Abstract
Background
A common sequel of tooth extraction is alveolar bone resorption. It makes the placement of dental implants difficult and creates an esthetic problem for the fabrication of conventional prostheses. Therefore, alveolar bone following tooth extraction should be preserved.
Aims and objectives
The present prospective study was conducted to evaluate the efficacy of the resorbable bioscaffold poly lactic co-glycolic acid (PLGA) in maintaining the alveolar height in post-extraction socket.
Material and methods
20 patients were selected based on inclusion and exclusion criteria and were randomly divided into two groups: cases and control comprising of 10 patients each. Atraumatic tooth extraction was done in all patients. PLGA bioscaffold was placed in cases and socket was closed with 3-0 vicryl. In control group, socket was directly closed with 3-0 vicryl. The patients were kept on follow-up and complications such as dry socket, pain, and swelling were recorded. IOPA were taken at 1st, 4th, 12th, and 24th week to record changes in the height of alveolar bone. The radiographic measurements were compared and the differences were statistically analyzed.
Results
Reduction in alveolar bone height after placement of PLGA bioscaffold was significantly less in cases as compared to controls at 4th, 12th, and 24th week following extraction. No complications were observed throughout the follow-up period.
Conclusion
PLGA scaffold significantly reduces bone resorption. Application is very simple and can be easily performed in a dental setup. However, PLGA scaffold adds significantly to the cost of treatment.
Keywords: Socket preservation, PLGA, Bioscaffold
1. Introduction
One of the most widely performed dental treatments today is tooth extraction.1 After a tooth is extracted, blood clot gets filled within extraction socket. It then slowly remodels and provides a suitable matrix for ingrowth of bone from the surrounding buccal bone.2 However, it is well documented that a significant dimensional change of the alveolar ridge is induced by this procedure.1
Thus, alveolar ridge resorption occurs commonly after tooth extraction.3 It is affected by load transferred to the overlying bone surface as well as from pressures within the bone.4
The overall reduction in height and width of the horizontal ridge has been reported to reach to about 40% and 60% of pre-extraction alveolar ridge height and width, respectively.5 Although the bone resorption continues over time after tooth extraction, during the first month, the most statistically significant loss of alveolar dimension occurs.6
Alveolar ridge resorption can create a clinical problem in two different ways: one, for the fabrication of conventional or implant-supported prostheses, an esthetic problem is created and second, it can make dental implants placement very difficult or sometimes even impossible. Therefore, following tooth extraction preservation of alveolar bone dimensions is always desirable.3
After the extraction, multiple types of bone graft materials including autograft, allograft, xenograft, and alloplastic materials can be used for socket preservation in combination with or without a barrier membrane.1, 2, 4, 5, 7, 8, 9, 10, 11, 12, 13, 14, 15 The gold-standard grafting material is the autografts but they suffer from disadvantages such as defect size limitation, increased surgical time, and also can cause donor site morbidity. Allografts and xenografts address disadvantages of autografts, but they are less osteoconductive, are not vascularized, exhibit poor mechanical properties and also carry an increased risk of infection. To overcome the limitations and drawbacks of allografts, autografts, and xenografts, synthetic bone grafting materials and composite materials such as hydroxyapatite and beta-tricalcium phosphate have been developed more recently. These materials are osteoconductive, but have a disadvantage of having a long degradation time thereby inhibiting full regeneration of the bone.2, 4, 16, 17, 18
Finally, some studies have also used synthetic polymers, such as PLA or PLGA, for bone augmentation. It can be used as a space filler10, 11, 19, 20, 21 or a membrane.2, 22, 23 PLGA is synthetic, biodegradable, biocompatible,24, 25, 26 and its clinical use is widespread.2, 27, 28 It is used in both oral-maxillofacial and general orthopedic applications and is capable of delivering drugs,26 proteins, and growth factors27 to enhance bone healing.2, 24, 27
Although used extensively, literature supporting the use of poly lactic co-glycolic acid (PLGA) in maintaining ridge height after tooth extraction is scant, thereby creating the need to assess its success clinically and radiographically.
2. Materials and methods
A prospective study was done on 20 patients requiring extraction based on exclusion and inclusion criteria.
Inclusion criteria include:
-
(1)
All healthy adult patients.
-
(2)
Age group 25–45 years.
-
(3)
Teeth to be extracted should have minimum periapical changes radiographically and probing depth not more than 2–3 mm clinically.
-
(4)
Teeth which can be extracted by intra-alveolar (forceps method) under local anesthesia.
Exclusion criteria include:
-
(1)
Medically compromised patients (systemic diseases affecting bone metabolism).
-
(2)
Teeth having radiographically evident large periapical changes (abscess/granuloma/cyst).
-
(3)
Teeth requiring transalveolar/open extraction.
All patients were explained of the procedure and after taking consent, patients were randomly divided into two groups: cases and control group consisting of 10 patients each.
Detailed case history was taken. Routine hematological investigations were carried out. A pre-operative IOPA using paralleling technique was taken before initiating the procedure.
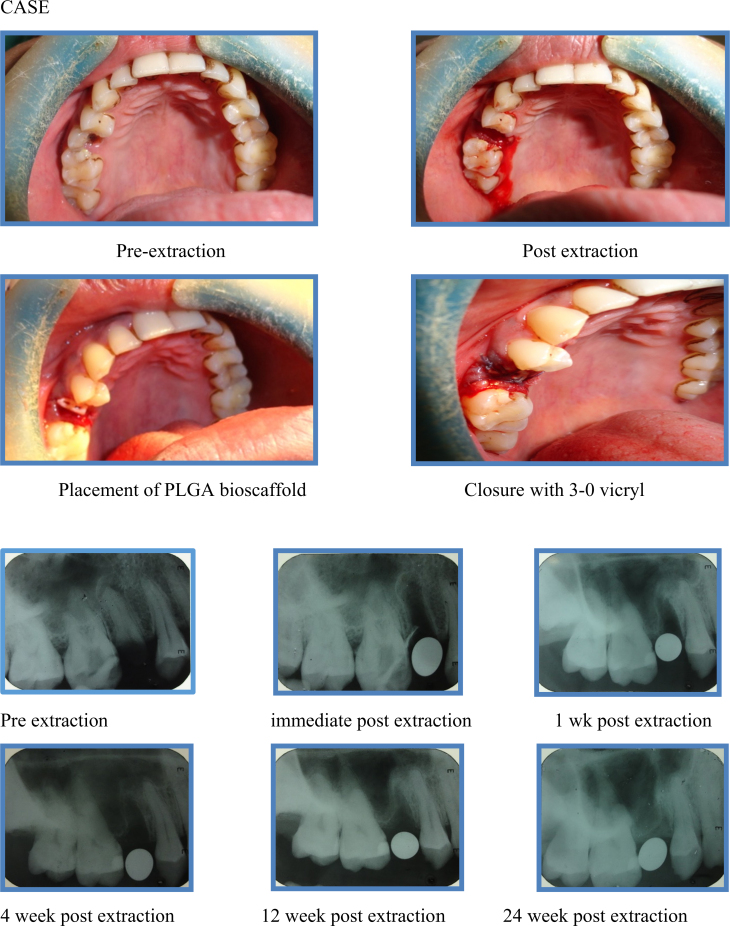
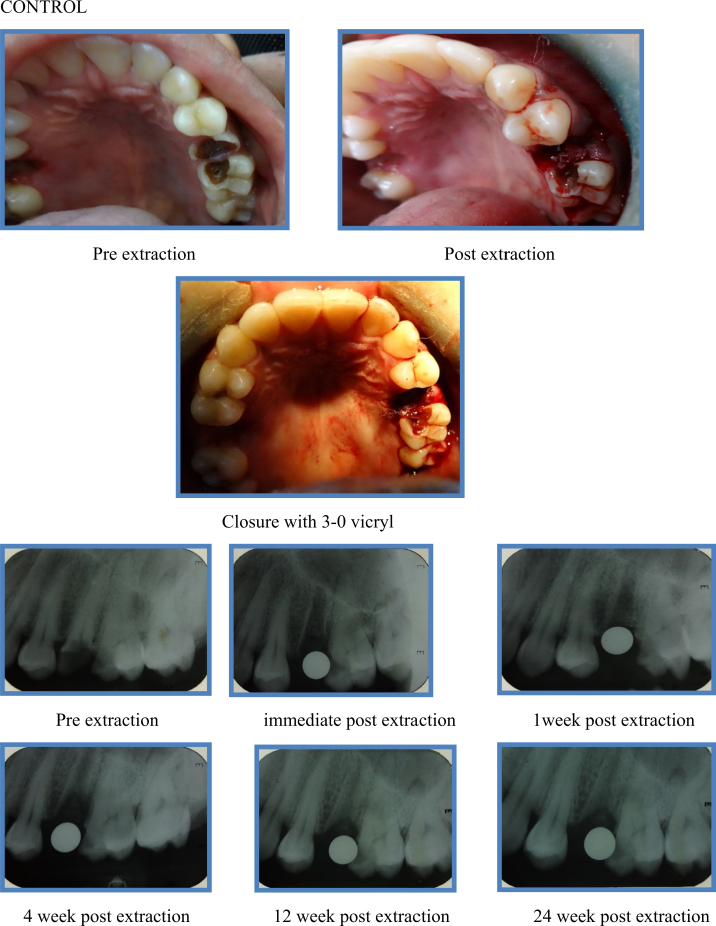
The procedure was performed under local anesthesia (lignocaine 2% with epinephrine 1:100,000) by the same surgeon to avoid bias. Tooth extraction was done carefully, with minimal soft tissue reflection and without causing any trauma to the underlying alveolar bone. The socket was then gently irrigated with normal saline and hemostasis was achieved. Immediately after the extraction, a sterile poly lactic co glycolic acid (PLGA) bioscaffold was placed into the socket in such a way that it fits snugly into the extraction socket till the level of the alveolar margins. Once it is placed, the socket was closed with 3-0 vicryl to prevent the graft from getting displaced. In control group, the socket was directly closed with 3-0 vicryl without using any graft material.
After extraction, patients were instructed to apply gentle pressure on the gauze pack over the operated site for a period of 30 min. Chemical plaque control with chlorhexidine gluconate solution (0.2% 1 min, TID) was advised 24 h following the procedure for the first post-operative week. Antibiotics (Cap. amoxycillin 500 mg + clavulanic acid 125 mg BD for five days) and anti-inflammatory drugs (Tab. ibuprofen 400 mg + paracetamol 325 mg TID for five days) were prescribed. The patients were recalled and complications such as dry socket, pain, and swelling, pus discharge, if any, were recorded. Post-operative IOPA were taken at 1st week, 4th week, 12th week, and 24th week. The radiographs were taken using the paralleling technique to eliminate bias due to radiographic elongation or foreshortening. Radiographs obtained were traced and this tracing was used to record the measurement of height of the alveolar socket. The measurements obtained using post-operative radiographs were compared and the differences were statistically analyzed.
2.1. Statistical analysis
The measurements were expressed using mean values and standard deviations. The differences between the measurements recorded in the cases and control groups were analyzed using a t-test. A p value of less than 0.05 was considered to be statistically significant.
3. Results
Out of 10 patients selected for cases, six patients were male (60%) and four were female (40%). In control group, five patients were male (50%) while five were female (50%). The mean age of patients for cases was 30.2 ± 7.62 (range 19–41 years) while for controls was 31.3 ± 7.53 years (range 20–42 years). There was no statistically significant difference between the two groups as regard to gender and age (p value for gender and age was 0.653 and 0.750 respectively).
Mean crestal bone height pre-operatively, immediately after extraction as well as one week after extraction was 12.95 ± 6.43 mm for cases while 12.7 ± 1.1 mm for controls. There was no statistically significant difference present between the two groups pre-operatively, immediate post-extraction, and at one week after extraction (p value 0.550).
At 4th week, the mean crestal bone height recorded for cases was 12.66 ± 0.61 mm while for controls it was 11.82 ± 1.09 mm. At 12th week, the mean crestal bone height was 12.34 ± 0.62 mm for cases and 11.14 ± 1.01 mm for controls while at 24th week it was 12.04 ± 0.63 mm for cases and 10.55 ± 1.03 mm for controls.
Difference between the two groups was statistically significant at 4th week, 12th week, and 24th week (p value was 0.047, 0.005, <0.001 respectively).
Difference in crestal bone height between pre-operatively and at 24 weeks after extraction was 0.91 ± 0.14 mm for cases and 2.15 ± 0.18 mm for controls. Statistically significant difference was present between the two groups (p value < 0.05).
Percent reduction in height between pre-operative and at 24 weeks after extraction for cases was 7.03 ± 1.13% while for controls it was 16.98 ± 1.44%. Difference between the two groups was statistically significant (p value < 0.05).
Clinically, complications such as pain, swelling, pus discharge, and dry socket were not observed throughout the follow-up period in any of the patients belonging to case as well as control group (Fig. 1, Fig. 2).
Fig. 1.
Case.
Fig. 2.
Control.
4. Discussion
PLGA is a synthetic, biodegradable copolymer of poly lactic acid (PLA) and poly glycolic acid (PGA).24, 26 This copolymer is degraded by hydrolysis and its byproducts are excreted in urine. It can be used as a space filler10, 11, 19, 20, 21 or a membrane.2, 22, 23 In our study, we used PLGA copolymer in the form of scaffolds. The scaffold serves to provide a temporary structure for cells to proliferate and maintain their differentiated function before being resorbed by the body.18, 24
The PLGA was not used as a membrane in our study as membrane technique presents some clinical disadvantages like difficulty in obtaining the complete coverage of the membrane, which if gets exposed to the oral environment and may be colonized by bacteria and the risk of collapse of the membrane in the socket if the membrane is not supported by filling graft materials.10
During the first week of the study, no major changes in the alveolar bone height were observed in both case and the control group. However, from 4th week onwards till the 24th week, it was clearly evident from the results that there was significant less bone resorption in cases (0.91 ± 0.14 mm) where a biodegradable, synthetic scaffold of PLGA was inserted following tooth extraction as compared to controls (2.15 ± 0.18 mm).
In cases, there was only 7.03 ± 1.13% of alveolar bone resorption at the end of 24 weeks as compared to controls where 16.98 ± 1.44% of alveolar bone resorption was seen which shows that the use of PLGA scaffolds after extraction significantly reduces alveolar ridge resorption.
The results of the study were in accordance with the study conducted by Serino et al. who had showed that alveolar bone resorption following tooth extraction may be prevented or reduced by the use of a bioresorbable synthetic sponge of polylactide–polyglycolide acid.10
During this study, we did not encounter any complications like pain, swelling, or pus discharge. This may be due to good oral hygiene exhibited by the patients. However, it also demonstrates that PLGA is biocompatible and is well accepted by the patient.
The main drawback of this study was that no histological analysis of the grafted site was done at the end of 24 weeks. So the quality of newly formed bone and the presence of remaining PLGA bioscaffold, if any, at the extraction site could not be determined.
5. Conclusion
The clinical use of the resorbable bioscaffold PLGA in post-extraction socket is highly predictable modality for maintaining the alveolar bone height. The placement of scaffold is very simple and can be easily mastered and performed in a dental setup. It must however be noted that patient selection and oral hygiene maintenance play a crucial role in the success of PLGA in post-extraction socket. Also, PLGA scaffolds add significantly to the cost of treatment.
Conflicts of interest
The authors have none to declare.
References
- 1.Horowitz R., Holtzclaw D., Rosen P.S. A review on alveolar ridge preservation following tooth extraction. J Evid Based Dent Pract. 2012;12(3):149–160. doi: 10.1016/S1532-3382(12)70029-5. [DOI] [PubMed] [Google Scholar]
- 2.Brown A., Zaky S., Ray H., Jr., Sfeir C. Porous magnesium/PLGA composite scaffolds for enhanced bone regeneration following tooth extraction. Acta Biomater. 2015;11:543–553. doi: 10.1016/j.actbio.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Camargo P.M., Lekovic V., Carnio J., Kenney E.B. Alveolar bone preservation following tooth extraction: a perspective of clinical trials utilizing osseous grafting and guided bone regeneration. Oral Maxillofac Surg Clin N Am. 2004;16(1):9–18. doi: 10.1016/j.coms.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Block M.S. Treatment of the single tooth extraction site. Oral Maxillofac Surg Clin N Am. 2004;16(1):41–63. doi: 10.1016/j.coms.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Kesmas S., Swasdison S., Yodsanga S., Sessirisombat S., Jansisyanont P. Esthetic alveolar ridge preservation with calcium phosphate and collagen membrane: preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(5):24–36. doi: 10.1016/j.tripleo.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Ten Heggeler J., Slot D., Van der Weijden G. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: a systematic review. Clin Oral Implant Res. 2011;22:779–788. doi: 10.1111/j.1600-0501.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 7.Gottlow J. Guided tissue regeneration using bioresorbable and non-resorbable devices: initial healing and long-term results. J Periodontol. 1993;64(11):1157–1165. doi: 10.1902/jop.1993.64.11s.1157. [DOI] [PubMed] [Google Scholar]
- 8.Lekovic V., Kenney E.B., Weinlaender M. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997;68:563–570. doi: 10.1902/jop.1997.68.6.563. [DOI] [PubMed] [Google Scholar]
- 9.Zubillaga G., Von Hagen S., Simon B., Deasy M. Changes in alveolar bone height and width following post-extraction ridge augmentation using a fixed bioabsorbable membrane and demineralized freeze-dried bone osteoinductive graft. J Periodontol. 2003;74:965–975. doi: 10.1902/jop.2003.74.7.965. [DOI] [PubMed] [Google Scholar]
- 10.Serino G., Biancu S., Iezzi G., Piattelli A. Ridge preservation following tooth extraction using a polylactide and polyglycolide sponge as space filler: a clinical and histological study in humans. Clin Oral Implants Res. 2003;14:651–658. doi: 10.1034/j.1600-0501.2003.00970.x. [DOI] [PubMed] [Google Scholar]
- 11.Nair P.N.R., Schug J. Observations on healing of human tooth extraction sockets implanted with bioabsorbable polylactic-polyglycolic acids (PLGA) copolymer root replicas: a clinical, radiographic, and histologic follow-up report of 8 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(5):559–569. doi: 10.1016/S1079210403006334. [DOI] [PubMed] [Google Scholar]
- 12.McAllister B., Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78(3):377–396. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]
- 13.Caiazzo A., Brugnami F., Mehra P. Buccal plate augmentation: a new alternative to socket preservation. J Oral Maxillofac Surg. 2010;68(10):2503–2506. doi: 10.1016/j.joms.2010.05.044. [DOI] [PubMed] [Google Scholar]
- 14.Eskow A.J., Mealey B.L. Evaluation of healing following tooth extraction with ridge preservation using cortical versus cancellous freeze-dried bone allograft. J Periodontol. 2014;85(4):514–524. doi: 10.1902/jop.2013.130178. [DOI] [PubMed] [Google Scholar]
- 15.Rothamel D., Schwarz F., Herten M. Dimensional ridge alterations following socket preservation using a nanocrystalline hydroxyapatite paste: a histomorphometrical study in dogs. Int J Oral Maxillofac Surg. 2008;37(8):741–747. doi: 10.1016/j.ijom.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Bertoldi C., Zaffe D., Consolo U. Polylactide/polyglycolide copolymer in bone defect healing in humans. Biomaterials. 2008;29(12):1817–1823. doi: 10.1016/j.biomaterials.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Laurie S.W., Kaban L.B., Mulliken J.B., Murray J.E. Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg. 1984;73(6):933–938. doi: 10.1097/00006534-198406000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y., Lim J., Teoh S.H. Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv. 2013;31(5):688–705. doi: 10.1016/j.biotechadv.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Serino G., Rao W., Iezzi G., Piattelli A. Polylactide and polyglycolide sponge used in human extraction sockets: bone formation following 3 months after its application. Clin Oral Implants Res. 2008;19(1):16–31. doi: 10.1111/j.1600-0501.2007.01311.x. [DOI] [PubMed] [Google Scholar]
- 20.Suhonen J., Meyer B. Polylactic acid (PLA) root replica in ridge maintenance after loss of a vertically fractured incisor. Endod Dent Traumatol. 1996;12:155–160. doi: 10.1111/j.1600-9657.1996.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 21.Suhonen J., Suuronen R., Hietanen J., Marinello C., Tormala P. Custom made polyglycolic acid (PGA)-root replicas placed in extraction sockets of rabbits. Dtsch Z Mund Kiefer Gesichts Chir. 1995;19:253–257. [Google Scholar]
- 22.Jung R., Kokovic V., Jurisic M., Yaman D., Subramani K., Weber F. Guided bone regeneration with a synthetic biodegradable membrane: a comparative study in dogs. Clin Oral Implants Res. 2011;22(8):802–807. doi: 10.1111/j.1600-0501.2010.02068.x. [DOI] [PubMed] [Google Scholar]
- 23.Lekovic V., Camargo P., Klokkevold P. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998;69(9):1044–1049. doi: 10.1902/jop.1998.69.9.1044. [DOI] [PubMed] [Google Scholar]
- 24.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 25.Ignatius A.A., Claes L.X. In vitro biocompatibility of bioresorbable polymers: poly(l, dl-lactide) and poly(l-lactide-co-glycolide) Biomaterials. 1996;17:631–639. doi: 10.1016/0142-9612(96)81421-9. [DOI] [PubMed] [Google Scholar]
- 26.Makadia H., Siegel S. Poly lactic-co-glycolic acid (PLGA) as a biodegradable controlled drug delivery carrier. Polymers. 2011;3(3):1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen H., Hu X., Yang F., Bei J., Wang S. An injectable scaffold: rhBMP-2-loaded poly(lactide-co-glycolide)/hydroxyapatite composite microspheres. Acta Biomater. 2010;6(2):455–465. doi: 10.1016/j.actbio.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Virlan M.J.R., Miricescu D., Totan A. Current uses of poly(lactic-co-glycolic acid) in the dental field: a comprehensive review. J Chem. 2015:1–12. [Google Scholar]