Abstract
ST8/SCCmecIV community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) has been a common threat, with large USA300 epidemics in the United States. The global geographical structure of ST8/SCCmecIV has not yet been fully elucidated. We herein determined the complete circular genome sequence of ST8/SCCmecIVc strain OC8 from Siberian Russia. We found that 36.0% of the genome was inverted relative to USA300. Two IS256, oppositely oriented, at IS256-enriched hot spots were implicated with the one-megabase genomic inversion (MbIN) and vSaβ split. The behavior of IS256 was flexible: its insertion site (att) sequences on the genome and junction sequences of extrachromosomal circular DNA were all divergent, albeit with fixed sizes. A similar multi-IS256 system was detected, even in prevalent ST239 healthcare-associated MRSA in Russia, suggesting IS256’s strong transmission potential and advantage in evolution. Regarding epidemiology, all ST8/SCCmecIVc strains from European, Siberian, and Far Eastern Russia, examined had MbIN, and geographical expansion accompanied divergent spa types and resistance to fluoroquinolones, chloramphenicol, and often rifampicin. Russia ST8/SCCmecIVc has been associated with life-threatening infections such as pneumonia and sepsis in both community and hospital settings. Regarding virulence, the OC8 genome carried a series of toxin and immune evasion genes, a truncated giant surface protein gene, and IS256 insertion adjacent to a pan-regulatory gene. These results suggest that unique single ST8/spa1(t008)/SCCmecIVc CA-MRSA (clade, Russia ST8-IVc) emerged in Russia, and this was followed by large geographical expansion, with MbIN as an epidemiological marker, and fluoroquinolone resistance, multiple virulence factors, and possibly a multi-IS256 system as selective advantages.
Introduction
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) is a class of MRSA, that has been reported since the 1990s [1–4]. CA-MRSA is primarily isolated in the community [2], but is also isolated in hospital settings [5]. CA-MRSA is generally associated with skin soft tissue infections (SSTI), but also life-threatening, severe, and invasive infections such as pneumonia (including necrotizing cases), sepsis, bloodstream infections, osteomyelitis, and lung (as well as pelvic and epidural) abscesses [1,3,4,6–8]. Large outbreaks of CA-MRSA include the USA300 epidemic with serious invasive infections in the United States in 2007 [2,4,9,10].
CA-MRSA exhibits heterogeneous genetic backgrounds, regarding multilocus sequence types (ST types), protein A gene (spa) types, or staphylococcal cassette chromosome mec (SCCmec) types [4,9,11–14]. The most characterized successful CA-MRSA include the ST8 lineage, such as ST8/SCCmecIV (USA300) [4,9,10–12,14,15], and also the lineages of ST30/SCCmecIV [3,4,8,16–18], ST59/SCCmecV or IV [4,8,19–24], and ST80/SCCmecIV [3,4,16,25,26]: each lineage includes diverse spa types.
MRSA achieves its dynamic evolution mainly through the action of mobile genetic elements, such as insertion sequences and transposons, plasmids, phages, and S. aureus pathogenicity islands (SaPIs), and also through mutations [4,10,13,14,27–31]. Successful CA-MRSA may have each characteristic genetic trait (or a combination) for virulence and/or drug resistance [4,10,13,14]: for example, USA300 has the Panton-Valentine leukocidin (PVL)-encoding phage and the two-cassette array of SCCmecIVa and the arginine catabolic mobile element (ACME) [15], and has become multidrug-resistant including resistance to fluoroquinolones [11,32]; and ST59/SCCmecV MRSA form Taiwan has the PVL-converting phage and the mobile element structure with IS1216V (MESPM1) encoding for multidrug resistance [22,23].
Regarding insertion sequences, IS256 was originally found in S. aureus as the terminal inverted repeat (IR) of transposon Tn4001, encoding for resistance to aminoglycosides (such as gentamicin) [33]. IS256 exists as multiple copies in a cell [33] with a preferred insertion site [34]. IS256 may affect virulence and drug resistance gene expression [30,34], and may also serve as a crossover point for homologous recombination [33]. IS256 is not common among S. aureus; for example, USA300 has no IS256 [30].
CA-MRSA possesses common bacteriological features, such as the elevated expression of cytolytic peptides (phenol-soluble modulins, PSMs, or δ-hemolysin, Hld) [9,35], less multidrug resistance [8,36], and low minimum inhibitory concentrations (MICs) for oxacillin and imipenem [36]. Moreover, CA-MRSA exhibits SCCmec type IV or V in many cases [4,8,13,14,37], and often produces PVL and carries ACME [4,8,14,15,37]. The ST types of globally distributed CA-MRSA include, for example, ST8, ST30, ST59, and ST80, as described above.
In Russia, Sidorenko’s group investigated the molecular characteristics (such as ST/spa/SCCmec types, drug resistance, and virulence genes) of MRSA obtained in a nation wide hospital MRSA surveillance. The prevalence of MRSA in Russia varies from 0 to 80% [38]. The prevalent CA-MRSA lineage exhibits ST8/SCCmecIVc, with spa types such as spa1(t008) and 363(t024) [38]. Prevalent healthcare-associated MRSA (HA-MRSA [2]) is the ST239/SCCmecIII lineage, with spa types such as spa3(t037) [38], which is one of the most globally distributed HA-MRSA lineages [39].
Our international (Japan-Russia, more recently Japan-Russia-Taiwan) joint MRSA studies started in 2006, and since then have isolated PVL+ ST30/spa19(t019)/SCCmecIVc CA-MRSA (strain RS08), only one precisely confirmed PVL+ case in Russia, from a 23-year-old female badminton player with furunculosis in Vladivostok, Far Eastern Russia [40]. We analyzed the whole genome structures, in terms of comparative genomics, of two unique Russian ST239/SCCmecIII lineages: spa351(t030)/SCCmecIIIR (strain 16K) from a case of urethritis in Vladivostok [41] and spa3(t037)/SCCmecIIIA (ST239Kras strain OC3) from a case of fatal pneumonia with sepsis in Krasnoyarsk, Siberian Russia [42]. The latter, ST239Kras, represented the Siberian Russian clade [42] of the globally important ST239 HA-MRSA lineage [39].
In the present study, we determined the complete circular genome sequence of prevalent ST8/SCCmecIVc CA-MRSA (ST8Kras strain OC8 [42]), which was isolated from a fatal pediatric pneumonia case in Krasnoyarsk, Siberian Russia. Based on OC8 data, we found that ST8/SCCmecIVc MRSA, which has widely spread in Russia, including European, Siberian, and Far Eastern regions, commonly carried a characteristic large (one-megabase) genomic inversion (MbIN), triggered by IS256 at hot spots, thereby establishing a novel unique clade (Russia ST8-IVc) of the global ST8/SCCmecIV CA-MRSA lineage. The evolution, potential virulence, and selective advantages of Russia ST8-IVc and also IS256’s spread and functions were discussed.
Materials and Methods
Ethics statement
The Ethics Review Boards of Krasnoyarsk State Medical University (Ethics Review Board No28/2010), Krasnoyarsk, Russia; Far Eastern Federal University School of Biomedicine, Vladivostok, Russia, together with the International Medical Education and Research Center, Niigata, Japan (Ethics Review Board No66-01-17/152) and the National Taiwan University College of Medicine, Taipei, Taiwan, specifically approved this study. Written informed consent was obtained from patients, where necessary.
Bacterial strains
Twenty-five MRSA strains were used in this study and data, including those described previously [11,41–43], are summarized in Table 1. The epidemiological definitions of CA-MRSA and HA-MRSA were based on the Centers for Disease Control and Prevention (CDC) criteria [2]. MRSA from Siberian Russia (Krasnoyarsk) included 10 strains of ST8/SCCmecIVc CA-MRSA (ST8Kras) from cases of SSTIs, community- or hospital-acquired pneumonia (CAP or HAP), sepsis, colitis, and healthy carriers (students and hospital workers) [42]; of these, strain OC8, which was isolated from a case of fatal pediatric CAP, was subjected to a complete genome sequence analysis in the present study. Strain OC3 of ST239/SCCmecIIIA HA-MRSA (ST239Kras), which was isolated from a case of fatal adult HAP with sepsis, and the comparative genome of which was analyzed [42], was also employed. MRSA from European Russia (Moscow, St. Petersburg, and Yaroslavl) were eight ST8/SCCmecIVc strains from cases of SSTIs, sepsis, osteomyelitis, fatal HAP, and a healthy carrier (hospital worker). MRSA from Far Eastern Russia (Vladivostok) included three strains of ST8/SCCmecIVc CA-MRSA isolated from cases of urethritis and SSTIs [41], and an additional ST8/SCCmecIVc strain from a case of nosocomial respiratory tract infection. ST239/SCCmecIIIR strain 16K, which was isolated from a case of urethritis and the comparative genome of which was analyzed [41], was also employed. ST30/spa19(t019)/SCCmecIVc strain RS08, which was isolated in 2006 in Vladivostok [40], was used as a reference strain of Russian CA-MRSA.
Table 1. Relevant characteristics of MRSA strainsa.
| Isolation | Strain | Isolation year | Genotype: | Gene for toxina, superantigen, adhesina | Resistancea,b | Patient | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| country, | ST, spaa, agr, | β-lactam agent | Non β-lactam agenta | Diseasea | Outcomea | Agea | Sexa | ||||||
| region, | SCCmeca, Coa | MIC (μg/ml) | |||||||||||
| citya | OXAa | IPMa | |||||||||||
| Russia | |||||||||||||
| Siberian | Ka | OC1 | 2010 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.13 | G, K, E Cl, L, Ch, Su | Skin abscess | R | 50Y | F | 40 |
| OC1C | 2011 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.25 | L, Ch | Colitis | R | 3Y | M | 40 | ||
| OC8 | 2007 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.25 | L, Ch | Pneumonia (CAP) | D | 1Y | M | 40 | ||
| OC11 | 2007 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.25 | L, Ch | Pneumonia, sepsis | D | 39Y | M | 40 | ||
| OC22 | 2008 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.5 | L, Ch | Pneumonia (CAP) | D | 41Y | M | 40 | ||
| OC23 | 2008 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 64 | 0.13 | L, Ch | Pneumonia (CAP) | D | 40Y | M | 40 | ||
| OC52 | 2008 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.25 | L, Ch | −c | −c | 34Y | F | 40 | ||
| OC59 | 2008 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.13 | G, K, L, Ch | Pneumonia | D | 4M | F | 40 | ||
| OC160 | 2011 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.5 | G, K, E, Clind, L, Ch | Wound infection, cellulitis | R | 53Y | M | 40 | ||
| OC217 | 2010 | 8, 1 (t008), 1, IVca, III | sea, psmα (↑)a | 32 | 0.13 | L | −d | −d | 19Y | F | 40 | ||
| OC3 | 2007 | 239, 3 (t037), 1, IIIAa, IV | tst, sek, seq, | ≥256 | 64 | G, K, E, Cl, L, T, Ch, | Pneumonia (HAP), sepsis | D | 46Y | M | 40 | ||
| psmα(↑)a, cna | R, Su, St | ||||||||||||
| European | Ma | M79 | 2011 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 32 | 0.25 | G, K, L | Wound infection | R | 20-60Ye | −e | This study |
| M185 | 2008 | 8, 1580 (t2648), 1, IVca, III | sea, psmα(↑)a | 32 | 0.25 | G, K, E, Cl, L, Ch | Sepsis | D | 20-60Ye | −e | This study | ||
| M257 | 2012 | 8, New (t1259), 1, IVca, III | sea, psmα(↑)a | 64 | 0.25 | E, Cl, L, Ch | −c | −c | 20-60Ye | −e | This study | ||
| Sa | S2 | 2011 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 64 | 0.25 | G, K, L, Ch | Burn wound | R | 20-60Ye | −e | This study | |
| S14 | 2011 | 8, 363 (t024), 1, IVca, III | sea, psmα(↑)a | 64 | 1 | G, K, E, Cl, L, Ch | Burn, sepsis | R | 20-60Ye | −e | This study | ||
| S65 | 2011 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | 64 | 0.25 | L, Ch | Wound, osteomyelitis | R | 20-60Ye | −e | This study | ||
| S214 | 2012 | 8, 363 (t024), 1, IVca, III | sea, psmα(↑)a | 64 | 4 | G, K, L, Ch | Osteomyelitis, pneumonia (HAP) | D | 20-60Ye | −e | This study | ||
| Ya | Y269 | 2011 | 8, 1 (t008), 1, IVca, III | sea, psmα(↑)a | ≥256 | 16 | G, K, E, Cl, L, Ch | Wound infection | R | 20-60Ye | M | This study | |
| Far Eastern | Va | 12K | 2008 | 8, 826 (tUK)a, 1, IVca, III | sea, psmα(↑)a | 32 | 0.06 | G, K, Ch | Urethritis | R | 21Y | M | 41, this study |
| 40K | 2008 | 8, 826 (tUK)a, 1, IVca, III | sea, psmα(↑)a | 32 | 0.06 | G, K, E, Cl, Ch | Wound infection | R | 19Y | M | 41, this study | ||
| RF57f | 2006 | 8, 826 (tUK)a, 1, IVca, III | sea, psmα(↑)a | 64 | 1 | G, K, E, Cl, Ch, R | Wound infection | R | 20-60Ye | −e | 41, this study | ||
| RF570 | 2010 | 8, 826 (tUK)a, 1, IVca, III | sea, psmα(↑)a | 64 | 1 | G, K, E, Cl, Ch, R | Nosocomial respiratory tract infection | R | 20-60Ye | −e | This study | ||
| 16K | 2008 | 239, 351 (t030), 1, IIIRa, IV | sea, sek, seq, cna | ≥256 | 64 | G, K, E, Cl, L, T, Ch, R, Su, St | Urethritis | R | 20Y | M | 41, this study | ||
| USA | USA300-0114 | -g | 8, 1 (t008), 1, IVaa, III | PVL+a, ACME+a, sek, seq, psmα(↑)a | 32 | 0.13 | K, E, Cii, T | -g | -g | -g | -g | 11, 42, 43 | |
aCity: K, Krasnoyarsk; M, Moscow; S, St. Petersburg; Y, Yaroslavl; V, Vladivostok. SCCmec: IVc, IV.3.1.2; IVa, IV.1.1.1; IIIA, III.1.1.2; IIIR, III.1.1.4. spa type: tUK (Unknown), unknown Ridom spa number. Gene: psmα(↑), elevated psmα expression (which was significantly higher than HA-MRSA expression levels, P<0.05). Other common adhesin genes include lukE-lukD, hla, hlg, hlg-v, hld, split hlb, and c12ag (icaA, icaD, eno, fnbA, fnbB, ebpS, clfA, clfB, fib, sdrC, sdrD, sdrE). PVL, Panton-Valentine leukocidin; ACME, arginine catabolic mobile element. Antimicrobial agent: OXA, oxacillin; IPM, imipenem; G, gentamicin; K, kanamycin; E, erythromycin; Cl, clindamycin; L, levofloxacin; Ci, ciprofloxacin; T, tetracycline; Ch, chloramphenicol; R, rifampicin; Su, sulfamethoxazole; St, streptomycin; Resistance: ind, inducible resistance; i, intermediate. Pneumonia: CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia. Outcome: R, recovery; D, death. Age: Y, year; M, month. Sex: F, female; M, male.
bLevofloxacin-resistant strains were also resistant to ciprofloxacin.
cHealthy carrier case (hospital worker).
dHealthy carrier case (student).
eFull personal (patient) information, not available.
fPrevious name, 57H.
gNo description.
USA300-0114, a type strain of ST8/SCCmecIVa CA-MRSA (USA300) from USA [11], was kindly provided by L. K. McDougal and L. L. McDonald.
Genotyping and virulence gene analysis
The molecular typing of MRSA, such as ST, clonal complex (CC), spa, agr, SCCmec [13], and Coagulase (Coa), was performed as described previously [42,44]. Regarding spa, allele numbers and types were determined using the public spa type databases, eGenomics (http://tools.egenomics.com/) and Ridom SpaServer (http://spaserver.ridom.de/). Forty-nine virulence genes were analyzed by PCR [44]: 3 leukocidin genes (lukPVSF, lukE-lukD, and lukM), 5 hemolysin genes (hla, hlb, hlg, hlg-v, and hld), the peptide cytolysin, PSMα (psmα), 19 staphylococcal superantigen (SAg) genes, named enterotoxin (SE) or enterotoxin-like (SEl) (tst, sea-e, seg-j, selk-r, and selu), staphylococcal exotoxin (set) genes, a staphylococcal superantigen-like gene cluster (ssl), 3 exfoliative toxin genes (eta/b and etd), the epidermal cell differentiation inhibitor gene (edin), 14 adhesin genes (icaA/D, eno, fib, fnbA/B, ebpS, clfA/B, sdrC-E, cna, and bbp), and the ACME-arcA gene.
Pulsed-field gel electrophoresis (PFGE) analysis
Bacterial DNA for PFGE was digested with SmaI and electrophoresed in 1.2% agarose with marker DNA (Lambda ladder; Bio-Rad Laboratories, Inc., Hercules, CA, USA), as described previously [41,42].
Susceptibility testing
Susceptibility testing of bacterial strains was performed using the agar dilution method with Mueller-Hinton agar [45]. Inducible clindamycin resistance was tested, as above, by using agar plates containing erythromycin at 1 μg/ml [42].
Genome analysis
The OC8 genome was analyzed by a long-read single-molecule real-time (SMRT) sequencing platform with P5/C3 chemistry using sequencing technology, a PacBio RS II system (Pacific Biosciences, Menlo Park, CA, USA), with the assembler software SMRT Analysis v2.3.0/hierarchical genome-assembly process (HGAP) pipeline [46]. Genome coverage (sequencing depth) was 259-fold of the genome size. Finishing of the genome contig to construct the complete circular genome sequence was performed by PCR and sequencing. The GenBank accession number for the OC8 complete circular genome sequence is AP017377.
Pairwise comparison between two genome sequences
In the inversion analysis, pairwise comparisons between two MRSA genome sequences were performed using WebACT (http://www.webact.org/WebACT/home).
Homology analysis
A homology analysis was performed using software BLAST (http://blast.ddbj.nig.ac.jp/top-e.html).
mRNA expression assay
The mRNA expression levels of the psmα gene and 16S rRNA genes were examined using an RT-PCR assay [42,47]. psmα expression levels were normalized to 16S rRNA expression levels. ST5/SCCmecII HA-MRSA strains (N315 and Mu50) were used as low psmα expression control strains, and the ST8/SCCmecIVa CA-MRSA type strain USA300-0114 and ST30/SCCmecIVc CA-MRSA strain RS08 were used as elevated psmα expression control strains [42].
Statistical analysis
Data were evaluated by Fisher’s exact test and an analysis of variance with repeated measurements for the mRNA expression assay. The level of significance was defined as a P value of <0.05.
Results
Molecular characteristics of ST8 MRSA in Russia
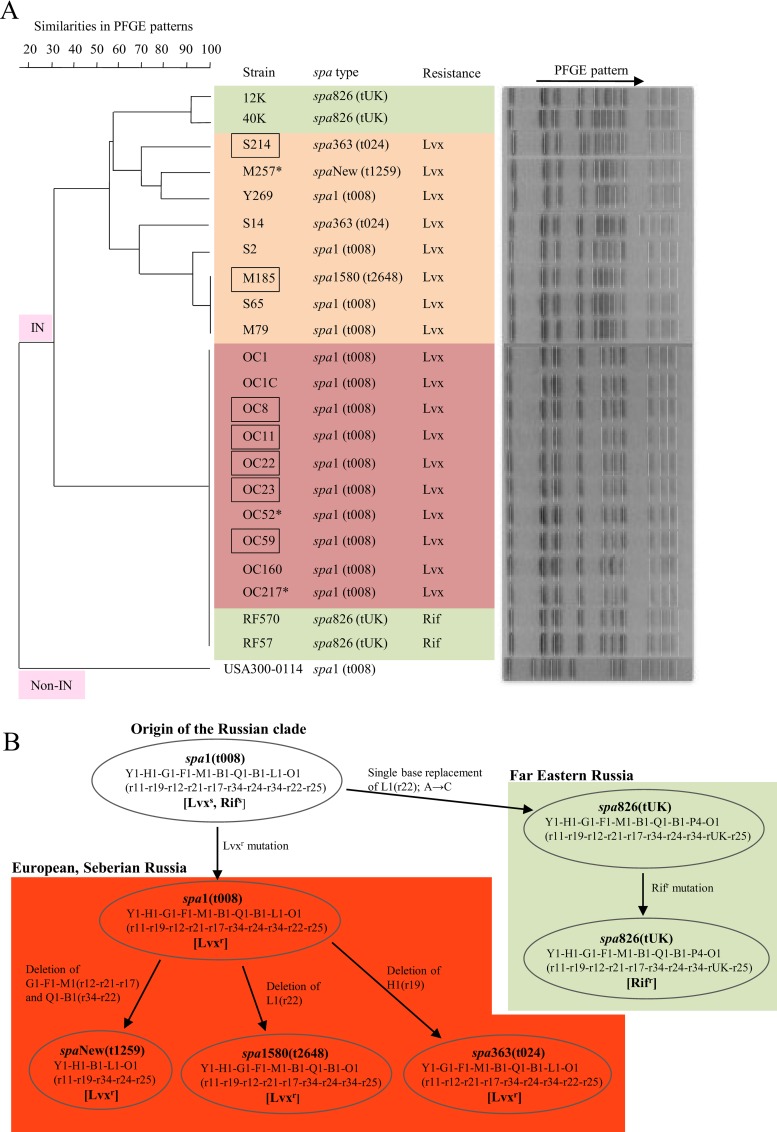
The molecular characteristics of ST8 MRSA strains from European Russia (Moscow, St. Petersburg, and Yaroslavl), Siberian Russia (Krasnoyarsk), and Far Eastern Russia (Valdivostok) are summarized in Table 1 and Fig 1. All ST8 strains exhibited the same genotypes for agr1, SCCmecIVc, and CoaIII. spa types were divergent depending on geographical locations. spa1(t008) was likely the common type, accounting for 100% (10/10) for Siberian Russia and 50% (4/8) for European Russia, but 0% (0/4) for Far Eastern Russia. Based on this result, together with previous findings showing that spa1(t008) was the most prevalent type [38], spa1(t008) may be the ancestral ST8 spa type (Fig 1B). All ST8 strains were positive for sea.
Fig 1.
Pulsed-field gel electrophoresis (PFGE) analysis (A) and phylogenetic spa type analysis of (B) of ST8/SCCmecIVc MRSA strains isolated in Russia. The MRSA strains shown are those described in Table 1. In A, strains were classified into two major groups: IN, those with a megabase inversion, and non-IN, those without a megabase inversion. The geographical location of MRSA isolation is colored: yellow, European Russia (Moscow, St. Petersburg, and Yaroslavl); red, Siberian Russia (Krasnoyarsk); green, Far Eastern Russia (Vladivostok). Square, isolated from a fatal case; asterisk, isolated from a healthy carrier. tUK, tUnknown (unknown Ridom spa number). Lvx, levofloxacin; Rif, rifampicin. In B, spa allele numbers and Ridom spa repeat numbers (in parentheses) are both shown. spa1(t008) represents the ancestral spa type for Russian ST8/SCCmecIVc MRSA; other spa types diverged directly from the common ancestral type. Lvxs, levofloxacin-susceptible; Rifs, rifampicin-susceptible; Lvxr, levofloxacin-resistant; Rifr, rifampicin-resistant.
All ST8 strains exhibited elevated psmα expression, similar to CA-MRSA (USA300 and RS08). Their MIC values for oxacillin and imipenem were lower than those of HA-MRSA (for example, OC3 and 16K), which is consistent with the characteristics of CA-MRSA [36], however, the MIC of imipenem for the Yaroslavl case was high (16 μg/ml). Therefore, the ST8 MRSA strains met the bacteriological criteria for CA-MRSA, although strains OC11, OC52, M257, S214, RF57, and RF570 were isolated from inpatients or hospital workers (healthy carriers), suggesting their spread even in hospitals.
All ST8 strains from Siberian and European Russia were resistant to levofloxacin (MICs, 4–8 μg/ml), while ST8 strains from Far Eastern Russia were susceptible. Only one strain from Siberian Russia (OC160) exhibited inducible clindamycin resistance; other cases of clindamycin resistance were constitutive. Rifampicin resistance (MICs, 4 μg/ml) was only detected in Far Eastern Russia. Chloramphenicol resistance (MICs, 64 μg/ml) was a common feature. Regarding diseases, of the ST8 strains analyzed, seven were from fatal cases of pneumonia or sepsis (Table 1).
In the PFGE analysis (Fig 1A), ST8 strains from Siberian Russia constructed a single cluster, suggesting the spread of a single type (ST8Kras). ST8 strains from European Russia were divergent from ST8Kras, and constituted some heterogeneous clusters. ST8 strains from Vladivostok included the ST8Kras type and a divergent type, which clustered within the European Russia types.
The circular genome structure of ST8Kras strain OC8
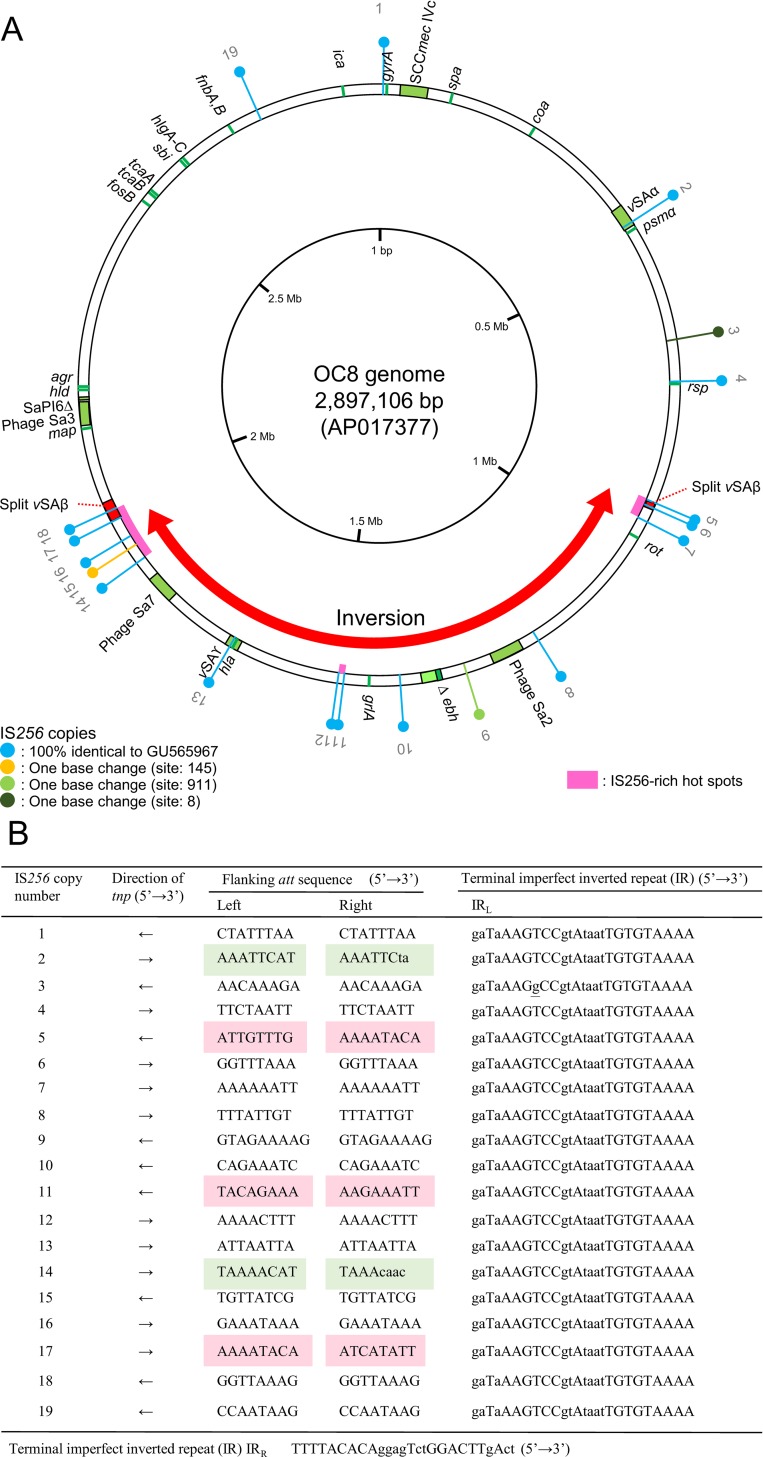
The OC8 genome was estimated to be 2,897,106 bp, sharing an approximately 99.9% homologous core region with the USA300 FPR3757 genome (GenBank Accession Number CP000255), albeit with highly diverged regions, such as phages and mobile genetic elements. Moreover, strain OC8 carried a 2,908-bp chloramphenicol resistance plasmid (pOC8) [42]. Based on the OC8 complete circular genome sequence, the OC8 circular genome map was constructed, as shown in Fig 2A, with a focus on phages, SaPIs, genomic islands, insertion sequences (particularly IS256 [48,49]), resistance genes or mutations, some virulence genes, some regulatory genes or regulons, and genes and genetic structures used for genotyping (spa, agr, coa, SCCmec). OC8 lacked drug resistance transposons; for example OC8 lacked Tn4001 and Tn554, in marked contrast to the ST237 HA-MRSA lineage in Russia (strains OC3 [42] and 16K [41]).
Fig 2.
OC8 circular genome map (A) and genetic status of IS256 copies on the genome (B). In A, OC8 genome information includes MRSA-typing targets, phages, SaPIs, mobile genetic elements, including IS256, virulence, drug resistance, and inversion. Genes (products) described on the genome map are: spa, protein A; coa, coagulase; psmα, phenol-soluble modulin α (cytolytic peptide); rsp, AraC family transcriptional regulator; rot, repressor of toxins; ebh, extracellular matrix-binding protein (very large surface-anchored protein/giant protein); grl, DNA topoisomerase IV (quinoplone resistance); hla, α-hemolysin (Hla); map, map protein; hld, δ-hemolysin (Hld); agr, accessory gene regulator; fos, fosfomycin resistance protein; tca, teicoplanin resistance-associated membrane protein; sbi, IgG-binding protein; hlg, γ-hemolysin (Hlg); fnb, fibronectin-binding protein; ica, intercellular adhesion protein A (biofilm formation); gyr, DNA gyrase (quinoplone resistance). The staphylococcal complement inhibitor (SCIN) gene (scn), staphylokinase (SAK) gene (sak), and superantigen SEA gene (sea) were carried by phage Sa3, and the β-hemolysin (Hlb) gene (hlb) was split by a phage Sa3 insertion. The OC8 genome carried 19 copies of IS256; they are numbered (① to ⑲), as shown in the figure. IS256-enriched hot spots are marked in pink. A large genomic inversion (MbIN), relative to USA300 FPR3757 (GenBank accession number CP000255), occurred between IS256⑤ and IS256⑰; the inverted region is marked with a red thick arrow. Due to MbIN, the genomic island vSAβ, which carried three IS256 (⑥, ⑰, and ⑱), was split into two parts located far from each other. In B, the direction of the IS256 insertion is shown by arrows. Attachment (att) site sequences appear on both sides of IS256 as direct repeats (DRs) upon insertion [48,49]; the att sequences of 19 IS256 copies were all divergent from each other. The att sequences in capital letters were present as att at the corresponding position of USA300 FPR3757, which lacked IS256. Regarding unusual att sets, the red mark (box) indicates heterogeneous att sequences on the left and right sides, and the green mark indicates the imperfect DRs of att. The 26-bp imperfect terminal inverted repeats of IS256 were identical for 19 IS256 copies, except for IS256③, which had one base change.
Regarding phages, the OC8 genome carried φSa2. φSa2 (OC8) was 45,781 bp in size and showed 86.5% homology to PVL-converting φSa2 (USA300 FPR3757), but lacked the PVL genes. The second phage was φSa7 of 44,446 bp in size. φSa7 (OC8) had no virulence genes. USA300 FPR3757 lacked φSa7. The third phage was φSa3 of 42,984 bp in size. φSa3 (OC8) was inserted into the hlb gene (Fig 2A). As shown in S1 Fig, φSa3 (OC8) had the immune evasion cluster (IEC) with the immune evasion genes scn (for staphylococcal complement inhibitor, SCIN) and sak (for staphylokinase, SAK) and also the SAg gene sea, on the left-end side, similar to ST239/SCCmecIII HA-MRSA TW20, which was isolated from a case of intensive care unit (ICU)-associated bacteremia in London [50,51]. Although the overall homology between φSa3 (OC8) and φSa3 (TW20) was 89.8%, similarities with the scn, sak, and sea genes were high at 99.4%, 99.8%, and 100%, respectively. The IEC of φSa3 (USA300 FPR3757) carried sak, chp (for the chemotaxis inhibitory protein of S. aureus, CHIPS), and scn, but lacked sea. φSa3 (OC8) and φSa3 (USA300 FPR3757) showed a homology of 81.8%.
Regarding SaPI, the OC8 genome carried SaPI6∆ with no SAg gene, and lacked SaPI5 carrying sek and seq, which was present in USA300 [10]. The OC8 genome lacked SaPI-carrying SAg genes.
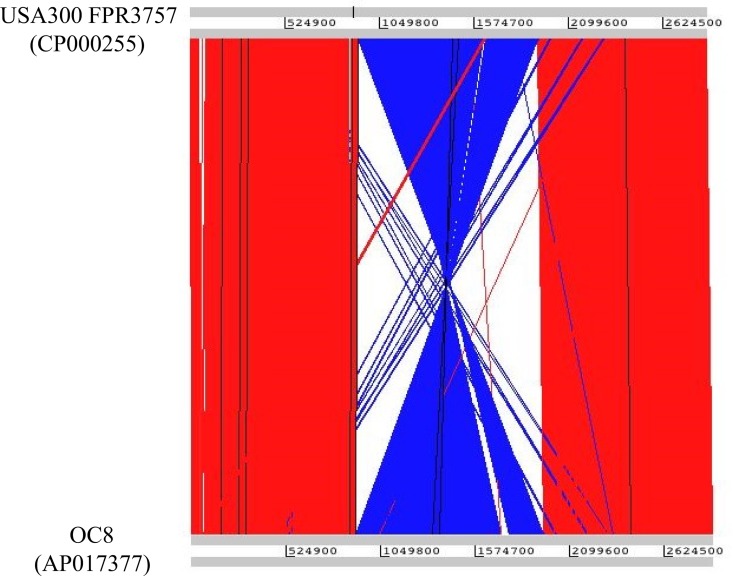
Regarding insertion sequences, 19 copies of IS256 were distributed along the OC8 genome. Their distribution was not random; there were three IS256-enriched regions, reflecting the gathered regions of IS256-preferred insertion sites (Fig 2A). These IS256-enriched regions may serve as recombination hot spots. This was in marked contrast to the USA300 FPR3757 genome, which did not have IS256 [30]. A large genomic inversion was identified relative to the USA300 FPR3757 genome; this event was triggered by two IS256 copies (⑤ and ⑰) in the hot spots, as shown in Fig 2A and visualized in Fig 3. The large genomic inversion was 1,042,885 bp in size, and corresponded to 36.0% of the OC8 genome; this approximately one-megabase genomic inversion was abbreviated as MbIN.
Fig 3. Sequence comparison between OC8 and USA300 FPR3757 genomes and visualization of a large genomic inversion.
Genomic sequence comparisons were performed using WebACT for the visualization of genomic inversions. The genome sequence of USA300 FPR3757 was from GenBank Accession number CP000255. The OC8 inverted region relative to USA300 FPR3757 is highlighted in blue.
In addition to IS256, the OC8 genome carried some other insertion sequences: IS431mec and ∆IS1272 in SCCmecIVc; two copies of IS1181 (of those, one copy had ∆tnp); and tree copies of IS200 family, which showed a 90.2% homology to ISSep3-like, therefore, suggesting that IS256 was the most prevalent insertion sequence on the OC8 genome. The OC8 genome did not have a ccrC-carrying unit, which was found in the ST59/SCCmecV(5C2&5) CA-MRSA from Taiwan [21] and also distributed to the ST239/SCCmecIIIR HA-MRSA lineage from Russia [41].
Regarding genomic islands, the OC8 genome carried 33,301-bp vSAα with an IS256② insertion. The second genomic island was vSAβ, which was 35,235 bp in size and showed 99.8% homology to vSAβ (USA300 FPR3757). vSAβ (OC8) contained three IS256 insertions (IS256⑥, IS256⑰, and IS256⑱). Moreover, vSAβ (OC8) was split into two parts by a MbIN event between IS256⑤ and IS256⑰ (Fig 2A). The third genomic island was a 21,319-bp vSAγ with an IS256⑬ insertion.
Toxin genes on the OC8 genome map included psmα, hla (in vSAγ), sea (in φSa3), hld, and hlg. hlb was split due to a φSa3 insertion. Immune evasion genes, included in the map, were spa, ebh, map, scn and sak (in φSa3), sbi, and fnbA, B. Of those, ebh (encoding for the giant protein Ebh [52,53]) had a nonsense mutation (G→T at position 11,029 bp) and was shortened due to a prenature stop codon (TAA); thus, the truncated product of ∆ebh was predicted to be only 3,676 aa long, corresponding to 35.3% of the entire ebh gene product (Ebh, 10,421 aa long [53]) of USA300 FPR3757 (S2 Fig); ∆ebh is marked by dark green in Fig 2A.
Regarding IS256 insertions, which may affect gene expression and regulation, IS256④ was inserted 521 bp upstream of rsp (a gene for the AraC family transcriptional regulator). There were no IS256 insertions in ica (biofilm-associated gene cluster, ica operon), rot (gene for the repressor of toxins), or their promotor regions; IS256 insertions in these genes (or promotor regions) were noted in terms of IS256-directed virulence alternations [30,34]. The location of rot on the OC8 genome was markedly divergent from rot (USA300 FPR3757) due to OC8 MbIN (Fig 2A).
Regarding drug resistance specified by the genome, the levofloxacin resistance of OC8 was due to gyrA (Ser84Leu) and grlA (Ser80Phe) mutations. OC8 carried the fosfomycin-inactivating enzyme gene (fosB [54]); however, the MIC of fosfomycin for OC8 was 1.0 μg/ml. There were no IS256 insertions in tcaA,B (glycopeptide resistance-related genes), the inactivation of which resulted in glycopeptide resistance phenotypes [55,56].
Status of multiple IS256 copies in OC8
IS256 has a 26-bp imperfect terminal IR, and is flanked by the direct repeat (DR) of 8 or 9-bp host att site sequences [27,48,49]. We analyzed the status of all 19 IS256 copies on the OC8 genome; data are summarized in Fig 2A, B. All IS256 copies shared the same or similar sequences; 16 out of the 19 copies were the same, and three had one nucleotide replacement (Fig 2A). Regarding 26-bp imperfect terminal IR sequences, only IS256③ had a single base change (Fig 2B).
The flanking att site sequences for 19 IS256 copies in OC8 were 8 or 9 bp, and are summarized in Fig 2B. These flanking att site sequences were all divergent and generally AT-rich.
Regarding the arrangement of att sequences on the left and right (attL and attR), in 14 out of the 19 IS256 copies (73.7%), attL and attR were the same (and directly oriented as DR), and such att sequences were present as an att site at the corresponding position of the USA300 FPR3757 genome, as expected. However, for two IS256 copies (② and ⑭) (10.5%), attL and attR were imperfect repeats, and although the attL sequence was present as an att site at the corresponding position of USA300 FPR3757, no attR sequence was present in USA300 FPR3757. In the remaining three IS256 copies (⑤, ⑪, and ⑰) (15.8%), attL and attR were heterogeneous; however, attL and attR were both present as att sites at the corresponding positions in USA300 FPR3757. The last three cases, IS256⑤, ⑪, and ⑰, were present in IS256-rich hot spots on the OC8 genome (Fig 2A).
Nine out of 19 IS256 copies (47.4%) were inserted in the opposite orientation, as shown with, for example, IS256⑤ vs. IS256⑰ (Fig 2B). The OC8 genome had no Tn4001, which had two terminal IS256 copies [33].
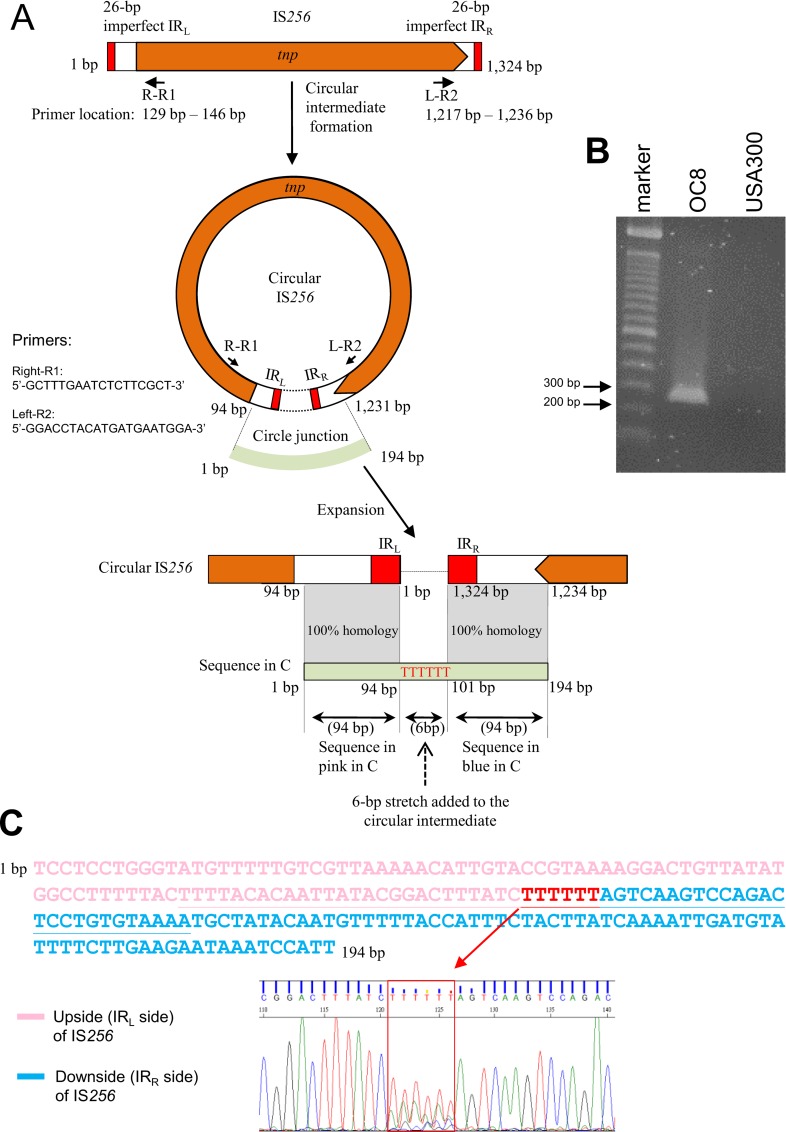
The S. aureus heritage of IS256 [27] includes an extrachromosomal IS256 circular molecule [48,49]. This extrachromosomal circular DNA of IS256 was present in OC8 (Fig 4A and 4B). The circle junction of the IS256 circular DNA in OC8 contained complete IS256 termini, including imperfect IRL and IRR, and an additional 6-bp nucleotide stretch. However, the 6-bp sequence determined was a mixture of distinct stretches, suggesting the presence of heterogeneous IS256 circular molecules in OC8, each with a 6-bp stretch of a distinct sequence (Fig 4C).
Fig 4. The structure of IS256 and its extrachromosomal circular DNA in OC8.
In A, the structure of IS256 (OC8) is based on the OC8 genome sequence (GenBank accession number AP017377); the structure was very similar to previously described IS256 structures [27,48,49]. PCR primers to detect an IS256 circular DNA were designed based on the OC8 genome sequence. In B, the PCR primer set (R-R1 and L-R2, shown in A) exactly detected IS256 circular DNA for OC8 (PCR product size, approximately 200 bp), while there were no amplified bands for strain USA300-0114, which lacked IS256. In C (and B), the 194-bp nucleotide sequence of the estimated PCR product, perfectly matched the IRL side and IRR side regions of IS256 (OC8), and contained a 6-bp stretch, marked in red; 26-bp imperfect IR sequences and 6-bp stretch sequences were underlined in C. However, the 6-bp stretch data showed a “mixed” result, with TTTTTT as the highest base content (followed by AAAAAA). Since the 6-bp stretch originates from a flanking att sequence [48] and OC8 carries 19 IS256 copies with distinct att sequences, the “mixed” 6-bp stretch reflects the presence of heterogeneous circular DNA (in terms of stretch sequences) in OC8. This observation is consistent with the AT-rich att sequences of 19 IS256 copies on the genome.
Genomic inversion and deletion triggered by IS256
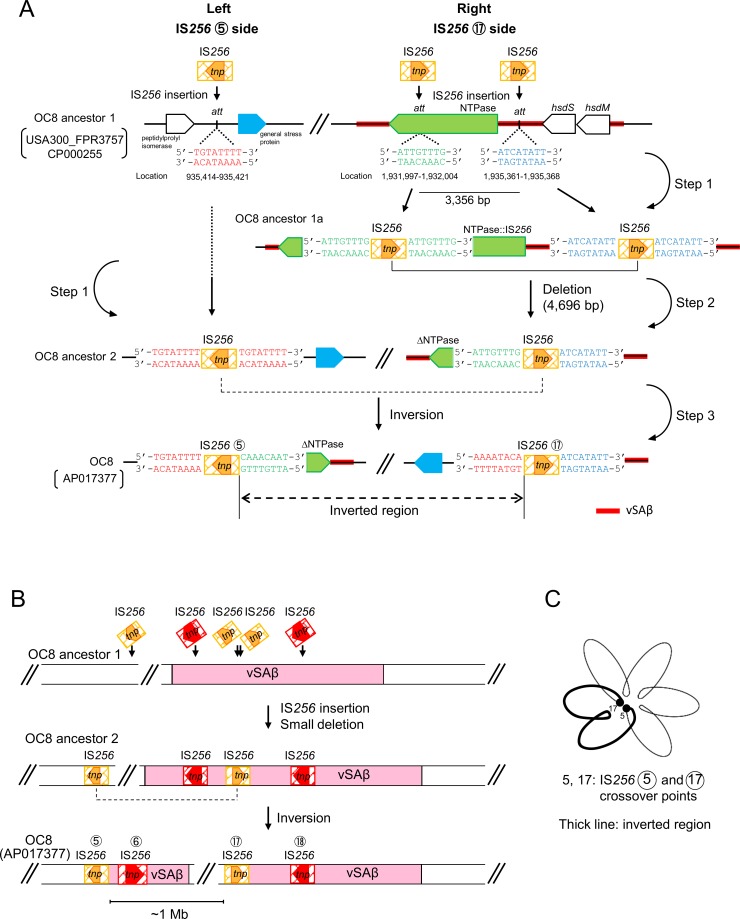
A possible model for the OC8 genomic inversion event triggered by two IS256 (⑤ and ⑰) is shown in Fig 5. These events included two major steps: a deletion between the DR sequences of IS256 and an inversion between the IR sequences of IS256. Furthermore, we hypothesized the presence of ancestor strains of OC8 (OC8 ancestor 1 and OC8 ancestor 2) in this model. On the left of Fig 5A, a single IS256 insertion event occurred at the att site (5’-TGTATTTT) of OC8 ancestor 1, which was also present at the corresponding position of USA300 FPR3757, generating IS256 flanked by DR of the att sequence (OC8 ancestor 2).
Fig 5. Possible mechanisms for the large genomic inversion in OC8.
In this model, shown in A, we hypothesized ancestor strains of OC8 for a one-megabase inversion (MbIN) and simultaneously-occurring deletion events. An initial ancestor strain (OC8 ancestor 1) lacks IS256, but has att site sequences, similar to USA300 FPR3757 (GenBank accession number CP000255); the size of OC8 ancestor 1 DNA flanked by two att sites on the right side of the figure was estimated to be 3,356 bp. The first step (step 1) includes three IS256 insertions at different att sites. As shown on the right side of the figure, a homogenous recombination (step 2) then occurs between the direct repeats of IS256 (in OC8 ancestor 1a), deleting a small region and leaving only one copy of IS256 (generating OC8 ancestor 2). In step 3, a homogenous recombination subsequently occurs between the inverted repeats of IS256 (on OC8 ancestor 2), with the one-megabase region being inverted, and generating OC8. The genes of NTPase, hsdS, and hsdM (on the top right side) were located in the genomic island vSAβ (marked with a red line). In B, figures focus on a vSAβ split event, which occurred simultaneously with MbIN. OC8 ancestor 1, OC8 ancestor 2, and OC8 are the same as those described in A. In C, a hypothetical folded chromosome structure with loop domains is illustrated, based on [57], to boost the crossover and subsequent MbIN events at the two genomic locations, which are far from each other. (The diagram is not to scale.)
On the right of OC8 ancestor 1, two IS256 insertion events possibly occurred at the two att sites (5’-ATTGTTTG and 5’-ATCATATT), which were also present at the corresponding positions of USA300 FPR3757, generating two IS256 copies, which were flanked by each att DR (as shown in OC8 ancestor 1a). This may have been followed by homologous recombination between the two IS256, directly oriented (as DR), resulting in one IS256 copy flanked by heterogeneous att sequences (5’-ATTGTTTG and 5’-ATCATATT), with a 4,696-bp deletion (3,356-bp OC8 ancestor 1 DNA plus 1,324-bp IS256 DNA plus 16-bp left and right att sequences), as shown in OC8 ancestor 2.
Homologous recombination may have occurred between two IS256, oppositely oriented (as IR), in OC8 ancestor 2, resulting in current OC8 with two IS256 copies (⑤ and ⑰), but with a 1,042,885-bp inside region inverted; this step essentially included the vSAβ split (Fig 5A and 5B). Fig 5C shows a hypothetically folded chromosome structure for OC8, potentially allowing for the crossover of two IS256 copies (⑤ and ⑰), which are located approximately a distance of 1 Mb from each other; a figure was illustrated based on [57].
IS256⑪, with heterogeneous attL and attR and located at hot spots (Fig 2A and 2B), may have been the result of homologous recombination between two hypothetical IS256 DR sequences in an OC8 ancestor strain, deleting a 1,403-bp region (63-bp OC8 ancestor 1 DNA plus 1,324-bp IS256 DNA plus 16-bp left and right att sequences), as shown in S3 Fig.
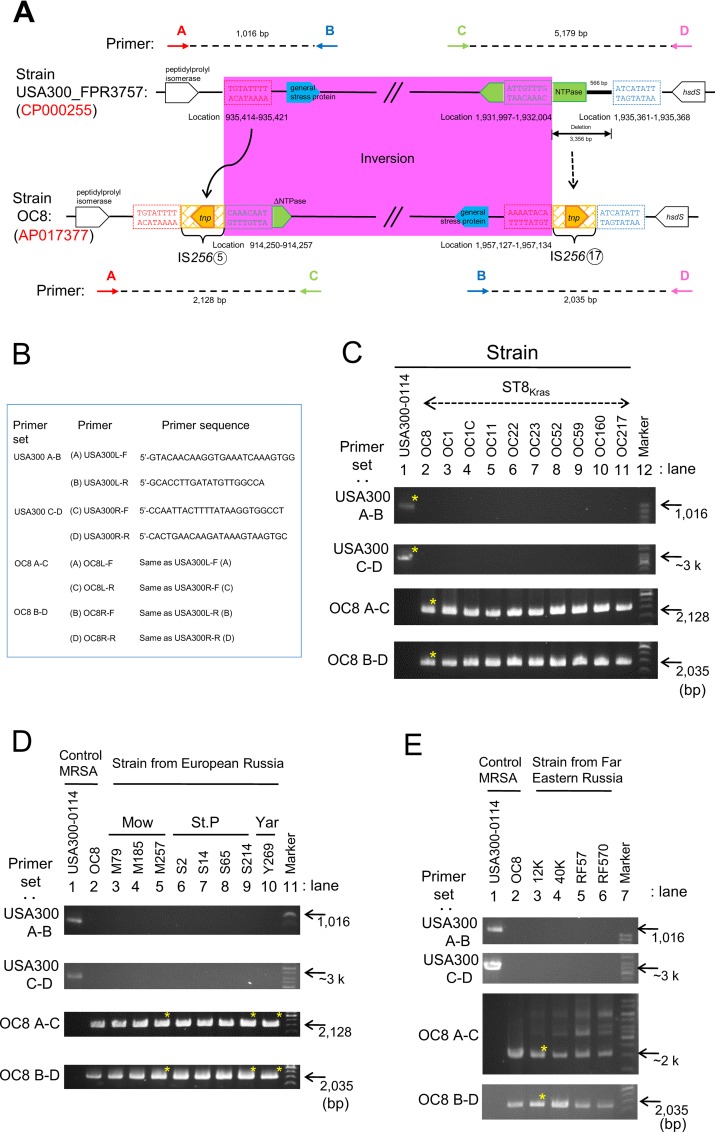
PCR detection and geographical distribution of MbIN
The OC8 genome has the characteristic junction regions of MbIN. In order to detect the left-side and right-side junction regions by PCR, PCR primers (A-C and B-D, respectively) were designed based on the OC8 complete genome sequence, as shown in Fig 6A and 6B. In order to detect the corresponding non-IN region of USA300 FPR3757, we designed PCR primers (A-B and C-D, respectively) based on the USA300 FPR3757 complete genome sequence (Fig 6A and 6B). PCR with the primer sets (A-B) and (C-D) gave positive bands for USA300-0114, as expected (Fig 6C), and the sequences of the PCR products were consistent with the USA300 FPR3757 sequences. The primer sets (A-B and C-D) produced negative results for OC8, as expected (Fig 6C). Instead, PCR with the primer sets (A-C) and (B-D) gave positive bands for the predicted sequences of OC8 and produced negative results for USA300-0114 (Fig 6C).
Fig 6. PCR targeting the OC8-type megabase inversion (MbIN).
In A and B, PCR primers targeting the junction sites of OC8 MbIN (A-C and B-D) and those targeting the corresponding region of USA300 FPR3757 (A-B and C-D) were designed based on the OC8 or USA300 FPR3757 complete genome sequence, respectively. The structures of the MbIN junction regions of OC8 and the corresponding regions of USA300 FPR3757 are from Fig 5. In C to E, PCR products with an asterisk were sequenced, and the sequences determined were consistent with the OC8 or USA300 FPR3757 genome sequence. ST8Kras is ST8/SCCmecIVc MRSA from Krasnoyarsk, Siberian Russia. The geographical location of MRSA isolated in European Russia: Mow, Moscow; St. P, St. Petersburg; Yar, Yaroslavl. Regarding Far Eastern Russia, MRSA was isolated in Vladivostok.
We then examined ST8/SCCmecIVc strains from European, Siberian, and Far Eastern Russia (Fig 6C to 6E). PCR with the primer sets (A-C) and (B-D) gave positive bands for the predicted sequences of all strains examined. PCR with the primer sets (A-B) and (C-D) produced negative results for all strains examined. These results strongly indicated that all ST8/SCCmecIVc strains from European, Siberian, and Far Eastern Russia carry MbIN, suggesting a common origin (with MbIN) in evolution.
Analysis of the Russian ST239 HA-MRSA lineage for MbIN and IS256 extrachromosomal circular DNA
Finally, the ST239 HA-MRSA lineage in Russia was examined for MbIN and IS256 circular DNA by PCR. Regarding MbIN, when ST239/spa3(t037)/SCCmecIIIA HA-MRSA (strain OC3 from Krasnoyarsk) and ST239/spa351(t030)/SCCmecIIIR HA-MRSA (strain 16K from Vladivostok) were examined, both strains were negative in PCR (A-C) and (B-D), but were positive in PCR (A-B) and (C-D), except for OC3, which was negative in PCR (C-D), most probably due to a mutation(s). Therefore, the Russian ST239 HA-MRSA lineage carried no OC8-type MbIN.
Regarding IS256 circular DNA, the circle junction of IS256 circular DNA in OC3 and 16K contained the complete IS256 termini, including imperfect IRL and IRR, and an additional 6-bp heterogeneous stretch (S4 Fig), similar to an OC8 case (albeit with distinct mixed sequence patterns due to a 6-bp heterogeneous stretch).
Discussion
Inversions occur through homologous recombination, in which two genetic structures with homologous sequences of 300 bp or more are present in opposite orientations (as IRs) [58,59]. These genomic inversions (intrachromosomal recombination) are events involved in evolution; the genes in the inverted segment are functional [60] and inversions may create a selective advantage for bacterial pathogenesis, as reported with Pseudomonas aeruginose [61]. Large genomic inversions have been reported in ribosomal RNA genes (rrn) in Escherichia coli [62], in prophage regions in enterohemorrhagic E. coli serotype O157:H7 [63], in IS6100 in P. aeruginose [61], in Salmonella Typhimurium [60], and also in S. aureus (MRSA USA800) [64]. S. aureus generally maintain the overall gene orders of the genome; however, in USA800 (ST5/SCCmecIV lineage), the genomic inversion relative to USA300 is approximately 500 kb in size, and may have occurred between IRs of IS1181 and a 73-bp sequence [64].
The inversion in the present study (OC8) was triggered by IRs of IS256 and was 1,042,885 bp in size, which was approximately two-fold larger than the USA800 genomic inversion, representing the largest genomic inversion in S. aureus (or MRSA). MbIN serves as an epidemiological marker in PCR targeting Russia ST8-IVc. The results of the present study also suggest that in addition to IRs of long homologous sequences, an additional factor, namely, IS256-enriched hot spots, is necessary for a large genomic inversion (MbIN) because even though there were several sets of IS256 IRs on the OC8 genome, MbIN only occurred between those in IS256-enriched hot spots.
Deletion events occur when two long homologous sequences are directly oriented (as DRs) [59]. We found the trace of two deletions for OC8 of 63 bp and 3,356 bp in size, compared to the USA300 FPR3757 genome. These relatively small deletions also occurred at hot spots, at which IS256 DRs were adjacent to each other. Therefore, an ancestral strain of OC8 (before deletion events) may have carried two more IS256 copies on the chromosome (a possible total of 21 copies per genome). Possible OC8 mutants with larger deletions may also have been eliminated during evolution.
Although IS256 has extensively been investigated [27,30,33,34,48,49,65], precise analysis of cell-to-cell spread and genome-wide/intracellular distribution of IS256 has not been reported before. Regarding the cell-to-cell spread of IS256, we speculate that one copy of IS256 was introduced into OC8 (its ancestral strain). We confirmed the presence of an IS256 extrachromosomal circular DNA in OC8, as reported previously [48,49]. It is conceivable that the circular form of IS256 is transferred from cell to cell, similar to the erythromycin resistance transposon Tn554 (in ST239/SCCmecIII HA-MRSA), which formed a circular DNA [41,66] and was successfully transferred by conjugation (in bacterial mixed cultures), as a “transmissible transposon” [41,42]. We clearly demonstrated that small plasmids, such as 2.9-kb chloramphenicol resistance plasmids, are transferred in S. aureus by conjugation (in bacterial mixed cultures) at a markedly higher frequency than “transmissible” large penicillinase (PCase) plasmids [41,42]. This mode of inter-bacterial transmission may also strongly stimulate the spread of IS256 among S. aureus. The circular form of IS256 is now being investigated to verify its cell-to-cell transfer (as a “transmissible insertion sequence”).
Regarding the inner-cellular spread of IS256, the behavior of IS256 was flexible. i) Although the notion that IS256 insertions may not occur randomly has been reported previously [34], we found three IS256-enriched recombination hot spots on the OC8 genome, in addition to a series of the single location of IS256. The molecular mechanisms underlying this gathered manner of the IS256 insertion have not yet been elucidated. However, hot spots may reflect gathered IS256-preferred insertion site sequences, may occur at “junk” regions on the genome, or hot spot regions may provide a unique topological circumstance that boosts the attack of IS256 transposase. ii) IS256-flanking att sequences (8 or 9 pb in size) detected on the OC8 genome were all divergent, suggests that att site selection is not strict (frequency of the appearance of the same att sequence, <5.3% [<1/19] or <4.8% [<1/21]). IS256 extrachromosomal circular DNA also existed in various forms, each with a 6-bp heterogeneous stretch. Therefore, the IS256 transposase, which is a DNA-binding protein (49), preferred sizes rather than fixed unique sequences. iii) IS256-flanking att sequences exhibited three distinct flanking manners: homogenous attL and attR as DR (73.7%), which are created upon insertions; heterogeneous attL and attR at hot spots (10.5%), which are created upon inversions or deletions, subsequent to insertions; and partially homologous attL and attR as imperfect flanking DR (15.8%), which are made upon insertions and subsequent attR mutation(s) through unknown mechanism(s). In the present study, therefore, heterogeneous attL and attR indicated the presence of inversions or deletions.
Regarding the role of IS256 in evolution, in addition to the above chromosomal rearrangements, we emphasize the successful spread of IS256 among MRSA in Russia. The prevalent ST8/SCCmecIVc CA-MRSA lineage (strain OC8), prevalent ST239/spa3(t037)/SCCmecIIIA HA-MRSA lineage (strain OC3) [42], and prevalent ST239/spa351(t030)/SCCmecIIIR HA-MRSA lineage (strain 16K) [41] all carried multiple IS256 copies, strongly suggests that an IS256 multicopy system confers a selective advantage on its host and boosts MRSA evolution.
Regarding genome sequencing technology and an IS256 analysis, in our previous comparative genomic analyses, on OC3 [42] and 16K [41], we used pyrosequencing technology without filling all the gaps between contigs (except for relevant genetic structures); therefore, a precise analysis of IS256-adjacent sequences or the large inversion was not performed. In the present study, since we used PacBio RS II system technology [46] and also completed making a complete circular genome sequence, we succeeded in conducting precise analysis on genome-wide IS256 distribution and the large genomic inversion. A small plasmid analysis was not available for the PacBio RS II system, because small DNA pieces, less than approximately 20 kb, were removed, and only large DNA pieces were employed for library construction. Therefore, for small plasmids, we isolated plasmid DNA in separate experiments for a complete plasmid sequence analysis using previously described methods.
Regarding the effects of IS256 on gene expression, IS256 has been considered to alter S. aureus virulence and drug resistance [30]. For example, the insertion of IS256 into the rot promoter has been shown to affect virulence levels [30]. In the present study, an IS256 insertion was not observed in the rot promotor region [30] or ica regions [34,65]. Regarding genomic islands, vSAα, vSAβ, and vSAγ all had an IS256 insertion. Of these, vSAβ was split into two parts by an MbIN event, with a small deletion. The possible association of IS256 insertions, MbIN, and deletions with virulence expression is under investigation.
Regarding the global geographical structures of MRSA, several continental clades and intercontinental spread have been reported for the ST239/SCCmecIII HA-MRSA lineage [39], albeit with no Russian isolates. Heterogeneous ST239/SCCmecIII sub-lineages are distributed in Russia [38], such as emerging ST239/spa351(t030)/SCCmecIIIR in Vladivostok [41] and ST239/spa3(t037)/SCCmecIIIA (ST239Kras) in Krasnoyarsk [42]. The latter, ST239Kras, represented the Siberian Russian clade of the ST239 HA-MRSA lineage, with the possible evolutionary routes of Brazil-Europe-Siberian Russia [42].
In the case of the globally disseminated ST8/SCCmecIV CA-MRSA lineage, USA300 (ST8/spa1[t008]/SCCmecIVa with PVL-encoding φSa2 and ACME linked to SCCmecIVa) is the most successful example [2,4,9–12,14,15]. USA300 caused the largest MRSA epidemic in the United States [4,11,12,14,32], exhibited intercontinental transmission [8,32,67], and has been the most precisely characterized among MRSA [4,9–12,14,15,37]. Many other geographical variants of the ST8/SCCmecIV CA-MRSA lineage have also been described [68]. We previously reported prevalent CA-MRSA with ST8/spa606(t1767)/SCCmecIVl (ST8 CA-MRSA/J) in Japan, which carried a unique Japanese subtype of SCCmecIV (SCCmecIVl) and novel SaPIj50 with the tst gene [69]; and also ST8 CA-MRSA with spa779(tUK: 11-19-12-21-17-34-24-24-34-24-24-34-22-25)/SCCmecIVx (subtype unknown) in Taiwan; however, the prevalence of ST8 MRSA in Taiwan was low (2.4%) [20]. In Asia, SCCmec types of the ST8 lineage may be highly divergent and unique.
In the present study, based on the OC8 complete genome data, we established the Russian clade of the ST8/SCCmecIV CA-MRSA lineage. Russia ST8-IVc had a genetic marker of unique MbIN (triggered by IS256 hot spots), spread widely to European, Siberian, and Far Eastern Russia with geographical microevolution, including spa types, and was associated with not only SSTIs, but also serious and invasive infections, such as pneumonia, sepsis, and bloodstream infections, in both community and hospital settings [this study, 41,42]. The global evolutionary route of Russia ST8-IVc remains to be elucidated.
Regarding multiple drug resistance (MDR), Russia ST8-IVc strains from European and Siberian regions exhibited levofloxacin (ciprofloxacin) resistance, providing a selective advantage for Russian ST8-IVc, similar to USA300 cases [11,32,67]. Common chloramphenicol resistance also appears to provide a selective advantage for Russia ST8-IVc because chloramphenicol is one of the most common drugs used in chemotherapy in Russia [42]. Rifampicin resistance may have been selected due to tuberculosis treatments [70] or by the geographical common use of rifampicin.
Concerning multiple virulence factors (MVFs) of MRSA, although PVL genes and ACME were not present, unlike USA300 [15], Russia ST8-IVc (including OC8) carried, for example, the SAg-SEA gene (sea), strongly expressed psmα, hla, and a series of immune evasion genes, such as spa, ebh, map, scn, sak, sbi, fnbA, and fnbB, which have been reported previously [4,8,9,10,12,30,42,71]. Although we have not fully analyzed gene mutations in OC8, the very large gene ebh (encoding for the giant protein Ebh [52,53]) had a premature stop codon; therefore its product, truncated Ebh (Ebh∆), was predicted to possess the N-terminal signal peptide, FIVAR repeats, and a part of the extension of FIVAR/GA modules, but lacked the bulk of the extension of FIVAR/GA modules, the transmembrane domain, and C-terminal-positive charges [52,53], thereby losing its function as a very large surface-anchored protein. The nonsense mutation in the ebh gene was unique to strain OC8 (ST8Kras). Other ST8Kras strains (OC11 and OC22), Russia ST8-IVc strains from St. Petersburg and Vladivostok (S214 and 12K), and ST239/SCCmecIII strains (OC3 and 16K) did not have the OC8-type nonsense mutation in the ebh gene; this point is further under investigation.
One IS256 insertion occurred 521-bp upstream of rsp (the gene for the AraC family transcriptional regulator [72]), suggesting an influence on the regulation system for virulence genes. In order to gain a more precise understanding of the gene expression and potential virulence of Russia ST8-IVc (OC8), further investigations are needed.
Regarding the ST8/SCCmecIV CA-MRSA lineage, factors associated with a successful clonal expansion in each region/country include i) MDR, not only resistance to globally important agents (e.g., fluoroquinolones) but also resistance to regionally common agents (e.g., chloramphenicol), ii) the ability of powerful adherence, colonization, and spread, and iii) enhanced MVFs.
In conclusion, we determined the complete circular genome sequence of ST8/spa1(t008)/SCCmecIVc CA-MRSA (ST8Kras strain OC8). This enabled us to gain novel insights into the following. i) Regarding large genomic rearrangements, OC8 had MbIN, the largest genomic inversion in MRSA, and vSAβ (OC8) essentially split. Its impact is unknown, however, since MbIN was a common feature of successful Russian ST8-IVc, it was not a fitness burden. MbIN was unambiguously diagnosed by PCR. ii) Regarding IS256’s spread and functions, it was of special interest that the ST8 CA- and ST239 HA-MRSA lineages in Russia all carried multi-IS256. We speculate that IS256 has strong transmission potential and epidemiological advantages. IS256 exhibited flexible manners at the integration stage and extrachromosomal DNA stage, and acted as a powerful trigger for MRSA evolution, for example, IS256 at its hot spots created MbIN. iii) Regarding MVFs, we found additional virulence factors of OC8, such as the truncated giant surface protein Ebh∆ and IS256 insertion related to pan-regulation. iv) Regarding global geographical structures, we assigned Russia ST8-IVc as a new powerful clade of the globally disseminated ST8/SCCmecIV CA-MRSA lineage. Russia ST8-IVc was geographically expanded in both community and hospital settings since approximately 2006, with characteristic MbIN as an epidemiological marker and fluoroquinolone resistance, increased MVFs, and possibly a multi-IS256 system as selective advantages. The evolutionary route of Russia ST8-IVc remains to be elucidated.
Supporting Information
φSa3 (OC8) exhibited the highest homology to φSa3 (T0103). The left-side immune evasion cluster (IEC) region of φSa3 (OC8) also exhibited high homology to φSa3 (TW20). Homologous regions are shaded in each comparison. Genes in IEC: scn, the staphylococcal complement inhibitor (SCIN) gene; sak, the staphylokinase (SAK) gene; sea, the staphylococcal enterotoxin A (ETA) gene. OC8 lacked chp, the chemotaxis inhibitory protein of the S. aureus (CHIPS) gene, unlike USA300 FPR3757 (GenBank accession number CP000255).
(TIF)
The nucleotide sequence of ebh and deduced amino acid sequence of Ebh were compared between USA300 FPR3757 (upper side) and OC8 (lower side) in A and B, respectively. In A, ebh (OC8) had three synonymous substitutions (black), two non-synonymous substitutions (red), and one nonsense mutation (blue). In order to confirm the nonsense mutation, we designed two primer sets, ebh1F and ebh1R (5'-GTGTTCAAACGGTTCAATCA and 5'-AATAATCGTTTCAGCAGCAG, generating a 170-bp product) and ebh2F and ebh2R (5'-ACTTAGATGGTACGCGTTTA and 5'-AACTATTCACTTGCTCTGCT, generating a 369-bp product) based on the OC8 genome (ebh) sequence. The PCR with those primers and OC8 DNA and subsequent sequencing perfectly confirmed the nonsense mutation (G→T at position 11,029 bp). Due to the nonsense mutation at B, the orf of ebh (OC8) was shortened, and corresponded to only 35.3% of ebh (USA300 FPR3757). In B, Ebh (USA300 FPR3757) was 10,421 aa in length, while truncated Ebh (OC8) was only 3, 676 aa, corresponding to 35.3% of Ebh (USA300 FPR3757). Truncated Ebh (OC8), Ebh∆, showed 100% homology to the corresponding region of Ebh (USA300 FPR3757), but lacked the bulk of FIVAR GA modules and transmembrane domain of Ebh [52,53].
(TIF)
In this model, we hypothesized ancestor strains of OC8 for a deletion event. An initial ancestor strain (OC8 ancestor 1) lacks IS256, but has att site sequences, similar to USA300 FPR3757; the size of OC8 ancestor 1 DNA flanked by two att sites was estimated to be 63 bp. The first step (step 1) includes two IS256 insertions at different att sites (generating OC8 ancestor 2). In step 2, a homogenous recombination occurs between direct repeats of IS256 (in OC8 ancestor 2), deleting a small region and leaving only one copy of IS256 (generating OC8 with IS256⑪).
(TIF)
In A, the structure of IS256 (OC8) is based on the OC8 genome sequence (GenBank accession number AP017377) and is the same as that described in Fig 4A. In A and B, strains OC3 and 16K were examined for the circle junction of IS256 circular molecules by PCR, using PCR primer set (R-R1 and L-R2). Their amplified bands were very similar to that of OC8. In C, the sequence of the PCR products, estimated, perfectly matched the IRL side and IRR side regions of IS256 (OC8), and contained the 6-bp stretch, marked in red; 26-bp imperfect IR sequences and 6-bp stretch sequences are underlined. The 6-bp stretch data showed a “mixed” result, with TTGTGT (for 16K) or TATTTT (for OC3) as a highest base content, most probably reflecting divergent att sequences on each genome.
(TIF)
Acknowledgments
We thank L. K. McDougal and L. L. McDonald for the USA300 type strain and K. Hiramatsu for ST5/SCCmecII HA-MRSA (the New York/Japan clone) reference strains.
Data Availability
Data are available from the DDBJ, there are two accession numbers LC129040 and AP017377.
Funding Statement
This study was supported by each institutional sources, including those to the 10th anniversary of the Japan-Russia joint research on infections from Far Eastern Federal University School of Biomedicine and Krasnoyarsk State Medical University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Centers for Disease Control and Prevention (CDC). Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. MMWR Morb Mortal Wkly Rep. 1999;48:707–710. [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 3.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. 10.1016/S0140-6736(09)61999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–687. 10.1128/CMR.00081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–656. 10.1086/499815 [DOI] [PubMed] [Google Scholar]
- 6.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. 10.1001/jama.290.22.2976 [DOI] [PubMed] [Google Scholar]
- 7.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Hung WC, Takano T, Nishiyama A. Genetic nature and virulence of community-associated methicillin-resistant Staphylococcus aureus. BioMedicine. 2013;3:2–18. [Google Scholar]
- 9.Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008;16:361–369. 10.1016/j.tim.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurlow LR, Joshi GS, Richardson AR. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). FEMS Immunol Med Microbiol. 2012;65:5–22. 10.1111/j.1574-695X.2012.00937.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenover FC, McDougal LK, Goering RV, Killgore G, Projan SJ, Patel JB, et al. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44:108–118. 10.1128/JCM.44.1.108-118.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy AD, Otto M, Braughton KR, Whitney AR, Chen L, Mathema B, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci USA. 2008;105:1327–1332. 10.1073/pnas.0710217105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009;53:4961–4967. 10.1128/AAC.00579-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhlemann AC, Otto M, Lowy FD, DeLeo FR. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus. Infect Genet Evol. 2014;21:563–574. 10.1016/j.meegid.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. 10.1016/S0140-6736(06)68231-7 [DOI] [PubMed] [Google Scholar]
- 16.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. 10.3201/eid0908.030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isobe H, Takano T, Nishiyama A, Hung WC, Kuniyuki S, Shibuya Y, et al. Evolution and virulence of Panton-Valentine leukocidin-positive ST30 methicillin-resistant Staphylococcus aureus in the past 30 years in Japan. Biomed Res. 2012;33:97–109. [DOI] [PubMed] [Google Scholar]
- 18.Harro JM, Daugherty S, Bruno VM, Jabra-Rizk MA, Rasko DA, Shirtliff ME. Draft genome sequence of the methicillin-resistant Staphylococcus aureus isolate MRSA-M2. Genome Announc. 2013. January;1(1). pii: e00037–12. 10.1128/genomeA.00037-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyle-Vavra S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel Staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J Clin Microbiol. 2005;43:4719–4730. 10.1128/JCM.43.9.4719-4730.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takano T, Higuchi W, Zaraket H, Otsuka T, Baranovich T, Enany S, et al. Novel characteristics of community-acquired methicillin-resistant Staphylococcus aureus strains belonging to multilocus sequence type 59 in Taiwan. Antimicrob Agents Chemother. 2008;52:837–845. 10.1128/AAC.01001-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higuchi W, Takano T, Teng LJ, Yamamoto T. Structure and specific detection of staphylococcal cassette chromosome mec type VII. Biochem Biophys Res Commun. 2008;377:752–756. 10.1016/j.bbrc.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 22.Hung WC, Takano T, Higuchi W, Iwao Y, Khokhlova O, Teng LJ, et al. Comparative genomics of community-acquired ST59 methicillin-resistant Staphylococcus aureus in Taiwan: novel mobile resistance structures with IS1216V. PLoS One. 2012;7:e46987 10.1371/journal.pone.0046987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang TW, Chen FJ, Miu WC, Liao TL, Lin AC, Huang IW, et al. Complete genome sequence of Staphylococcus aureus M013, a pvl-positive, ST59-SCCmec type V strain isolated in Taiwan. J Bacteriol. 2012;194:1256–1257. 10.1128/JB.06666-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawanobori E, Hung WC, Takano T, Hachuda K, Horiuchi T, Higuchi W, et al. Emergence of Panton-Valentine leukocidin-positive ST59 methicillin-susceptible Staphylococcus aureus with high cytolytic peptide expression in association with community-acquired pediatric osteomyelitis complicated by pulmonary embolism. J Microbiol Immunol Infect. 2015;48:565–573. 10.1016/j.jmii.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 25.Stegger M, Price LB, Larsen AR, Gillece JD, Waters AE, Skov R, et al. Genome sequence of Staphylococcus aureus strain 11819–97, an ST80-IV European community-acquired methicillin-resistant isolate. J Bacteriol. 2012;194:1625–1626. 10.1128/JB.06653-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fluit AC, Carpaij N, Majoor EA, Weinstein RA, Aroutcheva A, Rice TW, et al. Comparison of an ST80 MRSA strain from the USA with European ST80 strains. J Antimicrob Chemother. 2015;70:664–669. 10.1093/jac/dku459 [DOI] [PubMed] [Google Scholar]
- 27.Mahillon J, Chandler M. Insertion sequences. Microbiol Mol Biol Rev. 1998;62:725–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novick RP, Subedi A. The SaPIs: mobile pathogenicity islands of Staphylococcus. Chem Immunol Allergy. 2007;93:42–57. 10.1159/0000100857 [DOI] [PubMed] [Google Scholar]
- 29.Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8:541–551. 10.1038/nrmicro2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benson MA, Ohneck EA, Ryan C, Alonzo F 3rd, Smith H, Narechania A, et al. Evolution of hypervirulence by a MRSA clone through acquisition of a transposable element. Mol Microbiol. 2014;93:664–681. 10.1111/mmi.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia G, Wolz C. Phages of Staphylococcus aureus and their impact on host evolution. Infect Genet Evol. 2014;21:593–601. 10.1016/j.meegid.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 32.Tenover FC, Goering RV. Methicillin-resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother. 2009;64:441–446. 10.1093/jac/dkp241 [DOI] [PubMed] [Google Scholar]
- 33.Lyon BR, Gillespie MT, Skurray RA. Detection and characterization of IS256, an insertion sequence in Staphylococcus aureus. J Gen Microbiol. 1987;133:3031–3038. 10.1099/00221287-133-11-3031 [DOI] [PubMed] [Google Scholar]
- 34.Ziebuhr W, Krimmer V, Rachid S, Lössner I, Götz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Mol Microbiol. 1999;32:345–356. [DOI] [PubMed] [Google Scholar]
- 35.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. 10.1038/nm1656 [DOI] [PubMed] [Google Scholar]
- 36.Takano T, Higuchi W, Yamamoto T. Superior in vitro activity of carbapenems over anti-methicillin-resistant Staphylococcus aureus (MRSA) and some related antimicrobial agents for community-acquired MRSA but not for hospital-acquired MRSA. J Infect Chemother. 2009;15:54–57. 10.1007/s10156-008-0665-5 [DOI] [PubMed] [Google Scholar]
- 37.Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages SA, et al. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus. J Infect Dis. 2008;197:1523–1530. 10.1086/587907 [DOI] [PubMed] [Google Scholar]
- 38.Gostev V, Lazareva I, Sidorenko S. Molecular epidemiology of methicillin-resistant Staphylococcus aureus circulating in the Russian Federation. Abstracts of the 10th International Symposium on Antimicrobial Agents and Resistance. Int J Antimicrob Agents. 2015;45(S2):S97. [Google Scholar]
- 39.Harris SR, Feil EJ, Holden MT, Quail MA, Nickerson EK, Chantratita N, et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science. 2010;327:469–474. 10.1126/science.1182395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baranovich T, Potapov V, Yamamoto T. The first isolation of Panton-Valentine leukocidin (PVL) positive community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) in Russia. Euro Surveill. 2007;12:E070315.070314. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto T, Takano T, Higuchi W, Iwao Y, Singur O, Reva I, et al. Comparative genomics and drug resistance of a geographic variant of ST239 methicillin-resistant Staphylococcus aureus emerged in Russia. PLoS One. 2012;7:e29187 10.1371/journal.pone.0029187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khokhlova OE, Hung WC, Wan TW, Iwao Y, Takano T, Higuchi W, et al. Healthcare- and Community-Associated Methicillin-Resistant Staphylococcus aureus (MRSA) and Fatal Pneumonia with Pediatric Deaths in Krasnoyarsk, Siberian Russia: Unique MRSA's Multiple Virulence Factors, Genome, and Stepwise Evolution. PLoS One. 2015;10:e0128017 10.1371/journal.pone.0128017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Higuchi W, Mimura S, Kurosawa Y, Takano T, Iwao Y, Yabe S, et al. Emergence of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone in a Japanese child, demonstrating multiple divergent strains in Japan. J Infect Chemother. 2010;16:292–297. 10.1007/s10156-010-0051-y [DOI] [PubMed] [Google Scholar]
- 44.Takano T, Hung WC, Shibuya M, Higuchi W, Iwao Y, Nishiyama A, et al. A new local variant (ST764) of the globally disseminated ST5 lineage of hospital-associated methicillin-resistant Staphylococcus aureus (MRSA) carrying the virulence determinants of community-associated MRSA. Antimicrob Agents Chemother. 2013;57:1589–1595. 10.1128/AAC.01147-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinical and Laboratory Standards Institute. Performance standard for antimicrobial susceptibility testing: 25th informational supplement M100-S25, Wayne PA, USA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 46.Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. 10.1038/nmeth.2474 [DOI] [PubMed] [Google Scholar]
- 47.Khokhlova O, Tomita Y, Hung WC, Takano T, Iwao Y, Higuchi W, et al. Elderly infection in the community due to ST5/SCCmecII methicillin-resistant Staphylococcus aureus (the New York/Japan clone) in Japan: Panton-Valentine leukocidin-negative necrotizing pneumonia. J Microbiol Immunol Infect. 2015;48:335–339. 10.1016/j.jmii.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 48.Loessner I, Dietrich K, Dittrich D, Hacker J, Ziebuhr W. Transposase-dependent formation of circular IS256 derivatives in Staphylococcus epidermidis and Staphylococcus aureus. J Bacteriol. 2002;184:4709–4714. 10.1128/JB.184.17.4709-4714.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennig S, Ziebuhr W. Characterization of the transposase encoded by IS256, the prototype of a major family of bacterial insertion sequence elements. J Bacteriol. 2010;192:4153–4163. 10.1128/JB.00226-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgeworth JD, Yadegarfar G, Pathak S, Batra R, Cockfield JD, Wyncoll D, et al. An outbreak in an intensive care unit of a strain of methicillin-resistant Staphylococcus aureus sequence type 239 associated with an increased rate of vascular access device-related bacteremia. Clin Infect Dis. 2007;44:493–501. 10.1086/511034 [DOI] [PubMed] [Google Scholar]
- 51.Holden MT, Lindsay JA, Corton C, Quail MA, Cockfield JD, Pathak S, et al. Genome sequence of a recently emerged, highly transmissible, multi-antibiotic- and antiseptic-resistant variant of methicillin-resistant Staphylococcus aureus, sequence type 239 (TW). J Bacteriol. 2010;192:888–892. 10.1128/JB.01255-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuroda M, Tanaka Y, Aoki R, Shu D, Tsumoto K, Ohta T. Staphylococcus aureus giant protein Ebh is involved in tolerance to transient hyperosmotic pressure. Biochem Biophys Res Commun. 2008;374:237–241. 10.1016/j.bbrc.2008.07.037 [DOI] [PubMed] [Google Scholar]
- 53.Cheng AG, Missiakas D, Schneewind O. The giant protein Ebh is a determinant of Staphylococcus aureus cell size and complement resistance. J Bacteriol. 2014;196:971–981. 10.1128/JB.01366-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson MK, Keithly ME, Goodman MC, Hammer ND, Cook PD, Jagessar KL, et al. Structure and function of the genomically encoded fosfomycin resistance enzyme, FosB, from Staphylococcus aureus. Biochemistry. 2014;53:755–765. 10.1021/bi4015852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandenberger M, Tschierske M, Giachino P, Wada A, Berger-Bächi B. Inactivation of a novel three-cistronic operon tcaR-tcaA-tcaB increases teicoplanin resistance in Staphylococcus aureus. Biochim Biophys Acta. 2000;1523:135–139. [DOI] [PubMed] [Google Scholar]
- 56.Maki H, McCallum N, Bischoff M, Wada A, Berger-Bächi B. tcaA inactivation increases glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2004;48:1953–1959. 10.1128/AAC.48.6.1953-1959.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Snustad DP, Simmons MJ. Principles of Genetics, Binder Ready Version. 7th ed. New Jersey: Wiley; 2015. pp. 201–202. [Google Scholar]
- 58.Bi X, Liu LF. recA-independent and recA-dependent intramolecular plasmid recombination. Differential homology requirement and distance effect. J Mol Biol. 1994;235:414–423. 10.1006/jmbi.1994.1002 [DOI] [PubMed] [Google Scholar]
- 59.Grindley ND, Whiteson KL, Rice PA. Mechanisms of site-specific recombination. Annu Rev Biochem. 2006;75:567–605. 10.1146/annurev.biochem.73.011303.073908 [DOI] [PubMed] [Google Scholar]
- 60.Riley M, Anilionis A. Evolution of the bacterial genome. Annual Reviews in Microbiology. 1978;32:519–560. [DOI] [PubMed] [Google Scholar]
- 61.Kresse AU, Dinesh SD, Larbig K, Römling U. Impact of large chromosomal inversions on the adaptation and evolution of Pseudomonas aeruginosa chronically colonizing cystic fibrosis lungs. Mol Microbiol. 2003;47:145–158. [DOI] [PubMed] [Google Scholar]
- 62.Hill CW, Harnish BW. Inversions between ribosomal RNA genes of Escherichia coli. Proc Natl Acad Sci U S A. 1981;78:7069–7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iguchi A, Iyoda S, Terajima J, Watanabe H, Osawa R. Spontaneous recombination between homologous prophage regions causes large-scale inversions within the Escherichia coli O157:H7 chromosome. Gene. 2006;372:199–207. 10.1016/j.gene.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 64.Shukla SK, Kislow J, Briska A, Henkhaus J, Dykes C. Optical mapping reveals a large genetic inversion between two methicillin-resistant Staphylococcus aureus strains. J Bacteriol. 2009;191:5717–5723. 10.1128/JB.00325-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hennig S, Ziebuhr W. A transposase-independent mechanism gives rise to precise excision of IS256 from insertion sites in Staphylococcus epidermidis. J Bacteriol. 2008;190:1488–1490. 10.1128/JB.01290-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphy E. Properties of the site-specific transposable element Tn554 Molecular biology of the staphylococci. VCH Publishers, New York, NY; 1990. pp. 123–135. [Google Scholar]
- 67.Glaser P, Martins-Simões P, Villain A, Barbier M, Tristan A, Bouchier C, et al. Demography and Intercontinental Spread of the USA300 Community-Acquired Methicillin-Resistant Staphylococcus aureus Lineage. MBio. 2016;7:e02183–15. 10.1128/mBio.02183-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strommenger B, Bartels MD, Kurt K, Layer F, Rohde SM, Boye K, et al. Evolution of methicillin-resistant Staphylococcus aureus towards increasing resistance. J Antimicrob Chemother. 2014;69:616–622. 10.1093/jac/dkt413 [DOI] [PubMed] [Google Scholar]
- 69.Iwao Y, Ishii R, Tomita Y, Shibuya Y, Takano T, Hung WC, et al. The emerging ST8 methicillin-resistant Staphylococcus aureus clone in the community in Japan: associated infections, genetic diversity, and comparative genomics. J Infect Chemother. 2012;18:228–240. 10.1007/s10156-012-0379-6 [DOI] [PubMed] [Google Scholar]
- 70.Sekiguchi J, Fujino T, Araake M, Toyota E, Kudo K, Saruta K, et al. Emergence of rifampicin resistance in methicillin-resistant Staphylococcus aureus in tuberculosis wards. J Infect Chemother. 2006;12:47–50. 10.1007/s10156-005-0417-8 [DOI] [PubMed] [Google Scholar]
- 71.Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol. 2012;15:92–99. 10.1016/j.mib.2011.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li T, He L, Song Y, Villaruz AE, Joo HS, Liu Q, et al. AraC-Type Regulator Rsp Adapts Staphylococcus aureus Gene Expression to Acute Infection. Infect Immun. 2015;84:723–734. 10.1128/IAI.01088-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
φSa3 (OC8) exhibited the highest homology to φSa3 (T0103). The left-side immune evasion cluster (IEC) region of φSa3 (OC8) also exhibited high homology to φSa3 (TW20). Homologous regions are shaded in each comparison. Genes in IEC: scn, the staphylococcal complement inhibitor (SCIN) gene; sak, the staphylokinase (SAK) gene; sea, the staphylococcal enterotoxin A (ETA) gene. OC8 lacked chp, the chemotaxis inhibitory protein of the S. aureus (CHIPS) gene, unlike USA300 FPR3757 (GenBank accession number CP000255).
(TIF)
The nucleotide sequence of ebh and deduced amino acid sequence of Ebh were compared between USA300 FPR3757 (upper side) and OC8 (lower side) in A and B, respectively. In A, ebh (OC8) had three synonymous substitutions (black), two non-synonymous substitutions (red), and one nonsense mutation (blue). In order to confirm the nonsense mutation, we designed two primer sets, ebh1F and ebh1R (5'-GTGTTCAAACGGTTCAATCA and 5'-AATAATCGTTTCAGCAGCAG, generating a 170-bp product) and ebh2F and ebh2R (5'-ACTTAGATGGTACGCGTTTA and 5'-AACTATTCACTTGCTCTGCT, generating a 369-bp product) based on the OC8 genome (ebh) sequence. The PCR with those primers and OC8 DNA and subsequent sequencing perfectly confirmed the nonsense mutation (G→T at position 11,029 bp). Due to the nonsense mutation at B, the orf of ebh (OC8) was shortened, and corresponded to only 35.3% of ebh (USA300 FPR3757). In B, Ebh (USA300 FPR3757) was 10,421 aa in length, while truncated Ebh (OC8) was only 3, 676 aa, corresponding to 35.3% of Ebh (USA300 FPR3757). Truncated Ebh (OC8), Ebh∆, showed 100% homology to the corresponding region of Ebh (USA300 FPR3757), but lacked the bulk of FIVAR GA modules and transmembrane domain of Ebh [52,53].
(TIF)
In this model, we hypothesized ancestor strains of OC8 for a deletion event. An initial ancestor strain (OC8 ancestor 1) lacks IS256, but has att site sequences, similar to USA300 FPR3757; the size of OC8 ancestor 1 DNA flanked by two att sites was estimated to be 63 bp. The first step (step 1) includes two IS256 insertions at different att sites (generating OC8 ancestor 2). In step 2, a homogenous recombination occurs between direct repeats of IS256 (in OC8 ancestor 2), deleting a small region and leaving only one copy of IS256 (generating OC8 with IS256⑪).
(TIF)
In A, the structure of IS256 (OC8) is based on the OC8 genome sequence (GenBank accession number AP017377) and is the same as that described in Fig 4A. In A and B, strains OC3 and 16K were examined for the circle junction of IS256 circular molecules by PCR, using PCR primer set (R-R1 and L-R2). Their amplified bands were very similar to that of OC8. In C, the sequence of the PCR products, estimated, perfectly matched the IRL side and IRR side regions of IS256 (OC8), and contained the 6-bp stretch, marked in red; 26-bp imperfect IR sequences and 6-bp stretch sequences are underlined. The 6-bp stretch data showed a “mixed” result, with TTGTGT (for 16K) or TATTTT (for OC3) as a highest base content, most probably reflecting divergent att sequences on each genome.
(TIF)
Data Availability Statement
Data are available from the DDBJ, there are two accession numbers LC129040 and AP017377.