Abstract
Experimental data in rodents suggest that the effects of bisphenol A (BPA) on oocyte development may be modified by dietary methyl donors. Whether the same interaction exists in humans is unknown. We evaluated whether intake of methyl donors modified the associations between urinary BPA concentrations and treatment outcomes among 178 women who underwent 248 IVF cycles at a fertility center in Boston between 2007 and 2012. Participants completed a validated food frequency questionnaire and provided up to two urine samples per treatment cycle. High urinary BPA concentrations were associated with a 66% lower probability of implantation (p=0.007) among women who consumed <400μg/day of food folate, but not among women consuming ≥400μg/day (21% higher probability of implantation, p=0.18) (p,interaction=0.04). A similar pattern was observed for probability of clinical pregnancy (p,interaction=0.07) and live birth (p,interaction=0.16). These results are consistent with previous animal data but further evaluation in other human populations is needed.
Keywords: Bisphenol A, in vitro fertilization, folate, methyl donors
INTRODUCTION
Bisphenol A (BPA) is an endocrine disruptor that binds with androgen receptors, peroxisome proliferator–activated receptor γ, thyroid hormone receptor [1], and most importantly, with diverse estrogen receptors [2-7]. Specifically, BPA at very low concentrations can mediate the activities of endogenous estrogens in pituitary cells via several types of nongenomic signaling [5 8] acting via membrane estrogen receptors (mERα, mERβ, and GPER/GPR30 (G protein-coupled estrogen receptor)), and therefore modify functional responses, such as cell proliferation, prolactin release, and transporter function [5 9 10]. BPA is used in a variety of consumer products, including polycarbonate plastics and the epoxy lining of cans [11]. Exposure to BPA is primarily through dietary ingestion [12], however, emerging literature suggests that thermal receipt paper could also be a potential source of exposure to BPA [13-15], leading to widespread general population exposure [16]. BPA is subject to an efficient first-pass metabolism in the liver after oral administration and is rapidly conjugated and excreted due to the absence of enterohepatic circulation [17 18]. BPA was detected in over 90% of urine samples of a representative sample of U.S. residents [19 20]. As a consequence, the evaluation of potential adverse health effects of BPA has been an active area of research, including its association with diabetes, asthma, cancer [21-27], and in particular with reproductive health outcomes [28].
Identifying clear adverse health effects of this chemical in humans, however, has been elusive [28]. For example, while we initially reported adverse effects of BPA on intermediate treatment outcomes among women undergoing in vitro fertilization (IVF) treatments, [29-31] we did not find evidence of adverse effects of BPA on IVF outcomes in a recent, updated analysis with expanded enrollment and follow-up [32]. A possible explanation for these null findings in human studies is the lack of consideration of effect modification by other factors such as diet.
Experimental animal data suggest that the effects of BPA on mouse oocyte development may be modified by diet [33]. In the Agouti mouse model, maternal supplementation with either genistein, a phytoestrogen, or methyl donors (folic acid, vitamin B12, choline, betaine) modified the effects of BPA on the coat color distribution of their offspring, an epigenetically-determined trait [34]. Moreover, in mice placed on a phytoestrogen-free diet, there was a dose-response relationship between BPA and congression failure (defined as the failure of the chromosomes to properly align on an otherwise normal meiotic spindle), whereas BPA was unrelated to oocyte abnormalities in the animals fed a soy-based diet [35]. In agreement with these intriguing experimental findings, we recently found that pretreatment soy intake modified the relation between urinary BPA concentrations and IVF treatment outcomes [36]. Specifically, higher urinary BPA concentrations were associated with worse IVF outcomes among women who did not consume soy but there was no effect of BPA on IVF outcomes among women who regularly consumed soy [36]. We have previously shown that methyl donors were associated with improved ovulation among healthy women [37], lower risk of pregnancy loss [38], and improved treatment outcomes among women undergoing IVF in the same study as the current manuscript [39 40]. However, whether in humans an interaction between methyl donors and BPA exists as suggested by experimental data in rodents is unknown. In this manuscript, we explored whether intake of folate and other methyl donors modified the association between urinary BPA concentrations and IVF outcomes among women at an academic fertility medical center.
METHODS
Study population
Participants were women in couples seeking evaluation and treatment for infertility at the Massachusetts General Hospital (MGH) Fertility Center who enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort established in 2004 to evaluate environmental and dietary determinants of fertility [41]. Dietary assessment was introduced in 2007. Women between 18 and 45 years old were eligible to participate and approximately 80% of those contacted by the research nurses enrolled. Between 2004 and 2012, 256 women enrolled in the EARTH Study completed at least one IVF cycle at the MGH Fertility Center and provided at least one spot urine sample for the measurement of BPA per IVF cycle. Of those, 178 women completed a food frequency questionnaire (FFQ) for the assessment of the dietary folate intake between 2007 and 2012. Women were followed through all treatment cycles. IVF cycles in which women used an egg donor (n=12) and cryo-thaw cycles (n=18) were excluded. Moreover, 9 women contributing 11 IVF cycles were also excluded since the FFQ were completed after the start of their IVF cycle. The study was approved by the Human Studies Institutional Review Boards of the MGH, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC). Participants signed an informed consent after the study procedures were explained by a research nurse and all questions were answered.
Clinical management and assessment of outcomes
Clinical information was abstracted from the patient's electronic medical record by research nurses. Follicle stimulating hormone (FSH) was measured in the serum of a blood sample collected on the third day of the menstrual cycle using an automated electrochemiluminescence immunoassay at the MGH Core Laboratory as previously described (limit of detection (LOD)=0.1U/L) [30]. Infertility diagnosis was coded according to previously described definitions of the Society for Assisted Reproductive Technology (SART) [30]. The participant's date of birth was collected at entry, and weight and height were measured by the nurses. Body mass index (BMI) was calculated as weight (in kilograms) per height (in meters) squared.
Women underwent one of three controlled ovarian stimulation IVF treatment protocols on day 3 of induced menses after completing a cycle of oral contraceptives: 1) luteal phase GnRH-agonist protocol, 2) follicular phase GnRH-agonist/Flare protocol, or 3) GnRH-antagonist protocol. Protocols were chosen by the treating physician after taking into consideration age, basal FSH, AMH, BMI and prior response to gonadotropins. Antagonist and flare protocols were for the most part used for either older patients > 38 years or low responders [42-44]. Lupron dose was reduced at, or shortly after, the start of ovarian stimulation with FSH/hMG in the luteal phase GnRH-agonist protocol. FSH/hMG and GnRH-agonist or GnRH-antagonist was continued to the day of trigger with Human Chorionic Gonadotropin (hCG). Women were monitored for serum E2 levels (LOD=5pg/mL) (Elecsys Estradiol II reagent kit, Roche Diagnostics), follicle size measurements and counts, and endometrial thickness and pattern. hCG was administered intramuscularly approximately 36 hrs before the scheduled oocyte retrieval procedure to induce ovulation. Details of oocyte retrieval have been previously described [30]. In brief, peak serum E2 concentration was defined as the highest level of E2 preceding the oocyte retrieval and obtained on the day of hCG administration. Oocyte retrieval was completed with the presence of 3 or more lead follicles (≥16 mm in diameter) and the estradiol level reached at least 600 pg/mL.
Embryologists determined the total number of oocytes retrieved per cycle and classified them as germinal vesicle, metaphase I, metaphase II (MII) or degenerated. Oocytes underwent either conventional IVF or intracytoplasmic sperm injection (ICSI) as clinically indicated. The same cell culture medium [Advantage™ Cleavage and Blastocyst (QA, Sage) sequential media] was used during the entire study period. Embryologists determined fertilization rate 16-20 hours after insemination as the number of oocytes with two pronuclei divided by the number of MII oocytes that were either inseminated or injected. The resulting embryos were monitored for cell number and morphological quality (1 (best) to 5 (worst)) on day 2 and 3. Three measures of embryo quality were considered. Specifically, grade 3, 4 and 5 embryos on day 3 were considered poor quality, embryos with 5 cells or fewer on day 3 were considered to be slow cleavage, and embryos with 9 or more cells on day 3 were considered to have accelerated cleavage. We also derived a composite measure of quality and where embryos were considered of “best quality” if they had 4 cells on day 2, 8 cells on day 3, and a morphologic quality score of 1 or 2 on days 2 and 3. Clinical outcomes were assessed among women who underwent an embryo transfer. Implantation was defined as a serum β-hCG level > 6 mIU/mL, typically measured 17 days (range 15–20 days) after oocyte retrieval. An elevation in β-hCG with the confirmation of an intrauterine pregnancy on an ultrasound at 6 weeks was considered a clinical pregnancy. A live birth was defined as the birth of a neonate on or after 24 weeks gestation.
Urine sample collection and BPA measurements
Women provided up to two spot urine samples per IVF cycle, with the first one collected between Day 3 and Day 9 of the gonadotrophin phase, and the second one collected on the day of oocyte retrieval, prior to the procedure or administration of intravenous fluids. Urine was collected in a sterile polypropylene specimen cup. Specific gravity (SG), which was used to correct BPA concentrations for urine dilution, was measured at room temperature using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA) calibrated with deionized water before each measurement. The urine was divided into aliquots, frozen, and stored at −80 °C. Samples were shipped on dry ice overnight to the CDC where they were stored at or below −40 °C until analysis. The urinary concentrations of the sum of free and conjugated BPA species (total BPA) were measured using online solid-phase extraction (SPE) coupled with isotope dilution-high-performance liquid chromatography (HPLC)-tandem mass spectrometry (MS/MS), as described before [45]. First, 100 μL of urine was treated with β-glucuronidase/sulfatase (Helix pomatia, H1; Sigma Chemical Co, St. Louis, MO, USA) to hydrolyze the BPA-conjugated species. BPA was then retained and concentrated on a C18 reversed-phase size-exclusion SPE column (Merck KGaA, Germany), separated from other urine matrix components using a pair of monolithic HPLC columns (Merck KGaA), and detected by negative ion-atmospheric pressure chemical ionization-MS/MS. LOD for BPA was 0.4 μg/L. In addition to study samples, each analytical run included low-concentration and high-concentration quality control materials, prepared with spiked pooled human urine, and reagent blanks to assure the accuracy and reliability of the data [45]. BPA concentrations were corrected for urine dilution by SG using the following formula: Pc = P[(1.015 - 1)/SG - 1], where Pc is the SG-corrected BPA concentration (μg/L), P is the measured BPA concentration (μg/L), and 1.015 is the mean SG level in the study population [46]. The geometric mean of the SG-adjusted BPA concentrations from two spot urine samples collected during each IVF cycle was used as a measure of cycle-specific urinary BPA concentration. For cycles with only one urine sample (~20%), the BPA concentration for that single sample was used as the cycle-specific urinary BPA concentration. Samples with a BPA concentration below the LOD were assigned a value equal to the LOD divided by the square root of 2 prior to adjustment for urine dilution by SG as described previously [47].
Dietary assessment
Intake of folate and other methyl donors (vitamin B12, betaine and choline) was assessed using a validated food frequency questionnaire (FFQ) [48]. Participants were asked to report how often per day, on average, they consumed specified amounts of 131 food items during the previous year. Nutrient intakes were estimated by summing the nutrient contribution of all food and supplement items in the questionnaire, taking into consideration the brand, type and dose of dietary supplements used. Nutrient contents were obtained from the nutrient database of the US Department of Agriculture with additional information from manufacturers [49]. Total folate intake (Supplemental Table 1) was calculated using dietary folate equivalents (DFE) to account for differences in absorption between natural and synthetic folate [50]. From this questionnaire we also estimated dietary pattern adherence scores [51]. Folate intake with this questionnaire has been validated against prospectively collected diet records (r=0.71) [48] and against red blood cell (r=0.51) [52] and plasma folate levels (r=0.63) [53]. Participants also completed a detailed take-home questionnaire with additional questions on lifestyle factors, reproductive health, and medical history. This questionnaire included a section querying the frequency of consumption of 15 soy-based foods, a method previously found to produce valid estimates of intake [54-57].
Statistical analysis
Demographic and baseline reproductive characteristics of the women are presented using median ± interquartile ranges (IQRs) or percentages. Women were divided into quartiles of urinary BPA concentrations (based on their cycle-specific geometric mean of the SG-adjusted BPA as described above) with the lowest quartile considered as the reference group. We dichotomized intakes of folate (total, supplemental), vitamin B12, and other methyl donors (betaine and choline) at the median intake level of the study population. For folate from food sources, the median intake was 449 μg/day, and we thus decided to dichotomize intake at 400 μg/day to mirror established recommendations for women of reproductive age for the prevention of neural tube defects [58]. Associations between urinary BPA concentrations and demographics and baseline reproductive characteristics were evaluated using Kruskal–Wallis tests for continuous variables and chi-squared tests for categorical variables (data not shown). Multivariable generalized linear mixed models were used to evaluate the association between urinary BPA concentrations and IVF outcomes among women with low and high intake of dietary folate, with a random intercept to account for multiple IVF cycles in the same woman. A Poisson distribution and log link function were specified for oocyte counts, a normal distribution and identity link function were specified for endometrial wall thickness and E2 trigger levels, and a binomial distribution and logit link function were specified for embryo quality, fertilization rates, and clinical outcomes (implantation, clinical pregnancy and live birth). To test whether the associations between urinary BPA concentrations and IVF outcomes were modified by intake of methyl donors (interactions), a product of quartiles of urinary BPA concentrations and a dichotomized methyl donor intake was entered into the model. An interaction between urinary BPA concentrations and methyl donors was considered when the p-value of interaction term was <0.10. Tests for linear trends [59] were conducted using the median values of each quartile of urinary BPA concentration as a continuous variable. To allow for better interpretation of the results, population marginal means [60] are presented accounting for all the covariates in the model.
Confounding was assessed using prior knowledge on biological relevance and descriptive statistics from our study population through the use of directed acyclic graphs [61]. The variables considered as potential confounders included factors previously related to IVF outcomes in this and other studies, and factors associated with BPA exposure and IVF outcomes in this study, regardless of whether they had been previously described as predictors of IVF outcomes (Table 1). Final models were adjusted for age (continuous), BMI (continuous), race (white vs. nonwhite), day 3 FSH result (continuous), E2 trigger result (continuous), protocol type (luteal phase agonist, antagonist, and flare), infertility diagnosis (male, female and unexplained), intake of vitamin B12 (continuous), intake of folate from supplements (continuous), calorie intake (continuous), intake of soy foods (continuous) and adherence to data-derived dietary patterns (continuous). Statistical analyses were performed with SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
Table 1.
Demographic and reproductive characteristics of 178 women in the EARTH Study according to quartiles of specific gravity adjusted urinary BPA concentrations by intake of food folate.
| 74 women who consumed <400μg/day of food folate Median (IQR) or N (%) | 104 women who consumed ≥400μg/day of food folate Median (IQR) or N (%) | |||||
|---|---|---|---|---|---|---|
| BPA quartile Q1 (n=19) | BPA quartile Q4 (n=14) | P-valuea | BPA quartile Q1 (n=25) | BPA quartile Q4 (n=30) | P-valuea | |
| Food folate intake, μg /day | 320.3 (255.4, 351.8) | 328.0 (259.2, 360.1) | 0.51 | 617.3 (479.7, 822.2) | 552.9 (481.5, 638.4) | 0.05 |
| Urinary BPA, μg/L | 0.7 (0.6, 0.8) | 2.4 (2.0, 2.7) | <.0001 | 0.7 (0.6, 0.8) | 2.5 (2.0, 3.6) | <.0001 |
| Personal characteristics | ||||||
| Age (years) | 36.0 (32.0, 37.0) | 35.0 (33.0, 39.0) | 0.52 | 34.0 (32.0, 37.0) | 34.5 (32.0, 37.0) | 0.72 |
| Race/ethnicity, n (%) | 0.66 | 0.11 | ||||
| White | 13 (68.4) | 12 (85.7) | 21 (84.0) | 27 (90.0) | ||
| Black | 0 (0) | 1 (7.1) | 0 (0) | 1 (3.3) | ||
| Asian | 4 (21.1) | 1 (7.1) | 3 (12.0) | 0 (0) | ||
| Other | 2 (10.5) | 0 (0) | 1 (4.0) | 2 (6.7) | ||
| BMI (kg/m2) | 21.7 (20.4, 23.1) | 22.6 (21.8, 23.8) | 0.34 | 22.9 (21.2, 26.4) | 22.9 (21.0, 25.0) | 0.75 |
| Current smoker, n (%) | 0.21 | 0.97 | ||||
| Never smoker | 18 (94.7) | 10 (71.4) | 18 (72.0) | 20 (66.7) | ||
| Ever smoker | 1 (5.3) | 4 (28.6) | 7 (28.0) | 10 (33.3) | ||
| Baseline reproductive characteristics | ||||||
| Initial infertility diagnosis | 0.58 | 0.60 | ||||
| Male factor | 8 (42.1) | 7 (50.0) | 9 (36.0) | 11 (36.7) | ||
| Female | 4 (21.1) | 2 (14.3) | 8 (32.0) | 10 (33.3) | ||
| DOR | 0 (0) | 1 (7.1) | 2 (8.0) | 2 (6.7) | ||
| Endometriosis | 1 (5.3) | 0 (0) | 2 (8.0) | 1 (3.3) | ||
| Ovulatory | 1 (5.3) | 1 (7.1) | 2 (8.0) | 5 (16.7) | ||
| Tubal | 2 (10.5) | 0 (0) | 2 (8.0) | 2 (6.7) | ||
| Uterine | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Unexplained | 7 (36.8) | 5 (35.7) | 8 (32.0) | 9 (30.0) | ||
| Initial treatment protocol | 0.47 | 0.14 | ||||
| Antagonist | 3 (15.8) | 1 (7.1) | 1 (4.0) | 7 (23.3) | ||
| Flareb | 2 (10.5) | 3 (21.4) | 5 (20.0) | 4 (13.4) | ||
| Luteal phase agonistc | 14 (73.7) | 10 (71.4) | 19 (76.0) | 19 (63.3) | ||
| E2 Trigger Levels, pmol/L | 2244 (1630, 3643) | 2410 (2205, 2693) | 0.59 | 1944 (1516, 2434) | 1693 (1459, 2439) | 0.07 |
| Day 3 FSH Levels, IU/L | 6.8 (5.3, 8.7) | 7.5 (6.0, 8.1) | 0.69 | 6.4 (6.1, 8.5) | 7.4 (6.3, 8.6) | 0.09 |
| Embryo Transfer Day | 0.88 | 0.16 | ||||
| No embryos transferred | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Day 2 | 1 (5.9) | 1 (7.1) | 1 (4.0) | 1 (3.3) | ||
| Day 3 | 11 (64.7) | 6 (42.9) | 20 (80.0) | 22 (73.3) | ||
| Day 5 | 5 (29.4) | 7 (50.0) | 4 (16.0) | 7 (23.3) | ||
| Number of Embryos Transferred | 0.63 | 0.24 | ||||
| No embryos transferred | 2 (10.5) | 0 (0) | 0 (0) | 0 (0) | ||
| 1 embryo | 1 (5.3) | 1 (7.1) | 3 (12.0) | 3 (10.0) | ||
| 2 embryos | 12 (63.1) | 10 (71.4) | 15 (60.0) | 23 (76.7) | ||
| 3+ embryos | 4 (21.1) | 3 (21.4) | 7 (28.0) | 4 (13.3) | ||
From Kruskal-Wallis test for continuous variables and chi-squared tests (or Fisher's exact test where appropriate) for categorical variables.
Luteal phase GnRH-agonist protocol.
Follicular phase GnRH-agonist/Flare protocol.
RESULTS
This analysis includes 178 women who had a median age of 35.0 years (IQR: 32-38). Fifty-three women (30%) were overweight or obese (BMI≥25 kg/m2), and a minority were past (26%) or current (2%) smokers. The distribution of urinary BPA concentrations in this study population was from below the LOD (0.4 μg/L) to 10.5 μg/L, with a median of 1.3 μg/L (IQR: 0.9-1.9), and it was significantly correlated with intake of choline (r=0.14, p=0.03). No other correlations between BPA and intake of methyl donors were found (data not shown). The median (IQR) time between FFQ completion and first treatment cycle was 194 (95-333) days. These women had a median intake of 449 μg/day of folate from food sources (IQR: 332-547) and a median intake of 4.8 μg/day of vitamin B12 from food sources (IQR: 3.5-6.6). The corresponding figures for intake of folate and vitamin B12 from supplements were 571 μg/day (IQR: 400-806) and 6.0 μg/day (IQR: 4.6-12.0 μg/L). Among women with higher intake of folate from food sources, the median (IQR) day 3 FSH levels (IU/L) across quartiles of urinary BPA concentrations were 6.4 (6.1-8.5), 6.3 (5.1-7.5), 6.9 (5.3-7.6) and 7.4 (6.3-8.6) (p-value=0.09) and for E2 trigger levels (pmol/L) were 1944 (1516-2434), 1673 (1158, 2359), 2212 (1753, 3455) and 1693 (1459, 2439) (p-value=0.07). No other baseline characteristics were significantly related to urinary BPA concentrations among women who consumed lower and higher intakes of folate, respectively.
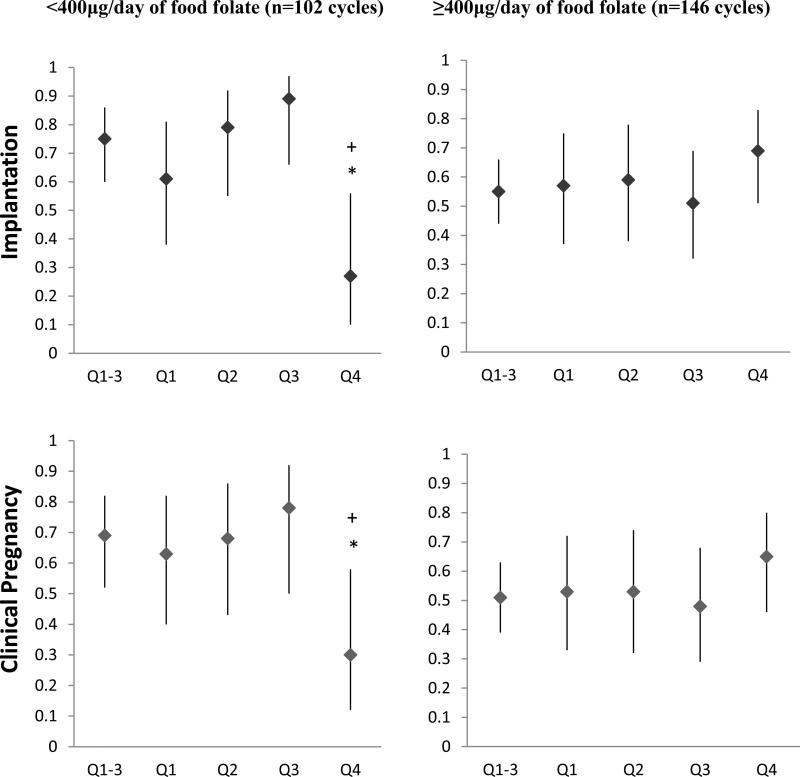
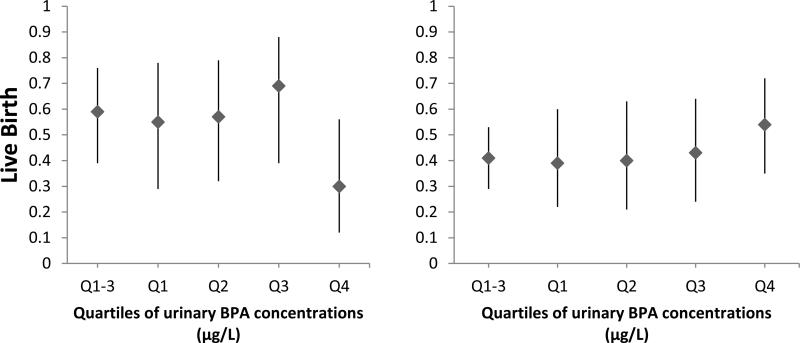
Intake of folate from food sources modified the association between urinary BPA concentrations with probability of implantation (p-interaction=0.04), and clinical pregnancy (p-interaction=0.07) in our study population (Figure 1). Women whose intake of folate from food sources was below 400 μg/day had lower probability of implantation (p, linear trend=0.07) and clinical pregnancy (p, linear trend =0.10) with increasing urinary BPA concentrations. On the other hand, urinary BPA concentrations were unrelated to these outcomes among women whose intake of folate from food sources was of 400 μg/day or higher. The difference in association between urinary BPA and clinical outcomes by levels of dietary folate intake were stronger in post hoc analyses where women in the top quartiles of urinary BPA were compared to women in the bottom quartile. Specifically, when compared to women in the three first quartiles of urinary BPA, women in the fourth quartile had significantly lower probability of implantation (−66%; p=0.007), clinical pregnancy (−58%; p=0.03) and live birth (−49%; p=0.06) when they also had an intake of folate from food sources below 400 mcg/d but not when folate from foods was higher (21% higher implantation, p=0.18; 22% higher clinical pregnancy, p=0.22; and 23% higher live birth, p=0.27). The tests for interaction of these post hoc analysis were statistically significant (p, interaction= 0.002 for implantation, 0.01 for clinical pregnancy and 0.07 for live birth). Results were nearly identical when food folate was dichotomized at the median (449 μg/day) rather than at 400μg/day, and also when year of urine sample was included in the models as a covariate (data not shown).
Figure 1. Effect modification of folate intake from food sources on the relation between implantation (p-interaction=0.04), clinical pregnancy (p-interaction=0.07) and live birth (p-interaction=0.16), with urinary BPA concentrations (μg/L) (n=248 fresh cycles).
The adjusted proportion of initiated cycles (95% CI) resulting in implantation, clinical pregnancy, and live birth across quartiles of specific gravity adjusted urinary BPA concentrations by folate intake are presented. Models are adjusted for age (continuous), BMI (continuous), race (white vs. nonwhite), day 3 FSH result (continuous), E2 trigger result (continuous), protocol type (luteal phase agonist, antagonist, and flare), infertility diagnosis (male, female and unexplained), intake of vitamin b12 (continuous), intake of folate from supplements (continuous), calorie intake (continuous), intake of soy foods (continuous) and adherence to data-derived dietary patterns (continuous). Implantation was defined as a serum β-hCG level > 6 mIU/mL typically measured 17 days (range 15–20 days) after egg retrieval, clinical pregnancy as the presence of an intrauterine pregnancy confirmed by ultrasound and live birth as the birth of a neonate on or after 24 weeks gestation. *Indicates a p-value<0.10 comparing the fourth quartile vs. first quartile. +Indicates a p-value<0.05 comparing the fourth quartile vs. the three bottom quartiles. Notes: Q1-3 was a unique group including Q1, Q2 and Q3.
There was no evidence of effect of modification by folate intake from food sources on the relation between urinary BPA concentrations and intermediate outcomes (Table 2). Specifically, urinary BPA concentrations were unrelated to total and mature oocyte yields, embryo quality and fertilization rates regardless of folate intake status. Similarly, there was no evidence of an interaction between BPA and intakes of folate from supplements, vitamin B12, choline or betaine on clinical outcomes of IVF (Supplemental Table 1).
Table 2.
Adjusteda mean (95% CI) pre-clinical outcomes according to specific gravity adjusted urinary BPA concentrations (μg/L) by intake of folate from food sources among 178 women (contributing to 248 fresh cycles)b from the EARTH Study.
| MII Oocyte Yield, n | Total Oocyte Yield, n | |||
|---|---|---|---|---|
| Ranges | <400μg/day of food folate | ≥400μg/day of food folate | <400μg/day of food folate | ≥400μg/day of food folate |
| Urinary BPA, μg/L | ||||
| (<LOD-0.90) | 8.86 (7.78, 10.10) | 9.10 (7.89, 10.50) | 10.40 (8.83, 12.24) | 10.61 (9.21, 12.21) |
| (0.91-1.35) | 8.61 (7.48, 9.92) | 8.28 (7.10, 9.66) | 10.62 (8.99, 12.55) | 10.11 (8.70, 11.76) |
| (1.36-2.15) | 9.50 (8.32, 10.85) | 8.68 (7.52, 10.01) | 11.00 (9.30, 13.00) | 9.76 (8.47, 11.25) |
| (2.20-10.45) | 8.80 (7.47, 10.36) | 8.29 (7.24, 9.50) | 9.54 (7.75, 11.74) | 9.89 (8.66, 11.30) |
| P-trendc | 0.92 | 0.46 | 0.46 | 0.54 |
| P-interaction | 0.71 | 0.70 | ||
| Proportion with >1 Best embryo qualityd | Fertilization rate, All cyclese | |||
|---|---|---|---|---|
| <400μg/day of food folate | ≥400μg/day of food folate | <400μg/day of food folate | ≥400μg/day of food folate | |
| Urinary BPA, μg/L | ||||
| (<LOD-0.90) | 0.30 (0.10, 0.62) | 0.47 (0.26, 0.69) | 0.79 (0.70, 0.85) | 0.71 (0.63, 0.78) |
| (0.91-1.35) | 0.21 (0.06, 0.51) | 0.27 (0.11, 0.51) | 0.75 (0.66, 0.83) | 0.76 (0.68, 0.83) |
| (1.36-2.15) | 0.32 (0.11, 0.63) | 0.45 (0.25, 0.67) | 0.68 (0.58, 0.77) | 0.72 (0.64, 0.79) |
| (2.20-10.45) | 0.37 (0.10, 0.76) | 0.36 (0.19, 0.56) | 0.76 (0.64, 0.85) | 0.71 (0.63, 0.77) |
| P-trendc | 0.59 | 0.66 | 0.61 | 0.66 |
| P-interaction | 0.15 | 0.71 | ||
Models are adjusted for age (continuous), BMI (continuous), race (white vs. nonwhite), day 3 FSH result (continuous), E2 trigger result (continuous), protocol type (luteal phase agonist, antagonist, and flare), infertility diagnosis (male, female and unexplained), intake of vitamin B12 (continuous), intake of folate from supplements (continuous), calorie intake (continuous), intake of soy foods (continuous) and adherence to data-derived dietary patterns (continuous).
102 fresh cycles were underwent among women who consumed <400 μg/d of folate and 146 fresh cycles women who consumed ≥400 μg/d of folate.
Tests for trend were performed using the median level of urinary bisphenol A level in each group as a continuous variable in the model.
We classified embryos as best quality if they had 4 cells on day 2, 8 cells on day 3, and a morphologic quality score of 1 or 2 on days 2 and 3.
Including both IVF and ICSI cycles.
Abbreviations: <LOD, below limit of detection (0.4 μg/L); n, number.
DISCUSSION
We evaluated whether intake of methyl donors previously related to improved infertility treatment outcomes modified the relationship between urinary BPA concentrations with intermediate and clinical outcomes among women undergoing IVF treatments. Our prospective cohort study including 178 women contributing 248 fresh IVF cycles showed that intake of folate from food sources modified the relation of urinary BPA with probability of implantation and clinical pregnancy. Specifically, among women who consumed less than 400 μg/day of food folate, BPA was inversely associated with probability of implantation and clinical pregnancy; however, BPA was unrelated to these outcomes among women who consumed ≥400μg/day of folate from foods. Nevertheless, we found no evidence of interaction with total or supplemental folate intake, nor with intake of vitamin B12, choline or betaine. While the significant interaction with food folate is consistent with previous experimental work in rodents and is suggestive of a true biological interaction across species, the lack of interaction with other methyl donors raises concerns that this may be a chance finding or the result of residual confounding.
To our knowledge, this is the first study to report an interaction between urinary BPA concentrations and dietary folate intake in a human population. These findings are consistent with previously published experimental data in rodents showing a protective effect of folate on the adverse reproductive effects of BPA [34 35]. Dolinoy et al. reported that maternal supplementation with methyl donors like folic acid restored the coat color distribution in BPA-exposed offspring [34]. Thirteen percent of BPA-exposed/methyl donor-supplemented offspring were classified as yellow compared with 21% of offspring exposed to BPA alone. Furthermore, almost 20% of BPA-exposed/methyl donor supplemented offspring were classified as pseudoagouti compared with only 9.6% of BPA-exposed offspring. While the consistency of our findings with studies in rodents suggests they may represent a true biological interaction, it is not possible to discern the underlying biological mechanism for effect modification of BPA exposure by folate intake. Research in rodents suggests that protective effects of folate on DNA methylation may be one of the mechanisms underlying this interaction. In the Agouti mouse model, maternal exposure to BPA results in decreased methylation at the CabpIAP metastable epiallele offspring, indicating that BPA-induced DNA hypomethylation is not locus-dependent [34]. Therefore, a hypermethylation effect based on nutritional supplementation with methyl donors or genistein may counteract BPA-induced DNA hypomethylation, resulting in a protective effect of diet against the adverse reproductive effects of BPA exposure. Moreover, there is evidence that genetic variation in the folic acid metabolism impacts ovarian responsiveness to gonadotropins as well as the basal and stimulated production of estradiol in the granulosa cell, [62 63] suggesting the existence of an unknown mechanism linking folic acid metabolism with the production of estrogens in the ovary. Whether these or other, yet to be described, biological mechanisms underlie the observed interaction is unknown, highlighting the need for additional study of potential interactions between diet and BPA.
The interest of interaction between nutrients and BPA on their relation with reproductive health arose when EPA and the National Institute of Environmental Health Sciences (NIEHS) provided funding for experimental studies in animals in order to better identify the lowest observed adverse effect levels (LOAEL) for BPA because results across experimental studies were not consistent [64-66]. One important factor potentially related with these heterogeneous findings for the effects of BPA was the maternal diet, considered a primary source of exposure to exogenous estrogenic substances [67 68]. Consequently, Thigpen et al. suggested that increased consideration should be given to the content of phytoestrogens and methyl donors in the maternal rodent diet when performing studies evaluating the estrogenic activity of EDCs such as BPA [69]. These experimental studies led us to investigate whether the inconsistent epidemiologic literature on the association of BPA with reproductive outcomes could be due to lack of consideration of diet, which may modify the effects of BPA.
Our study has several limitations worth noting. First, because all study participants were women undergoing infertility treatment with assisted reproduction, it is not possible to know whether our findings are generalizable to women trying to become pregnant without medical interventions. However, women in this study are comparable to women attending fertility clinics in the United States, suggesting that these findings may be applicable to other women seeking infertility treatment [70]. Second, measurement error and misclassification of both spot urine BPA concentrations and self-reported folate intake are possible, and this would likely attenuate associations [71]. However, tests for interaction, using the misclassified exposures, are valid provided the probability of misclassification satisfies certain bounds [72]. Measured exposures have correlation close to 0 and misclassification of them is likely independent, therefore, in similar scenarios [72], evidence for significant interaction remains. Strengths of our study include its prospective design and complete follow-up of participants, which minimizes the risk of reverse causation, the comprehensive adjustment of possible confounding variables made possible by standardized assessment of a wide range of participant characteristics and the adequately powered (80%) study which was able to detect clinically relevant differences of 32% in implantation, 29% in clinical pregnancy and 23% in live birth between women in the top and bottom quartiles of urinary BPA concentrations who consumed 400 μg/day of food folate as compared to those who did not.
In summary, we found that intake of folate from foods may modify the association between urinary BPA concentrations and certain reproductive outcomes among women undergoing IVF. This finding is consistent with previous experimental data and represents the first report of a possible interaction between folate and BPA in humans. However, there was no evidence of such interaction with intakes of total and supplemental folate or with intakes of vitamin B12, choline or betaine suggesting the possibilities that the observed interactions could be the result of a true interaction with an unmeasured factor strongly associated to intake of folate from food sources as well as the possibility of a chance finding. Therefore, it is important that the possibility that the reproductive health effects of BPA are modulated by intake of methyl donors requires further evaluation in other populations. More generally, our findings suggest that research addressing the more general hypothesis that the effect of environmental chemicals on health outcomes may be modified by diet and other lifestyle factors is warranted.
Supplementary Material
HIGHLIGHTS.
High urinary BPA concentrations were associated with lower probability of implantation, clinical pregnancy and live birth, among women who consumed <400μg/day of food folate.
Urinary BPA concentrations were not associated with probability of implantation, clinical pregnancy and live birth, among women who consumed ≥400μg/day of food folate.
We found no evidence of interactions with total or supplemental folate intake, nor with intake of vitamin B12, choline or betaine.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Xiaoyun Ye, Xiaoliu Zhou, Josh Kramer, and Tao Jia (CDC, Atlanta, GA) for measuring the urinary concentrations of BPA. We also acknowledge all members of the EARTH study team, specifically the Harvard T H Chan School of Public Health research nurses Jennifer B. Ford and Myra G. Keller, research staff Ramace Dadd and Patricia Morey, physicians and staff at Massachusetts General Hospital fertility center and a special thanks to all the study participants.
FUNDING: NIH grants R01ES022955, R01ES009718, R01ES000002 from the National Institute for Environmental Health Sciences, T32DK007703-16 and P30DK46200 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR'S CONTRIBUTION TO MANUSCRIPT: R.H. and J.E.C. were involved in study concept and design, and critical revision for important intellectual content of the manuscript. L.M.A drafted the manuscript, analyzed data and had a primary responsibility for final content; P.L.W contributed to method modification and provided statistical expertise. A.J.G. reviewed the statistical analysis; L.M.A, J.E.C., A.J.G., Y.H.C, P.L.W. and R.H. interpreted the data; I.S and A.M.C. were involved in acquisition of the data. All authors were involved in the critical revision of the manuscript and approved the final manuscript.
CONFLICT OF INTEREST: None of the authors has any conflicts of interest to declare. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
REFERENCES
- 1.Richter CA, Birnbaum LS, Farabollini F, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. doi: http://dx.doi.org/10.1016/j.reprotox.2007.06.004[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould JC, Leonard LS, Maness SC, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142(1-2):203–14. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 3.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–63. doi: 10.1210/endo.139.10.6216. doi: 10.1210/endo.139.10.6216[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 4.Dong S, Terasaka S, Kiyama R. Bisphenol A induces a rapid activation of Erk1/2 through GPR30 in human breast cancer cells. Environ Pollut. 2011;159(1):212–8. doi: 10.1016/j.envpol.2010.09.004. doi: 10.1016/j.envpol.2010.09.004[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 5.Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environmental health perspectives. 2005;113(4):431–9. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushima A, Kakuta Y, Teramoto T, et al. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J Biochem. 2007;142(4):517–24. doi: 10.1093/jb/mvm158. doi: 10.1093/jb/mvm158[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 7.Okada H, Tokunaga T, Liu X, et al. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environmental health perspectives. 2008;116(1):32–8. doi: 10.1289/ehp.10587. doi: 10.1289/ehp.10587[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochukov MY, Jeng YJ, Watson CS. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 somatomammotropes. Environmental health perspectives. 2009;117(5):723–30. doi: 10.1289/ehp.0800182. doi: 10.1289/ehp.0800182[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeng YJ, Watson CS. Combinations of physiologic estrogens with xenoestrogens alter ERK phosphorylation profiles in rat pituitary cells. Environmental health perspectives. 2011;119(1):104–12. doi: 10.1289/ehp.1002512. doi: 10.1289/ehp.1002512[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alyea RA, Watson CS. Nongenomic mechanisms of physiological estrogen-mediated dopamine efflux. BMC neuroscience. 2009;10:59. doi: 10.1186/1471-2202-10-59. doi: 10.1186/1471-2202-10-59[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NTP [April 28, 2016];National Toxicology Program. Brief on Bisphenol A [CAS NO. 80-05-07] 2008 http://ntp.niehs.nih.gov/ntp/ohat/bisphenol/bisphenol.pdf.
- 12.Carwile JL, Ye X, Zhou X, et al. Canned soup consumption and urinary bisphenol A: a randomized crossover trial. Jama. 2011;306(20):2218–20. doi: 10.1001/jama.2011.1721. doi: 10.1001/jama.2011.1721[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrlich S, Calafat AM, Humblet O, et al. Handling of Thermal Receipts as a Source of Exposure to Bisphenol A. Jama. 2014;311(8):859–60. doi: 10.1001/jama.2013.283735. doi: 10.1001/jama.2013.283735[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hormann AM, Vom Saal FS, Nagel SC, et al. Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA). PloS one. 2014;9(10):e110509. doi: 10.1371/journal.pone.0110509. doi: 10.1371/journal.pone.0110509[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thayer KA, Taylor KW, Garantziotis S, et al. Bisphenol A, Bisphenol S, and 4-Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) in Urine and Blood of Cashiers. Environmental health perspectives. 2016;124(4):437–44. doi: 10.1289/ehp.1409427. doi: 10.1289/ehp.1409427[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenberg LN, Hauser R, Marcus M, et al. Human exposure to bisphenol A (BPA). Reproductive toxicology (Elmsford, NY) 2007;24(2):139–77. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Volkel W, Bittner N, Dekant W. Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug metabolism and disposition: the biological fate of chemicals. 2005;33(11):1748–57. doi: 10.1124/dmd.105.005454. doi: 10.1124/dmd.105.005454[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 18.Volkel W, Colnot T, Csanady GA, et al. Metabolism and Kinetics of Bisphenol A in Humans at Low Doses Following Oral Administration. Chemical research in toxicology. 2002;15(10):1281–87. doi: 10.1021/tx025548t. doi: 10.1021/tx025548t[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 19.Calafat AM, Ye X, Wong LY, et al. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environmental health perspectives. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CDC CfDCaP . Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables (February, 2015) USA: Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2015. http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf. [Google Scholar]
- 21.Song Y, Hauser R, Hu FB, et al. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int J Obes (Lond) 2014;38(12):1532–7. doi: 10.1038/ijo.2014.63. doi: 10.1038/ijo.2014.63[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Q, Cornelis MC, Townsend MK, et al. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses' Health Study (NHS) and NHSII cohorts. Environ Health Perspect. 2014;122(6):616–23. doi: 10.1289/ehp.1307201. doi: 10.1289/ehp.1307201[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhandari R, Xiao J, Shankar A. Urinary Bisphenol A and Obesity in US Children. American journal of epidemiology. 2013;177(11):1263–70. doi: 10.1093/aje/kws391. doi: 10.1093/aje/kws391[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003–2006(). Environmental research. 2011;111(6):825–30. doi: 10.1016/j.envres.2011.05.014. doi: 10.1016/j.envres.2011.05.014[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. Jama. 2012;308(11):1113–21. doi: 10.1001/2012.jama.11461. doi: 10.1001/2012.jama.11461[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 26.Lakind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research. Crit Rev Toxicol. 2014;44(2):121–50. doi: 10.3109/10408444.2013.860075. doi: 10.3109/10408444.2013.860075[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 27.Rochester JR. Bisphenol A and human health: a review of the literature. Reproductive toxicology (Elmsford, NY) 2013;42:132–55. doi: 10.1016/j.reprotox.2013.08.008. doi: 10.1016/j.reprotox.2013.08.008[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 28.Peretz J, Vrooman L, Ricke WA, et al. Bisphenol A and Reproductive Health: Update of Experimental and Human Evidence, 2007–2013. Environmental health perspectives. 2014;122(8):775–86. doi: 10.1289/ehp.1307728. doi: 10.1289/ehp.1307728[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrlich S, Williams PL, Missmer SA, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environmental health perspectives. 2012;120(7):978–83. doi: 10.1289/ehp.1104307. doi: 10.1289/ehp.1104307[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mok-Lin E, Ehrlich S, Williams PL, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. International journal of andrology. 2010;33(2):385–93. doi: 10.1111/j.1365-2605.2009.01014.x. doi: IJA1014 [pii] 10.1111/j.1365-2605.2009.01014.x [doi][published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrlich S, Williams PL, Missmer SA, et al. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Human reproduction (Oxford, England) 2012;27(12):3583–92. doi: 10.1093/humrep/des328. doi: 10.1093/humrep/des328[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minguez-Alarcon L, Gaskins AJ, Chiu YH, et al. Urinary bisphenol A concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Human reproduction (Oxford, England) 2015;30(9):2120–8. doi: 10.1093/humrep/dev183. doi: 10.1093/humrep/dev183[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muhlhauser A, Susiarjo M, Rubio C, et al. Bisphenol A Effects on the Growing Mouse Oocyte Are Influenced by Diet. Biol Reprod. 2009 doi: 10.1095/biolreprod.108.074815. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhlhauser A, Susiarjo M, Rubio C, et al. Bisphenol A Effects on the Growing Mouse Oocyte Are Influenced by Diet. Biology of Reproduction. 2009;80(5):1066–71. doi: 10.1095/biolreprod.108.074815. doi: 10.1095/biolreprod.108.074815[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chavarro JE, Minguez-Alarcon L, Chiu YH, et al. Soy Intake Modifies the Relation Between Urinary Bisphenol A Concentrations and Pregnancy Outcomes Among Women Undergoing Assisted Reproduction. The Journal of clinical endocrinology and metabolism. 2016;101(3):1082–90. doi: 10.1210/jc.2015-3473. doi: 10.1210/jc.2015-3473[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaskins AJ, Mumford SL, Chavarro JE, et al. The impact of dietary folate intake on reproductive function in premenopausal women: a prospective cohort study. PloS one. 2012;7(9):e46276. doi: 10.1371/journal.pone.0046276. doi: 10.1371/journal.pone.0046276[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaskins AJ, Rich-Edwards JW, Hauser R, et al. Maternal prepregnancy folate intake and risk of spontaneous abortion and stillbirth. Obstet Gynecol. 2014;124(1):23–31. doi: 10.1097/AOG.0000000000000343. doi: 10.1097/aog.0000000000000343[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaskins AJ, Afeiche MC, Wright DL, et al. Dietary folate and reproductive success among women undergoing assisted reproduction. Obstetrics and gynecology. 2014;124(4):801–9. doi: 10.1097/AOG.0000000000000477. doi: 10.1097/aog.0000000000000477[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaskins AJ, Chiu YH, Williams PL, et al. Association between serum folate and vitamin B-12 and outcomes of assisted reproductive technologies. The American journal of clinical nutrition. 2015;102(4):943–50. doi: 10.3945/ajcn.115.112185. doi: 10.3945/ajcn.115.112185[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hauser R, Meeker JD, Duty S, et al. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology (Cambridge, Mass) 2006;17:682–91. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- 42.Polat M, Bozdag G, Yarali H. Best protocol for controlled ovarian hyperstimulation in assisted reproductive technologies: fact or opinion? Seminars in reproductive medicine. 2014;32(4):262–71. doi: 10.1055/s-0034-1375178. doi: 10.1055/s-0034-1375178[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 43.Pacchiarotti A, Selman H, Valeri C, et al. Ovarian Stimulation Protocol in IVF: An Up-to-Date Review of the Literature. Current pharmaceutical biotechnology. 2016;17(4):303–15. doi: 10.2174/1389201017666160118103147. [DOI] [PubMed] [Google Scholar]
- 44.Al-Inany HG, Youssef MA, Ayeleke RO, et al. The Cochrane database of systematic reviews. Vol. 4. Cd001750: 2016. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. doi: 10.1002/14651858.CD001750.pub4[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye X, Kuklenyik Z, Needham LL, et al. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Anal Chem. 2005;77:5407–13. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 46.Smith KW, Braun JM, Williams PL, et al. Predictors and Variability of Urinary Paraben Concentrations in Men and Women, Including before and during Pregnancy. Environmental health perspectives. 2012;120(11):1538–43. doi: 10.1289/ehp.1104614. doi: 10.1289/ehp.1104614[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meeker JD, Ehrlich S, Toth TL, et al. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reproductive toxicology (Elmsford, NY) 2010;30(4):532–9. doi: 10.1016/j.reprotox.2010.07.005. doi: S0890-6238(10)00250-9 [pii] 10.1016/j.reprotox.2010.07.005 [doi][published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. American journal of epidemiology. 1992;135(10):1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27-36. [DOI] [PubMed] [Google Scholar]
- 49.U.S. Department of Agriculture ARS USDA National Nutrient Database for Standard Reference, Release 25. Secondary USDA National Nutrient Database for Standard Reference, Release 25 2012. http://www.ars.usda.gov/ba/bhnrc/ndl.
- 50.Bailey LB. Dietary reference intakes for folate: the debut of dietary folate equivalents. Nutrition reviews. 1998;56(10):294–9. doi: 10.1111/j.1753-4887.1998.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 51.Gaskins AJ, Colaci DS, Mendiola J, et al. Dietary patterns and semen quality in young men. Human reproduction (Oxford, England) 2012;27(10):2899–907. doi: 10.1093/humrep/des298. doi: 10.1093/humrep/des298[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giovannucci E, Stampfer MJ, Colditz GA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med. 1998;129(7):517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 53.Jacques PF, Sulsky SI, Sadowski JA, et al. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57(2):182–9. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 54.Chun OK, Chung SJ, Song WO. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. Journal of the American Dietetic Association. 2009;109(2):245–54. doi: 10.1016/j.jada.2008.10.055. doi: 10.1016/j.jada.2008.10.055[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 55.Kirk P, Patterson RE, Lampe J. Development of a Soy Food Frequency Questionnaire to Estimate Isoflavone Consumption in US Adults. Journal of the American Dietetic Association. 1999;99(5):558–63. doi: 10.1016/S0002-8223(99)00139-X. [DOI] [PubMed] [Google Scholar]
- 56.Frankenfeld CL, Patterson RE, Kalhorn TF, et al. Validation of a soy food frequency questionnaire with plasma concentrations of isoflavones in US adults. Journal of the American Dietetic Association. 2002;102(10):1407–13. doi: 10.1016/s0002-8223(02)90313-5. [DOI] [PubMed] [Google Scholar]
- 57.Frankenfeld CL, Patterson RE, Horner NK, et al. Validation of a soy food-frequency questionnaire and evaluation of correlates of plasma isoflavone concentrations in postmenopausal women. The American journal of clinical nutrition. 2003;77(3):674–80. doi: 10.1093/ajcn/77.3.674. [DOI] [PubMed] [Google Scholar]
- 58.Willett WC. Folic acid and neural tube defect: can't we come to closure? Am J Public Health. 1992;82(5):666–8. doi: 10.2105/ajph.82.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosner B. Fundamentals of Biostatistics. 5th ed. Duxbury Press; Pacific Grove, CA: 2000. [Google Scholar]
- 60.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34(4):216–21. [Google Scholar]
- 61.Weng H-Y, Hsueh Y-H, Messam LLM, et al. Methods of Covariate Selection: Directed Acyclic Graphs and the Change-in-Estimate Procedure. American journal of epidemiology. 2009;169(10):1182–90. doi: 10.1093/aje/kwp035. doi: 10.1093/aje/kwp035[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 62.Thaler CJ, Budiman H, Ruebsamen H, et al. Effects of the common 677C>T mutation of the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene on ovarian responsiveness to recombinant follicle-stimulating hormone. American journal of reproductive immunology (New York, NY : 1989) 2006;55(4):251–8. doi: 10.1111/j.1600-0897.2005.00357.x. doi: 10.1111/j.1600-0897.2005.00357.x[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 63.Hecht S, Pavlik R, Lohse P, et al. Common 677C-->T mutation of the 5,10-methylenetetrahydrofolate reductase gene affects follicular estradiol synthesis. Fertility and sterility. 2009;91(1):56–61. doi: 10.1016/j.fertnstert.2007.11.011. doi: 10.1016/j.fertnstert.2007.11.011[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 64.Vom Saal FS, Prins GS, Welshons WV. Report of very low real-world exposure to bisphenol A is unwarranted based on a lack of data and flawed assumptions. Toxicological sciences : an official journal of the Society of Toxicology. 2012;125(1):318–20. doi: 10.1093/toxsci/kfr273. author reply 21-5 doi: 10.1093/toxsci/kfr273[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 65.vom Saal FS, Akingbemi BT, Belcher SM, et al. Flawed experimental design reveals the need for guidelines requiring appropriate positive controls in endocrine disruption research. Toxicological sciences : an official journal of the Society of Toxicology. 2010;115(2):612–3. doi: 10.1093/toxsci/kfq048. author reply 14-20 doi: 10.1093/toxsci/kfq048[published Online First: Epub Date]|. [DOI] [PubMed] [Google Scholar]
- 66.Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocrine reviews. 2012;33(3):378–455. doi: 10.1210/er.2011-1050. doi: 10.1210/er.2011-1050[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thigpen JE, Setchell KD, Ahlmark KB, et al. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Laboratory animal science. 1999;49(5):530–6. [PubMed] [Google Scholar]
- 68.Thigpen JE, Setchell KD, Saunders HE, et al. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2004;45(4):401–16. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- 69.Thigpen JE, Setchell KD, Kissling GE, et al. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. Journal of the American Association for Laboratory Animal Science : JAALAS. 2013;52(2):130–41. [PMC free article] [PubMed] [Google Scholar]
- 70.Stephen EH, Chandra A. Use of infertility services in the United States: 1995. Fam Plann Perspect. 2000;32(3):132–7. [PubMed] [Google Scholar]
- 71.Braun JM, Just AC, Williams PL, et al. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. Journal of exposure science & environmental epidemiology. 2014;24(5):459–66. doi: 10.1038/jes.2013.69. doi: 10.1038/jes.2013.69[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.VanderWeele TJ, Chen Y, Ahsan H. Inference for causal interactions for continuous exposures under dichotomization. Biometrics. 2011;67(4):1414–21. doi: 10.1111/j.1541-0420.2011.01629.x. doi: 10.1111/j.1541-0420.2011.01629.x[published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.