Abstract
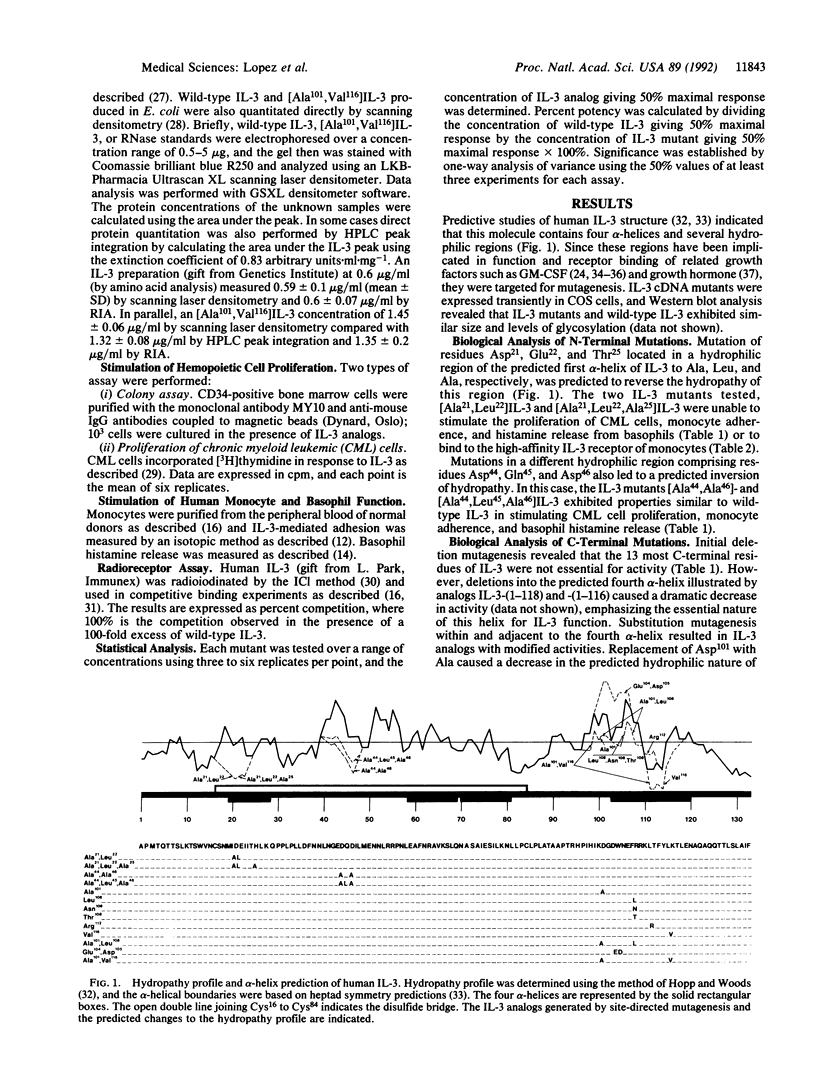
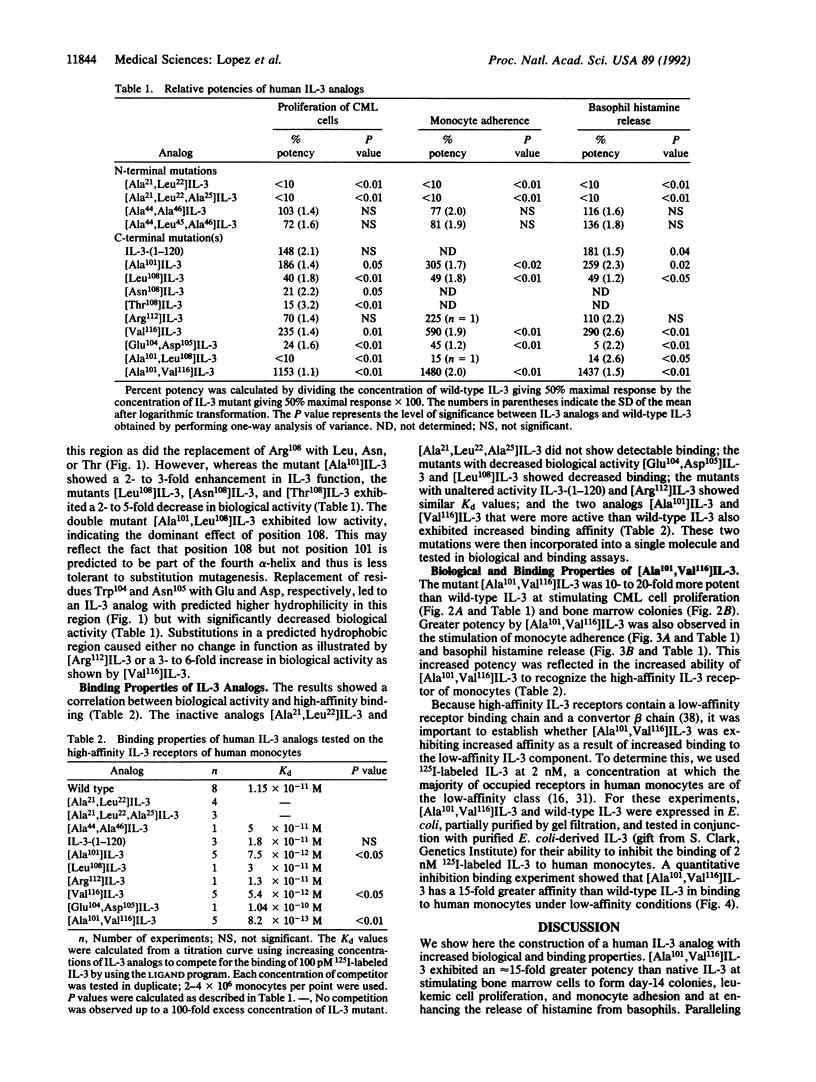
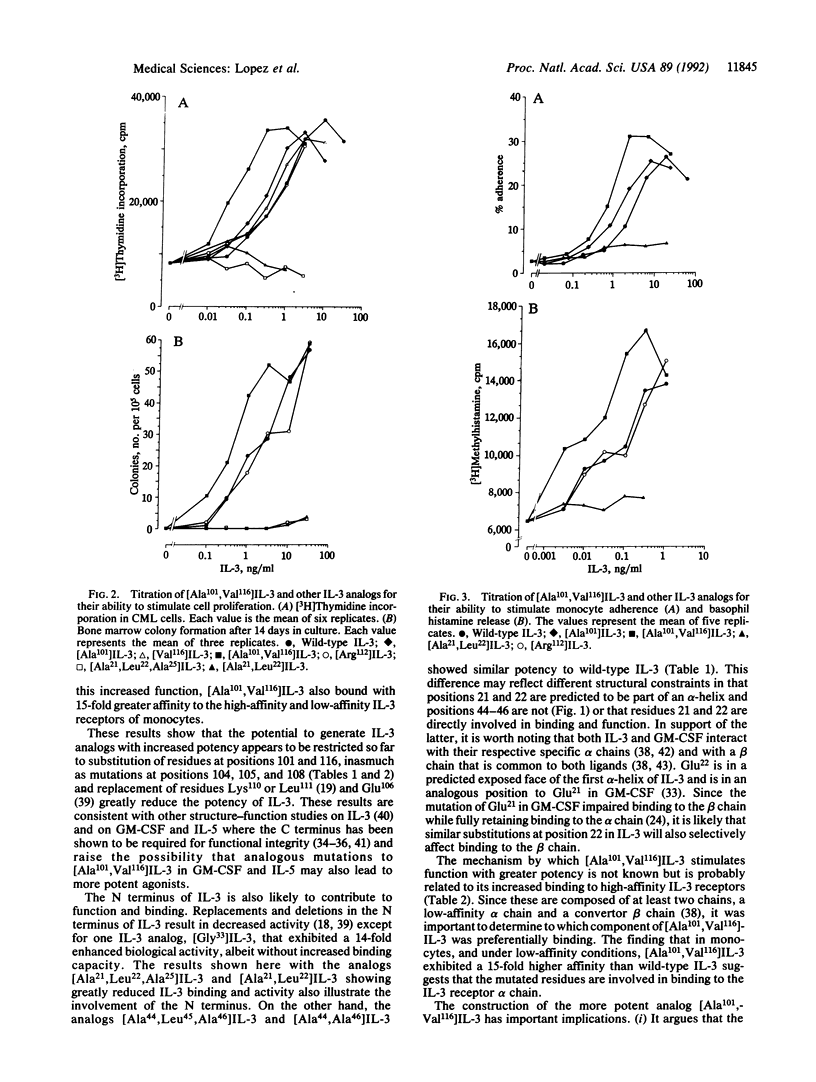
Human interleukin 3 (IL-3) variants generated by site-directed mutagenesis were analyzed in multiple biological and binding assays to identify residues critical for IL-3 activity. Two mutants carrying substitutions in the predicted hydrophilic region within the first alpha-helix, [Ala21,Leu22]IL-3 and [Ala21,Leu22,Ala25]IL-3 showed loss of biological activity and high-affinity binding. Mutants in a second predicted hydrophilic region, [Ala44,Leu45,Ala46]IL-3 and [Ala44,Ala46]IL-3, however, showed similar biological and binding activities to wild-type IL-3. Mutations in a C-terminal hydrophilic region that overlaps the fourth predicted alpha-helix led to either loss or gain of function. IL-3 analogs [Glu104,Asp105]-, [Leu108]-, [Asn108]-, [Thr108]-, and [Ala101,Leu108]IL-3 were less active than wild-type IL-3, whereas [Ala101]IL-3 and [Val116]IL-3 were 2- to 3-fold more potent. Significantly, the double mutant [Ala101,Val116]IL-3 exhibited a 15-fold greater potency than native IL-3. Receptor binding studies showed that [Ala101,Val116]IL-3 exhibited increased binding to the high- and low-affinity receptors of monocytes. These results show the generation of an IL-3 analog with increased biological and binding activities and support a model where the C terminus of IL-3 interacts with the alpha chain of the IL-3 receptor, making this region a useful focus for the development of more potent IL-3 agonists or antagonists.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt J., Briddell R. A., Srour E. F., Leemhuis T. B., Hoffman R. Role of c-kit ligand in the expansion of human hematopoietic progenitor cells. Blood. 1992 Feb 1;79(3):634–641. [PubMed] [Google Scholar]
- Clark-Lewis I., Lopez A. F., To L. B., Vadas M. A., Schrader J. W., Hood L. E., Kent S. B. Structure-function studies of human granulocyte-macrophage colony-stimulating factor. Identification of residues required for activity. J Immunol. 1988 Aug 1;141(3):881–889. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Clayberger C., Luna-Fineman S., Lee J. E., Pillai A., Campbell M., Levy R., Krensky A. M. Interleukin 3 is a growth factor for human follicular B cell lymphoma. J Exp Med. 1992 Feb 1;175(2):371–376. doi: 10.1084/jem.175.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras M. A., Bale W. F., Spar I. L. Iodine monochloride (IC1) iodination techniques. Methods Enzymol. 1983;92:277–292. [PubMed] [Google Scholar]
- Donahue R. E., Seehra J., Metzger M., Lefebvre D., Rock B., Carbone S., Nathan D. G., Garnick M., Sehgal P. K., Laston D. Human IL-3 and GM-CSF act synergistically in stimulating hematopoiesis in primates. Science. 1988 Sep 30;241(4874):1820–1823. doi: 10.1126/science.3051378. [DOI] [PubMed] [Google Scholar]
- Dorssers L. C., Mostert M. C., Burger H., Janssen C., Lemson P. J., van Lambalgen R., Wagemaker G., van Leen R. W. Receptor and antibody interactions of human interleukin-3 characterized by mutational analysis. J Biol Chem. 1991 Nov 5;266(31):21310–21317. [PubMed] [Google Scholar]
- Dunbar C. E., Smith D. A., Kimball J., Garrison L., Nienhuis A. W., Young N. S. Treatment of Diamond-Blackfan anaemia with haematopoietic growth factors, granulocyte-macrophage colony stimulating factor and interleukin 3: sustained remissions following IL-3. Br J Haematol. 1991 Oct;79(2):316–321. doi: 10.1111/j.1365-2141.1991.tb04540.x. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Moss J., Dottore M., Park L. S., Vadas M. A., Lopez A. F. Differential binding of IL-3 and GM-CSF to human monocytes. Growth Factors. 1992;6(1):15–29. doi: 10.3109/08977199209008868. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Cleland L. G., Gamble J. R., Lopez A. F. IL-3 and granulocyte-macrophage colony-stimulating factor stimulate two distinct phases of adhesion in human monocytes. J Immunol. 1990 Jul 1;145(1):167–176. [PubMed] [Google Scholar]
- Elliott M. J., Vadas M. A., Eglinton J. M., Park L. S., To L. B., Cleland L. G., Clark S. C., Lopez A. F. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989 Nov 15;74(7):2349–2359. [PubMed] [Google Scholar]
- Ganser A., Lindemann A., Seipelt G., Ottmann O. G., Eder M., Falk S., Herrmann F., Kaltwasser J. P., Meusers P., Klausmann M. Effects of recombinant human interleukin-3 in aplastic anemia. Blood. 1990 Oct 1;76(7):1287–1292. [PubMed] [Google Scholar]
- Ganser A., Lindemann A., Seipelt G., Ottmann O. G., Herrmann F., Eder M., Frisch J., Schulz G., Mertelsmann R., Hoelzer D. Effects of recombinant human interleukin-3 in patients with normal hematopoiesis and in patients with bone marrow failure. Blood. 1990 Aug 15;76(4):666–676. [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrayeb J., Kimura H., Takahara M., Hsiung H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984 Oct;3(10):2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak-Frendscho M., Arai N., Arai K., Baeza M. L., Finn A., Kaplan A. P. Human recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3 cause basophil histamine release. J Clin Invest. 1988 Jul;82(1):17–20. doi: 10.1172/JCI113567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammann M., Laufs J., Schell J., Gronenborn B. Rapid insertional mutagenesis of DNA by polymerase chain reaction (PCR). Nucleic Acids Res. 1989 Jul 11;17(13):5404–5404. doi: 10.1093/nar/17.13.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K., Shoemaker S. G., Alfaro S., Brown C. Hematopoietic activity of granulocyte/macrophage colony-stimulating factor is dependent upon two distinct regions of the molecule: functional analysis based upon the activities of interspecies hybrid growth factors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1213–1217. doi: 10.1073/pnas.86.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Sato N., Arai K., Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared beta subunit for the human IL-3 and GM-CSF receptors. Cell. 1991 Sep 20;66(6):1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Koshland D., Botstein D. Secretion of beta-lactamase requires the carboxy end of the protein. Cell. 1980 Jul;20(3):749–760. doi: 10.1016/0092-8674(80)90321-9. [DOI] [PubMed] [Google Scholar]
- Leary A. G., Yang Y. C., Clark S. C., Gasson J. C., Golde D. W., Ogawa M. Recombinant gibbon interleukin 3 supports formation of human multilineage colonies and blast cell colonies in culture: comparison with recombinant human granulocyte-macrophage colony-stimulating factor. Blood. 1987 Nov;70(5):1343–1348. [PubMed] [Google Scholar]
- Lokker N. A., Movva N. R., Strittmatter U., Fagg B., Zenke G. Structure-activity relationship study of human interleukin-3. Identification of residues required for biological activity by site-directed mutagenesis. J Biol Chem. 1991 Jun 5;266(16):10624–10631. [PubMed] [Google Scholar]
- Lokker N. A., Zenke G., Strittmatter U., Fagg B., Movva N. R. Structure-activity relationship study of human interleukin-3: role of the C-terminal region for biological activity. EMBO J. 1991 Aug;10(8):2125–2131. doi: 10.1002/j.1460-2075.1991.tb07746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Dyson P. G., To L. B., Elliott M. J., Milton S. E., Russell J. A., Juttner C. A., Yang Y. C., Clark S. C., Vadas M. A. Recombinant human interleukin-3 stimulation of hematopoiesis in humans: loss of responsiveness with differentiation in the neutrophilic myeloid series. Blood. 1988 Nov;72(5):1797–1804. [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Gillis D., Park L. S., Clark S., Vadas M. A. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7022–7026. doi: 10.1073/pnas.86.18.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Lyons A. B., Tapley P. M., To L. B., Park L. S., Clark S. C., Vadas M. A. Human interleukin-3 inhibits the binding of granulocyte-macrophage colony-stimulating factor and interleukin-5 to basophils and strongly enhances their functional activity. J Cell Physiol. 1990 Oct;145(1):69–77. doi: 10.1002/jcp.1041450111. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Shannon M. F., Hercus T., Nicola N. A., Cambareri B., Dottore M., Layton M. J., Eglinton L., Vadas M. A. Residue 21 of human granulocyte-macrophage colony-stimulating factor is critical for biological activity and for high but not low affinity binding. EMBO J. 1992 Mar;11(3):909–916. doi: 10.1002/j.1460-2075.1992.tb05129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., To L. B., Yang Y. C., Gamble J. R., Shannon M. F., Burns G. F., Dyson P. G., Juttner C. A., Clark S., Vadas M. A. Stimulation of proliferation, differentiation, and function of human cells by primate interleukin 3. Proc Natl Acad Sci U S A. 1987 May;84(9):2761–2765. doi: 10.1073/pnas.84.9.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie A. N., Barry S. C., Strath M., Sanderson C. J. Structure-function analysis of interleukin-5 utilizing mouse/human chimeric molecules. EMBO J. 1991 May;10(5):1193–1199. doi: 10.1002/j.1460-2075.1991.tb08060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner H. A., Yamasaki K., Jamal N., Minden M. M., Yang Y. C., Wong G. G., Clark S. C. Growth of human hemopoietic colonies in response to recombinant gibbon interleukin 3: comparison with human recombinant granulocyte and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6765–6769. doi: 10.1073/pnas.84.19.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Parry D. A., Minasian E., Leach S. J. Cytokine conformations: predictive studies. J Mol Recognit. 1991 Mar-Jun;4(2-3):63–75. doi: 10.1002/jmr.300040205. [DOI] [PubMed] [Google Scholar]
- Phillips J. A., Lopez A. F., Milton S. E., Vadas M. A., Shannon M. F. Synthesis and expression of the gene encoding human interleukin-3. Gene. 1989 Dec 14;84(2):501–507. doi: 10.1016/0378-1119(89)90527-1. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Ryan G. R., Milton S. E., Lopez A. F., Bardy P. G., Vadas M. A., Shannon M. F. Human interleukin-3 mRNA accumulation is controlled at both the transcriptional and posttranscriptional level. Blood. 1991 Mar 15;77(6):1195–1202. [PubMed] [Google Scholar]
- Saito H., Hatake K., Dvorak A. M., Leiferman K. M., Donnenberg A. D., Arai N., Ishizaka K., Ishizaka T. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2288–2292. doi: 10.1073/pnas.85.7.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt A. B., Johnson K. E., Kastelein R. A. Identification of critical amino acid residues in human and mouse granulocyte-macrophage colony-stimulating factor and their involvement in species specificity. J Biol Chem. 1991 Jul 25;266(21):13804–13810. [PubMed] [Google Scholar]
- Sieff C. A., Ekern S. C., Nathan D. G., Anderson J. W. Combinations of recombinant colony-stimulating factors are required for optimal hematopoietic differentiation in serum-deprived culture. Blood. 1989 Feb 15;73(3):688–693. [PubMed] [Google Scholar]
- Wagemaker G., van Gils F. C., Burger H., Dorssers L. C., van Leen R. W., Persoon N. L., Wielenga J. J., Heeney J. L., Knol E. Highly increased production of bone marrow-derived blood cells by administration of homologous interleukin-3 to rhesus monkeys. Blood. 1990 Dec 1;76(11):2235–2241. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]


