Abstract
Lung cancer is the leading cause of cancer death in the United States. The vast majority of patients are diagnosed with metastatic disease with a 5-year survival rate of less than 5%. After first-line chemotherapy or biomarker-matched targeted therapy, only suitable for a small group of patients, further systemic therapy options rendered very limited, if any, benefit until recently. Checkpoint inhibitors have significantly improved outcomes in patients with metastatic non-small cell lung cancer (NSCLC) and are currently an established second-line therapeutic option. In this manuscript, we review the mechanism of action of checkpoint inhibitors, present the available data with approved and experimental agents, discuss the progress that has already been made in the field, as well as toxicity awareness, and future perspectives.
Keywords: lung cancer, immunotherapy, checkpoint inhibitor
Introduction
Lung cancer is the leading cause of cancer death in the United States, with only 17.4% of patients being alive after 5 years [Howlader et al. 2015]. In 2015, an estimated number of 221,200 new cases were diagnosed and 158,040 deaths occurred [Siegel et al. 2015]. Approximately 85% of lung cancers can be classified as non-small cell lung cancer (NSCLC), divided into two major groups by histology: squamous (Sq) and nonsquamous (non-Sq). Early-stage disease is potentially curable, although curative-intent surgical resections are feasible in only 25–30% of patients. In some cases of locally advanced disease, definitive chemoradiation therapy offers a possibility of cure [Howington et al. 2013]. Unfortunately, 57% of patients have already distant metastatic disease at diagnosis with a 5-year survival rate of less than 5% [Howlader et al. 2015].
Much progress has been made recently to increase survival rates for patients with advanced disease. Targeted therapies against epidermal growth-factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and ROS1 have significantly improved outcomes for a molecularly defined subgroup of patients harboring respectively EGFR activating mutations, ALK or ROS1 translocations [Rosell et al. 2012; Solomon et al. 2014; Chan and Hughes, 2015; Khozin et al. 2015]. However, for the remaining majority of patients with nontargetable genomic alterations, platinum-based chemotherapy is still the backbone of first-line therapy in the metastatic setting [Leighl, 2012]. There can be benefit in non-Sq NSCLC from the addition of the vascular endothelium growth-factor (VEGF) inhibitor bevacizumab [Sandler et al. 2006; Zhou et al. 2015]. Upon progression, until recently, single-agent chemotherapy for patients with a good performance status was the therapy of choice, rendering response in up to 10% of patients and a median progression-free survival (PFS) of ~2.5 months, at the cost of significant toxicity [Leighl, 2012; Melosky, 2014; Thatcher et al. 2015]. This scenario was urging for new therapeutic options that would result in higher and durable responses and ultimately improve patient’s quality of life and outcomes.
Accompanying melanomas and kidney cancers [Larkin et al. 2015; Motzer et al. 2015; Robert et al. 2015], recent studies have shown encouraging activity of checkpoint inhibitors in NSCLC, changing the treatment paradigm of this disease. Two programmed death-1 (PD-1) inhibitors named nivolumab and pembrolizumab have been approved by the Food and Drug Administration (FDA) to treat metastatic disease in second line. Several clinical trials are ongoing to expand the indications for this class of drugs, including testing PD-1 and programmed death-ligand-1 (PD-L1) inhibitors as monotherapies in first line, combination trials with cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies, targeted therapy, chemotherapy, radiotherapy and vaccines. Studies are being conducted both in the refractory and front-line setting, aspiring to take over as protagonists in the fierce battle against lung cancer.
The objective of this review is to present the progress already made with checkpoint inhibition in lung cancer, outline the ongoing research in the field, and discuss the promising perspectives for the future.
Mechanism of action of programmed death-1 pathway
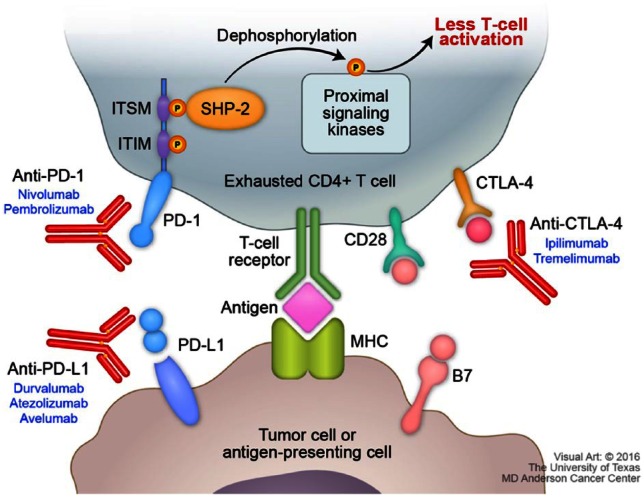
T cells require two signals to become fully activated [Lafferty et al. 1978]. The first signal comes from the interaction of T-cell receptors (TCR) with the antigen–peptide major-histocompatibility complex (MHC), which gives specificity to the immune response. To be fully activated, T cells need a costimulatory antigen-dependent signal that occurs through the interaction between CD28 on T cells and B7-1 and B7-2 on the antigen-presenting cells (APC). Expression of CTLA-4 by T cells represents one important mechanism to prevent overstimulation of the immune system. CTLA-4 has a 100-fold higher affinity with the B7 complex than CD28, and this interaction leads to an inhibitory effect on the cell [Pardoll, 2012]. Therefore, CTLA-4 inhibitors were developed to release these breaks. This class of drugs is currently approved for melanoma and being studied in lung cancer.
Another important mechanism of immune-response evasion is regulated by PD-L1 expression. PD-L1 binds to PD-1 on the T cells and thus initiates a dual mechanism of inhibition, by promoting apoptosis in antigen-specific T cells in lymph nodes and simultaneously reducing apoptosis in regulatory T cells (Tregs), which have a suppressor role (Figure 1). After the interaction takes place, PD-1 is phosphorylated on its two intracellular tyrosines and subsequently binds two phosphatases, SHP-1 and SHP-2. These two phosphatases can bind to the immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) of PD-1 downregulating antigen-receptor signaling. When ITSM alone is mutated, PD-1 loses its inhibitory function, making this tyrosine of pivotal role in PD-1 inhibition [Okazaki et al. 2001; Konishi et al. 2004; Sheppard et al. 2004; Keir et al. 2008]. It is important to notice that PD-L1, but not PD-L2, has greater affinity for B7-1 than CD28, which further increases the inhibitory effect on the pathway. In addition, PD-L2 expression was shown to be restricted to APC and Th2 cells [Lesterhuis et al. 2011]. Therefore, although PD-1 also binds PD-L2, several preclinical studies have shown that inhibiting PD-L2 does not result in effective T-cell activity as compared with PD-L1 inhibition [Keir et al. 2008; Lesterhuis et al. 2011].
Figure 1.
PD-1/PD-L1 pathway and immunotherapy targets.
ITSM, immunoreceptor tyrosine-based switch motif; ITIM, immunoreceptor tyrosine-based inhibitory motif; PD-1, programmed-death 1; PD-L1, programmed death-ligand 1; CD28, cluster of differentiation 28; MHC, major histocompatibility complex; SHP-2, Src homology 2 (SH2) domain containing non-transmembrane PTP; B7, B7 protein; CTLA-4, cytotoxic T-lymphocyte associated protein 4.
Current practice and completed or ongoing clinical trials
To date, there are two checkpoint inhibitors approved by the FDA as second-line therapy for NSCLC: nivolumab, approved for NSCLC independently of PD-L1 expression, and pembrolizumab, only approved for PD-L1-positive NSCLC. Below, we summarize the available data for the two approved inhibitors. In addition, we describe other agents that have shown clinical activity in lung cancer and are currently in clinical development (Table 1).
Table 1.
Completed trials with checkpoint inhibitors in non-small cell lung cancer.
| Author | Phase | Histology/line of treatment | Drug (dose) | Patients (n) | ORR (%) | PFS (%) | OS (%) |
|---|---|---|---|---|---|---|---|
| Brahmer et al. [2014]; Gettinger et al. [2015] | Ib | NSCLC/second | Nivolumab (1 mg/kg) | 33 | 3.0 | 1.8 | 9.2 |
| Nivolumab (3 mg/kg) | 37 | 24.3 | 1.9 | 14.9 | |||
| Nivolumab (10 mg/kg) | 59 | 20.3 | 3.7 | 9.2 | |||
| Rizvi et al. [2015c] | II | SqNSCLC/third | Nivolumab (3 mg/kg) | 117 | 14.5 | 1.9 | 8.2 |
| Rizvi et al. [2014] | I | EGFR-mutant/first | Nivolumab (3 mg/kg) + erlotinib (150 mg) | 21 | 19 | 6.8 | NR |
| Nishio et al. [2015] | II | SqNSCLC/second | Nivolumab (3 mg/kg) | 35 | 25.7 | 4.2 | NYR |
| Nishio et al. [2015] | II | Non-SqNSCLC/ second | Nivolumab (3 mg/kg) | 76 | 19.7 | 2.8 | NYR |
| Brahmer et al. [2015] | III | SqNSCLC/second | Nivolumab (3 mg/kg) | 135 | 20 | 3.5 | 9.2 |
| Borghaei et al. [2015] | III | Non-SqNSCLC/second | Nivolumab (3 mg/kg) | 292 | 19 | 2.3 | 12.2 |
| Antonia et al. [2014] | I | NSCLC/second | Nivolumab (1–3 mg/kg) + ipilimumab (1–3 mg/kg) | 49 | 11–33 | NR | NR |
| Garon et al. [2015] | I | NSCLC/first–fifth | Pembrolizumab (10 mg/kg) q3w | 287 | 19.2 | 3.7 | 12.0 |
| NSCLC/first–fifth | Pembrolizumab (10 mg/kg) q2w | 202 | 19.3 | 3.7 | 12.0 | ||
| Non-SqNSCLC/first–fifth | Pembrolizumab (2 mg/kg) q3w | 6 | 33.3 | 3.7 | 12.0 | ||
| Patnaik et al. [2015] | I/II | NSCLC/second | Pembrolizumab (2–10 mg/kg) + ipilimumab (1–3 mg/kg) | 18 | 39 | NR | NR |
| Herbst et al. [2015] | II/III | NSCLC/second | Pembrolizumab (2 mg/kg) | 345 | 18 | 3.9 | 10.4 |
| Pembrolizumab (10 mg/kg) | 346 | 18 | 4.0 | 12.7 | |||
| Horn et al. [2015] | I | NSCLC/second | Atezolizumab (1–20 mg/kg) | 88 | 23 | 4 | 16 |
| Spigel et al. [2015] | II | NSCLC/second (no brain metastases) | Atezolizumab (1200 mg) | 93 | 17 | 3.5 | 10.6 |
| NSCLC/second (previously treated brain metastases) | Atezolizumab (1200 mg) | 13 | 23 | 4.3 | 6.8 | ||
| Spira et al. [2015] | II | NSCLC/second | Atezolizumab (1200 mg) | 144 | 15 | 2.8 | 11.4 |
| Rizvi et al. [2015a] | I/II | NSCLC/first–third | Durvalumab (10 mg/kg) | 228 | 16 | NR | NR |
| Antonia et al. [2016a] | Ib | NSCLC/second | Durvalumab (3–20 mg/kg) + tremelimumab (1–10 mg/kg) | 102 | 27 | NR | NR |
| Gulley et al. [2015] | Ib | NSCLC/second | Avelumab (10 mg/kg) | 184 | 13.6 | 2.7 | 8.4 |
ORR, overall response rate; PFS, progression-free survival; OS, overall survival; NR, not reported; NYR, not yet reached; NA, nonapplicable; NSCLC, non-small cell lung cancer; Sq, squamous; EGFR, endothelial growth factor receptor; q3w, every three weeks; q2w, every two weeks.
Nivolumab
Nivolumab is a fully human IgG4 monoclonal antibody that binds to and blocks the activation of PD-1 by its ligand. It is currently approved as front-line monotherapy or in combination with ipilimumab for advanced melanoma, as second-line therapy for metastatic renal clear-cell carcinoma, and for advanced NSCLC that progressed on initial therapy.
Nivolumab was initially approved in March 2015 for advanced SqNSCLC based on an open-label, multicenter, randomized phase III trial (CheckMate 017) [Brahmer et al. 2015] that allocated patients to receive either nivolumab (n = 135), 3 mg/kg intravenously (IV) every 2 weeks, or docetaxel (n = 137), 75 mg/m2 IV every 3 weeks. The primary outcome, median overall survival (OS), was significantly higher in the nivolumab group (9.2 versus 6 months [hazard ratio (HR) 0.59; 95% CI, 0.44–0.79; p = 0.00025]. The median PFS was 3.5 months with nivolumab versus 2.8 months with docetaxel (HR for death or disease progression, 0.62; 95% CI, 0.47–0.81; p < 0.001). Response rates were also higher with nivolumab (20% versus 9%, p < 0.002).
The approval for non-SqNSCLC was issued in October 2015, based on demonstration of improvement in OS in an international, multicenter, open-label phase III clinical trial (CheckMate 057) [Borghaei et al. 2015] that randomized (1:1) patients to receive either nivolumab (n = 292), 3 mg/kg every 2 weeks or docetaxel (n = 290), 75 mg/m2 every 3 weeks. Overall survival was improved with a HR of 0.73 (95% CI, 0.60–0.89; p < 0.002). Median OS was 12.2 months in patients treated with nivolumab, compared with 9.4 months in the docetaxel group. Response rates were higher with nivolumab versus docetaxel (19% versus 12%, p = 0.02). Although PFS did not favor nivolumab over docetaxel (median 2.3 versus 4.2 months, respectively), the rate of PFS at 1 year was higher with nivolumab than with docetaxel (19% and 8%, respectively).
Nivolumab is currently being studied in ongoing phase III trials in the front-line setting [ClinicalTrials.gov identifiers: NCT02477826 and NCT02041533] and in the adjuvant setting [ClinicalTrials.gov identifier: NCT02595944]. Several other studies combining nivolumab with chemotherapy, immunotherapy and targeted therapies are ongoing, as listed in Table 2.
Table 2.
Ongoing phase III trials with PD-1 and PD-L1 inhibitors in lung cancer.
| Drug | Manufacturer | Study name | Primary endpoint | Histology/line of treatment | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| Nivolumab versus SOC | Bristol-Myers Squibb | CheckMate 026 | PFS | NSCLC PD-L1+/first | NCT02041533 |
| Nivolumab versus nivolumab + ipilimumab versus nivolumab + chemotherapy versus SOC | CheckMate 227 | OS/PFS | NSCLC/first | NCT02477826 | |
| Nivolumab versus SOC | CheckMate 331 | OS | SCLC/second | NCT02481830 | |
| Pembrolizumab versus SOC | Merck | KEYNOTE 024 | PFS | NSCLC PD-L1+/first | NCT02142738 |
| Pembrolizumab versus SOC | KEYNOTE 042 | OS | NSCLC PD-L1+/first | NCT02220894 | |
| SOC ± Pembrolizumab | KEYNOTE 189 | PFS | NSCLC/first | NCT02578680 | |
| Pembrolizumab versus placebo | KEYNOTE 091 | DFS | NSCLC/adjuvant | NCT02504372 | |
| Durvalumab ± tremelimumab versus SOC | AstraZeneca | MYSTIC | PFS | NSCLC/first | NCT02453282 |
| Osimertinib ± durvalumab | CAURAL | PFS | NSCLC EGFR-mutant/second | NCT02454933 | |
| Atezolizumab versus SOC | Roche/Genentech | IMpower 111 | PFS | NSCLC/first | NCT02409355 |
| SOC ± atezolizumab | IMpower 132 | PFS | NSCLC/first | NCT02657434 | |
| Avelumab versus SOC | EMD Serono | JAVELIN 100 | PFS | NSCLC PD-L1+/first | NCT02576574 |
| Avelumab versus docetaxel | JAVELIN 200 | OS | NSCLC/second | NCT02395172 |
NCT, national clinical trial; SOC, standard of care; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; EGFR, endothelial growth-factor receptor; OS, overall survival; PFS, progression-free survival; DFS, disease-free survival.
Pembrolizumab
Pembrolizumab is a humanized IgG4 PD-1-blocking antibody, currently approved for unresectable or metastatic melanoma as initial therapy or for refractory settings. It was granted accelerated approval for NSCLC, both Sq and non-Sq, based on the results of a randomized phase II/III trial (KEYNOTE-010) that included patients with previously treated advanced NSCLC who were PD-L1 positive in tumor cells by immunohistochemistry (⩾1%) [Herbst et al. 2015]. There were three arms in this trial: pembrolizumab at 2 mg/kg (n = 345), pembrolizumab at 10 mg/kg (n = 346), and docetaxel at 75 mg/m2 (n = 343) administered every 3 weeks. The median OS was 10.4 months for the lower dose of pembrolizumab, 12.7 months for the higher dose, and 8.5 months for docetaxel. OS was significantly longer for both doses of pembrolizumab when compared with docetaxel (pembrolizumab 2 mg/kg: HR, 0.71; 95% CI, 0.58–0.88; p = 0.0008) (pembrolizumab 10 mg/kg: HR, 0.61; 95% CI, 0.49–0.75; p < 0.0001). Response rate was 18% for both pembrolizumab groups against 9% for the docetaxel group.
Pembrolizumab carries the advantage of a slightly more convenient schedule as compared with nivolumab (every 3 weeks, rather than every 2 weeks). Response outcomes were comparable, however pembrolizumab’s study, KEYNOTE-010, was designed accruing only patients with PD-L1-positive tumors. This became a requirement for the FDA’s approval, which certainly decreases the eligibility for the drug, given that PD-L1 expression, although widely varied among published data (13–70%), is present in fewer than half of tumors in most cases [Kerr et al. 2015]. In addition, a second biopsy of the tumor would be often necessary, since PD-L1 expression is an adaptive maneuver by tumor cells to evade the immune system, usually associated with a more resistant line of cells.
Ongoing studies are assessing pembrolizumab as first-line therapy (KEYNOTE-024 [ClinicalTrials.gov identifier: NCT02142738] and KEYNOTE-042 [ClinicalTrials.gov identifier: NCT02220894]) and as adjuvant therapy (PEARLS [ClinicalTrials.gov identifier: NCT02504372]).
Durvalumab
Durvalumab (MEDI4736) is a selective, high-affinity human IgG1 monoclonal antibody that blocks PD-L1 binding to PD-1 and CD80, but does not bind to PD-L2, avoiding potential immune-related toxic effects due to PD-L2 inhibition, previously noted in animal models. Its safety and efficacy was reported in a phase I/II multicenter trial evaluating heavily pretreated patients with NSCLC [Rizvi et al. 2015a]. Durvalumab was administered at 10 mg/kg every 2 weeks until intolerable toxicity or disease progression for up to 12 months. A total of 149 patients were evaluable for response; overall response rate (ORR) was 14% (23% in PD-L1-positive tumors) and disease control rate (DCR) at 24 weeks was 24%. ORR was higher in Sq (21%) versus non-Sq (10%) histology. Responses were durable, with 76% ongoing at the time of the report.
A phase I/II dose-escalation and dose-expansion study reported its preliminary results on durvalumab as first-line therapy [Antonia et al. 2016b]. In 59 patients, it demonstrated an ORR of 25%, with 11 of the 12 responders having PD-L1 expression (the trial was amended to restrict enrollment to PD-L1+ tumors after initial poor response among the PD-L1-negative population). DCR was 56%. Responses were again noted to be durable, with nine ongoing responses (duration of response ranging from 5.7+ to 70.1+ weeks).
MYSTIC [ClinicalTrials.gov identifier: NCT02453282] is a phase III trial currently recruiting patients with stage IV NSCLC with no prior treatment to be randomized to the combination of durvalumab and tremelimumab (anti CTLA-4), durvalumab as monotherapy, or standard-of-care platinum-based chemotherapy. NEPTUNE [ClinicalTrials.gov identifier: NCT02542293] is another phase III open-label study recruiting patients to receive either durvalumab and tremelimumab or standard-of-care chemotherapy. The last ongoing phase III trial compares the efficacy and safety of durvalumab versus standard of care, and the combination of durvalumab and tremelimumab versus standard of care for patients who received at least two prior systemic therapies, including a platinum-based regimen [ClinicalTrials.gov identifier: NCT02352948]. Durvalumab is also being studied in phase III trials in the adjuvant setting for stages Ib, II or IIIA NSCLC [ClinicalTrials.gov identifier: NCT02273375]; and following concurrent chemoradiation in patients with stage III unresectable NSCLC [ClinicalTrials.gov identifier: NCT02125461].
Atezolizumab
Atezolizumab is a fully humanized monoclonal antibody of IgG1 isotype against PD-L1. Its safety is being assessed in phase I and II trials, currently with abstracts of partial results presented at oncology conferences. A phase II trial assessed safety and efficacy of atezolizumab at a dose of 1200 mg every 3 weeks in PD-L1-expressing tumors, with and without treated asymptomatic brain metastases [Spigel et al. 2015]. Overall response was 29% for chemo-naïve patients and 17% for patients who received two or more lines of systemic therapy.
A second phase II trial (POPLAR) randomly assigned 287 patients with NSCLC to receive atezolizumab 1200 mg or docetaxel 75 mg/m2 every 3 weeks [Spira et al. 2015]. OS was 11.4 months for atezolizumab and 9.5 months for docetaxel at a planned interim analysis (p = 0.11). PFS and ORR did not significantly differ between the two groups.
Several studies are ongoing evaluating atezolizumab in different settings and in combinations [ClinicalTrials.gov identifiers: NCT02657434, NCT02409355, NCT02366143, NCT02409342 and NCT02367794].
Avelumab
Avelumab is also a fully human monoclonal IgG1 PD-L1 antibody. A phase Ib expansion cohort including 184 NSCLC patients who progressed on platinum-based therapy and received avelumab at 10 mg/kg every 2 weeks demonstrated an ORR of 12%, stable disease in 38% of patients, and a median PFS of 11.6 weeks [Gulley et al. 2015].
Two phase III trials are ongoing, evaluating avelumab in PD-L1-positive tumors in the front-line setting [ClinicalTrials.gov identifier: NCT02576574] and in those who progressed on platinum-based chemotherapy, against docetaxel [ClinicalTrials.gov identifier: NCT02395172].
Programmed death-ligand 1 expression and mutational landscape
In the era of precision medicine and steadily increasing costs in healthcare, identifying a proper predictive biomarker is of utmost importance in selecting patients who will most likely benefit from specific therapies. Whether PD-L1-positive tumors have a higher chance of responding to PD-1 or PD-L1 inhibitors, and whether it should guide clinical decisions, however, is still unclear. Many of the published trials suggest significantly better response rates and survival correlating with higher levels of PD-L1 expression (KEYNOTE-001, CheckMate 057, POPLAR) [Borghaei et al. 2015; Garon et al. 2015; Spira et al. 2015]. In KEYNOTE-001, pembrolizumab rendered an ORR of 10.7% for less than 10% PD-L1 expression in cells (neoplastic and intercalated mononuclear inflammatory cells) against 45.2% for at least 50%. CheckMate 057, testing nivolumab for non-SqNSCLC, showed 36% ORR in patients with PD-L1 expression of at least 5%, and 37% in at least 10%, as compared with 10% and 11% in less than 5% and less than 10% PD-L1 expression, respectively. Interestingly, CheckMate 017 that evaluated nivolumab for SqNSCLC did not show significant differences in patients’ outcomes between the treatment groups based on PD-L1 expression, in agreement with several other early-phase trials [Brahmer et al. 2015].
There are some reasons that potentially explain the lack of concordance between trials. PD-L1 expression score is often measured through several different methods (distinct immunohistochemistry antibody clones, staining protocols, and platforms), different scoring systems, and arbitrary cutoff values (1%, 5%, 10% and 50%). Other reasons include the dynamic nature of PD-L1 expression, tumor histology, consideration of PD-L1 staining in the tumor microenvironment, and smoking status [Ji et al. 2015; Omori et al. 2015; Owonikoko et al. 2015].
Another important factor that might explain the difference in outcomes is the mutation burden of the tumor. The best responses of immunotherapy are noted in cancers with a high mutational load like melanomas, due to chronic exposure to ultraviolet light, and lung cancers, secondary to carcinogens from cigarette smoking [Herbst et al. 2014]. A study sequenced the exome from two independent cohorts of NSCLC patients treated with pembrolizumab and their matched normal deoxyribonucleic acid (DNA) [Rizvi et al. 2015b]. It was shown that a higher somatic nonsynonymous mutation burden was associated with the clinical efficacy of pembrolizumab. A total of 73% of patients with high nonsynonymous mutation burden experienced durable clinical benefit, as compared with 13% in the low mutation burden group (p = 0.04). ORR and PFS were also higher in patients with high nonsynonymous burden (ORR 63% versus 0%, p = 0.03; median PFS 14.5 versus 3.7 months, p = 0.01). The molecular signature of cigarette smoking also correlated with better outcomes. Most samples in this study were PD-L1-positive, therefore an association between mutation burden and PD-L1 expression could not be reliably assessed.
The observation that nonsynonymous mutation burden is associated with pembrolizumab efficacy is consistent with the hypothesis that, as a consequence of somatic mutations, neoantigens are expressed by tumor cells and recognized by the immune system. This finding could account for the discordance in outcomes by PD-L1 expression in the nivolumab’s Sq versus non-SqNSCLC populations. Sq lung cancers are more likely to be related to tobacco exposure and have higher mutational loads, so one could expect a more uniform response to immunotherapy, whereas non-Sq populations are more heterogeneous with regard to cigarette-smoking history and mutation burden, with a higher variance in responses.
Future directions: combination therapies
Several early-phase studies have already reported decent activity and an adequate safety profile in combining PD-1 or PD-L1 inhibitors with other classes of drugs, such as chemotherapy, targeted therapies and CTLA-4 inhibitors.
Chemotherapy
The rationale to combine immunotherapy with chemotherapy is to achieve a rapid and large initial response through the action of the cytotoxic drugs, thus releasing antigens to be recognized, and provide a later, however durable, response with checkpoint inhibition. KEYNOTE-021 evaluated the safety and activity of pembrolizumab combined with either: carboplatin and paclitaxel (cohort A); carboplatin, paclitaxel and bevacizumab (non-Sq, cohort B); or carboplatin and pemetrexed (non-Sq, cohort C) [Gadgeel et al. 2016]. A total of 74 treatment-naïve patients with advanced NSCLC were enrolled. ORR was 52% in cohort A, 48% in cohort B and 71% in cohort C. Grade 3/4 treatment-related adverse events (AEs) occurred in 36%, 46% and 42% of patients in cohorts A, B and C, respectively, with one treatment-related death (cohort B, pericardial effusion). Responses were regardless of PD-L1 status.
Another phase Ib study revealed an ORR of 67% through combinations of atezolizumab with different platinum-based doublets, again showing better results with pemetrexed. The safety profile was as predicted, with no unexpected toxicities. Phase III studies are ongoing with different checkpoint inhibitors in combination with chemotherapy in the front-line setting for advanced NSCLC.
Targeted therapy
Several tyrosine kinase inhibitors (TKIs) are approved as first-line therapies to treat ALK-translocated and EGFR-mutant non-SqNSCLC. EGFR pathway activation was shown to induce PD-1, PD-L1 and CTLA-4 upregulation, and increased markers of T-cell exhaustion [Akbay et al. 2013]. Therefore, a combined treatment strategy with checkpoint inhibitors was contemplated. Results of preclinical data, however, failed to demonstrate a synergistic effect, finding instead downregulation of PD-L1 expression after blockade of the EGFR pathway with TKIs [Akbay et al. 2013; Chen et al. 2015]. Findings suggested, on the other hand, that PD-1/PD-L1 blockade might play a role in EGFR-TKIs-resistant NSCLC patients.
Still, combination therapy with checkpoint inhibitors is being tested in several clinical trials, attempting to achieve durable responses and prolong survival among these molecularly defined patient subgroups. A phase I dose-escalation study with durvalumab and gefitinib showed grade 3/4 side effects in 3 out of 10 patients and the maximum tolerated dose was not reached [Creelan et al. 2015]. Phase I and II studies are ongoing, with nivolumab plus erlotinib or crizotinib for EGFR-mutated or ALK-translocated tumors, respectively [ClinicalTrials.gov identifiers: NCT01998126 and NCT02323126]. Pembrolizumab is being tested in combination with afatinib and crizotinib [ClinicalTrials.gov identifiers: NCT02364609, NCT02511184 and NCT02323126]. Atezolizumab is being combined with erlotinib or alectinib in a phase Ib trial [ClinicalTrials.gov identifier: NCT02013219].
Cytotoxic T-lymphocyte-associated antigen 4 inhibitors
Inhibiting the PD-1/PD-L1 pathway leads to PI3K activity reduction and downstream AKT activation [Patsoukis et al. 2012]. Anti-CTLA-4s, on the other hand, had no effect on the PI3K pathway, but inhibited AKT activation [Karman et al. 2012]. Because of the distinct mechanisms of regulating immune response, it was thought that combining both drug classes would provide a synergistic effect with high and durable responses. The anti-PD1 and anti-CTL4 combination was initially tested and approved in melanoma patients [Larkin et al. 2015]. Its use in lung cancer is being tested in the front-line and refractory settings.
CheckMate-012 is a phase I study that evaluated the safety and efficacy of nivolumab in combination with ipilimumab as first-line therapy in 148 patients with advanced NSCLC [Hellmann et al. 2016]. The trial consisted of four treatment groups, with doses varying from 1 to 3 mg/kg for both nivolumab and ipilimumab. The group selected for further exploration was nivolumab 3 mg/kg every 2 weeks plus ipilimumab 1 mg/kg every 6 weeks. In this group, any-grade AEs were seen in 69% of patients, and 28% were grades 3 or 4. ORR was 31% and median PFS was 8.3 months.
Another recent phase Ib study was conducted at five cancer centers in the US and evaluated durvalumab in combination with tremelimumab in metastatic NSCLC [Antonia et al. 2016a]. Durvalumab was given in doses of 3–20 mg/kg every 4 weeks, or 10 mg/kg every 2 weeks, and tremelimumab in doses of 1–10 mg/kg every 4 weeks for six doses and then every 12 weeks for three doses. The primary endpoint was safety. A total of 102 patients were enrolled into the dose-escalation phase with a median follow up of 18.8 weeks. The maximum tolerated dose was exceeded in the cohort receiving durvalumab 20 mg/kg plus tremelimumab 3 mg/kg every 4 weeks, with two (33%) of six patients having a dose-limiting toxicity. Treatment-related serious AEs occurred in 36% of patients and three deaths were related to treatment (attributed to myasthenia gravis, pericardial effusion and neuromuscular disorder). Evidence of clinical activity was noted both in PD-L1-positive and PD-L1-negative tumors. Response was noted in 17% of patients, and most of them were durable responses. The dose of 20 mg/kg of durvalumab and 1 mg/kg of tremelimumab administered IV every 4 weeks was considered safe and selected to be tested in phase III studies, which are ongoing.
Radiation therapy
The use of checkpoint inhibitors in nonmetastatic patient populations and in combination with radiation therapy is not established. Radiation is being administered with more accurate contouring, minimizing toxicities and increasing efficacy [Tang et al. 2014; Seyedin et al. 2015]. It is very commonly used in oligometastatic disease or oligoprogression, contributing to increased PFS and possibly OS [Siva et al. 2010; Iyengar et al. 2014; Xanthopoulos et al. 2015]. One of the reasons for radiation therapy to improve patients’ outcomes in the metastatic setting could be that by targeting specific lesions that might have acquired resistance to the current systemic therapy being administered, it allows for the same regimen to be given for a longer period of time.
Another extremely important mechanism by which radiotherapy can improve patients’ outcomes is through the release of antigens that follows tumor DNA damage. The antigenic stimulation can induce enhanced and tumor-specific responses from the immune system against tumor cells, through increased activity of cytotoxic T cells [Hiniker et al. 2012]. The effect is seen not only locally, but also systemically, with reports of complete remissions in metastasis at distant sites [Finkelstein et al. 2011; Postow et al. 2012; Golden et al. 2013]. This is known as the ‘abscopal effect’, and the immune system has been shown to play a crucial role in it. Preclinical data demonstrated that the immune response was tumor specific and the theory was corroborated by the demonstration that mice depleted of CD8+ T cells or T-cell-deficient mice had no abscopal effect following radiotherapy [Demaria et al. 2004; Liang et al. 2013].
Combining radiation with immunotherapy, therefore, became of great interest for researchers. More specifically, stereotactic body radiation therapy (SBRT) provides a high rate of local control, has a favorable toxicity profile, and can induce a more robust immune response when compared with conventionally fractioned radiotherapy by being able to deliver high doses of radiation in a more precisely delineated target [Seung et al. 2012; Amini et al. 2014]. Blocking immune checkpoints could augment the abscopal effect and tumor responses. Significant clinical responses were already reported from the combination of ipilimumab and radiotherapy in melanoma with temporal association. Several early-phase studies are being developed to evaluate the safety and efficacy of this approach in a variety of solid tumors, the majority of them utilizing SBRT [ClinicalTrials.gov identifiers: NCT02608385, NCT02407171, NCT02400814 and NCT02463994].
A phase I trial enrolled 25 patients with refractory advanced malignancies with lung or liver metastases to receive four doses of ipilimumab at 3 mg/kg, with either concurrent SBRT (starting the day after the first dose), or sequential SBRT (starting one week after the first dose), in a radiation-dose-escalation fashion [Tang et al. 2015]. Twelve patients completed all four cycles and nine patients completed planned radiographic evaluation after cycle four. Five of these exhibited decreased disease burden. In many instances, responding lesions were outside the radiation field. There were no grade 4/5 toxicities, and five patients experienced grade 3 AEs.
Small cell lung cancer
The population suffering from small cell lung cancer (SCLC) faces far worse survival outcomes than NSCLC patients, even at early stages [Byers and Rudin, 2015]. Treatment currently relies on platinum-based doublets, which usually provides good, however short, response [Noda et al. 2002]. Topotecan is the standard second-line therapy, which provides a short clinical benefit in a small percentage of patients [Von Pawel et al. 1999, 2014; O’Brien et al. 2006].
Following the same rationale of NSCLC, SCLC might be an immunologically manipulable neoplasm, given the high rate of somatic mutations, mostly associated with tobacco exposure [Peifer et al. 2012]. It was shown that significantly more immune effector T cells (Teff) were detected in limited-stage SCLC compared with extended-staged disease [Koyama et al. 2008]. Long-term survivors of SCLC maintained a high Teff to Treg cell ratio, whereas patients with recurrent disease exhibited a low Teff:Treg ratio.
Immunotherapy, as expected, is already showing promising results in early-phase trials, with a 25% response rate and a 31% DCR in a phase Ib trial with pembrolizumab administered in 16 patients with PD-L1-positive, platinum-refractory advanced disease [Ott et al. 2015].
Most recently, a phase I/II trial enrolled 216 patients that had disease progression after at least one previous platinum-containing regimen to receive either nivolumab alone at 3 mg/kg, or in combination with ipilimumab in different treatment groups, ranging from 1–3 mg/kg of both drugs [Antonia et al. 2016c]. ORR was 10% in the nivolumab group, 23% in the nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (N1 + I3) group and 19% in the nivolumab 3 mg/kg plus ipilimumab 1 mg/kg (N3 + I1). Grade 3 or 4 treatment-related AEs occurred in 13% in the nivolumab group, 30% in the N1 + I3 group and 19% in the N3 + I1 group, with discontinuation of therapy due to treatment-related AEs occurring in 6%, 11% and 7%, respectively. One patient died of pneumonitis in the N3 + I1 group.
On the basis of this trial, phase III studies are ongoing comparing nivolumab alone versus N1 + I3 every 3 weeks for two 42-day cycles followed by nivolumab versus placebo as maintenance therapy for those with disease control (CheckMate 451 [ClinicalTrials.gov identifier: NCT02538666]). Nivolumab is also being compared with single-agent chemotherapy as second-line therapy (CheckMate 331 [ClinicalTrials.gov identifier: NCT02481830]).
Toxicity
Checkpoint inhibitors are a unique class of drugs, not only in how they affect cancer, but also in how they affect the human body. Unfortunately, immunologic activation is nonspecific and can affect healthy tissues as a result of highly activated CD4 and CD8 T cells. Although a reduced rate of any-grade and grades 3/4 AEs were seen with anti-PD1 therapy when compared with chemotherapy (CheckMate 057 and 017 showed 58% and 69% of all grades in the nivolumab arm versus 86% and 88% in the docetaxel arm; 7% and 10% of grade 3–4 in the nivolumab arm versus 55% and 69% in the docetaxel arm), they can be dangerous and potentially fatal if not recognized and promptly treated. Most common AEs from any grade, ranging from 8% to 16% include fatigue, nausea, hyporexia, asthenia and diarrhea. Fatal events with checkpoint inhibitors are very rare (<1%), due to encephalitis, myasthenia gravis, pneumonitis and pericarditis (Table 3). Diarrhea or colitis and skin rashes can be severe. However, overall, these agents are much better tolerated than standard cytotoxic chemotherapy. Discontinuation of therapy due to AEs occurred in 3–10% with the PD-1 inhibitors nivolumab and pembrolizumab.
Table 3.
Treatment-related adverse events reported in phase II/III trials of checkpoint inhibitors in non-small cell lung cancer.
| Trial | Drug | Number of patients | Grades 1 and 2 (%) | Grades 3–5 (%) | Deaths | Discontinuations (%) |
|---|---|---|---|---|---|---|
| CheckMate 017 Brahmer et al. [2015] | Nivolumab | 131 | 58 | 7 | 0 | 3 |
| CheckMate 057 Borghaei et al. [2015] | Nivolumab | 287 | 69 | 10 | 1* | 5 |
| KEYNOTE 010 Herbst et al. [2015] | Pembrolizumab 2 mg/kg | 339 | 63 | 13 | 3$ | 4 |
| Pembrolizumab 10 mg/kg | 343 | 66 | 16 | 3¶ | 5 | |
| POPLAR Spira et al. [2015] | Atezolizumab | 142 | 67 | 11 | 1§ | 1 |
Death caused by encephalitis.
Deaths caused by pneumonitis (two patients) and pneumonia (one patient).
Deaths caused by myocardial infarction (one patient), pneumonitis (one patient) and pneumonia (one patient).
Death caused by cardiac failure.
Management of symptoms often requires treatment delays and the toxicity is managed differently from chemotherapy. Physicians need to be aware that early administration of oral or intravenous steroids when indicated is crucial to the appropriate management of these side effects, with a slow taper needed to allow recovery and safety in the administration of subsequent doses. Therefore, success of this class of drugs is strictly related to prompt recognition of immune-related toxicity and to strict adherence to guidelines for their management.
Conclusion
Immunotherapy research has been rapidly trailing an important path in cancer treatment, making its way to improve outcomes in several different cancer histologies.
There are still several unanswered questions such as how to best select patients who will benefit the most, how to most accurately assess response to treatment, and how to overcome resistance to checkpoint inhibitors. It is, nonetheless, an exciting time for research and drug development, as we are coming closer to further improving lung cancer patients’ outcomes and quality of life.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Gustavo Schvartsman, Division of Cancer Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Renata Ferrarotto, Department of Thoracic Head and Neck Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Erminia Massarelli, Department of Medical Oncology, City of Hope Comprehensive Cancer Center, 1500 East Duarte Road, Duarte, CA 91010, USA.
References
- Akbay E., Koyama S., Carretero J., Altabef A., Tchaicha J., Christensen C., et al. (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3: 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini A., Yeh N., Gaspar L., Kavanagh B., Karam S. (2014) Stereotactic body radiation therapy (SBRT) for lung cancer patients previously treated with conventional radiotherapy: a review. Radiat Oncol 9: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia S., Gettinger S., Chow L., Juergens R., Borghaei H., Shen Y., et al. (2014) Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line non-small cell lung cancer (NSCLC): interim phase I results. ASCO Annual Meeting Abstracts 32: 8023. [Google Scholar]
- Antonia S., Goldberg S., Balmanoukian A., Chaft J., Sanborn R., Gupta A., et al. (2016a) Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase Ib study. Lancet Oncol 17: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia S., Kim S., Spira A., Ahn M., Ou S., Stjepanovic N., et al. (2016b) Safety and clinical activity of durvalumab (MEDI4736), an anti-PD-L1 antibody, in treatment-naïve patients with advanced non-small-cell lung cancer. ASCO Annual Meeting Abstracts 34: 9029. [Google Scholar]
- Antonia S., López-Martin J., Bendell J., Ott P., Taylor M., Eder J., et al. (2016c) Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase I/II trial. Lancet Oncol 17: 883–895. [DOI] [PubMed] [Google Scholar]
- Borghaei H., Paz-Ares L., Horn L., Spigel D., Steins M., Ready N., et al. (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Horn L., Gandhi L., Spigel D., Antonia S., Rizvi N., et al. (2014) Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): Survival and clinical activity by subgroup analysis. ASCO Annual Meeting Abstracts 32: 8112. [Google Scholar]
- Brahmer J., Reckamp K., Baas P., Crino L., Eberhardt W., Poddubskaya E., et al. (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers L., Rudin C. (2015) Small cell lung cancer: where do we go from here? Cancer 121: 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B., Hughes B. (2015) Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 4: 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Fang W., Zhan J., Hong S., Tang Y., Kang S., et al. (2015) Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol 10: 910–923. [DOI] [PubMed] [Google Scholar]
- Creelan B., Chow L., Kim D., Kim S., Yeh T., Karakunnel J., et al. (2015) Safety and tolerability results from a phase I study of MEDI4736, a human IgG1 anti-programmed cell death-ligand-1 (PD-L1) antibody, combined with gefitinib in patients (pts) with non-small-cell lung cancer (NSCLC). ASCO Annual Meeting Abstracts 33: 3047. [Google Scholar]
- Demaria S., Ng B., Devitt M., Babb J., Kawashima N., Liebes L., et al. (2004) Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 58: 862–870. [DOI] [PubMed] [Google Scholar]
- Finkelstein S., Timmerman R., Mcbride W., Schaue D., Hoffe S., Mantz C., et al. (2011) The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol 2011: 439752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgeel S., Stevenson J., Langer C., Gandhi L., Borghaei H., Patnaik A., et al. (2016) Pembrolizumab (pembro) plus chemotherapy as front-line therapy for advanced NSCLC: KEYNOTE-021 cohorts A-C. ASCO Annual Meeting Abstracts 34: 9016. [Google Scholar]
- Garon E., Rizvi N., Hui R., Leighl N., Balmanoukian A., Eder J., et al. (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- Gettinger S., Horn L., Gandhi L., Spigel D., Antonia S., Rizvi N., et al. (2015) Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33: 2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden E., Demaria S., Schiff P., Chachoua A., Formenti S. (2013) An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 1: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley J., Spigel D., Kelly K., Chandler J., Rajan A., Hassan R., et al. (2015) Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: a phase Ib, open-label expansion trial in patients progressing after platinum-based chemotherapy. ASCO Annual Meeting Abstracts 33: 8034. [Google Scholar]
- Hellmann M., Gettinger S., Goldman J., Brahmer J., Borghaei H., Chow L., et al. (2016) CheckMate 012: safety and efficacy of first-line (1L) nivolumab (nivo; N) and ipilimumab (ipi; I) in advanced (adv) NSCLC. ASCO Annual Meeting Abstracts 34: 3001. [Google Scholar]
- Herbst R., Baas P., Kim D., Felip E., Perez-Gracia J., Han J., et al. (2015) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- Herbst R., Soria J., Kowanetz M., Fine G., Hamid O., Gordon M., et al. (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDl3280A in cancer patients. Nature 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiniker S., Chen D., Reddy S., Chang D., Jones J., Mollick J., et al. (2012) A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol 5: 404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn L., Spigel D., Gettinger S., Antonia S., Gordon M., Herbst R., et al. (2015) Clinical activity, safety and predictive biomarkers of the engineered antibody MPDl3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study. ASCO Annual Meeting Abstracts 33: 8029. [Google Scholar]
- Howington J., Blum M., Chang A., Balekian A., Murthy S. (2013) Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143: e278S–e313S. [DOI] [PubMed] [Google Scholar]
- Howlader N., Noone A., Krapcho M., Garshell J., Miller D., Altekruse S., et al. (2015) SEER Cancer Statistics Review, 1975–2012, National Cancer Institute: Bethesda, MD; Available at: http://seer.cancer.gov/csr/1975_2012/ (accessed 30 March 2016). Based on November 2014. SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- Iyengar P., Kavanagh B., Wardak Z., Smith I., Ahn C., Gerber D., et al. (2014) Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 32: 3824–3830. [DOI] [PubMed] [Google Scholar]
- Ji M., Liu Y., Li Q., Li X., Zhao W., Zhang H., et al. (2015) PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J Transl Med 13: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karman J., Jiang J., Gumlaw N., Zhao H., Campos-Rivera J., Sancho J., et al. (2012) Ligation of cytotoxic T lymphocyte antigen-4 to T cell receptor inhibits T cell activation and directs differentiation into Foxp3+ regulatory T cells. J Biol Chem 287: 11098–11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir M., Butte M., Freeman G., Sharpe A. (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr K., Tsao M., Nicholson A., Yatabe Y., Wistuba I., Hirsch F., et al. (2015) Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol 10: 985–989. [DOI] [PubMed] [Google Scholar]
- Khozin S., Blumenthal G., Zhang L., Tang S., Brower M., Fox E., et al. (2015) FDA approval: ceritinib for the treatment of metastatic anaplastic lymphoma kinase-positive non-small cell lung cancer. Clin Cancer Res 21: 2436–2439. [DOI] [PubMed] [Google Scholar]
- Konishi J., Yamazaki K., Azuma M., Kinoshita I., Dosaka-Akita H., Nishimura M. (2004) B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 10: 5094–5100. [DOI] [PubMed] [Google Scholar]
- Koyama K., Kagamu H., Miura S., Hiura T., Miyabayashi T., Itoh R., et al. (2008) Reciprocal CD4+ T-cell balance of effector CD62llow CD4+ and CD62lhighcD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res 14: 6770–6779. [DOI] [PubMed] [Google Scholar]
- Lafferty K., Warren H., Woolnough J., Talmage D. (1978) Immunological induction of T lymphocytes: role of antigen and the lymphocyte costimulator. Blood Cells 4: 395–406. [PubMed] [Google Scholar]
- Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J., Cowey C., Lao C., et al. (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighl N. (2012) Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol 19: S52–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesterhuis W., Steer H., Lake R. (2011) PD-L2 is predominantly expressed by Th2 cells. Mol Immunol 49: 1–3. [DOI] [PubMed] [Google Scholar]
- Liang H., Deng L., Chmura S., Burnette B., Liadis N., Darga T., et al. (2013) Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol 190: 5874–5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melosky B. (2014) Treatment algorithms for patients with metastatic non-small cell, non-squamous lung cancer. Front Oncol 4: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R., Escudier B., Mcdermott D., George S., Hammers H., Srinivas S., et al. (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio M., Hida T., Nakagawa K., Sakai H., Nogami N., Atagi S., et al. (2015) Phase II studies of nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients with advanced squamous (sq) or nonsquamous (non-sq) non-small cell lung cancer (NSCLC). ASCO Annual Meeting Abstracts 33: 8027. [Google Scholar]
- Noda K., Nishiwaki Y., Kawahara M., Negoro S., Sugiura T., Yokoyama A., et al. (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346: 85–91. [DOI] [PubMed] [Google Scholar]
- O’Brien M., Ciuleanu T., Tsekov H., Shparyk Y., Čučeviá B., Juhasz G., et al. (2006) Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 24: 5441–5447. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Maeda A., Nishimura H., Kurosaki T., Honjo T. (2001) PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A 98: 13866–13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori S., Kenmotsu H., Abe M., Watanabe R., Sugino T., Wakuda K., et al. (2015) Changes in PD-L1 expression in non-small cell lung cancer by immunohistochemical analysis. ASCO Annual Meeting Abstracts 33: e22118. [Google Scholar]
- Ott P., Fernandez M., Hiret S., Kim D., Moss R., Winser T., et al. (2015) Pembrolizumab (Mk-3475) in Patients (Pts) with Extensive-Stage Small Cell Lung Cancer (Sclc): Preliminary Safety and Efficacy Results from Keynote-028. ASCO Annual Meeting Abstracts 33: 7502. [Google Scholar]
- Owonikoko T., Kowalski J., Kim S., Dwivedi B., Chen Z., Behera M., et al. (2015) PD-L1, PD-1, and CTLA-4 as prognostic biomarkers in resected non-small cell lung cancer. ASCO Annual Meeting Abstracts 33: 7551. [Google Scholar]
- Pardoll D. (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik A., Socinski M., Gubens M., Gandhi L., Stevenson J., Bachman R., et al. (2015) Phase I study of pembrolizumab (pembro; MK-3475) plus ipilimumab (IPI) as second-line therapy for advanced non-small cell lung cancer (NSCLC): KEYNOTE-021 Cohort D. ASCO Annual Meeting Abstracts 33: 8011. [Google Scholar]
- Patsoukis N., Sari D., Boussiotis V. (2012) PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle 11: 4305–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M., Fernandez-Cuesta L., Sos M., George J., Seidel D., Kasper L., et al. (2012) Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 44: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M., Callahan M., Barker C., Yamada Y., Yuan J., Kitano S., et al. (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi N., Brahmer J., Ou S., Segal N., Khleif S., Hwu W., et al. (2015a) Safety and clinical activity of MEDI4736, an anti-programmed cell death-ligand 1 (PD-L1) antibody, in patients with non-small cell lung cancer (NSCLC). ASCO Annual Meeting Abstracts 33: 8032. [Google Scholar]
- Rizvi N., Chow L., Borghaei H., Shen Y., Harbison C., Alaparthy S., et al. (2014) Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC. ASCO Annual Meeting Abstracts 32: 8022. [Google Scholar]
- Rizvi N., Hellmann M., Snyder A., Kvistborg P., Makarov V., Havel J., et al. (2015b) Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi N., Mazieres J., Planchard D., Stinchcombe T., Dy G., Antonia S., et al. (2015c) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase II, single-arm trial. Lancet Oncol 16: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C., Schachter J., Long G., Arance A., Grob J., Mortier L., et al. (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., et al. (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase III trial. Lancet Oncol 13: 239–246. [DOI] [PubMed] [Google Scholar]
- Sandler A., Gray R., Perry M., Brahmer J., Schiller J., Dowlati A., et al. (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550. [DOI] [PubMed] [Google Scholar]
- Seung S., Curti B., Crittenden M., Walker E., Coffey T., Siebert J., et al. (2012) Phase I study of stereotactic body radiotherapy and interleukin-2—tumor and immunological responses. Sci Trans Med 4: 137ra74. [DOI] [PubMed] [Google Scholar]
- Seyedin S., Schoenhals J., Lee D., Cortez M., Wang X., Niknam S., et al. (2015) Strategies for combining immunotherapy with radiation for anticancer therapy. Immunotherapy 7: 967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard K., Fitz L., Lee J., Benander C., George J., Wooters J., et al. (2004) PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett 574: 37–41. [DOI] [PubMed] [Google Scholar]
- Siegel R., Miller K., Jemal A. (2015) Cancer statistics, 2015. CA Cancer J Clin 65: 5–29. [DOI] [PubMed] [Google Scholar]
- Siva S., Macmanus M., Ball D. (2010) Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol 5: 1091–1099. [DOI] [PubMed] [Google Scholar]
- Solomon B., Mok T., Kim D., Wu Y., Nakagawa K., Mekhail T., et al. (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371: 2167–2177. [DOI] [PubMed] [Google Scholar]
- Spigel D., Chaft J., Gettinger S., Chao B., Dirix L., Schmid P., et al. (2015) Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1-selected patients with non-small cell lung cancer (NSCLC). ASCO Annual Meeting Abstracts 33: 8028. [Google Scholar]
- Spira A., Park K., Mazieres J., Vansteenkiste J., Rittmeyer A., Ballinger M., et al. (2015) Efficacy, safety and predictive biomarker results from a randomized phase II study comparing MPDL3280A versus docetaxel in 2L/3L NSCLC (POPLAR). ASCO Annual Meeting Abstracts 33: 8010. [Google Scholar]
- Tang C., Naing A., De Groot P., Chang J., Massarelli E., Parkhurst K., et al. (2015) Phase I study of ipilimumab and stereotactic radiation targeting liver or lung lesions in patients with advanced malignancies. Int J Radiat Oncol 93: S208. [Google Scholar]
- Tang C., Wang X., Soh H., Seyedin S., Cortez M., Krishnan S., et al. (2014) Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher N., Hirsch F., Luft A., Szczesna A., Ciuleanu T., Dediu M., et al. (2015) Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase III trial. Lancet Oncol 16: 763–774. [DOI] [PubMed] [Google Scholar]
- Von Pawel J., Jotte R., Spigel D., O’Brien M., Socinski M., Mezger J., et al. (2014) Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol 32: 4012–4019. [DOI] [PubMed] [Google Scholar]
- Von Pawel J., Schiller J., Shepherd F., Fields S., Kleisbauer J., Chrysson N., et al. (1999) Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 17: 658–667. [DOI] [PubMed] [Google Scholar]
- Xanthopoulos E., Handorf E., Simone C., IInd, Grover S., Fernandes A., Sharma S., et al. (2015) Definitive dose thoracic radiation therapy in oligometastatic non-small cell lung cancer: a hypothesis-generating study. Pract Radiat Oncol 5: e355–e363. [DOI] [PubMed] [Google Scholar]
- Zhou C., Wu Y., Chen G., Liu X., Zhu Y., Lu S., et al. (2015) Beyond: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer.J Clin Oncol 33: 2197–2204. [DOI] [PubMed] [Google Scholar]