Abstract
Fibromyalgia syndrome is a chronic pain disorder and defies definitively efficacious therapy. In this review, we summarize the results from the early treatment research as well as recent research evaluating the pharmacological, interventional and nonpharmacological therapies. We further discuss future directions of fibromyalgia syndrome management; we specifically focus on the issues that are associated with currently available treatments, such as the need for personalized approach, new technologically oriented and interventional treatments, the importance of understanding and harnessing placebo effects and enhancement of patient engagement in therapy.
KEYWORDS : exercise, fibromyalgia, interventional treatment, multidisciplinary therapy, pharmacological treatment, placebo, treatment engagement
Practice points.
Fibromyalgia (FM) syndrome is a common chronic pain disorder which is difficult to treat.
Anti-inflammatory agents, selective serotonin reuptake inhibitors and opioids seem to have limited benefit for FM.
Agents targeting serotonin and norepinephrine uptake seem to help some FM patients.
Preliminary results from the trials evaluating various interventional approaches, such as occipital nerve stimulation, lidocaine infusion, hyperbaric oxygen and transcranial stimulation show some promises but need to be tested in larger, controlled trials.
Trials consistently show effectiveness of appropriately designed exercise programs in pain reduction, possibly mediated via immune and neural reregulation.
Multimodal treatment that include activation and psychological/behavioral approaches are beneficial and cost-effective treatments for FM.
Large placebo effects are commonly observed, which may complicate our understanding of clinical effectiveness, yet we need to develop ways to better understand and apply placebo effects in practice.
Patients’ active engagement in therapy is critical for successful rehabilitation. Incorporating approaches to help patients become committed to treatment will likely enhance our ability to effectively treat FM.
Fibromyalgia (FM) syndrome is a common chronic pain disorder. The cardinal features of FM are generalized body pain and diffuse hyperalgesia [1]. FM patients also commonly present a range of other problems, including persistent fatigue, dysregulated sleep, cognitive dysfunction, functional bowel disorder, paresthesias and mood disturbance [1]. It has been estimated that FM affects 3–5% of the population [2]. In the USA, the National Arthritis Data Working group has estimated that up to 5 million people are afflicted with FM [3].
Research in the past four decades suggests several factors that are contributory to FM. The strongest evidence points to dysregulation of pain modulation, based upon the results of experimentally induced pain testing yielding heightened processing and attenuated inhibitory process of noxious stimuli [4–7] as well as imaging studies showing increased neural response to pain [5,8–9]. Although there is no microscopic evidence of definitive pathology in the muscles tissues, peripheral abnormalities such as local hypoxia and ischemia may create a biochemical environment that contributes to peripheral sensitization, leading to central pain sensitization [10]. There is also some evidence that there is dysregulation in the sympatho-adrenal and hypothalamic–pituitary–adrenal systems in FM [11,12].
The primary purposes of this paper are to review current evidence for various approaches to treat FM and further discuss the future direction. We will review several notable pharmacological, nonpharmacological and interventional approaches that are commonly used for treating FM as well as those that received significant attention by the media and the public.
Early studies
Corticosteroids and other anti-inflammatory drugs were among the first agents to be studied, probably due to their common usage in pain management. A double-blind, cross-over trial of prednisone [13], however, failed to demonstrate any treatment benefit. The study used a dose of 15 mg per day; no other trial using steroid has been reported. NSAIDs are commonly used by FM patients [14]; however, double-blind, placebo-controlled trials evaluating NSAIDs for FM, such as naproxen and ibuprofen, have consistently failed to show efficacy [15–18].
Anxiolytics and hypnotics have also been used commonly for chronic pain to treat associated sleep disturbance and anxiety. However, controlled trials evaluating benzodiazepine, zolpidem and zopiclone [17,19–20] failed to show improvement in FM symptoms despite some improvement in sleep parameters. As the review on this topic by Moldofsky [21] indicates, whereas these drugs may improve subjective reports of sleep and fatigue, its effects on EEG sleep stages are limited. Furthermore, the long-term use of these agents may be associated with tolerance and dose escalation. Given the chronic nature of FM, chronic therapy with these drugs may raise some concerns.
Tricyclics were among the most studied drugs as the dysregulation of serotonin and norepinephrine were implicated in FM [22,23]. Low-dose amitriptyline and cyclobenzaprine are two agents that showed promising results in early studies. The trials show that low-dose amitriptyline (typically 25–50 mg per night, significantly lower than the dose for treating depression) improved pain and sleep [15,24]. Cyclobenzaprine is chemically close to amitriptyline although it is used as a muscle relaxant with no expected antidepressant effects. The use of cyclobenzaprine resulted in improvement in pain, sleep and fatigue in FM patients [25,26]. Recently, a multi-centered Phase II trial evaluating a small dose sublingual cyclobenzaprine for 12 weeks [27] showed promising benefit for FM and sleep symptoms relative to placebo. Phase III RCT is currently in progress for further investigation on this formulation.
The meta-analysis [28] provides some support that the use of tricyclics for FM is effective. However, not all patients benefit from these agents. It has been estimated that beneficial response is seen in 36 and 33% of the patients using amitriptyline and cyclobenzaprine, respectively [29]. A recent Cochrane review [30] reached the same conclusion that amitriptyline may be beneficial for a small portion of FM patients but is certainly not a panacea.
The introduction of selective serotonin reuptake inhibitors (SSRIs) was enthusiastically received as safer alternatives to tricyclics. However, the results of controlled trials were disappointing with marginal symptom reduction in FM [31,32]. A recent Cochrane review [33] notes overall poor quality of the research methods and concludes that there is no evidence that SSRIs are more efficacious than placebo. Given the high prevalence of depression in FM [34], although SSRI may be a good option to treat mood for FM patients, its effect on FM symptoms appears limited. The lack of efficacy with SSRI suggests that regulation of norepinephrine reuptake may play some role in FM symptoms. The dissapointing results from the SSRI trials and the promising early results with tricyclics led to further trials of selective serotonin-norepinephrine reuptake inhibitors (SNRIs) in the 2000s.
US FDA-approved medications
Pregabalin, a GABA analog and antiepileptic drug, was the first drug to be approed by US FDA in 2007. A multicenter, double blind, randomized controlled trial (RCT) with 750 patients showed greater improvement in pain and other functional outcomes in those taking pregabalin (300–600 mg a day) or placebo for 14 weeks [35]. A systematic review evaluating the use of pregabalin for FM [36] suggests the superior efficacy of pregabalin relative to placebo in pain reduction, improvement in sleep and quality of life measures but not mood. There also seems to be high placebo response rate, however. A meta-analysis of four RCTs with more than 3000 patients has shown that 40% of patients receiving pregabalin versus 28% of the patients receiving placebo reported greater than 30% pain reduction [37].
Duloxetine and milnacipran are two other FDA approved medications, both SNRIs. A double blind RCTs with duloxetine dose between 60 and 120 mg a day have typically shown greater improvement in pain and self-reported functional ability [38–40] and the effects can be maintained long term [41]. However, there is, as is the case with pregabalin, a substantial placebo effect; >30% pain reduction was report by 48% of the treated patients and 32% of patients receiving placebo [42].
Similarly, double blind RCTs [43–45] evaluating milnacipran (100–200 mg daily) showed significant improvement in pain reports and a range of symptoms compared with placebo group. A pooled data from two RCTs [46] showed 52–61% of treated patients reporting >30% pain reduction versus 36% of the placebo group. A recent report of the 3-year open-label study [47] suggests that the clinical benefit seems to be sustained for a long term.
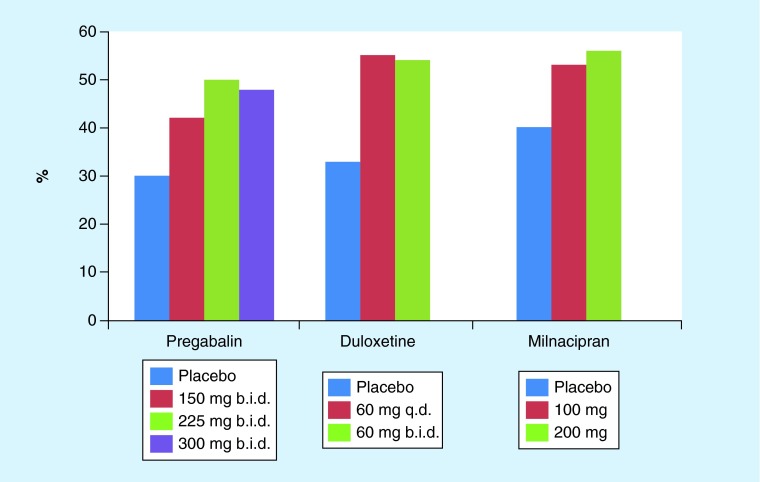
One of the concerns with these trials is the relatively high placebo response rates. For example, a trial with 30 mg duloxetine failed to show improvement in pain severity relative to placebo, mostly due to the high placebo effects [48], For the purpose of illustration, Figure 1 shows the results (% reporting >30% pain reduction) from the three trials [35,38,45] by dose. A recent systematic review [49] of 18 placebo-controlled trials with over 3500 patients estimates that approximately 50% of treatment response to these drugs can be attributed to the placebo effects. This certainly is a significant concern as, while placebo effects could last a long time, their efficacy could gradually diminishing over time, leading to decline in the overall treatment effects. Long sustained placebo effects for various conditions with a range of therapeutic approaches have been reported by many (e.g., [50–52]). We will further discuss the clinical implication of placebo later in the future direction section.
Figure 1. . Percentage of the patients reporting greater than 30% pain reduction by dose for pregabalin, duloxetine, and milnacipran.
b.i.d.: Twice daily; q.d.: Once daily.
Other pharmacotherapies
• Opioids
Prescribing opioids, particularly long-term use for those with chronic noncancer pain has been a controversial issue. Conclusive data showing the efficacy of chronic opioid therapy are by and large absent, although clinical debate continues as to the appropriateness of such therapy. Opioid use among FM patients appeared to be relatively modest in general. A large insurance database estimates about 11% of FM patients use chronic opioid therapy [53]. Opioid use among treatment seeking FM patients may be greater, up to 35% [54,55] and slightly higher if tramadol use was included [56]. A recent observational study with 1700 FM patients reports that those using chronic opioid users show significantly smaller degrees of improvement compared with nonopioid users in a range of outcome variables [56]. Another observational study [57] notes that opioid users tend to show more severe symptoms and poorer functional ability. Overall, the evidence does not seem to support the use of chronic opioids improving FM.
A note on the use of tramadol may be of interest. Tramadol is a weak μ-receptor agonist with SNRI effect, currently classified as a Schedule IV control substance. In one RCT [58] with over 300 FM patients, tramadol/acetaminophen (titrated up to 300 mg/2600 mg a day) resulted significantly greater benefit for improving FM and sleep symptoms relative to placebo. Given the promising results, further research on the effectiveness of tramadol for FM is warranted. Currently, a Phase III multicenter study is ongoing to evaluate the benefit of combining tramadol with cognitive–behavioral approach [59], but their results have not been released yet.
Interestingly, there are reports suggesting that low dose opioid antagonist, naltrexone, may improve FM symptoms. A small pilot, single blind cross over study [60] showed 33% symptom reduction with 4.5 mg daily for 8 weeks. A subsequent study [61] showed the similar effects of 29% pain reduction with naltrexone versus 18% reduction with placebo. The hypothesized mechanisms include the opioidergic reregulation and impact on the proinflammatory system but further research is needed to clarify the effect and mechanism of naltrexone to treat FM.
• Dopamine agonists
Dopamine agonists are frequently used to treat periodic limb movement disorder, which typically occurs in the deep sleep. Some FM patients also exhibit significant arousal interference during this time. Initial trials of these agents for treating FM showed promising results. The open trial with Ropinirole for FM [62] yielded greater than 50% pain reduction in 74% of the patients. A double blind RCT with pramipexole [63] also showed significant greater number of patients taking the drug showing at least 50% pain reduction (42%) than did those taking placebo (15%). However, subsequently trials have not obtained similar results. Three studies have been registered in clinicaltrials.gov. One study [64] with 229 patients evaluating rotigotine at two dose levels (4 mg/24 h, 8 mg/24 h) relative to placebo showed large attrition rates, 43, 74 and 39%, respectively, mostly due to adverse event or lack of efficacy, making it difficult to appreciate the results. Another Phase II, placebo controlled, multinational, multicenter study with ropinirole [65] with 160 patients is listed as completed but no study results are posted. Yet another Phase II, placebo-controlled trial [66] with 61 FM patients evaluating extended release pramipexole was terminated prior to completion. Thus, despite the early demonstration of efficacy, the problems with the subsequent trials suggest that the evidence of dopamine agonist for treating fibromyalgia cannot be established at this time.
• Cannabinoids
Cannabinoids have recently attracted significant attention as potentially effective analgesia for pain conditions. In the first double blind, placebo controlled RCT [67] with 40 FM patients evaluating synthetic cannabinoid (nabilone, titrated to 1 mg twice daily) for 4 weeks showed significant reduction in pain and other symptoms relative to placebo group at the post-treatment although the benefit was short lived. The nabilone group had a greater dropout rate (25%) versus the placebo group (10%) and reported greater side effects of drowsiness (50%), dry mouth (30%) and vertigo (27%). Ware et al. [68] compared nabilone to lose dose (10–20 mg at bedtime) amitriptyline in the crossover trial of 2 weeks trial. While side effects (dizziness, nausea, dry mouth, drowsiness) were more prominent with nabilone, both showed comparative decreases in sleep disturbance although how two drugs differ in the sleep outcome was not clear. Both groups showed marginal, if any, reduction in FM-related symptoms, however. Furthermore, the recruitment was difficult and approximately a half of approached subjects declined to participate in the trials, making it difficult to ascertain neither the generalizability of the results nor feasibility of the cannabinoid as a main stream choice of therapy. Overall, the trials seems to support the safety of the cannabis use for low-risk groups but the trials were short in duration; long-term benefit/risk of chronic cannabinoid therapy for FM is not well understood [69,70].
• Sodium oxybate
Sodium oxybate is a commercially produced form of sodium salt of γ-hydroxybutyric acid (GHB), a Schedule I control substance due to the high risk associated with its abuse potential. Sodium oxybate, on the other hand is a Schedule III drug, approved to treat narcolepsy. Illicit GHB is known to be used as a ‘date rape drug’ due to its effects causing amnesia and increased passivity. In general, however, abuse and misuse complications of sodium oxybate are relatively rare according to the post-marketing data [71,72].
Sodium oxybate has been approved by the FDA to treat excessive daytime sleepiness and cataplexy in narcolepsy. It has shown to restore and reregulate slow wave sleep [73] has led to a series of trials to evaluate the efficacy of sodium oxybate for treating FM. An early small, crossover RCT [74] showed that sodium oxybate 6 g a day at bedtime for 4 weeks significantly improved pain, fatigue and sleep compared with the placebo. More recently, the multicenter studies [75–77] have shown 4.5–6 g per night dose of sodium oxybate is superior to placebo in reduced pain and FM related symptoms. However, the dropout rates were also relatively high due to side effects, particularly for the higher dose group. Another concern for the use of sodium oxybate is its very short elimination half-life, about 30 min to 1 h [78]. Because of the short half-life, the dose had to be given twice during the night, making it necessary to awake patients 2.5–4 h after bedtime. Interrupting sleep of patients whose sleep is poor does not make sense [79], although a polysomnographic study [80] shows significant improvement in various sleep parameters in those patients taking sodium oxybate with this divided dosing approach.
However, the application for the FDA approval of sodium oxybate for treating FM was denied in 2010. The advisory committee's decision [81] appears to reflect although the available evidence suggests some benefit of sodium oxybate for a subset of FM patients, there is ongoing concern for a high abuse and misuse potential [82].
Nonpharmacological options
• Exercise
Physical deconditioning is common and may contribute to the maintenance of FM [83]. Generally, incorporation of some physical fitness program as a part of FM treatment is considered critical. There are a range of exercises available that vary in intensity and effects. Combination of land-based aerobic exercise with strengthening and stretching program shows beneficial effects in reducing FM symptoms and improving physical functioning [84,85]. A systematic review of various exercise trials including a total of 2494 patients [86] indicates that exercise overall shows significant effects in reducing pain and related FM symptoms. At least moderate level of exercise intensity is considered necessary to produce appreciative benefit [87]. However, recent evidence suggests that various mind/body exercises that do not require strenuous movement or raising heartrate may help reduce FM symptoms. For example, group qigong exercise [88,89], tai chi exercise [90,91] and yoga practice [92,93] showed significantly greater symptomatic improvement compared with the patients in the control group or waitlist. A meta-analysis [94] reports medium to high effect size in pain reduction from these exercises for FM.
Exercise is known to be beneficial for overall health. How exactly, however, it affects pain modulation is an interesting question. Recent evidence suggests that exercise may reregulate the immune and stress-related responses. Bote and colleagues [95] found that after completing 8 months of 60 min twice weekly water exercise program, sedentary FM patients showed significant decrease in pro-inflammatory cytokines and noradrenaline levels [95,96]. Even a single session of modest exercise appears to reduce the proinflammatory markers [97]. Furthermore, a recent functional imaging study [98] has demonstrated the normalization of functional connectivity in the brain regions implicated in pain perception for FM patients who underwent a 15-week exercise program. These results suggest that exercise may help improve the adaptation system of the immune and stress systems in FM.
One of the difficulties in helping patients become active, particularly for implementing relatively vigorous exercise for FM patients is long-term adherence with home exercise programs. Some patients are also intolerant to exercise; they experience severe a flare-up in response to exercise, discouraging them from continuing the program. The general recommendations for providing exercise therapy include starting at the low level where patients could engage without significant distress, gradual increase of the intensity level, incorporate different types of exercise and reduction of exercise intensity/duration if not tolerated but maintain the frequency of exercise [87,99]. Later in this paper, we will discuss ways to enhance patients’ engagement to exercise programs.
• Psychological & behavioral modalities: monotherapy
Several behavioral modalities that are commonly used to treat chronic pain patients have been evaluated although the methodological constrains and variations across studies make the quality of evidence rather weak. In general, technique specific therapy, such as biofeedback and hypnosis, may have limited efficacy as a sole modality; thus far, the empirical results show inconsistent and modest effects [100,101]. Mindfulness-based stress reduction (MBSR) is programmatic approach consisting of breathing, meditating and body awareness trainings. It has recently gained much attention and popularity to treat various chronic illnesses. For FM, an early study [102] showed greater reduction in pain and symptoms for the patients undergoing the MBSR training relative to those receiving supportive group sessions. However, the subsequent three-arm study comparing the MBSR training to active control and wait list group did not show significant effects on symptom reduction [103]. However, in this study, those receiving MBSR showed some improvement in QOL. Another RCT evaluating mindfulness exercise [104] has shown significant improvement in social and emotional functioning. Thus, this type of therapeutic effort may not necessarily improve pain but still may positively impact some important life domains of FM patients.
One of the most accepted behavioral therapy modalities in pain management is cognitive–behavioral therapy (CBT). CBT presents an inclusive therapeutic framework where it involves a range of both behavioral and cognitive modalities to address pain-related maladaptive coping, which is known to contribute to the pain modulatory system [105]. In general, research consistently shows that CBT, when given as a monotherapy, is effective in improving the specific target variables (e.g., maladaptive cognition, mood, QOL) [106–108] although the effects on the primary FM symptoms may be limited. A recently published study of a RCT comparing CBT to waitlist controls [109] also shows the superior results in the cognitive and affective components of FM, but not pain. Although these results are disappointing, CBT typically does much better as a part of a multimodal program. The concept of CBT has been applied and integrated to various rehabilitation approaches. CBT-based activation programs typically show very promising results for managing FM symptoms (see below).
• Multidisciplinary treatment
Given the complex, multifactorial nature of FM, experts generally stress the importance of employing therapy approaches that can address multiple factors associated with FM. [110,111]. Although there are some other variations, a typical trial testing a multidisciplinary approach includes education, exercise and psychological (typically cognitive–behavioral) therapy. Although education is not typically given as a sole modality, it has a vital role in promoting self-management and augmenting the effects of other therapeutic approaches. Education to address misconception about FM, setting appropriate expectation and acquiring accurate information about FM and treatments can set a stage for the treatment [112]. Although the treatment targeting multiple problem areas of FM does make sense in theory, the systematic evaluation of studies evaluating the multidisciplinary treatment for FM is difficult because there is large variability in the methodological vigor and what are included in therapy. It should also be mentioned that conducting a well controlled trial to evaluate the multimodal pain therapy is often not feasible due to its cost and labor intensiveness.
A systematic review [113] points to the methodological weakness yet provides some evidence the effectiveness of the approach for various chronic pain conditions including FM. The efficacy seems to be superior for those programs aiming at acquisition of coping and pain management skills to the others that mostly provide information/education [101]. The effectiveness seems to last after the therapy; reduction in pain and other symptoms were observed 12 months later [114]. A recent recommendation [115] by FM experts strongly emphasizes the importance of educating patients, establishing working goals and applying multimodal therapy approaches consisting of education, medications, exercise and CBT.
There has only been one published study thus far that specifically tested the combination therapy of CBT with medication trial [116]. In this trial, patients were randomized into a combination of CBT and milnacipran, drug monothearpy or CBT monotherapy. The results suggest that the combination therapy and CBT monothearpy were equally beneficial in reducing symptoms, suggesting that milnacipran added very little to the clinical benefit of CBT.
Multimodal therapy is resource intensive in nature and often not readily available. Concern about the cost in particular has made the availability of such program more difficult to sustain [117]; however, many agree that effective multi/interdisciplinary treatment is critical in management of chronic pain. Multidisciplinary approach has also been shown cost effective for FM care [118]. Furthermore, a recent retrospective and small case series [119–121] suggest that a brief course of multidisciplinary sessions for FM can be well accepted by the patients and show promising results. These results further suggest that less intensive, brief multidisciplinary treatment may be a sustainable and viable option as a clinical model of direct care.
Interventional approach
• Occipital nerve stimulation
Occipital nerve stimulation (ONC) is a form of an invasive neuromodulation therapy. A subcutaneous electrode is implanted to electronically stimulate the greater occipital nerve. Exact mechanism of how ONC work is not well understood but it is assumed to have an impact on multiple neural networks both at the spinal and supraspinal levels [122]. The ONC was first tried openly on a small number of FM women with chronic daily headaches who during the trial stimulation showed at least a 50% reduction of headache intensity along with significant reduction in various FM symptoms [123]. Subsequently, a randomized, crossover trials was conducted [124] with 11 patients with FM who failed prior treatment with tricyclics, physical therapy and supportive counseling. They underwent 5 weeks of sham stimulation (lowest amplitude possible) versus 5 weeks of ‘subthreshold’ (no paresthesia) treatment. The analyses yielded the significant treatment effects, although the effects were small. Pain VAS scores went down to 27 from 34 in the treatment group, whereas the sham stimulation achieved VAS of 30. The small effect may be due to the lack of paresthesia in the treatment group, which is considered critical for obtaining effective pain reduction. In their next study [125], in addition to the sham and subthreshold stimulation, they added suprathreshold amplitude where patients could adjust stimulus level. Thirty five patients completed the cross over trial of 2 weeks at each stimulation level. The baseline pain VAS was 41.97; the mean pain VAS scores were 38.54 after the sham stimulation, 37.34 after the subthreshold stimulation and 35.14 after the suprathreshold stimulation. The treatment effect of the suprathreshold stimulation was statistically significant. However, it was a weak effect with 7/100 pain reduction. As for the subthreshold stimulation, it was not different from the sham stimulation. Given the invasiveness, potential complications and cost of the procedure, the results may not support its feasibility at this time. However, since there is evidence of significant pain relief for some headache patients (e.g., [122,126]), some FM patients may benefit from the procedure. Research effort to identify who or what part of FM may benefit from it may enhance the efficacy of the treatment.
• Lidocaine infusion
Administration of intravenous lidocaine-like drugs to treat pain symptoms has a long history [127]. Studies in animals with experimental nerve injury and in humans with chronic neuropathic pain have shown that nerve injury leads to ectopic impulse generation in damaged and dysfunctional primary sensory neurons and their axons [127]. The development of ectopic hyperexcitability is thought to be caused by remodeling of the local electrical properties of the axon membrane by changes in sodium-channel distribution. Lidocaine suppresses abnormal discharge originating at nerve injury sites, associated dorsal root ganglia and spine by the blockade of sodium channels and NMDA receptors, and by reducing substance P, without affecting normal nerve conduction [128]. Intravenous lidocaine promotes a decrease in spinal sensitization, reducing spontaneous pain, dysesthesia, hyperalgesia and allodynia [129]. In controlled studies, intravenous lidocaine was effective in different dosages and regimens for the treatment of a variety of neuropathic pain states such as post-stroke pain, peripheral neuropathy, diabetic neuropathy and complex regional pain syndrome I and II [130].
For FM, the small uncontrolled studies in the 1990s [131,132] have shown promising results in pain reduction in those who received lidocaine infusion therapy. Another recent open trial [133] showed statistically significant, although small, reduction in pain and other FM symptoms at post-infusion as well as 30 days after five consecutive intravenous lidocaine infusion of escalating dose to 5 mg/kg. The results of the placebo controlled RCTs are inconsistent. McCleane et al. [134] showed that 63 patients had statistically significant pain reduction following intravenous lidocaine over 24 h when compared with their response to saline 3 weeks after the infusion, although the degree of pain reduction was small, about 10%. Another controlled RCT [135] compared 15 patients who received four weekly infusions to those received saline; both groups showed comparable pain reduction. The results are difficult to interpret as they all started to take small dose amitriptyline. Furthermore, although the inclusion of placebo condition may improve methodological soundness of the study, it is not entirely clear whether blinding was successful with the use of saline. Lidocaine infusion is known to produce fairly distinctive sensation experienced often as side effects such as hypotension, headaches, dizziness, drowsiness, numbness of lips or tunnel vision [136].
Overall, lidocaine infusion seems to lead to small reduction in pain for some FM patients. At this time, the available research is difficult interpret due to the large variability in the parameters of infusion (dose, duration, frequency). The use of saline as a placebo may also not present the best blind procedure. Furthermore, intravenous lidocaine infusion is not inexpensive. It is time-consuming and has to be given in a clinic with close hemodynamic monitoring and resuscitation equipment ready.
• Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) provides pure, 100% oxygen at a pressure that is much greater than the atmospheric level. HBOT has been used to treat a range of conditions and is currently approved by the FDA to treat 13 conditions including decompression sickness, carbon monoxide toxicity and arterial insufficiency. In animal model, HBOT has been shown to have an antinociceptive effect via activation of endogenous opioids [137] and inhibition of the inflammatory response [138]. HBOT has also been tried to treat chronic pain conditions; the studies tend to be small and preliminary, but have yielded promising effects on pain management [82]. The first trial of HBOT for FM was published in 2004 [139], that compared 26 patients receiving HBOT to 24 patients who received room air at the sea level pressure; both groups underwent 15 90-min sessions. Those in the HBOT group showed significantly greater pain reduction at the post-treatment than that of the control group. Unfortunately, their group assignments could present bias as the treatment condition could not be blind (much lower air pressure). Efrati et al. [140] used a wait-list approach, thus comparing both between and within groups of treatment vs no treatment. The HBOT was five 90 sessions per week for 8 weeks, which showed significant symptom reduction and some normalization of neural activity based upon SPECT imaging. However, it is noteworthy that patients often complained of symptom worsening during the first 4 weeks. This this study also could not blind patients. Although the results are promising, HBOT is not a benign approach; it carries a risk of oxygen toxicity and other side effects. Further risk–benefit analyses may be needed to assure the safety and efficacy of this approach.
• Transcranial stimulation
Given the likely importance of the dysregulated central activity in FM, approaches that can modify neural activities noninvasively have become of significant interest. Two types of transcranial stimulation, transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have been used to treat the cognitive and affective disorders [141]. They have also been tried for various chronic pain conditions, yielding some promising but variable results in pain reduction [142–144].
TMS sends a brief electrical current from an electromagnetic coil that is placed against patient's scalp, creating a regional magnetic field that affects neural activity. Boyer et al. [145] recently reported the results from the double-blind RCT in which 19 FM patients underwent a repeated TMS whereas 19 FM control patients received a series of sham stimulations. TMS treated patients showed significant reduction in the Fibromyalgia Impact Questionnaire (FIQ) score and the Mental component score of the SF-36, whereas there was no significant reduction in pain with the treatment. Similar results are reported by others and the meta-analytic review [146] concludes that TMS may modulate mood but the effects on pain may be marginal.
tDCS is another noninvasive brain stimulation method. It sends a weak electrical current through a pair of electrodes placed on the scalp in the region of interest. The current is considered to help modulate the neural activity, possibly in a polarity specific way [141]. There are only few studies that evaluated tDCS in FM. One is an open trial with 14 FM patients [147], yielding 50% reduction in the pain score in seven patients. The other study was double-blind RCT in which 24 patients received five sessions of tDCS, compared with 24 patients who received five sessions of sham stimulation. As in the TMS trials, there was a significant reduction in the FIQ scores for treated patients. A small degree of pain reduction was also observed (about 13%).
Overall, the results from these trials should be considered preliminary. Overall, it is difficult to ascertain how the stimulation actually affects the neural changes and/or symptoms of FM. Further studies with larger sample size are needed to delineate the treatment efficacy; such trials should ideally assess several cardinal symptoms of FM in addition to pain and mood, such as fatigue, sleep and cognitive functioning.
Management of FM in 2016: future perspective
In this section, we would like to discuss topics that that we think should complement the review. First, we will discuss the methodological issues. Although not directly relevant to daily practice, effective utilization of evidence-based FM care depends on understanding the scientific and methodological appropriateness of the published clinical trials.
Another important issue in FM management is comorbidity of other pain conditions in FM. FM can occur with other pain conditions that may complicate the way we approach FM management. Third, we will discuss the issue of placebo effect. Interestingly, although the outcomes of placebo-controlled studies for treating FM vary significantly, they are consistent in showing fairly large placebo effects. Certainly, the presence of large placebo effect is not FM specific; yet we have very little understanding of this ubiquitous effect. Lastly, we will focus on the difficulty associated with implementing effective rehabilitation program. The literature indicates that a multimodal approach involving activation is critical in rehabilitating FM patients, yet motivating patients to sustain active effort to adhere with the regimen is challenging. We will discuss some adjunct approaches that may enhance the activation therapy.
• Methodological issues
The importance of considering individual variations and developing a personalized approach cannot be overstated [148,149]. This is where we encounter a limit of the conventional research methodology using the RCTs. RCTs, of course, are capable of protecting internal validity of the study by controlling potentially confounding variables. However, reliance on RCTs and p-value-based statistical significance testing has recently been criticized [150–152]. In RCT, mean comparisons between groups are the main theme, thus helping us see if the treatment in question benefit ‘an average patient’, but it does not tell us whether the treatment would benefit a particular patient who is sitting in your exam room. Such methodological limitation needs to be reminded as healthcare providers seek evidence-based approaches. Essentially, the lack of the methodological flexibility to take patient heterogeneity into account devalues of study results, limiting our understanding of which treatment should work for whom.
Furthermore, there are treatment options, mostly interventional, that we may not be able to evaluate against ethically and scientifically sound controls. Such difficulty often leads to inconclusive evidence where practice using such modalities is considered not evidence based but rather anecdotally supported.
Recently, several innovative methodologies have been proposed. Particularly, modern statistical framework utilizing a causal modeling may help us determine the true effects of therapy for a specific patient (e.g., dynamically modified outcome) [153]. Adaptive analytical approach to determine the applicability of various treatment for a given patient may also prove to be helpful (e.g., sequential multiple assignment randomization trials [154]).
In addition to the analytical development, digital technology may also help us better obtain an in-depth view of patients’ FM conditions. The conventional practice is to conduct assessment of FM patients in the clinical setting where patients are asked to recall and summarize their symptom severity, quality and frequency. Such symptom assessment is quite vulnerable to recall bias, score anchoring and over- and underestimation due to various reasons (e.g., want to continue meds). However, technological advancement is making it easier for patients and clinicians to monitor the real time symptoms and functional status easily and feasibly. Commercial products to track physical activity and cell phone applications to monitor symptoms can be incorporated into clinical practice to obtain real-time assessment of symptoms/functions at home (i.e., natural environment) for treatment planning and outcome assessment. Such assessments also afford us a longitudinal view of the symptoms, thereby helping us determine how various symptoms impact one another. Sequential analyses of relevant FM symptoms have shown that exacerbation of one symptom tend to worsen others, although the degree to which change in one symptom drives another varies across individuals [155]. Thus, each patient may have a specific FM-related domain that drives other symptoms to worsen or positively influence. Of course, the relationship between symptoms is complex, likely bidirectional and reciprocal. However, there may be symptoms that are more influential to others in their relationship. For example, patient A's overall FM conditions worsen as her sleep gets poorer whereas it is not sleep for patient B but anxiety that seems to drive her pain and fatigue to worsen. Perhaps, it is this ‘driving factor’ in FM for each patient that needs to be a primary therapy target; in other words, identifying a patient-specific driving factor for his or her FM can help us identify a specific therapy modality that targets the factor. If we can identify and treat the dominant FM factor, we may have a better chance of controlling overall FM symptoms.
• Pain comorbidity in FM
It has been well recognized that FM patients commonly complain of other pain conditions such as temporomandibular joint pain and headaches as well as psychiatric problems [34,156–157]. Evidence also suggests that FM can occur in conjunction with a range of medical conditions. For example, a large, epidemiological study with over 1.2 million adults with pain conditions [158] reports that approximately 21% of patients with HIV associated pain and 14% of patients with multiple sclerosis are diagnosed with FM. FM also co-occurs with other muscloskeletal pain conditions, such as myofascial pain [159] and work related musculoskeletal disorders [160]. Obesity, one of the common pathways to various health issues, is also common in FM [55]; confounding obesity may also lead to the development of other pain problems such as osteoarthritis of knee [161]. Comorbid pain and other medical conditions complicate the already complex picture of FM management. Those comorbid conditions may represent additional or augmented pain pathways; without addressing them, effective FM management may not be achieved. There is some evidence of successful treatment of comorbid pain complaints carries over to better FM care [160,162]. Clearly, personalized care plans based upon the overall clinical picture of each patient are needed.
• Placebo effects: how should we deal with them?
Placebo effects are typically considered something bad, akin to error, in clinical research. Research strategies focus on how we can minimize placebo effects. On the other hand, in a clinical setting, whether symptom improvement is due to ‘true’ intended treatment effects and/or placebo does not matter much to clinicians or to patients, as long as patients are feeling better. As many of the review of the placebo-controlled trials show, FM patients tend to show a high level of placebo responses [49]; 30–40% response to placebo is not uncommonly observed. The discrepancy poses a difficult challenge; although it is treated as something undesirable in research, it is clinically entrenched in our daily practice yet very little is known what it is and how it works clinically.
High degrees of placebo responses are certainly not specific to FM. Placebo effects have been extensively studied in the field of antidepressant therapy. In the provocative and influential paper ‘Listening to Prozac but Hearing Placebo’ [163], they estimated the effect sizes of placebo versus active drug and concluded that 75% of the efficacy of the active drug may be attributed to placebo effects. There has been much debate as to how we can harness the placebo effects, since they are inheritedly a part of clinical effects [164,165]. Perhaps, one way is not just trying to eradicate the placebo effects in research but to study them more closely. We do not even know the very basic parameters of placebo effects, such as, how long does it last? Does it eventually wear out? How do we know the decline of treatment effects is due to patients’ tolerance or unresponsiveness to ‘true’ medication effects versus placebo effects wearing off? Placebo research strongly suggests that placebo effect is not inert but may lead to significant neurobiological changes, possibly enhancing treatment effects [166]. Research on placebo analgesia generally suggests that placebo effects involve an active process of analgesia, possibly involving dopaminergic, endocannabinoids and endogenous opioid systems [167].
• Exercise: how could we help patients get activated?
Our review, as well as others [85,87] consistently shows exercise is beneficial for FM patients. Exercise, for that matter, is beneficial for overall physical and mental health. However, it is commonly acknowledged that it is difficult to start and maintain. The traditional medical model of exercise treatment is that if patients are not motivated or compliant with the regimen, the burden is totally on the patients’ side and providers assume that the patient is not motivated to engage in the regimen, often telling patients, ‘come back when you are ready’. Interestingly, however, the extent of which how much a person intend to do something actually leads to behavioral commitment is surprisingly modest, approximately 20–35% of variance in engaging in goal behavior [168]. Another common assumption that past behavior is the best predictor is equally tenuous, about 26% of the variance [Sutton S, Sheeran P, Unpublished Data].
Research investigating factors that strengthen or weaken patients’ engagement to exercise and other self-management approaches to treat chronic pain has yielded interesting findings. For example, pain severity or functional status at the beginning of the treatment fail to predict adherence [169], whereas long-term exercise engagement is related to the cognitive factors such as self-efficacy [170] and belief about the benefit of the regimen [171]. The CDC report [172] regarding the common barriers to physical activity in general includes lack of time, fear of injury, lack of support, low self-efficacy for exercising, not liking exercise, lack of motivation and limited resources. For people with chronic pain, we must also consider actual pain flare after exercise [173,174] as well as fear of such exacerbation [175]. Treatment approaches that address these factors are likely to enhance patients’ engagement to exercise and self-management therapies, leading to better outcomes. We will now briefly discuss three strategies that may be used as a part of such approaches.
Education
Successful rehabilitation of FM often include exercise and self-management skill training, and these approaches require patients to have a paradigm shift from a passive patient role (treatment should be done to me) to an active role. This paradigm shift is critical in enhancing patients’ engagement in therapy. Providing information can also set a tone for appropriate expectation and goals, leading to better adherence with physical regimen [176,177]. Box 1 lists some examples of educational materials that can be used for FM patients.
Box 1. . Example educational contents for fibromyalgia patients.
Concept of chronic pain
What is fibromyalgia?
How is fibromyalgia different from acute injury or pain?
‘Gate control model’: pain isn't just one thing!
Hurt vs harm
Common myths about fibromyalgia
Treatment options for fibromyalgia
Medications
Procedures
Information
Exercise
Coping skill training
Rationales for multimodal approaches
How effective are they?
Can we ‘cure’ chronic pain?
Strategies to cope
‘But I hurt too much’ what to do?
Pain versus suffering
-
Habit change:
Goals
What to expect
Motivational enhancement therapy
Motivational enhancement therapy (MET) was originally developed for treating addiction behaviors [178] where difficult behavioral changes are necessary to achieve clinical benefit. Since then it has been adapted to help patients with a range of illnesses to promote behavioral changes. For people living with FM and more generally chronic pain, this also has a significant implication. MET can provide clinicians a therapeutic framework in which clinicians help patients clarify problems and desired goals, analyze therapeutic behaviors based upon patients’ personal values and internalize motivation via MET specific techniques. The details of MET approaches are beyond the scope of this paper; some reviews are available for MET in chronic pain [179–181]. Incorporation of MET in pain treatment is fairly young; however, the available evidence is encouraging. When pain therapy includes MET, patients tend to improve their pain and functional status [182–184]. MET also reduces the risk of opioid misuse in chronic pain patients [185]. When MET was combined with exercise therapy for chronic pain, it improves patients’ ability to actively engage in exercise [184].
Volitional approach
Implementation Intentions (IIS) is another approach to help patients attain therapeutic goals. IIS is a practical, volitional approach aiming to reduce the barriers to goal attainment with action planning and problem solving. IIS helps patients practice of various ‘if then’ contingencies to map out in advance how patients may strive for achieving their therapeutic goals. IIS typically involves two steps [186]: Action planning that determines when, where and how to do the target behavior and coping planning that offers a series of problem solving exercises that work by addressing common and personalized barriers that may jeopardize the chance of successful rehabilitation. General problem solving and behavioral skill training can be included as a part of the IIS practice. For example, self-management training for pain flare-ups, communication and social skill and stress management training can help patients to attain basic skills that can be applied to address specific barriers to specific therapy. Therapeutic course of IIS needs to be individualized to patients’ needs and circumstances; a generic of list of suggested IIS approaches is described in Table 1. The IIS approach is basically a practical, common sense approach and it is easily understood by patients. IIS has been shown to successfully improve the level of physical activity in a range of people including sedentary women [187], obese elderly people [186], cardiac patients [188] and diabetic patients [189]. The meta-analytic review of the implementation intentions on physical activity from 24 studies shows encouraging results with a pooled effect of 0.31 at post-treatment and 0.24 for follow-up visit with a higher effects shown with a program involving specific barrier management [186,190].
Table 1. . Implementation intentions: examples of action and coping planning to specific barriers.
| Barriers | Approaches |
|---|---|
| Time management | Clarifying values of exercise |
| If, then problem solving and action plans: | |
| – If there is not enough time to exercise because… | |
| – Apply problem solving | |
| – Develop action plans | |
| – Handling procrastination | |
| – How procrastination happen | |
| – Apply problem solving | |
| |
– Develop action plans |
| Flare-ups | Flare-up management: |
| – What can we do to prepare | |
| – Skill training for flare up management | |
| – If-then exercise | |
| |
– Develop action plans |
| Support from others | Interpersonal effectiveness: |
| – Communication training | |
| – Interpersonal effectiveness to improve relation with others | |
| – Asking support | |
| – If-then exercise | |
| |
– Develop action plans |
| Resource management | Available resources: |
| – What are available within 20 min from home | |
| – Parks, recreation centers, shopping area, trails | |
| – Weather, pain, stress, time, low motivation | |
| – If, then exercise | |
| – Develop action plans using available resources |
Clearly, treating a complex, chronic pain syndrome such as FM requires a collaborative effort between providers and patients. Patients’ own commitment and engagement in therapeutic efforts plays a key role in establishing the efficacy of many treatment options. In other words, whether given treatment works or not largely depend upon whether patients are willing to maintain their effort to adhere with the regimen. If this is the case, perhaps we should pay closer attention to develop ways to help patients becoming more ‘therapy ready’. In addition to the treatment itself, our clinical effort to provide such ‘meta-supportive therapy’, if you will, may work well for enhancing treatment effectiveness and helping patients restore the quality of life that has been compromised by this very difficult pain condition.
Conclusion
Despite extensive effort, FM continues to be a very difficult condition to successfully treat. The review of the literature consistently shows that most modalities may provide some efficacy for a minority of FM patient, but on average they yield unimpressive, rather marginal, outcomes. This may reflect the heterogeneity of FM patients. FM is by nature a multisymptom, possibly multimechanistic disorder; FM patients are known to be heterogeneous in their symptoms, functions and adaptation to their illness [191,192]. Thus, it should not be surprising that any modality with narrow scope is unlikely to achieve the reasonable level of efficacy for FM management. A recent systematic review of pharmacological and nonpharmacological therapies for FM [193] with 102 trials (n = 14,982 patients) reflects the disappointing results. The methodological flaws generally make it difficult to interpret the results. The efficacy appears greater with modalities that targets broader areas, such as multimodal therapy, exercise and CBT; these modalities are known to lead to lifestyle and behavioral changes that promote overall health. Although the exact mechanisms of these approaches are difficult to ascertain, their broader approaches have a greater chance of modifying areas that directly as well as indirectly associated with FM symptoms. Pharmacological and interventional approaches, on the other hand, tend to target more specific pathways. Perhaps, modalities can be combined to address both specific and broad aspects of FM, and match the approach to patient's clinical needs. We have also discussed several areas relevant to improve the quality of clinical trials and our ability to interpret the results. Issues of treating comorbidity, how to handle placebo effects and adherence with regimen all are essential factors in FM management. Although the field has made the great strides in the past 40 years in better understanding FM and testing a range of treatments, further innovation in FM management to help millions of people suffering from this difficult condition is warranted.
Footnotes
Financial & competing interests disclosure
A Okifuji has received a grant from the NIH (U34AR067378). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee [see comments] Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: direct health care costs of fibromyalgia syndrome in London, Canada. J. Rheumatol. 1999;26(4):885–889. [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arroyo JF, Cohen ML. Abnormal responses to electrocutaneous stimulation in fibromyalgia [see comments] J. Rheumatol. 1993;20(11):1925–1931. [PubMed] [Google Scholar]
- 5.Gibson SJ, Littlejohn GO, Gorman MM, Helme RD, Granges G. Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain. 1994;58(2):185–193. doi: 10.1016/0304-3959(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 6.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70(1):41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 7.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 8.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 9.Lorenz J, Grasedyck K, Bromm B. Middle and long latency somatosensory evoked potentials after painful laser stimulation in patients with fibromyalgia syndrome. Electroencephalogr. Clin. Neurophysiol. 1996;100(2):165–168. doi: 10.1016/0013-4694(95)00259-6. [DOI] [PubMed] [Google Scholar]
- 10.Staud R. Peripheral pain mechanisms in chronic widespread pain. Best Pract. Res. Clin. Rheumatol. 2011;25(2):155–164. doi: 10.1016/j.berh.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Light KC, White AT, Tadler S, Iacob E, Light AR. Genetics and gene expression involving stress and distress pathways in fibromyalgia with and without comorbid chronic fatigue syndrome. Pain Res. Treat. 2012;2012:427869. doi: 10.1155/2012/427869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Lavin M. Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia. Arthritis Res. Ther. 2007;9(4):216. doi: 10.1186/ar2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark S, Tindall E, Bennett RM. A double blind crossover trial of prednisone versus placebo in the treatment of fibrositis. J. Rheumatol. 1985;12(5):980–983. [PubMed] [Google Scholar]
- 14.Wolfe F, Anderson J, Harkness D, et al. Health status and disease severity in fibromyalgia: results of a six-center longitudinal study. Arthritis Rheum. 1997;40(9):1571–1579. doi: 10.1002/art.1780400905. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg DL, Felson DT, Dinerman H. A randomized, controlled trial of amitriptyline and naproxen in the treatment of patients with fibromyalgia. Arthritis Rheum. 1986;29(11):1371–1377. doi: 10.1002/art.1780291110. [DOI] [PubMed] [Google Scholar]
- 16.Yunus MB, Masi AT, Aldag JC. Short term effects of ibuprofen in primary fibromyalgia syndrome: a double blind, placebo controlled trial. J. Rheumatol. 1989;16(4):527–532. [PubMed] [Google Scholar]
- 17.Russell IJ, Fletcher EM, Michalek JE, Mcbroom PC, Hester GG. Treatment of primary fibrositis/fibromyalgia syndrome with ibuprofen and alprazolam. A double-blind, placebo-controlled study. Arthritis Rheum. 1991;34(5):552–560. doi: 10.1002/art.1780340507. [DOI] [PubMed] [Google Scholar]
- 18.Quijada-Carrera J, Valenzuela-Castano A, Povedano-Gomez J, et al. Comparison of tenoxicam and bromazepan in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial. Pain. 1996;65(2–3):221–225. doi: 10.1016/0304-3959(95)00199-9. [DOI] [PubMed] [Google Scholar]
- 19.Moldofsky H, Lue FA, Mously C, Roth-Schechter B, Reynolds WJ. The effect of zolpidem in patients with fibromyalgia: a dose ranging, double blind, placebo controlled, modified crossover study. J. Rheumatol. 1996;23(3):529–533. [PubMed] [Google Scholar]
- 20.Drewes AM, Andreasen A, Jennum P, Nielsen KD. Zopiclone in the treatment of sleep abnormalities in fibromyalgia. Scand. J. Rheumatol. 1991;20(4):288–293. doi: 10.3109/03009749109096802. [DOI] [PubMed] [Google Scholar]
- 21.Moldofsky H. The significance of dysfunctions of the sleeping/waking brain to the pathogenesis and treatment of fibromyalgia syndrome. Rheum. Dis. Clin. North Am. 2009;35(2):275–283. doi: 10.1016/j.rdc.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Russell IJ, Michalek JE, Vipraio GA, Fletcher EM, Javors MA, Bowden CA. Platelet 3H-imipramine uptake receptor density and serum serotonin levels in patients with fibromyalgia/fibrositis syndrome [see comments] J. Rheumatol. 1992;19(1):104–109. [PubMed] [Google Scholar]
- 23.Wolfe F, Russell IJ, Vipraio G, Ross K, Anderson J. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J. Rheumatol. 1997;24(3):555–559. [PubMed] [Google Scholar]
- 24.Ginsberg F, Mancaux A, Joos E, Vanhove P, Famaey J-P. A randomized placebo-controlled trial of sustained-release amitriptyline in primary fibromyalgia. J. Musculoskel. Pain. 1996;4:37–47. [Google Scholar]
- 25.Bennett RM, Gatter RA, Campbell SM, Andrews RP, Clark SR, Scarola JA. A comparison of cyclobenzaprine and placebo in the management of fibrositis. A double-blind controlled study. Arthritis Rheum. 1988;31(12):1535–1542. doi: 10.1002/art.1780311210. [DOI] [PubMed] [Google Scholar]
- 26.Santandrea S, Montrone F, Sarzi Puttini P, Boccassini L, Caruso I. A double-blind crossover study of two cyclobenzaprine regimens in primary fibromyalgia syndrome. J. Int. Med. Res. 1983;21:74–80. doi: 10.1177/030006059302100202. [DOI] [PubMed] [Google Scholar]
- 27.Liderman S, Clauw DJ, Gendreau J, et al. TNX-102 SL for the treatment of fibromyalgia: role of nonrestorative sleep on pain centralization. Ann. Rheum. Dis. 2015;74:313. [Google Scholar]
- 28.Arnold LM, Keck PE, Jr, Welge JA. Antidepressant treatment of fibromyalgia. A meta-analysis and review. Psychosomatics. 2000;41(2):104–113. doi: 10.1176/appi.psy.41.2.104. [DOI] [PubMed] [Google Scholar]
- 29.Carette S, Bell MJ, Reynolds WJ, et al. Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia. A randomized, double-blind clinical trial [see comments] Arthritis Rheum. 1994;37(1):32–40. doi: 10.1002/art.1780370106. [DOI] [PubMed] [Google Scholar]
- 30.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database Syst. Rev. 2015;7:CD008242. doi: 10.1002/14651858.CD008242.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldenberg D, Mayskiy M, Mossey C, Ruthazer R, Schmid C. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum. 1996;39(11):1852–1859. doi: 10.1002/art.1780391111. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe F, Cathey MA, Hawley DJ. A double-blind placebo controlled trial of fluoxetine in fibromyalgia. Scand. J. Rheumatol. 1994;23(5):255–259. doi: 10.3109/03009749409103725. [DOI] [PubMed] [Google Scholar]
- 33.Walitt B, Urrutia G, Nishishinya MB, Cantrell SE, Hauser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst. Rev. 2015;6:CD011735. doi: 10.1002/14651858.CD011735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernik M, Sampaio TP, Gandarela L. Fibromyalgia comorbid with anxiety disorders and depression: combined medical and psychological treatment. Curr Pain Headache Rep. 2013;17(9):358. doi: 10.1007/s11916-013-0358-3. [DOI] [PubMed] [Google Scholar]
- 35.Arnold LM, Russell IJ, Diri EW, et al. A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J. Pain. 2008;9(9):792–805. doi: 10.1016/j.jpain.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Hauser W, Bernardy K, Uceyler N, Sommer C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin – a meta-analysis of randomized controlled trials. Pain. 2009;145(1–2):69–81. doi: 10.1016/j.pain.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Straube S, Derry S, Moore RA, Mcquay HJ. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology (Oxford) 2010;49(4):706–715. doi: 10.1093/rheumatology/kep432. [DOI] [PubMed] [Google Scholar]
- 38.Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. 2005;119(1–3):5–15. doi: 10.1016/j.pain.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 39.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50(9):2974–2984. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 40.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136(3):432–444. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Mease PJ, Russell IJ, Kajdasz DK, et al. Long-term safety, tolerability, and efficacy of duloxetine in the treatment of fibromyalgia. Semin. Arthritis Rheum. 2010;39(6):454–464. doi: 10.1016/j.semarthrit.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Arnold LM, Clauw DJ, Wohlreich MM, et al. Efficacy of duloxetine in patients with fibromyalgia: pooled analysis of 4 placebo-controlled clinical trials. Prim. Care Companion J. Clin. Psychiatry. 2009;11(5):237–244. doi: 10.4088/PCC.08m00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitton O, Gendreau M, Gendreau J, Kranzler J, Rao SG. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Hum. Psychopharmacol. 2004;19(Suppl. 1):S27–S35. doi: 10.1002/hup.622. [DOI] [PubMed] [Google Scholar]
- 44.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin. Ther. 2008;30(11):1988–2004. doi: 10.1016/j.clinthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Mease PJ, Clauw DJ, Gendreau RM, et al. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J. Rheumatol. 2009;36(2):398–409. doi: 10.3899/jrheum.080734. [DOI] [PubMed] [Google Scholar]
- 46.Geisser ME, Palmer RH, Gendreau RM, Wang Y, Clauw DJ. A pooled analysis of two randomized, double-blind, placebo-controlled trials of milnacipran monotherapy in the treatment of fibromyalgia. Pain Pract. 2011;11(2):120–131. doi: 10.1111/j.1533-2500.2010.00403.x. [DOI] [PubMed] [Google Scholar]
- 47.Arnold LM, Palmer RH, Ma Y. A 3-year, open-label, flexible-dosing study of milnacipran for the treatment of fibromyalgia. Clin. J. Pain. 2013;29(12):1021–1028. doi: 10.1097/AJP.0b013e31828440ab. [DOI] [PubMed] [Google Scholar]
- 48.Arnold LM, Zhang S, Pangallo BA. Efficacy and safety of duloxetine 30 mg/d in patients with fibromyalgia: a randomized, double-blind, placebo-controlled study. Clin. J. Pain. 2012;28(9):775–781. doi: 10.1097/AJP.0b013e3182510295. [DOI] [PubMed] [Google Scholar]
- 49.Hauser W, Sarzi-Puttini P, Tolle TR, Wolfe F. Placebo and nocebo responses in randomised controlled trials of drugs applying for approval for fibromyalgia syndrome treatment: systematic review and meta-analysis. Clin. Exp. Rheumatol. 2012;30(6 Suppl. 74):78–87. [PubMed] [Google Scholar]
- 50.Goetz CG, Wuu J, Mcdermott MP, et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Mov. Disord. 2008;23(5):690–699. doi: 10.1002/mds.21894. [DOI] [PubMed] [Google Scholar]
- 51.Nickel JC. Placebo therapy of benign prostatic hyperplasia: a 25-month study. Canadian PROSPECT study group. Br. J. Urol. 1998;81(3):383–387. doi: 10.1046/j.1464-410x.1998.00554.x. [DOI] [PubMed] [Google Scholar]
- 52.Cherkin DC, Sherman KJ, Avins AL, et al. A randomized trial comparing acupuncture, simulated acupuncture, and usual care for chronic low back pain. Arch. Intern. Med. 2009;169(9):858–866. doi: 10.1001/archinternmed.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Painter JT, Crofford LJ, Talbert J. Geographic variation of chronic opioid use in fibromyalgia. Clin. Ther. 2013;35(3):303–311. doi: 10.1016/j.clinthera.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzcharles MA, Ste-Marie PA, Gamsa A, Ware MA, Shir Y. Opioid use, misuse, and abuse in patients labeled as fibromyalgia. Am. J. Med. 2011;124(10):955–960. doi: 10.1016/j.amjmed.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 55.Okifuji A, Donaldson GW, Barck L, Fine PG. Relationship between fibromyalgia and obesity in pain, function, mood, and sleep. J. Pain. 2010;11(12):1329–1337. doi: 10.1016/j.jpain.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng X, Robinson RL, Mease P, et al. Long-term evaluation of opioid treatment in fibromyalgia. Clin. J. Pain. 2015;31(1):7–13. doi: 10.1097/AJP.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 57.Fitzcharles MA, Faregh N, Ste-Marie PA, Shir Y. Opioid use in fibromyalgia is associated with negative health related measures in a prospective cohort study. Pain Res. Treat. 2013;2013:898493. doi: 10.1155/2013/898493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am. J. Med. 2003;114(7):537–545. doi: 10.1016/s0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 59.Combined Behavioral and Analgesic Trial for Fibromyalglia (COMBAT-FM) https://clinicaltrials.gov/ct2/show/NCT01598753
- 60.Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med. 2009;10(4):663–672. doi: 10.1111/j.1526-4637.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Younger J, Noor N, Mccue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65(2):529–538. doi: 10.1002/art.37734. [DOI] [PubMed] [Google Scholar]
- 62.Holman AJ. Ropinirole, open preliminary observations of a dopamine agonist for refractory fibromyalgia. J. Clin. Rheumatol. 2003;9(4):277–279. doi: 10.1097/01.rhu.0000081264.26484.e5. [DOI] [PubMed] [Google Scholar]
- 63.Holman AJ, Myers RR. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005;52(8):2495–2505. doi: 10.1002/art.21191. [DOI] [PubMed] [Google Scholar]
- 64.The use of rotigotine for treatment of reducing signs and symptoms of fibromyalgia in adults. https://clinicaltrials.gov/ct2/show/NCT00464737
- 65.Fibromyalgia study in adults. https://clinicaltrials.gov/ct2/show/NCT00256893
- 66.Pramipexole ER vs. placebo in fibromyalgia. https://clinicaltrials.gov/ct2/show/NCT00689052
- 67.Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J. Pain. 2008;9(2):164–173. doi: 10.1016/j.jpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth. Analg. 2010;110(2):604–610. doi: 10.1213/ANE.0b013e3181c76f70. [DOI] [PubMed] [Google Scholar]
- 69.Kahan M, Srivastava A, Spithoff S, Bromley L. Prescribing smoked cannabis for chronic noncancer pain: preliminary recommendations. Can. Fam. Physician. 2014;60(12):1083–1090. [PMC free article] [PubMed] [Google Scholar]
- 70.Hill KP. Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA. 2015;313(24):2474–2483. doi: 10.1001/jama.2015.6199. [DOI] [PubMed] [Google Scholar]
- 71.Carter LP, Pardi D, Gorsline J, Griffiths RR. Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse. Drug Alcohol Depend. 2009;104(1–2):1–10. doi: 10.1016/j.drugalcdep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Sodium oxybate: updates and correction to previously published safety data. J. Clin. Sleep Med. 2011;7(4):415–416. doi: 10.5664/JCSM.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pardi D, Black J. gamma-Hydroxybutyrate/sodium oxybate: neurobiology, and impact on sleep and wakefulness. CNS Drugs. 2006;20(12):993–1018. doi: 10.2165/00023210-200620120-00004. [DOI] [PubMed] [Google Scholar]
- 74.Scharf MB, Baumann M, Berkowitz DV. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J. Rheumatol. 2003;30(5):1070–1074. [PubMed] [Google Scholar]
- 75.Russell IJ, Perkins AT, Michalek JE. Sodium oxybate relieves pain and improves function in fibromyalgia syndrome: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Arthritis Rheum. 2009;60(1):299–309. doi: 10.1002/art.24142. [DOI] [PubMed] [Google Scholar]
- 76.Russell IJ, Holman AJ, Swick TJ, Alvarez-Horine S, Wang YG, Guinta D. Sodium oxybate reduces pain, fatigue, and sleep disturbance and improves functionality in fibromyalgia: results from a 14-week, randomized, double-blind, placebo-controlled study. Pain. 2011;152(5):1007–1017. doi: 10.1016/j.pain.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 77.Spaeth M, Bennett RM, Benson BA, Wang YG, Lai C, Choy EH. Sodium oxybate therapy provides multidimensional improvement in fibromyalgia: results of an international Phase 3 trial. Ann. Rheum. Dis. 2012;71(6):935–942. doi: 10.1136/annrheumdis-2011-200418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.2013. www.fda.gov/ohrms/dockets/dockets/05n0479/05N-0479-EC9-Attach-2.pdf Food and Drug Administration (04-03), Xyrem® (sodium oxybate)
- 79.Alarcon GS. Questioning the likelihood that sodium oxybate can be used to successfully treat fibromyalgia: comment on the article by Russell et al . Arthritis Rheum. 2009;60(9):2854. doi: 10.1002/art.24815. [DOI] [PubMed] [Google Scholar]
- 80.Moldofsky H, Inhaber NH, Guinta DR, Alvarez-Horine SB. Effects of sodium oxybate on sleep physiology and sleep/wake-related symptoms in patients with fibromyalgia syndrome: a double-blind, randomized, placebo-controlled study. J. Rheumatol. 2010;37(10):2156–2166. doi: 10.3899/jrheum.091041. [DOI] [PubMed] [Google Scholar]
- 81.US FDA. Bethesda, MD, USA: 2010. Summary Minutes of the Joint Meeting of the Aruthritis Advisory Committee and the Drug Safety and Risk Management Advisory Committee.www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM227134.pdf [Google Scholar]
- 82.Sutherland AM, Clarke HA, Katz J, Katznelson R. Hyperbaric oxygen therapy: a new treatment for chronic pain? Pain Pract. 2015 doi: 10.1111/papr.12312. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 83.Mengshoel AM, Vollestad NK, Forre O. Pain and fatigue induced by exercise in fibromyalgia patients and sedentary healthy subjects. Clin. Exp. Rheumatol. 1995;13(4):477–482. [PubMed] [Google Scholar]
- 84.Kelley GA, Kelley KS, Jones DL. Efficacy and effectiveness of exercise on tender points in adults with fibromyalgia: a meta-analysis of randomized controlled trials. Arthritis. 2011;2011:125485. doi: 10.1155/2011/125485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones KD, Adams D, Winters-Stone K, Burckhardt CS. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988–2005) Health Qual. Life Outcomes. 2006;4:67. doi: 10.1186/1477-7525-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hauser W, Klose P, Langhorst J, et al. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2010;12(3):R79. doi: 10.1186/ar3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Busch AJ, Webber SC, Brachaniec M, et al. Exercise therapy for fibromyalgia. Curr. Pain Headache Rep. 2011;15(5):358–367. doi: 10.1007/s11916-011-0214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu W, Zahner L, Cornell M, et al. Benefit of Qigong exercise in patients with fibromyalgia: a pilot study. Int. J. Neurosci. 2012;122(11):657–664. doi: 10.3109/00207454.2012.707713. [DOI] [PubMed] [Google Scholar]
- 89.Lynch M, Sawynok J, Hiew C, Marcon D. A randomized controlled trial of qigong for fibromyalgia. Arthritis Res. Ther. 2012;14(4):R178. doi: 10.1186/ar3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jones KD, Sherman CA, Mist SD, Carson JW, Bennett RM, Li F. A randomized controlled trial of 8-form Tai chi improves symptoms and functional mobility in fibromyalgia patients. Clin. Rheumatol. 2012;31(8):1205–1214. doi: 10.1007/s10067-012-1996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang C, Schmid CH, Rones R, et al. A randomized trial of tai chi for fibromyalgia. N. Engl. J. Med. 2010;363(8):743–754. doi: 10.1056/NEJMoa0912611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carson JW, Carson KM, Jones KD, Bennett RM, Wright CL, Mist SD. A pilot randomized controlled trial of the Yoga of Awareness program in the management of fibromyalgia. Pain. 2010;151(2):530–539. doi: 10.1016/j.pain.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oka T, Tanahashi T, Chijiwa T, Lkhagvasuren B, Sudo N, Oka K. Isometric yoga improves the fatigue and pain of patients with chronic fatigue syndrome who are resistant to conventional therapy: a randomized, controlled trial. Biopsychosoc. Med. 2014;8(1):27. doi: 10.1186/s13030-014-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mist SD, Firestone KA, Jones KD. Complementary and alternative exercise for fibromyalgia: a meta-analysis. J. Pain Res. 2013;6:247–260. doi: 10.2147/JPR.S32297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bote ME, Garcia JJ, Hinchado MD, Ortega E. An exploratory study of the effect of regular aquatic exercise on the function of neutrophils from women with fibromyalgia: role of IL-8 and noradrenaline. Brain Behav. Immun. 2014;39:107–112. doi: 10.1016/j.bbi.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Ortega E, Bote ME, Giraldo E, Garcia JJ. Aquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patients. Scand. J. Med. Sci. Sports. 2012;22(1):104–112. doi: 10.1111/j.1600-0838.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 97.Bote ME, Garcia JJ, Hinchado MD, Ortega E. Fibromyalgia: anti-inflammatory and stress responses after acute moderate exercise. PLoS ONE. 2013;8(9):e74524. doi: 10.1371/journal.pone.0074524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flodin P, Martinsen S, Mannerkorpi K, et al. Normalization of aberrant resting state functional connectivity in fibromyalgia patients following a three month physical exercise therapy. Neuroimage Clin. 2015;9:134–139. doi: 10.1016/j.nicl.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones KD, Burckhardt CS, Deodhar AA, Perrin NA, Hanson GC, Bennett RM. A six-month randomized controlled trial of exercise and pyridostigmine in the treatment of fibromyalgia. Arthritis Rheum. 2008;58(2):612–622. doi: 10.1002/art.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bernardy K, Fuber N, Klose P, Hauser W. Efficacy of hypnosis/guided imagery in fibromyalgia syndrome – a systematic review and meta-analysis of controlled trials. BMC Musculoskelet. Disord. 2011;12:133. doi: 10.1186/1471-2474-12-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okifuji A, Hare BD. Mangement of musculoskeletal pain. In: Ebert MH, Kerns RD, editors. Behavioral and Pharmacologic Pain Management. Cambridge University Press; Cambridge, UK: 2010. [Google Scholar]
- 102.Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of postintervention and 3-year follow-up benefits in well-being. Psychother. Psychosom. 2007;76(4):226–233. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. Pain. 2011;152(2):361–369. doi: 10.1016/j.pain.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 104.Davis MC, Zautra AJ. An online mindfulness intervention targeting socioemotional regulation in fibromyalgia: results of a randomized controlled trial. Ann. Behav. Med. 2013;46(3):273–284. doi: 10.1007/s12160-013-9513-7. [DOI] [PubMed] [Google Scholar]
- 105.Okifuji A, Turk DC. Behavioral and cognitive-behavioral approaches to treating patients with chronic pain: thinking outside the pill box. J. Rational Cog. Psychol. 2015;33(3):218–238. [Google Scholar]
- 106.Alda M, Luciano JV, Andres E, et al. Effectiveness of cognitive behaviour therapy for the treatment of catastrophisation in patients with fibromyalgia: a randomised controlled trial. Arthritis Res. Ther. 2011;13(5):R173. doi: 10.1186/ar3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wicksell RK, Kemani M, Jensen K, et al. Acceptance and commitment therapy for fibromyalgia: a randomized controlled trial. Eur. J. Pain. 2013;17(4):599–611. doi: 10.1002/j.1532-2149.2012.00224.x. [DOI] [PubMed] [Google Scholar]
- 108.Bernardy K, Fuber N, Kollner V, Hauser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome – a systematic review and metaanalysis of randomized controlled trials. J. Rheumatol. 2010;37(10):1991–2005. doi: 10.3899/jrheum.100104. [DOI] [PubMed] [Google Scholar]
- 109.Karlsson B, Burell G, Anderberg U, Svardsudd K. Cognitive behaviour therapy in women with fibromyalgia: a randomized clinical trial. Scand. J. Pain. 2015;9:11–21. doi: 10.1016/j.sjpain.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 110.Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know, and what we need to know. Best Pract. Res. Clin. Rheumatol. 2003;17(4):685–701. doi: 10.1016/s1521-6942(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 111.Bennett RM. Multidisciplinary group programs to treat fibromyalgia patients. Rheum. Dis. Clin. North Am. 1996;22(2):351–367. doi: 10.1016/s0889-857x(05)70276-3. [DOI] [PubMed] [Google Scholar]
- 112.Goldenberg DL, Clauw DJ, Fitzcharles MA. New concepts in pain research and pain management of the rheumatic diseases. Semin. Arthritis Rheum. 2011;41(3):319–334. doi: 10.1016/j.semarthrit.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 113.Scascighini L, Toma V, Dober-Spielmann S, Sprott H. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology (Oxford) 2008;47(5):670–678. doi: 10.1093/rheumatology/ken021. [DOI] [PubMed] [Google Scholar]
- 114.Martin J, Torre F, Padierna A, et al. Six-and 12-month follow-up of an interdisciplinary fibromyalgia treatment programme: results of a randomised trial. Clin. Exp. Rheumatol. 2012;30(6 Suppl. 74):103–111. [PubMed] [Google Scholar]
- 115.Arnold LM, Clauw DJ, Dunegan LJ, Turk DC. A framework for fibromyalgia management for primary care providers. Mayo Clin. Proc. 2012;87(5):488–496. doi: 10.1016/j.mayocp.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ang DC, Jensen MP, Steiner JL, Hilligoss J, Gracely RH, Saha C. Combining cognitive–behavioral therapy and milnacipran for fibromyalgia: a feasibility randomized-controlled trial. Clin. J. Pain. 2013;29(9):747–754. doi: 10.1097/AJP.0b013e31827a784e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loeser JD. Comprehensive pain programs versus other treatments for chronic pain. J. Pain. 2006;7(11):800–801. doi: 10.1016/j.jpain.2006.09.008. discussion 804–806. [DOI] [PubMed] [Google Scholar]
- 118.Alaujan S, Elliott R, Knaggs R. Systematic review of economic evaluations in multidisciplinary pain management services for managing people with fibromyalgia or chronic widespread pain. Value Health. 2015;18(7):A660. [Google Scholar]
- 119.Hooten WM, Townsend CO, Sletten CD, Bruce BK, Rome JD. Treatment outcomes after multidisciplinary pain rehabilitation with analgesic medication withdrawal for patients with fibromyalgia. Pain Med. 2007;8(1):8–16. doi: 10.1111/j.1526-4637.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 120.Jones KD, Bennett RM, Ward RL, Deodhar AA. Description of a half-day interprofessional fibromyalgia clinic with an evaluation of patient satisfaction. Am. J. Phys. Med. Rehabil. 2011;90(10):825–833. doi: 10.1097/PHM.0b013e31821f6ed3. [DOI] [PubMed] [Google Scholar]
- 121.Pfeiffer A, Thompson JM, Nelson A, et al. Effects of a 1.5-day multidisciplinary outpatient treatment program for fibromyalgia: a pilot study. Am. J. Phys. Med. Rehabil. 2003;82(3):186–191. doi: 10.1097/01.PHM.0000046625.72055.35. [DOI] [PubMed] [Google Scholar]
- 122.Magis D, Jensen R, Schoenen J. Neurostimulation therapies for primary headache disorders: present and future. Curr. Opin. Neurol. 2012;25(3):269–276. doi: 10.1097/WCO.0b013e3283532023. [DOI] [PubMed] [Google Scholar]
- 123.Thimineur M, De Ridder D. C2 area neurostimulation: a surgical treatment for fibromyalgia. Pain Med. 2007;8(8):639–646. doi: 10.1111/j.1526-4637.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 124.Plazier M, Dekelver I, Vanneste S, et al. Occipital nerve stimulation in fibromyalgia: a double-blind placebo-controlled pilot study with a six-month follow-up. Neuromodulation. 2014;17(3):256–263. doi: 10.1111/ner.12121. [DOI] [PubMed] [Google Scholar]
- 125.Plazier M, Ost J, Stassijns G, De Ridder D, Vanneste S. C2 Nerve field stimulation for the treatment of fibromyalgia: a prospective, double-blind, randomized, controlled cross-over study. Brain Stimul. 2015;8(4):751–757. doi: 10.1016/j.brs.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 126.Chen YF, Bramley G, Unwin G, et al. Occipital nerve stimulation for chronic migraine-a systematic review and meta-analysis. PLoS ONE. 2015;10(3):e0116786. doi: 10.1371/journal.pone.0116786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bonica J. The Management of Pain. Lea & Febiger; PA, USA: 1953. [Google Scholar]
- 128.Petersen KL, Rowbotham MC. Will ion-channel blockers be useful for management of nonneuropathic pain? J. Pain. 2000;1(3 Suppl.):26–34. doi: 10.1054/jpai.2000.9822. [DOI] [PubMed] [Google Scholar]
- 129.Finnerup NB, Biering-Sorensen F, Johannesen IL, et al. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102(5):1023–1030. doi: 10.1097/00000542-200505000-00023. [DOI] [PubMed] [Google Scholar]
- 130.Tremont-Lukats IW, Challapalli V, Mcnicol ED, Lau J, Carr DB. Systemic administration of local anesthetics to relieve neuropathic pain: a systematic review and meta-analysis. Anesth. Analg. 2005;101(6):1738–1749. doi: 10.1213/01.ANE.0000186348.86792.38. [DOI] [PubMed] [Google Scholar]
- 131.Bennett MI, Tai YM. Intravenous lignocaine in the management of primary fibromyalgia syndrome. Int. J. Clin. Pharmacol. Res. 1995;15(3):115–119. [PubMed] [Google Scholar]
- 132.Posner I. Treatment of fibromyalgia syndrome with intravenous lidocaine: a prospective, randomized pilot study. J. Musculoskel. Pain. 1994;2:55–65. [Google Scholar]
- 133.Schafranski MD, Malucelli T, Machado F, et al. Intravenous lidocaine for fibromyalgia syndrome: an open trial. Clin. Rheumatol. 2009;28(7):853–855. doi: 10.1007/s10067-009-1137-8. [DOI] [PubMed] [Google Scholar]
- 134.Mccleane G. Does intravenous lidocaine reduce fibromyalgia pain? A rancomized double-blind, placebo controlled cross-over study. Pain Clinics. 2000;12(3):181–185. [Google Scholar]
- 135.Vlainich R, Issy AM, Gerola LR, Sakata RK. Effect of intravenous lidocaine on manifestations of fibromyalgia. Pain Pract. 2010;10(4):301–305. doi: 10.1111/j.1533-2500.2010.00362.x. [DOI] [PubMed] [Google Scholar]
- 136.Raphael JH, Southall JL, Treharne GJ, Kitas GD. Efficacy and adverse effects of intravenous lignocaine therapy in fibromyalgia syndrome. BMC Musculoskelet. Disord. 2002;3:21. doi: 10.1186/1471-2474-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gibbons CR, Liu S, Zhang Y, et al. Involvement of brain opioid receptors in the anti-allodynic effect of hyperbaric oxygen in rats with sciatic nerve crush-induced neuropathic pain. Brain Res. 2013;1537:111–116. doi: 10.1016/j.brainres.2013.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wilson HD, Wilson JR, Fuchs PN. Hyperbaric oxygen treatment decreases inflammation and mechanical hypersensitivity in an animal model of inflammatory pain. Brain Res. 2006;1098(1):126–128. doi: 10.1016/j.brainres.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 139.Yildiz S, Kiralp MZ, Akin A, et al. A new treatment modality for fibromyalgia syndrome: hyperbaric oxygen therapy. J. Int. Med. Res. 2004;32(3):263–267. doi: 10.1177/147323000403200305. [DOI] [PubMed] [Google Scholar]
- 140.Efrati S, Golan H, Bechor Y, et al. Hyperbaric oxygen therapy can diminish fibromyalgia syndrome – prospective clinical trial. PLoS ONE. 2015;10(5):e0127012. doi: 10.1371/journal.pone.0127012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Elder GJ, Taylor JP. Transcranial magnetic stimulation and transcranial direct current stimulation: treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimers Res. Ther. 2014;6(9):74. doi: 10.1186/s13195-014-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Galhardoni R, Correia GS, Araujo H, et al. Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature. Arch Phys. Med. Rehabil. 2015;96(4 Suppl.):S156–S172. doi: 10.1016/j.apmr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 143.O'Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst. Rev. 2014;4:CD008208. doi: 10.1002/14651858.CD008208.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Luedtke K, Rushton A, Wright C, Geiss B, Juergens TP, May A. Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: a systematic review and meta-analysis. Clin. J. Pain. 2012;28(5):452–461. doi: 10.1097/AJP.0b013e31823853e3. [DOI] [PubMed] [Google Scholar]
- 145.Boyer L, Dousset A, Roussel P, et al. rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology. 2014;82(14):1231–1238. doi: 10.1212/WNL.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 146.Knijnik LM, Dussan-Sarria JA, Rozisky JR, et al. Repetitive transcranial magnetic stimulation for fibromyalgia: systematic review and meta-analysis. Pain Pract. 2015;16(3):294–304. doi: 10.1111/papr.12276. [DOI] [PubMed] [Google Scholar]
- 147.Castillo-Saavedra L, Gebodh N, Bikson M, et al. Clinically effective treatment of fibromyalgia pain with high-definition transcranial direct current stimulation: Phase II open-label dose optimization. J. Pain. 2015;17(1):14–26. doi: 10.1016/j.jpain.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Okifuji A, Donaldson GW. Use of ecological momentary assessment to monitor fibromyalgia. Pain Manag. 2011;1(3):195–197. doi: 10.2217/pmt.11.15. [DOI] [PubMed] [Google Scholar]
- 149.Rusu A, Boersma K, Turk DC. Ubgroups of pain patients – the potential of customizing treatments. In: Hasenbring M, Rusu A, Turk DC, editors. From Acute to Chronic Back Pain: Risk Factors, Mechanisms, and Clinical Implications. Oxford University Press; Oxford, UK: 2012. [Google Scholar]
- 150.Ventegodt S, Andersen NJ, Brom B, Merrick J, Greydanus DE. Evidence-based medicine: four fundamental problems with the randomized clinical trial (RCT) used to document chemical medicine. Int. J. Adolesc. Med. Health. 2009;21(4):485–496. doi: 10.1515/ijamh.2009.21.4.485. [DOI] [PubMed] [Google Scholar]
- 151.Carey TA, Stiles WB. Some problems with randomized controlled trials and some viable alternatives. Clin. Psychol. Psychother. 2015;23(1):87–95. doi: 10.1002/cpp.1942. [DOI] [PubMed] [Google Scholar]
- 152.Wasserstein RL, Lazar NA. The ASA's statement on p-values: context, process, and purpose. Am. Stat. 2016 Epub ahead of print. [Google Scholar]
- 153.Donaldson GW, Nakamura Y, Moinpour C. Mediators, moderators, and modulators of causal effects in clinical trials – Dynamically Modified Outcomes (DYNAMO) in health-related quality of life. Qual. Life Res. 2009;18(2):137–145. doi: 10.1007/s11136-008-9439-x. [DOI] [PubMed] [Google Scholar]
- 154.Almirall D, Compton SN, Gunlicks-Stoessel M, Duan N, Murphy SA. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Stat. Med. 2012;31(17):1887–1902. doi: 10.1002/sim.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Okifuji A, Bradshaw DH, Donaldson GW, Turk DC. Sequential analyses of daily symptoms in women with fibromyalgia syndrome. J. Pain. 2011;12(1):84–93. doi: 10.1016/j.jpain.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Balasubramaniam R, De Leeuw R, Zhu H, Nickerson RB, Okeson JP, Carlson CR. Prevalence of temporomandibular disorders in fibromyalgia and failed back syndrome patients: a blinded prospective comparison study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007;104(2):204–216. doi: 10.1016/j.tripleo.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 157.Kato K, Sullivan PF, Evengard B, Pedersen NL. Chronic widespread pain and its comorbidities: a population-based study. Arch Intern Med. 2006;166(15):1649–1654. doi: 10.1001/archinte.166.15.1649. [DOI] [PubMed] [Google Scholar]
- 158.Davis JA, Robinson RL, Le TK, Xie J. Incidence and impact of pain conditions and comorbid illnesses. J. Pain Res. 2011;4:331–345. doi: 10.2147/JPR.S24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cakit BD, Taskin S, Nacir B, Unlu I, Genc H, Erdem HR. Comorbidity of fibromyalgia and cervical myofascial pain syndrome. Clin. Rheumatol. 2010;29(4):405–411. doi: 10.1007/s10067-009-1342-5. [DOI] [PubMed] [Google Scholar]
- 160.Hartzell MM, Neblett R, Perez Y, Brede E, Mayer TG, Gatchel RJ. Do comorbid fibromyalgia diagnoses change after a functional restoration program in patients with chronic disabling occupational musculoskeletal disorders? Spine (Phila. Pa 1976) 2014;39(17):1393–1400. doi: 10.1097/BRS.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 161.Moss P, Knight E, Wright A. Subjects with knee osteoarthritis exhibit widespread hyperalgesia to pressure and cold. PLoS ONE. 2016;11(1):e0147526. doi: 10.1371/journal.pone.0147526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Giamberardino MA, Affaitati G, Fabrizio A, Costantini R. Effects of treatment of myofascial trigger points on the pain of fibromyalgia. Curr. Pain Headache Rep. 2011;15(5):393–399. doi: 10.1007/s11916-011-0205-3. [DOI] [PubMed] [Google Scholar]
- 163.Kirsch I, Sapirstein G. Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prev. Treat. 1998;1(2):2a. [Google Scholar]
- 164.Colloca L, Miller FG. Harnessing the placebo effect: the need for translational research. Philos. Trans. R Soc. Lond. B Biol. Sci. 2011;366(1572):1922–1930. doi: 10.1098/rstb.2010.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roose SP, Schatzberg AF. The efficacy of antidepressants in the treatment of late-life depression. J. Clin. Psychopharmacol. 2005;25(4 Suppl. 1):S1–S7. doi: 10.1097/01.jcp.0000162807.84570.6b. [DOI] [PubMed] [Google Scholar]
- 166.Schmidt L, Braun EK, Wager TD, Shohamy D. Mind matters: placebo enhances reward learning in Parkinson's disease. Nat. Neurosci. 2014;17(12):1793–1797. doi: 10.1038/nn.3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pecina M, Zubieta JK. Molecular mechanisms of placebo responses in humans. Mol. Psychiatry. 2015;20(4):416–423. doi: 10.1038/mp.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gollwitzer PM, Sheeran P. Implementation intentions and goal achievement: a meta-analysis of effects and processes. Adv. Exp. Soc. Psychol. 2006;38:69–119. [Google Scholar]
- 169.Glombiewski JA, Hartwich-Tersek J, Rief W. Attrition in cognitive-behavioral treatment of chronic back pain. Clin. J. Pain. 2010;26(7):593–601. doi: 10.1097/AJP.0b013e3181e37611. [DOI] [PubMed] [Google Scholar]
- 170.Thompson E, Broadbent J, Bertino MD, Staiger PK. Do pain-related beliefs influence treatment adherence? a systematic review. Clin. J. Pain. 2015;32(2):164–178. doi: 10.1097/AJP.0000000000000235. [DOI] [PubMed] [Google Scholar]
- 171.Burton LC, Shapiro S, German PS. Determinants of physical activity initiation and maintenance among community-dwelling older persons. Prev. Med. 1999;29(5):422–430. doi: 10.1006/pmed.1999.0561. [DOI] [PubMed] [Google Scholar]
- 172.Centers for Disease Control and Prevention: Overcoming Barriers to Physical Activity. 2011. www.cdc.gov/physicalactivity/basics/adding-pa/barriers.html
- 173.Richards SC, Scott DL. Prescribed exercise in people with fibromyalgia: parallel group randomised controlled trial. BMJ. 2002;325(7357):185. doi: 10.1136/bmj.325.7357.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schachter CL, Busch AJ, Peloso PM, Sheppard MS. Effects of short versus long bouts of aerobic exercise in sedentary women with fibromyalgia: a randomized controlled trial. Phys. Ther. 2003;83(4):340–358. [PubMed] [Google Scholar]
- 175.Bair MJ, Matthias MS, Nyland KA, et al. Barriers and facilitators to chronic pain self-management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med. 2009;10(7):1280–1290. doi: 10.1111/j.1526-4637.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Coppack RJ, Kristensen J, Karageorghis CI. Use of a goal setting intervention to increase adherence to low back pain rehabilitation: a randomized controlled trial. Clin. Rehabil. 2012;26(11):1032–1042. doi: 10.1177/0269215512436613. [DOI] [PubMed] [Google Scholar]
- 177.Escolar-Reina P, Medina-Mirapeix F, Gascon-Canovas JJ, Montilla-Herrador J, Valera-Garrido JF, Collins SM. Self-management of chronic neck and low back pain and relevance of information provided during clinical encounters: an observational study. Arch. Phys. Med. Rehabil. 2009;90(10):1734–1739. doi: 10.1016/j.apmr.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 178.Miller W. Motivational interviewing with problem drinkers. Behav. Psychother. 1983;11:147–172. [Google Scholar]
- 179.Okifuji A, Turk DC. Engagement of patients in the self-management of pain. In: O'Donohue W, James L, Snipes C, editors. Practical Strategies and Tools to Promote Treatment Engagemen. Springer; NY, USA: In Press. [Google Scholar]
- 180.Jensen MP. Enhancing motivation to change in pain treatment. In: Turk DC, Gatchel RJ, editors. Psychological Approaches to Pain Management: a Practitioner's Handbook. Guilford Press; NY, USA: 2002. pp. 71–93. [Google Scholar]
- 181.Okifuji A, Turk DC. Motivating pain patients for behavioral change. In: Fishman SM, Ballantyne JC, Rathmell JP, editors. Bonica's Management of Pain. Lippincott Williams & Wilkins Media; PA, USA: 2009. [Google Scholar]
- 182.Ang DC, Kaleth AS, Bigatti S, et al. Research to encourage exercise for fibromyalgia (REEF): use of motivational interviewing, outcomes from a randomized-controlled trial. Clin. J. Pain. 2013;29(4):296–304. doi: 10.1097/AJP.0b013e318254ac76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tse MM, Vong SK, Tang SK. Motivational interviewing and exercise programme for community-dwelling older persons with chronic pain: a randomised controlled study. J. Clin. Nurs. 2013;22(13–14):1843–1856. doi: 10.1111/j.1365-2702.2012.04317.x. [DOI] [PubMed] [Google Scholar]
- 184.Vong SK, Cheing GL, Chan F, So EM, Chan CC. Motivational enhancement therapy in addition to physical therapy improves motivational factors and treatment outcomes in people with low back pain: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2011;92(2):176–183. doi: 10.1016/j.apmr.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 185.Chang YP, Compton P, Almeter P, Fox CH. The effect of motivational interviewing on prescription opioid adherence among older adults with chronic pain. Perspect. Psychiatr. Care. 2014;51(3):211–219. doi: 10.1111/ppc.12082. [DOI] [PubMed] [Google Scholar]
- 186.Belanger-Gravel A, Godin G, Bilodeau A, Poirier P. The effect of implementation intentions on physical activity among obese older adults: a randomised control study. Psychol. Health. 2013;28(2):217–233. doi: 10.1080/08870446.2012.723711. [DOI] [PubMed] [Google Scholar]
- 187.Arbour KP, Martin Ginis KA. A randomised controlled trial of the effects of implementation intentions on women's walking behaviour. Psychol. Health. 2009;24(1):49–65. doi: 10.1080/08870440801930312. [DOI] [PubMed] [Google Scholar]
- 188.Sniehotta FF, Scholz U, Schwarzer R. Action plans and coping plans for physical exercise: a longitudinal intervention study in cardiac rehabilitation. Br. J. Health Psychol. 2006;11(Pt 1):23–37. doi: 10.1348/135910705X43804. [DOI] [PubMed] [Google Scholar]
- 189.Thoolen BJ, De Ridder D, Bensing J, Gorter K, Rutten G. Beyond good intentions: The role of proactive coping in achieving sustained behavioural change in the context of diabetes management. Psychol. Health. 2009;24(3):237–254. doi: 10.1080/08870440701864504. [DOI] [PubMed] [Google Scholar]
- 190.Belanger-Gravel A, Godin G, Amireault S. A meta-analytic review of the effect of implementation intentions on physical activity. Health Psychol. Rev. 2013;7(1):23–54. [Google Scholar]
- 191.Wilson HD, Starz TW, Robinson JP, Turk DC. Heterogeneity within the fibromyalgia population: theoretical implications of variable tender point severity ratings. J. Rheumatol. 2009;36(12):2795–2801. doi: 10.3899/jrheum.090432. [DOI] [PubMed] [Google Scholar]
- 192.Thieme K, Turk DC. Heterogeneity of psychophysiological stress responses in fibromyalgia syndrome patients. Arthritis Res. Ther. 2006;8(1):R9. doi: 10.1186/ar1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nuesch E, Hauser W, Bernardy K, Barth J, Juni P. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: network meta-analysis. Ann. Rheum. Dis. 2013;72(6):955–962. doi: 10.1136/annrheumdis-2011-201249. [DOI] [PubMed] [Google Scholar]