Abstract
During HIV+HCV+ co-infection CD14brightCD16--monocytes produce soluble immune-activation markers that predict disease-progression and poor IFNα-treatment response. We evaluated relationships among immune-activation, monocyte phenotype, CD4-memory T-cells and HCV-, CMV- and CMV/EBV/Influenza (CEF)-specific IFNγ-response, before and during IFNα-treatment. Effector-memory and central-memory CD4-T-cell frequencies were lower in HCV+HIV+ than uninfected-donors, and correlated negatively with HCV-level, CD14brightCD16--monocytes and plasma sCD14. sCD14 and CD14brightCD16- monocytes negatively correlated with IFNα-dependent HCV-decline. sCD14 negatively associated with and CD4 effector-memory T-cells positively-associated with CEF-specific IFNγ-response. These data support a role for memory-CD4 T-cells in HCV-containment, and link immune-activation and CD14brightCD16--monocyte frequency to failure of interferon-dependent HCV-clearance.
Keywords: HIV, hepatitis C, CMV, cellular immunity, monocyte, T cell
Introduction
Immune-activation predicts morbidity during HIV-infection [1, 2]. Soluble (s)CD14, IL-6, IP-10 and sCD163 are plasma markers of immune-activation in chronic viral-infection [2-8]. Elevated sCD14 levels negatively predict response to HCV IFN-therapy during HCV+HIV+ co-infection [3, 4, 9]. Monocyte-activation is also negatively associated with response to HCV-therapy [10], and monocytes contribute to immune-activation via mechanisms that include elaboration of sCD14, IP-10, sCD163 and IL-6 in HIV and HCV infections [11-14].
Containment and clearance of HCV is dependent on CD4 T-cells [15-17], and positively associated with IFNγ and cytolytic function [15, 18-21]. HCV-specific memory T-cells are positively associated with resolution of acute HCV-infection [16, 22]. Monocytes may partly shape this HCV-directed response, via direct-contact and polarizing cytokines [20, 21]. Our prior findings indicate soluble-factors of immune-activation, including IL-6 and sCD14, can impair CD4 T-cell responses [23, 24]. We extend these studies here to evaluate the role of immune-activation, monocyte-subset frequency, and CD4-memory T-cells in host control of HCV and IFNα-treatment-induced HCV-clearance during HCV+HIV+co-infection.
Methods
AIDS Clinical Trials Group (ACTG) A5294 was a phase-3 trial evaluating efficacy of boceprevir/pegylated-interferon (PegIFN)/ribavirin (rbv) in the setting of HCV+HIV+ co-infection. PegIFNα/rbv lead-in for 4 weeks preceded addition of boceprevir. Sixty-four HCV-treatment-naïve participants from A5294 whose entry criteria included at least 8 weeks on antiretroviral-therapy (ART) with CD4 count >200/mm3, genotype 1 HCV-infection, HIV-1 RNA <50 copies/mL and HCV RNA data were selected. After obtaining IRB-approved consent, peripheral blood mononuclear cell (PBMC) and plasma samples were prepared at each clinical-site, cryopreserved, and sent to a central-storage facility. Cryopreserved PBMCs from baseline (week 0), and plasma from baseline and week 4 of boceprevir/PegIFNα/rbv treatment were analyzed. Plasma and PBMC from uninfected control (n=25), and HCV+ mono-infected (n=34) participants were obtained at the Cleveland VA hospital under a separate IRB-approved protocol.
Plasma was evaluated for sCD14, IL-6, IP-10 and sCD163 by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota).
Cryopreserved PBMCs were thawed and stained with monoclonal antibodies. Viable lymphocyte-gated cells that were CD3+CD8-CD4+CD27-/+CD45RO-/+ were analyzed. For monocyte phenotype, we quantified proportions of viable-gated cells that were CD14bright/dimCD16-/+. CD86 expression was recorded as mean fluorescence intensity, compared to isotype control.
HCV-peptides (n=441, 18aa each, overlapping by 11aa) representing the entire HCV-1a H77 sequence, CMV/EBV/Influenza (CEF) peptide pool (32 immunodominant CD8 epitopes) and CMV (pool of 138 15-mer peptides of the pp65 protein, overlapping by 11 aa) were supplied by the National Institutes of Health AIDS Research and Reference Reagent Program (Division of AIDS, NIAID). HCV peptides were pooled together into 10 pools (27–61 peptides/pool) according to viral protein region (core peptides 1–27; E1 peptides 28–55; E2 peptides 56–107; NS2 plus P7 peptides 108–147; NS3-1 peptides 148–193; NS3-2 peptides 194–239; NS4 peptides 240–287; NS5A peptides 288–348; NS5B-1 peptides 349–394; and NS5B-2 peptides 395–441). Peptide-pools were utilized at a final concentration of 2.7μg/mL each peptide (≤0.5% DMSO; Sigma). PBMCs (3×105 /well) were plated onto 96-well IFN-γ ELISPOT plates in presence or absence of CEF-, CMV-peptide, or each of the 10 HCV-peptide pools, incubated for 20hrs at 37°C, developed and analyzed as described [25-29]. A response was characterized as IFN-γ-production frequency 3-fold greater than mean-background frequency with ≥15 spot-forming units (sfu) per well, as described [30]. Using similar criteria, we have previously observed no responses to HCV-peptide pools in healthy control or disease control subjects [25-29, 31].
We evaluated associations between continuous variables using Spearman rank correlation coefficients and inter-group comparisons by Mann-Whitney U test, both rank-based methods. All tests of significance were two-sided and P-values ≤ .05 were considered significant. Analyses were performed using SPSS for Windows v. 20.0 (IBM Corp, Armonk, New York).
Results
Baseline characteristics of the study participants are summarized in Supplemental Table 1. HCV+HIV+ co-infected participants had higher AST and ALT levels, higher and APRI score than uninfected controls. CD4 T-cell subsets were defined by expression of CD27 and CD45RO, withcentral-memory (CM) CD27+CD45RO+ and effector-memory (EM) CD27-CD45R0+, as previously described [32]. The data demonstrate that CD4CM and EM frequencies were lower in HCV+HIV+ co-infected compared to uninfected participants and HCV+ mono-infected participants; while HCV mono-infected participants had higher CD4CM and EM-T-cell frequencies compared to uninfected participants (Fig. 1a-b). While it is well documented that T-cell-mediated immunity is vital for HCV-clearance [15, 16, 18, 19, 22], the relationship between frequencies of CD4-memory T-cell subsets and HCV-control during HCV+HIV+ co-infection has not been investigated. We observed negative correlations between CD4CM T-cell frequency and HCV-level (r= -0.320, p=0.012), as well as CD4EM frequency and HCV-level (r= -0.368, p=0.004) prior to HCV-therapy in HCV+HIV+ co-infected patients (Fig. 1c). We noted similar negative associations between CD8CM and EM-T-cell frequencies and HCV-level prior to start of HCV-therapy (r= -0.268, p= 0.039; r= -0.368, p= 0.004 respectively, not shown). CD4CM and EM-T-cell absolute counts also negatively correlated with HCV-level (r= -0.273, p= 0.033; r= -0.33, p= 0.008, respectively, not shown). In HCV mono-infection, there was no association between CD4CM and EM frequencies and pre-treatement HCV viral load. These results are consistent with CD4CM and EM T-cells playing a role in HCV-control during HCV+HIV +co-infection.
Figure 1. CD4CM and EM Cells are reduced in HCV+HIV+ co-infection and negatively correlate with HCV-level and classical monocyte frequency.
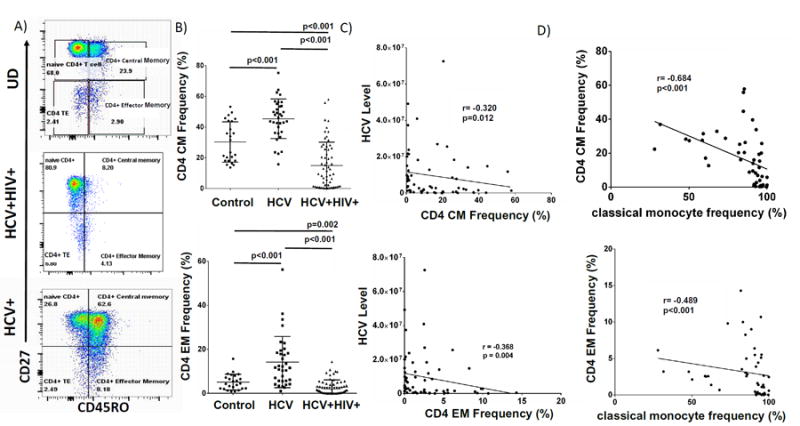
A) Representative CD4 T cell subset gating strategy of cryopreserved PBMC of HCV+HIV+co-infected, HCV-infected and uninfected subjects (controls) which were stained with Yellow Live/Dead stain (Invitrogen, Grand Island, New York), anti-CD3-AlexaFluor700 (clone UCHT1), anti-CD14-Alexaflour700 (M5E2), anti-CD16-APC-H7 (3G8), anti-CD4-PE (RPA-T4), anti-CD8-PerCP (53-6.7), anti-CD27-PE-Cy7(M-T271), anti-CD45RO-FITC (UCHL1), anti-CD86-PE-Cy7 (IT2.2) or isotype controls. Flow cytometry data were acquired on a BD LSRII flow cytometer (BD Biosciences), and analyzed using FlowJo (TreeStar). Live cells were identified by forward and side scatter and viability. B) Week 0 CD4+CM and CD4+EM T cell frequencies (%) of each group. C) Baseline (Week 0) CD4 CM (top) and EM (bottom) frequencies of HCV+HIV+co-infected participants in relation to HCV level in absence of exogenous IFN. D) Week 0 classical monocyte (CD14brightCD16-) frequency (%) of HCV+HIV+co-infected participants (n=49) in relation to CD4 CM and EM frequencies.
To understand potential relationships among monocytes, HCV-level and CD4-memory T-cell populations, classical CD14brightCD16-, intermediate CD14brightCD16+ and patrolling CD14dimCD16+ monocytes) were analyzed as described [33]. In HCV+HIV+ co-infected participants, classical-monocyte frequency negatively correlated with CD4CM and EM frequencies (Fig 1d)and absolute counts (r= -0.609, p< 0.001; r= -0.478, p< 0.001, respectively, not shown). Upon activation, monocytes produce soluble markers of immune-activation, including sCD14, IP-10, IL-6 and sCD163 [11], known to be elevated in patients with chronic HCV and HIV-infections [11-14]. Expectedly, serum sCD163, IP-10, and IL-6 were significantly higher in HCV+HIV+ co-infected participants compared to uninfected controls (not shown). These soluble immune-activation markers positively correlated with each other. Specifically, IL-6 levels were positively correlated with both sCD163 and sCD14 levels (Supp Fig 1a-b), and sCD163 directly correlated with IP-10 levels (Supp Fig 1c). sCD163 and IP-10 positively correlated with APRI score (r=0.571, p<0.001; r=0.300, p=0.017, respectively), a marker of liver fibrosis (Supp Fig1d-e). . There was a trend towards classical-monocyte frequency positively correlating with levels of IP-10 (r=0.281, p=0.053), IL-6 (r=0.481, p=0.055), and sCD14 (r= 0.214, p=0.100) in HCV+HIV+. co-infected participants. No relationship was observed between classical-monocyte frequency and soluble markers in uninfected controls.
Upon treatment with Peg-IFNα/rbv, we observed an increase in sCD14, similar to our previous findings [3], and an increase in IL-6 during 4 weeks of therapy (not shown). In contrast, levels of sCD163 and IP-10 decreased during treatment (not shown). Baseline classical-monocyte frequency correlated negatively with HCV-decline at week 4 of PegIFNα/rbv-therapy (r=-0.33, p=0.020, Fig. 2a). We found a similar trend between classical-monocyte frequency and HCV-decline at 4 weeks of therapy in HCV mono-infected subjects (r= -0.250, p=0.180, not shown). Baseline sCD14 negatively correlated with HCV-decline (Fig. 2b), as we have previously reported [3]. CD4EM baseline frequency (Fig. 2c) and absolute count (not shown) positively correlated with greater HCV-decline (r=0.300, p=0.016; r=0.273, p=0.036) and negatively correlated with sCD14 levels at baseline (r= -0.254, p=0.048, Fig. 2d). In addition, there was a negative correlation between IL-6 level and CD4EM T-cell frequency (r= -0.301, p=0.018 not shown), indicating soluble immune-activation markers negatively associate with CD4 EM frequency. The negative association found between CD4CM and EM frequencies and HCV prior to therapy (Fig 1b), was upheld at week 4 of therapy (not shown).
Figure 2. Classical Monocyte frequency and sCD14 are negatively associated with HCV decline, while CD4EM frequency is positively associated with HCV decline during exogenous IFN.
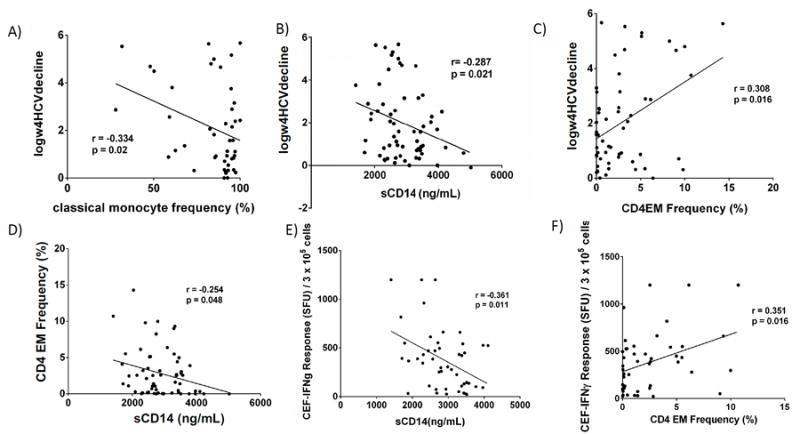
We next examined whether anti-viral effector T-cells play a role in these relationships. We evaluated CEF-, CMV- and HCV-specific T-cell frequency by IFNγ ELISPOT. 52% of HIV+HCV+co-infected participant PBMC samples responded to HCV-peptides, while 82% and 84% responded to CEF and CMV peptide pools, respectively. There was no association between cumulative frequency of HCV-specific T-cell responses and HCV-level or therapy-response. However, previous work has indicated that the response to therapy may be more related to the breadth of the response to HCV than magnitude of response [16, 17, 34, 35]. When the number of HCV-peptide pools targeted was examined, we observed a modest trend for enhanced IFNα-dependent HCV-decline among subjects who responded to 5 or more peptide pools (5 was the median number of pools targeted) (p=0.200). Given that CEF-peptide responses are CD8 T-cell-driven, we evaluated potential associations between CD8 T-cells and CMV- or CEF-specific IFNγ-responses. As anticipated, we observed a positive trend between CD8CM and EM T-cell frequency and CEF-specific IFNγ-response (r=0.258, p=0.080; r=0.215, p=0.150). sCD14 negatively associated with CEF-specific T-cell responses (r= -0.361, p=0.011, Fig. 2e). We also found CD4EM T-cell frequency and count positively associated with CEF-specific IFNγ-response (r=0.351, p=0.016; r=0.302, p=0.040), the former shown in Fig. 2f. These results support the role of CD4EM T-cells in anti-viral-specific immune-function and suggest that sCD14 may be a negative predictor of anti-viral immune function.
Discussion
T-cell-mediated immunity plays a vital role in HCV-clearance [15, 17, 18], presumably by cytolysis of infected cells and IFNγ-secretion [15, 18, 19]. Consistent with prior studies, our data show a negative relationship between both CD4CM and EM T-cell frequencies and HCV-level during HCV+HIV+ co-infection (Fig. 1b). There were similar negative associations between CD8CM and EM T-cell frequencies and HCV-level before start of HCV-therapy. We observed a positive association between CD4EM frequency and CEF-specific T-cell response (Fig. 2f), and a trend towards an association between HCV-specific T-cell breadth and IFNα-induced HCV-therapy response, supporting a role for CD4-memory T-cells in anti-viral immune-function. Furthermore, there was a positive relationship between CD4EM T-cell frequencies and greater IFNα-induced HCV-decline magnitude (Fig. 2d). An alternative to CD4 T-cells directly playing a role in viral-control would be that HCV directly or indirectly has a negative effect on CD4CM and EM numbers. To address the effect of HIV, we evaluated these cells during HCV+ mono-infection, where we observed heightened CD4CM and EM frequencies compared to those in uninfected participants (Fig 1a-b), and no relation between CD4CM or EM frequency and HCV-level. The latter suggests that the relationship may be restricted to persons with HIV-infection, possibly uncovering an interaction only when immune function is impaired, as is the case when CD4 numbers and function are reduced during HIV co-infection.
CD4CM and EM T-cell frequencies negatively associated with sCD14 (Fig. 2b) and IL-6, which in turn negatively predict HCV-therapy response [3, 4, 9]. These associations would be consistent with a negative impact of these factors on T-cell function. In fact, IL-6 has been shown to impair CD4 T-cell induction of pro-survival factor BCL-2 upon IL-7-stimulation [23], and there is at least one report that sCD14 can directly inhibit CD4 T-cell IL-2-production [24].
For the first time, we show that classical-monocyte frequency is negatively associated with both CD4CM and EM frequencies (Fig. 1c) and HCV-decline magnitude at week 4 of PegIFNα/rbv-therapy (Fig. 2a), consistent with a potential negative role for classical-monocytes in IFNα-dependent T-cell-mediated HCV-clearance. No association was seen between patrolling- or intermediate-monocytes and HCV-levels, suggesting specificity of the finding. Circulating monocytes have an overlapping phenotype with Kuppfer cells, the resident liver-macrophages, both characterized by expression of CD14 [36]. Circulating monocytes are also capable of infiltrating into the liver and serving as precursors to Kuppfer cells [36]. Upon stimulation, classical-monocytes shed CD14 [11], CD163, and produce IP-10, IL-6 and TNF [12, 14]. Here sCD14 negatively associated with CEF-specific IFNγ-response (Fig. 2e). Together, these data suggest negative roles for classical-monocytes and sCD14 in anti-viral immune-function.
Our results extend our understanding of endogenous and exogenous mechanisms of IFNα-action and immune-mechanisms of HCV-control. In the IFN-free HCV-treatment era, the majority of treated subjects will achieve sustained virologic response (SVR) [37]. However, a treatment-resistant population exists [37], and HCV- eradication worldwide will likely require a preventative vaccine. Further evaluation of the role of T-cell and monocyte immune-function will inform refinement of therapeutic-strategies towards further improved viral-control and vaccine designs.
Supplementary Material
Acknowledgments
Grant support: This work was supported by VA Merit 110BX001894-01, U01AI068636, and AI068634 from the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The Authors have no known conflicts of interest
References
- 1.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 2.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–1226. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony DD, Conry SJ, Medvik K, Sandhya Rani MR, Falck-Ytter Y, Blanton RE, et al. Baseline levels of soluble CD14 and CD16+56- natural killer cells are negatively associated with response to interferon/ribavirin therapy during HCV-HIV-1 coinfection. J Infect Dis. 2012;206:969–973. doi: 10.1093/infdis/jis434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchetti G, Nasta P, Bai F, Gatti F, Bellistri GM, Tincati C, et al. Circulating sCD14 is associated with virological response to pegylated-interferon-alpha/ribavirin treatment in HIV/HCV co-infected patients. PLoS One. 2012;7:e32028. doi: 10.1371/journal.pone.0032028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campillo-Gimenez L, Assoumou L, Valantin MA, Pajanirassa P, Villemonteix J, Soulie C, et al. Switch to maraviroc/raltegravir dual therapy leads to an unfavorable immune profile with low-level HIV viremia. AIDS. 2015;29:853–856. doi: 10.1097/QAD.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 6.Ramirez LA, Arango TA, Thompson E, Naji M, Tebas P, Boyer JD. High IP-10 levels decrease T cell function in HIV-1-infected individuals on ART. J Leukoc Biol. 2014;96:1055–1063. doi: 10.1189/jlb.3A0414-232RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payer BA, Reiberger T, Aberle J, Ferenci P, Holzmann H, Rieger A, et al. IL28B and interferon-gamma inducible protein 10 for prediction of rapid virologic response and sustained virologic response in HIV-HCV-coinfected patients. Eur J Clin Invest. 2012;42:599–606. doi: 10.1111/j.1365-2362.2011.02623.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230. 1230 e1221–1223. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartigan-O’Connor DJ, Lin D, Ryan JC, Shvachko VA, Cozen ML, Segal MR, et al. Monocyte activation by interferon alpha is associated with failure to achieve a sustained virologic response after treatment for hepatitis C virus infection. J Infect Dis. 2014;209:1602–1612. doi: 10.1093/infdis/jit801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiki N, Berger D, Prigl C, Boelke E, Wiedeck H, Seidelmann M, et al. Endotoxin binding and elimination by monocytes: secretion of soluble CD14 represents an inducible mechanism counteracting reduced expression of membrane CD14 in patients with sepsis and in a patient with paroxysmal nocturnal hemoglobinuria. Infect Immun. 1998;66:1135–1141. doi: 10.1128/iai.66.3.1135-1141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villacres MC, Literat O, DeGiacomo M, Du W, Frederick T, Kovacs A. Defective response to Toll-like receptor 3 and 4 ligands by activated monocytes in chronic hepatitis C virus infection. J Viral Hepat. 2008;15:137–144. doi: 10.1111/j.1365-2893.2007.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS. 2015;29:1263–1265. doi: 10.1097/QAD.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel-Hakeem MS, Bedard N, Murphy D, Bruneau J, Shoukry NH. Signatures of protective memory immune responses during hepatitis C virus reinfection. Gastroenterology. 2014;147:870–881. e878. doi: 10.1053/j.gastro.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grakoui A, Shoukry NH, Woollard DJ, Han JH, Hanson HL, Ghrayeb J, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 18.Gruner NH, Gerlach TJ, Jung MC, Diepolder HM, Schirren CA, Schraut WW, et al. Association of hepatitis C virus-specific CD8+ T cells with viral clearance in acute hepatitis C. J Infect Dis. 2000;181:51528–1536. doi: 10.1086/315450. [DOI] [PubMed] [Google Scholar]
- 19.Shoukry NH, Grakoui A, Houghton M, Chien DY, Ghrayeb J, Reimann KA, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Saha B, Kodys K, Szabo G. IFN-gamma production by human natural killer cells in response to HCV-infected hepatoma cells is dependent on accessory cells. J Hepatol. 2013;59:442–449. doi: 10.1016/j.jhep.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong HY, Lee YJ, Seo SK, Lee SW, Park SJ, Lee JN, et al. Blocking of monocyte-associated B7-H1 (CD274) enhances HCV-specific T cell immunity in chronic hepatitis C infection. J Leukoc Biol. 2008;83:755–764. doi: 10.1189/jlb.0307168. [DOI] [PubMed] [Google Scholar]
- 22.Golden-Mason L, Burton JR, Castelblanco N, Klarquist J, Benlloch S, Wang C, et al. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44:1098–1109. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- 23.Shive CL, Mudd JC, Funderburg NT, Sieg SF, Kyi B, Bazdar DA, et al. Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis. 2014;210:619–629. doi: 10.1093/infdis/jiu125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rey Nores JE, Bensussan A, Vita N, Stelter F, Arias MA, Jones M, et al. Soluble CD14 acts as a negative regulator of human T cell activation and function. Eur J Immunol. 1999;29:265–276. doi: 10.1002/(SICI)1521-4141(199901)29:01<265::AID-IMMU265>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Ott PA, Berner BR, Herzog BA, Guerkov R, Yonkers NL, Durinovic-Bello I, et al. CD28 costimulation enhances the sensitivity of the ELISPOT assay for detection of antigen-specific memory effector CD4 and CD8 cell populations in human diseases. J Immunol Methods. 2004;285:223–235. doi: 10.1016/j.jim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 27.Anthony DD, Post AB, Valdez H, Peterson DL, Murphy M, Heeger PS. ELISPOT analysis of hepatitis C virus protein-specific IFN-gamma-producing peripheral blood lymphocytes in infected humans with and without cirrhosis. Clin Immunol. 2001;99:232–240. doi: 10.1006/clim.2001.5018. [DOI] [PubMed] [Google Scholar]
- 28.Karulin AY, Hesse MD, Tary-Lehmann M, Lehmann PV. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J Immunol. 2000;164:1862–1872. doi: 10.4049/jimmunol.164.4.1862. [DOI] [PubMed] [Google Scholar]
- 29.Anthony DD, Valdez H, Post AB, Carlson NL, Heeger PS, Lehmann PV. Comprehensive determinant mapping of the hepatitis C-specific CD8 cell repertoire reveals unpredicted immune hierarchy. Clin Immunol. 2002;103:264–276. doi: 10.1006/clim.2001.5193. [DOI] [PubMed] [Google Scholar]
- 30.Yonkers NL, Rodriguez B, Post AB, Asaad R, Jones L, Lederman MM, et al. HIV Coinfection Impairs CD28-Mediated Costimulation of Hepatitis C Virus-Specific CD8 Cells. J Infect Dis. 2006;194:391–400. doi: 10.1086/505582. [DOI] [PubMed] [Google Scholar]
- 31.Valdez H, Carlson NL, Post AB, Asaad R, Heeger PS, Lederman MM, et al. HIV long-term non-progressors maintain brisk CD8 T cell responses to other viral antigens. Aids. 2002;16:1113–1111. doi: 10.1097/00002030-200205240-00004. [DOI] [PubMed] [Google Scholar]
- 32.Yonkers NL, Sieg S, Rodriguez B, Anthony DD. Reduced Naive CD4 T Cell Numbers and Impaired Induction of CD27 in Response to T Cell Receptor Stimulation Reflect a State of Immune Activation in Chronic Hepatitis C Virus Infection. J Infect Dis. 2011;203:635–645. doi: 10.1093/infdis/jiq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyk-Pearson S, Tester IA, Klarquist J, Palmer BE, Pawlotsky JM, Golden-Mason L, et al. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol. 2008;82:1827–1837. doi: 10.1128/JVI.01581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tester I, Smyk-Pearson S, Wang P, Wertheimer A, Yao E, Lewinsohn DM, et al. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J Exp Med. 2005;201:1725–1731. doi: 10.1084/jem.20042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boltjes A, Movita D, Boonstra A, Woltman AM. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol. 2014;61:660–671. doi: 10.1016/j.jhep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 37.Pawlotsky JM. Hepatitis C Virus Resistance to Direct-Acting Antiviral Drugs in Interferon-Free Regimens. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


