Abstract
Background
Childhood obesity characterized by excessive fat in the body is one of the most serious health problems worldwide due to the social, medical, and physiological complications. Obesity and associated diseases are triggering factors for oxidative stress and inflammation. The aim of this study was to explore the possible association between childhood obesity and inflammatory and oxidative status.
Material/Methods
Thirty-seven obese children and 37 healthy controls selected from among children admitted to BLIND University Paediatrics Department were included in the study. Anthropometric measurements were performed using standard methods. Glucose, lipid parameters, CRP, insulin, total oxidant status (TOS), total anti-oxidant status (TAS) levels, and total thiol levels (TTL) were measured in serum. HOMA index (HOMA-IR) were calculated. The differences between the groups were evaluated statistically using the Mann-Whitney U test.
Results
Body mass index was significantly higher in the obese group (median: 28.31(p<0.001). Glucose metabolism, insulin, and HOMA-IR levels were significantly higher in the obese group (both p<0.001). Total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels were significantly higher in the obese group (p<0.001). TAS (med: 2.5 μmol Trolox eq/L (1.7–3.3)) and TOS (med: 49.1 μmol H2O2 eq/L (34.5–78.8)) levels and TTL (med: 0.22 mmol/L (0.16–0.26)) were significantly higher in the obese group (p=0.001). CRP levels showed positive correlation with TOS and negative correlation with TTL levels (p=0.005, r=0.473; p=0.01, r=−0.417; respectively). TTL levels exhibited negative correlation with TOS levels (p=0.03, r=−0.347).
Conclusions
In conclusion, obese children were exposed to more oxidative burden than children with normal weight. Increased systemic oxidative stress induced by childhood obesity can cause development of obesity-related complications and diseases. Widely focussed studies are required on the use of oxidative parameters as early prognostic parameters in detection of obesity-related complications.
MeSH Keywords: Antioxidants, Oxidative Stress, Pediatric Obesity, Sulfhydryl Reagents
Background
Childhood obesity characterized by excessive fat deposition in the body is one of the emerging and critical health issues worldwide due to the social, medical, and physiological complications. Although the prevalence of obesity in children varies among countries, it is rapidly increasing worldwide. The World Health Organization reported that in 2012 around 44 million (6.7%) of the world’s children aged less than 5 years old were overweight or obese. The rate of childhood obesity has increased from 31 million (5%) in 1990. Childhood obesity predisposes to chronic adulthood diseases such as cardiovascular diseases, diabetes mellitus, metabolic syndrome, and cancer [1,2].
Low-grade inflammation, metabolism, and oxidative stress are the main underlying mechanisms of pathogenesis in obesity. Adipose tissue is not only an energy reserve, it is also metabolically active and secretes hormones and cytokines. Excessive fat tissue generates reactive oxygen species (ROS), which are triggers for oxidative stress, dysregulation of adipokine mechanism, and pro-inflammatory cytokine release [3,4]. Inordinate increase of serum free fatty acids (FFA) in obese children leads to impaired glucose metabolism, which augments oxidative stress parameters and mitochondrial overload by accumulation of energy substrates [5].
Oxidative stress is a condition associated with changes in the balance between oxidant and antioxidant systems in favor of the oxidative system [6]. Oxidants occur in normal metabolic processes; however, increased levels are produced in pathophysiological conditions. The onset of formation of reactive oxygen radicals is prevented by enzymatic activity or natural antioxidants. The expression ‘thiol’ refers to the sulphur-containing constituents. Intracellular and plasma protein thiol groups act as buffers against oxidation by virtue of their highly reduced state. Total thiol level (TTL) shows antioxidant effects by arranging the redox potential [7,8]. In addition, total oxidant status (TOS), total antioxidant status (TAS), and the ratio between them (oxidative stress index) were reported in many studies [9,10] to be suitable and practical parameters representing oxidative status. The aim of the present study was to explore the association between obesity and inflammatory and oxidative parameters.
Material and Methods
Study population
We consecutively selected 37 obese adolescents (median age: 11 years, age range 6–16) from obese children who visited the BLIND University Pediatric Endocrinology Clinic between November 2014 and September 2015. Additionally, 37 healthy children (median age: 11 years, age range 7–16) were included as the control group. In the obese group there were 19 males (51.4%) and 18 females (48.6%), and in the control group there were 21 males (56.8%) and 16 females (43.2%). The study protocol was approved by the BLIND University Medical Faculty Ethics Committee. The study was carried out in accordance with the principles set forth in the Declaration of Helsinki, as amended in 2009.
Participants with inflammatory chronic diseases, viral infections (hepatitis A, B, and C, cytomegalovirus, and Epstein-Barr virus), endocrine disorders, and any systemic diseases (affecting the hepatic, renal or respiratory systems) were excluded from both groups.
Anthropometric measurements
All children’s weight and height measurements were performed by the same person who used the same equipment. The age at admission, height (cm), weight (kg), body mass index (BMI) (kg/m2), and BMI Z-score (Cole’s least mean-square method was used for calculating BMI standard deviation score) [11] were calculated. Height measurements were performed with sensitivity of 1 mm. Weight measurements were performed while standing upright and stationary on the scale without shoes and with light underwear; measurements were performed with sensitivity of 100 g. BMI was calculated as: body weight (kg)/height (meters) squared. We included children in the ≥95th percentile according to BLIND children BMI references.
Blood samples
Serum samples were obtained after 10–12 h of fasting and were used for measurements of all biochemical parameters. Serum specimens were kept at −80°C until the tests were analyzed. Glucose, alanine transaminase (ALT), C-reactive protein (CRP), total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride levels were quantified using an Abbott Architect CI16200 device (New Jersey, USA) by spectrophotometric method. HOMA index was calculated by the formula: fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5. Insulin levels were analyzed by chemiluminescence method (Siemens, Advia centaur, 2006, Germany). Hemoglobin (Hb), hematocrit (Hct), and mean cell volume (MCV), which are complete blood parameters, were measured with an Abbott CELL-DYN 3700 device.
Quantification of total oxidant status
Plasma TOS was analyzed using a procedure improved by Erel [12]. Oxidants that occur in serum oxidize the ferrous ion of an o-dianisidine compound to ferric ion. Oxidation provides the ferric ion forms as colored compounds with xylenol orange in acidic circumstances. Color density of xylenol orange is directly proportional to oxidant levels. Hydrogen peroxide was used to calibrate the analysis and results are represented in micromoles of hydrogen peroxide equivalent per liter (μmol H2O2 equiv/l).
Quantification of total antioxidant status
Plasma TAS was analyzed using a procedure improved by Erel [13]. The method of analysis is based on the measurement of the amount of hydroxyl radicals. (Fe+2 + O-dianisidine) compound constitute Fenton type reaction with hydrogen peroxide, which generates the OH radicals. Hydroxyl radicals react with O-dianisidine molecules that form yellow-brown dianisidyl radicals. Existing antioxidants reduce the color formation by suppressing oxidant reactions. The reaction was performed using spectrophotometry method at 240 nm by use of an automated analyzer (Architect CI16200, Abbott, New Jersey, USA). Trolox, which is a water-soluble analogue of vitamin E, was used as the calibrator. Results are shown as μmol Trolox equivalent/L.
Oxidative stress index
Oxidative stress index (OSI) is calculated as the ratio of serum TOS levels to TAS levels. OSI is an indicator of the degree of oxidative stress and the formula is: OSI (arbitrary unit) = (TOS, μmol H2O2 eq/L)/(TAS, μmol Trolox eq/L) [12].
Quantification of total thiol
Measurement of the TTL (sulfhydryl groups) levels was performed using the methods described by Ellman [14] as improved by Hu et al. [15]. The technique was performed by an automated analyser. In the mixture, thiols react with 5.5-dithiobis-2-nitrobenzoic acid (DTNB), turning into a dark-colored anion (5-thio-2-nitrobenzoic acid) with an absorbance peak at 412 nm. Reduced glutathione was used as the calibrator for analysis. The quantification was analyzed by an auto-analyser (Architect CI16200, Abbott, New Jersey, USA) and the results are shown in mmol/L.
Statistics
Constant variables that do not present normal distribution were compared with the Mann-Whitney U test, which is a non-parametric test for comparison of 2 independent groups. The Pearson chi-square test was used for comparison of categorical variables between 2 independent groups. Relation of non-parametric variables was evaluated by the Spearman-Rho correlation method. Median (Md) and min-max values were used for descriptive statistics that do not exhibit normal distribution. The SPSS program (version 17.0) (SPSS, Chicago, IL, USA) was used for statistical analysis. p<0.05 values were accepted as statistically significant.
Results
Demographical findings
The control and patient groups were matched for age and were not significantly different (p=0.47). Body mass index of the obese group was 28.3 kg/m2 (range 22.2–53.1), which was significantly higher than the 18.3 kg/m2 (range 4.7–23.8) in the control group (p<0.001) (Table 1).
Table 1.
Oxidative parameters, lipid and glucose metabolism of obese and control groups.
| Obese group (n=37) Median (Min–Max) |
Control group (n=37) Median (Min–Max) |
p values | |
|---|---|---|---|
| N (female/male) | 37 (19/18) | 37 (21/16) | |
| Age (years) | 11 (6–16) | 11 (7–16) | NS |
| Height (cm) | 151.4 (122–171) | 146 (118–173) | NS |
| Weight (kg) | 69.3 (38–146) | 40 (20–65) | <0.001 |
| BMI (kg/m2) | 28.3 (22.2–53.1) | 18.3 (4.7–23.8) | <0.001 |
| BMI-sds | 2.1 (1.4–3.3) | −0.1 (−1.9–2.2) | <0.001 |
| ALT (U/L) | 26 (11–162) | 14 (6–33) | <0.001 |
| CRP (mg/L) | 0.2 (0–8.8) | 0.1 (0–2.9) | 0.03 |
| Hemoglobin (g/dl) | 13.1 (10.4–14.5) | 12.6 (10.2–16.3) | NS |
| Hematocrite | 39.1 (33.2–42.5) | 37.9 (32.9–46.4) | NS |
| MCV (fl) | 78.6 (68.7–89.3) | 77.7 (65.9–86.3) | NS |
| Fasting glucose (mg/dl) | 96 (77–114) | 94 (64–104) | NS |
| Fasting insulin (μIU/mL) | 20.5 (7.5–66.4) | 11.8 (2.6–26.1) | <0.001 |
| HOMA-IR | 5.2 (1.7–15.5) | 2.7 (0.55–6.47) | <0.001 |
| Total cholesterol (mg/dL) | 166.5 (18–254) | 148.5 (90–204) | 0.01 |
| HDL cholesterol (mg/dL) | 46 (18–63) | 56.5 (26–107) | <0.001 |
| LDL cholesterol (mg/dL) | 110 (40–171) | 83 (45–250) | <0.001 |
| Triglyserides (mg/dL) | 117 (38–220) | 67.5 (22–192) | <0.001 |
| TAS (μmol Trolox eq/L) | 2.5 (1.7–3.3) | 2.2 (1.6–2.8) | <0.001 |
| TOS (μmol H2O2 eq/L) | 49.1 (34.5–78.8) | 42.3 (34.7–133) | 0.006 |
| Thiol (mmol/L) | 0.22 (0.16–0.26) | 0.24 (0.20–0.31) | 0.001 |
| OSI (TOS/TAS) | 0.20 (0.13–0.35) | 0.19 (0.05–0.70) | NS |
NS – Non significant; Significant difference at p<0.05 level; TAS – total antioxidant status; TOS – total oxidant status; HOMA-IR – homeostatic model assessment-insulin resistance; BMI-sds – body mass index-standard deviation score; MCV – mean corpuscular volume.
Lipid profile
Total cholesterol, triglyceride, and LDL, which reflect the lipid profile, were significantly higher in the obese group than in the control group, in which HDL levels were significantly lower (p=0.01, p<0.001, p<0.001, and p<0.001, respectively) (Table 1).
Glucose metabolism
No statistically significant difference in serum glucose levels between the groups was observed (p=0.13). Insulin and HOMA-IR levels, the indicator of insulin resistance [Insulin Md=20.5 μIU/mL (7.5–66.4), HOMA-IR Md=5.2 (1.7–15.5)] of the obese group were significantly higher than in the control group [Insulin Md=11.8 μIU/mL (2.6–26.1), HOMA-IR Md=2.7 (0.55–6.47)] (p<0.001) (Table 1).
Serum ALT, Cortisol, and CRP levels
Serum ALT and CRP levels were significantly higher in the obese group (p<0.001, p=0.03, respectively). Serum cortisol levels were not different between the groups. CRP levels exhibited significant positive correlation with TOS (p=0.024; r=0.473) levels and negative correlation with TTL levels (p=0.027; r=−0.417) in the obese group (Table 2).
Table 2.
Correlation in obese group.
| Correlations coefficents (r) | |||||||
|---|---|---|---|---|---|---|---|
| LDL | TG | Insulin | HOMA-IR | CRP | BMI | Hb | |
| TAS | −0.04 | 0.102 | 0.222 | 0.268 | 0.137 | 0.016 | 0.260 |
| TOS | 0.423* | 0.441* | 0.196 | 0.164 | 0.473* | 0.118 | −0.448* |
| OSI | 0.394* | 0.337* | 0.168 | 0.162 | 0.227 | 0.080 | −0.361* |
| TTL | −0.124 | −0.162 | −0.050 | −0.076 | −0.417* | −0.332* | 0.601* |
TAS – total antioxidant status; HOMA-IR – homeostatic model assessment-insulin resistance; CRP – C-reactive protein; TOS – total oxidantsStatus; BMI – body mass index; Hb – haemoglobin.
Significant difference at p<0.05 level.
Oxidative parameters
The oxidative stress parameters TOS and TAS were significantly higher in the obese group [TOS Md=49.1 (34.5–78.8), TAS Md=2.5 (1.7–3.3)] than in the control group [TOS Md=423 (347–1338), TAS Md=2.2 (1.6–2.8)] (p=0.006, p<0.001, respectively) (Figure 1). No difference was observed between OSI levels of both groups. TTL levels were significantly higher in the control group (p=0.001) (Figure 2).
Figure 1.
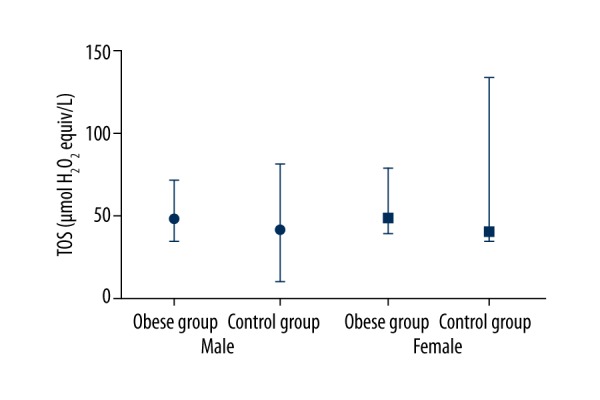
TOS median, min, max levels between groups.
Figure 2.
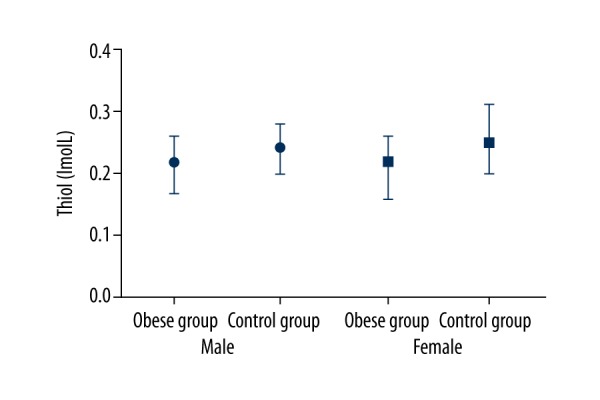
Thiol median, min, max levels between groups.
Correlations
TTL exhibited significant positive correlation with Hb, Hct, and MCV levels in the obese group (p<0.003, r=0.601, r=0.469, r=0.523) and negative correlation with CRP and TOS levels (p=0.02; r=−0.363, p=0.03, r=−0.347). We found a significant negative correlation between BMI and MCV levels in the obese group (p=0.02, r=−0,365). BMI levels had a significant positive correlation with insulin and HOMA levels (p=0.01, r=0.402; p=0.01, r=0.387, respectively). In the obese group, OSI values showed a significant negative correlation with Hb values (p=0.02, r=−0.361) and a positive correlation with LDL and TG levels (r=0.394, r=0.337).
Effect of sex
We did not find any significant differences between groups regarding sex. In the obese female subgroup, TAS levels were positively correlated with ALT (p=0.01; r=0.553) and CRP (p=0.01; r=0.586) levels, but we did not recover any difference in obese males and controls. Hb (p=0.013) and HDL (p=0.031) levels were significantly different between obese male and females.
Discussion
Seventy percent of obese adolescents grow up to be obese adults; therefore, pediatric obesity is important because it gives rise to metabolic syndrome, insulin resistance, and type 2 diabetes [16]. The underlying mechanism of these diseases is closely related to oxidative stress and inflammation, which worsen in obesity [17–19]. Triggering systemic inflammation is one aspect of obesity and the other the immune system-impairing effect [20]. In addition, adipose tissue not only stores excess fat, but also secretes many bioactive molecules. Pre-adipocytes and macrophages are found in adipose tissue as well as adipocytes [21].
Obesity-related proliferation and growth (hyperplastic/hypertrophic) of adipocytes result in increased levels of beta-3 adrenergic receptors. Increased beta receptor levels facilitate the passage of monocytes to visceral adipose stroma, thereby causing onset of a pro-inflammatory cycle between adipocytes and monocytes [3]. In particular, activated macrophages are increased in visceral adipose tissue; these macrophages are called M1, and are the classically activated macrophages; they are up to 40–60% greater in obese adipose tissue rather than in non-obese adipose tissue. Activated macrophages increase the secretion of pro-inflammatory cytokines TNF-α, IL-1B, and IL-6. Augmentation of pro-inflammatory cytokines increases the oxidative stress and causes insulin resistance [22,23]. In the present study we found that CRP levels (as primary inflammatory parameters), insulin, and HOMA-IR levels (as index for insulin resistance) were significantly higher in the obese group than in the control group (p=0.03, <0.001, and <0.001, respectively).
Long-term accumulation of energy substrates (lipid and glucose) causes FFA passage to the blood, especially from visceral adipose tissue. In our study, lipid profiles in the obese group were dysregulated and were significantly higher than in the control group. Increased fatty acids (predominantly in muscle and liver tissues) causes increased cell differentiation and mitochondrial workload, thereby increasing the release of free radicals and ROS products, which are especially harmful for specific organelles and DNA [5]. ALT level, which is an indicator of cellular destruction due to DNA damage in the liver, was significantly higher in obese adolescents in the study performed by Prigon et al. [24], and ALT levels were also significantly higher (p<0.001).
Secretion of pro-inflammatory cytokines, adhesion molecules, and growth factors are increased by enhanced ROS production. ROS accomplishes this effect through redox-sensitive transcription factors [25], especially NF-KB and NADPH oxidase (NOX) pathways. NOX4 is an enzyme complex that is the major source for ROS production in adipocytes [26]. ROS that is produced by NOX targets mitochondria, and this external ROS increase upregulates the mitochondrial redox-sensitive system [27]. Antioxidants are increased due to an increased oxidative load in mitochondria attempting to prevent mitochondrial oxidative damage and impairment [28].
Oliver et al. showed that obese children had increased concentrations of F2-isoprostanes (oxidants), whereas antioxidants (SOD and glutathione) were moderately reduced, but not significantly [29]. Prigon et al. also suggested that TOS and OSI levels were higher in obese children with non-alcoholic fatty liver disease than in non-obese children [24]. In a study performed by Faenzia et al., diacron-reactive oxygen metabolite levels (an oxidative stress indicator) of obese children were significantly higher than in non-obese children, but blood anti-oxidant capacity were significantly lower [30]. In our study, oxidative stress markers TOS and TAS were significantly higher in the obese group compared to the control (p=0.006, p<0.001, respectively). Oxidative stress index (OSI) was not significantly different between the groups. In contrast to the other studies, we found that increased TAS levels were the result of increased TOS levels in the attempt to balance oxidation, perhaps because younger people have more active anti-oxidant systems.
Females are reported to have more resistance against oxidative and inflammatory events than males [31]. Sobieska et al. suggested that girls are more resistant to the negative effect of increased adiposity status compared to males [32]. However, we did not find any difference between oxidative and inflammatory parameters between males and females.
Increased oxidative burden leads to reduced redox potential in mitochondria. Inadequate redox potential impairs formation of –SH (disulphide) bonds in organelles such as the endoplasmic reticulum. This condition damages 3D structure of active physiological proteins and hence might play a role in diseases such as type 2 diabetes mellitus [33,34]. Thiol groups, which contain sulphur components, play an important role in the non-enzymatic anti-oxidant cascade; they eliminate ROS products and free radicals produced by both enzymatic and non-enzymatic pathways [35,36]. Proteins have the greatest anti-oxidant capacity in the body; they achieve most of their effect by the sulphur groups they contain [37]. Hemoglobin, more than the other plasma proteins, exhibits an anti-oxidant effect through sulphur groups and enzymes [38]. In parallel, we found that TTL (an indicator of total disulphide bonds) was significantly lower in the obese group than in the controls, explaining the shortage of disulphide anti-oxidant activity (p=0.001). In addition, TTL had a significant positive correlation with Hb, Hct, and MCV in the obese group (p<0.001; r=0.601, r=0.469, r=0.523).
The increase in oxidative stress augments insulin resistance in muscle and adipose tissue. Increased insulin resistance decreases insulin release from the pancreas. Advanced glycation end-products (AGEs) are produced due to hyperglycemia; these products cause low-grade inflammation by binding to receptors, creating a vicious cycle. Increase in pro-inflammatory cytokines, oxidative stress, and related hyperglycemia raise the risk of type 2 diabetes, atherosclerosis, and cardiac diseases, particularly in obese children (39, 40). In this study we found no significant difference in glucose levels between the 2 groups. However, insulin and HOMA-IR levels were significantly higher in the obese group than in the control group, and their levels were positively correlated with increasing BMI (r=0.402, r=0.387, respectively).
The study has several limitations. Because this was a pilot study, we did not evaluate the diversity of cytokines or their levels. The study population was small. Finally, we did not evaluate specific enzymatic antioxidants such as superoxide dismutase, catalase, and glutathione.
Conclusions
We found a significant burden of oxidative stress as indicated by TAS, TOS, and TTL levels in the obese children. This finding may contribute to understanding the underlying triggering mechanisms of obesity-related diseases of adults who were obese in childhood. The potential pathological effects on the oxidant system should be evaluated, as well as glycogen metabolism, to improve our understanding of the effects of childhood obesity on health.
Footnotes
Conflict of interest
There is no conflict of interest to be declared.
Source of support: Departmental sources
References
- 1.World Health Statistics full report. World Health Organization; 2014. http://www.who.int/gho/publications/world_health_statistics/2014/en/ [Google Scholar]
- 2.Reilly JJ, Methven E, McDowell ZC, et al. Health consequences of obesity. Arch Dis Child. 2003;88(9):748–52. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011;12(5):3117–32. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hensley K, Robinson KA, Gabbita SP, et al. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28(10):1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 5.Rzheshevsky AV. Fatal “triad”: Lipotoxicity, oxidative stress, and phenoptosis. Biochemistry (Mosc) 2013;78(9):991–1000. doi: 10.1134/S0006297913090046. [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–83. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen CK. Redox signaling and the emerging therapeutic potential of thiol antioxidants. Biochem Pharmacol. 1998;55(11):1747–58. doi: 10.1016/s0006-2952(97)00672-2. [DOI] [PubMed] [Google Scholar]
- 8.Atmaca G. Antioxidant effects of sulfur-containing amino acids. Yonsei Med J. 2004;45(5):776–88. doi: 10.3349/ymj.2004.45.5.776. [DOI] [PubMed] [Google Scholar]
- 9.Clapp BR, Hingorani AD, Kharbanda RK, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64(1):172–78. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab. 2000;26(3):163–76. [PubMed] [Google Scholar]
- 11.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44(1):45–60. [PubMed] [Google Scholar]
- 12.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38(12):1103–11. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37(2):112–19. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Hu ML, Louie S, Cross CE, et al. Antioxidant protection against hypochlorous acid in human plasma. J Lab Clin Med. 1993;121(2):257–62. [PubMed] [Google Scholar]
- 16.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: a systematic review. International journal of obesity and related metabolic disorders: Int J Obes Relat Metab Disord. 1999;23(Suppl 8):S1–107. [PubMed] [Google Scholar]
- 17.Marseglia L, Manti S, D’Angelo G, et al. Oxidative stress in obesity: A critical component in human diseases. Int J Mol Sci. 2015;16(1):378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6(7):438–46. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, Lima FB. Adipose tissue as an endocrine organ: from theory to practice. J Pediatr (Rio J) 2007;83(5 Suppl):S192–203. doi: 10.2223/JPED.1709. [DOI] [PubMed] [Google Scholar]
- 22.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirgon O, Bilgin H, Cekmez F, et al. Association between insulin resistance and oxidative stress parameters in obese adolescents with non-alcoholic fatty liver disease. J Clin Res Pediatr Endocrinol. 2013;5(1):33–39. doi: 10.4274/Jcrpe.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp Gerontol. 2000;35(5):521–32. doi: 10.1016/s0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 26.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 27.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51(7):1289–301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55(3):798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 29.Oliver SR, Rosa JS, Milne GL, et al. Increased oxidative stress and altered substrate metabolism in obese children. Int J Pediatr Obes. 2010;5(5):436–44. doi: 10.3109/17477160903545163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faienza MF, Francavilla R, Goffredo R, et al. Oxidative stress in obesity and metabolic syndrome in children and adolescents. Horm Res Paediatr. 2012;78(3):158–64. doi: 10.1159/000342642. [DOI] [PubMed] [Google Scholar]
- 31.Kerksick C, Taylor Lt, Harvey A, Willoughby D. Gender-related differences in muscle injury, oxidative stress, and apoptosis. Med Sci Sports Exerc. 2008;40(10):1772–80. doi: 10.1249/MSS.0b013e31817d1cce. [DOI] [PubMed] [Google Scholar]
- 32.Sobieska M, Gajewska E, Kalmus G, Samborski W. Obesity, physical fitness, and inflammatory markers in Polish children. Med Sci Monit. 2013;19:493–500. doi: 10.12659/MSM.883959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson JD. Type 2 diabetes as a redox disease. Lancet. 2014;383(9919):841–43. doi: 10.1016/S0140-6736(13)62365-X. [DOI] [PubMed] [Google Scholar]
- 34.Csala M, Margittai E, Banhegyi G. Redox control of endoplasmic reticulum function. Antioxid Redox Signal. 2010;13(1):77–108. doi: 10.1089/ars.2009.2529. [DOI] [PubMed] [Google Scholar]
- 35.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 36.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 37.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–85. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Reischl E, Dafre AL, Franco JL, Wilhelm Filho D. Distribution, adaptation and physiological meaning of thiols from vertebrate hemoglobins. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146(1–2):22–53. doi: 10.1016/j.cbpc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Bonnefont-Rousselot D. Obesity and oxidative stress: Potential roles of melatonin as antioxidant and metabolic regulator. Endocr Metab Immune Disord Drug Targets. 2014;14(3):159–68. doi: 10.2174/1871530314666140604151452. [DOI] [PubMed] [Google Scholar]
- 40.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]


