Abstract
Rationale: Whether sleep-disordered breathing (SDB) severity and diminished lung function act synergistically to heighten the risk of adverse health outcomes remains a topic of significant debate.
Objectives: The current study sought to determine whether the association between lower lung function and mortality would be stronger in those with increasing severity of SDB in a community-based cohort of middle-aged and older adults.
Methods: Full montage home sleep testing and spirometry data were analyzed on 6,173 participants of the Sleep Heart Health Study. Proportional hazards models were used to calculate risk for all-cause mortality, with FEV1 and apnea–hypopnea index (AHI) as the primary exposure indicators along with several potential confounders.
Measurements and Main Results: All-cause mortality rate was 26.9 per 1,000 person-years in those with SDB (AHI ≥5 events/h) and 18.2 per 1,000 person-years in those without (AHI <5 events/h). For every 200-ml decrease in FEV1, all-cause mortality increased by 11.0% in those without SDB (hazard ratio, 1.11; 95% confidence interval, 1.08–1.13). In contrast, for every 200-ml decrease in FEV1, all-cause mortality increased by only 6.0% in participants with SDB (hazard ratio, 1.06; 95% confidence interval, 1.04–1.09). Additionally, the incremental influence of lung function on all-cause mortality was less with increasing severity of SDB (P value for interaction between AHI and FEV1, 0.004).
Conclusions: Lung function was associated with risk for all-cause mortality. The incremental contribution of lung function to mortality diminishes with increasing severity of SDB.
Keywords: sleep apnea, impaired lung function, mortality
At a Glance Commentary
Scientific Knowledge on the Subject
Lung function and sleep-disordered breathing severity are independently associated with an increased risk for all-cause mortality.
What This Study Adds to the Field
This study seeks to determine whether measures of lung function and sleep-disordered breathing severity interact to alter the risk of all-cause mortality in a community cohort. The findings of this study indicate that the incremental impact of lung function impairment on all-cause mortality lessens with increasing degree of sleep-disordered breathing.
Several studies published over the past few decades have shown that level of lung function below predicted values is associated with an increased risk for mortality (1–5). The increase in risk has been demonstrated not only in clinical samples with established lung disease and associated impairment of lung function (1, 2), but also in several general population samples with a wide distribution of lung function and without overt respiratory disease (3–5). In addition to lung function, sleep-disordered breathing (SDB) and measures of its severity, such as apnea–hypopnea index (AHI), have also been strongly associated with all-cause and cause-specific mortality (6–8). Although the clinical consequences associated with the co-occurrence of SDB and chronic obstructive pulmonary disease (COPD) have been the topic of substantial research in recent years (9–11), the influence of lower lung function on the risk for all-cause mortality associated with SDB has not been well studied, especially in community-based populations with a relatively normal spectrum of lung function.
The biologic mechanisms by which lower lung function and SDB may synergistically augment the risk of adverse outcomes are not entirely clear. People with SDB and lung disease, specifically COPD, have more pronounced nocturnal hypoxemia when compared with those with SDB or COPD alone (11, 12). Greater sleep-related hypoxemia in SDB from impaired lung function may augment the potential mechanisms through which SDB has been linked with clinical sequelae including activation of the sympathetic nervous system, increase in oxidative stress, and systemic inflammation (13). Although more severe nocturnal desaturation was demonstrated in the Sleep Heart Health Study (SHHS) among participants with SDB and mild airways obstruction compared with those not having airways obstruction (11), that finding has not been consistently observed in other studies (14). Thus, the current study sought to characterize whether having low lung function modifies the effects of SDB on all-cause mortality in middle-aged and older adults in a community-based setting. Using the Sleep Heart Health Study data, we hypothesized that the association between lower lung function and mortality would be stronger in those with increasing severity of SDB.
Methods
Study Design and Population
The SHHS is a prospective cohort study of cardiovascular consequences of SDB. Details of the study design have been reported previously (15). Briefly, between 1995 and 1998 potential subjects were recruited from prospective cohort studies including the Framingham Offspring and Omni Study, the Atherosclerosis Risk in Communities Study, the Cardiovascular Health Study, the Strong Heart Study, and the cohort studies of respiratory disease in Tucson and of hypertension in New York. Eligible participants were at least 40 years of age and were not being treated for SDB with positive pressure therapy, oral appliance, oxygen, or tracheostomy. A total of 6,441 subjects completed the baseline examination. Written consent was obtained from all participants and the study protocol was approved by the institutional review board of each participating field site.
Data Collection
Participants completed a baseline examination that included a detailed health interview, a full-montage unattended home polysomnogram, measurements of blood pressure and anthropometry, and assessments of sleep habits and prescription medication use. Prevalent cardiovascular disease was defined as history of physician-diagnosed angina, heart failure, myocardial infarction, stroke, and coronary revascularization, determined by adjudicated surveillance data provided by the parent cohorts or by self-report at enrollment. Information on other health behaviors, such as smoking, was obtained by self-report. The sleep study was conducted using a portable monitor (P-Series; Compumedics, Abbotsville, Australia). Details of the polysomnographic equipment, hook-up procedures, failure rates, scoring, and quality assurance and control have been published (15).
Apneas were identified if airflow was absent or nearly absent for at least 10 seconds. Hypopneas were identified when there was at least 30% reduction in airflow or thoracoabdominal movement below baseline values for at least 10 seconds that was associated with a 4% decrease in oxygen saturation. Apneas were further classified as obstructive if movement on either the chest or abdominal inductance channels was noted, or as central if no displacement was observed on both of these channels. The AHI was defined as the number of apneas and hypopneas, each associated with a 4% decrease in oxygen saturation, per hour of sleep. Lung function was measured using spirometry as per the American Thoracic Society criteria (16). Spirometry was performed based on parent study protocols when available, otherwise protocols designed for SHHS were used, as previously described (11). FEV1, FVC, and FEV1/FVC ratio were available for the current analysis.
Death from any cause, the primary endpoint for this report, was identified and confirmed for the cohort using multiple concurrent approaches including follow-up interviews, written annual questionnaires or telephone contacts with study subjects or next-of-kin, surveillance of local hospital records and community obituaries, and linkage with the Social Security Administration Death Master File (6). Using these methods, over an average follow-up of 10.9 years (maximum, 15.9), 1,457 deaths were identified in the incident cohort with a censoring date of December 7, 2011.
Statistical Analysis
The AHI was categorized using the following commonly used clinical cut-points: less than 5.0, 5.0–14.9, 15.0–29.9, and greater than or equal to 30.0 events per hour (6). The FEV1 was modeled as a continuous variable scaling FEV1 such that derived coefficients represent difference in risk for a 200-ml difference in FEV1 (i.e., difference between and not within participants because lung function was not assessed during the follow-up period). Mortality rates were calculated by dividing number of deaths by accumulated number of person-years at risk. Poisson regression was used to determine the age-adjusted incidence rate ratios for mortality within strata of FEV1 and AHI using the underlying age distribution of the cohort at enrollment. Proportional hazards regression models were then constructed to calculate unadjusted and adjusted relative hazard ratios for mortality. Age, sex, race, and body mass index (BMI) were considered as covariates individually and in combination.
To additionally account for potential confounding, prevalent hypertension, cardiovascular disease (angina, heart failure, myocardial infarction, stroke, and coronary revascularization), and smoking status (current, former, or never) at enrollment were considered as covariates. Interaction terms were constructed between the AHI (categorical) and FEV1 (continuous) and both of these variables were also included in the model along with the interaction term while adjusting for other covariates as mentioned. Several complementary parameterizations were used for FEV1 including linear and nonlinear terms within the context of proportional hazards regression. Likelihood ratio tests were used to determine the significance of AHI-FEV1 interaction term in the multivariable models. The proportional hazards assumption was assessed for FEV1 and AHI using the method proposed by Grambsch and Therneau (17) and was not violated for either variable. Analyses were conducted by AHI categories to examine strata-specific effect of FEV1 on all-cause mortality. The STATA 12.0 (College station, TX) and the R-statistical packages (“survival” package) were used for all analyses.
Results
Follow-up data on all-cause mortality were available on 6,441 participants, with only 4% having missing data on FEV1, providing an analytical sample size of 6,173. Participants were, on average, 62.9 years old at baseline (SD, 10.9), 47% male, 76% non-Hispanic white, 8% African American, and had an average BMI of 28.5 kg/m2 (SD, 5.4). Forty-six percent of the sample was classified as never smokers, 43% as former smokers, and 11% as current smokers, with 53% having prevalent hypertension and 17% having prevalent cardiovascular disease. Significant differences between those with and without spirometry data were observed in age, race, and smoking status but the cumulative mortality rates in the two subgroups were not significantly different. The study sample was categorized based on common AHI clinical cut-points (Table 1). Significant differences in baseline characteristics were noted, with the group without SDB (AHI <5.0 events/h) being younger and having higher adjusted lung function, lower BMI, a higher proportion of never-smokers, and a larger number of women than those with SDB. Not surprisingly, the group without SDB had less prevalent comorbidity including hypertension and cardiovascular disease, and had a lower overall all-cause mortality rate compared with the groups with higher AHI values. Comparisons were also made using quartiles of FEV1 (see Table E1 in the online supplement), and results were similar, with expected differences in age, mortality rate, comorbidities, and a higher proportion of females in the lowest FEV1 group.
Table 1.
Baseline Characteristics of the Sleep Heart Health Study Cohort by AHI
| AHI (Events/h) |
||||
|---|---|---|---|---|
| <5 (n = 3,461) | 5–14.9 (n = 1,831) | 15–19.9 (n = 749) | ≥30 (n = 400) | |
| Age, yr | 61.3 (11.1) | 64.8 (10.6) | 65.0 (10.4) | 64.3 (10.7) |
| FEV1, L | 2.63 (0.81) | 2.65 (0.84) | 2.71 (0.77) | 2.75 (0.75) |
| FVC, L | 3.51 (1.05) | 3.51 (1.08) | 3.57 (1.00) | 3.58 (0.98) |
| FEV1/FVC, % | 75.0 (8.1) | 75.7 (7.4) | 76.2 (7.7) | 76.8 (7.1) |
| BMI, kg/m2 | 27.0 (4.5) | 29.6 (5.4) | 30.8 (5.9) | 32.5 (6.1) |
| Sex, n (%) | ||||
| Women | 2,182 (63) | 835 (46) | 271 (36) | 117 (29) |
| Men | 1,279 (37) | 996 (54) | 478 (64) | 283 (71) |
| Race, n (%) | ||||
| White | 2,681 (77) | 1,400 (76) | 561 (75) | 296 (74) |
| African American | 288 (8) | 131 (7) | 63 (8) | 37 (9) |
| Native American | 269 (8) | 205 (11) | 91 (12) | 48 (12) |
| Hispanic | 167 (5) | 68 (4) | 30 (4) | 15 (4) |
| Other | 56 (2) | 27 (1) | 4 (1) | 4 (1) |
| Smoking status, n (%) | ||||
| Never | 1,641 (48) | 790 (43) | 331 (45) | 168 (42) |
| Former | 1,321 (38) | 873 (48) | 340 (46) | 203 (51) |
| Current | 474 (145) | 159 (9) | 72 (10) | 25 (6) |
| Hypertension, n (%) | 1,611 (47) | 1,061 (58) | 447 (60) | 268 (68) |
| Cardiovascular disease, n (%)* | 422 (13) | 350 (20) | 162 (23) | 91 (24) |
| Deaths, n (%) | 691 (20) | 468 (26) | 233 (31) | 117 (29) |
| Follow-up time, person-years | 38,338.7 | 19,554.6 | 7,830.5 | 4,164.0 |
| Mortality rate, per 1,000 person-years | 18.0 | 23.9 | 29.8 | 28.1 |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index.
P < 0.0001 for comparisons of age, FEV1/FVC, BMI, sex, race, smoking status, hypertension, diabetes, cardiovascular disease across AHI categories using chi-square and analysis of variance to compare categorical and continuous variables, respectively. P = 0.01 for comparison of FEV1 across categories. Values represent mean (SD) unless otherwise indicated.
Cardiovascular disease defined as presence of angina, heart failure, myocardial infarction, stroke, or any coronary revascularization procedure.
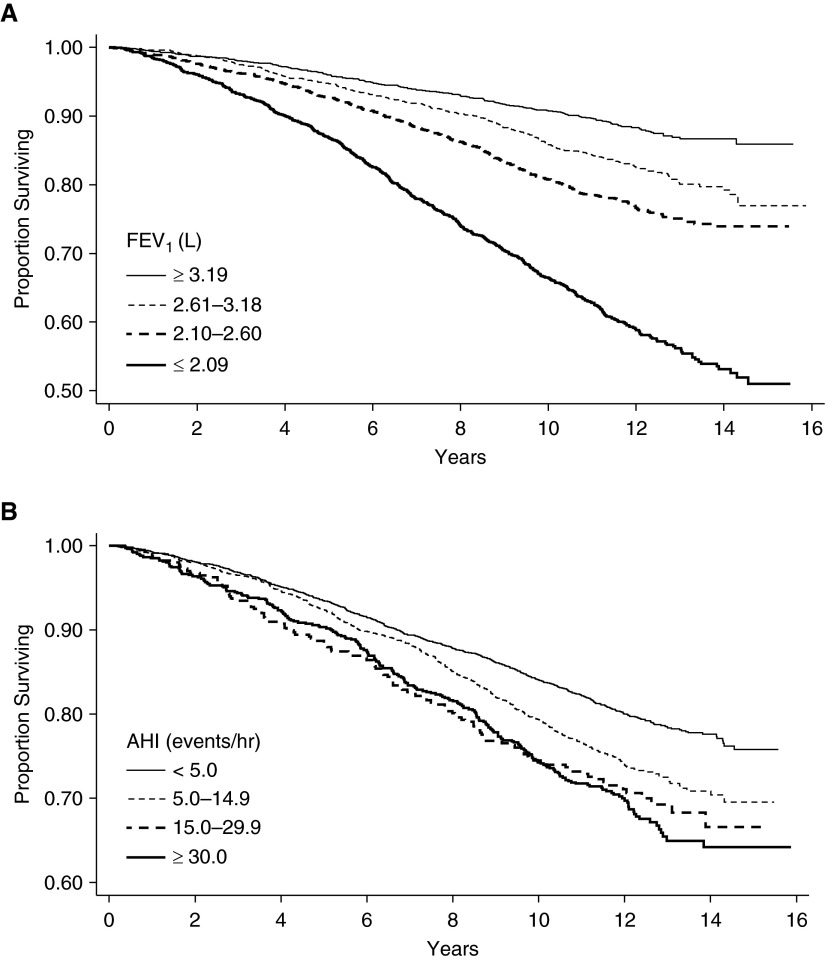
For the proportional hazards model for all-cause mortality, linear and nonlinear approaches were used to assess the shape of the association between FEV1 and all-cause mortality. These analyses revealed that irrespective of whether the whole cohort was used or whether SDB subgroups were examined, no statistically significant differences were noted between linear or nonlinear approaches for parameterizing FEV1 in any of the models. Thus, all analyses herein include FEV1 as a linear term. Furthermore, to ease exposition, quartiles of FEV1 were also used. Unadjusted Kaplan-Meier analysis revealed that lower lung function and higher AHI were both associated with all-cause mortality (Figure 1). The mortality rate in those with SDB (AHI ≥5 events/h) was higher than in the group without (26.9 vs. 18.2 deaths per 1,000 person-years of follow-up; P < 0.001). Age-adjusted mortality rates were also examined and compared across FEV1 quartiles and AHI categories. In people without SDB (AHI <5 events/h), a dose–response association was noted between a lower FEV1 and higher risk for mortality (Table 2). In contrast, in people with moderate to severe SDB (AHI, 15.0–29.9 and ≥30.0 events/h), a lower FEV1 was not associated with an increase in mortality risk when compared with a higher FEV1.
Figure 1.
Kaplan–Meier survival curves for (A) FEV1 and (B) apnea–hypopnea index (AHI) categories.
Table 2.
Age-adjusted Incidence Rate Ratios for Mortality among Categories of AHI and FEV1
| AHI (Events/h) | FEV1 Quartile |
||||
|---|---|---|---|---|---|
| ≥3.19 L | 2.61–3.18 L | 2.10–2.60 L | ≤2.09 L | P Value for Trend* | |
| <5 | 1.00 | 1.29 | 1.52 | 1.88 | <0.001 |
| 5.0–14.9 | 1.33 | 1.46 | 1.63 | 1.78 | 0.015 |
| 15.0–29.9 | 1.71 | 2.36 | 1.94 | 2.19 | 0.520 |
| ≥30 | 2.44 | 1.77 | 1.86 | 2.11 | 0.821 |
Definition of abbreviation: AHI = apnea–hypopnea index.
Age-adjusted incidence rate ratios for mortality were computed using Poisson regression models using AHI <5 events/h and FEV1 ≥3.19 L as the reference group. Incidence mortality rate ratios represent the relative difference in mortality rates comparing a specific AHI–FEV1 group with the reference group.
P value for a trend across FEV1 quartile within each AHI category.
Proportional hazards models were subsequently constructed to characterize the independent and combined effects of SDB (i.e., AHI) and lung function (FEV1) on all-cause mortality. The first model was an unadjusted model and included the FEV1 as a continuous variable, the AHI with categorical variables, and a set of interaction terms. The second model added demographic (age, sex, race), smoking status, and anthropometrics variables (BMI) as covariates. The final model additionally included variables of prevalent medical comorbidity that have been associated with all-cause mortality (e.g., hypertension, cardiovascular disease, and diabetes). Table 3 shows the results of these models with the AHI dichotomized at a cut-point of five events per hour (no-SDB vs. SDB). For every 200-ml decrease in FEV1 there was a 10% increase in all-cause mortality (hazard ratio [HR], 1.10; 95% confidence interval [CI], 1.08–1.13) in those with an AHI less than five events per hour, whereas there was only a 6% (HR, 1.06; 95% CI, 1.04–1.09) increase in all-cause mortality in those with an AHI greater than or equal to five events per hour. The difference in the influence of FEV1 on all-cause mortality was statistically significant comparing participants with and without SDB with lower FEV1 being less strongly associated in those with an AHI greater than or equal to five events per hour (P = 0.008 for interaction between FEV1 and AHI).
Table 3.
Adjusted Hazard Ratios for the Association of FEV1 (per Decrease of 200 ml) with All-Cause Mortality
| AHI (Events/h) |
||
|---|---|---|
| <5 | ≥5 | |
| N | 3,318 | 2,825 |
| Person-years | 36,765 | 29,983 |
| Deaths | 670 | 777 |
| Mortality rate* | 18.2 | 26.9 |
| Hazard ratios for FEV1/200 ml | ||
| Model 1† | 1.21 (1.19–1.24) | 1.13 (1.12–1.16) |
| Model 2‡ | 1.11 (1.09–1.14) | 1.08 (1.05–1.10) |
| Model 3§ | 1.10 (1.08–1.13) | 1.06 (1.04–1.09) |
Definition of abbreviation: AHI = apnea–hypopnea index.
P values for the interaction term between AHI and FEV1 in models 1, 2, and 3: <0.0001, 0.029, and 0.015.
Chi-square values for likelihood ratio test for inclusion of interaction term in models 1, 2, and 3 were 18.8 (P < 0.0001), 6.12 (P < 0.013), and 7.10 (P < 0.0077).
Mortality rate per 100,000 person-years.
Model 1: FEV1 (continuous), AHI (categorical), FEV1 (continuous) × AHI (categorical) interaction.
Model 2: Includes the variables from model 1 along with age, sex, race, body mass index, and smoking status.
Model 3: Includes the variables from model 2 along with hypertension and cardiovascular disease.
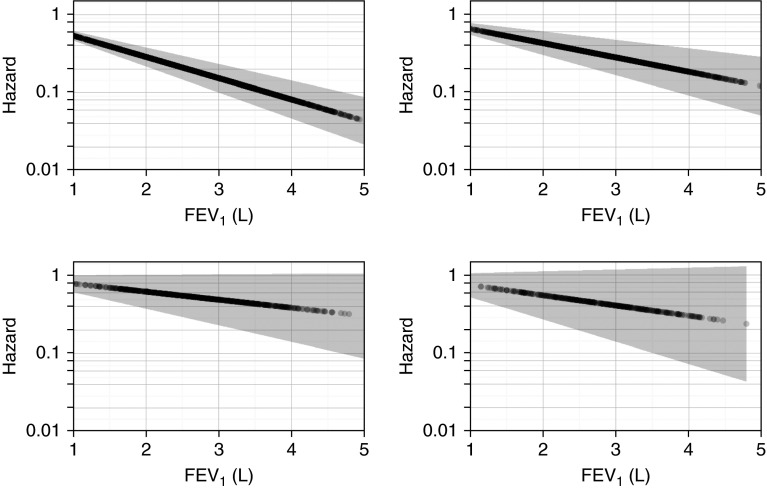
Effect modification of AHI by FEV1 on all-cause mortality was further examined as a function of SDB severity (Table 4). In subjects without SDB (AHI <5 events/h), the hazard ratio for all-cause mortality per 200-ml decrease in FEV1 was 1.10 (95% CI, 1.08–1.13). In contrast, the adjusted HRs for mild (AHI, 5.0–14.9 events/h), moderate (AHI, 15.0–29.9 events/h), and severe SDB (AHI, ≥30 events/h) were 1.07 (95% CI, 1.04–1.10), 1.06 (95% CI, 1.02–1.1), and 1.03 (95% CI, 0.98–1.09). A significant negative interaction was noted again between FEV1 and AHI categories. Figure 2 displays the hazard for mortality associated with FEV1 stratified by AHI group demonstrating that the effect of a lower FEV1 on all-cause mortality decreases with increasing AHI. Additional analyses revealed that age did not alter the interaction between AHI and FEV1 (data not shown). Similarly, sex also did not have a material impact on the magnitude of the AHI-FEV1 interaction.
Table 4.
Adjusted Hazard Ratios for FEV1 (per Decrease of 200 ml) Associated with All-Cause Mortality
| AHI (Events/h) |
||||
|---|---|---|---|---|
| <5.0 | 5.0–14.9 | 15.0–29.9 | ≥30.0 | |
| N | 3,314 | 1,755 | 718 | 386 |
| Person-years | 36,739 | 18,754 | 7,544 | 4,041 |
| Deaths | 667 | 452 | 224 | 114 |
| Mortality rate* | 18.2 | 24.1 | 29.7 | 28.2 |
| Hazard ratio for FEV1/200 ml |
||||
| Model 1† | 1.21 (1.19–1.24) | 1.15 (1.12–1.18) | 1.13 (1.09–1.17) | 1.10 (1.05–1.16) |
| Model 2‡ | 1.11 (1.09–1.14) | 1.09 (1.06–1.12) | 1.07 (1.03–1.11) | 1.05 (0.99–1.11) |
| Model 3§ | 1.10 (1.08–1.13) | 1.07 (1.04–1.10) | 1.06 (1.02–1.11) | 1.03 (0.98–1.09) |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index.
P values for interaction between AHI categories and FEV1 as a trend in models 1, 2 and 3: <0.001, 0.009, and 0.004.
Chi-square values for likelihood ratio test for inclusion of interaction term in models 1, 2, and 3 were 20.77 (P < 0.0001), 7.56 (P < 0.05), and 8.94 (P < 0.03).
Mortality rate per 100,000 person-years.
Model 1: FEV1 (continuous), AHI (categorical), FEV1 × AHI interaction.
Model 2: FEV1 (continuous), AHI (categorical), FEV1 × AHI interaction, age, sex, race, BMI, smoking status.
Model 3: FEV1 (continuous), AHI (categorical), FEV1 × AHI interaction, age, sex, race, BMI, smoking status, hypertension, and cardiovascular disease.
Figure 2.
Hazard for all-cause mortality for FEV1 (L) by category of apnea–hypopnea index (AHI). (Top left) AHI <5.0 events per hour. (Top right) AHI 5.0–14.9 events per hour. (Bottom left) AHI 15.0–29.9 events per hour. (Bottom right) AHI ≥30.0 events per hour.
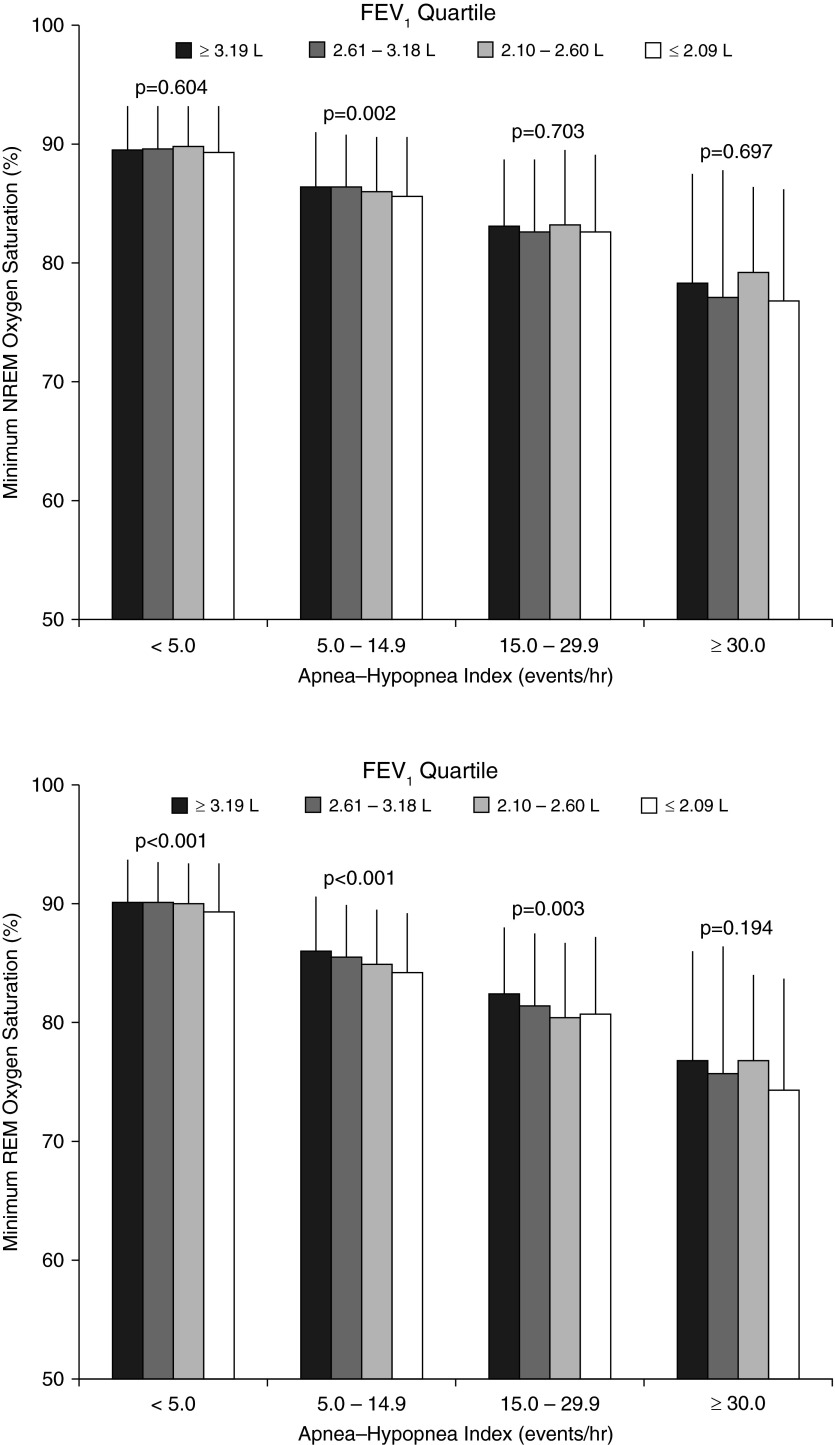
To further characterize the potential influence of sleep-related hypoxemia on all-cause mortality, metrics of nocturnal oxygen saturation were examined across FEV1 and AHI categories. Oxygen saturation nadir and the percent of total sleep time below an oxygen saturation of 90% during REM and non-REM sleep are shown in Figure 3. During REM and non-REM sleep, the nadir of oxygen saturation during sleep was the lowest in participants in the lowest FEV1 quartile. Not surprisingly, the nadir oxygen saturation was also related to SDB severity with progressively decreasing values with increasing AHI. The inverse association between AHI and nadir oxygen saturation during REM or non-REM sleep was less pronounced in those with a lower versus higher FEV1 indicating the marked influence of AHI and marginal impact of FEV1 on nocturnal oxygen saturation values. Similarly, total sleep time with an oxygen saturation below 90% was also associated with FEV1 and AHI. As with the nadir oxygen saturation data, the magnitude of the trend for AHI was far more remarkable than that for FEV1 (Table 5). These results collectively indicate that, as the AHI increases, the relative impact of a lower FEV1 on nocturnal hypoxemia decreases.
Figure 3.
Minimum oxygen saturation as a function of FEV1 quartiles and apnea–hypopnea index categories during non-REM (NREM) sleep (upper panel) and REM sleep (lower panel). P values for trend across FEV1 quartiles within each apnea–hypopnea index category are indicated in the figure. P values for trend across apnea–hypopnea index categories within each FEV1 quartile were all <0.001.
Table 5.
Percentage of Time Spent with Oxygen Saturation Below 90% Stratified by FEV1 Quartiles and AHI Categories
| FEV1 Quartile | AHI (Events/h) |
||||
|---|---|---|---|---|---|
| <5.0 | 5.0–14.9 | 15.0–29.9 | ≥30.0 | P Value* | |
| ≥ 3.19 L | 0.90 (5.33) | 2.32 (6.20) | 5.91 (10.60) | 15.88 (16.73) | <0.001 |
| 2.61–3.18 L | 0.68 (4.06) | 2.32 (6.74) | 6.61 (10.73) | 18.17 (19.94) | <0.001 |
| 2.10–2.60 L | 0.95 (5.430) | 3.88 (10.56) | 7.89 (12.99) | 19.85 (21.65) | <0.001 |
| ≤2.09 L | 2.30 (10.38) | 4.84 (11.68) | 9.96 (16.11) | 18.70 (20.29) | <0.001 |
| P value† | <0.001 | <0.001 | 0.002 | 0.240 | |
Definition of abbreviation: AHI = apnea–hypopnea index.
P value for trend across AHI categories, within each FEV1 quartile.
P value for trend across FEV1 quartiles, within each AHI category.
Analyses were also undertaken to examine whether the FVC and FEV1/FVC ratio modified the effects of AHI on all-cause mortality risk. As with the FEV1, FVC was modeled continuously (scaled by 200 ml). Similar to results of the FEV1 analyses, the incremental contribution of 200-ml lower FVC to all-cause mortality was more in individuals without SDB (AHI <5 events/h; HR, 1.07; 95% CI, 1.05–1.09) compared with those with (AHI ≥5 events/h; HR, 1.05; 95% CI, 1.03–1.07) in fully adjusted models (see Table E2). Furthermore, the relative contribution of a decrease in FVC on all-cause mortality decreased with increasing SDB severity such that the additional full-adjusted risk for all-cause mortality decreased from 7% (HR, 1.07; 95% CI, 1.05–1.07) in the group without SDB to 3% (HR, 1.03; 95% CI, 0.99–1.08) in the group with an AHI greater than or equal to 30 events per hour (see Table E3). In models including both FEV1 and FVC separately, estimates of mortality risk associated with lung function parameters were attenuated given the high degree of collinearity (r = 0.94) between FEV1 and FVC (see Figure E1). The interaction between FEV1/FVC ratio and AHI was much weaker, with most estimates of incremental all-cause mortality associated with lower FEV1/FVC ratio being nonsignificant, likely caused by the narrow distribution of this value and the limited number of participants with low FEV1/FVC values (see Tables E4 and E5).
To examine the potential effects of survival bias on the reported findings, several additional sensitivity analyses were conducted. Because exclusion of cohort members who died before the SHHS inception cohort could lead to the problem of left truncation, the effects of survival bias were assessed by examining subsets of the inception cohort by including only those participants who survived up to different time points after the initial enrollment (i.e., 2, 4, 6, and 8 yr after establishing the baseline cohort). The resulting sample subsets exclude participants who experience the mortality endpoint before the simulated enrollment date and thus exacerbate the problem of left truncation. Analyses of the resulting sample subsets showed no differences when compared with the full sample and thus survival bias does not explain the negative interaction between AHI and FEV1.
Discussion
This analysis of the SHHS data showed that lower lung function was associated with a higher risk for all-cause mortality. However, counter to our prior hypothesis, the relative contribution of lower lung function to mortality risk varied with severity of SDB. In fact, the relative effects of FEV1 on all-cause mortality were less with increasing AHI values. The finding of the association between lower lung function, as assessed by the absolute FEV1, and mortality has been demonstrated in several previous studies (18–20). Our findings based on the SHHS, which is a community-based cohort study not enriched by sampling for people with lung disease, confirm that impairments in lung function that do not fall in the range associated with clinical diagnoses are nevertheless associated with a higher risk for all-cause mortality.
Previous studies have shown that patients with “overlap syndrome” (COPD and SDB) have a high predisposition for adverse sequelae including COPD exacerbations and poor patient-reported outcomes (10, 21). Based on such evidence, it was hypothesized that SDB and lung function (i.e., lower FEV1) would synergistically augment risk for mortality. Although a statistical interaction was indeed noted between SDB severity and lung function level, the direction was counter to what was initially hypothesized. Several possible reasons could explain the observed negative interaction between AHI and FEV1. First, it is plausible that SDB imposes sufficiently high risk for all-cause mortality such that the contributions of other coexisting physiologic impairments, such as a lower FEV1, do not have the same influence as it does in the absence of SDB. Second, it is possible that survival bias could also explain the negative interaction observed between SDB severity and lower levels of lung function. Because increasing SDB severity and impaired lung function individually confer such high additive risk for all-cause mortality, those that are most susceptible to their effects may have experienced early mortality and thus not been included in the study sample. Therefore, participants with moderate to severe SDB and lower levels of lung function enrolled in SHHS cohort represent survivors with a lower predisposition to the consequences from either of the two conditions. However, simulated samples from the original SHHS cohort that explored the problem of left truncation showed that survival bias was not a likely reason for the negative interaction between AHI and FEV1.
Third, it is conceivable that a positive interaction would have been uncovered if a substantial number of participants with moderate to severe SDB and COPD (i.e., “overlap syndrome”) were longitudinally assessed. However, the SHHS cohort was not enriched with participants with significant lung disease and thus cannot provide evidence on health-related outcomes, such as all-cause mortality, in patients that have SDB and COPD. Finally, the focus of the current analysis was on all-cause mortality. Assessment of other clinically relevant endpoints, such as incident cardiovascular disease, may reveal that a high AHI and a low FEV1 do, in fact, augment the attributable risk of each factor alone. Despite the aforementioned plausible explanations, the counterintuitive finding of a negative interaction may indeed represent a true biologic phenomenon. Assessment of the nadir oxygen saturation values during REM and non-REM sleep and percentage of time spent with an oxygen saturation below 90% showed that SDB is a greater contributor to nocturnal hypoxemia than impaired lung function. Thus, if heightened health risk in patients with SDB and impaired lung function is from the cumulative effects of nocturnal hypoxemia, it is then not surprising to find that with increasing SDB severity, the relative influence of FEV1 is progressively less. The importance of SDB with regard to mortality risk and nocturnal hypoxemia, as we have previously shown (6), is therefore supported by findings of the current study.
There are several limitations of the current analysis that warrant consideration. First, spirometry measurements were missing in approximately 5% of the study sample. Although there were some demographic differences between participants with and without spirometry data, there were no significant differences in mortality between the two groups and thus any bias introduced from missing data is likely to be small. Second, as previously mentioned, the study sample was not enriched for lung disease, which limits the generalizability of inferences to those with severe obstructive lung disease and SDB. Nonetheless, the results presented herein do highlight the combined effects of two well-established risk factors (i.e., SDB and lung function) on all-cause mortality in a community sample of middle-aged and older adults. Additionally, cause-specific mortality (e.g., cancer death, stroke death) was not available for the cohort and thus inferences regarding how SDB and impaired lung function increase mortality are not possible.
Finally, neither lung function nor relevant covariates were assessed in a longitudinal fashion. These limitations notwithstanding, there are also numerous strengths in the current analysis. These include a study sample that included a large number of community-based adults with an equal representation of men and women. Moreover, the availability of objective data on SDB severity from overnight polysomnography along with information garnered from spirometry offered the unique opportunity to examine the effects of SDB and lung function on all-cause mortality. Finally, the availability of long-term follow-up data along with the ability to adjust for potential confounding factors facilitated a rigorous assessment of the independent and interactive effects of SDB and lung function on an endpoint of clinical and public health value.
In conclusion, lung function impairment is associated with a higher risk of death in this large community cohort of middle-aged and older adults. However, with increasing SDB severity, the incremental addition of lung function to mortality risk decreases. Given the finding that impairment in lung function has less of an impact as SDB severity increases, an appropriate follow-up is to assess cohorts that are enriched with people with lung disease, such as COPD, while also examining other endpoints. Such analyses would extend the evidence base on defining whether other physiologic impairments augment the established effects of SDB on health outcomes.
Supplementary Material
Footnotes
The Sleep Heart Health Study was supported by NHLBI cooperative agreements U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53934 (University of Minnesota), U01HL53931 (New York University), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL63463 (Case Western Reserve University), and U01HL63429 (Missouri Breaks Research). N.P. is supported by National Institutes of Health grant K23-HL123594 and the Johns Hopkins University School of Medicine Clinician Scientist Award. N.M.P. is supported by National Institutes of Health grants R01 HL075078 and R01 HL117167.
Author Contributions: Study design and collection of data, J.S., S.F.Q., D.J.G., S.R., and N.M.P. Analysis of data, N.P., C.C., G.N., and N.M.P. Interpretation of findings, N.P., C.C., G.N., J.S., S.F.Q., D.J.G., S.R., and N.M.P. Preparation of manuscript, N.P., C.C., G.N., J.S., S.F.Q., D.J.G., S.R., and N.M.P.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201511-2178OC on April 22, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 2.Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Antó JM, Agustí AG, Gómez FP, Rodríguez-Roisín R, Moons KG, Kessels AG, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374:704–711. doi: 10.1016/S0140-6736(09)61301-5. [DOI] [PubMed] [Google Scholar]
- 3.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 4.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–715, discussion 715–716. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schünemann HJ, Dorn J, Grant BJ, Winkelstein W, Jr, Trevisan M. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118:656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 9.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;151:82–86. doi: 10.1164/ajrccm.151.1.7812577. [DOI] [PubMed] [Google Scholar]
- 10.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182:325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 11.Sanders MH, Newman AB, Haggerty CL, Redline S, Lebowitz M, Samet J, O’Connor GT, Punjabi NM, Shahar E Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 12.Azuma M, Chin K, Yoshimura C, Takegami M, Takahashi K, Sumi K, Nakamura T, Nakayama-Ashida Y, Minami I, Horita S, Oka Y, et al. Associations among chronic obstructive pulmonary disease and sleep-disordered breathing in an urban male working population in Japan. Respiration. 2014;88:234–243. doi: 10.1159/000366064. [DOI] [PubMed] [Google Scholar]
- 13.Lee R, McNicholas WT. Obstructive sleep apnea in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2011;17:79–83. doi: 10.1097/MCP.0b013e32834317bb. [DOI] [PubMed] [Google Scholar]
- 14.Shiina K, Tomiyama H, Takata Y, Yoshida M, Kato K, Nishihata Y, Matsumoto C, Odaira M, Saruhara H, Hashimura Y, et al. Overlap syndrome: additive effects of COPD on the cardiovascular damages in patients with OSA. Respir Med. 2012;106:1335–1341. doi: 10.1016/j.rmed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J, Samet JM, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- 16.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 17.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 18.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58:388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannino DM, Diaz-Guzman E, Buist S. Pre- and post-bronchodilator lung function as predictors of mortality in the Lung Health Study. Respir Res. 2011;12:136. doi: 10.1186/1465-9921-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan G, Knuiman MW, Divitini ML, James A, Musk AW, Bartholomew HC. Decline in lung function and mortality: the Busselton Health Study. J Epidemiol Community Health. 1999;53:230–234. doi: 10.1136/jech.53.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaya FT, Lin PJ, Aljawadi MH, Scharf SM. Elevated economic burden in obstructive lung disease patients with concomitant sleep apnea syndrome. Sleep Breath. 2009;13:317–323. doi: 10.1007/s11325-009-0266-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.