Abstract
Aims
Longitudinal determinants of aortic stiffness (AS) measured by magnetic resonance imaging (MRI) have not been assessed in a large community-based population. Our aim was to examine the determinants of change in thoracic AS over 10 years of follow-up in a multi-ethnic population of individuals 45 years of age and older measured by MRI.
Methods and results
We studied 1160 participants (mean age = 60 ± 9 years at baseline, 45% male) with aortic MRI at both the MESA Year 0 and Year 10 examinations. Ascending and descending aorta distensibility (AAD/DAD) and aortic arch pulse-wave velocity (PWV) were measured using MRI. Determinants of the change in AS parameters over 10 years were assessed using linear regression adjusted for baseline values, demographic variables, baseline risk factors and change in risk factors, and chronic risk exposure. AAD and DAD decreased slightly (5% decrease in median for AAD: 1.33–1.26 mmHg−1 · 10−3, P = 0.008; 5% decrease in median for DAD: 1.73–1.64 mmHg−1 · 10−3, P < 0.001), and PWV increased over 10 years (18% increase in median: 6.8–8.0 m/s P < 0.001). Baseline age was related to a reduction in AAD and DAD and an increase in PWV throughout the follow-up period. Baseline and change in mean blood pressure and continued smoking were associated with a reduction in AAD and an increase in PWV. Furthermore, baseline heart rate was also related to a reduction in AAD and DAD. Blood pressure normalization was related to less aortic stiffening throughout the follow-up period.
Conclusions
In our longitudinal, community-based cohort study of adult individuals aged 45 years or greater, greater mean blood pressure and a history of smoking history were associated with increased aortic stiffening over 10 years as assessed by MRI.
Keywords: aortic stiffness, magnetic resonance imaging, aortic distensibility, pulse-wave velocity, cardiovascular risk
Introduction
Arterial stiffness is associated with cardiovascular morbidity and mortality.1–3 Stiffening of large arteries is a common feature of aging and is also accelerated with exposure to traditional cardiovascular risk factors, such as hypertension3 and diabetes.4
Magnetic resonance imaging (MRI) has the unique ability to evaluate large arterial stiffness non-invasively with a great level of accuracy and reproducibility, including aortic distensibility (AD) and arch pulse-wave velocity (PWV).5–7 Aortic distensibility, reflecting local biomechanical arterial wall alterations, has been shown to be a sensitive marker of age-related aortic stiffening in the general population, especially before the fifth decade.5 It is measured as changes in aortic diameter or cross-sectional area relative to blood pressure changes during the cardiac cycle. On the other hand, arterial PWV reflects more advanced alterations of material properties involving the entire vessel. Carotid-femoral PWV (cf-PWV) using arterial applanation tonometry has emerged as the gold standard method because of its relative ease in determination and its perceived reliability.3,8 However, cf-PWV ignores stiffness of the aortic arch that provides nearly half of total arterial compliance.9 In this regard, MRI allows measurement of the aortic arch PWV.10
Some cross-sectional studies have assessed the correlates of aortic stiffness (AS) assessed by different methods;11,12 however, there are few studies to assess the correlates of aortic arch PWV assessed by MRI. In addition, no previous studies have evaluated the longitudinal determinants of AS by MRI in a large community-based population. The availability of MRI aortic studies in the large population of the multi-ethnic study of atherosclerosis (MESA) allows longitudinal assessment of AS in relation to risk factor exposure and other subclinical and clinical variables. This knowledge is crucial to elucidate the mechanisms that underlie arterial stiffening and may be useful in primary prevention and risk stratification for cardiovascular events. Therefore, the aim of the present study was to evaluate the change in AS assessed by MRI over 10 years and to define its relationship with risk factor exposure and clinical evolution in MESA. These assessments could also provide important information for clinical care of patients with cardiovascular diseases.
Method
Study population
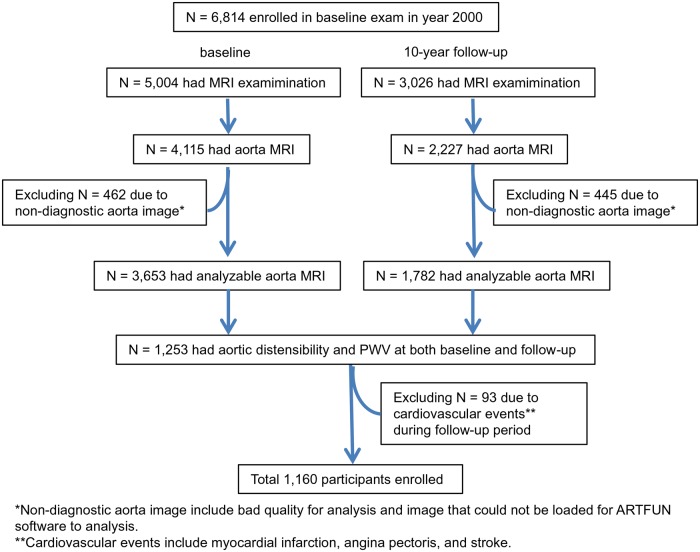
The MESA is described elsewhere.13 In summary, between 2000 and 2002, 6814 men and women 45–84 years of age without clinical cardiovascular disease (CVD) who identified themselves as white, African American, Hispanic, or Chinese were recruited from 6 US communities (Winston-Salem, New York, Baltimore, Minneapolis, Chicago, and Los Angeles). In the longitudinal follow-up of the fifth examination (from April 2010 to February 2012), 3015 participants underwent cardiac magnetic resonance (CMR) imaging. For this analysis, we included 1160 MESA participants with adequate aortic MRI at both the baseline and 10-year follow-up visit after excluding those who had cardiovascular events during follow-up (Figure 1). All participants gave informed consent for the study protocol, which was approved by the institutional review boards of all MESA field centres and the CMR reading centre.
Figure 1.
Participant enrolment diagram for the present study.
Aortic MRI
MRI images at both baseline and 10-year follow-up were acquired with 1.5 T scanners. Gradient echo phase-contrast cine MRI (PC-CMR) was performed to evaluate aortic flow and aortic area. Images of the ascending and descending aorta were obtained in the transverse plane perpendicular to the aortic lumen at the level of the right pulmonary artery. Imaging parameters were the following: repetition time: 45 ms, echo time: 3.5 ms, flip angle: 20°, field of view: 300 mm, slice thickness: 8 mm, matrix: 256 × 256 for baseline and 128 × 128 interpolated to 256 × 256 for follow-up, temporal resolution: 50 ms for baseline and 20 ms for follow-up per cardiac cycle, encoding velocity: 150 cm/s, and bandwidth: 245 Hz/pixel.
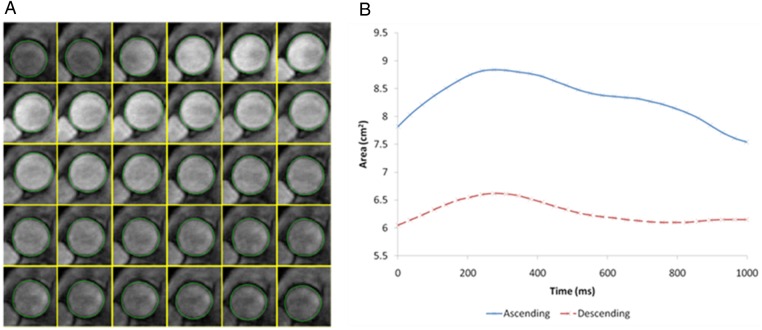
To determine aortic strain and distensibility, the maximum and minimum cross-sectional areas of the ascending and descending aorta were measured with automated software (ARTFUN, INSERM LIM) as described by Herment et al.14 and used by Redheuil et al.5 (Figure 2). Aortic strain and distensibility were then calculated as follows:
Figure 2.
Aortic strain assessment with MRI. (A) Automatic tracking of aortic contour on transverse plane at the level of the right pulmonary artery. (B) Temporal curve of aortic areas obtained after automatic tracking.
PPmri is the brachial pulse pressure (PP) measured from the averaged systolic and diastolic pressure measured before and after MRI acquisition.
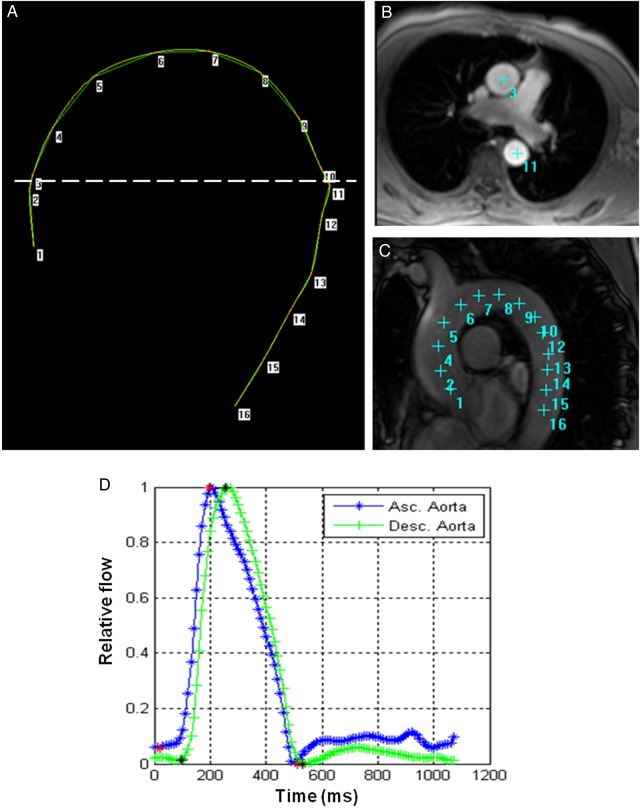
Transit time was calculated as the average time difference, using the least squares estimate, between all data points on the systolic upslope of the ascending and descending aortic flow curves after peak flow normalization. Using the oblique sagittal view acquired with a black blood pulse sequence through the thoracic aorta, the distance between ascending and descending aorta was obtained at the precise locations where the through plane velocities were measured (Figure 2). Aortic arch PWV was then calculated as follows (Figure 3):
Figure 3.
Aortic arch PWV assessment with MRI. (A) Ascending and descending flow curves before peak flow normalization (upper) and after peak flow normalization (lower). Δt was estimated as the average time difference using the least squares estimate between all data points on the systolic slope of the ascending and descending aortic flow curves after peak normalization. (B) Measurement of the transit distance (D) in the aortic arch. Numbers are corresponding to those in c and d. Arch length is measured as the distance from 3–10 in this case. (C) Aortic arch view with black blood sequence. (D) PC cine transverse view on phase-contrast cine MRI.
Intra- and inter-observer reproducibility of AAD, DAD, and PWV was excellent with intraclass correlation coefficient (ICC) ranging from 0.85 for AAD, 0.94 for DAD, 0.96 for PWV in intra-observer, and 0.70 for AAD, 0.82 for DAD, and 0.90 for PWV in inter-observer reproducibility in 30 random subjects. The previous study in MESA demonstrated that inter-study reproducibility of measurements for AS was acceptable.15
Measures of CVD risk factors
All participants completed standardized questionnaires to obtain information about smoking history, medication usage, diagnosis of high cholesterol, and diabetes. Height and weight were measured, and resting systolic (SBP) and diastolic (DBP) blood pressures were measured three times with participants in the seated position with an automated oscillometric sphygmomanometer. The average of the last two measurements was used in analysis. Mean brachial blood pressure (MBP) was calculated as (2 × DBP + SBP)/3. Brachial PP was calculated as (SBP – DBP). Hypertension was defined as SBP ≥140 mmHg, DBP ≥90 mmHg, or current use of antihypertensive medications. Low- (LDL) and high-density lipoprotein (HDL) and glucose levels were measured from blood samples obtained after a 12 h fast. Diabetes mellitus was defined as fasting glucose ≥126 mg/dl or use of insulin or oral hypoglycaemic medication.
Statistical analysis
Continuous variables were shown as mean ± SD unless otherwise specified and categorical variables as percentages. Normality was evaluated by the Shapiro–Wilk test and quantile–quantile plots. Comparisons between groups were assessed using Student's t-test for normally and the Mann–Whitney U test for non-normally distributed data. Distributions of AD and PWV were skewed, so they were presented as median and interquartile range and logarithmically transformed for linear regression models (logAAD, logDAD, and logPWV, respectively). Categorical variables were compared between groups using the χ2 test.
Determinants of change in AS parameters were assessed using multivariable linear regression. Dependent variables were changes in AS (△logAAD: Year 10 logAAD – Year 0 logAAD; △logDAD: Year 10 logDAD – Year 0 logDAD; △logPWV: Year 10 logPWV – Year −0 logPWV). Models were constructed as follows: change in AS = demographic variables (age, gender, race) + baseline risk factors (mean blood pressure, BMI, heart rate, LDL, HDL) + change in risk factors (i.e. 10-year difference in each values) + chronic risk exposure (antihypertensive drug use, smoking, diabetes) + baseline AS value. To assess the potential confounding effect of aortic area on relationship of change in AS with risk factors, further adjustments for baseline and change in aortic area were done. Models that alternatively included SBP or DBP instead of mean blood pressure were also evaluated. Multiple linear regression models were also used to assess the relationship of change in PWV with change in AD (AAD and DAD, separately).
Further analysis was performed to assess the relationship of blood pressure change with change in AS. We used 140 mmHg for SBP as the cut-off point based on the Joint National Committee-6 criteria for systolic hypertension. Four categories were defined: < 140 mmHg at both Year 0 (baseline) and Year 10 (follow-up) (consistently normal BP; reference), <140 mmHg at Year 0 and ≥140 mmHg at Year 10 (normal to high BP), ≥140 mmHg at Year 0 and <140 mmHg at Year 10 (high to normal BP), and ≥140 mmHg at both Year 0 and Year 10 (consistently high BP). AS parameters were compared across categories using one-way ANOVA and posterior multiple comparisons analysis with the Bonferroni correction. Adjusted means for change in AS in each category were determined using the multiple linear regression models described above. Finally, we assessed the relationship of the each type of antihypertensive medication (angiotensin converting enzyme inhibitors: ACEI, angiotensin II receptor blockers: ARB, calcium-channel blocker: CCB, beta-blocker, and diuretic) with change in AS using the multiple linear regression model. We also assess the relationship of use of lipid-lowering drug with change in AS.
A two-tailed P-value of <0.05 was considered statistically significant. All statistical analyses were performed using STATA version 12.0 (Stata Corp LP, College Station, TX).
Results
MESA participant characteristics
A total of 1160 participants included in the study was younger (60 vs. 63 years), tended to be more Chinese (21 vs. 10%) and less Hispanic (9 vs. 24%), and overall to have a lower risk profile such as hypertension (38 vs. 46%), diabetes (7 vs. 14%), or current smoking (9 vs. 14%) than the subgroup of 5654 participants who were not included. Demographics and clinical characteristics for the cohort at baseline and at the Year 10 follow-up examination are presented in Table 1. During the 10-year follow-up period, there was an increase in the prevalence of hypertension and diabetes mellitus, an increase in anti-hypertension medication use, and a decrease in the prevalence of tobacco use. LDL cholesterol decreased and HDL increased during follow-up in association with increased use of lipid-lowering therapy. SBP, DBP, and mean blood pressure decreased in association with the increase in antihypertensive therapy, whereas PP increased accompanied by a marked reduction in DBP compared with SBP.
Table 1.
Population characteristics (n = 1160)
| Characteristics | Baseline | 10-Year follow-up | P-value |
|---|---|---|---|
| Age, year | 59.5 (9.4) | 69.0 (9.3) | |
| Men, % | 527 (45%) | – | |
| Ethnicity, % | |||
| White | 485 (42%) | – | |
| Chinese | 240 (21%) | – | |
| Black | 326 (28%) | – | |
| Hispanic | 109 (9%) | – | |
| BMI, kg m−2 | 27.4 (5.0) | 27.6 (5.2) | 0.01 |
| Hypertension, % | 445 (38%) | 648 (56%) | <0.001 |
| Antihypertensive medication, % | 371 (32%) | 607 (52%) | <0.001 |
| ACEI/ARB | 162 (14%) | 349 (30%) | <0.001 |
| CCB | 126 (11%) | 207 (18%) | <0.001 |
| Beta-blockers | 91 (8%) | 185 (16%) | <0.001 |
| Alpha-blockers | 49 (4%) | 36 (3%) | <0.001 |
| Diuretics | 129 (11%) | 220 (19%) | <0.001 |
| Lipid-lowering medication, % | 173 (15%) | 410 (35%) | <0.001 |
| Diabetes status, % | 87 (7%) | 188 (16%) | <0.001 |
| Current smoking status, % | 106 (9%) | 80 (7%) | <0.001 |
| LDL cholesterol, mg/dl | 116.2 (30.2) | 108.3 (31.5) | <0.001 |
| HDL cholesterol, mg/dl | 51.5 (15.0) | 56.9 (17.0) | <0.001 |
| Blood pressures | |||
| SBP, mmHg | 123.8 (20.4) | 122.6 (20.2) | 0.06 |
| DBP, mmHg | 71.7 (10.4) | 67.9 (10.0) | <0.001 |
| MBP, mmHg | 89.1 (12.5) | 86.2 (11.7) | <0.001 |
| PP, mmHg | 52.1 (15.8) | 54.7 (16.9) | <0.001 |
| HR, bpm | 61.9 (8.7) | 64.1 (10.3) | <0.001 |
| Aortic parameters | |||
| Minimum ascending aortic area, cm2 | 7.6 (1.9) | 8.4 (2.0) | <0.001 |
| AAD, mmHg−1 · 10−3 | 1.34 (0.83–2.22) | 1.28 (0.84–1.95) | 0.003 |
| Minimum descending aortic area, cm−2 | 4.2 (1.2) | 4.7 (1.2) | <0.001 |
| DAD, mmHg−1 · 10−3 | 1.75 (1.11–2.82) | 1.64 (1.13–2.40) | <0.001 |
| Aortic arch PWV, m/s | 6.7 (5.2–9.3) | 8.1 (6.5–10.0) | <0.001 |
Values are median (interquartile range) for AAD, DAD, and PWV, and mean (SD) for others, or %. BMI indicates body mass index; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CCB, calcium-channel blocker; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; PP, pulse pressure; AAD, ascending aorta distensibility; DAD, descending aorta distensibility; PWV, pulse-wave velocity.
Areas of both ascending and descending aorta increased during follow-up. Aortic distensibility decreased slightly at both ascending and descending levels (5% decrease in median for AAD: 1.34–1.28 mmHg−1 · 10−3, P = 0.003; 5% decrease in median for DAD: 1.75–1.64 mmHg−1 · 10−3, P < 0.001). PWV increased over the 10-year follow-up period (18% increase in median: 6.7–8.1 m/s, P < 0.001) (Table 1).
Longitudinal analysis of the relationship between change in aortic stiffness and risk factors
Determinants of change in AS from linear regression models are presented in Table 2. Older age at baseline was a significant determinant for decreased AAD/DAD and increased PWV (B = −0.14, SE = 0.02, P < 0.01 for △logAAD; B = −0.10, SE = 0.02, P < 0.01 for △logDAD; B = 0.12, SE = 0.01, P < 0.01 for △logPWV). Higher mean blood pressure at baseline was also significantly associated with a reduction of AAD and increase in PWV (B = −0.08; SE = 0.02; P < 0.01 for △logAAD, B = 0.04; SE = 0.01; P < 0.01 for △logPWV), while higher heart rate (HR) was associated with decreased AAD and DAD (B = −0.06, SE = 0.02, P < 0.01 for △logAAD; B = −0.04, SE = 0.02, P < 0.01 for △logDAD). Increasing mean blood pressure during follow-up was associated with a reduction in AAD and an increase in PWV (B = −0.06, SE = 0.02, P < 0.01 for △logAAD; B = 0.04, SE = 0.01, P < 0.01 for △logPWV). Continued smoking was also associated with a reduction in AAD and an increase in PWV. Participants with diabetes mellitus at baseline—but not with newly diagnosed diabetes mellitus—was associated with less increase in PWV compared with those without diabetes (B = −0.103, SE = 0.038, P < 0.01 for △logPWV). Baseline AS values were also significant determinants of further progressive impairment of aortic function (B = −0.833, SE = 0.028, P < 0.01 for △logAAD; B = −0.802, SE = 0.031, P < 0.01 for △logDAD; B = −0.813, SE = 0.023, P < 0.01 for △logPWV). Use of antihypertensive medication and cholesterol levels were not associated with change in AS during the follow-up. These findings were consistent even after further adjustment baseline and change in aortic area (data not shown).
Table 2.
Association of change in AS over 10 years with demographics, baseline, and change in risk factors in multivariate analysis
| Change in AAD (△logAAD, units) |
Change in DAD (△logDAD, units) |
Change in PWV (△logPWV, units) | |
|---|---|---|---|
| Baseline factors | |||
| Age per 10 years | −0.14 (0.02)** | −0.10 (0.02)** | 0.12 (0.01)** |
| Male | 0.016 (0.040) | −0.008 (0.040) | 0.014 (0.022) |
| Race (Ref. White) | |||
| Chinese | 0.056 (0.050) | 0.060 (0.049) | 0.043 (0.028) |
| Black | 0.101 (0.044)* | 0.025 (0.043) | 0.064 (0.024)** |
| Hispanic | 0.015 (0.063) | 0.167 (0.062)** | 0.068 (0.035) |
| BMI per 10 kg/m2 | 0.08 (0.04) | −0.06 (0.04) | −0.02 (0.02) |
| MBP per 10 mmHg | −0.08 (0.02)** | −0.02 (0.02) | 0.04 (0.01)** |
| HR per 10 beats/60 s | −0.06 (0.02)** | −0.04 (0.02)* | −0.01 (0.01) |
| LDL per 10 mg/dl | 0.004 (0.007) | 0.002 (0.007) | 0.004 (0.003) |
| HDL per 10 mg/dl | 0.02 (0.01) | 0.02 (0.01) | −0.01 (0.01) |
| DM at 0 y | 0.014 (0.069) | −0.059 (0.068) | −0.103 (0.038)* |
| New DM at 10 y | 0.002 (0.063) | −0.045 (0.062) | −0.013 (0.035) |
| BP medication use | |||
| No 0 y, No 10 y (n = 523) | Ref | Ref | Ref |
| No 0 y, Yes 10 y (n = 266) | 0.033 (0.049) | −0.001 (0.048) | 0.006 (0.027) |
| Yes 0 y, No 10 y (n = 30) | 0.158 (0.116) | 0.155 (0.114) | 0.089 (0.064) |
| Yes 0 y, Yes 10 y (n = 342) | 0.093 (0.049) | −0.022 (0.048) | −0.016 (0.027) |
| Current smoking status | |||
| No 0 y, No 10 y (n = 1039) | Ref | Ref | Ref |
| No 0 y, Yes 10 y (n = 15) | 0.226 (0.156) | 0.097 (0.154) | 0.049 (0.086) |
| Yes 0 y, No 10 y (n = 41) | −0.060 (0.094) | 0.102 (0.092) | 0.081 (0.052) |
| Yes 0 y, Yes 10 y (n = 65) | −0.167 (0.077)* | 0.076 (0.075) | 0.216 (0.042)** |
| 10 year difference (10y–0y) | |||
| BMI difference per 10 kg/m2 | 0.08 (0.08) | −0.12 (0.08) | −0.07 (0.04) |
| MBP difference per 10 mmHg | −0.06 (0.02)** | −0.02 (0.02) | 0.004 (0.001)** |
| HR per 10 beat/60s | −0.04 (0.02) | −0.007 (0.0017) | 0.005 (0.011) |
| LDL difference per 10 mg/dl | 0.01 (0.01) | 0.004 (0.006) | −0.001 (0.004) |
| HDL difference per 10 mg/dl | −0.009(0.0017) | −0.03 (0.02) | 0.002 (0.009) |
| Baseline AS indices | |||
| logAAD, per 1 unit | −0.833 (0.028)** | NA | NA |
| logDAD, per 1 unit | NA | −0.802 (0.031)** | NA |
| logPWV, per 1 unit | NA | NA | −0.813 (0.023)** |
Coefficient and standard error (in brackets) for multivariate linear regression model are expressed. 0 y indicate baseline; 10 y, 10 year follow-up; logAAD, log-transformed AAD; logDAD, log-transformed DAD; logPWV, log-transformed PWV; △logAAD, change in logAAD (10y–0y); △logDAD, change in logDAD; △logPWV, change in logPWV; AS, aortic stiffness; NA, not available; Ref., reference category. Other abbreviation as in Table 1.
*P < 0.05.
**P < 0.01.
Similar results were obtained in models using SBP or DBP instead of mean blood pressure. Both baseline values and change in SBP and DBP as well as MBP were associated with corresponding changes in AAD and PWV in separate models. Higher SBP at baseline and an increase in SBP were associated with a reduction of DAD (Supplementary data online, Table S1).
Change in PWV was negatively associated with change in AAD (B = −0.131, SE = 0.055, P = 0.017), but not with change in DAD (B = −0.016, SE = 0.054, P = 0.77).
Change in aortic stiffness with blood pressure during follow-up
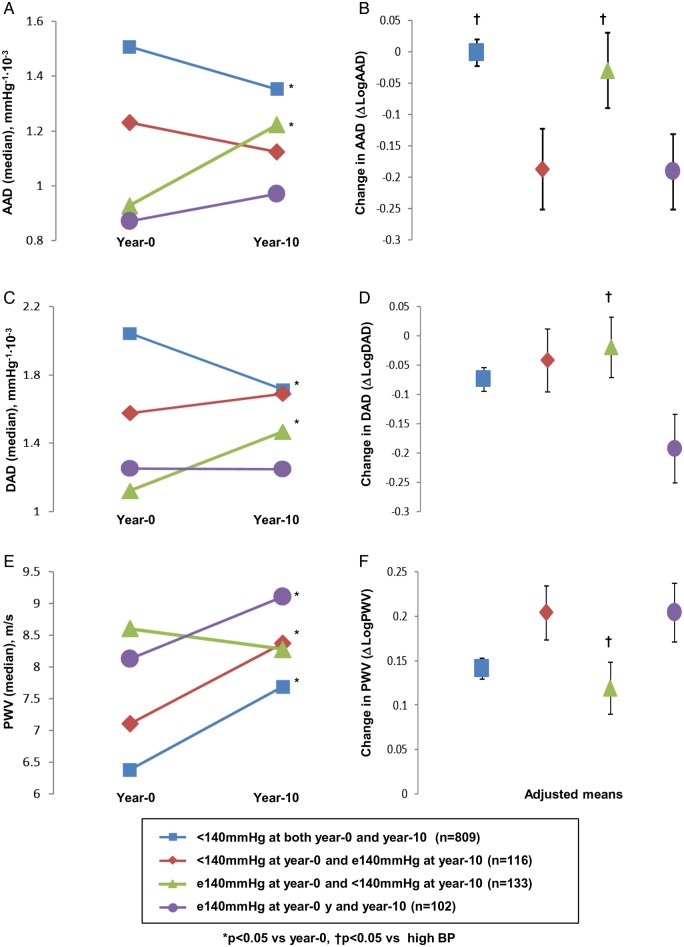
Changes in AS parameters stratified by categories of blood pressure change are shown in Figure 4. The group with ≥140 mmHg at Year 0 and <140 mmHg at Year 10 (high to normal BP) experienced an increase in AAD/DAD (P < 0.05 vs. baseline, respectively), whereas AAD/DAD in other groups decreased (consistently normal BP) or remained similar (normal to high BP and consistently high BP) during follow-up. PWV showed no change in the group with decreasing BP but increased significantly in all other groups. The 10-year AS values among participants with high to normal BP trended towards the values obtained from those with consistently normal BP (<140 at both baseline and follow-up). Even after adjustment for baseline AS values, risk factors and antihypertensive medication use, less aortic stiffening was seen in the group with high to normal BP compared with the consistently high BP (≥140 at Year 0 and Year 10) (Figure 4).
Figure 4.
Change in AS over 10 years stratified by SBP change. Left panel (A, C and E) shows each AS parameter (median value) both at Year 0 and Year 10 stratified by categories of change in SBP. Right panel (B, D and F) shows adjusted mean for change in each AS parameter calculated using linear regression models. Participants were stratified by SBP: those with SBP < 140 mmHg at Year 0 and Year 10, <140 mmHg at Year 0 and >140 mmHg at Year 10, >140 mmHg at Year 0 and <140 mmHg at Year 10, and >140 mmHg at both Year 0 and Year 10. Abbreviation as in Tables 1 and 2.
Change in aortic stiffness with the use of class in antihypertensive medication
Participants with the use of CCB at baseline showed less decrease in AAD even after adjustment for baseline and change in blood pressure compared with those without CCB use (B = 0.11, P < 0.05). Other drugs including ACE-I/ARB, beta-blocker, and diuretics were not associated with change in AS (Supplementary data online, Table S2). There was no significant association between the use of lipid-lowering drugs with change in AS (Supplementary data online, Table S3).
Discussion
The present study demonstrated the relationships between AS parameters assessed by MRI and demographic/risk factors in a longitudinal analysis using a large multi-ethnic population. Reduction in AD and a steep increase in aortic PWV were documented over 10 years of follow-up. The decrease in AAD and increase in PWV were associated with age, higher baseline blood pressure, increase in blood pressure through follow-up, and continuous smoking. A higher baseline HR was associated with a decrease in AAD and DAD. Importantly, reduction of blood pressure to an adequate level during follow-up was associated with less aortic stiffening. To the best of our knowledge, the present study is the first to evaluate longitudinal determinants of AS parameters assessed by MRI in a large general population cohort.
AAD and DAD, markers of local AS, decreased slightly (5% decrease in median for both AAD and DAD), and PWV that indicate regional AS, increased over 10 years (18% increase in median) in a large population with the mean age of 60 years at baseline. These results in longitudinal observation are consistent with previous cross-sectional study indicating that AD decreases more pronounced in young adulthood and middle age (<50 years) compared with the elderly, whereas steep increase in PWV are seen after 50 years.5
In our study, the longitudinal determinants for AAD and PWV were to some extent similar: older age, higher blood pressure at baseline, increase in blood pressure during the follow-up period, and continuous smoking represented the main determinants of adverse aortic function in the second half of the human lifespan. On the other hand, mean blood pressure and smoking status were not as associated with change in DAD as they were for change in AAD. Furthermore, change in PWV was associated with change in AAD, but not with change in DAD. Different structural components in different aortic segments might underlie the variation in the determinants of AAD vs. DAD. The ascending aorta has greater amounts of elastic fibres than the descending aorta, which in turn has greater density of smooth muscle cells compared with the ascending aorta.16 Moreover, mechanical load and support vary along the aortic length, which could also account for regional variation in the response to risk factor exposure. In any case, these segmental differences in the determinants of AS may be relevant to our understanding of vascular diseases and vascular aging, and might suggest different targets for therapy in the future depending on the type of aortic disease.
Few studies assessed the determinants for longitudinal change of cf-PWV.17–21 A recent report from Baltimore longitudinal study of aging showed that age and SBP are the longitudinal determinants of cf-PWV, and other cardiovascular risk factors were not associated with longitudinal change in cf-PWV in general community-based population.20 These results are consistent with the systemic review of prior cross-sectional studies that showed the contribution of risk factors other than age and blood pressure to cf-PWV is small or insignificant.22 On the other hand, continuous smoking as well as age and blood pressure was associated with change in PWV in the present study. The difference in findings between the present study and aforementioned longitudinal study might be a result of improved precision in PWV assessment by MRI and ignorance of arch PWV stiffness in cf-PWV. On the other hand, this could also be because of differences in population characteristics.
Relationship of age to aortic stiffness
Age was the main determinant of increased AS using multivariate linear regression model in the present study. Older age at baseline implied more decreased distensibility and increased PWV at follow-up compared with a younger age. An accelerated increased rate of PWV with advancing age is consistent with previous longitudinal analysis using tonometry.20 Advancing age is associated with thinning and fragmentation of elastin fibres caused by pulsatile stress as well as accumulation of collagen fibres and proteoglycans, resulting in aortic stiffening.16
Relation of blood pressure with aortic stiffness
The association of blood pressure with AS has been reported in cross-sectional23 and longitudinal studies.20,21 The present study showed, however, that higher baseline and change in blood pressure over 10 years were both associated with reduction of AAD and an increase in PWV. With the results of other studies demonstrating that a higher PWV predicts a longitudinal increase in BP,17 the association between BP and AS could be conceived as a vicious cycle.
Interestingly, less development of AS, demonstrated as maintained distensibility and PWV, was seen in the group of participants with high to normal BP (≥140 mmHg at Year 0 and <140 mmHg at Year 10) compared with those in the consistently high BP group (≥140 at Year 0 and Year 10). Aortic stiffness parameters at the end of the follow-up in the high to normal BP group approximated those of the group with consistently normal BP (<140 at both baseline and follow-up). This important finding is consistent with previous studies that show less aortic stiffening can be seen in well-treated hypertensive patients21 and may indicate the importance of blood pressure control for preventing aortic stiffening. Reduction of BP to adequate levels might prevent the aortic stiffening and the development or further worsening of hypertension that might accelerate progression of vascular aging.
Use of antihypertensive medication was not associated with change in the present study. One of the reasons for this finding might be a mediation of decreased BP by antihypertensive mediation, because use of antihypertensive medication was weakly associated (relationship not significant) in models without blood pressure measures as covariates. Interestingly, use of CCB was associated with less decrease in AAD, while others BP medications did not have the same effect. These findings are consistent with previous studies that demonstrated the beneficial effect by different classes of antihypertensive drugs alone or in combination.24–26 The variation of effect in classes of antihypertensive drug on AS might also attribute to no association of antihypertensive medication with change in AS. Future interventional studies are needed to assess the beneficial effects on AS of different drugs.
Impact of other risk factors on aortic stiffness
Continuous smoking was associated with increased AS (both AAD and PWV) in the present study. During cigarette smoking, there is augmentation of arterial stiffness due to the stimulation of sympathetic nerves by nicotine, which results in blood pressure increase, endothelial dysfunction, and constriction of vascular smooth muscle cells.27 Chronic smoking may also induce activated vascular inflammation and increased oxide LDL, leading to chronic reduced production of nitric oxide with consequent endothelial dysfunction. Studies have demonstrated that smoking cessation reversed these effects of smoking on impaired arterial stiffness.28
In cross-sectional studies, HR was a significant determinant of arterial stiffness in patients with hypertension.23,29 An increased HR exerts a greater number of pulsatile cycles on the arterial wall, leading to increased fatigue and fracture of elastic fibres in the arterial wall, which is an important mechanism of age-related arterial stiffening.16 In the present study, a higher baseline HR was associated with a decrease in ascending aorta distensibility, but not with PWV.
In the present study, the increase in PWV during the follow-up period was less in those with diabetes than in those without diabetes. Such observations are in disagreement with previous reports showing that abnormal AS is associated with impaired glucose metabolism.12 One potential explanation for these apparently paradoxical results may be medical interventions on DM participants. They received more antihypertensive drugs such as ACE-I/ARB and CCB than non-diabetic participants, which might have improved AS.24,26 In addition, anti-diabetic drugs might have beneficial effects on AS.30 Future prospective studies are needed to confirm whether and how these interventions may alter AS in DM and non-DM individuals.
Study limitations
The present study has limitations. First, because our population consisted of middle-aged and older individuals (mean 60 years of age at baseline), it is not possible to generalize these results to younger adults. Second, differences on MRI settings between the baseline and follow-up examinations might have influenced the evaluation of AS parameters in our study. Third, we used brachial PP for the calculation of AD instead of central PP. However, given the age of our population in the two exams, the differences between brachial and central PP are likely to have been smaller than those seen among younger individuals due to the amplification phenomenon. Last, we did not assess other variables such as the augmentation index or PP amplification, which result from wave reflections, in addition to AD and PWV. Future studies should be conducted to assess the effect of other related factors on aortic stiffening to fully elucidate the complex nature of aortic biomechanics. The strengths of our study include its large sample size, the assessment of regional proximal AS by sophisticated methodology using MRI, and the ability to assess the relationship of AS with baseline and follow-up characteristics on a 10-year longitudinal design.
Conclusions
The present study demonstrates that age, blood pressure, and smoking are the main determinants of AS in a large, adult, multi-ethnic population aged 45 years and older at baseline. Furthermore, not only elevated baseline blood pressure but also increase in blood pressure during the 10-year follow-up period were associated with progressive AS. Finally, our findings suggest that blood pressure control is effective in halting the progression of aortic stiffening. Future investigations are needed to assess the possible impact of therapeutic strategies aimed at reducing AS on the risk of subsequent cardiovascular disease.
Supplementary data
Supplementary data are available at European Heart Journal—Cardiovascular Imaging online.
Funding
This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, and N01-HC-95168 from the National Heart, Lung, and Blood Institute.
Acknowledgements
We thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Conflict of interest: none declared.
References
- 1.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010;121:505–11. PubMed PMID: 20083680. Pubmed Central PMCID: 2836717. Epub 2010/01/20.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Col Cardiol 2010;55:1318–27. PubMed PMID: 20338492. Epub 2010/03/27.eng. [DOI] [PubMed] [Google Scholar]
- 3.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001;37:1236–41. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshank K. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085–90. [DOI] [PubMed] [Google Scholar]
- 5.Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension 2010;55:319–26. PubMed PMID: 20065154. Pubmed Central PMCID: 3035625. Epub 2010/01/13.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers WJ, Hu Y-L, Coast D, Vido DA, Kramer CM, Pyeritz RE et al. Age-associated changes in regional aortic pulse wave velocity. J Am Col Cardiol 2001;38:1123–9. [DOI] [PubMed] [Google Scholar]
- 7.Grotenhuis HB, Westenberg JJ, Steendijk P, van der Geest RJ, Ottenkamp J, Bax JJ et al. Validation and reproducibility of aortic pulse wave velocity as assessed with velocity-encoded MRI. JMRI 2009;30:521–6. PubMed PMID: 19711407. Epub 2009/08/28.eng. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;28:1462–536. PubMed PMID: 17562668. Epub 2007/06/15.eng. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res 2009;3:56–64. PubMed PMID: 20161241. Pubmed Central PMCID: 2705910. Epub 2010/02/18.Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redheuil A. Cardiovascular aging: Insights from local and regional measures of aortic stiffness using magnetic resonance imaging. Artery Res 2014;8:66–72. [Google Scholar]
- 11.Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA et al. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertension 2002;15(1 Pt 1):16–23. PubMed PMID: 11824854. Epub 2002/02/05.eng. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA et al. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation 2007;115:2628–36. PubMed PMID: 17485578. Epub 2007/05/09.eng. [DOI] [PubMed] [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. PubMed PMID: 12397006. Epub 2002/10/25.eng. [DOI] [PubMed] [Google Scholar]
- 14.Herment A, Kachenoura N, Lefort M, Bensalah M, Dogui A, Frouin F et al. Automated segmentation of the aorta from phase contrast MR images: validation against expert tracing in healthy volunteers and in patients with a dilated aorta. JMRI 2010;31:881–8. PubMed PMID: 20373432. Epub 2010/04/08.eng. [DOI] [PubMed] [Google Scholar]
- 15.Noda C, Ambale Venkatesh B, Ohyama Y, Liu CY, Chamera E, Redheuil A et al. Reproducibility of functional aortic analysis using magnetic resonance imaging: the MESA. Eur Heart J Cardiovasc Imaging 2016;17:909–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Col Cardiol 2007;50:1–13. PubMed PMID: 17601538. Epub 2007/07/03.eng. [DOI] [PubMed] [Google Scholar]
- 17.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012;308:875–81. PubMed PMID: 22948697. Pubmed Central PMCID: 3594687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benetos A, Adamopoulos C, Bureau JM, Temmar M, Labat C, Bean K et al. Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 2002;105:1202–7. PubMed PMID: 11889014. [DOI] [PubMed] [Google Scholar]
- 19.Crichton GE, Elias MF, Robbins MA. Cardiovascular health and arterial stiffness: the Maine-Syracuse Longitudinal Study. J Hum Hypertension 2014;28:444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 2013;62:934–41. PubMed PMID: 24001897. Pubmed Central PMCID: 3880832. Epub 2013/09/05.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ait-Oufella H, Collin C, Bozec E, Laloux B, Ong KT, Dufouil C et al. Long-term reduction in aortic stiffness: a 5.3-year follow-up in routine clinical practice. J Hypertension 2010;28:2336–41. PubMed PMID: 20683338. Epub 2010/08/05.eng. [DOI] [PubMed] [Google Scholar]
- 22.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension 2009;54:1328–36. PubMed PMID: 19884567. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA et al. Cross-sectional correlates of increased aortic stiffness in the community: The Framingham heart study. Circulation 2007;115:2628–36. [DOI] [PubMed] [Google Scholar]
- 24.Karalliedde J, Smith A, DeAngelis L, Mirenda V, Kandra A, Botha J et al. Valsartan improves arterial stiffness in type 2 diabetes independently of blood pressure lowering. Hypertension 2008;51:1617–23. PubMed PMID: 18426991. [DOI] [PubMed] [Google Scholar]
- 25.Boutouyrie P, Achouba A, Trunet P, Laurent S. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR study. Hypertension 2010;55:1314–22. PubMed PMID: 20404219. Epub 2010/04/21.eng. [DOI] [PubMed] [Google Scholar]
- 26.Seidlerova J, Filipovsky J, Mayer O, Wohlfahrt P, Cifkova R. Positive effects of antihypertensive treatment on aortic stiffness in the general population. Hypertens. Res. 2014;37:64–8. PubMed PMID: 24048486. Epub 2013/09/21.eng. [DOI] [PubMed] [Google Scholar]
- 27.Mahmud A, Feely J. Effect of Smoking on Arterial Stiffness and Pulse Pressure Amplification. Hypertension 2002;41:183–7. [DOI] [PubMed] [Google Scholar]
- 28.Jatoi NA, Jerrard-Dunne P, Feely J, Mahmud A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension 2007;49:981–5. PubMed PMID: 17372029. Epub 2007/03/21.eng. [DOI] [PubMed] [Google Scholar]
- 29.Malayeri AA, Natori S, Bahrami H, Bertoni AG, Kronmal R, Lima JA et al. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2008;102:491–6. PubMed PMID: 18678312. Pubmed Central PMCID: 2586608. Epub 2008/08/06.eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh N, Ogawa Y, Usui T, Tagami T, Kono S, Uesugi H et al. Antiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effect. Diabetes Care 2003;26:2493–9. PubMed PMID: 12941708. Epub 2003/08/28.eng. [DOI] [PubMed] [Google Scholar]