Abstract
Rules linking patterns of olfactory receptor neuron activation in the nose to activity patterns in the brain and ensuing odor perception remain poorly understood. Artificially stimulating olfactory neurons with electrical currents and measuring ensuing perception may uncover these rules. We therefore inserted an electrode into the nose of 50 human volunteers and applied various currents for about an hour in each case. This induced assorted non-olfactory sensations but never once the perception of odor. To validate contact with the olfactory path, we used functional magnetic resonance imaging to measure resting-state brain activity in 18 subjects before and after un-sensed stimulation. We observed stimulation-induced neural decorrelation specifically in primary olfactory cortex, implying contact with the olfactory path. These results suggest that indiscriminate olfactory activation does not equate with odor perception. Moreover, this effort serendipitously uncovered a novel path for minimally invasive brain stimulation through the nose.
Keywords: olfactory perception, olfactory cortex, olfactory epithelium, default-mode network, resting-state activity, brain stimulation
Introduction
Direct electrical stimulation of receptor surfaces served in the early study of vision and audition, and in both cases, these efforts also led to sensory prostheses, still in development in vision (Lewis et al. 2015), and in advanced application in audition (Kan and Litovsky 2014). There are several reasons to pursue a similar path in olfaction. First, the principles underlying olfactory coding remain poorly understood (Haddad et al. 2010; Spors et al. 2012; Mainland et al. 2014; Nunez-Parra et al. 2014). If we could measure perception following systematic titration of spatial and/or temporal electrical stimulation parameters, this may reveal important aspects of the olfactory code (Wander and Rao 2014). Moreover, several investigations into this code have historically relied on artificial stimulation of the rodent olfactory system at various levels using either electrophysiology or more recently optogenetics (Ottoson 1959; Spors et al. 2012; Smear et al. 2013; Li et al. 2014). Given that rodents cannot explicitly report perception, characterization of the path from artificial stimulation to explicit perception is important. In turn, this effort may also have value beyond the probing of basic principles alone. Although loss of olfaction is not as devastating to humans as loss of audition and vision, it nevertheless carries a meaningful deleterious impact (Pinto et al. 2014; Croy et al. 2014a). If we could artificially generate olfactory perception, this may aid in sensory restoration (Fleiner et al. 2012). Finally, electrical stimulation of olfactory sensory neurons may be technically simple because these neurons are in fact quite accessible in the human nose. This accessibility has allowed for live biopsies of these neurons (Feron et al. 1998; Leopold et al. 2000; Rawson and Ozdener 2013), and for recording odorant-induced local field potentials directly from the epithelial surface of subjects who concurrently reported olfactory perception (Hummel et al. 1996; Lapid et al. 2009, 2011; Lapid and Hummel 2013).
Despite the above, there have been only few efforts to electrically stimulate olfactory sensory neurons in order to generate odor perception, and these efforts yielded mixed results. One early study reported assorted olfactory sensations following electrical stimulation of the nasal mucosa, including electrically induced perception of the odors of almond, bitter almond, vanilla, and a general burnt odor or stench (Uziel 1973). Later efforts, however, failed to replicate this outcome (Straschill et al. 1983; Ishimaru et al. 1997). This failure to artificially generate perception persisted despite concurrent electrical stimulation-related evoked potentials recorded directly from the olfactory tract (Sato et al. 1996), and from the scalp (attributed by the authors to the olfactory bulb) (Ishimaru et al. 1997). These peripheral measurements, however, do not determine whether electrical stimulation in the nose at all reached the cortex in general, and olfactory structures in particular. Given the potential above-described value of such stimulation, and relying on recent advances in endoscopically guided electrode placement (Lapid et al. 2011), we decided to again try and generate olfactory perception through electrical stimulation of the human olfactory mucosa. Critically, here we also used functional magnetic resonance imaging (fMRI) in order to ask whether such stimulation is reflected in brain activity.
Materials and Methods
Subjects
Overall, we studied 60 subjects (27 F, mean age: 26.3 ± 5.1), who participated in a total of 156 separate experimental sessions after providing written informed consent to procedures approved by the Wolfson Hospital Helsinki Committee. Because tolerance for the endoscopic procedures we used in this study increases with experience, several subjects participated in Experiments 1, 2, and 3. All subjects were screened for good general physical condition, self-reported intact olfaction, no history of psychiatric disease, and non-use of chronic medication of any kind. All experiments were preceded by an endoscopic examination that assured a clear and undamaged nasal passage.
Electrical Stimulation
A pure-silver stimulating macro-electrode (diameter: 700 ÷ 1000 µm) was placed under endoscopic guidance at the target intranasal tissue location (Fig. 1A). In different experiments, we targeted different locations at the ventral surface of the middle turbinate, the superior turbinate, the septum dorsum, and the olfactory cleft (Fig. 1B). Stimulation was generated using a custom made and constructed battery-powered electronic stimulator (voltage source 0–3.2 V, peak to peak), driven by an electro-optically isolated function generator (Supplementary Figure 2). Biphasic sine wave electrical pulses were delivered in separate experiments in either continuous or burst mode. Because current is dependent on the naturally fluctuating mucosal impedance and electrode contact, currents were measured continuously in real time using a battery-powered current-to-voltage converter circuit. This allowed us to assure electrode contact throughout the experiment, and to reposition electrodes in cases of lost contact due to motion.
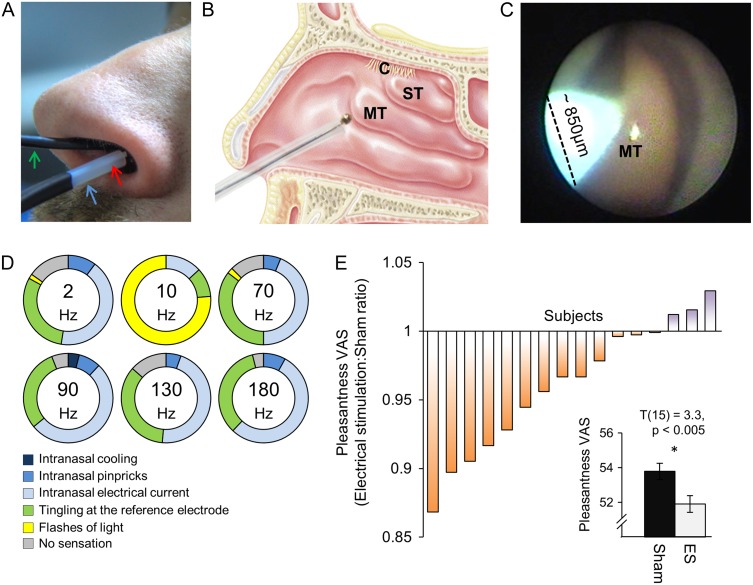
Figure 1.
Intranasal electrical stimulation modifies but does not generate olfactory perception. (A) An endoscope (green arrow) was used to guide the electrode through an endoscopic working channel. Two Teflon tubes were positioned at the entrance of the stimulated nostril: one for delivering odors from an olfactometer (blue arrow), and another for measuring nasal airflow (red arrow). (B) A schematic of electrode placement within a sagittal section of the human nasal cavity, including the middle turbinate (MT), the superior turbinate (ST), and the olfactory cleft (C). (C) An endoscopic view inside the right nostril. The shiny pear-shaped body in the nostril is the MT. The pure-silver stimulating macro-electrode shows up as green in the image. (D) Perception induced by electrical stimulation. Percentage of stimulated subjects (n = 50) that reported: no sensation (gray); flashes of light (yellow); tingling at the reference electrode (green); or generalized intranasal somatosensory sensations such as electrical current (light blue), pinpricks (blue), and cooling (dark blue). Not one subject reported perception of odor. (E) Odor perception altered by electrical stimulation (n = 16). Each bar reflects the ratio between mean pleasantness rating given by a subject during electrical stimulation and sham. A mean of 1 implies no difference between the terms. An average lower than 1 implies reduced pleasantness during electrical stimulation. Inset contains the averaged data in bar representation, error bars are standard error of the mean (s.e.m).
Experiment 1: Electrical Stimulation Aimed at Evoking the Perception of Odor
In this experiment, we test the hypothesis that electrical stimulation of the nasal mucosa will induce olfactory percepts.
Subjects
Fifty subjects (24 F, age: 23–33 years) participated in 70 separate experimental sessions. Fourteen subjects came back on different days for stimulation of different parts of the ventral surface of the middle turbinate. In six subjects, in addition to the middle turbinate, the stimulating electrode was placed on the superior turbinate, the septum dorsum, and the olfactory cleft. Notably, power analysis using the only study that reported stimulation-induced perception (Uziel 1973) implies that 22 subjects provide α = 0.05 and power = 0.8. The current cohort far exceeds this, and is in fact larger than all other studies of electrical stimulation in the nose combined. In 28 out of 70 experiments, we applied a topical nasal decongestant (Otrivin spray, Xylometazoline) prior to endoscopy because of significantly occluded nasal passages.
Procedures
Subjects were comfortably seated in a dental chair, and the electrode was placed at the target region under endoscopic guidance (Fig. 1C). The reference electrode was placed in different experiments either on the forehead, nose, or scalp (Fz or Cz). Subjects were asked to close their eyes and breathe through their nose. Electrical stimulation onset was preceded by an auditory cue, and triggered concurrently with nasal inhalation such that it mimicked natural sniff-dependent olfaction (Mainland and Sobel 2006; Kepecs et al. 2007; Carey et al. 2009; Carey and Wachowiak 2011; Smear et al. 2011; Rojas-Libano and Kay 2012). We used stimulation durations of 0.5, 1, 2, and 3 s, and inter stimulus interval (ISI) was 30–50 s. For each set of stimulation parameters, we generated the initial stimulation at 50 mV, and then incrementally increased the voltage until a sensation of any kind was reported. Based on our hypothesis and piloting efforts, after each trial, we also specifically probed for the following sensations: a sense of odor; electrical current in the nose; pinpricks in the nose; cooling in the nose; tingling at the reference electrode; and flashes of light (phosphenes). Subjects were asked to report these sensations along an intensity scale ranging from 1 (low) to 5 (high) (Supplementary Figure 1A). Once a sensation was reported, we repeated the above stimulation protocol but without the preceding auditory cue, in order to evaluate spontaneous self-reported sensation (i.e., subjects were unaware of stimulation onset). The stimulation parameters we tested using this design were a continuous sine wave delivered at 5 frequencies: 2, 10, 70, 90, 130, and 180 Hz, and a burst mode (5 cycles, 100-µs pulse width) delivered at 90 or 180 Hz, all applied at currents ranging from 50 up to 800 µA, at typically 10 µA intervals. A typical experiment lasted about 1 h.
Experiment 2: Electrical Stimulation Aimed at Altering the Perception of Odor
In this experiment, we test the hypothesis that electrical stimulation delivered to the olfactory mucosa concurrent with odor stimulation will alter odor perception.
Subjects, Odorants, and Stimulation
In the first of two paradigms with odor, a total of 16 subjects participated (7 F, age: 23–29 years) in different sessions with the pleasant smelling pure molecule 2-phenylethanol (PEA, CAS: 60-12-8, from Sigma) (n = 11), and two complex odorants; a pleasant smelling “chocolate” odor (n = 10) and an unpleasant smelling “manure” odor (n = 10) (both from Sensale). In a second paradigm, 15 subjects (5 F, age: 23–29 years) participated in an experiment where each trial contained a binary mixture of PEA and “manure” odor. The odorants were delivered into the stimulated nostril using a computer-controlled air-dilution olfactometer (Johnson and Sobel 2007) that embedded the odorant pulse within a constant stream of clean air at 5.5 L per min, 80% humidity, 37°C. Electrical stimulation was as before, using a stimulating electrode placed on the ventral surface of the middle turbinate and a reference electrode placed on the forehead, in order to deliver a continuous 2-Hz sine wave. Current amplitude was individually set for each participant by first delivering incremental currents until a sensation of any kind was reported, and then reverting to the highest yet un-sensed current. This assured the use of the highest possible current yet without awareness for the stimulation process. The resulting average current applied was 200 µA (min = 50 µA, max = 640 µA).
Procedures
After determining individual current settings, we commenced with odor delivery. Each session contained 20 trials of the same odorant, ISI between trials was 30 s. Half of the trials (random order) were delivered together with electrical stimulation that started 4 s prior to odor onset, continued during 1.5 s of odor delivery, and lasted 2.5 s after odor termination (Supplementary Figure 1B). Following each trial, subjects used a visual-analog scale (VAS) to answer three questions (Q1–Q3): rate odor intensity (Q1), rate odor pleasantness (Q2), and estimate whether an electrical current was delivered, or not (Q3) (Supplementary Figure 1C). Finally, in a second paradigm, rather than rate pleasantness, subjects were asked to estimate mixture content, ranging from 100% PEA to 100% “manure” odor (each of the two odors was introduced alone twice before experiment onset, where subjects named the odor and rated its intensity and pleasantness). Note that unbeknown to participants, the mixture was always 50/50. In all cases, VAS values were normalized to a scale ranging from 0 to 100, and these values were submitted to an analysis of variance (ANOVA). In the analysis, we excluded trials where odor was not sensed at all (1.6% of trials in electrical stimulation and 2.6% of sham), where it was rated at below or above two standard deviations from the mean intensity (0.7% of trials in electrical stimulation and 2.9% of sham), and trials where the electrical stimulation was potentially detected (19.7% of trials). This exclusion protected against cognitive influence on the result. Notably, the effects later reported materialized in full when repeating the analysis without the above exclusion criteria.
Experiment 3: Electrical Stimulation Aimed at Altering Neural Activity in Olfactory Cortex
In this experiment, we test the hypothesis that electrical stimulation delivered to the olfactory mucosa will alter neural activity in olfactory cortex.
Subjects
A total of 20 healthy subjects (9 F, age: 22–33 years) participated in the fMRI study.
Procedures
Although electrical stimulation has been safely used in the imaging environment (Iannilli et al. 2008), our paradigm poses added risks. First, the electrode is hidden inside the nose such that we have limited monitoring in the scanner. Second, unlike skin surface electrodes, here the electrode is in contact with the physiologically variable mucosa. This results in fluctuating impedance that may result in current spikes. With these safety considerations in mind, we opted for a design where we electrically stimulated in laboratory and then went directly to the scanner (adjacent building) in order to assess influence of stimulation on ensuing resting-state brain connectivity (Harmelech and Malach 2013; Power et al. 2014). Each subject participated twice, exactly one week apart, once with electrical stimulation, and once with sham stimulation. Subjects were blind to condition (sham or electrical stimulation), which was counterbalanced across subjects for order. On a given day, subjects were first scanned, then stimulated (sham or electrical stimulation), and then scanned again. The entire procedure, including transfer back and forth from scanner to laboratory to scanner, lasted ~2.5 h. Electrical stimulation in laboratory was administered according to procedures described earlier, using alternating ON (2 min) and OFF (0.5 min) periods in burst mode (5 cycles, 100-μs pulse width, 18 Hz) for a total of 10 min (4 ON periods) (Supplementary Figure 1D). During stimulation, subjects were asked to close their eyes. Ten subjects were stimulated in the right nostril and 10 in the left. The sham experiments were identical in all respects to the stimulation experiments, yet no current was driven through the electrode. To verify that stimulation was sub-threshold, subjects used an intensity scale to estimate after each session whether they thought an electrical current was delivered or not. In the scanner, we conducted two 6-min resting-state functional scans. Subjects were asked to keep their eyes open while fixating on a red cross, presented on a black screen. After the final resting state scan of each day, we conducted a sniffing task that was used as an independent localizer to generate functional regions of interest (fROIs) of primary olfactory cortex. The sniffing task was selected because sniffing in the absence of odor is a powerful activator of primary olfactory structures (Sobel et al. 1998). Here subjects closed their eyes and were cued by an auditory tone to sniff for 2 s once every 20–24 s (jittered) for 30 times. After each trial, subjects pressed a button denoting whether an odor was present or not. Subjects were misleadingly instructed in advance that we may use very low concentration odorants in this scan, yet in practice no odorants were delivered. This approach was taken so as to maximize attention to olfaction during this localizer (Zelano et al. 2005, 2011; Bensafi et al. 2007).
Imaging Setup
Images were acquired on a 3-T Trio Magnetom Siemens scanner. Functional data were collected using a T2*-weighted gradient-echo planar imaging sequence. The first resting scan was used to define fROIs that are part of the default-mode network (DMN). This scan consisted of 180 repetitions comprising 35 axial slices titled toward the anterior commissure–posterior commissure (AC–PC) plane (repetition time [TR] = 2000 ms, echo time [TE] = 25 ms, flip angle = 75°, field of view (FOV) 216 mm, matrix size 72 × 72, slice thickness of 3 mm with 0.3 mm gap, and 3 × 3 in-plane resolution), covering the full brain. The second resting scan and the sniffing-localizer task scan were limited to the ventral aspects of the brain, and acquired at higher spatial resolution: 180 repetitions comprising 31 axial slices titled toward the AC–PC plane (TR = 2000 ms, TE = 25 ms, flip angle = 75°, FOV 216 mm, matrix size 86 × 86, with 7/8 phase partial Fourier. Slice thickness of 2.5 mm with 0.25 mm gap, and 2.5 × 2.5 in-plane resolution). Anatomical images were acquired at the end of each fMRI session using a 3-dimensional (3D) T1-weighted magnetization prepared rapid gradient-echo sequence at high resolution (1 × 1 × 1 mm voxel, TR = 2300 ms, TE = 2.98 ms, inversion time = 900 ms, and a flip angle = 9°). Nasal airflow was precisely monitored throughout the scans using a nasal cannula (1103, Teleflex medical) linked to a spirometer (ML141, ADInstruments), and instrumentation amplifier (Power-Lab 16SP, ADInstruments) (Johnson et al. 2006).
Data Analysis and Preprocessing
Data from two subjects were excluded from further analyses: one due to excessive head motion, and the second due to failure to place the electrode. All raw fMRI data are publically posted at:
http://www.weizmann.ac.il/neurobiology/worg/materials.html.
fMRI data were analyzed using the BrainVoyager QX version 2.8 software package (Brain Innovation) and Matlab software (MathWorks). The first two images of each functional scan were discarded to avoid T1 saturation effects. Functional scan preprocessing included 3D head motion correction, slice scan time correction, and linear trend removal. The functional images were superimposed on 2D anatomical images and incorporated into the 3D data sets through trilinear interpolation. The complete data set was transformed into Talairach space.
For the resting-state scans, functional images were subjected to standard additional processing steps (Fox et al. 2005; Van Dijk et al. 2010): band-pass filtering (0.01–0.11 Hz), then removing sources of noninterest variance (i.e., motion and physiological noise) by regressing out the six motion parameters obtained during the motion correction procedure, and the mean signal from the lateral ventricles. Finally, the functional images were spatially smoothed with a Gaussian kernel with a full width at half maximum of 8 mm.
Localizer Task
The sniffing-localizer task was performed as the final scan on each day. Statistical analysis was based on the general linear model (GLM), with sniffs as a predictor of interest, and the six motion parameters as nuisance predictors. This sniffing predictor was defined as the time period of both the actual sniff, and the auditory cue for sniff onset in order to account for the anticipatory response in primary olfactory cortex (Zelano et al. 2005). The sniffing boxcar regressor was convolved with the canonical hemodynamic response function. Analysis was conducted in Talarich space after fixed-effects concatenation of the two localizer-scans of each subject. First, we used a random-effects GLM multi-subject analysis to define fROIs that were activated by sniffing. Next, we demarked individual fROIs for each subject maintaining a similar number of voxels for each region across subjects.
Resting State-Homotopic Inter-hemispheric Connectivity
Spontaneous brain activity tends to be highly correlated across homotopic regions in opposite hemispheres (Salvador et al. 2005; Nir et al. 2008; Stark et al. 2008). We hypothesized that unilateral nostril stimulation may change the neural activity of the affected hemisphere differently than the opposite hemisphere, and thus reduce the inter-hemispheric synchronization. To test this hypothesis, we applied two independent analyses: the first was a region-specific hypothesis-driven analysis. In this analysis, fROIs were independently defined based on the independent sniffing-localizer task, then Pearson correlations were computed across homotopic regions, and finally, following Fisher transformation these values were submitted to a repeated measures ANOVA. The second approach was an exploratory analysis using a voxel-wise image analysis method called voxel-mirrored homotopic connectivity (Zuo et al. 2010; Dinstein et al. 2011; Hahamy et al. 2015). This approach examines the functional connectivity between each pair of homologous voxels in the brain. Specifically, for each subject separately, we tested the Pearson correlation coefficient between the time course of each voxel with the time course of its mirrored voxel in the opposite hemisphere (as determined by the x coordinates in Talarich space). Note that the relatively extensive spatial smoothing applied to the data accounted for possible voxel-wise anatomical asymmetries between the two hemispheres. However, as this smoothing may cause the mixing of blood-oxygen level dependent (BOLD) signals from the two cerebral hemispheres along the midline, we did not test near-midline correlations (Hahamy et al. 2015). Following Fisher transformation of the single-subject correlation values, we generated a statistical parametric map of the t-values of post-sham versus post-electrical-stimulation. As a control, we conducted the same analysis on the pre-sham and pre-electrical-stimulation scans. We corrected for multiple comparisons by establishing a minimum cluster size for voxels showing a significant effect using the Neuroelf analysis package (www.neuroelf.net) (Forman et al. 1995). The cluster-size threshold was determined using a Monte-Carlo simulation by creating 10 000 3D images containing normally distributed random noise. The simulated data were used to determine the likelihood of observing, by chance, clusters of contiguous voxels that were significant on a per-voxel threshold of P = 0.05. With a per-voxel threshold of P = 0.05 (uncorrected), a cluster size that was evident in less than 5% of the simulated runs started at 60 contiguous functional voxels, and this value was set as threshold for a corrected P value of 0.05.
Connectivity in the DMN
One of the main potential applications of electrical stimulation is the treatment of neurological disorders, such as temporal lobe epilepsy (TLE). Given that the hippocampus is a key component in the network abnormality associated with TLE (James et al. 2013; Haneef et al. 2014), we tested if electrical stimulation alters hippocampal connectivity with core seeds of the DMN. For defining core seeds, we used the first scan that covers the full brain, and then tested connectivity in the second scan. Individual-level independent component analysis (ICA) and self-organizing group-level ICA (Sog-ICA) were applied to the functional time series using two “plug-in” extensions of BrainVoyager QX (Esposito et al. 2005; Goebel et al. 2006). Sog-ICA was used to summarize relevant independent components at the group level. The cluster size, which is the number of individual components grouped into one group-level component, was set to 20. Cluster “group” components were calculated as random-effects maps using the random-effects analysis of covariance module in BrainVoyager QX. After performing a sog-ICA run of the pre-sham and pre-stimulation runs of each subject, we selected the DMN component cluster. Using this component, we defined the precuneus/posterior cingulate cortex (PCC) (63 functional voxels), medial prefrontal cortex (mPFC) (40 functional voxels), and bilateral hippocampus (right = 31 functional voxels, left = 32 voxels).
Results
Electrical Stimulation Failed to Evoke Perception of Odor
We first asked whether electrically stimulating the nasal mucosa would generate olfactory perception. Before testing the naive subjects reported in this study, we conducted extensive pilot testing on co-author N.S. This included testing stimulating electrode placement at multiple locations in the nasal cavity, testing reference electrode placement at multiple locations, stimulating during breath-holding or concurrent with sniffing, and testing different stimulation parameters (i.e., current vs. voltage source, repeated anodic vs. cathodic current, sine wave vs. square pulses, and different frequencies). These extensive efforts generated assorted sensations and perceptions, but never once a perception of odor. This outcome subsequently replicated in all of the naive subjects tested: we used an endoscopically guided stimulating electrode to deliver incremental increases in current to the nasal mucosa of 50 subjects in areas known to contain olfactory sensory neurons (Fig. 1A–C, Online Video 1). Currents were triggered concurrently with nasal inhalation so as to match natural conditions (Mainland and Sobel 2006; Kepecs et al. 2007; Carey et al. 2009; Carey and Wachowiak 2011; Smear et al. 2011; Rojas-Libano and Kay 2012), and subjects were probed for their perception following each trial. Despite the use of various currents and frequencies (see Materials and Methods) in 70 1-h-long electrical stimulation runs, we never once successfully induced perception of odor. In turn, currents increased into the range of 100–800 µA evoked intranasal sensations (either a sense of electrical current, pinpricks, or cooling inside the nostril) as well as an itching sensation on the skin around the reference electrode (forehead/nose/scalp). Moreover, stimulation at 10 Hz drove visual perception of light flashes in 76% of runs (detection between 55 and 450 µA) using this frequency (Fig. 1D) (Supplementary Table 1 lists all sensations by all subjects). Most stimulations were at the middle turbinate where olfactory local field potentials are readily obtained (Lapid and Hummel 2013). Considering results from epithelial biopsies regarding the spread of olfactory mucosa (Feron et al. 1998; Leopold et al. 2000; Rawson and Ozdener 2013), we also stimulated at the superior turbinate (n = 5), septum dorsum (n = 3), and olfactory cleft (n = 3), yet failed to generate perception of odor.
Un-Sensed Electrical Stimulation had a Mild but Statistically Significant Impact on Concurrent Odor Perception
Lack of olfactory perception may reflect failure to place the stimulating electrode in the vicinity of olfactory sensory neurons. To further gauge whether we at all influenced the olfactory path, we set out to ask whether such electrical stimulation has any influence on concurrent odor-induced perception as implied in a previous report (Straschill et al. 1983). We used 2 tasks (Supplementary Figure 1). In the first task, we asked whether electrical current influences the perception of odorant pleasantness, a primary axis of human olfactory perception (Schiffman 1974; Yeshurun and Sobel 2010). Here we delivered 20 brief pulses of odor, a random half concurrent with un-sensed (see Materials and Methods) electrical stimulation, and then asked subjects to rate odorant pleasantness. We tested this in 16 subjects using three odors; the pleasant smelling “rose” and “chocolate” and an unpleasant odor of “manure”. A repeated measures ANOVA on pleasantness ratings, with levels of odor (“rose/chocolate/manure”) and current (ON/OFF) revealed a main effect of current (F(1,28) = 7.23, P = 0.012), a main effect of odor (F(2,28) = 3.65, P = 0.04), and no interaction (F(2,28) = 0.072, P = 0.93). Given the lack of interaction, we combined the odors for planned comparisons that revealed that undetected electrical stimulation reduced the perceived pleasantness of odors (mean rating with stimulation = 51.2 ± 15.8, without stimulation = 53.1 ± 15.89, t(15) = 3.3, P < 0.005) (Fig. 1E). In a second task, we delivered 20 brief pulses of binary odor mixtures, a random half concurrent with un-sensed electrical stimulation, and then asked subjects to rate the proportion of each component in the mixture. Given a patchy distribution of human epithelial representation (Lapid et al. 2011), we hypothesized that stimulation at a given location might influence perception of one component more than the other. We tested this in 15 subjects using mixtures of “rose” and “manure”. We observed no effect of stimulation on the perception of binary mixtures (t(14) = 0.51, P = 0.62).
The above experiments with odors were attempted in order to validate that we contacted olfactory neurons, yet they came short of providing a convincing answer. Although currents had a statistically significant impact on perception, this impact was very minimal. Moreover, this very minimal effect may reflect the intended contact with the olfactory path, but it also may reflect non-neuronal mechanisms. For example, the currents may have influenced aspects related to mucosal sorption of the odorants (Scott et al. 2014), before any receptor activation, and this alone may account for the significant but small effects we observed. In turn, we are restricted in our ability to improve this experiment for the following reason: ideally, we would like to increase the currents in order to more effectively probe for an influence. However, if we increase the currents, then the currents will also be sensed as such, and this alone will always remain an alternative explanation for altered perception. With this limitation in mind, we next used fMRI in order to test for evidence of this stimulation in olfactory cortex.
Un-Sensed Electrical Stimulation Functionally Decorrelated Left from Right-Hemisphere Olfactory Cortex
Ideally, we would have liked to stimulate within the magnetic resonance imaging (MRI) scanner in order to measure an event-related stimulation-evoked response. We decided against this due to safety considerations (see Materials and Methods). Instead, we opted for estimating the influence of electrical stimulation on ensuing resting-state activity. In contrast to traditional task-based fMRI studies, resting-state studies examine BOLD fluctuations in the absence of any explicit stimulus or behavior, while subjects simply rest in the scanner (Harmelech and Malach 2013; Power et al. 2014). Importantly, resting-state activity has been used as a measure following brain stimulation (Fox et al. 2014). We hypothesized that because of the largely (although not exclusively (Wilson 1997)) unilateral projection pattern in olfaction, stimulation in one nostril should primarily influence one hemisphere, thus reducing the functional synchronization between homolog regions. Twenty subjects were scanned during an eyes-open resting state (2 × 6 min) four times: before and after real yet undetectable unilateral electrical stimulation, and one week apart, before and after unilateral sham stimulation (total of 80 scanning sessions, counterbalanced for order, subjects blind to condition). Each subject also conducted a sniffing task (see Materials and Methods) for functionally localizing the olfactory structures (Sobel et al. 1998). The two sessions were identical in all respects but electrical current. Two subjects were excluded from continued analysis (see Materials and Methods).
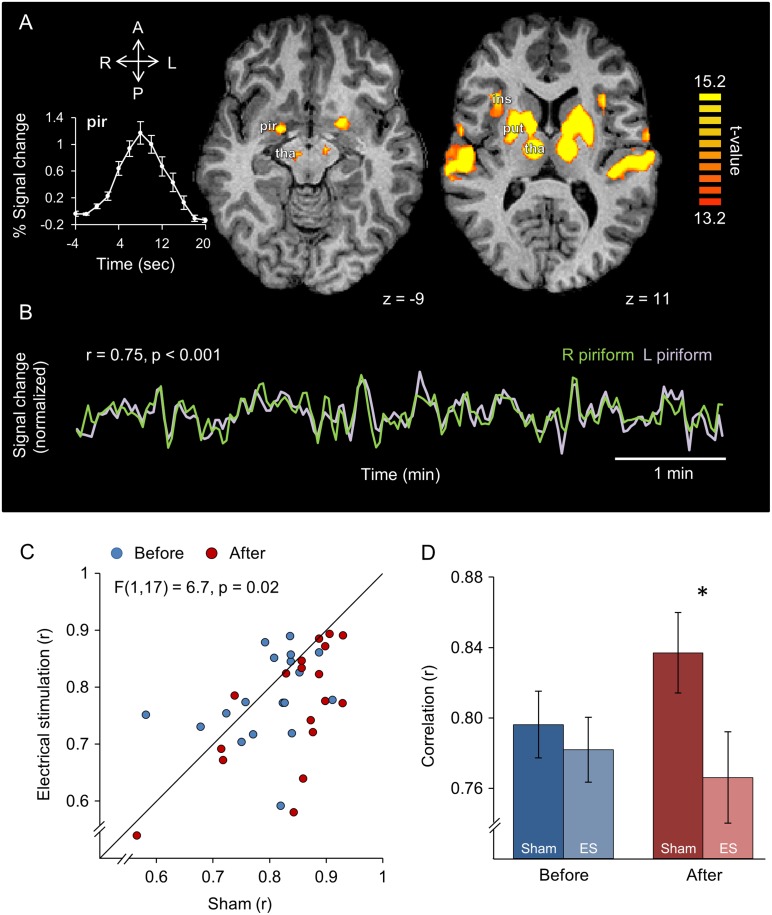
Consistent with our intended sub-detection-threshold stimulation, analysis of post-experimental ratings revealed that subjects were unable to determine which scanning session followed real electrical stimulation and which followed sham (rating of perceived current on a 10-point scale: sham = 1.8 ± 2, electrical stimulation = 2.1 ± 2.6, Wilcoxon signed-rank test Z = 26, P = 0.56). Thus, we are able to assign any ensuing differences to stimulation alone. Group analysis of the sniff localizer task revealed powerful group activations induced by sniffing (Fig. 2A). Ideally, we would have liked to test for influence of stimulation at the first synapse following the epithelium, namely at the olfactory bulb. The bulb, however, is not currently measurable with fMRI, so we concentrated on the next synapse, namely piriform cortex. We used the sniffing task to highlight an fROI in left and right piriform cortices for each subject. In agreement with previous reports on the robustness of homotopic inter-hemispheric correlation of neural activity (Nir et al. 2008), we observed that the average resting-state correlation between left and right piriform cortices was r = 0.8 ± 0.09 (Fig. 2B). To ask whether electrical stimulation in one nostril influenced this correlation, we conducted an ANOVA on the Fisher z-transformed correlation coefficients between left and right hemispheres with levels of time (before/after treatment), condition (sham/electrical stimulation) and stimulated nostril (right/left nostril). This revealed a significant main effect of condition (F(1,16) = 9.6, P = 0.007), and a time × condition interaction (F(1,16) = 6.7, P = 0.02) (Fig. 2C). Given a lack of interaction of condition × time × side of stimulation (F(1,16) = 0.57, P = 0.46), we combined left- and right-stimulated subjects for continued analysis. Planned comparisons revealed that whereas the process of this experiment (scan—followed by real/sham stimulation session—followed by scan) ended in increased connectivity across hemispheres (average r before sham = 0.8 ± 0.08, after sham = 0.84 ± 0.09, (t(17) = 2.65, P < 0.05), electrical stimulation completely prevented this course (average r before stimulation = 0.78 ± 0.08, after stimulation = 0.77 ± 0.11, (t(17) = 0.41, P > 0.05), such that correlation across piriform cortices was significantly lower after electrical stimulation compared to after sham (average r after sham stimulation = 0.84 ± 0.09, average r after real stimulation = 0.77 ± 0.11, (t(17) = 4.01, P < 0.001) (Fig. 2D). This implies that the electrical stimulation we applied in the nose indeed contacted the olfactory path. To test whether this reduced connectivity was not merely a reflection of a brain-wide phenomenon, we replicated this analysis in several additional regions that were activated by sniffing. We found that the time × condition interaction was not evident in the insula (F(1,17) = 0.41 , P = 0.53), putamen (F(1,17) = 3.8, P = 0.68), cerebellum (F(1,17) = 0.008, P = 0.93), or thalamus (F(1,17) = 2.15, P = 0.16). Finally, to estimate whether any subliminal trigeminal sensations mediated this result, we tested for a correlation between subject-wise perceived current differences and piriform cortex correlation differences, and observed no link between the 2 (Spearman's rho = 0.15, P = 0.54). In other words, un-sensed electrical stimulation in the nostrils selectively influenced correlation across left and right primary olfactory cortex.
Figure 2.
Un-sensed unilateral intranasal electrical stimulation reduced correlation across left and right piriform cortex. (A) Group activation map (n = 18) for the sniffing-localizer task; Increased BOLD signal was detected in the piriform cortex (pir), thalamus (tha), putamen (put), and insula (ins). Inset: event-related averaging of the sniff-induced response in left and right piriform uncovers a typical hemodynamic response. L, left; R, right; A, anterior; P, posterior. (B) Example from one subject depicting the time course of right (green) and left (light purple) piriform cortex during a 6-min resting-state scan. (C) Correlation across left and right piriform cortices before (blue) and after (red) sham and electrical stimulation (n = 18). The diagonal is the unit slope line. Each point reflects one subject. The points mostly fall under the line, implying reduced connectivity after electrical stimulation. (D) The data from (C) presented in averaged form. Note that the majority of red dots in (C) fall under the unit slope line, but also that Panels (C) and (D) do not start at zero. Together this implies a very consistent, but small effect. Error bars are s.e.m. *P < 0.05.
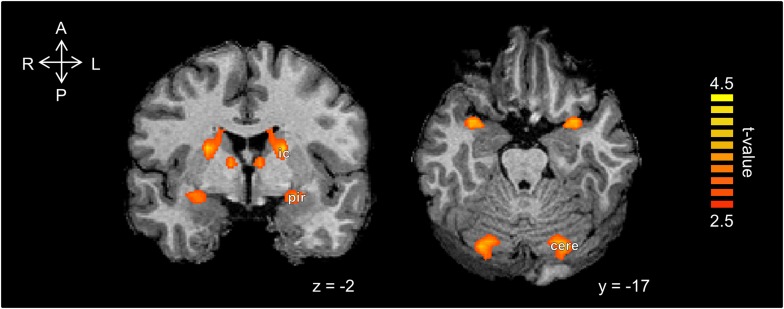
The above hypothesis-driven exploration targeted fROIs in olfactory regions. To ask whether electrical stimulation in the nose influenced brain activity beyond the classic olfactory structures, we conducted an exploratory analysis of the entire brain (see Materials and Methods). This revealed a post-stimulation decrease in inter-hemispheric connectivity again mainly in the piriform cortex, and also in the caudate/internal capsule, and the cerebellum (Fig. 3). Importantly, there were no differences in functional connectivity between the before sham and the before electrical stimulation conditions. The convergence of altered activity in piriform cortex across the exploratory and hypothesis-directed analyses, the latter relying on independently obtained fROIs, together lends strength to this result in piriform cortex.
Figure 3.
Un-sensed unilateral intranasal electrical stimulation reduced homotopic correlations in several deep brain regions. Statistical parametric maps of voxel-mirrored homotopic connectivity differences between post-sham and post-electrical stimulation. There was a significant decrease in inter-hemispheric connectivity mainly in the piriform cortex (pir), caudate/internal capsule (ic), and the cerebellum (cere).
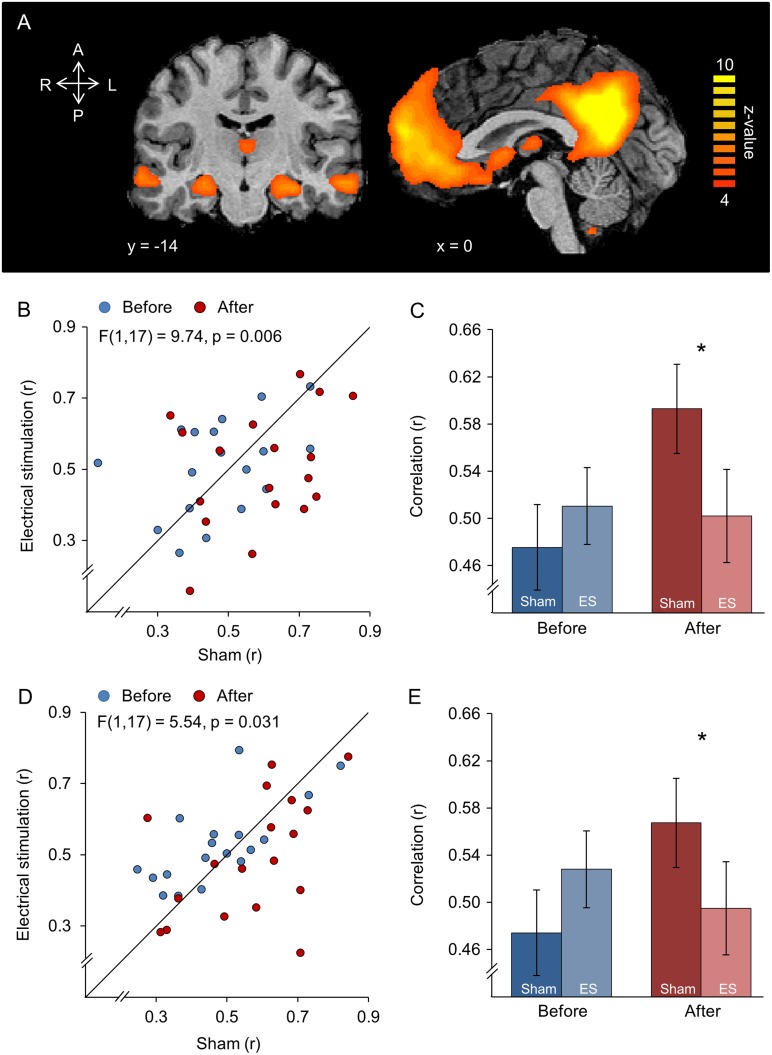
A major area of interest in brain stimulation is management of epilepsy (Kringelbach et al. 2007; Lozano and Lipsman 2013). Given that piriform cortex where we observed the strongest effects of stimulation is anatomically and functionally predisposed to involvement in focal epilepsy (Vaughan and Jackson 2014), we set out to ask whether stimulation had any additional impact consistent with relevance for epilepsy. Beyond involvement of ventral structures, TLE also involves decreased connectivity between the hippocampus and regions of the DMN (James et al. 2013; Haneef et al. 2014). DMN connectivity is also influenced by brain stimulation (Halko et al. 2014). To evaluate whether electrical stimulation in the nostrils influenced connectivity between the hippocampus and regions of the DMN, we calculated the correlation values between the hippocampi and 2 main hubs of the DMN: the PCC and the mPFC (Fig. 4A, see Materials and Methods). An ANOVA with levels of time (before/after treatment), condition (sham/electrical stimulation), and hippocampus side (right/left hemisphere) revealed a significant time × condition interaction only between mPFC and hippocampi (F(1,17) = 9.74, P = 0.006). The time × condition interaction between precuneus/PCC and hippocampi showed the same trend; however, it did not survive correction for multiple comparisons (F(1,17) = 5.54, P = 0.031) (family-wise error rate for 3 comparisons α < 0.017) (Fig. 4B,D). There was no interaction of condition × time × hippocampus side (mPFC and hippocampi: F(1,17) = 2.08, P = 0.17; precuneus/PCC and hippocampi: F(1,17) = 0.31, P = 0.58).
Figure 4.
Un-sensed unilateral intranasal electrical stimulation reduced functional connectivity in the DMN. (A) Group map of the component that reflected the DMN during the pre-sham and pre-stimulation resting state. (B) Correlation between the hippocampus (hp) and mPFC. The diagonal is the unit slope line. Each point reflects one subject. The points mostly fall under the line, implying reduced connectivity after electrical stimulation. (C) The data from (B) presented in averaged form. (D) Correlation between the hippocampus (hp) and precuneus/PCC. The diagonal is the unit slope line. Each point reflects one subject. The points mostly fall under the line, implying reduced connectivity after electrical stimulation. (E) The data from (D) presented in averaged form. Note that the majority of red dots in (C) fall under the unit slope line, but also that Panels (C) and (D) do not start at zero. Together, this implies a very consistent, but small effect. Error bars are s.e.m, *P < 0.05.
Discussion
Electrical stimulation failed to generate olfactory perception, slightly altered odor-induced perception, and significantly decorrelated left from right primary olfactory cortex and hippocampal connectivity within the DMN. In other words, we obtained both negative and positive results, and these have diverse implications for both basic and clinical neuroscience.
For basic neuroscience, this result has implications for coding in the olfactory system. A natural odorant likely activates specific subsets of olfactory sensory neurons as a reflection of the receptor subtypes they express (Firestein 2001). Moreover, natural odorants may excite some receptors while inhibiting others (Duchamp-Viret et al. 1999; Delay and Restrepo 2004; Su et al. 2011), and it is this fine interplay that characterizes a given odorant. In contrast, our stimulation likely indiscriminately influenced large populations of these cells, populations never activated in concert by natural stimuli. Such activation patterns are likely not recognized as odor by the olfactory system. Consistent with this notion, when a single receptor subtype is significantly overexpressed in mice, those mice fail to detect the cognate odorant activating that receptor (Fleischmann et al. 2008). In other words, extensive indiscriminate activation in the olfactory system is not necessarily perceived as odor. Finally, natural odors may also drive a temporal pattern of activation (Gire et al. 2013; Haddad et al. 2013; Rebello et al. 2014) that was likely missing from our stimulation regimen. Thus, although artificially stimulating peripheral olfactory endings may be highly informative (Ottoson 1959; Sato et al. 1996; Xu et al. 1999; Wei et al. 2003; Li et al. 2014), even if accompanied by altered activity in downstream olfactory targets, this does not necessarily imply induction of odor perception.
The serendipitous implications of these results for clinical neuroscience are that we may be able to take advantage of the olfactory nerve as a path from the external world directly to the deepest ventral aspects of the brain. Deep brain stimulation (DBS) with electrical currents is used to treat conditions ranging from movement disorders to intractable depression and epilepsy (Kringelbach et al. 2007; Lozano and Lipsman 2013). Typical DBS entails drilling the skull and placing an electrode at target locations of interest. Given possible side effects of this procedure, alternatives include noninvasive stimulation by magnetic or direct-current fields (Fregni and Pascual-Leone 2007; Najib et al. 2011) or minimally invasive targeting of cranial nerves as an entryway into the brain (Shiozawa et al. 2014). The olfactory nerve is a particularly appealing novel target in this respect because it has readily accessible nerve endings in the nasal passage that are one synapse away from deep brain structures. This may be especially relevant for epilepsy, as seizures can be prevented (Ebert and Löscher 2000; Jaseja 2008) or more rarely caused (Ilik and Pazarli 2015) by smelling odors, implying an intimate link between olfaction and epilepsy. Indeed, piriform cortex, where we observed the strongest effects of stimulation, is anatomically and functionally predisposed to involvement in focal epilepsy (Vaughan and Jackson 2014). Moreover, the impact on hippocampal connectivity within the DMN is also consistent with impacting brain mechanisms relevant to epilepsy (James et al. 2013; Haneef et al. 2014).
In turn, we would like to clearly acknowledge the limitations of this study. First, we remain ignorant as to the precise impact of stimulation in neural terms. Did stimulation increase or decrease excitability? Did it change baseline firing-rates? Perhaps stimulation altered local levels of Adenosine triphosphate (Bekar et al. 2008)? These and other alternatives often remain unanswered in human brain stimulation studies, and this remains true here as well. More specifically, the extensive effort we report (80 fMRI scanning sessions) uncovers the potential complexity of these mechanisms. Had we merely conducted a within-subjects experiment where each subject was scanned twice, once following a sham procedure and once following a real procedure (a legitimate experimental design), we would have concluded that stimulation reduces functional brain connectivity. However, because we also scanned each individual before each experiment as well, we learned that this reduction was in fact a prevented increase that would otherwise occur throughout the process of spending 2 h in an MRI magnet with a break between scans (the sham). Moreover, although we contacted deep brain structures, we might have acted on them in a manner that is inconsistent with clinical intervention, at least for epilepsy. Specifically, when a single receptor subtype is significantly overexpressed in mice, not only (as noted above) do those mice fail to detect the cognate odorant activating that receptor (Fleischmann et al. 2008), the cognate odorant can also cause seizures (Nguyen and Ryba 2012). Thus, the type of generalized olfactory activation we may have mimicked here may be the opposite of the type of activation relevant for epilepsy treatment and prevention. Indeed, the impact we observed on the DMN was consistent with the presence rather than absence of epilepsy (James et al. 2013; Haneef et al. 2014). Thus, we can conclude that we demonstrated contact with the relevant brain substrates, but that we do not understand what it is we altered in neural terms. In turn, we can remain optimistic in this respect because as previously noted odors can both increase (Ilik and Pazarli 2015) or decrease (Ebert and Löscher 2000; Jaseja 2008) seizures, so we predict that ultimately development will allow intranasal electrical currents to do the same.
A second limitation relevant to the interpretation of this study is that the trigeminal nerve may have mediated a portion of the effects we observed, especially the very small reduction in concurrent odor pleasantness (Bensafi et al. 2013). We think it is unlikely, however, that we failed to contact the olfactory path all together for the following reasons: first, trigeminal stimulation is typically associated with sensation (Croy et al. 2014b), yet here subjects were unable to detect the electrical stimulation used in the imaging and odor experiments. Moreover, the asymmetries in sensation they reported were unrelated to asymmetries in functional connectivity. Second, tissue studies identified olfactory sensory neurons at the locations we stimulated (Feron et al. 1998; Leopold et al. 2000; Rawson and Ozdener 2013), and others and we have measured an odor evoked local field potential using an electrode placed at these very same locations (Hummel et al. 1996; Lapid et al. 2009, 2011; Lapid and Hummel 2013). Third, and most critically in this respect, currents altered activity selectively in primary olfactory cortex. The full-brain exploratory analyses reiterated this alteration in primary olfactory cortex, yet did not uncover any such effects in the core brain regions associated with the trigeminal network (Hummel and Livermore 2002; Brand 2006; Albrecht et al. 2010; Bensafi et al. 2012; Croy et al. 2014b). If currents arrived at the brain through the trigeminal nerve, we would expect primary effects in primary trigeminal afferents. Indeed, when the trigeminal nerve is directly stimulated, such trigeminal patterns are observed (Albrecht et al. 2010), yet no such patterns were observed here (note that trigeminal stimulation also activates piriform, but it primarily activates brainstem, ventrolateral posterior thalamic nucleus, anterior cingulate cortex, insula, precentral gyrus, as well as primary and secondary somatosensory cortices, most of which were not influenced here at all). Given all this, we think that our results reflect influence through the olfactory path. That said, if we are wrong and the effects reported here were partially trigeminally mediated through some indirect path (Daiber et al. 2013), this remains a novel approach, as trigeminal endings in the nasal mucosa have not been targeted for electrical stimulation. Unlike in typical trigeminal transcutaneous stimulation of the supraorbital and infraorbital divisions, stimulation through the nose opens the option of developing a nasally implanted stimulator. Notably, the maxillary sinus provides plenty of space for such a stimulator that can be wired directly to the middle turbinate. If such a nasally implanted stimulator will alter activity patterns in piriform cortex without driving perception of any kind, this remains equally valuable regardless of the neural path subserving this effect. Thus, we conclude that indiscriminate electrical stimulation of the olfactory mucosa does not generate olfactory perception but does alter activity in deep brain structures, suggesting a novel entry path through the nose for electrically altering human brain function in brain areas relevant to conditions such as epilepsy and beyond.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Grant #662629 (NanoSmell) from EC Horizon 2020 and grant #2014905 from NSF-BSF joient program in brain research.
Supplementary Material
Notes
We thank Ofer Perl for help with figures. Conflict of Interest: The Weizmann Institute Office of Technology Licensing is preparing an application for patent on “brain stimulation through the nose”.
References
- Albrecht J, Kopietz R, Frasnelli J, Wiesmann M, Hummel T, Lundstrom JN.. 2010. The neuronal correlates of intranasal trigeminal function-an ALE meta-analysis of human functional brain imaging data. Brain Res Rev. 62:183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekar L, Libionka W, Tian GF, Xu Q, Torres A, Wang X, Lovatt D, Williams E, Takano T, Schnermann J, et al. 2008. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med. 14:75–80. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Iannilli E, Poncelet J, Seo HS, Gerber J, Rouby C, Hummel T.. 2012. Dissociated representations of pleasant and unpleasant olfacto-trigeminal mixtures: an FMRI study. PLoS One. 7:e38358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensafi M, Iannilli E, Schriever VA, Poncelet J, Seo HS, Gerber J, Rouby C, Hummel T. 2013. Cross-modal integration of emotions in the chemical senses. Front Hum Neurosci. 7:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensafi M, Sobel N, Khan RM.. 2007. Hedonic-specific activity in piriform cortex during odor imagery mimics that during odor perception. J Neurophysiol. 98:3254–3262. [DOI] [PubMed] [Google Scholar]
- Brand G. 2006. Olfactory/trigeminal interactions in nasal chemoreception. Neurosci Biobehav Rev. 30:908–917. [DOI] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pirez N, Wachowiak M.. 2009. Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J Neurophysiol. 101:1073–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Wachowiak M.. 2011. Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb. J Neurosci. 31:10615–10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Nordin S, Hummel T.. 2014. a. Olfactory disorders and quality of life—an updated review. Chem Senses. 39:185–194. [DOI] [PubMed] [Google Scholar]
- Croy I, Schulz M, Blumrich A, Hummel C, Gerber J, Hummel T.. 2014. b. Human olfactory lateralization requires trigeminal activation. NeuroImage. 98:289–295. [DOI] [PubMed] [Google Scholar]
- Daiber P, Genovese F, Schriever VA, Hummel T, Mohrlen F, Frings S.. 2013. Neuropeptide receptors provide a signalling pathway for trigeminal modulation of olfactory transduction. Eur J Neurosci. 37:572–582. [DOI] [PubMed] [Google Scholar]
- Delay R, Restrepo D.. 2004. Odorant responses of dual polarity are mediated by cAMP in mouse olfactory sensory neurons. J Neurophysiol. 92:1312–1319. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, Courchesne E.. 2011. Disrupted neural synchronization in toddlers with autism. Neuron. 70:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp-Viret P, Chaput MA, Duchamp A.. 1999. Odor response properties of rat olfactory receptor neurons. Science. 284:2171–2174. [DOI] [PubMed] [Google Scholar]
- Ebert U, Löscher W.. 2000. Strong olfactory stimulation reduces seizure susceptibility in amygdala-kindled rats. Neurosci Lett. 287:199–202. [DOI] [PubMed] [Google Scholar]
- Esposito F, Scarabino T, Hyvarinen A, Himberg J, Formisano E, Comani S, Tedeschi G, Goebel R, Seifritz E, Di Salle F.. 2005. Independent component analysis of fMRI group studies by self-organizing clustering. NeuroImage. 25:193–205. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, McGrath JJ, Mackay-Sim A.. 1998. New techniques for biopsy and culture of human olfactory epithelial neurons. Arch Otolaryngol--Head Neck Surg. 124:861–866. [DOI] [PubMed] [Google Scholar]
- Firestein S. 2001. How the olfactory system makes sense of scents. Nature. 413:211–218. [DOI] [PubMed] [Google Scholar]
- Fleiner F, Lau L, Goktas O.. 2012. Active olfactory training for the treatment of smelling disorders. Ear Nose Throat J. 91:198–203, 215. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Shykind BM, Sosulski DL, Franks KM, Glinka ME, Mei DF, Sun Y, Kirkland J, Mendelsohn M, Albers MW, Axel R. 2008. Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron. 60:1068–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC.. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 33:636–647. [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A.. 2014. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA. 111:E4367–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME.. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A.. 2007. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 3:383–393. [DOI] [PubMed] [Google Scholar]
- Gire DH, Restrepo D, Sejnowski TJ, Greer C, De Carlos JA, Lopez-Mascaraque L.. 2013. Temporal processing in the olfactory system: can we see a smell?. Neuron. 78:416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E.. 2006. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 27:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Lanjuin A, Madisen L, Zeng H, Murthy VN, Uchida N.. 2013. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat Neurosci. 16:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad R, Weiss T, Khan R, Nadler B, Mandairon N, Bensafi M, Schneidman E, Sobel N.. 2010. Global features of neural activity in the olfactory system form a parallel code that predicts olfactory behavior and perception. J Neurosci. 30:9017–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahamy A, Behrmann M, Malach R.. 2015. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nat Neurosci. 18:302–309. [DOI] [PubMed] [Google Scholar]
- Halko MA, Farzan F, Eldaief MC, Schmahmann JD, Pascual-Leone A.. 2014. Intermittent theta-burst stimulation of the lateral cerebellum increases functional connectivity of the default network. J Neurosci. 34:12049–12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J Jr, Stern JM.. 2014. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia. 55:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmelech T, Malach R.. 2013. Neurocognitive biases and the patterns of spontaneous correlations in the human cortex. Trends Cogn Sci. 17:606–615. [DOI] [PubMed] [Google Scholar]
- Hummel T, Knecht M, Kobal G.. 1996. Peripherally obtained electrophysiological responses to olfactory stimulation in man: electro-olfactograms exhibit a smaller degree of desensitization compared with subjective intensity estimates. Brain Res. 717:160–164. [DOI] [PubMed] [Google Scholar]
- Hummel T, Livermore A.. 2002. Intranasal chemosensory function of the trigeminal nerve and aspects of its relation to olfaction. Int Arch Occup Environ Health. 75:305–313. [DOI] [PubMed] [Google Scholar]
- Iannilli E, Del Gratta C, Gerber J, Romani G, Hummel T.. 2008. Trigeminal activation using chemical, electrical, and mechanical stimuli. Pain. 139:376–388. [DOI] [PubMed] [Google Scholar]
- Ilik F, Pazarli AC.. 2015. Reflex epilepsy triggered by smell. Clin EEG Neurosci. 46:263–265. [DOI] [PubMed] [Google Scholar]
- Ishimaru T, Shimada T, Sakumoto M, Miwa T, Kimura Y, Furukawa M.. 1997. Olfactory evoked potential produced by electrical stimulation of the human olfactory mucosa. Chem Senses. 22:77–81. [DOI] [PubMed] [Google Scholar]
- James GA, Tripathi SP, Ojemann JG, Gross RE, Drane DL.. 2013. Diminished default mode network recruitment of the hippocampus and parahippocampus in temporal lobe epilepsy. J Neurosurg. 119:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaseja H. 2008. Scientific basis behind traditional practice of application of “shoe-smell” in controlling epileptic seizures in the eastern countries. Clin Neurol Neurosurg. 110:535–538. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Russell C, Khan RM, Sobel N.. 2006. A comparison of methods for sniff measurement concurrent with olfactory tasks in humans. Chem Senses. 31:795–806. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Sobel N.. 2007. Methods for building an olfactometer with known concentration outcomes. J Neurosci Methods. 160:231–245. [DOI] [PubMed] [Google Scholar]
- Kan A, Litovsky RY.. 2014. Binaural hearing with electrical stimulation. Hear Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF.. 2007. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 98:205–213. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ.. 2007. Translational principles of deep brain stimulation. Nat Rev Neurosci. 8:623–635. [DOI] [PubMed] [Google Scholar]
- Lapid H, Hummel T.. 2013. Recording odor-evoked response potentials at the human olfactory epithelium. Chem Senses. 38:3–17. [DOI] [PubMed] [Google Scholar]
- Lapid H, Seo HS, Schuster B, Schneidman E, Roth Y, Harel D, Sobel N, Hummel T.. 2009. Odorant concentration dependence in electroolfactograms recorded from the human olfactory epithelium. J Neurophysiol. 102:2121–2130. [DOI] [PubMed] [Google Scholar]
- Lapid H, Shushan S, Plotkin A, Voet H, Roth Y, Hummel T, Schneidman E, Sobel N.. 2011. Neural activity at the human olfactory epithelium reflects olfactory perception. Nat Neurosci. 14:1455–1461. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Hummel T, Schwob JE, Hong SC, Knecht M, Kobal G.. 2000. Anterior distribution of human olfactory epithelium. Laryngoscope. 110:417–421. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Ackland HM, Lowery AJ, Rosenfeld JV.. 2015. Restoration of vision in blind individuals using bionic devices: a review with a focus on cortical visual prostheses. Brain Res. 1595C:51–73. [DOI] [PubMed] [Google Scholar]
- Li A, Gire DH, Bozza T, Restrepo D.. 2014. Precise detection of direct glomerular input duration by the olfactory bulb. J Neurosci. 34:16058–16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Lipsman N.. 2013. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron. 77:406–424. [DOI] [PubMed] [Google Scholar]
- Mainland J, Sobel N.. 2006. The sniff is part of the olfactory percept. Chem Senses. 31:181–196. [DOI] [PubMed] [Google Scholar]
- Mainland JD, Lundstrom JN, Reisert J, Lowe G.. 2014. From molecule to mind: an integrative perspective on odor intensity. Trends Neurosci. 37:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najib U, Bashir S, Edwards D, Rotenberg A, Pascual-Leone A.. 2011. Transcranial brain stimulation: clinical applications and future directions. Neurosurg Clin N Am. 22:233–251, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MQ, Ryba N.. 2012. A smell that causes seizure. PloS one. 7(7):e41899 doi:10.1371/journal.pone.0041899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard-Sagiv H, Kipervasser S, Andelman F, Neufeld MY, et al. 2008. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci. 11:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez-Parra A, Li A, Restrepo D.. 2014. Coding odor identity and odor value in awake rodents. Prog Brain Res. 208:205–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoson D. 1959. Olfactory bulb potentials induced by electrical stimulation of the nasal mucosa in the frog. Acta Physiol Scand. 47:160–172. [DOI] [PubMed] [Google Scholar]
- Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK.. 2014. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 9:e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE.. 2014. Studying brain organization via spontaneous fMRI signal. Neuron. 84:681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson NE, Ozdener MH.. 2013. Primary culture of the human olfactory neuroepithelium. Methods Mol Biol. 945:81–93. [DOI] [PubMed] [Google Scholar]
- Rebello MR, McTavish TS, Willhite DC, Short SM, Shepherd GM, Verhagen JV.. 2014. Perception of odors linked to precise timing in the olfactory system. PLoS Biol. 12:e1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Libano D, Kay LM.. 2012. Interplay between sniffing and odorant sorptive properties in the rat. J Neurosci. 32:15577–15589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E.. 2005. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 15:1332–1342. [DOI] [PubMed] [Google Scholar]
- Sato M, Kodama N, Sasaki T, Ohta M.. 1996. Olfactory evoked potentials: experimental and clinical studies. J Neurosurg. 85:1122–1126. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. 1974. Physicochemical correlates of olfactory quality. Science. 185:112–117. [DOI] [PubMed] [Google Scholar]
- Scott J, Sherrill L, Jiang J, Zhao K.. 2014. Tuning to odor solubility and sorption pattern in olfactory epithelial responses. J Neurosci. 34(6):2025–2036. doi:10.1523/JNEUROSCI.3736-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa P, Silva ME, Carvalho TC, Cordeiro Q, Brunoni AR, Fregni F.. 2014. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: a systematic review. Arq Neuropsiquiatr. 72:542–547. [DOI] [PubMed] [Google Scholar]
- Smear M, Resulaj A, Zhang J, Bozza T, Rinberg D.. 2013. Multiple perceptible signals from a single olfactory glomerulus. Nat Neurosci. 16:1687–1691. [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O'Connor R, Bozza T, Rinberg D.. 2011. Perception of sniff phase in mouse olfaction. Nature. 479:397–400. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD.. 1998. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 392:282–286. [DOI] [PubMed] [Google Scholar]
- Spors H, Albeanu DF, Murthy VN, Rinberg D, Uchida N, Wachowiak M, Friedrich RW.. 2012. Illuminating vertebrate olfactory processing. J Neurosci. 32:14102–14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, Gee DG, Roy AK, Banich MT, Castellanos FX, et al. 2008. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 28:13754–13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straschill M, Stahl H, Gorkisch K.. 1983. Effects of electrical stimulation of the human olfactory mucosa. Appl Neurophysiol. 46:286–289. [DOI] [PubMed] [Google Scholar]
- Su CY, Martelli C, Emonet T, Carlson JR.. 2011. Temporal coding of odor mixtures in an olfactory receptor neuron. Proc Natl Acad Sci USA. 108:5075–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel A. 1973. Stimulation of human olfactory neuro-epithelium by long-term continuous electrical currents. J Physiol (Paris). 66:409–422. [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL.. 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DN, Jackson GD.. 2014. The piriform cortex and human focal epilepsy. Front. Neurol. 5:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wander JD, Rao RP.. 2014. Brain-computer interfaces: a powerful tool for scientific inquiry. Curr Opin Neurobiol. 25:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Han D, Cai Z, Yang L, Liu X, Xian M, Zhang X.. 2003. [Experimental study on olfactory evoked potential in rabbits]. Lin chuang er bi yan hou ke za zhi = J Clin Otorhinolaryngol.. 17:618–620. [PubMed] [Google Scholar]
- Wilson DA. 1997. Binaral interactions in the rat piriform cortex. J Neurophysiol. 78:160–169. [DOI] [PubMed] [Google Scholar]
- Xu C, Ni D, Li F.. 1999. [Olfactory evoked potentials produced by electrical stimulation of the olfactory mucosa]. Zhonghua er bi yan hou ke za zhi. 34:224–226. [PubMed] [Google Scholar]
- Yeshurun Y, Sobel N.. 2010. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu Rev Psychol. 61:219–241. [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N.. 2005. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 8:114–120. [DOI] [PubMed] [Google Scholar]
- Zelano C, Mohanty A, Gottfried JA.. 2011. Olfactory predictive codes and stimulus templates in piriform cortex. Neuron. 72:178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang YF, Castellanos FX, et al. 2010. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 30:15034–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.