Abstract
The global burden of chronic obstructive pulmonary disease (COPD) continues to grow in part due to better outcomes in other major diseases and in part because a substantial portion of the worldwide population continues to be exposed to inhalant toxins. However, a disproportionate burden of COPD occurs in people of low socioeconomic status (SES) due to differences in health behaviors, sociopolitical factors, and social and structural environmental exposures. Tobacco use, occupations with exposure to inhalant toxins, and indoor biomass fuel (BF) exposure are more common in low SES populations. Not only does SES affect the risk of developing COPD and etiologies, it is also associated with worsened COPD health outcomes. Effective interventions in these people are needed to decrease these disparities. Efforts that may help lessen these health inequities in low SES include 1) better surveillance targeting diagnosed and undiagnosed COPD in disadvantaged people, 2) educating the public and those involved in health care provision about the disease, 3) improving access to cost-effective and affordable health care, and 4) markedly increasing the efforts to prevent disease through smoking cessation, minimizing use and exposure to BF, and decreasing occupational exposures. COPD is considered to be one the most preventable major causes of death from a chronic disease in the world; therefore, effective interventions could have a major impact on reducing the global burden of the disease, especially in socioeconomically disadvantaged populations.
Keywords: health disparities, COPD, interventions, prevention, management, international, socioeconomic status
Introduction
Health disparities are a major contributor in chronic obstructive pulmonary disease (COPD) around the world.1,2 COPD is not unique, as health disparities are found with other chronic diseases and throughout the continuum of care. However, the influence of health disparities and socioeconomic status (SES) on the etiology and outcomes of major noncommunicable chronic diseases is more evident with COPD. Environmental risk factors for developing COPD are more common in low SES people, which include tobacco smoking, environmental tobacco smoke (ETS), indoor and outdoor air pollution, and biomass fuel (BF) exposure.1 Furthermore, genetic tendencies for asthma3 and nicotine addiction4 may contribute to disparities in COPD since some race/ethnic/tribal groups are overrepresented in low SES stratum in different countries. According to the World Health Organization (WHO), >90% of COPD deaths occur in low-income and middle-income countries (LMICs).5 In an effort to reduce the magnitude of COPD in populations that bear a disproportionate burden of disease, initiatives by national and international organizations are presently targeting disparities in respiratory diseases. Continuity in disease prevention and delivery of health care as well as access to appropriate medications for COPD needs to be provided to all segments of the population, particularly the disadvantaged. This article provides perspective on the health disparities related to SES in COPD and strategies that may help in efforts to provide quality health care in this population.
Methods
We conducted systematic searches of the literature in English or articles with English abstracts using bibliographic databases of PubMed/Medline (National Library of Medicine and National Institutes of Health), Embase, and the Cochrane database. We used the keywords “COPD” or “emphysema” or “chronic bronchitis”, “socioeconomic status”, “poverty”, “health disparities”, “prevalence”, and “outcomes”.
Definitions of health disparities and SES
For all chronic diseases, multiple socioeconomic factors contribute to health disparities including income, education, residential segregation, genetic susceptibility, stress, access to health care, social and physical environment, and employment. Unequal access to quality health care is the foundation of health disparities. However, in general, health disparities are not only a function of whether an individual can afford to buy goods and services that aid good health but also include health behaviors related to lifestyle and the environment. Unhealthy behaviors such as tobacco smoking often provide pleasure and relaxation that help regulate mood,6 particularly in low SES people who have significant stressors in life.
Disparities exist when differences in health outcomes or health determinants are observed between populations. A number of organizations have defined health-related disparities including the WHO,7 the United States (US) Office of Disease Prevention and Health Promotion,6 and international pulmonary organizations such as the American Thoracic Society (ATS), and European Respiratory Society (ERS).8 The WHO defines health disparities as:
Inequalities that exist when members of certain population groups do not benefit from the same health status as other groups. Further, these disparities occur in the provision of healthcare and access to healthcare across different racial, ethnic and socioeconomic groups.7
In Healthy People 2020, the US Office of Disease Prevention and Health Promotion describes disadvantaged populations as:
Groups of people who have experienced greater obstacles to health based on their racial or ethnic group; religion, SES, gender, age, occupation, mental health, cognitive, sensory or physical disability or other characteristics linked to discrimination or exclusion.6
A joint 2013 ATS/ERS committee published a policy statement broadly defining disparities in respiratory health similar to the Healthy People 2020 definition – specifically “significant differences in respiratory health that are closely linked to racial ancestry, social, economic and/or environmental differences”.8
SES is a composite assessment of a person’s economic and social position in a society based on several factors including income, occupation, home and neighborhood environment, and educational attainment. These factors are correlated, and it is difficult to identify the independent contribution of each factor on the composite assessment of SES. However, many investigators use a single component of SES, for example, educational attainment or annual income, to represent a person’s overall economic and social position in their country. In studies evaluating the SES, education is often defined by years of educational attainment (ie, less than high school (secondary school), high school (secondary school graduate), some college/university, or college/university graduate.
Income is usually defined by individual annual income or cumulative household income. Since the median annual income varies widely between countries, the income-based definition of “low SES” can also vary widely across the globe. The WHO defines extreme poverty as an individual income less than or equal to the equivalent of 2.00 US dollar (USD) per day.9 The United Kingdom, like most of the developed world, defines poverty as 60% of median income in a country. However, in the US, poverty is defined by the US Census Bureau by comparing income to be three times the cost of a minimum food diet in 1963, which is updated annually for inflation and adjusted for family size, composition (ie, number of adults and children in a household), and age of householders.10 For example, in 2014, the US poverty level for a family of five persons was ~29,000 USD annual income, which was 53.7% of the 54,000 USD median household income.
Other infrequently used measures of SES include geographical area deprivation which categorizes neighborhoods by global positioning system and median income; number of persons living in household; and occupation (eg, manual labor in industry, service industry, office worker, agricultural worker, housewife, professional worker, and machine operator).11
Health disparities in respiratory diseases
Health disparities are more commonly seen for respiratory diseases. The lowest socioeconomic groups are up to 14 times more likely to have respiratory disease than the highest group.8 An individual’s socioeconomic position usually has continuous and numerous health effects that accumulate over a lifetime, and these long-term environmental exposures lead to diseases such as COPD. Disparities related to SES are evident for a number of respiratory diseases including asthma, COPD, lung cancer, and respiratory tract infections such as tuberculosis.8 Lung cancer is clearly more likely to occur in people of low SES, even when adjusting for tobacco use.10 In some countries, treated tuberculosis is a relatively common cause of COPD in people of low SES living in low-income, multiple person dwellings.1
Compared to other common causes of morbidity and death, COPD appears to be more socioeconomically related than others. In a study considering various causes of death, the association was stronger for COPD than cardiac disease.11 This is principally true because smoking and environment play leading roles in lung disease development – and people of lower SES tend to have substantially greater exposures to both of these factors. Although asthma affects all ethnic groups, suboptimal outcomes are disproportionately shared by certain minority groups and economically disadvantaged persons.12,13 However, low income has a greater influence on COPD prevalence than on asthma prevalence,14 this may be because asthma is more genetically driven whereas COPD is more environmentally induced.
According to the WHO, the social determinants of health are mostly responsible for the health inequities seen within and between countries.15 The WHO defines social determinants of health as the conditions in which people are born, grow, work, live, age, and the wider set of forces and systems shaping the conditions of daily life. Whitehead described the principal determinants of health disparities arising from multiple sources; Table 1 provides the correlations relative to COPD.16
Table 1.
Whitehead’s principal determinants of health disparities and COPD correlates
| General determinants of health disparities | Corresponding determinants for COPD |
|---|---|
| Natural biologic variation | Genetic susceptibilities |
| Health-damaging behavior of individuals | Smoking tobacco and/or biomass fuel use |
| Transient health advantage for people who adopt new behaviors first | Educated people making behavioral changes more readily than those with lower levels of education |
| Health-damaging behavior in which the degree of choice of lifestyles is severely restricted | People being born into and remaining in impoverished and low education settings |
| Exposure to unhealthy living and working conditions | Exposure to environmental (second-hand) smoke and indoor biomass fuels |
| Inadequate access to health care | People in lower socioeconomic status and having less health care access |
| Natural selection leading to the tendency for the sick to move down the social hierarchy | People with advanced COPD become disabled due to disease |
Note: Data adapted from Whitehead M. The concepts and principles of equity and health. Health Promotion Int. 1991;6:217–228.16
Abbreviation: COPD, chronic obstructive pulmonary disease.
Health disparities in COPD
As shown in Tables 2–4, there is a strong relationship between COPD and SES.17–58 Environmental factors contributing to the development or worsening of COPD include use of tobacco products, occupation, prenatal and childhood exposures, respiratory tract infections, air pollution, and housing conditions including the use of BF, all of which are more likely to be present in low SES people. In developed countries, the major risk factor for COPD is long-term tobacco product use, whereas in less developed countries, the conditions of the home (BF) and work setting (dust, fumes, and vapors) often have a more substantial impact on health than either personal position in society or tobacco exposure. Consistent with this, a multinational study from the 1970s and 1980s showed there was a poor correlation between COPD mortality and cigarette smoking data among 31 countries.59 More recently, the Burden of Obstructive Lung Disease (BOLD) study showed a better correlation of COPD prevalence and tobacco use; however, the relationship was not strong in all settings.60
Table 2.
Relationship between socioeconomic status and respiratory measures
| References | Population (description and N) | Socioeconomic status measure(s) | Outcome measure(s) | Main findings |
|---|---|---|---|---|
| Trinder et al17 | General practices in UK (N=4,237) | Occupation of householder | Respiratory symptoms | Severity of respiratory symptoms worse in people with manual occupation in the presence of tobacco use |
| Shohami et al18 | Adults in UK attending general medical practices (N=22,675) | Occupation, education level, and area deprivation | Lung function impairment | Occupation, educational level, and living in area of deprivation associated with worse lung function |
| Welle et al19 | Norwegian general population survey (N=1,275) | Educational level | DLCO | DLCO related to education in men, not women |
| Schikowski et al20 | Germany (N=1,251, women only) | Education level, occupation, and residence | Lung function, respiratory symptoms, and air particulate matter | Low education more likely to suffer from low FEV1 and were occupationally exposed to particulate matter >10 ppm |
| Smith et al21 | Chinese population (never smokers) in ten regions (N=307,000) | Household income and education level | Prevalence of AO | AO associated with lower education and income |
| Kurmi et al22 | Cross-section of adults in ten diverse populations across China (N=500,000 adults) | Household annual income | Prevalence of AO and respiratory symptoms | AO inversely related to annual income |
| Liu et al23 | Cross-sectional survey in one US state (N=4,300 adults) | Education level | Prevalence of respiratory symptoms | Low educational level associated with higher frequency of respiratory symptoms, including frequent productive cough, dyspnea, and SOB affects ADLs |
Abbreviations: ADLs, activities of daily life; AO, airflow obstruction; DLCO, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; ppm, parts per million; SOB, shortness of breath.
Table 3.
Relationship between socioeconomic status and COPD prevalence
| References | Population, description, and N | Socioeconomic status measure(s) | Outcome measure(s) | Main findings |
|---|---|---|---|---|
| Bakke et al24 | Cross-sectional study of general adult population in Norway (N=1,512) | Occupation | Asthma and COPD prevalence | OR of 3.6 for obstructive lung disease in people with high degree of airborne exposure |
| Eachus et al25 | Adults from 40 general practices in the UK (N=28,080) | Deprivation score based on residence | Disease prevalence including COPD | Emphysema and chronic bronchitis relative index of 2.72 and 2.27, respectively (values higher than most other conditions) |
| Chen et al26 | National population survey in Canada (N=7,210) | Income | COPD disease prevalence | For low income persons OR = 3.7 for males and 2.4 for females |
| Marmot et al27 | Civil servants in London, UK (N=10,308) | Occupation (employment grade) | Chronic bronchitis prevalence | OR for CB for men 1.44 and women 1.21 |
| Montnemery et al28 | Adults in Sweden (N=12,071) | Occupation, social position, and residence location | Prevalence of CBE and respiratory symptoms | CBE more common in unskilled and semiskilled workers, low social position |
| Lindberg et al29 | Sweden (N=1,165) | Occupation | COPD incidence over 10 years in subjects with respiratory symptoms | Manual workers had an OR = 1.78 vs professionals. Low education level had an OR = 1.73 |
| Ellison-Loschmann et al30 | European Community respiratory health Survey in Europe, Australia, New Zealand, and the US | Educational level and occupational class | Prevalence and incidence of chronic bronchitis | Low educational and occupational levels (prevalence ratio =1.9 and 1.8, respectively) |
| Halvorsen and Matrinussen32 | Norwegian prescription database of COPD patients (N=62,882) | Educational level and level of unemployment in community | COPD prevalence | Communities with low educational levels and unemployment associated with higher risk of COPD |
| Karnevisto et al33 | Finland – national population-wide survey (N=6,525) | Education and household income | COPD and asthma prevalence | Education significant risk factor for COPD, whereas low household income was a risk factor for asthma |
| Lovasi et al34 | Multi-ethnic study of artherosclerosis at multiple sites in the US (N=3,706) | Education, household income, and wealth indicators | Degree of emphysema on computed tomography scan | Higher percent of emphysema in people with lower high school education, annual income, and wealth |
| Yin et al35 | People’s Republic of China (31 provinces), (N=49,363) | Education and household income | COPD prevalence by self-report | SES predictive of COPD risk independent of smoking and rural vs urban residence |
| Herrick et al36 | Cross-sectional population study in one US state (N=25,986) | Annual household income and highest level of education | COPD prevalence | COPD prevalence threefold greater between highest and lowest income levels as well as between lowest and highest education levels |
| Burney et al60 | Multicenter (n=22 countries), international study burden of obstructive lung disease (N=15,355) | Poverty as measured by GNI of countries | COPD prevalence | COPD prevalence fivefold greater between highest and lowest income levels as well as between lowest and highest education levels |
| Kainu et al37 | Finnish population (N=8,000, COPD N=628) | Occupation | COPD prevalence | Prevalence higher in manual than nonmanual occupations |
| Golec et al38 | Polish farmers (N=64) | Size of farm | COPD prevalence | Lower SES in COPD patients |
| Hagstad et al39 | Swedish never-smokers with obstructive lung disease (N=967) | Education level, occupation | Proportion of nonsmokers with COPD who had occupational exposures | OR of COPD related to occupation = 0.72 in college graduates vs those with less than high-school education |
| Lee et al40 | Korean never smokers with COPD (N=3,473) | Educational level, occupation | COPD prevalence | Low education level and manual labor were risk factors for COPD |
| Tan et al41 | Canadian cross-sectional study in general adult population (N=5,176) | Educational level | COPD prevalence in ever and never-smokers | Low education level associated with higher prevalence in both never and ever-smokers |
Abbreviations: CB, chronic bronchitis; CBE, chronic bronchitis/emphysema; COPD, chronic obstructive pulmonary disease; GNI, gross national income; OR, odds ratio; SES, socioeconomic status.
Table 4.
Effect of socioeconomic status on respiratory-related outcomes in chronic obstructive pulmonary disease
| References | Population | SES measure | Outcome measure(s) | Main findings |
|---|---|---|---|---|
| Prescott et al43 | Copenhagen, Denmark general population (N=14,223) | Educational level and household income | Hospitalization for COPD | Higher rates of hospitalization related to income and education levels (independent of smoking history) |
| Van Rossum et al42 | the Netherlands (N=18,001) | Occupation | Mortality | COPD had highest rate of increased mortality related to occupation compared with other common causes of death |
| Steenland et al11 | Adults in 27 states in the US, American Cancer Society population (N=1,330,886) | Occupation | All cause and cause-specific mortality | SES gradient most substantial for all specific causes of death |
| Huisman et al44 | European data from numerous countries (N=1,000,000 deaths) | Education level | Mortality rate in low-educational groups expressed as a proportion of mortality rate in high-educational groups | Low education groups had highest mortality including COPD, cancer, and heart disease |
| Antonelli-Incazi et al45 | Elderly in Rome, Italy | Income based upon census tract estimate for residence | Hospitalization rate of COPD | Relative risk for females with COPD 3.3 and males 4.3 (higher than other diseases) |
| Blanc et al46 | US population survey of COPD patients (N=427) | Educational level and annual income | Tiotropium use | Less use of tiotropium with lower SES (OR =0.3) |
| Reilly et al47 | National survey of 30 provinces in the People’s Republic of China (N=169,871) | Education, residence (urban vs rural) | Mortality | Relative risk of death 2.37 and 2.47 for men and women, respectively. RR for urban vs rural residence 2.14 and 1.79, respectively |
| Schane et al48 | National cross-sectional US survey (N=18,858 total N=1,736 COPD patients) | Income and education | Risk factors for depression in COPD vs non-COPD | Less than HS education showed OR =1.63 for depression |
| Wong et al49 | Data from St Paul’s Hospital in Vancouver, BC, Canada | Marital status and need for social work consultation while in hospital | Hospital LOS and readmission rate in AECOPD patients | Marital status and need for social work intervention associated with prolonged LOS and readmission for AECOPD |
| Lewis et al10 | National Longitudinal Mortality Study in the US (N=184,924) | Marital status, education, health insurance, poverty level, and occupation | Mortality in a general adult population | Education, marital status, and income predictive of mortality, not seen with insured vs uninsured |
| Arne et al50 | Sweden, survey of 55 municipalities (N=1,475) | Education level, employment status, and social support | Health status and quality of life in COPD vs non-COPD subjects | Lack of social support and low economic status associated with poorer health status in COPD |
| Calderón-Larrañaga et al51 | UK, national cross-sectional study (N=53,676,021) | Deprivation index | Hospitalizations for COPD | Deprivation and smoking prevalence were variables with highest explanatory power, accounting for 59.3% and 51.4% of the total variance, respectively |
| Miravitlles et al52 | Spain, nationwide survey (N=4,574) | Education level and occupation | HrQOL in COPD patients | Worse HrQOL in low education level and in unskilled workers |
| Eisner et al31 | CA, in the US (N=1,202) (insured COPD patients) | Education and income levels | Physical impairment (6-minute walk), pulmonary function, and disease severity including BODE index | Low SES associated with worse physical impairment, pulmonary, function, and disease severity in a COPD population with broad access to health care |
| Omachi et al53 | CA, in the US, population survey in persons >55 years (N=277) | Health literacy | COPD-related health status and COPD-related ED or hospitalizations using multifactorial analysis adjusted for income and educational levels | Poorer health literacy associated with worse health status, HrQOL, and ED and hospitalizations for COPD |
| McAllister et al54 | All Scottish residents (UK) | Scottish Index of Multiple Deprivation (measure using multiple domains such as income, housing, access, education) | Hospitalization rates in COPD associated with deprivation index and winter season | SES and winter act synergistically on rate of COPD hospitalizations |
| Gershon et al55 | ON, Canada | Average household income based on residence | Mortality of COPD | Although overall COPD mortality decreased between 1966 and 2012, differences in COPD mortality between low and high income widened over the study period |
| Lange et al56 | Copenhagen, Denmark (N=6,590) adults with COPD | Education <8 years, 8–10 years, >10 years with some college or completed college | AECOPD, hospital admissions, mortality | Highest risk of AECOPD, low lung function, and highest respiratory symptoms |
| Trachtenberg et al57 | Administrative database in Winnipeg, Canada N=34,741 asthma and COPD) | Census-based household income | Hospitalizations for asthma or COPD | Lower SES associated with higher risk of hospitalizations |
| Sharma et al58 | US Medicare beneficiaries with COPD | Socioeconomic status based on if Medicaid eligible (low SES) | Burn injuries related to oxygen use | Twofold risk of oxygen-related burn injuries in low SES people |
Abbreviations: AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; ED, emergency department; HrQOL, health-related quality of life; LOS, length of stay; OR, odds ratio; RR, relative risk; SES, socioeconomic status.
SES and respiratory symptoms
One would expect that if SES is a risk factor for developing COPD, it would also increase the risk of respiratory symptoms. Studies over the last 2 decades have shown that people of low SES exhibit lower lung function and more respiratory symptoms than those in higher SES groups – typically at least a several-fold difference (Table 2). Whether using forced expiratory volume in 1 second (FEV1), the presence of airflow obstruction (AO), or respiratory symptoms, all the studies show more impairment in people with low SES and whether it is based on income, education, or occupation.
SES and COPD prevalence
Table 3 shows studies of the relationship between SES and COPD prevalence, often showing >1.5 to three-fold greater in disadvantaged people; this holds true whether income or educational levels are used to define SES. The prevalence of emphysema, because it is more common in smokers than those exposed to BF, may exhibit a different relationship to SES depending on the country.61 The BOLD and PLATINO studies have been the most extensive studies of COPD internationally – both support the relationship between poverty and higher COPD prevalence.60,62
In most countries, people of low SES account for the majority of all COPD patients. In the US, although low SES people constitute the minority of the overall population (<20%), they account for nearly two-thirds of all patients with COPD63,64 or AO.22 Similarly, in a study of the Chinese population, people with low educational level accounted for 43% of the survey population in rural areas but 81% of those were with COPD.65 Finish33 and Korean40 studies reported that two-thirds of subjects with COPD were in the lowest SES group based on education.
Risk factors for COPD in the lower SES population
Tobacco use
Long-term tobacco use is the most common cause of COPD in developed countries – this is most evident in people with low SES due to relatively high rates of tobacco use. In the US, where overall current use of tobacco is <20% of adults, rates of current smoking are three- to fivefold higher in those with less than a high school education compared with college graduates.66 One of the authors of this article undertook a quality improvement project in four indigent medical clinics and found that current smoking rates were >70% among adult patients (R Pleasants, Duke Asthma, Allergy, and Airways Center, Duke University School of Medicine, personal communication, June, 2013). Often, these exposures begin early in life, as children in low SES settings are more likely to be exposed to environmental tobacco smoke from household members and these same people are more likely to eventually smoke tobacco products.
Some epidemiologic studies (for example, Twyman et al67) have shown that low education is associated with lower odds of quitting tobacco compared with persons of higher educational attainment. Some hypotheses include 1) impoverished people are less likely to seek treatment for tobacco addiction because of significant life stressors, 2) smoking is often socially accepted among their family and peers, 3) they have lower levels of knowledge of benefits of smoking cessation drug therapies, 4) health care providers believe that this population is less interested in quitting smoking, 5) they have higher rates of mental health issues including multisubstance abuse, and 6) genetics. Though these are hypothesized explanations for the disparity in smoking cessation rates in low SES groups, there are no randomized controlled data to support a causal relationship. In addition, many epidemiologic studies often leave out key variables in their analysis. One study found that smoking behaviors including age of initiation, intensity of tobacco use, and smoking cessation capabilities appear to be genetically related.68 In the COPD Gene trial, it was found that in a genetic analysis of >3,000 COPD patients, the age of onset of tobacco use and smoking cessation behavior were related to nicotinic receptors and hepatic metabolism of nicotine.4 Neither study examined the relationship of genetics, smoking, and SES.
However, in less-developed countries with very wide social gradients, the poorest are unable to afford tobacco products, and therefore, long-term tobacco use is less likely to be the leading cause of COPD. In Peru where tobacco use is very low in the adult population (3.3%), smoking accounts for <10% of all COPD cases. COPD was more likely to be due to posttuberculosis infection and indoor BF; the latter accounting for 60% of the COPD cases.69 In some countries such as Uganda, some of the poor grow their own tobacco and therefore have ready access to tobacco.
Occupation
In the US, it is estimated that ~15% of COPD cases could be attributed to inhaled particles at work such as production of plastics, textiles and leather, building and construction, military service, food, crops, chemicals, petroleum, and coal mines.70 Disadvantaged people tend to be the ones working in these environments. However, occupation-related COPD often does not occur in isolation, as many at-risk people have exposures to harmful particles in the household and outdoor environments. A greater risk of COPD has been reported with combined occupational and smoking exposures. The odds ratios ranged from 4.0 to 6.2 for COPD among occupationally exposed smokers, compared with 1.4–3.2 for occupationally exposed never-smokers.71,72 In another study, the difference between occupationally exposed never-smokers and occupationally exposed ever-smokers was reported to be even greater (14-fold).73
In a National Health and Nutrition Examination Survey study in the US, occupations associated with an increased risk of COPD were freight, stock, material handlers, records processing and distribution clerks, sales, transportation-related occupations, machine operators, construction trades, and waitresses.74 The fraction of COPD attributable to work was estimated as 19.2% overall and 31.1% among never smokers. The majority of workers in high-risk occupations had low income and education levels. In a more recent Swiss study of working adults, occupational exposures to biological dusts, mineral dusts, and gases/fumes were associated with two- to fivefold higher incidence of COPD of at least moderate severity; however, SES measures were not reported.75
Household and outdoor pollution
Almost 3 billion of the world’s poorest rely upon solid fuels (wood, animal dung, charcoal, crop wastes, and coal) burned in inefficient, polluting stoves for cooking, lighting, and heating – leading to premature deaths from respiratory and cardiovascular diseases and cancer.76 It has been suggested that BF is a more common cause of COPD than cigarette smoke globally.77 The use of gas stove and solid fuels for cooking, lighting, and heating78,79 and air pollution have been linked to increased respiratory symptoms, impaired lung function,80–82 and development of COPD.83,84 Although the role of air pollution in causing COPD is not clear, high levels of outdoor pollution are an important contributor to an increased risk of acute exacerbations of COPD85 and possibly mortality.86
Much of the combustible fuel exposure is related to poor housing conditions such as overcrowding and poor ventilation when using BF. In addition to consistently conferring a higher risk of the emergence of COPD, it also increases the risk of acute respiratory infections.87 Women appear to be particularly at risk to develop biomass-related COPD, although environmental tobacco smoke may play a role.80 Meta-analyses have reported approximately twofold greater odds of developing COPD in people exposed to BF.84,88
In a cohort study in the US, use of wood for indoor heating in fireplaces or woodstoves increases the risk of COPD among current smokers, Caucasians, and men.89 These effects remained even when controlling for age, smoking, and educational level. Wood smoke may lead to greater risk of developing COPD,90 whereas indoor use of coal for heating may be more likely to increase the risk of lung cancer.91
Outdoor air pollution is also an important factor that causes lung diseases in people of low SES as they tend to live in poor housing, less desirable areas in close proximity to highways, and pollution-producing manufacturers and are therefore exposed to disproportionate amounts of inhalant toxins. In the US, the estimated proportion of the population living near a major highway was 4.2% for the poor, 3.7% for the near-poor, and 3.5% for the nonpoor categories.92 In a longitudinal study of 4,757 women in Germany, it was found that chronic exposure to PM10 and nitric dioxide and living near a major highway led to an increased risk of COPD (1.8-fold).20
Intrauterine and childhood exposures
Exposures, beginning with the fetus continuing throughout childhood, can have an impact on the trajectory of lung function development and ultimately long-term respiratory health. Intrauterine exposure to tobacco as well as second-hand smoke in young people are important risk factors for developing both asthma and COPD, especially in disadvantaged people.93–99 In the US, smoking rates in women during the 3 months prior to pregnancy are similar to the general population (~20%), decline to ~10% of women while pregnant, and then increase close to pre-pregnancy rates postpartum.100 Women of low SES tend to smoke more during and after pregnancy. In a UK study, women of low SES were three times more likely to smoke during pregnancy than higher SES women.101 One study found a positive association between fetal size at 10 weeks gestation and FEV1 in 10-year olds.95 Another study found that smoking during pregnancy, particularly in first trimester, is associated with worse respiratory complications in children.102
Lung function impairment and respiratory symptoms after birth and through childhood are also associated with asthma occurrence in later years,95–97 ultimately increasing the risk of developing COPD later in life.100,103 In children, chronic exposure to air pollution is associated with a reduction in age-related forced vital capacity and lung growth.104 However, other studies found that there was no effect or inconclusive evidence for longitudinal changes in lung function.94,105,106 A Swedish study of 2,278 children looked at the effect of air quality during the first year of life on lung function at 16 years of age.93 Early life exposures to traffic-related air pollution during the first year of life resulted in lower FEV1, especially in males. Biomass smoke is also an independent risk factor for COPD in people exposed in early life.84 Lastly, impaired lung function in early adulthood is also associated with an increased risk of developing COPD.107
It is also known that recurrent childhood respiratory tract infections can lead to the development of lung diseases later in life108–111 and that low SES increases the risk of childhood respiratory tract infections. In one study early childhood respiratory infections as well as a history of asthma in the family were significant determinants of COPD risk.110 A recent 50-year longitudinal study of a cohort of children beginning at 10–15 years of age found that asthma and wheezy bronchitis associated with viral infections led to increased risk of developing COPD, while considering smoking history.111
Health care access
Disparities in health care access persist despite efforts for many years to improve care for underserved patients, ethnic and racial minorities, and the underinsured. Access to health care providers, diagnostic testing, and medications are often more limited in disadvantaged people, although universal health insurance in some countries lessen health disparities. In addition to access being an issue in the low SES people, lower literacy levels also impact their health status and other outcomes.53
Shortage of primary care and specialist physicians in some communities is an important factor for the unequal provision of health care quality and access to health care services.112,113 In the US, Spain, and many developing countries, relatively few pulmonologists and specialty services such as spirometry and pulmonary rehabilitation are available to disadvantaged people.8 In the US, minority patients with asthma are less likely to be referred to specialists than whites.114
Access to health care is not the only important factor in health disparities; the Black Report emphasized that despite free health care provision through the National Health Service, health disparities in mortality rates by social class were evident.115 A US study showed that in people provided with similar health insurance, those with low SES had worse health outcomes than those at with high SES.31 In contrast, a Canadian study found that there was no difference in COPD mortality between people of low and high education – this was felt to be due to universal health coverage and the greater acute health care utilization by low SES people.116 Therefore, it is not just a matter of providing health care resources at a low cost to people of low SES, but it must involve other additional strategies to promote healthy behaviors in these patients and other contributors that affect health outcomes.
Disparities also exist in tobacco cessation advice from health care providers.117,118 In a US study, compared to Caucasian smokers, African–American and Hispanic smokers were approximately two-thirds less likely to be asked by their doctor about tobacco use and not have used a cessation medication. These differences were unexplained even when factors known to impact these rates were considered.117 In another study, low SES people were less likely to receive provider-assisted assistance in stopping smoking than higher SES people.118 New quality outcome measures requiring screening for tobacco use by health care providers in the US have decreased this disparity.
Affordability and access to effective COPD medications is a major barrier in many disadvantaged people. In some impoverished countries, the national formularies have very limited choices for the treatment of COPD. One US study showed that low SES people were only one-third as likely to have used their inhaled tiotropium.46 An important problem with COPD medication costs is the lack of availability of cheap generics; in part because regulatory agencies, such as the US Food and Drug Administration, have higher expectations for inhaled generic equivalents than for oral generics.119
Clinical characteristics of COPD in people of low SES
Both biomass and tobacco smoke are generated from the combustion of plant materials, which produce complex carbon-based particles and organic compounds such as hydrocarbons and irritant gases. Biomass smoke increases the expression of some matrix metalloproteinases similar to tobacco smoke.120,121 Like tobacco smoke, BF leads to chronic inflammation, increased oxidative stress, and respiratory symptoms.122 Combined exposures to tobacco smoke and BF have additive effects on the risk of developing lung diseases.90
Clinical features tend to differ between tobacco smokers and people exposed to BF.61,123–126 In a study of women with severe COPD, comparing wood smoke-related and tobacco smoke-related COPD, there were no significant differences between the two groups in terms of AO, resistance, or hyperinflation. However, bronchial hyper-reactivity was more evident in the former.123 In another study, those exposed to BF scored lower on the symptom and activity scores using a quality of life assessment.61 This study also showed that women in the biomass group had significantly more air trapping than the tobacco group (radiologist score was 2.6 and 1.5, respectively) and had lower oxygen saturation at rest and during exercise. In another study in Columbia, South American women with chronic bronchitis secondary to long-term wood smoke inhalation, dyspnea, AO, air trapping, and increased airway resistance were common.125 Additional research is needed in this area as tobacco smoking becomes less prevalent and other causes of COPD become more common.
Several studies have examined the radiologic and pathologic characteristics of biomass vs tobacco smoke-related COPD.34,61,125–127 One study, using 3,706 multiethnic subjects enrolled from six distinct US locations (Multi-Ethnic Study of Atherosclerosis), examined the relationship among SES, emphysema scores, and other measures.34 One-half of the subjects were current or former smokers, one-third had occupational dust exposure histories, and one-third were born outside the US. Those who did not complete high school had more AO than those with a graduate degree, whereas, unexpectedly, the less well-educated people had a lesser extent of emphysema. However, to what extent biomass exposures contributed to this discrepancy was unclear. In a study of 43 Mexican women, grouped by tobacco smoking or BF exposure, computed tomography (CT) scans showed that the tobacco group had significantly more emphysema than the biomass group (radiologist score was 2.3 vs 0.7; emphysema on CT 27% vs 19%, and a larger size of emphysematous spaces).61 In another study of Mexican women with COPD secondary to wood smoke inhalation (n=12) vs smoking-related COPD (n=10), pathological evidences of anthracosis, chronic bronchitis, centrilobular emphysema, and pulmonary hypertension were present in most patients exposed to wood smoke.125 In an autopsy study of 48 Mexican COPD patients, emphysema was less likely to be severe in BF patients than in smokers.127 Small airways fibrosis was more evident with BF, whereas increased goblet cell hyperplasia and emphysema were seen in smokers. Emphysema was more evident in the smokers. In terms of survival, a 7-year follow-up study showed that women with biomass-induced COPD have similar survival rates as men with tobacco-related COPD.124
Effect of SES on COPD outcomes
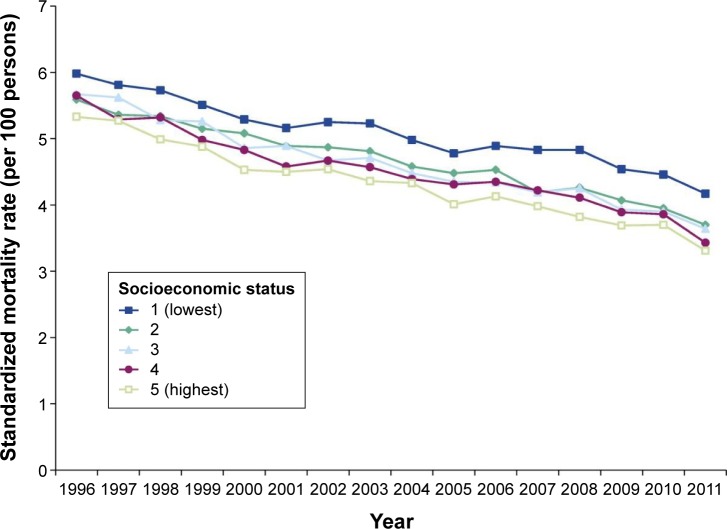
Table 4 lists the studies that show a strong association between low SES and worse outcomes in COPD patients including death, emergency department (ED) visits, hospitalizations, medication use, and health-related quality of life.11,43,44,54–56,60,128 There may be sex differences as one study reported that occupation and education were predictors of death in men, whereas only education was significant in women.43 In a systematic review, among several common causes of death (COPD, diabetes, heart disease, all cancers, and stroke), the relationship with SES was strongest for COPD-related deaths.55 However, with regard to lung cancer (vs all cancers), the association between SES and risk was also quite strong,128 therefore how various causes of death are categorized may affect the reported association between mortality and SES. A BOLD study analysis found that smoking is the most important cause of AO, but airflow restriction was more associated with mortality in poor countries.60 With regard to COPD mortality, people in lower SES groups comprise between one-half to three-fourths of all deaths where COPD is reported to be the primary cause.10,11,129 A recent Canadian study showed that the mortality gradient between low and high SES is slowly widening (Figure 1).55 In countries where current smoking rates continue to decline in higher SES groups such as the US and Canada, although rates decline more slowly in disadvantaged people, this gradient will likely widen even further. However, the impact of SES on mortality is not seen with all COPD patient types. A UK study found that mortality in alpha-1 antitrypsin patients was no different as a function of educational status or occupation,130 likely because it is a genetically driven cause of COPD.
Figure 1.
All-cause mortality rate by socioeconomic status among COPD patients in Canada from 1996/1997 to 2011/2012.
Note: Reprinted with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society. Gershon AS, Hwee J, Victor JC, Wilton AS, To T. 2014. Trends in socioeconomic status-related differences in mortality among people with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 11:1195–1202. The Annals of the American Thoracic Society is an official journal of the American Thoracic Society.55
Abbreviation: COPD, chronic obstructive pulmonary disease.
Other outcome measures that are affected by SES in COPD patients are health-related quality of life,52 depression,48 medication (tiotropium and albuterol) use, ED visits, hospitalizations, and burn injuries from home supplemental oxygen.58 These disparities existed even in people who have ready access to health care.10,31 Hospital readmission for acute exacerbation of COPD (AECOPD), now a quality measure in the US hospitals, is also more common in disadvantaged COPD patients.49 A UK study, examining the relationship between air pollution and albuterol prescriptions by primary care physicians, found that deprivation was associated with greater prescribing.131 Conversely, maintenance therapy use is decreased in low SES people.46
Strategies to aid the diagnosis and management of COPD patients with low SES
There is an old adage in the US supposedly quoted from a famous bank robber named Willie Sutton that evolved into Sutton’s law used in clinical medicine. Sutton reputedly replied to a reporter’s inquiry why he robbed banks that “because that’s where the money is”.132 Translated into where the greatest opportunities and challenges exist for COPD, it is targeting low SES populations and that is where the money and other resources need to be directed.
In reality, to make substantive changes in COPD risk factors and social determinants of health, health equity strategies must be part of larger, multifaceted efforts that will always depend on societal changes that can be very difficult to achieve. Broadly speaking, there are three important principles that should be included in the strategies to improve COPD health equity: 1) include all stakeholders in the development and implementation of interventions; 2) adapt interventions to address the unique barriers and facilitators of the disparate population; and 3) create implementation strategies targeting all levels of the socioecologic framework – individual, interpersonal, organizational, community, and public policy. Stakeholders can vary from government agencies, health care organizations such as WHO, ATS, ERS, health insurers, health care institutions, research organizations, health care providers, patients, and community groups. The remainder of this section focusses approaches that may be applied by individuals or in local settings.
Some strategies that might be employed include 1) prioritize population screening and case-finding in people of low SES to identify symptomatic people with exposure histories in this high-risk population; 2) undertake educational efforts in people with low SES using culturally sensitive strategies in local communities; optimally beginning at a young age regarding the use of tobacco products, indoor biomass, second-hand smoke, and COPD; 3) provide alternatives to the use of indoor biomass cooking; 4) identify cost-effective strategies for health care in this population; and 5) identify existing and potential strategies to save medication costs and optimize adherence.
Organizational strategies
The WHO, ATS, ERS, Forum of International Respiratory Societies, and European COPD Coalition are among the health care organizations that are responding to the problems surrounding COPD and SES. Some key ATS efforts targeting health disparities include establishing a group called the Lung Corp,133 publishing a perspective of respiratory health equality in the US,134 and a joint policy statement with ERS on disparities in respiratory health.8 The ATS created the Lung Corps to reduce health disparities through the promotion of research, training, disseminating best practices and monitoring, and advocating lung health to the public. In addition, an ATS subcommittee reported that attainment of respiratory health equality necessitates the end of disparities, which would require a multidisciplinary effort targeting environmental exposures, encouraging healthy lifestyles, and optimizing the prevention, screening, diagnosis, and management of lung diseases.136 A joint ATS/ERS policy statement8 describes actions including developing educational programs for health care providers and policy makers to reduce disparities, holding conferences addressing health care disparities, and collaborating with other organizations such as the WHO. ATS/ERS jointly plans to decrease disparities by 1) guiding research agendas, 2) increasing healthcare provider awareness of disparities, 3) advocating for appropriate policies, and 4) tracking progress with disparities. Nearly 2 decades ago, ATS developed the Methods in Clinical and Operational Research program to increase the numbers of public health, academic, and clinical leaders to facilitate research and its application in public health and health care to respiratory diseases in various countries. The recent ATS/ERS research priorities publication did not address health disparities as one of the research priorities, but did state that the natural history of the disease should be studied with regard to race, sex, and SES.135
The Forum of International Respiratory Societies developed a guide on preventing and treating lung diseases, including COPD.136 Principal recommendations include increase public awareness, target environment and tobacco, increase public health and clinical research, and train more health care providers globally to deal with lung diseases. It also recommended developing cost-effective management protocols for COPD in low-income settings. The European COPD Coalition has published a call for action among countries in the European Union to better target COPD.137 Among multiple recommendations, it was stated that the European Union should support and initiate research on social and environmental determinants of health to better understand factors that increase the risk of developing COPD in Europe.
The WHO has a number of publications addressing COPD and SES including an essential medicine list, tobacco prevention strategies, guidelines to reduce indoor BF exposure, prevention of chronic respiratory tract diseases, and managing COPD in low SES settings.138–143 WHO recommendations for the diagnosis and management of COPD in low SES settings by primary care providers have been published.142 Considering the lack of availability of spirometry in many low-income settings, WHO recommends the use of clinical history and peak expiratory flow measurement to guide the diagnosis of COPD. Severity of COPD is judged based on symptoms. Education of the patient and family is recommended regarding biomass fuels, occupational exposures, and tobacco smoke. Recommended drug therapies included short-acting β2-agonists and low dose oral theophylline. They also state that ipratropium may be an option, but may be too expensive.142 Regarding tobacco, WHO embraces the MPOWER acronym approach for tobacco: Monitor tobacco use and prevention policies, Protect people from tobacco smoke, Offer help to quit tobacco use, Warn about the dangers of tobacco, Enforce bans on tobacco advertising, promotion, and sponsorship, and lastly Raise taxes on tobacco.139 Goals of the WHO Strategy for Prevention and Control of Chronic Respiratory Diseases are to improve 1) surveillance to map and analyze the determinants with particular reference to poor and disadvantaged populations and to monitor the future trends, 2) primary prevention to reduce the level of exposure of individuals and populations, and 3) secondary and tertiary prevention to strengthen health care for people with chronic respiratory diseases.141 WHO also provides a list of essential medicines for many diseases that is updated every 2 years.138 Additionally, the WHO has begun a noncommunicable chronic diseases monitoring system for >190 countries.143
Surveillance
Opportunities for surveillance include expanding epidemiologic studies in more low SES settings and using these data to aid efforts such as to screen for undiagnosed disease. Despite the tremendous global burden of chronic respiratory disease, there are relatively limited data on its prevalence, natural history, and associated morbidity and mortality of COPD in LMICs.144 However, there has been significant progress in defining the prevalence and impact of COPD globally through surveillance studies – particularly with the newer BOLD and PLATINO international studies, additional studies in Africa,145 and in the US with the expansion of the Behavioral Risk Factor Surveillance System for COPD prevalence.146,147
Ideally, each surveillance system should be capable of describing disease prevalence and disease impact in disparate people as well as the overall population. Furthermore, the information must be communicated to appropriate people including policy makers, health care providers, and the population from which the data were derived. This is largely a role of the public health sector in collaboration with others involved in health care provision. In general, the lay public’s knowledge of COPD is far less than that of other diseases such as asthma, cancer, and heart diseases;148,149 therefore, it is imperative to educate the general public about COPD, partially relying on these surveillance data. Ideally, this education should occur throughout the population including the educational systems of young people.
One proposed strategy is to provide annual reports on lung health surveillance data from vulnerable, disparate populations.150 The WHO has recently begun a multinational surveillance of chronic diseases to better monitor low SES populations.143 In the US, a Nationwide Framework for Surveillance of Cardiovascular and Chronic Lung Diseases recommends to provide such data through surveillance to analyze disparities in disease prevalence, risk factors, outcomes, and health care delivery. It was recommended to integrate various surveillance systems into a central system that is able to function nationally and locally.151 In addition to the use of the Behavioral Risk Factor Surveillance System health survey, the US Centers for Disease Control and Prevention also recently expanded surveillance for COPD through the use of chronic disease indicators.152 The chronic disease indicators are used by epidemiologists at local, state, and national levels in the US to monitor many major chronic diseases. The updated indicators are 1) mortality of COPD as a primary or secondary cause, 2) COPD prevalence, 3) ED visits with COPD as a primary or secondary cause, and 4) hospitalizations as a primary or secondary cause. Previously, only mortality was included in these measures; inclusion of COPD as comorbidity in surveillance of other diseases will provide greater perspective of its impact across health care.
Primary prevention
Of all possible interventions, primary prevention for COPD through the avoidance of exposure to inhalant toxins would have the most impact on this disease; however, this is difficult to achieve in disadvantaged people. Both strategies and interventions are necessary to target low SES communities by improving housing conditions, providing education on tobacco in people at a young age, reducing tobacco use, and reducing other detrimental environment exposures, however, interventions are not often implemented.
To have an impact on tobacco use in the low SES population, it will require societal changes and efforts by health care organizations, public health, and individual providers. Societal interventions that can impact tobacco use include raising taxes on cigarettes, banning the use of tobacco in certain settings such as health care facilities, schools, government facilities, restaurants, and bars, and restricting advertisement.100 A study conducted in one US state showed that communities with smoke-free public policies had significantly lower rates of COPD hospitalizations.153 A recent Cochrane Review reported that national smoking bans in 21 countries led to improvement in multiple outcome measures.154 Because people who are more educated smoke tobacco at much lower rates, it is logical that improving secondary and tertiary education programs for the low SES population would contribute significantly to lower smoking rates. Education access and performance are heavily determined by family background, including family income and environment, which can strongly influence cognitive and noncognitive abilities.
As a whole, health care providers perform suboptimally in assisting patients to quit smoking. A 2007 report by the American Association of Medical Colleges on physician behavior showed that most physicians consistently ask patients about their smoking status and advise them to stop (86%) if a smoker, but steps afterward were infrequent.155 Physicians indicated the tools, training, and resources to aid patients effectively in stopping smoking are inadequate.
There are limited studies evaluating smoking cessation interventions in low-income people.156,157 One study used a systematic, telephone-based intervention involving counseling, nicotine replacement therapy, and community resources, to double the quit rates (17% vs 8%).156 Identifying and reaching out to poor people in their local communities was an important part of this program. In reality, typical interventions should be effective, but there is such a large number of smokers who are of low SES, and they are less inclined to quit, therefore the task is large. A national smoking cessation approach in Denmark was able to achieve reasonable abstinence rates in low SES people, however were still 30% lower in the low education vs the high education group.157
Perhaps idealistic, the best approach for a physician to effectively facilitate smoking cessation is as follows: 1) ensure determination of smoking status, 2) the provider and other team members advise smoker to quit, 3) if the patient is willing, the prescriber initiates further steps with a simple prescriptive order, where drug therapy and counseling resources are available and are provided directly to the patient thereafter, and 4) the provider sees the patient in follow-up. Unfortunately, there are multiple barriers inhibiting this optimistic model in many settings, much of it are resource based. Of note, the WHO essential medicine list includes nicotine replacement therapy. In the US, the cost of nicotine replacement therapy is comparable to the costs of smoking 20 cigarettes per day; therefore, cost of this therapy should be no different than that of smoking for most people.
Household air pollution is a modifiable exposure for which specific interventions such as the use of improved fuels (higher on the fuel ladder) for cook stoves, gas lighting, or heaters may be effective in lowering the risk of COPD.158 The WHO provides guidelines for lowering indoor exposure to burning BF.140 Some of the key recommendations include 1) exposure to fine particulate matter (PM 2.5) should not exceed 0.23 mg/min when unvented (eg, without a chimney or hood) and 0.80 mg/min when vented (eg, with a chimney or hood); 2) amount of carbon monoxide should not exceed 0.16 g/min for unvented devices and 0.59 g/min for vented devices; 3) unprocessed coal should not be used as an indoor fuel; and 4) kerosene should not be used as an indoor fuel. The WHO recommends biogas, ethanol, liquefied petroleum gas, and natural gas as safer alternative fuels. In addition to cleaner fuels, vented, more efficient biomass stoves might also serve to decrease indoor exposure. There is an international effort to bring safer BF in homes by the UN Foundation Global Alliance for Clean Cookstoves and the Climate and Clean Air Coalition.159 Unfortunately, the less expensive fuel options are generally less efficient fuels, produce more smoke, and are used by people with the most poorly designed homes. Cleaner fuels such as propane or liquid petroleum gas burn very cleanly, but are often too expensive for many impoverished households.
A number of studies show that reducing BF exposures improves respiratory outcomes. In 2011, a randomized, controlled study (RESPIRE 6) showed that a reduction in disease activity is possible – in this case, there was a decrease in severe pneumonia in children after a chimney stove intervention to reduce indoor air pollution.160 Romieu et al conducted a trial in >500 Mexico women to evaluate the effect of a chimney wood stove intervention vs the traditional open fire stove on respiratory symptoms and lung function.161 Although the adherence was poor (50%), use of the chimney stoves reduced respiratory symptoms (rate ratio of 0.29 for wheezing) and decline in lung function (31 vs 62 mL) over a 1-year period compared with those using open fire.161 In another study, investigators from the People’s Republic of China followed participants for up to 9 years and found that using biogas instead of biomass for cooking reduced the annual decline of FEV1 by 12 mL per year and improved kitchen ventilation reduced the decline by 13 mL per year, compared with those who took up neither intervention.162 One study in Columbia, South America, showed that conversion to natural gas heating led to a reduction in outpatient visits and hospitalizations for COPD.163 In the US, the Environmental Protection Agency has new recommendations on indoor wood stoves to decrease particulate emissions.164
Although ambient air pollution has well-documented the adverse effects on patients with COPD, the evidence for air pollution as a cause of COPD is inconclusive.87 A study before and during the 2008 Beijing Olympics showed that the risk of asthma outpatient visits declined with lower ozone levels that occurred with changes in automobile use and manufacturing preceding and during the games.165 Regarding air pollution, minimizing exposure on poor air quality periods may aid in lessening the symptoms or preventing the exacerbations in COPD patients. Therefore, having daily, easily accessible air quality reports to the general population including people with lung disease is valuable, particularly for those with severe disease.
Prevention through appropriate immunizations can improve outcomes in the COPD population from influenza and pneumococcus infections.166 Pertussis can be associated with AECOPD,167 but there is no evidence that vaccination can decrease the risk of AECOPD. Like many other aspects of health care in low SES, immunizations are less likely to be administered appropriately in disadvantaged people in most,168,169 but not in all settings.170 Countries with fully funded immunization programs have higher rates of utilization.170
Health care access
Access to health care is a common barrier to good health in disadvantaged people because of the lack of resources and local availability of primary care and specialists. This is particularly true in rural areas, where a significant segment of the COPD population resides. Too often, care for disadvantaged people occurs in EDs, where care is very costly and not optimal as the principal site of health care for COPD patients. In the UK, a survey showed that the majority of patient contact with health services occurs via either the general practitioner or an emergency admission to hospital.171 The development of a National Service Framework for COPD in the UK has set goals to improve the quality and access to COPD services as well as to reduce inequalities and health care utilization costs.172 A study by Lisspers et al in Sweden showed that incorporating specialist nurses in asthma/COPD clinics decreased AECOPD in patients without additional secondary specialist care.173 Researchers from Plymouth University in the UK are undertaking a project in several LMICs to improve access to health care to prevent and manage chronic lung diseases including nontraditional pulmonary rehabilitation clinics, smoking cessation programs, and involving local midwives.174 The project, FRESH AIR, will adapt and test innovative ways to implement evidence-based practice in the prevention, diagnosis, and treatment of chronic lung disease in several LMICs. A study in Pakistan plans to target low resource settings using various approaches such as providing COPD care in the existing tuberculosis clinics.175
Studies looking at the impact of telemedicine have been equivocal in improving access to health care and outcomes in COPD.176 Telemedicine is more challenging in low SES people for various reasons. For example, the poor and elderly tend to use cheaper, less “smart” phones, and therefore, technology may not be compatible with the COPD patients’ technological capabilities.
Educating more respiratory specialists, including minority providers, is needed as rural and poor areas lack pulmonary specialists in many countries. Little has been published regarding the geographic distribution of specialists and therefore availability of care for low SES COPD patients.8 The ATS, ERS, and Forum of International Respiratory Societies support better education and availability of providers available to COPD patients. They want to ensure that medical and other health care students are taught about the association between COPD and SES. Postgraduate education regarding disparities is also needed at multi- and interdisciplinary conferences for health care providers, public health practitioners, health agencies, and others in every country to address SES and lung diseases in an ongoing basis.
Screening and diagnosis
Since the majority of people with undiagnosed COPD have low SES, undertaking population screening or case-finding in targeted communities or health care settings is desirable. There are a number of screening and case-finding questionnaires that can identify people at high risk of COPD.177–181 Although smoking history is always addressed, most do not include questions about BF or occupational exposures.183 A COPD screening questionnaire was developed in the People’s Republic of China, which included both smoking and exposure to BF from cooking but did not include occupational exposures.181 The BOLD and PLANTINO studies’ questionnaires included occupational exposures and smoking history, but not BF.62,182 To make COPD case-finding or population screening most applicable globally, it is imperative to develop and validate such questionnaires that include all three major categories of COPD risk factors – tobacco, occupation, and BF exposures. In general, the sensitivity of such screening/case-finding questionnaires can be improved with portable flow meters that measure FEV1 and FEV6.183
Diagnostic resources for COPD in LMICs are poor; therefore, diagnoses are often made on the basis of clinical features alone, which is a distinct limitation for a disease that by the strictest definition requires documentation of AO. One Chinese study did use discriminant function analysis, not relying on spirometry, by using nine patient characteristics including smoking history, occupation, and rural vs urban residence, to screen for COPD in impoverished areas. The sensitivity was reported to be ~90% based upon AO measured by spirometry. One study showed the benefits of screening for undiagnosed COPD in different socioeconomic practice settings.184
The WHO published specific guidelines to aid primary care providers in low-income populations for the diagnosis and management (Figure 2) of COPD.142 In situations where diagnostic spirometry is limited, WHO recommends to make a diagnosis based on age, clinical history (persistent respiratory symptoms and chronic productive cough), and exposure history (>1 ppd × 15 years). Although debatable, it was also recommended that peak flow meters can be used to determine AO reversibility and thus help differentiate between asthma and COPD. However, portable spirometers that can measure peak flows, FEV1, FEV6, and/or forced vital capacity are now fairly inexpensive.
Figure 2.
World Health Organization drug therapy guidelines for managing acute exacerbations of COPD and stable management in resource limited settings.
Note: Reprinted from World Health Organization. Prevention and control of non-communicable diseases: guidelines for primary health care in low-resource settings. Copyright 2012.142
Abbreviations: ADRs, adverse drug reactions; AECOPD, acute exacerbation of COPD; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; ICS, inhaled corticosteroid; IV, intravenous.
Treatment
The care offered to low SES patients with chronic respiratory disease is not always based on research or best practices. Often, access to optimal COPD treatments, such as availability to primary and specialist providers, smoking cessation counseling, supplemental oxygen, and noninvasive ventilation, is limited in these people. The WHO published guidelines for the treatment of asthma and COPD by primary care providers in resource-limited settings.142 It was designed to aid easy access and facilitate implementation in busy community clinics and small hospitals. It provides recommendations for acute and chronic management of COPD. Patient education regarding preventive measures such as stopping smoking tobacco products, use of well-ventilated areas for cooking and heating, as well as awareness about high-risk occupations is recommended. The recommended drug therapies are few and by most developed country’s standards would probably be considered to be suboptimal.
One important goal to optimally treat COPD patients is to properly diagnose and manage comorbidities common to this population. Early identification and optimal treatment of heart disease, lung cancer, depression, and obstructive sleep apnea can improve outcomes. Heart disease and lung cancers are more common in COPD, which more likely occur in low SES people. Cardio-selective B-blockers, when prescribed for some types of heart disease, reduce the overall mortality in COPD.185 Unfortunately, too often practitioners are reluctant to prescribe these agents for COPD, although the cardio-selective agents have a low risk of worsening AO.186 These agents are available generically and are relatively affordable. In the US, low-dose chest CT screening has commenced in high-risk people to identify early lung cancer and decrease the associated mortality; however, this is quite expensive.187 Obstructive sleep apnea occurs in COPD at about the same rate as the comparable patients in general population but is more common in low SES patients.188 Patients with coexisting COPD and sleep apnea typically develop more severe oxygen desaturation during sleep and have worse outcomes.189 Treatment of severe obstructive sleep apnea is most effectively done with noninvasive ventilation, although costs may be prohibitive.
Efforts to educate the general public and COPD patients are sorely lacking, particularly for impoverished people. Only recently, education about COPD to the general public has been undertaken in the US, whereas much more has been done to educate the public about asthma. Education about smoking, BFs, and the disease COPD should start as early as primary schools; however, health literacy is a major issue in some of these children and their families. Education about COPD and its risk factors should be culturally sensitive and at the appropriate education level for the target population. A study in a primary care setting showed that COPD patients with a high-school or less education, capable of communicating or writing, were able to be successfully educated about their disease.190
Poor access to medications and noncompliance are major factors that limit the optimal management of obstructive lung diseases.191,192 Identifying cost-effective drug therapy strategies will vary among different settings and countries; health care providers should explore what the options are in their local settings. Essentially, all evidence-based COPD medications are more limited in resource sparse settings and even the most basic medications have variable availability. This is true despite the overall cost-effectiveness of interventions such as smoking cessation counseling combined with drug therapy or maintenance drugs that can decrease AECOPD. It is well documented that maintenance medications for COPD can improve respiratory symptoms and quality of life and decrease AECOPD1 and that medication adherence can improve mortality.193 There is little definitive data supporting that these medications can modify the course of COPD; however, it could be a function of identifying phenotypes and genotypes with which drug therapies could slow down the progression of the disease and improve survival. Currently, interventions with the most mortality benefits relevant to COPD include 1) long-term supplemental oxygen, 2) smoking cessation, 3) lung transplantation, and 4) optimizing prevention or management of key comorbidities such as heart disease – the latter three include medications.
Annually, the WHO publishes an updated essential medicine list that includes the most basic drug therapies relevant to the acute and chronic management of COPD.194 Even for these therapies, disadvantaged persons have substantial challenges in accessing and paying for COPD medications as well as being adherent with their therapies. The most basic drug therapy, albuterol, can account for a high portion of personal income in LMICS, often as much as several days wages.144 In the US, drug costs can also be quite substantial for a person living in poverty. In an advanced COPD patient, a triple drug regimen could cost nearly half of a monthly personal income without health insurance subsidies (Table 5). Even when subsidized by insurers, the poor often choose between basic living necessities vs appropriately using their prescribed COPD medications. One strategy in the US to decrease drug costs for retired or disabled COPD patients, covered by the federal Medicare insurance program, is to prescribe nebulized albuterol, ipratropium, formoterol, arformoterol, and/or budesonide if they fail the same drugs through an inhaler. Different settings may have resources available to uniquely help lower the drug costs.
Table 5.
Drug costs relative to days of work in low-income people in the US
| Measure | Albuterol MDI (200 inhalations) | ICS/LABA inhalera | LAMA inhalera | Albuterol + ICS/LABA + LAMA |
|---|---|---|---|---|
| Retail drug costsb | $55.00 | $340.00 | $320.00 | $715.00 |
| Days of work required to pay for medication(s) using ~ minimum wagec | 0.86 days | 5.3 days | 5 days | 11.2 days |
Notes:
One month supply.
Approximate retail costs with no subsidies were obtained from www.goodrx.com. Accessed February 1, 2016.197
Annual income of $16,640 (based on a minimum wage salary of $8/hour at 40 hours/week).
Abbreviations: MDI, metered dose inhaler; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting antimuscarinic agent.
An important contributor to unaffordability of COPD medications is the lack of generic versions for the majority of drugs used to chronically manage COPD. In contrast, many of the prominent maintenance medications for cardiovascular diseases and other major chronic diseases often have cheaper generic drugs. In the US, this is affected by Food and Drug Administration requirements for new “generic” inhalers. Somewhat extensive and expensive studies are mandated for generic inhalers by the US Food and Drug Administration, often taking years to bring to market. The European Medicines Agency has less strict requirements to bring a generic inhaler to the market. It has been suggested that regulatory agencies among different countries share and accept submitted pharmaceutical company dossiers among themselves through harmonizing regulations. This has been the major impediment toward declaring a “global worldwide reference product” that generic pharmaceutical companies could use in their development.119
It is well-documented that people with low SES have significantly worse medication adherence, including for COPD. There are multiple contributors including commercial availability, costs, and health behaviors that affect adherence. Medication adherence is a function of numerous factors, and in general people of a low SES tend to be the most noncompliant.195 This is partially due to lack of resources in some people, however, occurs even in an insured indigent. In a study of a US health system in adults with health care insurance, it was found that medication adherence was poorest in people with low education level; and further patients with asthma or COPD had the worse adherence compared with chronic diseases such as depression and diabetes mellitus.31 In 2006, one study found that tiotropium was used less frequently in people with low vs high SES (odds ratio =0.3),46 whereas in another study albuterol use was prescribed more often in highly polluted areas where the poor live.131 An excellent review on access to essential medicines for obstructive lung diseases and related issues in disparate settings was published in 2015 by the Forum of International Respiratory Societies.196
Conclusion
Across the globe, COPD disproportionately affects ethnic minorities and low socioeconomic groups. Vulnerable populations have differential exposure to indoor and outdoor pollution, occupational and environmental hazards, and tobacco smoke, which contribute to disparities in prevalence of COPD and outcomes. However, social determinants of health (ie, access to health care, education, economic stability, social and community context, and neighborhood/built environment) add an additional barrier to establishing health equity. Consequently, interventions that are designed to improve health equity should be adapted for the socioeconomic and political context in which the vulnerable problems exist. Each setting, whether it is the individual clinic setting, health care system, community, region, and country, has its unique influences and resources that will determine the best approaches to lessen the burden of health disparities related to COPD. Whether it is at the health care provider–patient level, the health care organization level, or societal level – it has been and will continue to be a challenge to lessen the disparities associated with COPD and SES, if no other reason, the availability of adequate resources. No one sector will solve the issues with disparities regarding COPD. Eventually, COPD will be a disease of predominantly the low SES segment of the population, whether caused by tobacco or biomass or occupation. The question is going to be how significant a role the disease plays, which depends on resources, programmatic approaches, and individual health care and public health providers and societies. However, when it comes to health care costs, being poor is expensive. We have largely “diagnosed the syndrome of health disparities in COPD”, and evidence-based strategies that address SES are greatly needed to decrease disease burden.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease . Guidelines: Summarising clinical guidelines for primary care. COPD diagnosis, management and prevention; [Accessed March 8, 2016]. Available from: https://www.guidelines.co.uk/gold/copd. [Google Scholar]
- 2.Gershon AS, Dolmage TE, Stephenson A, Jackson B. Chronic obstructive pulmonary disease and socioeconomic status: a systematic review. COPD. 2012;9:216–226. doi: 10.3109/15412555.2011.648030. [DOI] [PubMed] [Google Scholar]
- 3.Holloway JW, Arshad SH, Holgate ST. Using genetics to predict the natural history of asthma. J Allergy Clin Immunol. 2010;126:200–209. doi: 10.1016/j.jaci.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Siedlinski M, Michael H, Cho MH, et al. Genome-wide association study of smoking behaviors in COPD patients. Thorax. 2011;66:894–902. doi: 10.1136/thoraxjnl-2011-200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Chronic obstructive pulmonary disease (COPD); Fact sheet. Mar, 2015. [Accessed January 23, 2016]. Available from: https://www.who.int/mediacentre/factsheets/fs315/en/
- 6.Healthy People 2020. Office of Disease Prevention and Health Promotion. US Department of Health and Human Services Phase I Report: Recommendations for the Framework and Format of Healthy People 2020. Section IV. Advisory Committee Findings and Recommendations. [Accessed January 23, 2016]. Available from: https://www.healthypeople.gov/2020/about/advisory/Reports.
- 7.World Health Organization The economics of social determinants of health and health inequalities: a resource book. [Accessed January 23, 2016]. Available from: http://apps.who.int/iris/handle/10665/84213.
- 8.Schraufnagel DE, Blasi F, Kraft M, et al. An official American Thoracic Society/European Respiratory Society policy statement: disparities in respiratory health. Am J Respir Crit Care Med. 2013;188:865–871. doi: 10.1164/rccm.201308-1509ST. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization health topics – poverty. World Health Organization; [Accessed January 23, 2016]. Available from: http://www.who.int/topics/poverty/en/ [Google Scholar]
- 10.Lewis DR, Clegg LX, Johnson NJ. Lung disease mortality in the United States: the National Longitudinal Mortality Study. Int J Tuberc Lung Dis. 2009;13:1008–1014. [PMC free article] [PubMed] [Google Scholar]
- 11.Steenland K, Hu S, Walker J. All-cause and cause-specific mortality by socioeconomic status among employed persons in 27 US states, 1984–1997. Am J Public Health. 2004;94:1037–1042. doi: 10.2105/ajph.94.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forno E, Celedon JC. Health disparities in asthma. Am J Respir Crit Care Med. 2012;185:1033–1035. doi: 10.1164/rccm.201202-0350ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crocker D, Brown C, Moolenaar R, et al. Racial and ethnic disparities in asthma medication usage and health-care utilization. Chest. 2009;136:1063–1071. doi: 10.1378/chest.09-0013. [DOI] [PubMed] [Google Scholar]
- 14.Pleasants R, Ohar J, Croft JB, et al. Chronic obstructive pulmonary disease and asthma – patient characteristics and health impairment. COPD. 2014;7:1–11. doi: 10.3109/15412555.2013.840571. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Commission on social determinants of health – final report. Closing the gap in a generation: health equity through action on the social determinants of health. [Accessed January 23, 2016]. Available from: http://www.who.int/social_determinants/thecommission/finalreport/en/
- 16.Whitehead M. The concepts and principles of equity and health. Health Promotion Int. 1991;6:217–228. doi: 10.2190/986L-LHQ6-2VTE-YRRN. [DOI] [PubMed] [Google Scholar]
- 17.Trinder PM, Croft PR, Martyn Lewis M. Social class, smoking and the severity of respiratory symptoms in the general population. J Epidemiol Comm Health. 2000;54:340–343. doi: 10.1136/jech.54.5.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shohami S, Welch A, Bingham S, et al. Area deprivation predicts lung function independently of education and social class. Eur Respir J. 2004;24:157–161. doi: 10.1183/09031936.04.00088303. [DOI] [PubMed] [Google Scholar]
- 19.Welle I, Eide GE, Gulsvik A, Bakke PS. Pulmonary gas exchange and educational level: a community study. Eur Respir J. 2004;23:583–588. doi: 10.1183/09031936.04.00106704. [DOI] [PubMed] [Google Scholar]
- 20.Schikowski T, Sugiri D, Reimann V, Pesch B, Ranft U, Kramer U. Contribution of smoking and air pollution exposure in urban areas to social differences in respiratory health. BMC Public Health. 2008;8:179. doi: 10.1186/1471-2458-8-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith M, Li L, Augustyn M, Kurmi O. Prevalence and correlates of airflow obstruction iñ317,000 never-smokers in China. Eur Respir J. 2014;44:66–77. doi: 10.1183/09031936.00152413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurmi OP, Li L, Wang J, et al. COPD and its association with smoking in the mainland China: a cross-sectional analysis of 0.5 million men and women from ten diverse areas. Int J Chron Obstruct Pulmon Dis. 2015;10:655–665. doi: 10.2147/COPD.S75454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Pleasants RA, Croft J, et al. Smoking duration, respiratory symptoms, and COPD in adults aged $45 years with a smoking history. Int J Chron Obstruc Pulmon Dis. 2015;10:1–8. doi: 10.2147/COPD.S82259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakke PS, Baste V, Hanoa R, Gulsvik A. Prevalence of obstructive lung disease in a general population: relation to occupational title and exposure to some airborne agents. Thorax. 1991;46:863–870. doi: 10.1136/thx.46.12.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eachus J, Williamw M, Chan P, et al. Deprivation and cause specific morbidity: evidence from the Somerset and Avon survey of health. BMJ (Clin Res) 1996;212:287–292. doi: 10.1136/bmj.312.7026.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Breithaupt K, Muhajarine N. Occurrence of chronic obstructive pulmonary disease among Canadians and sex-related factors. J Clin Epidemiol. 2000;53:755–761. doi: 10.1016/s0895-4356(99)00211-5. [DOI] [PubMed] [Google Scholar]
- 27.Marmot M, Shipley M, Brunner E, et al. Relative contribution of early life and adult socioeconomic factors to adult morbidity in the Whitehall II study. J Epidemiol Commun Health. 2001;55:301–307. doi: 10.1136/jech.55.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montnemery P, Bengtsson P, Elliott A, et al. Prevalence of obstructive lung diseases and respiratory symptoms in relation to living environment and socio-economic group. Respir Med. 2001;95:744–752. doi: 10.1053/rmed.2001.1129. [DOI] [PubMed] [Google Scholar]
- 29.Lindberg A, Jonnson AC, Ronmark E, et al. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127:1544–1552. doi: 10.1378/chest.127.5.1544. [DOI] [PubMed] [Google Scholar]
- 30.Ellison-Loschmann L, Sunyer J, Plana E, et al. Socioeconomic status, asthma, and chronic bronchitis in a large community-based study. Eur Respir J. 2007;29:897–905. doi: 10.1183/09031936.00101606. [DOI] [PubMed] [Google Scholar]
- 31.Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race, and COPD health outcomes. J Epidemiol Commun Health. 2011;65:26–34. doi: 10.1136/jech.2009.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halvorsen T, Matrinussen PA. The geography of chronic obstructive pulmonary disease: a population-based study of Norway. Soc Sci Med. 2014;111:25e34. doi: 10.1016/j.socscimed.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Karnevisto M, Vasankari T, Laitinen T, et al. Low socioeconomic status is associated with chronic obstructive airway diseases. Respir Med. 2011;105:1140–1146. doi: 10.1016/j.rmed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Lovasi GS, Diex Roux AV, Hoffman EA, et al. Socioeconomic status is positively associated with percent emphysema on CT scan: the MESA lung study. Acad Radiol. 2011;18:199–204. doi: 10.1016/j.acra.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin P, Zhang M, Li Y, et al. Prevalence of COPD and its association with socioeconomic status in China: findings from China chronic disease factor surveillance 2007. BMC Public Health. 2011;11:586. doi: 10.1186/1471-2458-11-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrick H, Pleasants R, Wheaton A, et al. Chronic obstructive pulmonary disease and associated health-care resource use – North Carolina 2007 and 2009. Morbid Mortal Wkly Rep. 2012;61:143–146. [PubMed] [Google Scholar]
- 37.Kainu A, Rouhos A, Sovijarvi A, et al. COPD in Helinski, Finland: socioeconomic status based on occupation has an important impact on prevalence. Scand J Public Health. 2013;41:570–578. doi: 10.1177/1403494813484554. [DOI] [PubMed] [Google Scholar]
- 38.Golec M, Skorska C, Mackiewicz B, et al. Relationship between COPD and lower socioeconomic status in farmers from south-eastern Poland (Lublin region) Rural Remote Health. 2014;14:2531. [PubMed] [Google Scholar]
- 39.Hagstad S, Backman H, Anders Bjerg A, et al. Prevalence and risk factors of COPD among never-smokers in two areas of Sweden: occupational exposure to gas, dust or fumes is an important risk factor. Respir Med. 2015;109:1439e1445. doi: 10.1016/j.rmed.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Kim SW, Kong KA, et al. Risk factors for chronic obstructive pulmonary disease among never-smokers in Korea. Int J Chron Obstruct Pulmon Dis. 2015;10:497–506. doi: 10.2147/COPD.S77662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70:822–829. doi: 10.1136/thoraxjnl-2015-206938. [DOI] [PubMed] [Google Scholar]
- 42.Van Rossum CT, Shipley MJ, van de Mheen H, et al. Employment grade differences in cause specific mortality. A 25 year followup of civil servants from the first Whitehall study. J Epidemiol Commun Health. 2000;54:178–184. doi: 10.1136/jech.54.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prescott E, Godtferden N, Vestbo J, Osler M. Social position and mortality from respiratory diseases in males and females. Eur Respir J. 2003;21:821–826. doi: 10.1183/09031936.03.00047502. [DOI] [PubMed] [Google Scholar]
- 44.Huisman M, Kunst AE, Bopp M, et al. Educational inequalities in cause-specific mortality in middle-aged and older men and women in eight western European populations. Lancet. 2005;365:493–500. doi: 10.1016/S0140-6736(05)17867-2. [DOI] [PubMed] [Google Scholar]
- 45.Antonelli-Incazi R, Ancona C, Forastiere F, et al. Socioeconomic status and hospitalization in the very old: a retrospective study. BMC Public Health. 2007;7:227. doi: 10.1186/1471-2458-7-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanc PD, Eisner MD, Yelin EH, et al. Socioeconomic gradients in tiotropium use among adults with COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:483–490. doi: 10.2147/copd.s3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reilly KH, Gu D, Duan X, et al. Risk factors for chronic obstructive pulmonary disease mortality in Chinese adults. Am J Epidemiol. 2008;167:998–1004. doi: 10.1093/aje/kwm393. [DOI] [PubMed] [Google Scholar]
- 48.Schane RE, Walter LC, Dinno A, Covinsky KE, Woodruff PG. Prevalence and risk factors for depressive symptoms in persons with chronic obstructive pulmonary disease. J Gen Intern Med. 2008;23:1757–1762. doi: 10.1007/s11606-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong AWM, Gan WQG, Burns J, et al. Acute exacerbation of chronic obstructive pulmonary disease: influence of social factors in determining length of hospital stay and readmission rates. Can Respir J. 2008;15:361–364. doi: 10.1155/2008/569496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arne M, Lundin F, Boman G, Janson C, Janson S, Emtner M. Factors associated with good self-rated health and quality of life in subjects with self-reported COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:511–519. doi: 10.2147/COPD.S24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calderón-Larrañaga A, Carney L, Soljak M. Association of population and primary healthcare factors with hospital admission rates for chronic obstructive pulmonary disease in England: national cross-sectional study. Thorax. 2011;66:191–196. doi: 10.1136/thx.2010.147058. [DOI] [PubMed] [Google Scholar]
- 52.Miravitlles M, Naberan K, Cantoni J, Azpeitia A. Socioeconomic status and health-related quality of life of patients with chronic obstructive pulmonary disease. Respiration. 2011;82:402–408. doi: 10.1159/000328766. [DOI] [PubMed] [Google Scholar]
- 53.Omachi TA, Sarkar U, Yelin EH, et al. Lower health literacy is associated with poorer health status and outcomes in chronic obstructive pulmonary disease. J Gen Intern Med. 2012;28:74–81. doi: 10.1007/s11606-012-2177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAllister DA, Morling JR, Fischbacher CM. Socioeconomic deprivation increases the effect of winter on admissions to hospital with COPD: retrospective analysis of 10 years of national hospital data. Prim Care Res J. 2013;22:296–299. doi: 10.4104/pcrj.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gershon AS, Hwee J, Victor JC, Wilton AS, To T. Trends in socioeconomic status-related differences in mortality among people with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:1195–1202. doi: 10.1513/AnnalsATS.201403-094OC. [DOI] [PubMed] [Google Scholar]
- 56.Lange P, Marott JL, Vestbo J, et al. Socioeconomic status and prognosis in Denmark. COPD. 2014;11:431–437. doi: 10.3109/15412555.2013.869580. [DOI] [PubMed] [Google Scholar]
- 57.Trachtenberg AJ, Dik N, Chateau D, et al. Inequities in ambulatory care and the relationship between socioeconomic status and respiratory hospitalizations: a population-based study of a Canadian city. Ann Fam Med. 2014;12:402–407. doi: 10.1370/afm.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma G, Meena R, Goodwin J, et al. Burn injury associated with home oxygen use in patients with chronic obstructive pulmonary disease. Mayo Clin Proc. 2015;90:492–499. doi: 10.1016/j.mayocp.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown CA, Crombie AK, Tunstall-Pedoe H. Failure of cigarette smoking to explain international differences in mortality from chronic obstructive pulmonary disease. J Epidemiol Community Health. 1994;48:134–139. doi: 10.1136/jech.48.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burney P, Jithoo, Kato B, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty – a BOLD analysis. Thorax. 2014;69:465–473. doi: 10.1136/thoraxjnl-2013-204460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camp PG, Alejandra Ramirez-Venegas A, Sansores RH, et al. COPD phenotypes in biomass smoke-versus tobacco smoke-exposed Mexican women. Eur Respir J. 2014;43:725–734. doi: 10.1183/09031936.00206112. [DOI] [PubMed] [Google Scholar]
- 62.Menezes AMB, Perez-Padilla R, Roberto J, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 63.Pleasants R, Ohar J, Croft JB, et al. Chronic obstructive pulmonary disease and asthma – patient characteristics and health impairment. J Chron Obstruct Pulmon Dis. 2014;7:1–11. [Google Scholar]
- 64.Kosacz N, Punturieri A, Croxton TL, et al. Chronic obstructive pulmonary disease among adults – United States, 2011. Morb Mortal Wkly Rep. 2012;61:938–943. [PubMed] [Google Scholar]
- 65.Yin P, Jiang C, Cheng K, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet. 2007;370:751–757. doi: 10.1016/S0140-6736(07)61378-6. [DOI] [PubMed] [Google Scholar]
- 66.Aguku IT, King BA, Dube SR. Current cigarette smoking among adults – United States 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- 67.Twyman L, Bonevski B, Paul C, Bryant J. Perceived barriers to smoking cessation in selected vulnerable groups: a systematic review of the qualitative and quantitative literature. Br Med J Open. 2014;4:e006414. doi: 10.1136/bmjopen-2014-006414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Batra V, Patkar AA, Berrettini WH, et al. The genetic determinants of smoking. Chest. 2003;123:1730–1739. doi: 10.1378/chest.123.5.1730. [DOI] [PubMed] [Google Scholar]
- 69.Jaganath D, Miranda JJ, Gilman RH, et al. Prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res. 2015;16:40. doi: 10.1186/s12931-015-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balmes JR. Occupational contribution to the burden of chronic obstructive pulmonary disease. J Occup Environ Med. 2005;47:154–160. doi: 10.1097/01.jom.0000152923.07801.e1. [DOI] [PubMed] [Google Scholar]
- 71.Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J. 2003;22:462–469. doi: 10.1183/09031936.03.00094203. [DOI] [PubMed] [Google Scholar]
- 72.de Meer G, Kerkhof M, Kromhout H, Schouten JP, Heederik D. Interaction of atopy and smoking on respiratory effects of occupational dust exposure: a general population-based study. Environ Health. 2004;3:6. doi: 10.1186/1476-069X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinmann S, Vollmer WM, Breen V, et al. COPD and occupational exposures: a case-control study. J Occup Environ Med. 2008;50:561–569. doi: 10.1097/JOM.0b013e3181651556. [DOI] [PubMed] [Google Scholar]
- 74.Hnizdo E, Sullivan PA, Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the third National Health and Nutrition Examination Survey. Am J Epidermiol. 2002;156:738–746. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 75.Mehta AJ, Miedinger D, Keidel D, et al. Occupational exposure to dusts, gases, and fumes and incidence of chronic obstructive pulmonary disease in the Swiss Cohort Study on air pollution and lung and heart diseases in adults. Am J Respir Crit Care Med. 2012;185:1292–300. doi: 10.1164/rccm.201110-1917OC. [DOI] [PubMed] [Google Scholar]
- 76.World Health Organization Household air pollution and health fact sheet N°292. Feb, 2016. [Accessed March 15, 2016]. Available from: http://www.who.int/mediacentre/factsheets/fs292/en/
- 77.Salvi S, Barnes P. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138:3–6. doi: 10.1378/chest.10-0645. [DOI] [PubMed] [Google Scholar]
- 78.Torres-Duque C, Maldonado D, Perez-Padilla R, Ezzati M, Viegi G. Biomass fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc. 2008;5:577–590. doi: 10.1513/pats.200707-100RP. [DOI] [PubMed] [Google Scholar]
- 79.Viegi G, Simoni M, Scognamiglio A, et al. Indoor air pollution and airway disease. Int J Tuberc Lung Dis. 2004;8:1401–1415. [PubMed] [Google Scholar]
- 80.Liu S, Zhou Y, Wang X, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax. 2007;62:889–897. doi: 10.1136/thx.2006.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orozco-Levi M, Garcia-Aymerich J, Villar J, et al. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:542–546. doi: 10.1183/09031936.06.00052705. [DOI] [PubMed] [Google Scholar]
- 82.Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environ Health Perspect. 2007;115:848–855. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y, Zou Y, Li X, et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med. 2014;11:e1001621. doi: 10.1371/journal.pmed.1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurmi OP, Semple S, Simkhaha P, Smith WCS, Ayres JG. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax. 2010;65:221–228. doi: 10.1136/thx.2009.124644. [DOI] [PubMed] [Google Scholar]
- 85.Liu Y, Lee K, Perez-Padilla R, Hudson NL, Mannino DM. Outdoor and indoor air pollution and COPD-related diseases in high- and low-income countries. J Int Tuberc Lung Dis. 2012;12:115–127. [PubMed] [Google Scholar]
- 86.Sint T, Donohue JF, Ghio AJ. Ambient air pollution particles and the acute exacerbation of chronic obstructive pulmonary disease. Inhal Toxicol. 2008;20:25–29. doi: 10.1080/08958370701758759. [DOI] [PubMed] [Google Scholar]
- 87.Sunyer J, Schwartz J, Tobias A, Macfarlane D, Garcia J, Anto JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol. 2000;151:50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- 88.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232–239. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 89.Hu G, Zhou Y, Tian Y, et al. COPD from exposure to biomass fuel: a meta-analysis. Chest. 2010;138:20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 90.Sood A, Petersen H, Blanchette CM, et al. Wood smoke exposure and gene promoter methylation are associated with increased risk for COPD in smokers. Am J Respir Crit Care Med. 2010;182:1098–1104. doi: 10.1164/rccm.201002-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim C, Chapman RS, Hu W, et al. Smoky coal, tobacco smoking, and lung cancer risk in Xunwei, China. Lung Cancer. 2014;84:31–35. doi: 10.1016/j.lungcan.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boehmer TK, Foster SL, Henry JR, Woghiren-Akinnifesi EL, Yip FY, et al. Residential proximity to major highways - United States, 2010. MMWR. 2013;62(03)(Suppl):46–50. [PubMed] [Google Scholar]
- 93.Schultz ES, Hallberg J, Bellander T, et al. Early-life exposure to traffic-related air pollution and lung function in adolescence. Am J Respir Crit Care Med. 2016;193:171–177. doi: 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 94.Schikowski T, Mills IC, Anderson HR, et al. Ambient air pollution: a cause of COPD? Eur Respir J. 2014;43:250–263. doi: 10.1183/09031936.00100112. [DOI] [PubMed] [Google Scholar]
- 95.Turner S, Prabhu N, Daneilan P, et al. First- and second-trimester fetal size and asthma outcomes at 10 years of age. Am J Respir Crit Care Med. 2011;184:407–413. doi: 10.1164/rccm.201012-2075OC. [DOI] [PubMed] [Google Scholar]
- 96.Young S, Arnott J, O’Keeffe PT, Le Souef PN, Landau LI. The association between early life lung function and wheezing during the first 2 yrs of life. Eur Respir J. 2000;15:151–157. doi: 10.1183/09031936.00.15115100. [DOI] [PubMed] [Google Scholar]
- 97.Tager IB, Hanrahan JP, Tosteson TD, et al. Lung function, pre- and post-natal smoke exposure, and wheezing in the first year of life. Am Rev Respir Dis. 1993;147:811–817. doi: 10.1164/ajrccm/147.4.811. [DOI] [PubMed] [Google Scholar]
- 98.Håland G, Carlsen KC, Sandvik L. Reduced lung function at birth and the risk of asthma at 10 years of age. N Engl J Med. 2006;355:1682–1689. doi: 10.1056/NEJMoa052885. [DOI] [PubMed] [Google Scholar]
- 99.Johannessen A, Bakke PS, Hardie JA, Eagan TM. Association of exposure to environmental tobacco smoke in childhood with chronic obstructive pulmonary disease and respiratory symptoms in adults. Respirology. 2012;17:499–505. doi: 10.1111/j.1440-1843.2012.02129.x. [DOI] [PubMed] [Google Scholar]
- 100.US Department of Health and Human Services The health consequences of smoking – 50 years of progress: a report of the Surgeon General. 2014. [Accessed January 23, 2016]. Available from: http://www.surgeongeneral.gov/library/reports/50-years-of-progress/
- 101.Delpisheh A, Kelly Y, Rizwan S, Brabin BJ. Socio-economic status, smoking during pregnancy and birth outcomes: an analysis of cross-sectional community studies in Liverpool (1993–2001) J Child Health Care. 2006;10:140–148. doi: 10.1177/1367493506062553. [DOI] [PubMed] [Google Scholar]
- 102.Prabhu N, Smith N, Campbell D, et al. First trimester maternal tobacco smoking habits and fetal growth. Thorax. 2010;65:235–240. doi: 10.1136/thx.2009.123232. [DOI] [PubMed] [Google Scholar]
- 103.Eisner MD, Balmes J, Katz PP, Trupin L, Yelin EH, Blanc PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health. 2005;4:7. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berend N. Contribution of air pollution to COPD and small airway dysfunction. Respirology. 2016;21:10. doi: 10.1111/resp.12644. [DOI] [PubMed] [Google Scholar]
- 105.Sunyer J. Urban air pollution and chronic obstructive pulmonary disease: a review. Eur Respir J. 2001;17:1024–1033. doi: 10.1183/09031936.01.17510240. [DOI] [PubMed] [Google Scholar]
- 106.Ko FW, Hui DS. Air pollution and chronic obstructive pulmonary disease. Respirology. 2012;17:395–401. doi: 10.1111/j.1440-1843.2011.02112.x. [DOI] [PubMed] [Google Scholar]
- 107.Lange P, Celli B, Agusti A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 108.Svanes C, Sunyer S, Plana E, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65(1):14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 109.Tennant PWE, Gibson GJ, Parker L, Pearce MS. Childhood respiratory illness and lung function at ages 14 and 50 years: childhood respiratory illness and lung function. Chest. 2010;137(1):146–155. doi: 10.1378/chest.09-0352. [DOI] [PubMed] [Google Scholar]
- 110.de Marco R, Accordini S, Marcon A, et al. Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2011;183:891–897. doi: 10.1164/rccm.201007-1125OC. [DOI] [PubMed] [Google Scholar]
- 111.Tagiyeva N, Deveruex G, Fiedling S, et al. Outcomes of childhood asthma and wheezy bronchitis a 50-year cohort study. Am J Respir Crit Care Med. 2016;193:23–30. doi: 10.1164/rccm.201505-0870OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feinstein JS. The relationship between socioeconomic status and health; a review of the literature. Milbank Q. 1993;71:279–322. [PubMed] [Google Scholar]
- 113.Mackenbach J, Stronks K, Kunst AE. The contribution of medical care to inequalities in health. Soc Science Med. 1989;29:369–376. doi: 10.1016/0277-9536(89)90285-2. [DOI] [PubMed] [Google Scholar]
- 114.Krishman JA. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med. 2001;161:1660–1668. doi: 10.1001/archinte.161.13.1660. [DOI] [PubMed] [Google Scholar]
- 115.Gray AM. Inequalities in health. The Black Report: a summary and comment. Int J Health Serv. 1982;12:349–380. doi: 10.2190/XXMM-JMQU-2A7Y-HX1E. [DOI] [PubMed] [Google Scholar]
- 116.Veugelers P, Yip A. Socioeconomic disparities in health care use: does universal coverage reduce inequalities in health? J Epidemiol Commun Health. 2003;57:424–428. doi: 10.1136/jech.57.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cokkinides VE, Halpern MT, Barbeau EM, et al. Racial and ethnic disparities in smoking-cessation interventions: analysis of the 2005 national health interview survey. Am J Prev Med. 2008;34:404–412. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 118.Browning KK, Ferketich AK, Salsberry PJ, et al. Socioeconomic disparity in provider-delivered assistance to quit smoking. Nicotine Tob Res. 2008;10:55–61. doi: 10.1080/14622200701704905. [DOI] [PubMed] [Google Scholar]
- 119.Al-Numani D, Colucci P, Ducharme MP. Rethinking bioequivalence and equivalence requirements of orally inhaled drug products. Asian J Pharm Sci. 2015;10:461–471. [Google Scholar]
- 120.Montaño M, Beccerril C, Ruiz V, Ramos C, Sansores RH, González-Avila G. Matrix metalloproteinases activity in COPD associated with wood smoke. Chest. 2004;125:466–472. doi: 10.1378/chest.125.2.466. [DOI] [PubMed] [Google Scholar]
- 121.Moran-Mendoza O, Pérez-Padilla JR, Salazar-Flores M, Vazquez-Alfaro F. Wood smoke-associated lung disease: a clinical, functional, radiological and pathological description. Int J Tuberc Lung Dis. 2008;12:1092–1098. [PubMed] [Google Scholar]
- 122.Perez-Padilla R, Ramirez-Venegas A, Sansores-Martinez R. Clinical characteristics of patients with biomass smoke-associated COPD and chronic bronchitis. J Chron Obstruct Pulm Dis. 2014;1:23–32. doi: 10.15326/jcopdf.1.1.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gonzalez-Garcia M, Tores-Duque CA, Bustos A, et al. Bronchial hyperresponsiveness in women with chronic obstructive pulmonary disease. Int J Chron Obstruc Pulmon Dis. 2012;7:367–373. doi: 10.2147/COPD.S30410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramirez-Venegas A, Sansores RH, Perez-Padilla R, et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med. 2006;173:393–397. doi: 10.1164/rccm.200504-568OC. [DOI] [PubMed] [Google Scholar]
- 125.González-García M, Maldonado Gomez D, Torres-Duque CA, et al. Tomographic and functional findings in severe COPD: comparison between the wood smoke-related and smoking-related disease. J Bras Pneumol. 2013;39:147–154. doi: 10.1590/S1806-37132013000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guarnieri MJ, Diaz JV, Basu C, et al. Effects of woodsmoke exposure on airway inflammation in rural Guatemalan women. PLoS One. 2014;9:e88455. doi: 10.1371/journal.pone.0088455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rivera RM, Cosio MG, Ghezzo H, Salazar M, Pérez-Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12:972–977. [PubMed] [Google Scholar]
- 128.Aldrich MC, Munro HM, Mumma M, et al. Chronic obstructive pulmonary disease and subsequent overall and lung cancer mortality in low-income adults. PLoS One. 2015;10:e0121805. doi: 10.1371/journal.pone.0121805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pleasants R, Herrick H, Liao W. Chronic obstructive lung disease in North Carolina: prevalence, characteristics and impact. N C Med J. 2013;74:376–383. [PubMed] [Google Scholar]
- 130.Dawkins P, Wood A, Nightingale P, Stockley R. Mortality in alpha-1-antitrypsin deficiency in the United Kingdom. Respir Med. 2009;103:1540–1547. doi: 10.1016/j.rmed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Sofianopoulou E, Rushton SP, Diggle PJ, Pless-Ulloli T. Association between respiratory prescribing, air pollution and deprivation, in primary health care. J Public Health. 2013;35:502–509. doi: 10.1093/pubmed/fdt107. [DOI] [PubMed] [Google Scholar]
- 132.Sutton W, Linn E. Where the Money Was: The Memoirs of a Bank Robber. New York: Viking Press; 1976. p. 160. [Google Scholar]
- 133.Thakur N, Mcgarry ME, Oh SS, et al. The Lung Corps’ approach to reducing health disparities in respiratory disease. Ann Am Thorac Soc. 2014;11:655–660. doi: 10.1513/AnnalsATS.201402-061AR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Celedón JC, Roman J, Schraufnagel DE, Thomas A. Respiratory health equality in the United States. The American Thoracic Society Perspective. Ann Am Thorac Soc. 2014;11:473–479. doi: 10.1513/AnnalsATS.201402-059PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Celli B, Decramer M, Wedzicha JA, et al. An official American Thoracic Society/European Respiratory Society statement: research questions in COPD. Eur Respir J. 2015;45:879–905. doi: 10.1183/09031936.00009015. [DOI] [PubMed] [Google Scholar]
- 136.Respiratory diseases of the world: realities of today – opportunities for tomorrow. [Accessed January 20, 2016]. Available from: http://www.thoracic.org/global-health-firs-report-respiratory-diseases-in-the-world/index.php.
- 137.European COPD Coalition Call to action on COPD. [Accessed January 28, 2016]. Available from: http://www.copdcoalition.eu/wp-content/uploads/2015/09/ECC-Call-to-Action-010915-BD.pdf.
- 138.19th World Health Organization Model List of Essential Medicines. 2015 Apr; [Google Scholar]
- 139.World Health Organization framework convention on tobacco control. 2005 [Google Scholar]
- 140.World Health Organization guidelines for indoor air quality: household fuel combustion. 2014 [PubMed] [Google Scholar]
- 141.World Health Organization 2008–2013 action plan for the global strategy for prevention and control of noncommunicable diseases [Google Scholar]
- 142.World Health Organization Prevention and control of non-communicable diseases: guidelines for primary health care in low-resource settings. 2012 [PubMed] [Google Scholar]
- 143.A global monitoring framework for noncommunicable diseases. [Accessed January 23, 2016]. Available from: http://www.who.int/nmh/events/2012/discussion_paper3.pdf.
- 144.Beran D, Zar HJ, Perrin C. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir Med. 2015;3:159–170. doi: 10.1016/S2213-2600(15)00004-1. [DOI] [PubMed] [Google Scholar]
- 145.Salvi S. The silent epidemic of COPD in Africa. Lancet Glob Health. 2015;3(1):e6–e7. doi: 10.1016/S2214-109X(14)70359-6. [DOI] [PubMed] [Google Scholar]
- 146.Ford ES, Croft J, Mannino DM, et al. COPD Surveillance – United States 1999–2011. Chest. 2013;144:284–305. doi: 10.1378/chest.13-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pleasants R, Heidari K, Wheaton J, et al. Targeting chronic obstructive pulmonary disease by state-based surveillance. J Chron Obstruct Pulmon Dis. 2015;12:680–689. [Google Scholar]
- 148.Carré PC, Roche N, Neukirch F, et al. The effect of an information leaflet upon knowledge and awareness of COPD in potential sufferers. A randomized controlled study. Respiration. 2008;76:53–60. doi: 10.1159/000115947. [DOI] [PubMed] [Google Scholar]
- 149.Braido F, Baiardini I, Sumberesi M, Blasi F, Canonica GW. Obstructive lung diseases and inhaler treatment: results from a national public pragmatic survey. Respir Res. 2013;14:94. doi: 10.1186/1465-9921-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Finn PW, Malhotra A. Health equality for pulmonary, critical care, and sleep medicine – opportunities for professional societies. Glob Heart. 2014;9:359–360. doi: 10.1016/j.gheart.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Institute of Medicine (US) Committee on a National Surveillance System for Cardiovascular and Select Chronic Diseases. Washington (DC): National Academies Press (US); 2011. A Nationwide Framework for Surveillance of Cardiovascular and Chronic Lung Diseases. [PubMed] [Google Scholar]
- 152.Holt J, Huston SL, Heidari K, et al. Indicators for chronic disease surveillance – United States, 2013. Morb Mortal Wkly Rep. 2015;64(RR01):1–15. [PubMed] [Google Scholar]
- 153.Hahn EJ, Rayens MK, Adkins S, et al. Fewer hospitalizations for chronic obstructive pulmonary disease in communities with smoke-free public policies. Am J Public Health. 2014;104:1059–1065. doi: 10.2105/AJPH.2014.301887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frazer K, Callinan JE, McHugh J, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev. 2016;2:CD005992.pub3. doi: 10.1002/14651858.CD005992.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Physician behavior and practice patterns related to smoking cessation summary report. Association of American Medical Colleges; [Accessed January 23, 2016]. Available from: https://www.aamc.org/download/55438/data/ [Google Scholar]
- 156.Haas JS, Linder JA, Park ER, et al. Proactive tobacco cessation outreach to smokers of low socioeconomic status. a randomized clinical trial. JAMA Intern Med. 2015;175:218–226. doi: 10.1001/jamainternmed.2014.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neumann T, Rasmussen M, Ghith N, et al. The Gold Standard Programme: smoking cessation interventions for disadvantaged smokers are effective in a real-life setting. Tob Control. 2013;22:e9. doi: 10.1136/tobaccocontrol-2011-050194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gordon SB, Bruce NG, Grigg J, et al. Respiratory risks from household air pollution in low and middle income countries. Lancet Respir Med. 2014;2:823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.United Nations Foundation Global Alliance for Clean Cookstoves and the Climate and Clean Air Coalition. [Accessed January 23, 2016]. Available from: http://www.unfoundation.org/what-we-do/campaigns-and-initiatives/cookstoves/
- 160.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–1726. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 161.Romieu I, Riojas-Rodríguez H, Marrón-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med. 2009;180:649–656. doi: 10.1164/rccm.200810-1556OC. [DOI] [PubMed] [Google Scholar]
- 162.Chapman BS, He X, Blair AE, Lan Q. Improvement in household stoves and risk of chronic obstructive pulmonary disease in Xuanwei, China: retrospective cohort study. Br Med J. 2005;331:1050. doi: 10.1136/bmj.38628.676088.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alvis-Guzmán N, Alvis-Estrada L, Fernando de L. The cost of connecting poor households to natural gas in Colombia and its impact on health. Rev Salud Pública. 2014;14:1. doi: 10.1590/s0124-00642012000100003. [DOI] [PubMed] [Google Scholar]
- 164.US Environmental Protection Agency Standards of Performance for New Residential Wood Heaters, New Residential Hydronic Heaters and Forced-Air Furnaces. [Accessed January 23, 2016]. Available from: https://federalregister.gov/a/2015-03733.
- 165.Wang T, Niel W, Gao J, et al. Air quality during the 2008 Beijing Olympics: secondary pollutants and regional impact. Atmos Chem Phys. 2010;10:7603–7615. [Google Scholar]
- 166.Varkey JB, Varkey AB, Varkey B. Prophylactic vaccinations in chronic obstructive pulmonary disease: current status. Curr Opin Pulm Med. 2009;15:90–99. doi: 10.1097/MCP.0b013e3283218356. [DOI] [PubMed] [Google Scholar]
- 167.Bonhoeffer J, Bär G, Riffelmann M, Solèr M, Heininger U. The role of Bordetella infections in patients with acute exacerbation of chronic bronchitis. Infection. 2005;33:13–17. doi: 10.1007/s15010-005-4004-9. [DOI] [PubMed] [Google Scholar]
- 168.Damiani G, Federico B, Visca M, Agostini F, Ricciardi W. The impact of socioeconomic level on influenza vaccination among Italian adults and elderly: a cross-sectional study. Prev Med. 2007;45:373–379. doi: 10.1016/j.ypmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 169.Galarce EM, Minsky S, Viswanath K. Socioeconomic status, demographics, beliefs and A (H1N1) vaccine uptake in the United States. Vaccine. 2011;29:5284–5289. doi: 10.1016/j.vaccine.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 170.de Lataillade C, Auvergne S, Delannoy I. 2005 and 2006 seasonal influenza vaccination coverage rates in 10 countries in Africa, Asia Pacific, Europe, Latin America and the Middle East. J Public Health Policy. 2009;30:83–101. doi: 10.1057/jphp.2008.40. [DOI] [PubMed] [Google Scholar]
- 171.Report 4 of The National Chronic Obstructive Pulmonary Disease Audit 2008 Patient Survey. The Royal College of Physicians of London, British Thoracic Society and British Lung Foundation; Dec, 2008. [Accessed January 23, 2016]. Available from: https://www.rcplondon.ac.uk/file/ [Google Scholar]
- 172.Department of Health [UK] Chronic Obstructive Pulmonary Disease National Service Framework: Development of the national service framework for COPD. [Accessed January 23, 2016]. Last modified August 13, 2007. Available from: http://collection.europarchive.org/tna/20080107220102/dh.gov.uk/en/Policyandguidance/Healthandsocialcaretopics/DH_4138532.
- 173.Lisspers K, Johansson G, Jansson C, et al. Improvement in COPD management by access to asthma/COPD clinics in primary care: data from the observational PATHOS study. Respir Med. 2014;108:1345–1354. doi: 10.1016/j.rmed.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 174.Plymouth University FRESH AIR international research programme: Collaborating internationally to address the problem of lung disease. [Accessed January 23, 2016]. Available from https://www.plymouth.ac.uk/research/primarycare/fresh-air.
- 175.Khan MA, Ahmed M, Anil S, Walley J. Strengthening the delivery of asthma and chronic obstructive pulmonary disease care at primary health-care facilities: study design of a cluster randomized controlled trial in Pakistan. Glob Health Action. 2015;8:28225. doi: 10.3402/gha.v8.28225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goldstein RS, O’Hoski S. Telemedicine in COPD: time to pause. Chest. 2014;145:945–949. doi: 10.1378/chest.13-1656. [DOI] [PubMed] [Google Scholar]
- 177.Price D, Tinkelman DG, Nordyke RJ, Isonaka S, Halberty RJ. Scoring system and clinical application of COPD diagnostic questionnaires. Chest. 2006;129:1531–1539. doi: 10.1378/chest.129.6.1531. [DOI] [PubMed] [Google Scholar]
- 178.Martinez FJ, Raczek AE, Seifer FD, et al. Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS) Chron Obstruct Pulmon Dis. 2008;5:85–95. doi: 10.1080/15412550801940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hanania NA, Mannino DM, Yawn BP, et al. Predicting risk of airflow obstruction in primary care: validation of the lung function questionnaire (LFQ) Respir Med. 2010;104:1160–1170. doi: 10.1016/j.rmed.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 180.Calverley PM, Nordyke RJ, Halbert RJ, Isonaka S, Nonikov D. Development of a population-based screening questionnaire for COPD. Chron Obstruct Pulmon Dis. 2005;2:225–232. [PubMed] [Google Scholar]
- 181.Zhou YM, Chen SY, Tian J, et al. Development and validation of a chronic obstructive pulmonary disease screening questionnaire in China. Int J Tuberc Lung Dis. 2013;17:1645–1651. doi: 10.5588/ijtld.12.0995. [DOI] [PubMed] [Google Scholar]
- 182.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 183.Haroon S, Jordan R, Takwoingi Y, Adab P. Diagnostic accuracy of screening tests for COPD: a systematic review and meta-analysis. Br Med J Open. 2015;5(10):e008133. doi: 10.1136/bmjopen-2015-008133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dirven JAM, Tange HJ, Muris JWM, et al. Early detection of COPD in general practice: patient or practice managed. A randomized controlled trial of two strategies in different socioeconomic environments. Prim Care Res. 2013;22:331–337. doi: 10.4104/pcrj.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Salpeter SS, Ormiston T, Salpeter E, Poole P, Cates C. Cardioselective beta-blockers for chronic obstructive pulmonary disease (Cochrane Review) Cochrane Database Syst Rev. 2005;4:CD003566. doi: 10.1002/14651858.CD003566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lammers JW, van Herwaarden CL. Ventilatory effects of long-term treatment with pindolol and metoprolol in hypertensive patients with chronic obstructive lung disease. Br J Clin Pharmacol. 1985;20:205–210. doi: 10.1111/j.1365-2125.1985.tb05062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chopra I, Chopra A, Bias TK. Reviewing risks and benefits of low-dose computed tomography screening for lung cancer. Postgrad Med. 2016;6:1–8. doi: 10.1080/00325481.2016.1134023. [DOI] [PubMed] [Google Scholar]
- 188.Rall FM, Grigg-Damberger M. Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Curr Opin Pulm Med. 2012;18:568–573. doi: 10.1097/MCP.0b013e328358be05. [DOI] [PubMed] [Google Scholar]
- 189.Marin JM, Soriano JB, Carrizo SJ, Boldava A, Celli B. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 190.Hill K, Mangovski-Alzamora S, Blouin M, et al. Disease-specific education in the primary care setting increases the knowledge of people with chronic obstructive pulmonary disease: a randomized controlled trial. Patient Educ Couns. 2010;81:14–18. doi: 10.1016/j.pec.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 191.Bazargani YT, de Boer A, Leufkens H, Mantel-Teeuwisse AK. Essential medicines for COPD and asthma in low and middle income countries. Thorax. 2014;69:1149–1151. doi: 10.1136/thoraxjnl-2014-205249. [DOI] [PubMed] [Google Scholar]
- 192.Babar Z, Lessing C, Mace C, Bissell K. The availability, pricing, and affordability of three essential medicines in 52 low- and middle-income countries. Pharmacoeconomics. 2013;31:1063–1082. doi: 10.1007/s40273-013-0095-9. [DOI] [PubMed] [Google Scholar]
- 193.Vestbo J, Anderson JA, Calverley PM, Celli B. Adherence to inhaled therapy, mortality and hospital admission in COPD. Thorax. 2009;64:939–943. doi: 10.1136/thx.2009.113662. [DOI] [PubMed] [Google Scholar]
- 194.19th WHO Model List of Essential Medicines (April 2015) World Health Organization; [Accessed January 26, 2016]. Available from: http://www.who.int/medicines/publications/essentialmedicines/EML2015_8-May-15.pdf. [Google Scholar]
- 195.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 196.Beran D, Zar HJ, Perrin C, Menezes AM, Burney P. Burden of asthma and chronic obstructive pulmonary disease and access to essential medicines in low-income and middle-income countries. Lancet Respir Med. 2015;3:159–170. doi: 10.1016/S2213-2600(15)00004-1. [DOI] [PubMed] [Google Scholar]
- 197.GoodRx, Inc. [homepage] [Accessed February 1, 2016]. Available at: http://www.goodrx.com/