ABSTRACT
Organophosphate pesticides used in agriculture can pose health risks to humans and wildlife. We hypothesized that dietary supplementation with Lactobacillus, a genus of commensal bacteria, would reduce absorption and toxicity of consumed organophosphate pesticides (parathion and chlorpyrifos [CP]). Several Lactobacillus species were screened for toleration of 100 ppm of CP or parathion in MRS broth based on 24-h growth curves. Certain Lactobacillus strains were unable to reach stationary-phase culture maxima and displayed an abnormal culture morphology in response to pesticide. Further characterization of commonly used, pesticide-tolerant and pesticide-susceptible, probiotic Lactobacillus rhamnosus strain GG (LGG) and L. rhamnosus strain GR-1 (LGR-1), respectively, revealed that both strains could significantly sequester organophosphate pesticides from solution after 24-h coincubations. This effect was independent of metabolic activity, as L. rhamnosus GG did not hydrolyze CP and no difference in organophosphate sequestration was observed between live and heat-killed strains. Furthermore, LGR-1 and LGG reduced the absorption of 100 μM parathion or CP in a Caco-2 Transwell model of the small intestine epithelium. To determine the effect of sequestration on acute toxicity, newly eclosed Drosophila melanogaster flies were exposed to food containing 10 μM CP with or without supplementation with live LGG. Supplementation with LGG simultaneously, but not with administration of CP 3 days prior (prophylactically), mitigated CP-induced mortality. In summary, the results suggest that L. rhamnosus may be useful for reducing toxic organophosphate pesticide exposure via passive binding. These findings could be transferable to clinical and livestock applications due to affordability and practical ability to supplement products with food-grade bacteria.
IMPORTANCE The consequences of environmental pesticide pollution due to widespread usage in agriculture and soil leaching are becoming a major societal concern. Although the long-term effects of low-dose pesticide exposure for humans and wildlife remain largely unknown, logic suggests that these chemicals are not aligned with ecosystem health. This observation is most strongly supported by the agricultural losses associated with honeybee population declines, known as colony collapse disorder, in which pesticide usage is a likely trigger. Lactobacilli are bacteria used as beneficial microorganisms in fermented foods and have shown potentials to sequester and degrade environmental toxins. This study demonstrated that commonly used probiotic strains of lactobacilli could sequester, but not metabolize, organophosphate pesticides (parathion and chlorpyrifos). This Lactobacillus-mediated sequestration was associated with decreased intestinal absorption and insect toxicity in appropriate models. These findings hold promise for supplementing human, livestock, or apiary foods with probiotic microorganisms to reduce organophosphate pesticide exposure.
INTRODUCTION
Organophosphate pesticides are a class of insecticide under scrutiny for being linked to toxic effects in both humans and wildlife (1). However, these compounds are still commonly used in agriculture and pest control programs. Organophosphate pesticides include parathion, malathion, methyl parathion, chloropyrifos (CP), diazinon, dichlorvos, phosmet, fenitrothion, tetrachlorvinphos, azamethiphos, and azinphos-methyl. Organophosphate pesticides irreversibly inhibit acetylcholinesterase to induce excessive cholinergic stimulation (2, 3). The potential off-target health consequences of organophosphate pesticide exposure for humans include neonatal developmental abnormalities (4, 5), endocrine disruption (6), neurodegeneration, cancer (7, 8), metabolic disruption (9), heart disease (10), chronic kidney disease, and other less common pathologies. In addition, organophosphate pesticides are reported to have negative impacts on honeybee colonies, which are critical pollinators for numerous agricultural products (11–13). Although the evidence is mixed and often correlative, the original use of these organophosphates as nerve agents strongly suggests that these pesticides are not aligned with human or wildlife health.
Despite negative consequences, the affordability and need to prevent crop losses associated with insect infestations suggest that organophosphate pesticide use will continue in the near future. One long-considered counter to these effects is the use of microbes to detoxify organophosphate pesticide-contaminated environments (14–20). Classical bioremediation efforts have identified numerous strains of soil bacteria that contain genetically diverse phosphotriesterases capable of organophosphate degradation (15). Moreover, the symbiotic relationship between Burkholderia strains and the bean bug, Riportus pedestris, has been shown to confer upon these insects resistance to organophosphate insecticides (21). Taken together, these observations suggest that microorganisms could be used to reduce the toxic effects of organophosphate insecticides in vivo.
There have been developments within bioremediation research to investigate the potential of transitory food-grade bacteria to prophylactically prevent the absorption of environmental toxins, such as pesticides (22), heavy metals (23), and aflatoxin (24, 25). Many lactobacilli are commonly used in fermented foods, such as yogurt, cheese, sauerkraut, pickles, beer, wine, cider, kimchi, cocoa, and kefir (26). Lactobacilli have also been shown to be natural and beneficial gastrointestinal microbiota members in humans, livestock, honeybees (27), and fish (28). Notably, lactobacilli have been shown to reduce organophosphate pesticide contamination of dairy products (29, 30). The mechanism of action used by lactobacilli against organophosphate pesticides remains unclear and is commonly attributed to phosphatase capabilities. However, one kimchi isolate, Lactobacillus brevis WCP902, was shown to contain the opdB gene, which conferred the active ability to degrade CP (31). Similarly, the common probiotic Lactobacillus rhamnosus strain GG (LGG) has a predicted hydrolase/phosphotriesterase (LGG_RS02045) with sequence similarity to the experimentally validated parathion hydrolase (opd) found in Brevundimonas diminuta.
The aim of this study was to better characterize the in vivo bioremediation potential of probiotic food-grade bacteria that interact with organophosphate pesticides. We hypothesized that the direct binding or metabolism of organophosphate pesticides by L. rhamnosus strains would reduce toxin absorption and downstream organism toxicity.
MATERIALS AND METHODS
Chemicals.
CP (catalog number 45395) and parathion (catalog number 45607) were obtained from Sigma-Aldrich. Stock solutions were prepared at 100 mg/ml or 10 mg/ml in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and stored frozen at −20°C until usage.
Bacterial strains and culture.
Lactobacillus species and strains used were L. rhamnosus GG, L. rhamnosus GR-1, L. casei ATCC 393, L. delbrueckii DSM 20074, L. plantarum ATCC 14917, L. crispatus ATCC 33820, L. fermentum ATCC 11739, L. johnsonii DSM 20053, L. reuteri ATCC 2773, and L. rhamnosus ATCC 7469.
A pET20b vector (EMD Millipore) containing the organophosphate insecticide-degrading PTE (phosphotriesterase) gene inserted between NdeI and EcoRI sites (20) was obtained as a gift from Frank M. Raushel (Texas A&M University, USA). Three hundred nanograms of pET20b/PTE vector was transformed into 50 μl of chilled (on ice for 15 min) chemically competent Escherichia coli BL21(DE3) cells by 2 min of heat shock at 42°C followed by incubation on ice for 2 min. The transformation mixture was serially diluted and plated for positive selection on Luria-Bertani agar (LB, catalog number DF0446173; BD Difco) plates containing 300 μg ampicillin/ml. Plasmids were isolated from transformants grown at 37°C aerobically overnight using the PureLink Quick plasmid miniprep kit according to the manufacturer's instructions (catalog number K210010; Invitrogen). Positive transformants were confirmed by sequencing the plasmids with the T7 promoter (TAATACGACTCACTATAGGG) and T7 terminator (GCTAGTTATTGCTCAGCGG) primers with the Applied Biosystems 3730 Analyzer platform at the London Regional Genomics Centre (Robart's Research Institute, London, Canada).
E. coli BL21(DE3)(pET20b/PTE) cells were inoculated from LB agar plates into LB broth, both supplemented with 300 μg ampicillin/ml of culture medium and incubated overnight (18 h) at 37°C aerobically. Lactobacilli were inoculated from Lactobacilli MRS agar (de Man, Rogosa, and Sharpe agar [catalog number 288130]; BD Difco) plates into MRS broth. Inoculated MRS broth cultures were subcultured and incubated overnight (18 h) at 37°C anaerobically and statically for experimental procedures. Bacteria were heat killed by incubation at 56°C for 90 min when necessary. Bacterial killing was confirmed by spread plating 100 μl of bacterial culture on MRS agar plates and verifying the absence of colony growth after 3 days (37°C anaerobically).
Pesticide tolerance assay.
Overnight broth cultures (stationary phase, 109 CFU/ml) were subcultured (1:100 dilution) into 96-well plates (catalog number 351177; Falcon) containing MRS broth with or without the addition of 100 ppm CP or parathion or vehicle (DMSO). Plates were sealed with optically clear multiwell plate-sealing films and incubated at 37°C and read every 30 min for 24 h at a wavelength of 600 nm by using a Labsystems Multiskan Ascent microplate reader.
Pesticide hydrolysis assay.
Semiqualitative assessment of bacterial CP hydrolysis was determined based on a modified protocol previously described (21). Briefly, 1 μl of overnight broth culture (106 CFU) of LGG or E. coli BL21(DE3)(pET20b/PTE) (positive control) was spot plated onto brain heart infusion (catalog number B11059; DB Difco) agar plates containing 1,000 ppm (1 mg/ml) of emulsified CP. After 48 h of anaerobic or aerobic incubation at 37°C for LGG and E. coli BL21(DE3) pET20b/PTE, respectively, the radius of halo formation (zone of clearing) was determined.
Pesticide binding and metabolism assay.
Overnight bacterial cultures were pelleted at 5,000 × g, washed, and resuspended in 50 mM HEPES (pH 6.8; 109 CFU/ml). Bacteria-buffer or buffer-alone solutions were incubated with 100 ppm of parathion or CP protected from light at 37°C for 24 h with gentle shaking at 200 rpm. Cultures were pelleted and supernatant was collected. Pellets were washed in 50 mM HEPES, resuspended in methanol, and sonicated for 15 min. Supernatants were again collected for assessment of organophosphate levels in pellets.
Pesticide levels were determined by high-performance liquid chromatography (HPLC) using a Poroshell 120 column (100 by 4.6 mm, 2.7 μm; Agilent, Mississauga, Ontario, Canada) and a UV detector, based on a modified protocol (32). CP and parathion were detected at 288- and 275-nm wavelengths, respectively. The solvent flow rate was 1 ml/min for solvent A (H2O plus 0.1% trifluoroacetic acid) and solvent B (acetonitrile plus 0.1% trifluoroacetic acid). A solvent gradient was set to 25, 25, 100, 100, 25, and 25% B at 0, 2, 9, 11, 11.5, and 13 min, respectively. Sample peak area units were compared to a linear relationship of appropriate standards of known concentrations. The limits of detection for parathion and CP were 0.001 ppm and 0.02 ppm, respectively. The percentage of pesticide remaining was calculated as the concentration (parts per million) of biological replicate divided by the concentration (parts per million) of the mean pesticide-only control replicates × 100.
Caco-2 cell culture and Transwell experimentation.
Caco-2 cells were obtained as a gift from Brad Urquhart (Western University, London, Canada). Caco-2 cells were routinely maintained at 37°C under atmospheric conditions with 5% CO2. Cells were cultured in Dulbecco's modified Eagle medium (catalog number 11960044; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 1 mM sodium pyruvate (catalog number 11360070; Gibco), 1% MEM nonessential amino acids (catalog number 11140050; Gibco), 4 mM l-glutamine (catalog number 25030081; Gibco), and 100 U/ml penicillin-streptomycin (catalog number 15140122; Gibco). Cells were used experimentally between passages 35 and 45.
Cells were seeded onto 12-mm Transwell plates (12-well plates with a 0.4-μm-pore-size polyester membrane insert, catalog number 3460; Corning) at a concentration of 1.5 × 105 cells/insert. Cells were differentiated by culture for 21 days as previously described (33). Prior to absorption experimentation, apical and basolateral compartments were washed 3 times and resuspended in Hanks' balanced salt solution (catalog number 14025092; Gibco). Cells were treated apically with 100 μM parathion or CP in the presence or absence of 109 CFU/ml of LGG or LGR-1. Basolateral sampling (100 μl) with replacement was performed at 30 min, 1 h, and 2 h. Following absorption experimentation, Caco-2 monolayer integrity was confirmed in a Lucifer yellow (CH lithium salt, catalog number 80015; Biotium) rejection assay according to the manufacturer's instructions.
Drosophila melanogaster husbandry.
Wild-type Canton-S (stock number 1) stocks were obtained from the Bloomington Drosophila Stock Center at Indiana University. Drosophila flies were maintained in medium containing 1.5% (wt/vol) agar, 1.73% (wt/vol) yeast, 7.3% (wt/vol) cornmeal, 7.6% (vol/vol) corn syrup, and 0.58% (vol/vol) acid mix at 22°C with 12-h light/dark cycles. For experimental procedures, media were supplemented with or without CP at concentrations of 1, 10, or 100 μM prior to solidification. Food was stored at 4°C and used within a week. Experiments with adult Drosophila used newly eclosed flies. All experiments were performed in polypropylene Drosophila vials (GEN32-121 and GEN49-102; Diamed Lab Supplies Inc.) containing 10 ml of food with 15 to 25 flies/tube.
Adult Drosophila survival assays.
Fifteen to 25 newly eclosed Drosophila flies were anesthetized with CO2 and randomly transferred (mixed sex) into standard vials at mid-light cycle (9 a.m.) for each replicate. Drosophila flies were confirmed to be alive following anesthetization and subsequently monitored daily (9 a.m.) for survival. Medium was supplemented with a single dose of 100 μl (109 CFU) of washed and concentrated LGG or phosphate-buffered saline vehicle when experimentally appropriate and allowed to air dry before use.
Drosophila negative geotaxis assay.
Negative geotaxis assays were performed as previously described (34). The mean distances climbed (after 3 s) of 5 replicates from 3 independent experiments were determined.
Drosophila microbiota analysis.
Whole Drosophila flies were surface sterilized with 70% ethanol and homogenized with a handheld motorized pestle in 0.01 M phosphate-buffered saline. Serially diluted fly homogenates were spot plated onto MRS agar plates. Plates were incubated anaerobically at 37°C, and CFU were enumerated after 48 h of incubation.
Statistical analyses.
All statistical analyses were performed using GraphPad Prism 6 with one-way analysis of variance tests with Tukey's multiple comparisons test or the Kruskal-Wallis test with Dunn's multiple-comparisons test. Alternatively, Mantel-Cox and Gehan-Breslow-Wilcoxon tests (in which early time points are weighted more strongly) were used for Drosophila survival analyses.
RESULTS
Lactobacilli vary in their ability to tolerate high levels of organophosphate pesticides.
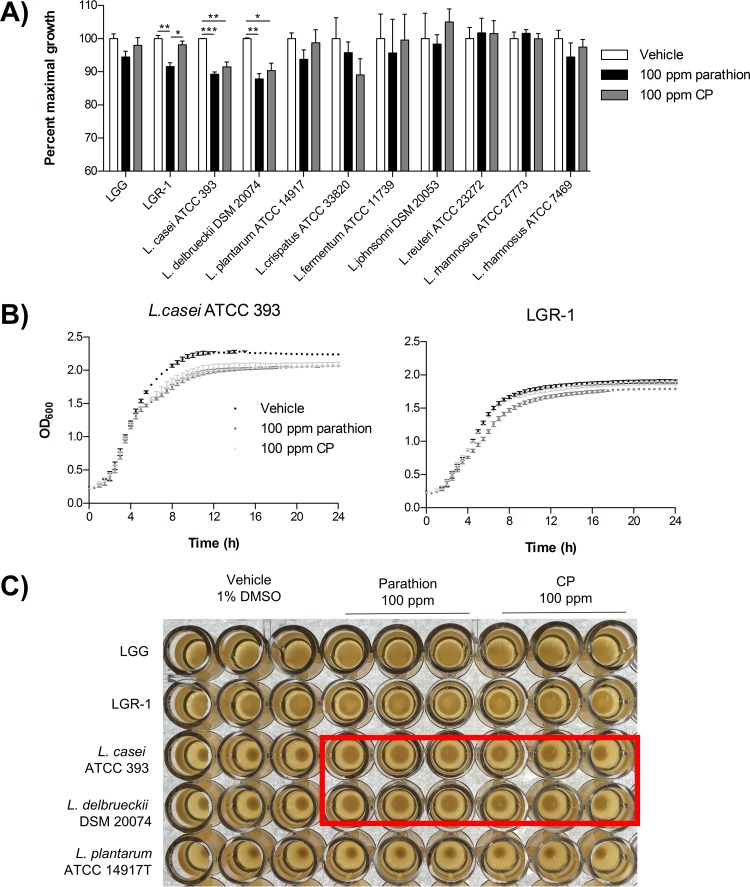
In an effort to determine a strain of Lactobacillus able to tolerate high levels of organophosphate pesticides, lactobacilli were screened for the ability to tolerate 100 ppm of parathion or CP via growth curves. In general, lactobacillus growth was largely unaltered by the presence of parathion or CP relative to the vehicle control. Based on the solubility maxima of parathion and CP at 37°C, pesticide concentrations greater than 100 ppm were not evaluated. However, LGR-1, L. casei ATCC 393, and L. delbrueckii DSM 20074 demonstrated significantly reduced growth (P < 0.05) in the presence of parathion or CP (Fig. 1A). Organophosphate pesticide treatment significantly reduced the stationary-phase growth maxima of these strains of lactobacilli compared to the vehicle control treatment (Fig. 1B). The organophosphate-induced growth deficiency was associated with abnormal shiny and mucoid culture morphologies for L. casei ATCC 393 and L. delbrueckii DSM 20074 (Fig. 1C). However, quantification of biofilm formation in a microtiter dish assay did not demonstrate any observable differences in biofilm formation for LGG, LGR-1, L. casei ATCC 393, or L. delbrueckii DSM 20074 following a 24-h exposure to 100 ppm of parathion or CP (see Fig. S1 in the supplemental material).
FIG 1.
Lactobacilli vary in their ability to tolerate high levels of organophosphate pesticides. (A) Percent maximal growth was calculated from 24-h growth curve data (based on the optical densities at 600 nm) using the area under the growth curve of pesticide-treated bacteria relative to that of vehicle treatment of each bacterial strain. (B) Representative growth curves of Lactobacillus casei ATCC 393 and Lactobacillus delbrueckii DSM 20074. (C) Representative image of lactobacillus cultures following 24 h of treatment with 100 ppm parathion or CP or vehicle. Red box, L. casei ATCC 393 and L. delbrueckii DSM 20074 cultures with differential morphologies following organophosphate pesticide treatment, compared to the vehicle cultures. Data are mean (± standard error of the mean) results from 3 independent experiments with triplicate technical replicates.
Hydrolase (WP_014569076.1) in L. rhamnosus GG was computationally identified as a potential organophosphate hydrolase.
The National Center for Biotechnology Information's align sequences protein Basic Local Alignment Search Tool (standard settings) was used to compare the query WP_014569076.1 (hydrolase corresponding to the gene LGG_RS02045 or php) to the experimentally confirmed parathion hydrolase present in Brevundimonas diminuta (UniProtKB P0A434). A significant alignment was observed (E = 10−26) with the standard compositional matrix adjustment method with the following characteristics: score = 94.4 bits, identities = 73/268, positives = 134/268 (50%), and gaps = 30/268 (11%). The standard settings for T-Coffee (version 11.00.d625267; 2016-01-11, 15:25:41, revision d625267, build 507) (35) were used to generate a multiple-sequence alignment between the hydrolase WP_014569076.1 from LGG and the experimentally confirmed parathion hydrolases from B. diminuta and Sphingobium fuliginis ATCC 27551 (UniProtKB P0A433) (Fig. 2). In addition, protein modeling with the Protein Homology/analogY Recognition Engine software version 2.0 (36) best matched the LGG hydrolase WP_014569076.1 as a likely member of the metallo-dependent hydrolases and phosphotriesterase-like protein superfamily and family, respectively, with 100% confidence and 52% identity.
FIG 2.
Multiple-sequence alignment of the LGG hydrolase, WP_014569076.1, and experimentally confirmed parathion hydrolases (Opd) from B. diminuta and S. fuliginis ATCC 27551.
L. rhamnosus strains GG and GR-1 can bind, but not metabolize, organophosphate pesticides.
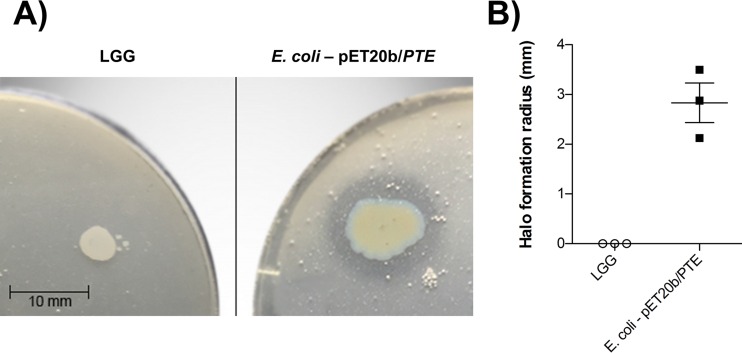
The functional potential of the hydrolase WP_014569076.1 from LGG was assessed in the semiquantitative pesticide hydrolysis assay with CP. Compared to the positive control, E. coli BL21(DE3)(pET20b/PTE), LGG did not demonstrate any CP hydrolysis under the aforementioned culture conditions, as evidenced by the lack of halo formation (Fig. 3). These results suggested that the hydrolase WP_014569076.1 likely does not function as an organophosphate pesticide hydrolase.
FIG 3.
The LGG hydrolase WP_014569076.1 does not hydrolyze organophosphate pesticides. (A) Representative image of results for the semiquantitative pesticide hydrolysis assay following 48-h incubations. (B) The radius of halo formation (pesticide hydrolysis) was quantified following 48-h incubations. Data are presented as means ± standard errors of the means from results of 3 independent experiments containing 3 or 4 technical replicates.
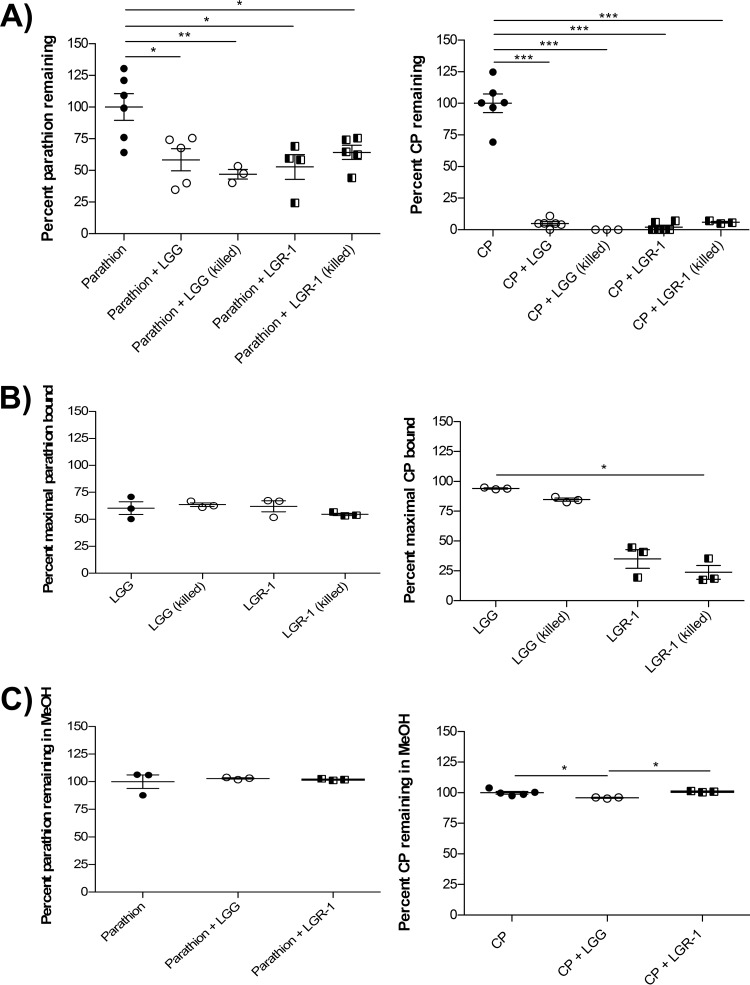
The commonly used commercial probiotics, LGG and LGR-1, were also characterized for organophosphate pesticide binding or metabolism, since they displayed opposite phenotypes of unimpaired and impaired growth in organophosphate-containing media, respectively. Both LGG and LGR-1 significantly sequestered parathion and CP from solution (P < 0.05); however, this effect was much more pronounced with CP (Fig. 4A). Interestingly, organophosphate sequestration was observed with LGG and LGR-1 even after heat killing, and the extent of binding was similar to that for live bacteria (Fig. 4A). This finding further supported the idea that the predicted LGG organophosphate hydrolase gene, WP_014569076.1, is not involved in organophosphate pesticide metabolism. The binding of organophosphate pesticides by LGG and LGR-1 was further supported by the confirmation of parathion and CP in the bacterial pellets following experimentation (Fig. 4B). The amount of CP observed in LGG pellets was considerably higher than that in LGR-1 pellets. A discrepancy between the amount of CP sequestered by LGR-1 and that found in LGR-1 pellets was likely due to weak LGR-1–CP binding interactions that were perturbed by washing the pellets prior to lysis. This preferential interaction of LGG, rather than LGR-1, with CP was not observed with parathion. Furthermore, disrupting bacterial membranes and surface proteins by using methanol as a solvent (37) for binding experiments prevented any notable observations of bacterium-organophosphate binding (Fig. 4C).
FIG 4.
LGG and LGR-1 can bind, but not metabolize, organophosphate pesticides. (A) Percentages of parathion and CP metabolized were determined in stationary-phase live and heat-killed LGG and LGR-1 cultures, relative to results in pesticide-only controls following 24-h coincubations in 50 mM HEPES. (B) Percent maximal parathion and CP bound relative to 100 ppm input was determined in bacterial pellets following 24-h pesticide coincubations in 50 mM HEPES. (C) Percentages of parathion and CP metabolized were determined in stationary-phase chemically killed LGG and LGR-1 cultures, relative to pesticide-only controls, following 24-h coincubations in methanol. Data are means ± standard deviations of results from at least 3 independent experiments *, P < 0.05; **, P < 0.01; ***, P < 0.001.
L. rhamnosus GG and GR-1 reduced the absorption of organophosphate pesticides in a Caco-2 Transwell model.
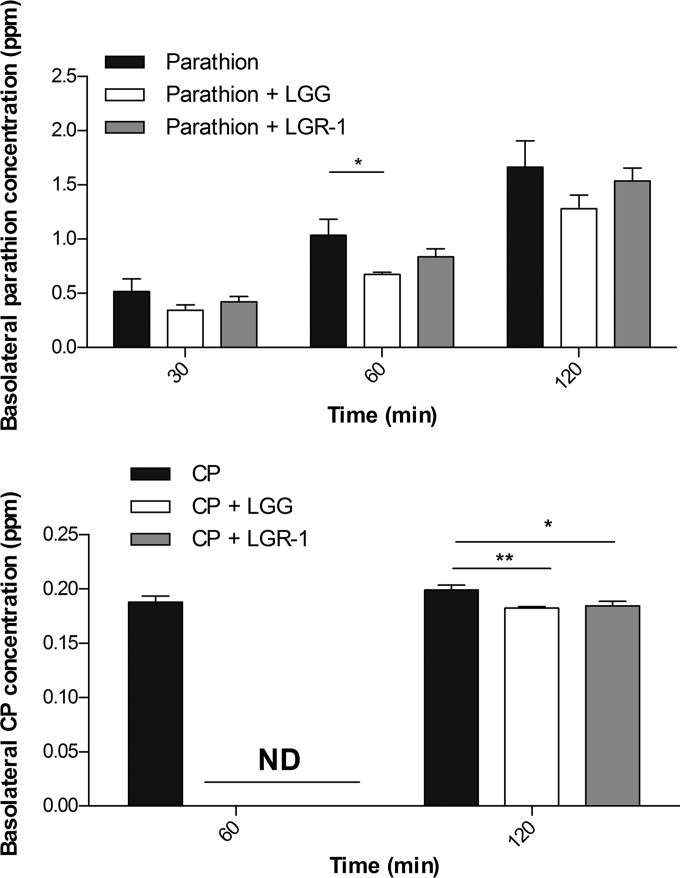
The ability of LGG- and LGR-1-mediated organophosphate pesticide sequestration was tested for the potential to reduce intestinal absorption of these compounds via a Caco-2 Transwell model of the small intestine. LGG and LGR-1 moderately reduced the Caco-2 apical-basolateral translocation of both parathion and CP compared to unsupplemented controls (Fig. 5). Similar to earlier binding experiments, reduction of organophosphate pesticide absorption was more pronounced with LGG than with LGR-1, and lactobacillus binding was more prominent with CP than with parathion. Basolateral levels of parathion increased kinetically (30 min > 60 min > 120 min) post-apical exposure for cells exposed to CP with or without supplementation of LGG or LGR-1. However, there was an insignificant trend (other than that for CP compared to CP-LGG at 1 h) of decreased basolateral parathion levels in LGG- or LGR-1-supplemented cells at all time points. Most notably, at 1 h post-apical exposure, CP apical-basolateral absorption was undetectable in CP-LGG and CP-LGR-1 simultaneously treated cells compared to cells treated with CP alone. However, by 2 h post-apical exposure, basolateral CP levels in CP-LGG– and CP–LGR-1–treated cells increased notably but remained significantly lower (P < 0.05) than that for cells treated with CP alone. These findings support earlier observations of potentially biologically relevant interactions of probiotic lactobacilli with consumed organophosphate pesticides that seem to vary in effect based on subtle differences in molecular structure.
FIG 5.
LGG and LGR-1 reduced the absorption of organophosphate pesticides in a Caco-2 Transwell model. Caco-2 cells, differentiated into small intestinal-like epithelium on Transwells, were exposed apically to 100 μM parathion or chlorpyrifos with or without 109 CFU/ml LGG or LGR-1. The basolateral compartment was kinetically analyzed for parathion or CP. Data displayed are means ± standard deviations of results from 4 independent experiments. *, P < 0.05; **, P < 0.01.
L. rhamnosus GG supplementation reduced the toxicity of chlorpyrifos to Drosophila.
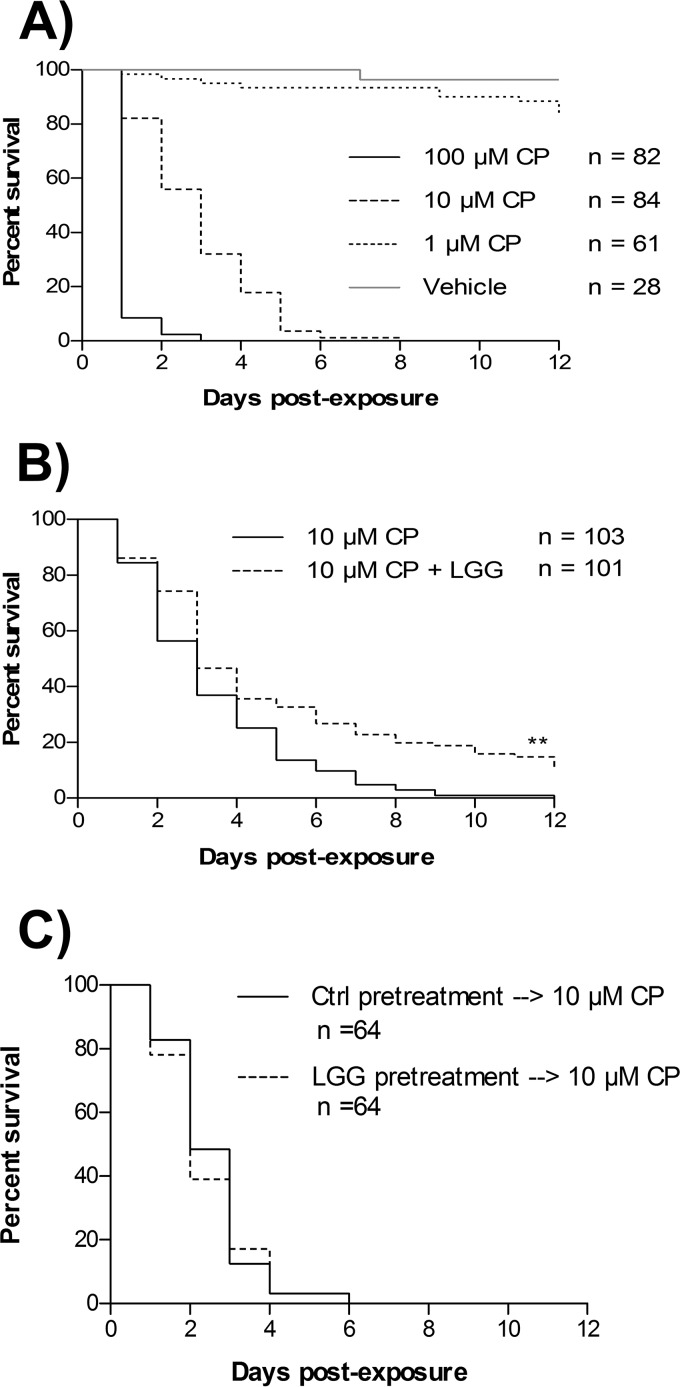
Since LGG displayed both the greatest sequestration and reduction of in vitro intestinal absorption of CP, the ability of oral LGG supplementation to mitigate mortality of Drosophila melanogaster exposed to food containing a lethal amount of CP was tested. Adult Drosophila flies were exposed to CP-containing food at various concentrations to determine an optimal CP dosage for intervention testing. Drosophila flies were sensitive to CP-induced mortality in a dose-dependent manner (Fig. 6A). Median survival of Drosophila was determined to be 1 day for 100 μM CP, 3 days for 10 μM CP, and undeterminable for 1 μM CP or vehicle. Based on these dose-mortality experiments, the 10 μM CP concentration was chosen to investigate the ability of LGG to mitigate acute CP-induced mortality. Drosophila flies that were simultaneously exposed to LGG and 10 μM CP had a median survival comparable to that of Drosophila flies that received just 10 μM CP (for both, median survival was 3 days) (Fig. 6B). However, the Drosophila flies simultaneously supplemented with both LGG and 10 μM CP displayed significantly prolonged overall survival (log rank test [Mantel-Cox], chi-square = 15.45, degrees of freedom [df] = 1; P < 0.0001) and fewer early deaths (Gehan-Breslow-Wilcoxon test, chi-square = 7.361, df = 1; P < 0.01) than those treated with 10 μM CP alone (Fig. 6B). Notably, at the experimental endpoint (day 12), 9.901% of Drosophila flies that were supplemented with LGG were still alive; alternatively, 0% of Drosophila flies exposed to 10 μM CP alone survived to 10 days postexposure. These observations suggest that prophylactic priming and continual supplementation of Drosophila with LGG may maximize Drosophila survival by preventing early deaths associated with CP exposure.
FIG 6.
Lactobacillus rhamnosus GG supplementation reduces the toxicity of chlorpyrifos to Drosophila melanogaster. (A) Survival curves for freshly eclosed Drosophila melanogaster flies exposed to media containing 1, 10, or 100 μM CP or vehicle control. (B) Survival curves for freshly eclosed Drosophila melanogaster flies exposed to media containing 10 μM CP with or without simultaneous supplementation with live LGG. (C) Survival curves for freshly eclosed Drosophila melanogaster flies exposed to LGG or vehicle for 3 days and then subsequent exposure to media containing 10 μM CP. Data displayed are from at least 3 independent experiments. **, P < 0.01.
Further experimentation was conducted in an attempt to elucidate if the prosurvival effects observed in CP-exposed Drosophila flies supplemented with LGG were due to direct LGG-CP interactions or to LGG modification of host responses to pesticide toxicity. To test this, Drosophila flies were pretreated with vehicle or LGG for 3 days prior to being transferred to medium containing 10 μM CP. No overall survival benefit (log rank test [Mantel-Cox], chi-square = 0.1419, df = 1; P = 0.7064) or survival benefit at an early time point (Gehan-Breslow-Wilcoxon test; chi-square = 0.4920, df = 1, P = 0.4830) was observed in Drosophila treated with LGG prior to 10 μM CP exposure (Fig. 6C). Together, these results suggest that LGG likely interacts directly with CP to mitigate CP-induced toxicity.
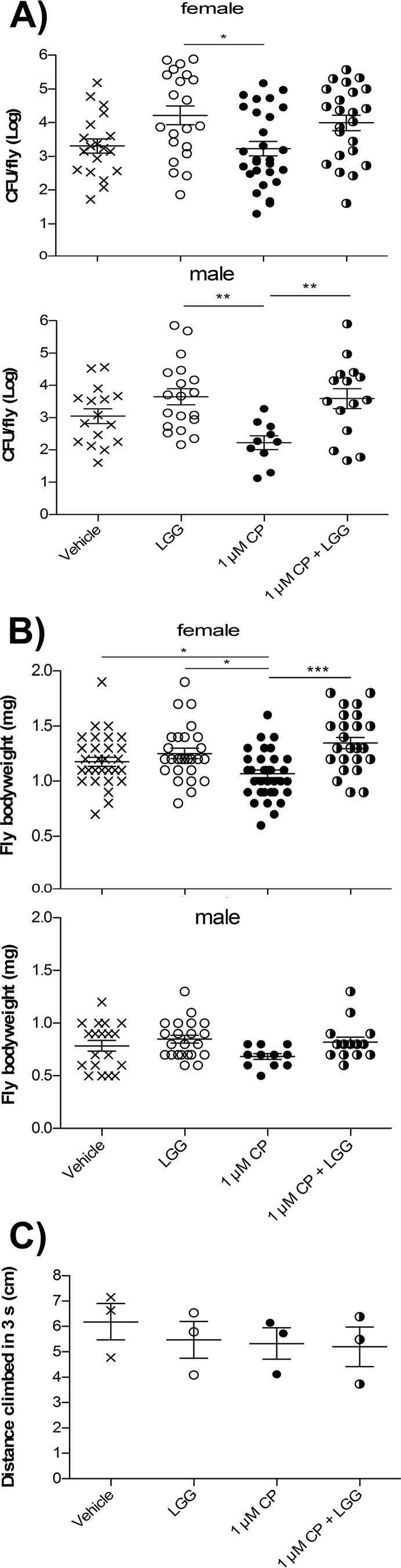
The direct effect of LGG supplementation on Drosophila microbiota composition and physiological responses following 3 days of exposure to 1 μM CP (a concentration that is not acutely lethal) were further characterized. Since lactobacilli are the dominant member of the Drosophila microbiota, lactobacilli were enumerated from Drosophila supplemented with LGG with or without simultaneous exposure to 1 μM CP. Both female and male Drosophila flies treated with LGG had significantly higher levels of lactobacilli than did Drosophila treated with 1 μM CP alone (Fig. 7A). Although 1 μM CP treatment did not significantly reduce Drosophila lactobacillus levels, they were notably lower in male flies and appeared to follow a bimodal response in females (some reduced, some unaffected) exposed to CP compared to vehicle controls. Furthermore, female Drosophila flies exposed for 3 days to medium containing 1 μM CP and LGG were significantly rescued (P < 0.05) from CP-mediated reductions in body weight (Fig. 7B). There was an insignificant trend toward similar body weight rescue in male flies. Moreover, since CP impacts insect locomotion due to exacerbated cholinergic stimulation, the ability of LGG supplementation to rescue Drosophila motor deficits following 3 days of 1 μM CP challenge was assessed in negative geotaxis assays. However, no differences in Drosophila locomotion (distance climbed after 3 s) were observed among the treatment groups of vehicle, LGG, 1 μM CP, and LGG with 1 μM CP (Fig. 7C). Together, these results suggest that (i) LGG supplementation can enhance the Drosophila microbiota with lactobacilli, and (ii) low-dose (1 μM) CP may cause developmental, rather than motor, deficits that can be rescued by LGG supplementation.
FIG 7.
Lactobacillus rhamnosus GG supplementation promotes body weight gain and lactobacillus enhancement in chlorpyrifos-exposed Drosophila but does not affect locomotion. Freshly eclosed Drosophila melanogaster flies were treated for 3 days with media containing vehicle, 1 μM CP, LGG, or 1 μM CP plus LGG. (A) Surface-sterilized Drosophila flies were homogenized and drop plated on MRS agar, and the CFU per Drosophila fly were enumerated. (B) Drosophila body weights were determined. (C) Drosophila locomotion was tested in a negative geotaxis assay in which average distance climbed after 3 s (of 15 to 25 flies) in 5 replicate experiments was quantified. Data displayed are means ± standard errors of the means from at least 3 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
DISCUSSION
Lactobacillus strains were found to grow in medium containing high levels of parathion and CP. Since the commonly used probiotic LGG and LGR-1 strains displayed unaltered and altered growth, respectively, in the presence of organophosphate pesticide, we further examined their activity. We found that the LGG hydrolase WP_014569076.1 was not responsible for organophosphate pesticide degradation. Although genetic cloning was not performed to definitively demonstrate that LGG_RS02045 was nonfunctional, LGG was not able to hydrolyze CP while E. coli BL21(DE3)(pET20b/PTE) was able to do so. Furthermore, heat-killed LGG had the same capability to sequester parathion and CP from solution as live LGG. This was also found with LGR-1 and suggests that metabolic activity is not required for bacterial organophosphate sequestration in these strains. Thus, unlike the findings reported by Cho et al. (38), we were unable to demonstrate degradation of the organophosphate pesticides parathion and CP by strains LGG and LGR-1. Differences in our experimental approach compared to the use of fermentation in organophosphate-contaminated dairy food products may explain this finding (29, 30). We suspect that the higher temperatures (42°C compared to 37°C) and acidic conditions present in the other in vitro fermentation studies promoted more rapid pesticide breakdown.
The finding of lactobacillus binding to organophosphate pesticides is congruent with reports of similar interactions with environmental toxins, such as aflatoxin (24, 25, 39), paralytic shellfish toxins (37), and metals (38–43). LGG had a better ability to retain CP sequestration—as measured by CP levels retained in bacterial pellets—from solution than LGR-1. This was also found in a Caco-2 Transwell model that simulated organophosphate absorption in the small intestine. This finding was surprising, given the genetic similarity between strains. One major difference between LGG and LGR-1 is that LGR-1 has a unique exopolysaccharide biosynthesis cluster (unpublished data). The abilities of lactobacillus-derived exopolysaccharide to bind toxins such as lead (44), cadmium (45), and aluminum (45) have been described previously. Furthermore, sediment microbe-produced exopolysaccharide has been shown to strongly interact with CP (46, 47). It is interesting to speculate that differences in bacterial exopolysaccharide may confer bacterium-organophosphate binding phenotypes in lactobacilli.
Results with Drosophila suggest that dietary supplementation of transitory bacteria can be important for conferring host benefits that are not present within the commensal microbiota. Specifically, prolonged interaction between CP and LGG may lead to increased survival. Unlike Drosophila exposed simultaneously to CP and LGG, those treated with LGG prior to pesticide exposure did not exhibit a survival benefit. These findings further support our hypothesis that LGG supplementation prevents downstream organophosphate pesticide toxicity by preventing absorption. Admittedly, we cannot completely rule out the possibility that LGG exposure results in priming of host detoxification pathways relevant to organophosphate pesticides. Results from Kamaladevi et al. (48) suggest that the survival benefit experienced by CP-challenged Caenorhabditis elegans supplemented with L. casei may be due to upregulation of host phase II detoxification genes. However, this does not appear to be the mechanism of LGG-mediated prosurvival in Drosophila lethally challenged with CP (this study).
LGG supplementation was able to significantly rescue weight loss in female flies exposed to 1 μM CP. This trend was also observed in male flies; however, findings in males were not significant. The outcome corresponded with significantly increased lactobacillus CFU per fly after treatment with LGG relative to findings with CP treatment alone. Lactobacillus is the dominant genus in the Drosophila microbiota and is important for many host physiological processes (49, 50). Lactobacillus plantarum isolated from Drosophila has been shown to promote host growth in nutrient-depleted media via improved protein assimilation (50). Thus, similar to the findings reported by Blum et al. (51), we have shown that human probiotic LGG may be capable of conferring beneficial effects to host flies in a similar fashion as the native microbiota.
In summary, this study has shown that the commonly used probiotic organisms LGG and LGR-1 are able to bind, but not metabolize, organophosphate pesticides and reduce intestinal absorption in vitro. Furthermore, LGG reduced mortality and growth deficits in Drosophila exposed lethally and subchronically to CP, respectively. This work expands upon our previous study's findings, which demonstrated that L. rhamnosus supplementation could reduce heavy metal bioaccumulation in pregnant Tanzanian women (23). Transitory food-grade bacteria have the potential to act like a nonspecific “sponge”—absorbing toxins and reducing their uptake by the host. This approach holds promise for supplementing human, livestock, or apiary foods with probiotic microorganisms and reducing downstream toxicity from organophosphate pesticides.
Supplementary Material
ACKNOWLEDGMENTS
We thank Brad Urquhart and Tom Velenosi for their guidance and generosity with equipment for Caco-2 experiments. We also thank Frank M. Raushel and Andrew N. Bigley for providing the pET20b/PTE plasmid. We gratefully acknowledge Alexandra Sull's assistance with quantification in negative geotaxis experiments.
This research was supported by a grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada. M.T. holds an NSERC CGS-M scholarship.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01510-16.
REFERENCES
- 1.Mesnage R, Defarge N, Spiroux de Vendômois J, Séralini GE. 2014. Major pesticides are more toxic to human cells than their declared active principles. Biomed Res Int 2014:179691. doi: 10.1155/2014/179691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karczmar AG. 1948. Anticholinesterase and toxic effects of parathion (O-O-diethyl p-nitrophenyl thiophosphate). Anat Rec 101:739. [PubMed] [Google Scholar]
- 3.Kwong TC. 2002. Organophosphate pesticides: biochemistry and clinical toxicology. Ther Drug Monit 24:144–149. doi: 10.1097/00007691-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Quirós-Alcalá L, Alkon AD, Boyce WT, Lippert S, Davis NV, Bradman A, Barr DB, Eskenazi B. 2011. Maternal prenatal and child organophosphate pesticide exposures and children's autonomic function. Neurotoxicology 32:646–655. doi: 10.1016/j.neuro.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Wang P, Tian Y, Wang XJ, Gao Y, Shi R, Wang GQ, Hu GH, Shen XM. 2012. Organophosphate pesticide exposure and perinatal outcomes in Shanghai, China. Environ Int 42:100–104. doi: 10.1016/j.envint.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Lacasaña M, López-Flores I, Rodríguez-Barranco M, Aguilar-Garduño C, Blanco-Muñoz J, Pérez-Méndez O, Gamboa R, Bassol S, Cebrian ME. 2010. Association between organophosphate pesticides exposure and thyroid hormones in floriculture workers. Toxicol Appl Pharmacol 243:19–26. doi: 10.1016/j.taap.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Surajudeen YA, Sheu RK, Ayokulehin KM, Olatunbosun AG. 2014. Oxidative stress indices in Nigerian pesticide applicators and farmers occupationally exposed to organophosphate pesticides. Int J Appl Basic Med Res 4(Suppl 1):S37–S40. doi: 10.4103/2229-516X.140730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lerro CC, Koutros S, Andreotti G, Friesen MC, Alavanja MC, Blair A, Hoppin JA, Sandler DP, Lubin JH, Ma X, Zhang Y, Beane Freeman LE. 2015. Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study. Occup Environ Med 72:736–744. doi: 10.1136/oemed-2014-102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debost-Legrand A, Warembourg C, Massart C, Chevrier C, Bonvallot N, Monfort C, Rouget F, Bonnet F, Cordier S. 2016. Prenatal exposure to persistent organic pollutants and organophosphate pesticides, and markers of glucose metabolism at birth. Environ Res 146:207–217. doi: 10.1016/j.envres.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Hung DZ, Yang HJ, Li YF, Lin CL, Chang SY, Sung FC, Tai SC. 2015. The long-term effects of organophosphates poisoning as a risk factor of CVDs: a nationwide population-based cohort study. PLoS One 10:e0137632. doi: 10.1371/journal.pone.0137632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, Vanengelsdorp D, Pettis JS. 2010. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One 5:e9754. doi: 10.1371/journal.pone.0009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettis JS, Lichtenberg EM, Andree M, Stitzinger J, Rose R, Vanengelsdorp D. 2013. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS One 8:e70182. doi: 10.1371/journal.pone.0070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urlacher E, Monchanin C, Rivière C, Richard FJ, Lombardi C, Michelsen-Heath S, Hageman KJ, Mercer AR. 2016. Measurements of chlorpyrifos levels in forager bees and comparison with levels that disrupt honey bee odor-mediated learning under laboratory conditions. J Chem Ecol 42:127–138. doi: 10.1007/s10886-016-0672-4. [DOI] [PubMed] [Google Scholar]
- 14.Munnecke DM, Hsieh DP. 1975. Microbial metabolism of a parathion-xylene pesticide formulation. Appl Microbiol 30:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer R, Iken B, Damania A. 2013. A comparison of organophosphate degradation genes and bioremediation applications. Environ Microbiol Rep 5:787–798. doi: 10.1111/1758-2229.12095. [DOI] [PubMed] [Google Scholar]
- 16.Bird SB, Sutherland TD, Gresham C, Oakeshott J, Scott C, Eddleston M. 2008. OpdA, a bacterial organophosphorus hydrolase, prevents lethality in rats after poisoning with highly toxic organophosphorus pesticides. Toxicology 247(2–3):88–92. doi: 10.1016/j.tox.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daughton CG, Hsieh DP. 1977. Accelerated parathion degradation in soil by inoculation with parathion-utilizing bacteria. Bull Environ Contam Toxicol 18:48–56. doi: 10.1007/BF01686304. [DOI] [PubMed] [Google Scholar]
- 18.Richins RD, Kaneva I, Mulchandani A, Chen W. 1997. Biodegradation of organophosphorus pesticides by surface-expressed organophosphorus hydrolase. Nat Biotechnol 15:984–987. doi: 10.1038/nbt1097-984. [DOI] [PubMed] [Google Scholar]
- 19.Serdar CM, Gibson DT, Munnecke DM, Lancaster JH. 1982. Plasmid involvement in parathion hydrolysis by Pseudomonas diminuta. Appl Environ Microbiol 44:246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai PC, Fox N, Bigley AN, Harvey SP, Barondeau DP, Raushel FM. 2012. Enzymes for the homeland defense: optimizing phosphotriesterase for the hydrolysis of organophosphate nerve agents. Biochemistry 51:6463–6475. doi: 10.1021/bi300811t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi Y, Hayatsu M, Hosokawa T, Nagayama A, Tago K, Flukatsu T. 2012. Symbiont-mediated insecticide resistance. Proc Natl Acad Sci U S A 109:8618–8622. doi: 10.1073/pnas.1200231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinder M, Bisanz JE, Burton JP, Reid G. 2015. Probiotic lactobacilli: a potential prophylactic treatment for reducing pesticide absorption in humans and wildlife. Benef Microbes 6:841–847. doi: 10.3920/BM2015.0022. [DOI] [PubMed] [Google Scholar]
- 23.Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, Reid G. 2014. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 5(5):e01580-14. doi: 10.1128/mBio.01580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gratz S, Wu QK, El-Nezami H, Juvonen RO, Mykkänen H, Turner PC. 2007. Lactobacillus rhamnosus strain GG reduces aflatoxin B1 transport, metabolism, and toxicity in Caco-2 cells. Appl Environ Microbiol 73:3958–3964. doi: 10.1128/AEM.02944-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbès S, Salah-Abbès JB, Sharafi H, Jebali R, Noghabi KA, Oueslati R. 2013. Ability of Lactobacillus rhamnosus GAF01 to remove AFM1 in vitro and to counteract AFM1 immunotoxicity in vivo. J Immunotoxicol 10:279–286. doi: 10.3109/1547691X.2012.718810. [DOI] [PubMed] [Google Scholar]
- 26.Chilton S, Burton JP, Reid G. 2015. Inclusion of fermented foods in food guides around the world. Nutrients 7:390–404. doi: 10.3390/nu7010390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vásquez A, Forsgren E, Fries I, Paxton RJ, Flaberg E, Szekely L, Olofsson TC. 2012. Symbionts as major modulators of insect health: lactic acid bacteria and honeybees. PLoS One 7:e33188. doi: 10.1371/journal.pone.0033188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, Wang W, Liu W, Gatlin DM III, Zhang Y, Yao B, Ringø E. 2012. Identification of highly-adhesive gut Lactobacillus strains in zebrafish (Danio rerio) by partial rpoB gene sequence analysis. Aquaculture 370–371(11):150–157. [Google Scholar]
- 29.Zhang YH, Xu D, Liu JQ, Zhao XH. 2014. Enhanced degradation of five organophosphorus pesticides in skimmed milk by lactic acid bacteria and its potential relationship with phosphatase production. Food Chem 164:173–178. doi: 10.1016/j.foodchem.2014.05.059. [DOI] [PubMed] [Google Scholar]
- 30.Zhou XW, Zhao XH. 2015. Susceptibility of nine organophosphorus pesticides in skimmed milk towards inoculated lactic acid bacteria and yogurt starters. J Sci Food Agric 95:260–266. doi: 10.1002/jsfa.6710. [DOI] [PubMed] [Google Scholar]
- 31.Islam SM, Math RK, Cho KM, Lim WJ, Hong SY, Kim JM, Yun MG, Cho JJ, Yun HD. 2010. Organophosphorus hydrolase (OpdB) of Lactobacillus brevis WCP902 from kimchi is able to degrade organophosphorus pesticides. J Agric Food Chem 58:5380–5386. doi: 10.1021/jf903878e. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Qare AW, Abou-Donia MB. 2001. Quantification of nicotine, chlorpyrifos and their metabolites in rat plasma and urine using high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 757:295–300. doi: 10.1016/S0378-4347(01)00161-X. [DOI] [PubMed] [Google Scholar]
- 33.Hubatsch I, Ragnarsson EG, Artursson P. 2007. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc 2:2111–2119. doi: 10.1038/nprot.2007.303. [DOI] [PubMed] [Google Scholar]
- 34.Nichols CD, Becnel J, Pandey UB. 2012. Methods to assay Drosophila behavior. J Vis Exp 61:e3795. doi: 10.3791/3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang JM, Taly JF, Notredame C. 2011. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res 39:W13–W17. doi: 10.1093/nar/gkr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schleifer KH. 1985. Analysis of chemical composition and primary structure of murein. Methods Microbiol 18:123–156. [Google Scholar]
- 38.Cho KM, Math RK, Islam SMA, Lim WJ, Hong SY, Kim JM, Yun MG, Cho JJ, Yun HD. 2009. Biodegradation of chlorpyrifos by lactic acid bacteria during kimchi fermentation. J Agric Food Chem 57:1882–1889. doi: 10.1021/jf803649z. [DOI] [PubMed] [Google Scholar]
- 39.Fazeli MR, Hajimohammadali M, Moshkani A, Samadi N, Jamalifar H, Khoshayand MR, Vaghari E, Pouragahi S. 2009. Aflatoxin B1 binding capacity of autochthonous strains of lactic acid bacteria. J Food Prot 72:189–192. [DOI] [PubMed] [Google Scholar]
- 40.Vasama M, Kumar H, Salminen S, Haskard C. 2014. Removal of paralytic shellfish toxins by probiotic lactic acid bacteria. Toxins (Basel) 6:2127–2136. doi: 10.3390/toxins6072127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monachese M, Burton JP, Reid G. 2012. Bioremediation and tolerance of humans to heavy metals through microbial processes: a potential role for probiotics? Appl Environ Microbiol 78:6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian F, Zhai Q, Zhao J, Liu X, Wang G, Zhang H, Zhang H, Chen W. 2012. Lactobacillus plantarum CCFM8661 alleviates lead toxicity in mice. Biol Trace Elem Res 150:264–271. doi: 10.1007/s12011-012-9462-1. [DOI] [PubMed] [Google Scholar]
- 43.Bhakta JN, Ohnishi K, Munekage Y, Iwasaki K, Wei MQ. 2012. Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents. J Appl Microbiol 112:1193–1206. doi: 10.1111/j.1365-2672.2012.05284.x. [DOI] [PubMed] [Google Scholar]
- 44.Feng M, Chen X, Li C, Nurgul R, Dong M. 2012. Isolation and identification of an exopolysaccharide-producing lactic acid bacterium strain from Chinese Paocai and biosorption of Pb(II) by its exopolysaccharide. J Food Sci 77:T111–T117. doi: 10.1111/j.1750-3841.2012.02734.x. [DOI] [PubMed] [Google Scholar]
- 45.Polak-Berecka M, Szwajgier D, Waśko A. 2014. Biosorption of Al(+3) and Cd(+2) by an exopolysaccharide from Lactobacillus rhamnosus. J Food Sci 79:T2404–T2408. doi: 10.1111/1750-3841.12674. [DOI] [PubMed] [Google Scholar]
- 46.Lundqvist A, Bertilsson S, Goedkoop W. 2010. Effects of extracellular polymeric and humic substances on chlorpyrifos bioavailability to Chironomus riparius. Ecotoxicology 19:614–622. doi: 10.1007/s10646-009-0430-2. [DOI] [PubMed] [Google Scholar]
- 47.Widenfalk A, Lundqvist A, Goedkoop W. 2008. Sediment microbes and biofilms increase the bioavailability of chlorpyrifos in Chironomus riparius (Chironomidae, Diptera). Ecotoxicol Environ Saf 71:490–497. doi: 10.1016/j.ecoenv.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 48.Kamaladevi A, Ganguli A, Balamurugan K. 2016. Lactobacillus casei stimulates phase-II detoxification system and rescues malathion-induced physiological impairments in Caenorhabditis elegans. Comp Biochem Physiol C Toxicol Pharmacol 179:19–28. doi: 10.1016/j.cbpc.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Erkosar B, Storelli G, Defaye A, Leulier F. 2013. Host-intestinal microbiota mutualism: “learning on the fly.” Cell Host Microbe 13:8–14. doi: 10.1016/j.chom.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F. 2011. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 51.Blum JE, Fischer CN, Miles J, Handelsman J. 2013. Frequent replenishment sustains the beneficial microbiome of Drosophila melanogaster. mBio 4(6):e00860-13. doi: 10.1128/mBio.00860-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.