega-3 fatty acids: Evidence from a randomized clinical trial |
n = 266; postmenopausal women between the ages of 35–75 with a breast density ≥ 25% |
Raloxifene 60 mg; Raloxifene 30 mg; Lovaza 4gm; Lovaza 4gm + Raloxifene 30 mg/day for 2 years |
Reduction in breast density only illustrated in women with a BMI > 29. No effect was seen in normal weight women |
| Signori et al. (2012) | Administration of omega-3 fatty acids and Raloxifene to women at high risk of breast cancer: interim feasibility and biomarkers analysis from a clinical trial |
Postmenopausal women between the ages of 35–75 years with a breast density > 25% |
4.0 g Lovaza; 4.0 g Lovaza + Raloxifene 30 mg/day for 2 years |
Decreased serum triglycerides and increased high-density lipoprotein (HDL) |
| Yee et al. (2010) | ω-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition |
n = 48 women with increased breast cancer risk |
0.84, 2.52, 5.04, and 7.56 g EPA + DHA/day for 6 months |
Increased serum and breast adipose tissue concentration with EPA + DHA supplements of 2.52, 5.04, and 7.56g |
| Davidson et al. (2007) | Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceride patients: an 8-week, randomized, double-blind, placebo-controlled study |
n = 254 men and women with previous history use of statin and TG levels > 200 and < 500 mg/dL |
4.0 g/day for 8 weeks | Significantly decreased circulating levels of CRP |
| Thorsdottir et al. (2007) | Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content |
n = 324 men/women aged 20–40 with BMI 27.5–32.5 kg/m2 |
9,000 mg/day for 8 weeks | Individuals who were supplemented with omega –3 achieved significantly greater weight loss and decrease in waist circumference vs. unsupplemented |
| Anti et al. (1992) | Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer |
n = 20; patients with sporadic adenomatous colorectal polyps |
4.1 g EPA/day; 3.6 g DHA/day for 12 weeks |
Decreased serum arachidonic acid levels and cellular proliferation of colonic crypts |
1.1. Omega-3 polyunsaturated fatty acids
Omega-3 and omega-6 PUFAs are essential nutrients, meaning they cannot be synthesized in the body and must be obtained from the diet. The three main dietary forms of omega-3 PUFAs are the marine-derived eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and the plant-derived alpha-linolenic acid (ALA). Omega-6 PUFAs include linoleic acid (LA), found in high concentrations in many vegetable oils. The ratio of omega-3 to omega-6 PUFAs is inversely associated with the pathogenesis of many diseases such as cardiovascular disease, rheumatoid arthritis, and many cancers [10]. Western diets have a particularly low omega-3:omega-6 PUFA ratio, thereby potentially increasing the risk of these chronic diseases. Clinical studies have shown that an omega-3:omega-6 PUFA ratio of 4:1 was associated with a 70% decrease in total mortality of CVD patients, a ratio of 2.5:1 suppressed cell proliferation in colon cancer, and a ratio of 2–3:1 decreased rheumatoid arthritis-associated inflammation [8].
Eicosanoids are the products of omega-3 and omega-6 PUFA cleavage from cell membrane phospholipids by phospholipase A2. The enzymes cyclooxygenase (COX) and lipoxygenase (LOX) metabolize PUFAs to produce these eicosanoids, which include pro-inflammatory prostaglandins, leukotrienes and thromboxanes as well as anti-inflammatory resolvins and protectins. COX and LOX produce proliferative and pro-inflammatory eicosanoid mediators from arachidonic acid (AA), an omega-6 PUFA and a derivative of LA, whereas anti-inflammatory eicosanoid products are produced from the omega-3 PUFAs EPA and DHA, which can be derived from ALA. A difference between the effects of EPA and DHA has been shown with organ specificity. DHA is more readily incorporated in organs such as the brain, liver, and retina whereas EPA are seen at higher concentrations in red blood cells [10–12].
There may be an optimal omega-3:omega-6 PUFA ratio to be achieved in the blood and tissues in order to reduce enzymatic conversion of LA to AA and increase substrate availability of COX and LOX to act on EPA and DHA [13]. The absorption and incorporation of omega-3 PUFAs into phospholipid membranes serves to inhibit the COX and LOX pathway utilization of AA, thereby decreasing production of pro-inflammatory prostaglandin E2 (PGE2), thromboxane A2, and leukotriene B4 metabolites [14–16]. These anti-inflammatory actions are thought to be responsible for the beneficial health effects seen with higher omega-3 PUFA consumption, including a reduction in the risk of obesity-associated cancer incidence and mortality.
1.2. Obesity-associated inflammation and cancer
1.2.1. Obesity and cancer
Obesity promotes an increased risk of many cancers and a worse cancer outcome after diagnosis. Obesity is an established risk factor for endometrial, colorectal, breast (postmenopausal), esophageal (adenocarcinoma subtype), liver, kidney, gallbladder, pancreatic, uterine, and ovarian cancer [17]. Obesity also worsens the prognosis of each these cancers as well several others, including prostate cancer, premenopausal breast cancer, thyroid cancer, and some leukemias. Morbid obesity (BMI > 40 kg/m2) is associated with a markedly higher risk of dying from cancer, increasing rates by 52% in men and 62% in women [18]. The exact mechanisms underlying the obesity-cancer link remain unclear, but abundant evidence suggests that they involve increased adipose tissue inflammation and metabolic dysfunction.
1.2.2. Adipose tissue inflammation
The local secretion of inflammatory adipocytokines from adipose tissue, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), resistin, and monocyte chemotactic protein-1 (MCP-1), is increased in obese individuals compared to their normal weight counterparts [19–21]. These pro-inflammatory cascades stem from an overabundance of immature pre-adipocytes, which recruit activated macrophages to the adipose tissue [22]. These adipose tissue macrophages (ATMs) are a primary source of pro-inflammatory cytokines, which are involved in paracrine and endocrine signaling and often have potent pro-tumor effects [22]. Cytokines promote tumor growth in the microenvironment by increasing angiogenesis and fostering an immunosuppressive environment, which works against the body’s anti-tumor immunity. IL-6 inhibits the maturation of dendritic cells, thus reducing the population of cytotoxic T-cells, which kill cancer cells [23]. IL-1β promotes tumor growth by inducing angiogenic factors, including vascular endothelial growth factor, which support the tumor with a nutrient rich blood supply [24].
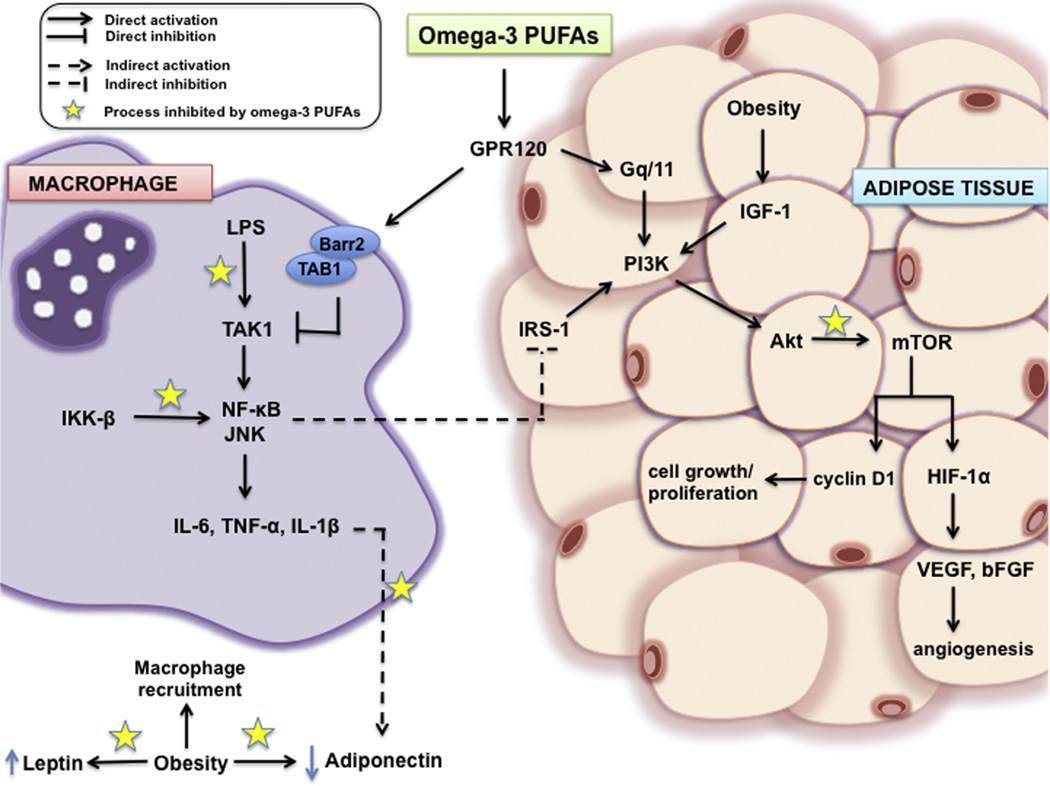
Activation of the transcription factor nuclear factor (NF)-κB through the phosphorylation of its upstream activator IkB kinase-β (IKK-β) induces increased gene expression pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β (Fig. 1).
Fig. 1.
Hypothesized mechanism of omega-3 PUFA infiltration into macrophages and adipose tissue and its role in targeting key mechanistic activators in inflammatory and cancer-related signaling such as inhibition of LPS, IKK-β, and Akt signaling as well as decreasing circulating leptin concentrations and TNF-α stimulated reductions in adiponectin levels. Solid arrows indicate direct activation, solid bar-headed arrows indicate direct inhibition, dashed arrows indicate indirect activation, and dashed bar-headed arrows indicate indirect inhibition. Stars indicate process inhibited by omega-3 PUFAs.
TNF-α is known to increase tumor cell proliferation, tumor stage, and systemic metastatic growth [25,26]. In addition, TNF-α and IKK-β activate c-Jun NH2-terminal kinase (JNK), which promotes proliferation and survival of tumor cells [27,28]. TNF-α also contributes to insulin resistance by increasing insulin receptor substrate 1 (IRS-1) phosphorylation at serine 307, which impairs its ability to initiate downstream signaling and consequently blocks the biological actions of insulin [29]. Like TNF-α, IL-6 also affects insulin signaling by inhibiting the gene transcription of IRS-1 and glucose transporter type 4 (GLUT-4), resulting in decreased insulin sensitivity [30]. Moreover, this increased secretion of cytokines from adipose tissue can lead to genomic instability through the shortening of telomeres. Telomere shortening is widely accepted as a biological occurrence of accelerating aging and aging-related diseases such as cancer. This is mediated through increased oxidative stress, DNA damage, and telomere shortening activation of p53 and p21 that upregulates secretion of TNF-α and IL-6 further leading to insulin resistance [31].
Various animal studies have shown that omega-3 PUFA supplementation can inhibit IKK-βphosphorylation via lipopolysaccharide (LPS) and transforming growth factor beta-activated kinase 1 (TAK1), an upstream activator of IKK-β and JNK, stimulation and decrease NF-κB activity, which in turn decreases cytokine production (Fig. 1) [32–35]. Omega-3 PUFAs can also act directly on cytokine synthesis, which is elevated in obese individuals. In particular, in vivo and in vitro experiments have shown decreased TNF-α, IL-6, and IL-1β production from macrophages and peripheral-blood mononuclear cells with omega-3 PUFA treatment [36–38]. Decreased cytokine production and synthesis due to omega-3 PUFA supplementation can be a critical component to reducing obesity-associated telomere shortening and clinically relevant for identifiable at risk populations.
1.2.3. Role of secretory adipokines, leptin and adiponectin
The adipokines leptin and adiponectin are primarily thought of as regulators of calorie intake and energy expenditure, however they are also involved in modulating inflammation and insulin resistance. Leptin signaling promotes satiety, thereby decreasing food intake. However, the onset of obesity causes individuals to become leptin resistant despite excess leptin production by the adipose tissue. In many obese individuals, leptin acts directly on macrophages to stimulate the synthesis of pro-inflammatory cytokines like TNF-α and IL-6. Adiponectin acts as an antagonist to leptin’s pro-inflammatory effects in part by activating AMP-dependent protein kinase (AMPK), which increases fatty acid oxidation and glucose uptake in skeletal muscle and decreased hepatic gluconeogenesis [39]. However, TNF-α, IL-6 and other pro-inflammatory mediators suppress adiponectin secretion from adipocytes, and the obese population typically has low adiponectin levels [39].
Leptin secretion from adipocytes has been shown to induce tumor growth as well as cancer cell invasion and angiogenesis, suggesting the role of adipose tissue as a catalyst to cancer development and progression. Interestingly, leptin antagonist treatment reduces the growth of triple negative breast tumors in mice through decreased VEGF, pSTAT3, and cyclin D1 levels, highlighting leptin’s pro-carcinogenic potential. Alternatively, clinical studies have shown circulating levels of adiponectin are inversely correlated with obesity-related malignancies such as breast cancer. Adiponectin functions to decrease endothelial cell proliferation and migration, induce apoptosis, and decrease tumor vascularization. Clinically, the leptin:adiponectin ratio (L:A) is increasingly accepted as a biomarker for metabolic syndrome [40]. Additionally, in colorectal cancer patients the L:A ratio is as much as eight fold greater (1.090) compared to cancer-free controls (0.065) and also serves as an independent predictor for adverse outcomes in colorectal cancer [41].
Omega-3 PUFAs have been shown to correlate with adiponectin levels, but this relationship has proven modifiable in both rodent models of obesity and human obese subjects. EPA supplementation consistently increases adiponectin secretion through blockage of inflammatory TNF-α signaling, despite established macrophage infiltration into the adipose tissue (Fig. 1). In vitro, omega-3 PUFAs have shown to upregulate secretion of adiponectin in murine and human adipocytes through decreased expression of cytokines and macrophage adipose tissue infiltration [42,43]. Furthermore, in vivo initiating a high dose EPA/DHA (12% of dietary lipids) in obese mice decreased plasma leptin levels compared to their non-supplemented counterparts [44].
1.2.4. Insulin signaling in obesity
High BMI levels are associated with increased insulin secretion, leading to hyperinsulinemia. This metabolic dysfunction contributes to the decreased insulin sensitivity that is also induced by obesity-associated chronic inflammation. Elevated levels of insulin have been associated with cancer progression through insulin-like growth factor (IGF) signaling. Increased circulating levels of IGF-1 have been correlated with increased risk of prostate, breast, and colorectal cancer [45]. Insulin itself is thought to have anti-inflammatory effects in healthy individuals, as it can decrease reactive oxygen species (ROS) in mononuclear cells and suppress MCP-1 and plasminogen activator inhibitor-1 (PAI-1) levels and intranuclear NF-κB binding [46–50]. Thus, adequate insulin signaling is needed to repair the inflammatory state of obesity, otherwise continuous secretion of TNF-α, IL-6, and C-reactive protein (CRP) will occur [51]. Omega-3 PUFAs can serve as therapeutic agents to help restore the insulin signaling pathway. A study published by Oh et al. [52] determined that omega-3 PUFAs improve insulin sensitivity through a G protein-coupled receptor 120 (GPR120)-mediated anti-inflammatory pathway (Fig. 1). This mechanism involved decreasing pro-inflammatory macrophage (M1) production of TNF-α, IL-6, and IL-1β and increasing anti-inflammatory macrophage (M2) production of IL-10, arginase, macrophage galactose-type C-type lectin 1 (MGL1), and macrophage mannose receptor (MMR) in adipose tissue [52]. Clinically, omega-3 PUFAs have been able to significantly improve insulin sensitivity after eight weeks of supplementation and reduce circulating levels of CRP and IL-6 in twelve healthy men and women 60–75 years of age by 64% and 39%, respectively [51].
1.3. Omega-3 PUFAs and their anticancer effects
1.3.1. Pre-clinical studies
Pre-clinical data suggest omega-3 PUFAs possess the ability to blunt the pro-tumorigenic effects of obesity. In mouse models of postmenopausal triple-negative breast cancer, Ford et al. [53] found that omega-3 PUFA supplementation reduced pro-inflammatory eicosanoid concentrations in the mammary tumors of obese mice to levels similar to normal weight control mice. Omega-3 PUFAs also suppressed mammary tumor growth to the same levels seen in control mice and normalized several metabolic hormones [53]. Similarly, Chung et al. [54] found decreased expression of pro-inflammatory genes MCP-1 and TNF-α in omega-3 PUFA supplemented mice as well as a significant 28% reduction in mammary tumor burden compared to non-supplemented obese mice. In vitro experiments have shown that omega-3 PUFAs inhibit Py230 mammary tumor cell growth by stimulating apoptosis [54]. Omega-3 PUFAs were also able to increase gene expression of the tumor suppressor gene BRCA1 by 60%, leading to decreased mammary tumorigenesis in rats [55]. Furthermore, omega-3 PUFAs can cooperate with selective estrogen receptor modulators (SERMs) to suppress rat mammary carcinogenesis. Normal dosing of the SERMs tamoxifen and raloxifene resulted in similar chemoprotective effects in rats as observed with a reduced SERM dose supplemented with omega-3 PUFAs. These findings may present an important opportunity to reduce the toxicity associated with SERM treatment [56].
Omega-3 PUFA supplementation has also been effective in reducing tumor burden in models of other cancer types. In a rat model of colon carcinogenesis, supplementation of a high-fat diet with omega-3 PUFAs led to a lower incidence of colon adenocarcinomas in comparison to both the non-supplemented high-fat and control group [57]. Another animal study has shown similar results when a high-fat diet is supplemented with omega-3 PUFAs, with decreases in colon cancer progression relative to high-fat diets alone through a reduction of PGE2 levels in colon mucosa [58]. The benefits of omega-3 supplementation also extend to prostrate cancer, the most frequently diagnosed cancer among men [59]. In a prostrate PTEN-knockout mice model, the addition of omega-3 PUFAs to a high-fat diet decreased prostrate tumor growth and increased overall tumor-free survival [60].
1.3.2. Clinical studies
Findings from clinical research suggest that obesity is associated with lower levels of plasma omega-3 PUFAs [61]. The ratio of omega-3 to omega-6 PUFAs consumed is inversely associated with breast cancer risk [62]. Thus, supplementation within the obesogenic diet may be able to offset the reduced plasma levels of omega-3 PUFAs associated with obesity and subsequently decrease the risk of cancer development and progression. Supporting this premise is a study showing the effect of an energy restricted dietary intervention with daily supplementation of 9,000 mg omega-3 PUFAs in men and women age 20–40 years old with a BMI of 27.5–32.5 [63]. The individuals that received the supplement experienced significantly greater weight loss and decreases in waist circumference within 8 weeks in comparison to the energy restricted diet without supplementation [63].
In a randomized trial, individuals diagnosed with hyperlipidemia and supplemented with omega-3 PUFAs displayed a reduction in CRP levels, a surrogate for systemic inflammation, which has been linked with increased risk of breast and lung cancer [64–66]. Additionally, high dose omega-3 PUFAs supplementation (3,360 mg) over a six month period normalized adiponectin concentrations and decreased serum TNF-α and breast MCP-1 levels in postmenopausal women at risk of hyperplasia of the breast due to family history and/or an abnormal breast biopsy [67]. Omega-3 PUFA supplementation has also displayed potential in combating colon and prostate cancer. For example, omega-3 PUFAs decreased rectal mucosal cell proliferation in colonic crypts (a marker of colon carcinogenesis) after two weeks of supplementation [68]. In addition, high levels of serum omega-3 PUFAs correlated with reduced risk of prostate cancer after a ten-year follow-up in a case-control study consisting of 476 men between the ages of 40–84 years [69].
Furthermore, telomere shortening attributed to obesity-induced cytokine secretion contributes to genome instability that may in part lead to increased cancer risk. However, omega-3 PUFAs can help to combat tumor carcinogenesis and progression. In particular, a study conducted by Farzaneh-Far et al. [70] indicates that a diet containing omega-3 PUFAs is associated with a reduced rate of telomere shortening, whereas a lack of omega-3 PUFAs correlates with an increased rate of telomere attrition in study participants. Moreover, Shen et al. [71] followed omega-3 PUFA levels in blood and telomere length in at-risk women of breast cancer over a 5-year period. The results illustrated an inverse correlation of omega-3 PUFAs and telomere length, suggesting that omega-3 PUFAs reduce the rate of telomere shortening. Similarly, the women participants of the study who consumed a diet lacking omega-3 PUFAs had shorter telomeres and a moderate risk for development of breast cancer. However, those that had a diet enriched with omega-3 PUFAs had longer telomeres and decreased risk of breast cancer [71] Thus, these results indicate the possible genomic stability associated with omega-3 PUFA supplementation.
Multiple studies have captured the increased anti-inflammatory effects of omega-3 PUFA supplementation in obese compared to normal weight individuals. It is plausible that the chronic-low grade inflammation observed in obesity sets the stage for a successful anti-inflammatory intervention. Alternatively, in normal weight individuals where low grade inflammation is generally absent, benefits of an anti-inflammatory intervention may be more subtle. A prospective intervention study investigated the effects of omega-3 PUFA supplementation on reproductive hormones utilizing the drug Lovaza, a purified and concentrated source of ethyl esters (EPA 55.4%/DHA 44.6%). Researchers found that 18–42 year old women given 4.0 g Lovaza daily had lower levels of follicle stimulating hormone (FSH) only if they were normal weight. However, serum cytokines IL-1β and TNF-α were reduced only in the obese women on study (by 72% and 56% respectively) [72]. A preclinical study also utilizing Lovaza in normal weight and obese mice found decreased mammary tumor growth in obese mice supplemented with the drug, whereas no differences in mammary tumor growth were observed between normal weight mice with or without supplementation [53]. Another clinical study illustrated a BMI-dependent effect of omega-3 PUFA supplementation on markers of breast cancer risk. Subjects aged >18 years with a high risk of breast cancer assessed by family background, atypical hyperplasia, and/or genetic susceptibility were randomly assigned to receive omega-3 PUFAs at doses of 0.84, 2.52, 5.04, or 7.56 g daily for six months. Breast tissue samples obtained by fine needle aspiration and serum collected at three and six months after initiation of supplementation showed significant increases over baseline in omega-3 PUFA content for the three highest doses [73]. However, a randomized clinical trial focusing on 266 postmenopausal women with high breast density (BD), an established risk factor for breast cancer, was only changed by omega-3 PUFA supplementation in the women who had a BMI > 29. These results suggest that omega-3 PUFA’s beneficial effects may only mitigate biological perturbations present in overweight or obese individuals, and thus potentially less relevant to those at a healthy weight [74].
1.4. Proposed risk of omega-3 PUFA oversupplementation
While omega-3 PUFAs can potentially reduce obesity-associated chronic inflammation and cancer development, these fatty acids can also contribute to adverse health effects. Unfortunately, there is very little data in humans on this topic, and the limited preclinical data suggests additional work is warranted. Daenen et al. [75] note in their preclinical mouse studies that omega-3 supplementation during chemotherapy resulted in chemoresistance and reduced cancer survival. It is imperative that the impact of omega-3 PUFAs on chemotherapy response be further examined, as this may have critical implications for treatment efficacy. Likewise, immunomodulation has also been linked to excessive omega-3 PUFA intake. Overuse of omega-3 PUFAs was associated with impairment of lymphocyte and innate immune responses [76]. Animal studies have linked increased use to decreased bacterial and viral clearance from the body, resulting in infection-induced inflammation and increased susceptibility to pathogens, which can subsequently lead to cancer [77–81]. In a SMAD3 colitis mouse model infected with Helicobacter hepaticus, a high-dose DHA supplementation (6% in the diet) was found to exacerbate levels of inflammation and dysplasia compared to the control diets [82]. Four weeks after H. hepaticus infection, mice consuming DHA had a significantly higher mortality rate and increased lean body mass loss. This was correlated with impaired immune function due to altered CD8+ cell populations, CD69+ activation, and increased expression of FoxP3 and L-selectin. However, low dose DHA (0.75%) did not significantly reduce colitis progression compared to the control diets [82]. Thus, these studies highlight the importance of determining the correct dosage for omega-3 PUFA supplementation in preventing cancer mortality and immune susceptibility.
1.5. The need to identify individual variations in response
Single nucleotide polymorphisms (SNPs), which occur when a single nucleotide is interchanged on a DNA strand, can alter an individual’s response to various nutrition-related factors, including omega-3 PUFA supplementation. Consequently, identification of specific SNPs may help researchers predict patients’ response to omega-3 PUFA supplementation as well as their cancer mortality risk, furthering physicians’ efforts to provide personalized therapies to patients at risk of cancer and those who are already diagnosed. For example, the Singapore Chinese Health Study found a positive association between marine omega-3 PUFA intake and rectal cancer risk for those who carry the PARP codon 762 Ala allele [83]. Similarly, another study highlights the effects of glutathione S-transferase polymorphisms on postmenopausal breast cancer risk in the Singapore Chinese Health Study. A case-control comparison was made between 258 breast cancer patients and 670 cohort controls. The researchers concluded that marine omega-3 PUFAs had a greater protective effect in women with genetic polymorphisms causing minimal enzymatic activity of GSTM1, GSTT1 and/or GSTP1 [84]. Lenihan-Geels et al. [85] notes that SNPs associated with the cytochrome c oxidase and arachidonate lipoxygenase genes alter the levels of eicosanoids produced from AA and EPA. Thus, heterogeneity in these metabolizing genes may be a critical determinant of differential anticancer responses to omega-3 PUFAs.
2. Conclusions
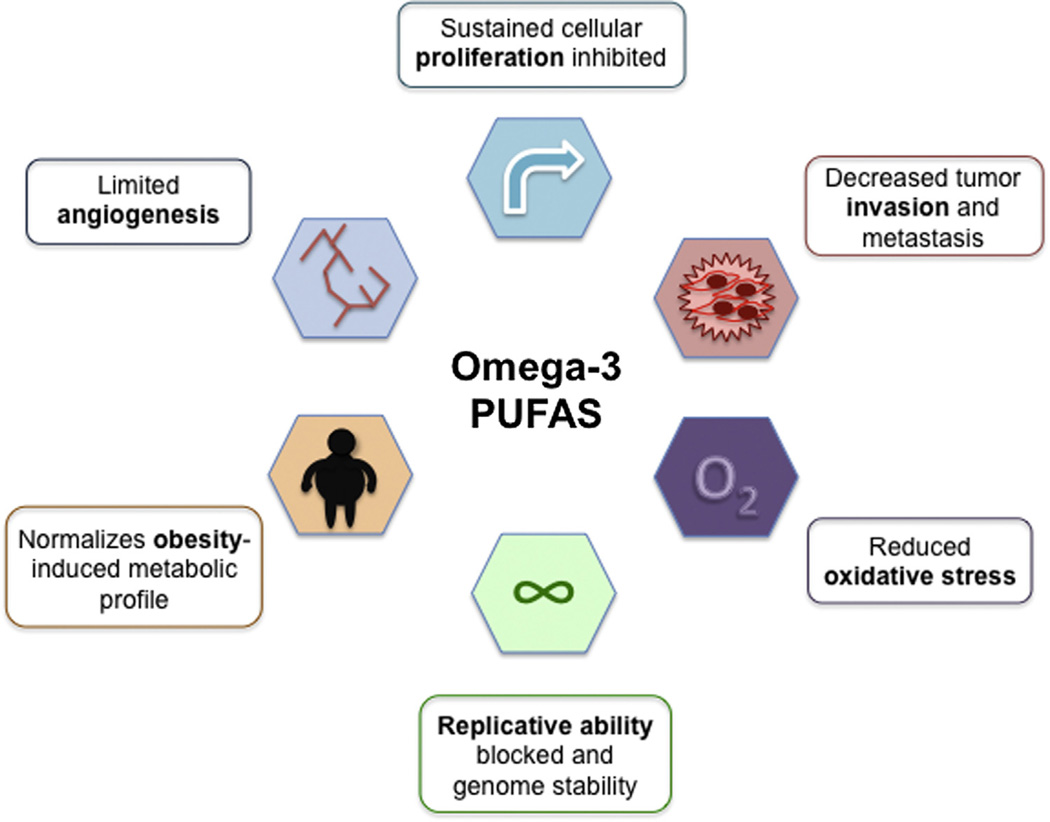
Animal and human studies have repeatedly demonstrated that omega-3 PUFA supplementation blunts many of the hallmarks of cancer (Fig. 2).
Fig. 2.
Possible roles of omega-3 PUFAs protecting against many of the hallmarks of cancer. Bolded words in each of the boxes correspond to the meaning of each symbol.
Omega-3 PUFA supplementation in obese individuals normalizes serum levels of several metabolic hormones, including insulin, leptin and adiponectin, and reduces cytokine production to levels seen in normal weight counterparts. This improved metabolic and inflammatory profile suppresses angiogenesis via VEGF inhibition and decreases ROS production. Reduced leptin signaling and cytokine-induced NF-κB activation also promotes apoptosis and inhibits cyclin D1 expression, respectively, which prevents sustained cellular proliferation. In addition, omega-3 PUFAs have the potential to decrease tumor invasion and metastasis by increasing LKB1, a tumor suppressor gene. This leads to AMPK phosphorylation and inhibition of the mTOR (mammalian target of rapamycin) pathway, which integrates growth signals and can regulate tumor growth, angiogenesis, and metastasis [86,87]. Finally, omega-3 PUFAs intake is inversely associated with rate of telomerase shortening, suggesting that they can enhance genome stability.
The clinical relevance of increased omega-3 PUFA intake as a strategy for reducing obesity-induced inflammatory mechanisms underlying cancer risk and progression is significant. The anti-inflammatory properties of omega-3 PUFAs can help to reduce the obesity-induced secretion of pro-inflammatory cytokines such as TNF-α, IL-6 and, IL-1β that often accompanies poor cancer prognosis. Continued preclinical and clinical studies are essential to the optimization of omega-3 PUFAs’ anticancer effects in order to break the obesity-cancer link. In particular, delineating the role and precise mechanisms of omega-3 PUFAs in suppressing obesity-induced inflammation will help identify promising key targets to reduce the human suffering caused by obesity-associated cancers.
Abbreviations
- GPR120
G-protein coupled receptor 120
- Barr2
beta-arrestin2
- TAB1
TGF-beta activated kinase 1/MAP3K7 Binding Protein 1
- LPS
lipopolysaccharide
- TAK1
transforming growth factor beta-activated kinase 1
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- JNK
c-Jun N-terminal kinases
- IKK-β
I-kappaB kinase-beta
- IL–6
interleukin-6
- TNF-α
tumor necrosis factor-alpha
- IL-1β
interleukin-1 beta
- Gq/11
Gq protein
- PI3K
phosphoinositide 3-kinase
- IRS–1
insulin receptor substrate-1
- IGF–1
insulin growth factor-1
- Akt
protein Kinase B
- mTOR
mammalian target of rapamycin
- HIF-1α
hypoxia inducible factor-1 alpha
- VEGF
vascular endothelial growth factor
- bFGF
basic fibroblast growth factor
Footnotes
Conflict of interest
The authors disclose no potential conflicts of interest.
Contributor Information
Subreen A. Khatib, Email: skhatib@live.unc.edu.
Emily L. Rossi, Email: emilylro@live.unc.edu.
Laura W. Bowers, Email: lwbowers@email.unc.edu.
Stephen D. Hursting, Email: hursting@email.unc.edu.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in body mass index among US adults 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. http://dx.doi.org/10.1001/jama.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eanes L. Obesity a persistent global health problem. Int. Arch. Nurs. Health Care. 2015;1:1–3. [Google Scholar]
- 3.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. J. Am. Med. Assoc. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. http://dx.doi.org/10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. 10 Facts on Obesity, 2014. [accessed 12.01.16]; http://www.who.int/features/factfiles/obesity/en/
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. 10 1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 8.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. http://dx.doi.org/10.1016/S0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 9.Harris WS. Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol. Res. 2007;55:217–223. doi: 10.1016/j.phrs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 11.Browning LM, Walker CG, Mander AP, West AL, Madden J, Gambell JM, et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012;96:748–758. doi: 10.3945/ajcn.112.041343. http://dx.doi.org/10.3945/ajcn.112.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopecky J, Rossmeisl M, Flachs P, Kuda O, Brauner P, Jilkova Z, et al. n-3 PUFA: bioavailability and modulation of adipose tissue function. Proc. Nutr. Soc. 2009;68:361–369. doi: 10.1017/S0029665109990231. http://dx.doi.org/10.1017/S0029665109990231. [DOI] [PubMed] [Google Scholar]
- 13.Fabian CJ, Kimler BF, Hursting SD. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015;17:1–11. doi: 10.1186/s13058-015-0571-6. 1-.1186/s13058-015-0571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardman WE. Omega-3 fatty acids to augment cancer therapy. J. Nutr. 2002;132:3508S–3512S. doi: 10.1093/jn/132.11.3508S. [DOI] [PubMed] [Google Scholar]
- 15.Hirai A, Hamazaki T, Terano T, Nishikawa T, Tamura Y, Kumagai A, et al. Eicosapentaenoic acid and platelet function in Japanese. Lancet. 1982;8204:1132–1133. doi: 10.1016/s0140-6736(80)92558-1. [DOI] [PubMed] [Google Scholar]
- 16.Black KL, Culp B, Madison D, Randall OS, Lands WE. The protective effects of dietary fish oil on focal cerebral infarction. Prostaglandins Med. 1979;3:257–268. doi: 10.1016/0161-4630(79)90067-3. [DOI] [PubMed] [Google Scholar]
- 17.American Institute for Cancer Research, Updated Estimate on Obesity-Related Cancers, 2014. [accessed 12.01.16]; http://www.aicr.org/cancer-research-update/2014/march_19/cru-updated-estimate-on-obesity-related-cancers.html. [Google Scholar]
- 18.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. http://dx.doi.org/10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 19.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. http://dx.doi.org/10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. http://dx.doi.org/10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 21.Samad F, Uysal KT, Wiesbrock SM, Pandey M, Hotamisligil GS, Loskutoff DJ. Tumor necrosis factor alpha is a key component in the obesity-linked elevation of plasminogen activator inhibitor 1. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6902–6907. doi: 10.1073/pnas.96.12.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. http://dx.doi.org/10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 23.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumorcells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 24.Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, Maemondo M, et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J. Immunol. 2002;169:469–475. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- 25.Cui LF, Guo XJ, Wei J, Liu FF, Fan Y, Lang RG, et al. Overexpression of TNF-α and TNFRII in invasive micropapillary carcinoma of the breast: clinicopathological correlations. Histopathology. 2008;53:381–388. doi: 10.1111/j.1365-2559.2008.03128.x. http://dx.doi.org/10.1111/j.1365-2559.2008.03128.x. [DOI] [PubMed] [Google Scholar]
- 26.Sheen-Chen SM, Chen WJ, Eng HL, Chou FF. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res. Treat. 1997;43:211–215. doi: 10.1023/a:1005736712307. [DOI] [PubMed] [Google Scholar]
- 27.Bubici C, Papa S. JNK signaling in cancer: in need of new, smarter therapeutic targets. Br. J. Pharmacol. 2014;171:24–37. doi: 10.1111/bph.12432. http://dx.doi.org/10.1111/bph.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu. Rev. Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. http://dx.doi.org/10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- 29.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. U. S. A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistance subjects. J. Biol. Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. http://dx.doi.org/10.1074/jbc.m301977200. [DOI] [PubMed] [Google Scholar]
- 31.Monickaraj F, Aravind S, Nandhini P, Prabu P, Sathishkumar C, Mohan V, et al. Accelerated fat cell aging links oxidative stress and insulin resistance in adipocytes. J. Biosci. 2013;38:113–122. doi: 10.1007/s12038-012-9289-0. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya A, Chandrasekar B, Rahman MM, Banu J, Kang JX, Fernandes G. Inhibition of inflammatory response in transgenic fat-1 mice on a calorierestricted diet. Biochem. Biophys. Res. Commun. 2006;349:925–930. doi: 10.1016/j.bbrc.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 33.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. http://dx.doi.org/10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 35.Nowak J, Weylandt KH, Habbel P, Wang J, Dignass A, Glickman JN, et al. Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n-3 fatty acids. Carcinogenesis. 2007;28:1991–1995. doi: 10.1093/carcin/bgm166. http://dx.doi.org/10.1093/carcin/bgm166. [DOI] [PubMed] [Google Scholar]
- 36.Babcock TA, Helton WS, Hong D, Espat NJ. Omega-3 fatty acid lipid emulsion reduces LPS-stimulated macrophage TNF-alpha production. Surg. Infect. (Larchmt) 2002;3:145–149. doi: 10.1089/109629602760105817. [DOI] [PubMed] [Google Scholar]
- 37.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 38.Schmöcker C, Weylandt KH, Kahlke L, Wang J, Lobeck H, Tiegs G, et al. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology. 2007;45:864–869. doi: 10.1002/hep.21626. http://dx.doi.org/10.1002/hep.21626. [DOI] [PubMed] [Google Scholar]
- 39.Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. (Lausanne) 2013;4:1–13. doi: 10.3389/fendo.2013.00071. http://dx.doi.org/10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falahi E, Khalkhali Rad AH, Roosta S. What is the best biomarker for metabolic syndrome diagnosis? Diabetes Metab. Syndr. 2015;9:366–372. doi: 10.1016/j.dsx.2013.06.014. http://dx.doi.org/10.1016/j.dsx.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Guadagni F, Roselli M, Martini F, Spila A, Riondino S, D’Alessandro R, et al. Prognostic significance of serum adipokine levels in colorectal cancer patients. Anticancer Res. 2009;29:3321–3327. [PubMed] [Google Scholar]
- 42.Oster RT, Tishinsky JM, Yuan Z, Robinson LE. Docasahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPAR% mRNA, in 3T3-L1 adipocytes. Appl. Physiol. Nutr. Metab. 2010;35:783–789. doi: 10.1139/H10-076. [DOI] [PubMed] [Google Scholar]
- 43.Tishinsky JM, Ma DW, Robinson LE. Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and PPARγ-dependent manner in human adipocytes. Obes. (Silver Spring) 2010;19:262–268. doi: 10.1038/oby.2010.186. [DOI] [PubMed] [Google Scholar]
- 44.Ruzickova J, Rossmeisl M, Prazak T, Flachs P, Sponarova J, Vecka M, et al. Omega-3 PUFA of marine orgin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids. 2004;19:1177–1185. doi: 10.1007/s11745-004-1345-9. [DOI] [PubMed] [Google Scholar]
- 45.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. http://dx.doi.org/10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 46.Després JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–459. [PubMed] [Google Scholar]
- 47.Després JP. The insuilin resistance-dyslipidemic syndrome of visceral obesity: effect on patients’ risk. Obes. Res. 1998;6:8S–17S. doi: 10.1002/j.1550-8528.1998.tb00683.x. http://dx.doi.org/10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 48.Dandona P, Aljada A, Bandyopadhyay A. Inflammation The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. http://dx.doi.org/10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, et al. Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J. Clin. Endocrinol. Metab. 2001;86:3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 50.Aljada A, Ghanim H, Mohanty P, Kapur N, Dandona P. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (Egr)-1 expression in mononuclear cells (MNC) and reduces plasma tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) concentrations. J. Clin. Endocrinol. Metab. 2002;87:1419–1422. doi: 10.1210/jcem.87.3.8462. [DOI] [PubMed] [Google Scholar]
- 51.Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction. Atherosclerosis, Thromb. Vasc. Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 52.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. http://dx.doi.org/10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ford NA, Rossi EL, Barnett K, Yang P, Bowers LW, Hidaka BH. Omega-3-acid ethyl esters block the protumorigenic effects of obesity in mouse models of postmenopausal basal-like and claudin-low breast cancer. Cancer Prev. Res. (Phila.) 2015;8:796–806. doi: 10.1158/1940-6207.CAPR-15-0018. http://dx.doi.org/10.1158/1940-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung H, Lee YS, Mayoral R, Oh DY, Siu JT, Webster NJ. Omega-3 fatty acids reduce obesity-induced tumor progression independent of GPR120 in a mouse model of postmenopausal breast cancer. Oncogene. 2015;34:3504–3513. doi: 10.1038/onc.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jourdan ML, Mahéo K, Barascu A, Goupille C, De Latour MP, Bougnoux P, et al. Increased BRCA1 protein in mammary tumours of rats fed marine ω-3 fatty acids. Oncol. Rep. 2007;17:713–719. [PubMed] [Google Scholar]
- 56.Manni A, Richie JP, Xu H, Washington S, Aliaga C, Bruggeman R, et al. Influence of omega-3 fatty acids on tamoxifen-induced suppression of rat mammary. Int. J. Cancer. 2014;134:1549–1557. doi: 10.1002/ijc.28490. http://dx.doi.org/10.1002/ijc.28490. [DOI] [PubMed] [Google Scholar]
- 57.Lindner MA. A fish oil diet inhibits colon cancer in mice. Nutr. Cancer. 1991;15:1–11. doi: 10.1080/01635589109514105. http://dx.doi.org/10.1080/01635589109514105. [DOI] [PubMed] [Google Scholar]
- 58.Rao CV, Reddy BS. Modulating effect of amount and types of dietary fat on ornithine decarboxylase, tyrosine protein kinase and prostaglandins production during colon carcinogenesis in male F344 rats. Carcinogenesis. 1993;14:1327–1333. doi: 10.1093/carcin/14.7.1327. [DOI] [PubMed] [Google Scholar]
- 59.Wynder EL, Mabuchi K, Whitmore WF., Jr Epidemiology of cancer of the prostate. Cancer. 1971;28:344–360. doi: 10.1002/1097-0142(197108)28:2<344::aid-cncr2820280214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 60.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. http://dx.doi.org/10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micallef M, Munro I, Phang M, Garg M. Plasma n-3 Polyunsaturated Fatty Acids are negatively associated with obesity. Br. J. Nutr. 2009;102:1370–1374. doi: 10.1017/S0007114509382173. http://dx.doi.org/10.1017/S0007114509382173. [DOI] [PubMed] [Google Scholar]
- 62.Hidaka BH, Li S, Harvey KE, Carlson SE, Sullivan DK, Kimler BF, et al. Omega-3 and omega-6 fatty acids in blood and breast tissue of high-risk women and association with atypical ctomorphology. Cancer Prev. Res. (Phila.) 2015;8:359–364. doi: 10.1158/1940-6207.CAPR-14-0351. http://dx.doi.org/10.1158/1940-6207. [DOI] [PubMed] [Google Scholar]
- 63.Thorsdottir I, Tomasson H, Gunnarsdottir I, Gisladottir E, Kiely M, Parra MD, et al. Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int. J. Obes. (Lond.) 2007;31:1560–1566. doi: 10.1038/sj.ijo.0803643. http://dx.doi.org/10.1038/sj.ijo.0803643. [DOI] [PubMed] [Google Scholar]
- 64.Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, Shalwitz RA. Efficacy and tolerability of adding prescription Omega-3 fatty acids 4 g/d to Simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week randomized, double-blind, placebo-controlled study. Clin. Ther. 2007;29:1354–1367. doi: 10.1016/j.clinthera.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 65.Fabian CJ, Kimler BF. Marine-derived omega-3 fatty acids: fishing for clues for cancer prevention. Am. Soc. Clin. Oncol. Educ. Book. 2013:97–101. doi: 10.14694/EdBook_AM.2013.33.97. [DOI] [PubMed] [Google Scholar]
- 66.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH. Circulating inflammation markers and prospective risk for lung cancer. J. Natl. Cancer Inst. 2013;105:1871–1880. doi: 10.1093/jnci/djt309. http://dx.doi.org/10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fabian CJ, Kimler BF, Phillips TA, Nydegger JL, Kreutzjans AL, Carlson SE, et al. Modulation of breast cancer risk biomarkers by high-dose omega-3 fatty acids: phase II pilot study in postmenopausal women. Cancer Prev. Res. 2015;8:922–931. doi: 10.1158/1940-6207.CAPR-14-0336. http://dx.doi.org/10.1158/1940-6207.CAPR-14-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anti M, Marra G, Armelao F, Bartoli GM, Ficarelli R, Percesepe A, et al. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103:883–891. doi: 10.1016/0016-5085(92)90021-p. [DOI] [PubMed] [Google Scholar]
- 69.Chavarro JE, Stampfer MJ, Li H, Campos H, Kurth T, Ma J. A prospective study of polyunsaturated fatty acid levels in blood and prostate cancer risk. Cancer Epidemiol. Biomarkers Prev. 2007;16:1364–1370. doi: 10.1158/1055-9965.EPI-06-1033. http://dx.doi.org/10.1158/1055-9965.EPI-06-1033. [DOI] [PubMed] [Google Scholar]
- 70.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. http://dx.doi.org/10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen J, Gammon MD, Terry MB, Wang Q, Bradshaw P, Teitelbaum SL, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int. J. Cancer. 2009;124:1637–1643. doi: 10.1002/ijc.24105. http://dx.doi.org/10.1002/ijc.24105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Safi ZA, Liu H, Carlson NE, Chosich J, Harris M, Bradford AP, et al. Omega-3 fatty acid supplementation lowers serum FSH in normal weight but not obese women. J. Clin. Endocrinol. Metab. 2016;101:324–333. doi: 10.1210/jc.2015-2913. http://dx.doi.org/10.1210/jc.2015-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am. J. Clin. Nutr. 2010;91:1185–1194. doi: 10.3945/ajcn.2009.29036. http://dx.doi.org/10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sandhu N, Schetter SE, Liao J, Hartman TJ, Richie JP, McGinley JN, et al. Influence of obesity on breast density reduction by omega-3 fatty acids: evidence from a randomized clinical trial. Cancer Prev. Res. (Phila.) 2015 doi: 10.1158/1940-6207.CAPR-15-0235. http://dx.doi.org/10.1158/1940-6207. [DOI] [PubMed] [Google Scholar]
- 75.Daenen LGM, Cirkel GA, Houthuijzen JM, Gerrits J, Oosterom I, Roodhart JML, et al. Increased plasma levels of chemoresistance-inducing fatty acid 16:4(n-3) after consumption of fish and fish oil. JAMA Oncol. 2015;1:1–12. doi: 10.1001/jamaoncol.2015.0388. http://dx.doi.org/10.1001/jamaoncol.2015.0388. [DOI] [PubMed] [Google Scholar]
- 76.Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002;56(Suppl. 3):S14–S19. doi: 10.1038/sj.ejcn.1601478. http://dx.doi.org/10.1038/sj.ejcn.1601478. [DOI] [PubMed] [Google Scholar]
- 77.Fenton JI, Hord NG, Ghosh S, Gurzell EA. Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins Leukot. Essent. Fat. Acids. 2013;89:379–390. doi: 10.1016/j.plefa.2013.09.011. http://dx.doi.org/10.1016/j.plefa.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwerbrock NMJ, Karlsson EA, Shi Q, Sheridan PA, Beck MA. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 2009;139:1588–1594. doi: 10.3945/jn.109.108027. http://dx.doi.org/10.3945/jn.109.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McFarland CT, Fan YY, Chapkin RS, Weeks BR, McMurray DN. Dietary polyunsaturated fatty acids modulate resistance to Mycobacterium tuberculosis in guinea pigs. J. Nutr. 2008;138:2123–2128. doi: 10.3945/jn.108.093740. 138/11/2123[pii]\r10.3945/jn.108.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cruz-Chamorro L, Puertollano MA, Puertollano E, Álvarez de Cienfuegos G, de Pablo MA. Examination of host immune resistance against Listeria monocytogenes infection in cyclophosphamide-treated mice after dietary lipid administration. Clin. Nutr. 2007;26:631–639. doi: 10.1016/j.clnu.2007.06.012. http://dx.doi.org/10.1016/j.clnu.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 81.Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, et al. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010;70:7960–7969. doi: 10.1158/0008-5472.CAN-10-1396. http://dx.doi.org/10.1158/0008-5472.CAN-10-1396. [DOI] [PubMed] [Google Scholar]
- 82.Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Kangohr I, Gardner EM, et al. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010;70:7960–7969. doi: 10.1158/0008-5472.CAN-10-1396. [DOI] [PubMed] [Google Scholar]
- 83.Stern MC, Butler LM, Corral R, Joshi AD, Yuan JM, Koh W, et al. Polyunsaturated fatty acids, DNA repair single nucleotide polymorphisms and colorectal cancer in the Singapore Chinese study. J. Nutrigenetics Nutrigenomics. 2010;2:273–279. doi: 10.1159/000308467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gago-Dominguez M, Castelao JE, Sun CL, Van Den Berg D, Koh WP, Lee HP, et al. Marine n-3 fatty acid intake, glutathione S-transferase polymorphisms and breast cancer risk in postmenopausal Chinese women in Singapore. Carcinogenesis. 2004;25:2143–2147. doi: 10.1093/carcin/bgh230. [DOI] [PubMed] [Google Scholar]
- 85.Lenihan-Geels G, Bishop KS, Ferguson LR. Cancer risk and eicosanoid production: interaction between the protective effect of long chain omega-3 polyunsaturated fatty acid intake and genotype. J. Clin. Med. 2016;5:1–12. doi: 10.3390/jcm5020025. http://dx.doi.org/10.3390/jcm5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andrade-Vieria R, Han JH, Marignani PA. Omega-3 polyunsaturated fatty acid promotes the inhibition of glycolytic enzymes and mTOR signaling by regulating the tumor suppressor LKB1. Cancer Biol. Ther. 2013;14:1050–1058. doi: 10.4161/cbt.26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pópulo H, Lopes JM, Soares P. The mTOR signaling in human cancer. Int. J. Mol. Sci. 2012;13:1886–1918. doi: 10.3390/ijms13021886. http://dx.doi.org/10.3390/ijms13021886. [DOI] [PMC free article] [PubMed] [Google Scholar]