Abstract
Mitochondria affect numerous aspects of mammalian reproduction. We investigated whether the decrease in oocyte quality associated with aging is related to altered mitochondria.
Oocytes from old (12 months) and young (9 weeks) C57BL/6J mice were compared in relation to: mitochondria morphology and dynamics (mitochondria density, coverage, size and shape) throughout folliculogenesis; levels of mitochondrial DNA (mtDNA); mitochondrial stress reflected in the expression of mitochondrial unfolded protein response (mt-UPR) genes; and levels of reactive oxygen species (ROS) under baseline conditions and following H2O2 treatment.
In old mice, mitochondria of primary follicle-enclosed oocytes were smaller, with lower mitochondria coverage (total mitochondria µm2/µm2 cytosol area) (p<0.05). Other follicular stages showed a similar trend, but the changes were not significant. Mature oocytes (Metaphase II – MII) from old mice had significantly less mtDNA (p<0.01), and elevated mt-UPR gene Hspd1 expression (p<0.05), compared with those from young mice. ROS levels in aged MII oocytes were also higher following pretreatment with H2O2 (p<0.05).
Aging is associated with altered mitochondrial morphological parameters and decreased mtDNA levels in oocytes, as well as an increase in ROS under stressful conditions and elevated expression of mitochondrial stress response gene Hspd1. Delineation of the mechanisms underlying mitochondrial changes associated with ageing may help in the development of diagnostic and therapeutic tools in reproductive medicine.
Keywords: Reproduction, Aging, Oocyte, Mitochondria
1. Introduction
Mitochondria are double membrane-bound organelles that are ubiquitously present in the cytoplasm of eukaryotic cells. Mitochondria play a number of important roles in the cell, including energy production, calcium homeostasis, fatty acid oxidation, and apoptosis. Among these, the generation of adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS) is arguably their most important and best-delineated function.
During OXPHOS, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. In eukaryotes, these redox reactions are carried out by the electron transport chain (ETC), which consists of 5 protein complexes located in the inner mitochondrial membrane. The energy released by electrons flowing through the ETC is used to transport protons across the inner mitochondrial membrane, creating a proton gradient. When protons flow back across the inner mitochondrial membrane and down this gradient, ATP is generated form adenosine triphosphate (ADP) in a phosphorylation reaction. While OXPHOS is a vital part of metabolism, some premature electron leak inevitably occurs at the ETC, producing reactive oxygen species (ROS) such as superoxide and hydrogen peroxide, leading to propagation of free radicals, damaging cells and contributing to disease, and, possibly, aging.
Mitochondria are unique organelles, as they contain their own DNA, which is circular, double stranded, and 15–17 kb in length (reviewed in [1, 2]). It is estimated that 2–10 copies of mitochondrial DNA (mtDNA) are present in each mitochondrion, and a total of 100–10,000 copies in most somatic cells. In mammals, mtDNA contains 37 genes, 22 of which encode for transfer RNAs (tRNAs), and 2 encode for small and large ribosomal RNA (rRNA) subunits. The remaining 13 genes encode for proteins that are all part of the ETC, which consists of approximately 80 proteins. Consequently, the functional competence of ETC requires coordinated transcription from both mitochondrial and nuclear DNAs.
The free radical theory of aging proposed a central role for the mitochondrion as the principle source of intracellular ROS leading to mtDNA mutations [3]. The mutation rate of the mitochondrial genome has been estimated to be significantly higher than that of the nuclear genome due its close proximity to ETC, its lack of protective histones, and the seemingly limited DNA repair mechanisms available in the mitochondria. Therefore ROS was proposed as a major source of acquired mtDNA mutations associated with aging [4, 5]. Consistent with this theory, acquired mtDNA mutations and corresponding decline in mitochondrial function have been extensively reported in normal human aging (Reviewed in [6]). In addition, animal models with accelerated mtDNA mutation are associated with significantly reduced longevity, and a phenotype consistent with human aging, including cardiomyopathy, hemopoietic stem cell decline, reduced fertility, and frailty [7, 8]. Based on these observations, the free radical theory of aging postulated that the accumulation of mtDNA mutations would lead to abnormalities in mitochondrial respiratory chain proteins, causing partial uncoupling of the respiratory chain. This in turn would lead to further increased ROS and more mtDNA mutations, resulting in an exponential increase in mtDNA mutation burden. However, mtDNA mutation burden does not significantly increase during human aging, suggesting that a model based on ROS fails to explain the rate of accumulation and impact of mtDNA mutations throughout human life.
More recent findings suggest that the role of mitochondrial impairment in ageing is more complex than originally postulated. Multiple mitochondrial signaling pathways can induce ageing and cellular senescence (reviewed in [9]). Among these are impaired function of ETC and/or bioenergetic imbalance, altered mitochondrial dynamics (fusion and fission), accumulation of mitochondrial metabolites, and loss of mitochondrial calcium hemostasis (reviewed in [9]).
Female reproductive aging has been shown to be associated with increased rates of infertility, spontaneous abortion, and birth defects [10–12]. Age-related increase in unfavorable reproductive outcomes is believed to be secondary to decreased oocyte quality (reviewed in [13]). Alterations in mitochondrial DNA copy number, mitochondrial mutation load and mitochondrial function have been demonstrated in aged mammalian oocytes and thought to be either contributory to or a hallmark of oocyte aging [14–19].
In the current study, we aimed to characterize morphologic and molecular changes associated with ageing in oocyte mitochondria. We assessed mitochondria morphology and dynamics throughout folliculogenesis, determined mtDNA quantity, and evaluated mitochondrial stress reflected by the expression of mitochondrial unfolded protein response (mt-UPR) genes and levels of ROS under baseline conditions and following H2O2 treatment.
2. Materials and Methods
2.1 Electron microscopy and mitochondrial analysis
C57BL/6J retired breeder, 7–8 months old, female mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were maintained according to the Yale University animal research requirements. Food and water were provided ad libitum and animals were housed under a 12-hour light-dark cycle. The Institutional Animal Care and Use Committee approval was obtained prior to the initiation of the study (protocol number 2011–11207). All animal experiments complied with National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). Once these mice reached 12 months of age, 9 weeks old young female mice were purchased from Jackson Laboratories.
Five young (9 weeks) and 5 old (12 months) female mice were deeply anesthetized and perfused with saline containing heparin followed by fixative solution (Paraformaldehyde 4%, gluteraldehyde 0.1%, picric acid 15%, in phosphate buffer (PB) 0.1 M, pH = 7.4). Both ovaries were removed and fixed overnight at 4°C with the same fixative without gluteraldehyde and then dehydrated using ethanol gradient. Ovaries were then embedded in Durcupan, cut in an ultramicrotome and collected in grids for ultrastructural analysis with Tecnai 12 Biotwin electron microscope. Oocytes at different stages of follicular development were imaged at 1900× magnification. For larger oocytes, several images covering the entire oocyte were taken and Adobe Photoshop software was used to montage these photos into a single image. Image J software was used to manually outline each individual mitochondrion, nucleus and cell membrane in oocytes.
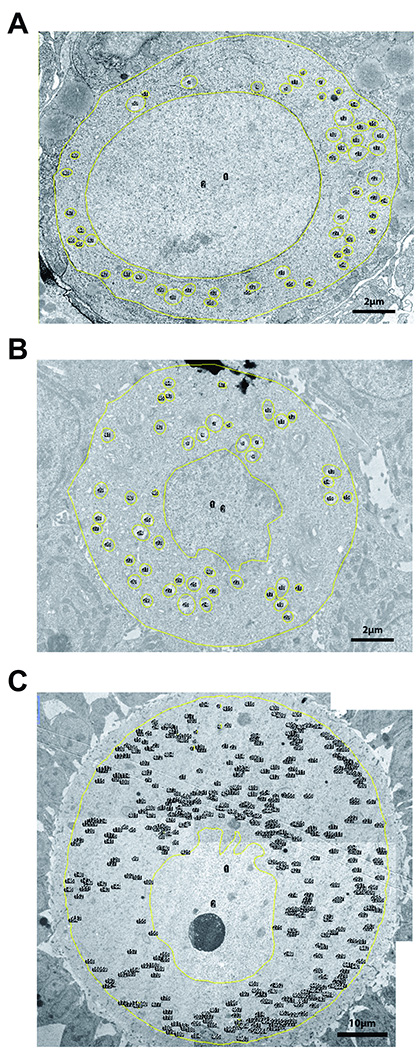
Mitochondria density and coverage were calculated by dividing the number and total area of mitochondria to the cytoplasm area, respectively. Mitochondria cross-sectional area was used as the measurement of mitochondria size. Mitochondrion aspect ratio was used as an index of mitochondrion shape (AR, major axis divided by minor axis, minimum value is 1.0) (Figure 1). Two investigators blindly estimated these parameters. A total of 9 primordial, 6 primary, 3 preantral and 8 antral follicles were examined in the young group. These numbers were 6, 5, 9 and 7 for the old group, respectively.
Figure 1. Representative images of mouse ovarian follicles used for electron microscopic studies.
A) primordial B) primary C) early antral follicle. Yellow lines outline cell membrane, nucleus and mitochondria. Cytosol area is calculated by subtracting nucleus area from whole cell area. Mitochondria density is calculated by dividing the total number of mitochondria to cytosol area. Mitochondria coverage is calculated by dividing total mitochondria area to cytosol area. Mitochondrion area (average mitochondrion size) is calculated by dividing the total mitochondria area to total mitochondria number. Mitochondrion aspect ratio is calculated by dividing major mitochondrion axis by minor mitochondrion axis (minimal value is 1.0). For larger follicles (C) several photos of the follicle were taken to cover the entire oocyte area at desired resolution to be able to visualize mitochondria. These photographs were then montaged into a single image before analysis.
2.2 Quantification of mtDNA levels in mouse somatic tissues and oocytes
For somatic tissues, the relative amount of mitochondrial DNA to nuclear genome was calculated. Total DNA was extracted from young (7–8 weeks) and old (10–12 months) mice tissues with Phenol:Chloroform:Isoamyl Alcohol (25:24:1, v/v) (Invitrogen) using standard protocol. Mitochondrial and nuclear DNA copy number were determined by qPCR of mitochondrial Cox3 gene and nuclear Ndufv1 gene (for the list of primers see Table 1). Quantitative PCRs were carried out on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories). Reactions were performed in triplicates. Each 15 µl reaction contained 7.5 µl of SYBR Green supermix (Bio-Rad Laboratories), 0.4 µM of each primer, and 1 µl of DNA. Amplifications were carried out using 40 cycles of PCR, in which the initial 5 min for denaturation at 95°C was followed by 40 cycles of 95°C for 10 s and 60°C for 60 s. A standard curve for each set of primers was first used to determine the linear dynamic range of each reaction and PCR efficiency. A melting curve analysis was used to exclude nonspecific amplifications. The 2−ΔΔCT method was used for analysis.
Table 1.
The list of primers used for quantitative RT-PCR.
| Gene | TaqMan assay number or Primer sequences (5' to 3'; F, forward; R, reverse) |
|---|---|
| β-actin | Mm00607939_s1 |
| Clpp | Mm00489940_m1 |
| Dnaja3 | Mm00469723_m1 |
| Gapdh | Mm99999915_ g1 |
| Hspd1 | Mm00849835_g1 |
| Hspe1 | Mm00434083_m1 |
| Cox3 | F: TTTGCAGGATTCTTCTGAGC R: TGAGCTCATGTAATTGAAACACC |
| Ndufv1 | F: CTTCCCCACTGGCCTCAAG R: CCAAAACCCAGTGATCCAGC |
Abbreviations: Clpp, caseinolytic peptidase P; Dnaja3, DnaJ heat shock protein family member A3; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Hspd1 heat shock 60 kDa protein 1 (Chaperonin 60); Hspe1 heat shock 10 kDa protein 1 (Chaperonin 10); Ndufv1, NADH dehydrogenase (ubiquinone) flavoprotein 1.
To quantify mtDNA levels in mature, metaphase II (MII) oocytes Cox3 fragment was amplified using the primers in Table 1 and subcloned into pCR™2.1-TOPO® - cloning vector (Invitrogen). One Shot TOP10 Chemically Competent E. coli were transformed and grown overnight at 37°C. Recombinant plasmids were purified using Qiagen plasmid isolation kit and the inserted mtDNA fragment was confirmed by DNA sequence analysis. Plasmid DNA was quantified using NanoDrop 2000 spectrophotometer (Thermo Scientific). A standard curve from 108 to 101 plasmid molecules was generated by serial 10-fold dilutions.
Single MII oocyte was lysed in 10 µl lysis solution containing 125 µg/ml Proteinase K and 17 µM SDS in sterile water by incubating at 55°C for 2 hours. Then Proteinase K was inactivated by heating the lysis mix at 95°C for 10 min and the mix was used directly for downstream PCR. Reactions were performed in triplicates. Each 10 µl reaction contained 5 µl of SYBR Green supermix (Bio- Rad Laboratories), approximately 0.3 µM of each primer, and 1/3 of total oocyte DNA. Oocyte mtDNA copy numbers were extrapolated from the standard curve. Overall, 52 oocytes from 6 young mice, and 33 oocytes from 7 old mice were tested in 4 independent experiments.
2.3 Mouse oocyte and granulosa cell collection
To collect granulosa cells, young (8–10 weeks) and old (10–12 months) mice were intraperitoneally injected with pregnant mare serum gonadotropin (PMSG, Sigma-Aldrich). Mice were euthanized via CO2 inhalation 44 hours after injection; ovaries were dissected in PBS and cleaned from surrounding fat tissue. Antral follicles were then punctured with 26.5-gauge needle under the dissecting microscope (Olympus SZH-ILLK) in M-199 medium and granulosa cells were separated from ovarian tissue using a 0.40-µm strainer and collected by centrifugation at 1500g for 5 min. Granulosa cells from 2 (young) or 3 (old) mice were pooled into one group and there were three groups for both young and old mice in each experiment. Experiments were repeated 3 times.
To collect germinal vesicle (GV) stage oocytes, the same procedures above were performed and GV oocytes were collected following antral follicle puncture. Oocytes from 2 (young) or 3–4 (old) mice were pooled into one group and there were three groups for both young and old mice in each experiment. Experiments were repeated 3 times.
To collect MII oocytes, young and old mice were injected with 5 IU of PMSG, followed by 5 IU of human chorionic gonadotropin (hCG, Sigma-Aldrich) after 48 hours. MII oocytes were collected from the oviducts 14 hours after hCG injections.
2.4 RNA extraction, Reverse transcription, and quantitative real time Polymerase chain reaction (qPCR)
Total RNA was isolated from GV oocytes and granulosa cells using Trizol (Invitrogen) according to the manufacturer’s instructions, dissolved in 20 µl of DEPC-water, and kept at −80°C until use. The quality and concentration of the RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific). RNA samples were treated with DNase I (Sigma-Aldrich, cat # AMPD1) according to the manufacturer’s recommendations. Two µg of total RNA were reverse transcribed to cDNA using oligo-dT priming at 37°C for 1 hour (Omniscript; QIAGEN). Quantitative real-time PCR was used to detect the expression of Clpp, Dnaja3, Hspd1, and Hspe1 (for the list of primers see Table 1). cDNA was assayed in triplicate. TaqMan Gene expression assays (Life Technologies) were used following manufacturer’s instructions. Briefly, each 20 µl reaction contained 1 µl of 20× TaqMan gene expression assay, 10 µl of 2× TaqMan Gene expression master mix, 4 µl of cDNA template and 5 µl of H2O. Quantitative RT-PCRs were carried out on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories), in which the initial 10 min for denaturation at 95°C was followed by 40 cycles of 95°C/15 sec and 60°C/1 min. Expression of the target gene was normalized to two housekeeping genes: Gapdh and β-actin. The 2−ΔΔCT method was used to calculate relative gene expression.
2.5 Determination of ROS levels in mouse oocytes
6-carboxy-2',7'-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) (Life Technologies, cat # c-400) was used to asses ROS levels in mouse oocytes. This nonfluorescent chemical is a general oxidative stress indicator. It passes through the oocyte plasma membrane and converts to green fluorescent form by oxidation with ROS. It stays inside the cell for a prolonged period because of its negative charges [20]. First, we conducted proof of principle experiment by exposing oocytes to H2O2 and assessed subsequent H2DCFDA fluorescence, which demonstrated that this method could indeed detect increased ROS levels in oocytes (data not shown). Second, we incubated GV and MII oocytes from young (7–8 weeks) and old (10–12 months) mice with 30 µM H2DCFDA in HEPES buffered MEMα for 20 min to compare baseline ROS levels in young and old oocytes. Third, we induced ROS generation by exposing both GV and MII oocytes to 20mM H2O2 for 5 min and then incubated these oocytes with 30 µM H2DCFDA in HEPES buffered MEMα for 20 min. In both experiments, oocytes were washed 3 times in H2DCFDA-free media and images were captured on Zeiss 510 confocal microscope (Carl Zeiss Microscopy, Thornwood, NY). ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantify fluorescence.
2.6 Statistical analysis
D'Agostino&Pearson omnibus and Shapiro-Wilk tests were used to determine normality. Unpaired t test, Mann-Whitney test or One-way ANOVA with Tukey or Dunnett’s post tests were performed to compare two or more groups, respectively. Posttest for linear trend was used following one-way ANOVA to determine changes in oocyte mitochondrial parameters with follicular development. All tests were performed using GraphPad Prism software version 6.0. Statistical significance was defined as p<0.05.
3. Results
3.1 Mitochondrial morphologic parameters change with follicular development in young and old mouse oocytes
Mitochondria affect all aspects of mammalian reproduction, including oocyte maturation, fertilization and embryonic development [13]. It was proposed that decreased oocyte quality and increased aneuploidy rate with aging could be explained with abnormal mitochondria numbers and/or function [10, 13, 14, 17–19, 21, 22]. Therefore, we set out to determine mitochondria dynamics in oocytes and compare young and old mice oocytes at different stages of follicular development using various electron microscopic parameters.
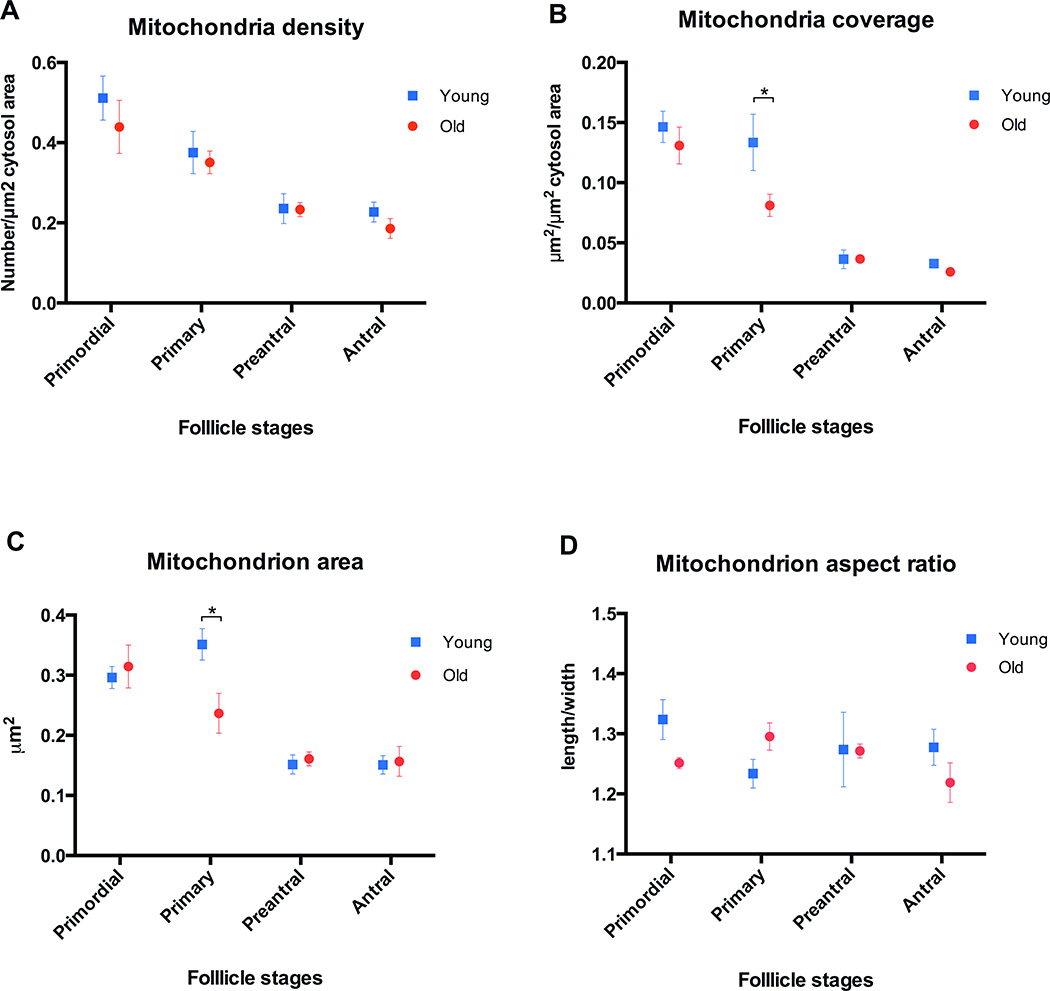
Testing for linear trend demonstrated that oocyte mitochondria density (number/µm2 cytosol area) decreases in both young and old mice with follicular development (p<0.05). Comparison of oocytes in follicles at the same stage of development between young and old groups showed that there is a trend towards decreased density in old mouse oocytes, especially in the antral follicles. However, these differences did not reach statistical significance (Figure 2A).
Figure 2. Characterization of mitochondrial parameters of young and old mice oocytes at different stages of follicular development.
A) Mitochondria density (number/µm2 cytosol area) decreases in both young and old mice oocytes with follicular development from primordial to antral stage (p<0.01). Although there is a trend toward decreased density in aged oocytes compared to the young oocytes at the same developmental stage, it does not reach statistical significance B) Mitochondria coverage (total mitochondria µm2/µm2 cytosol area) decreases in both young and old mice oocytes with follicular development (p<0.01). Mitochondria coverage of aged mouse oocytes in primary follicles is decreased compared to young oocytes at the same developmental stage (p<0.05). Although there is a trend toward decreased mitochondria coverage in old oocytes at other stages compared to the young oocytes, it does not reach statistical significance. C) Mitochondrion area (average mitochondrion size) decreases in both young and old mice oocytes with follicular development (p<0.01). Mitochondria of oocytes in primary follicles from aged mice are significantly smaller than their young counterparts (p<0.05). D) The shape (aspect ratio) of mitochondria is not significantly different between young and old oocytes at the same stage of follicular development, and there is not a significant trend in mitochondrial shape changes with follicular development in either group. Error bars represent standard error of the mean. Asterisks indicate statistical significance (p<0.05). Post test for linear trend following one-way ANOVA was used to determine statistically significant changes in mitochondrial parameters associated with follicular development. Unpaired t-test was used to compare young and old oocytes at the same developmental stage.
Mitochondria coverage (total mitochondria µm2/µm2 cytosol area) of mouse oocytes decreased in both young and old mice with follicular development, as was demonstrated by one-way ANOVA post linear trend testing (p<0.05). Moreover, mitochondria coverage of old mouse oocytes in primary follicles was reduced in comparison to young ones (p<0.05). As in the case for mitochondrial density, there was a trend towards decreased mitochondrial coverage in aged oocytes at other follicular development stages, but it did not reach statistical significance (Figure 2B).
Mitochondria became smaller as follicles grew from primordial to antral stage in both young and old mice (p<0.05). Moreover, old oocyte mitochondria size from primary follicles was significantly smaller than their young counterparts (p<0.05) (Figure 2C). The shape (aspect ratio) of the mitochondria did not differ significantly between old and young groups (Figure 2D).
3.2 Aging is associated with altered mtDNA levels in mouse somatic tissues and oocytes
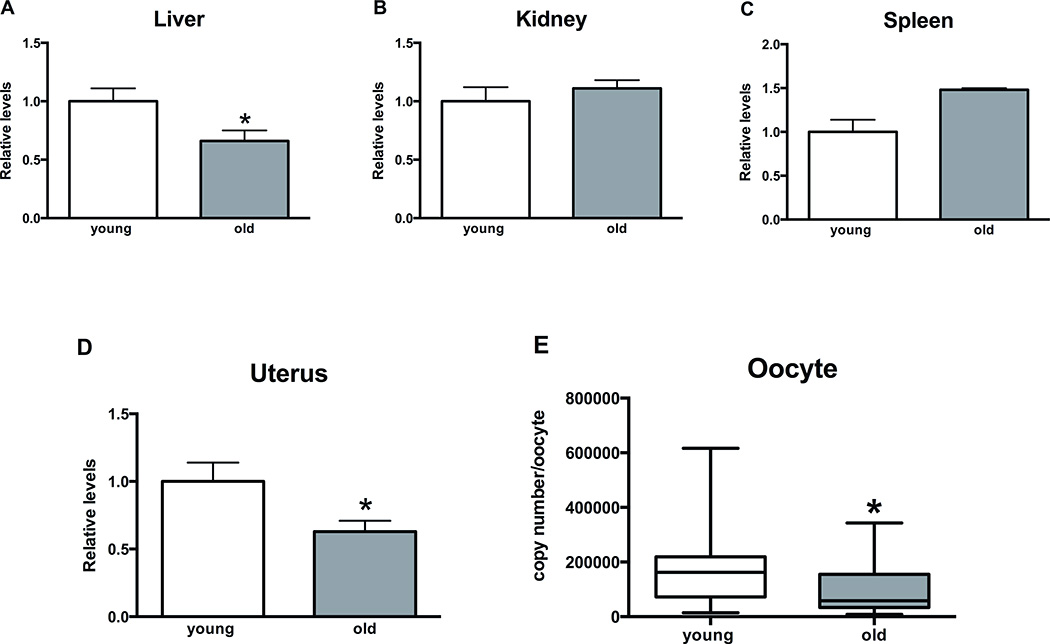
mtDNA levels in mammalian oocytes seem to correlate with their developmental potential [23–29]. Therefore, we analyzed mtDNA levels in aged mouse oocytes and somatic tissues. mtDNA levels were decreased by approximately 35% in old mice liver and uterus compared to young ones (p<0.05) (Figure 3A, D). Kidney mtDNA levels did not change with aging (Figure 3B). Although there was a trend towards increase in mtDNA levels in spleen of aged mice, it did not reach statistical significance (Figure 3C). MII oocytes demonstrated significant decrease in mtDNA copy numbers with aging (p<0.01) (Figure 3E). The median for young oocytes was 162106 copies/oocyte and the median for old oocytes was 58222 copies/oocyte. Considerable variation was observed in mtDNA copy numbers among individual oocytes with standard error of the mean of 19302 and 15429 for young and old groups, respectively.
Figure 3. Changes in mitochondrial DNA (mtDNA) levels in mice tissues (relative) and oocytes (absolute) with aging.
A, D) mtDNA levels decrease by approximately 35% in mice livers and uteri with aging (p<0.05). B, C) Aging does not affect mtDNA levels in mice kidneys and spleens. E) mtDNA copy numbers decrease significantly in mice MII oocytes with aging. Median for young and old oocytes was 162106 and 58222, (p<0.01). Unpaired t-test and Mann-Whitney test were used for statistical analysis for somatic tissues and oocytes, respectively. Asterisks indicate statistical significance (p<0.05). Error bars represent standard error of the mean for graphs A–D. Whiskers represent minimum to maximum values for graph E.
3.3 Hspd1 demonstrates increased expression in aged mouse oocytes, and Hspe1 demonstrates increased expression in aged mouse granulosa cells
It was demonstrated that mitochondrial unfolded protein response (mt-UPR) is involved in lifespan regulation in C. elegans and mammals [30–32]. We analyzed the expression levels of mt-UPR associated genes in aged mouse oocytes and granulosa cells, to dissect mechanisms implicated in follicular aging.
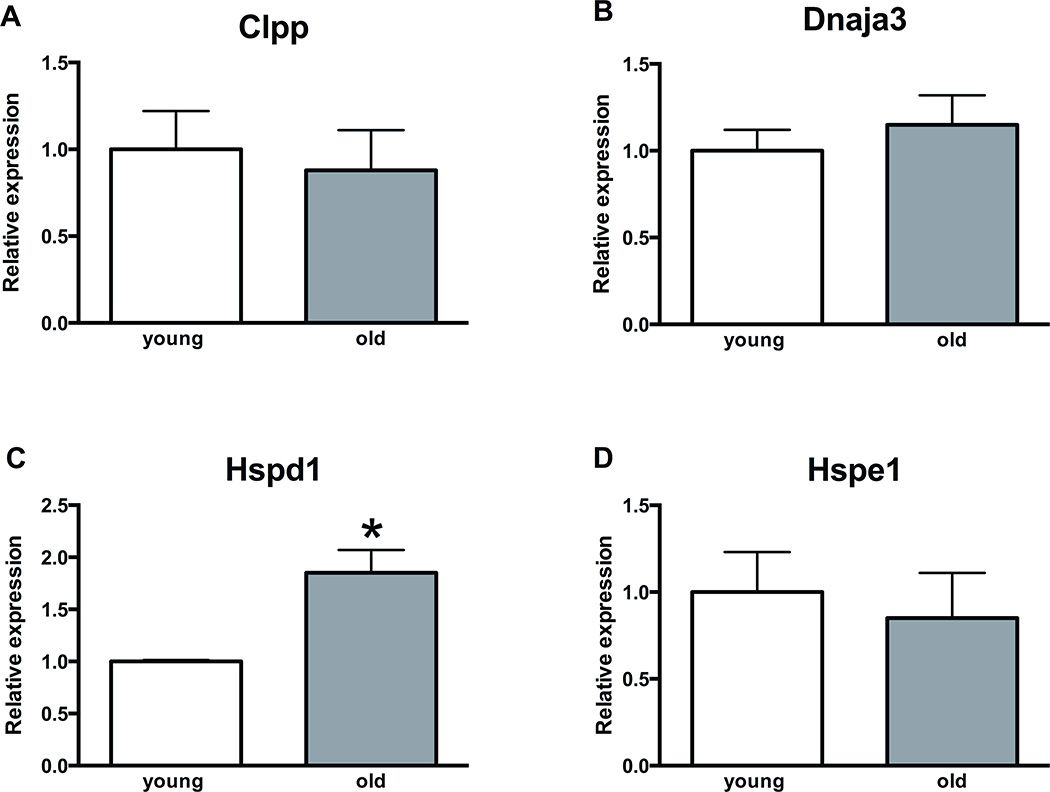
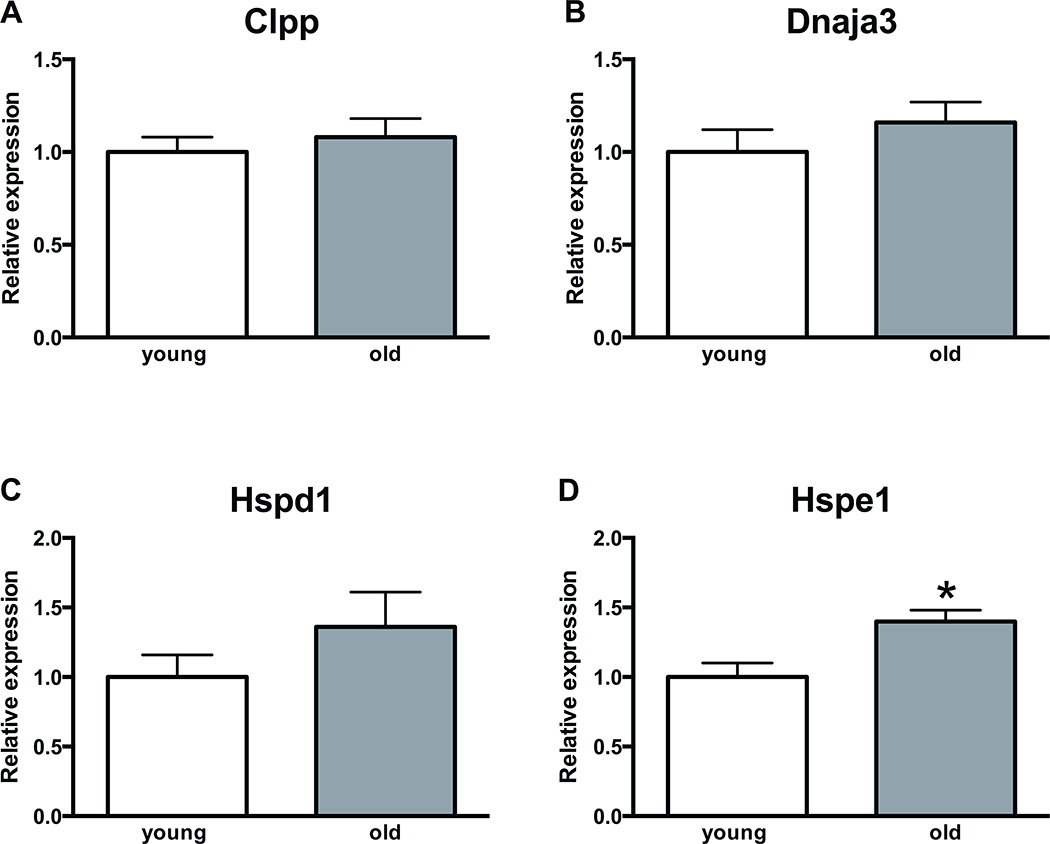
Gene expression analysis of mt-UPR genes in mouse oocytes and granulosa cells demonstrated that aging is associated with upregulated transcript levels of Hspd1 (p<0.05) in mouse oocytes and Hspe1 in mouse granulosa cells (p<0.05). Other mt-UPR associated genes, Clpp and Dnaja3, did not demonstrate altered expression (Figure 4, 5).
Figure 4. Expression of mitochondrial unfolded protein response genes in aged mice oocytes.
A, B, D) The levels of Clpp, Dnaja3 and Hspe1 do not change in mice oocytes with aging. C) Hspd1 demonstrates approximately 2-fold increase in transcript levels in aged mice oocytes (p<0.05). Error bars represent standard error of the mean. Asterisk indicates statistical significance. Unpaired t-test was used for statistical analysis.
Abbreviations: Clpp, caseinolytic peptidase P; Dnaja3, DnaJ heat shock protein family member A3; Hspd1: heat shock 60 kDa protein 1 (Chaperonin 60); Hspe1: heat shock 10 kDa protein 1 (Chaperonin 10)
Figure 5. Expression of mitochondrial unfolded protein response genes in aged mice granulosa cells.
A, B, C) The levels of Clpp, Dnaja3 and Hspd1 do not change in mice granulosa cells with aging. D) Hspe1 demonstrates approximately 1.5-fold increase in transcript levels in aged mice granulosa cells (p<0.05). Gene names are as in Figure 4. Error bars represent standard error of the mean. Asterisk indicates statistical significance. Unpaired t-test was used for statistical analysis.
Abbreviations: Clpp, caseinolytic peptidase P; Dnaja3, DnaJ heat shock protein family member A3; Hspd1: heat shock 60 protein 1; Hspe1: heat shock protein 1 (chaperonin 10)
3.4 Aging negatively affects anti-oxidant response of mouse oocytes
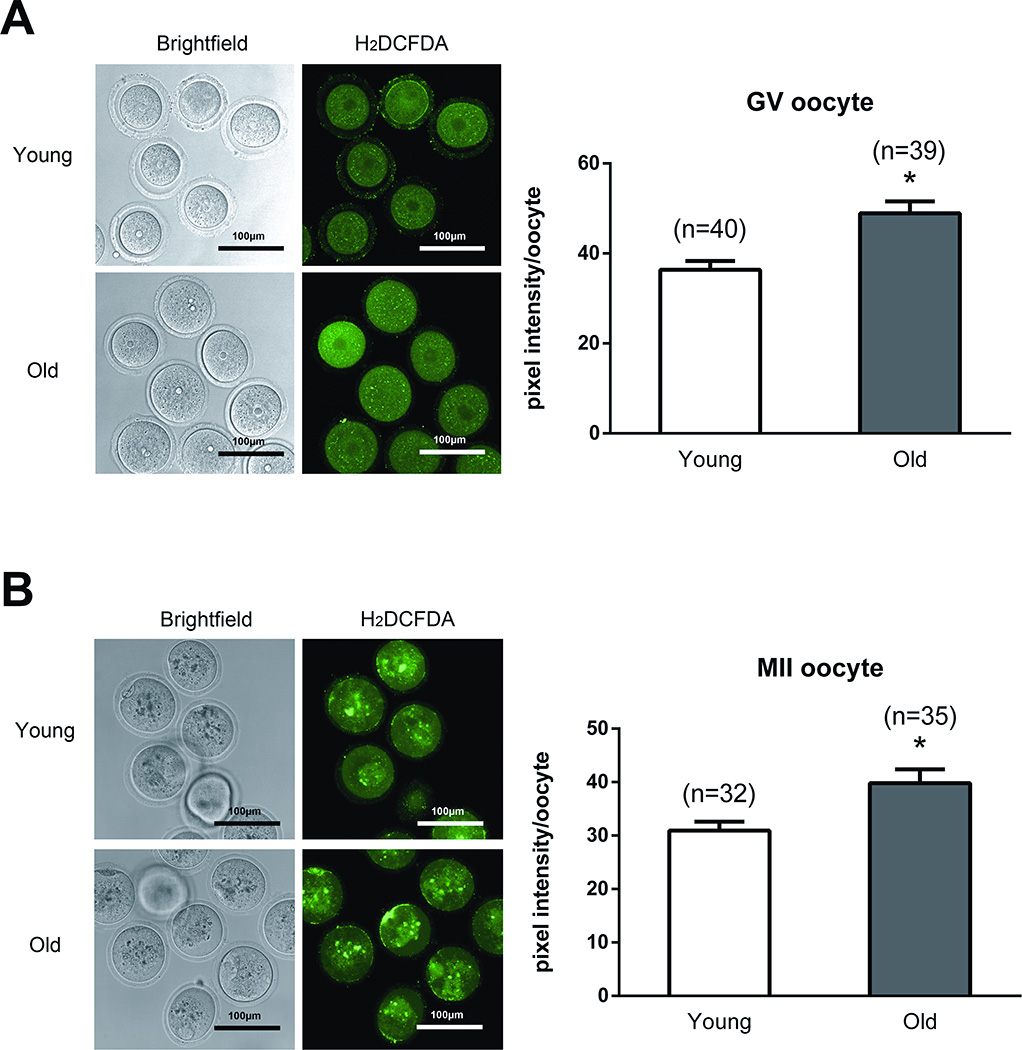
Studies have demonstrated that ROS can cause mtDNA damage and induce cellular senescence [9, 33]. We investigated ROS levels in mice oocytes under baseline conditions and following exposure to high levels of H2O2, one of the major ROS within the cell [9]. The intensity of ROS detecting fluorescence was not different in young and old GV and MII oocytes under baseline conditions (data not shown). However, ROS levels were significantly increased in old GV and MII oocytes compared to young ones after exposure to H2O2 (Figure 6). This difference observed in the intensity of ROS detecting fluorescence between the young and old oocytes can be explained by compromised antioxidant defense mechanisms of older oocytes.
Figure 6. ROS levels in young and old mice oocytes after treatment with H2O2.
A. ROS levels are significantly elevated in old mice germinal vesicle stage oocytes following H2O2 treatment. B. ROS levels are significantly elevated in old mice MII oocytes after treatment with H2O2. Fluorescence intensity of Carboxy-H2DCFDA was used to measure ROS levels. Asterisks indicate statistical significance. Unpaired t-test was used for statistical analysis.
Abbreviations: ROS, reactive oxygen species; H2O2: hydrogen peroxide; carboxy-H2DCFDA: 6-carboxy-2',7'-dichlorodihydrofluorescein diacetate.
4. Discussion
The number of women delivering their first child after the age of 35 increased from 1/100 to 1/12 from 1970 to 2006 [34]. Pregnancy rates decrease progressively with increasing female age both following intra-uterine insemination with donor sperm [35] and after in-vitro fertilization [36]. The rates of spontaneous abortions also increase with advanced maternal age [37, 38]. Delayed childbearing is associated with aged oocytes at conception, which are of poor quality primarily due to increased aneuploidy rates [11, 12]. Altered mitochondrial function and quantity are thought to be contributory to decreased oocyte quality in mammals (reviewed in [13]).
Multiple studies have demonstrated that 10–15-month-old C57BL/6J (our model) and other female mouse strains demonstrate significant decrease in fertility compared to 3 month-old or younger mice. They have fewer follicles present at all stages of follicular development; ovulate significantly decreased numbers of total and matures oocytes; have significantly increased numbers of degenerate/abnormal oocytes; demonstrate significantly decreased yield of embryos; have increased rates of oocyte aneuploidy, spindle abnormalities and chromosomal misalignment; demonstrate altered gene expression profiles; and show dramatic decrease in synchronous chromosomal segregation during meiosis [10, 39–41]. Importantly, a study by Selesniemi et al., analyzed 12-month-old C57BL/6J female mice compared to 3 month-old and showed dramatic changes in multiple reproductive parameters, including follicle, oocyte, embryo numbers, aneuploidy rate and spindle abnormalities, all of which negatively affect fertility [10]. In our study, we chose 12 month-old C57BL/6J female mouse as a model of reproductive aging, because this allowed us to investigate the effects of aging by providing the sufficient number of follicles/oocytes, as well as they were “old enough” to demonstrate these effects. We could have obtained more follicles/oocytes with younger mice (<10 months), but the effects of aging on reproduction of these mice are either unknown or not as profound. On the other hand, older mice >12 months-old would have likely demonstrated more profound effects of aging on mice reproductive tissues, but our sample size won’t be large enough to draw definitive conclusions.
In this study, we found that mitochondrial dynamics of follicle-enclosed oocytes from old mice show differences compared to those obtained from young mice, and mature oocytes from old mice have significantly lower amount of mtDNA compared to young ones. In addition, the expression of mt-UPR gene Hspd1, and ROS levels following pretreatment with H2O2 are elevated in aged oocytes. Our findings build upon a number of previous reports, and establish a difference between young and old female germlines regarding the morphology, molecular landscape, and stress response of mitochondria.
To characterize the differences between young and old mice oocyte mitochondria, we first assessed their morphology using electron microscopy. Previous studies had shown that mitochondria of mature MII oocytes differ from those of somatic cells as they are small (<1 µm in diameter), spherical, and contain fewer and truncated cristae surrounding a dense matrix [42]. During pre-implantation embryo development, mitochondria become more elongated (2.5 µm in diameter in 8-cell human embryos [42]), with higher number of cristae and lower density matrix [43]. This morphologic transition is completed by the time of the first embryonic cellular differentiation, which results in the formation of trophoectoderm (TE) and inner cell mass (ICM), at 5 to 6 days post-fertilization in humans [44].
In the current study, we assessed mitochondria of follicle-enclosed oocytes from young and old mice at different stages of follicular development and applied recently established mitochondrial morphologic parameters for each stage (Figure 1 and 2). The size and mitochondrial coverage [total area covered by mitochondria (µm2) in an oocyte/total area of the oocyte cytosol (µm2)] was smaller in oocytes from older mice. The difference was statistically significant in primary follicles, while the other stages showed a trend that did not reach statistical significance. A similar trend was observed for a decreased mitochondria density [mitochondria number/cytosol area (µm2)] in oocytes from older mice. Considering similar trend at all follicular stages, we believe increased population size could lead to significant differences. Importantly, we observed a decrease in mitochondrial size, coverage, and density throughout follicular development (from primordial to antral) in both old and young groups. Our findings establish parameters for oocyte mitochondrial dynamics throughout folliculogenesis, and suggest that mitochondria from older mice oocytes show a trend toward being smaller and covering a lesser portion of the cytoplasmic surface area. The differences in oocyte mitochondrial dynamics observed between young and old mice could reflect a potential decrease in the overall functional capacity of mitochondria associated with ageing. Alternatively, observed differences could be due to an altered energetic state of the ageing oocyte.
Human and mouse mature oocytes are estimated to contain between 50,000 to 500,000 mtDNA copies, with a significant degree of variation between studies as well as samples [26, 45–48]. The mtDNA copy number found in oocytes remains unchanged during fertilization and cleavage divisions, and mtDNA replication only resumes at the time of blastocyst formation (reviewed in [1, 2]). Studies investigating age-associated changes in mtDNA copy number in human oocytes reported conflicting findings, some suggesting a decrease [48], and others an increase [45, 49] associated with ageing. Most recently, an elegant study by Fragouli et al. [50] showed that in reproductively older women (average age 39.8 years, range 38–42), mtDNA copy number is decreased in cleavage stage embryos, and increased in blastocysts compared to reproductively younger women (average age 34.8 years, range 26–37). Our findings in the mouse model are consistent with those of Fragouli et al. as oocytes reflect cleavage stage embryo mtDNA content due to the later start of mtDNA replication at the blastocyst stage.
In compartmentalized eukaryotic cells various molecular pathways ensure the integrity of the protein-folding environments in the cytosol, endoplasmic reticulum (ER) and mitochondria. These pathways have evolved independently and sense unfolded protein stress in compartment specific manner, and signal to the nucleus for induction of the expression of UPR genes. The expression of these genes is essential for proteostasis [39]. The mt-UPR is a stress response that activates transcription of nuclear-encoded mitochondrial chaperone genes to promote protein homeostasis within the organelle. We tested the expression of four known mediators of mt-UPR in oocytes and granulosa cells of young and old mice at baseline (in the absence of induced stress), and detected an increased expression of Hspd1 in oocytes and Hspe1 in granulosa cells, while Clpp and Dnaja3 were unchanged (Figures 4 and 5). We then tested the ROS levels in young and old oocytes under baseline conditions and following exposure to high levels of H2O2. The intensity of ROS detecting fluorescence was similar in young and old GV and MII oocytes under baseline conditions, while ROS levels were significantly increased in old GV and MII oocytes compared to young ones after exposure to H2O2 (Figure 6). Collectively, these findings suggest compromised antioxidant defense mechanisms in older oocytes, associated with an elevated baseline mt-UPR stress response.
We find that aging is associated with a significant increase in ROS levels in oocytes under stressful conditions and elevated expression of mitochondrial stress response genes. Importantly, aged mouse oocytes have lower mtDNA levels and several of their mitochondrial morphological parameters seem to be altered. Our findings help further characterize cellular and molecular changes in oocyte mitochondria associated with ageing. The objective parameters established in this study may enable better assessment of the role of candidate genes in mitochondrial biology and ageing in animal models and potentially contribute to our understanding of human reproductive ageing.
Highlights.
Aging is associated with altered mitochondrial morphological parameters and decreased mtDNA levels in oocytes
Expression of mitochondrial stress response gene Hspd1 is increased in aged oocytes
Levels of reactive oxygen species in response to stressful conditions are elevated in aged oocytes
Acknowledgments
Funding
This research was funded by Award R01HD059909 from the National Institute of Health (NIH) and the National Natural Science Foundation of China (81501247).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
ES has full access to all of the data in the study, takes responsibility for the data, the analyses and interpretation, and the conduct of the research, and has the right to publish any and all data, separate and apart from the attitudes of the sponsor.
ES and EB are responsible for the study concept and design.
TW, KS-B and TH performed the electron microscopy and mitochondrial analysis.
TW and EB quantified the mtDNA levels in somatic tissues and oocytes.
EB, TW and KL did the oocyte and granulosa cell collection.
EB and KL performed RNA extraction, reverse transcription, and qPCR.
TW detected ROS levels in oocytes.
TW and EB performed the data analysis.
ES, HST and TH are responsible for interpretation of the data.
ES and EB drafted the manuscript.
ES, TH and HST undertook critical revision of the manuscript for important intellectual content.
ES provided administrative, technical, and material support, and was responsible for study supervision.
All of the authors reviewed the manuscript prior to submission.
The two lead authors, EB and TW, made an equal contribution.
Conflict of interest
None declared.
Ethical approval
The Institutional Animal Care and Use Committee approval was obtained prior to the initiation of the study (protocol number 2011–11207). All animal experiments complied with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Provenance and peer review
This article has undergone peer review.
REFERENCES
- 1.St John J. The control of mtDNA replication during differentiation and development. Biochim Biophys Acta. 2014;1840:1345–1354. doi: 10.1016/j.bbagen.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 2.St John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16:488–509. doi: 10.1093/humupd/dmq002. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 4.Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995;27:647–653. doi: 10.1016/1357-2725(95)00025-k. [DOI] [PubMed] [Google Scholar]
- 5.Greaves LC, Beadle NE, Taylor GA, Commane D, Mathers JC, Khrapko K, Turnbull DM. Quantification of mitochondrial DNA mutation load. Aging Cell. 2009;8:566–572. doi: 10.1111/j.1474-9726.2009.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne BA, Chinnery PF. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim Biophys Acta. 2015;1847:1347–1353. doi: 10.1016/j.bbabio.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 8.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlöf S, Oldfors A, Wibom R, Törnell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler DV, Wiley CD, Velarde MC. Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell. 2015;14:1–7. doi: 10.1111/acel.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging- associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- 12.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24:86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Babayev E, Seli E. Oocyte mitochondria function and reproduction. Curr Opin Obstet Gynecol. 2015;27:175–181. doi: 10.1097/GCO.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, Yoshimura Y. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30:1367–1375. doi: 10.1007/s10815-013-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells D, Kaur K, Grifo J, Glassner M, Taylor JC, Fragouli E. Clinical utilisation of a rapid low-pass whole genome sequencing technique for the diagnosis of aneuploidy in human embryos prior to implantation. J Med Genet. 2014;51:553–562. doi: 10.1136/jmedgenet-2014-102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chappel S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet Gynecol Int. 2013;2013:183024. doi: 10.1155/2013/183024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simsek-Duran F, Li F, Ford W, Swanson RJ, Jones HWJ, Castora FJ. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS One. 2013;8:e64955. doi: 10.1371/journal.pone.0064955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rambags BP, van Boxtel DC, Tharasanit T, Lenstra JA, Colenbrander B, Stout TA. Advancing maternal age predisposes to mitochondrial damage and loss during maturation of equine oocytes in vitro. Theriogenology. 2014;81:959–965. doi: 10.1016/j.theriogenology.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Rebolledo-Jaramillo B, Su MS, Stoler N, McElhoe JA, Dickins B, Blankenberg D, Korneliussen TS, Chiaromonte F, Nielsen R, Holland MM, Paul IM, Nekrutenko A, Makova KD. Maternal age effect and severe germ-line bottleneck in the inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 2014;111:15474–15479. doi: 10.1073/pnas.1409328111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66:143–152. doi: 10.1002/mrd.10341. [DOI] [PubMed] [Google Scholar]
- 21.Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online. 2004;2004:45–58. doi: 10.1016/s1472-6483(10)60497-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Wu XQ, Lu S, Guo YL, Ma X. Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 2006;16:841–850. doi: 10.1038/sj.cr.7310095. [DOI] [PubMed] [Google Scholar]
- 23.El Shourbagy SH, Spikings EC, Freitas M, St John JC. Mitochondria directly influence fertilisation outcome in the pig. Reproduction. 2006;131:233–245. doi: 10.1530/rep.1.00551. [DOI] [PubMed] [Google Scholar]
- 24.Viet Linh N, Kikuchi K, Nakai M, Tanihara F, Noguchi J, Kaneko H, Dang-Nguyen TQ, Men NT, Van Hanh N, Somfai T, Nguyen BX, Nagai T, Manabe N. Fertilization ability of porcine oocytes reconstructed from ooplasmic fragments produced and characterized after serial centrifugations. J Reprod Dev. 2013;59:549–556. doi: 10.1262/jrd.2013-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Reynier P, May-Panloup P, Chrétien MF, Morgan CJ, Jean M, Savagner F, Barrière P, Malthièry Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Hum Reprod. 2001;7:425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- 27.Lee SK, Zhao MH, Kwon JW, Li YH, Lin ZL, Jin YX, Kim NH, Cui XS. The association of mitochondrial potential and copy number with pig oocyte maturation and developmental potential. J Reprod Dev. 2014;60:128–135. doi: 10.1262/jrd.2013-098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge H, Tollner TL, Hu Z, Dai M, Li X, Guan H, Shan D, Zhang X, Lv J, Huang C, Dong Q. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol Reprod Dev. 2012;79:392–401. doi: 10.1002/mrd.22042. [DOI] [PubMed] [Google Scholar]
- 29.Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill S, Van Remmen H. Mitochondrial stress signaling in longevity: a new role for mitochondrialfunction in aging. Redox Biol. 2014;2:936–944. doi: 10.1016/j.redox.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz AM, Haynes CM. UPR(mt)-mediated cytoprotection and organismal aging. Biochim Biophys Acta. 2015;1847:1448–1456. doi: 10.1016/j.bbabio.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen MB, Jasper H. Mitochondrial proteostasis in the control of aging and longevity. Cell Metab. 2014;20:214–225. doi: 10.1016/j.cmet.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barja G. The mitochondrial free radical theory of aging. Prog Mol Biol Transl Sci. 2014;127:1–27. doi: 10.1016/B978-0-12-394625-6.00001-5. [DOI] [PubMed] [Google Scholar]
- 34.Cil AP, Turkgeldi L, Seli E. Oocyte Cryopreservation as a Preventive Measure for Age-Related Fertility Loss. Semin Reprod Med. 2015;33:429–435. doi: 10.1055/s-0035-1567819. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz D, Mayaux MJ. Female fecundity as a function of age: results of artificial insemination in 2193 nulliparous women with azoospermic husbands. N Engl J Med. 1982;306:404–406. doi: 10.1056/NEJM198202183060706. [DOI] [PubMed] [Google Scholar]
- 36.SART. National summary and fertility clinic reports. USA: Centers for disease control; 2010. Assisted reproductive technology success rates. [DOI] [PubMed] [Google Scholar]
- 37.Balasch J, Gratacos E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol. 2012;24:187–193. doi: 10.1097/GCO.0b013e3283517908. [DOI] [PubMed] [Google Scholar]
- 38.Farr SL, Schieve LA, Jamieson DJ. Pregnancy loss among pregnancies conceived through assisted reproductive technology, United States, 1999–2002. Am J Epidemiol. 2007;165:1380–1388. doi: 10.1093/aje/kwm035. [DOI] [PubMed] [Google Scholar]
- 39.Tarín JJ, Pérez-Albalá S, Cano A. Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod. 2001;65:141–150. doi: 10.1095/biolreprod65.1.141. [DOI] [PubMed] [Google Scholar]
- 40.Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, Jessberger R, Kirkwood TB, Höög C, Herbert M. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–1521. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motta PM, Nottola SA, Makabe S, Heyn R. Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod. 2000;15(suppl 2):129–147. doi: 10.1093/humrep/15.suppl_2.129. [DOI] [PubMed] [Google Scholar]
- 43.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Sathananthan AH, Trounson AO. Mitochondrial morphology during preimplantational human embryogenesis. Hum Reprod. 2000;15(Suppl 2):148–159. doi: 10.1093/humrep/15.suppl_2.148. [DOI] [PubMed] [Google Scholar]
- 45.Steuerwald N, Barritt JA, Adler R, Malter H, Schimmel T, Cohen J, Brenner CA. Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote. 2000;8:209–215. doi: 10.1017/s0967199400001003. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Prosser R, Simonetti S, Sadlock J, Jagiello G, Schon EA. Rearranged mitochondrial genomes are present in human oocytes. Am J Hum Genet. 1995;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- 47.Pikó L, Taylor KD. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987;123:364–374. doi: 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- 48.Chan CC, Liu VW, Lau EY, Yeung WS, Ng EH, Ho PC. Mitochondrial DNA content and 4977 bp deletion in unfertilized oocytes. Mol Hum Reprod. 2005;11:843–846. doi: 10.1093/molehr/gah243. [DOI] [PubMed] [Google Scholar]
- 49.Müller-Höcker J, Schäfer S, Weis S, Münscher C, Strowitzki T. Morphological–cytochemical and molecular genetic analyses of mitochondria in isolated human oocytes in the reproductive age. Mol Hum Reprod. 1996;2:951–958. doi: 10.1093/molehr/2.12.951. [DOI] [PubMed] [Google Scholar]
- 50.Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, Kokocinski F, Cohen J, Munne S, Wells D. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11:e1005241. doi: 10.1371/journal.pgen.1005241. [DOI] [PMC free article] [PubMed] [Google Scholar]