Significance
Lysophospholipid acyltransferase (LPLAT) proteins are required for many essential biological activities involving the transfer of acyl chains. One LPLAT, LpxM, is necessary for the biosynthesis of lipid A, which comprises the outer leaflet of the outer membrane in Gram-negative bacteria. Lipid A is important because it is a potent activator of the innate immune system and because of its role in preventing xenobiotics from permeating Gram-negative bacteria. In this work, we structurally and mechanistically characterize LpxM, providing insights that may enable the targeted discovery of inhibitors that prevent lipid A maturation; these might potentiate the uptake of extant antibiotics whose clinical efficacy is hitherto limited by poor permeability. Our insights into the mechanism of LpxM may facilitate the study of diverse LPLATs.
Keywords: LpxM, acyltransferase, lipid A, RapidFire mass spectrometry, phosphopantetheine ejection assay
Abstract
Gram-negative bacteria possess a characteristic outer membrane, of which the lipid A constituent elicits a strong host immune response through the Toll-like receptor 4 complex, and acts as a component of the permeability barrier to prevent uptake of bactericidal compounds. Lipid A species comprise the bulk of the outer leaflet of the outer membrane and are produced through a multistep biosynthetic pathway conserved in most Gram-negative bacteria. The final steps in this pathway involve the secondary acylation of lipid A precursors. These are catalyzed by members of a superfamily of enzymes known as lysophospholipid acyltransferases (LPLATs), which are present in all domains of life and play important roles in diverse biological processes. To date, characterization of this clinically important class of enzymes has been limited by a lack of structural information and the availability of only low-throughput biochemical assays. In this work, we present the structure of the bacterial LPLAT protein LpxM, and we describe a high-throughput, label-free mass spectrometric assay to characterize acyltransferase enzymatic activity. Using our structure and assay, we identify an LPLAT thioesterase activity, and we provide experimental evidence to support an ordered-binding and “reset” mechanistic model for LpxM function. This work enables the interrogation of other bacterial acyltransferases’ structure–mechanism relationships, and the assay described herein provides a foundation for quantitatively characterizing the enzymology of any number of clinically relevant LPLAT proteins.
Among the most intractable of the challenges in the development of new antimicrobial therapeutics is finding chemical matter that can permeate the Gram-negative cellular envelope. Such bacteria pose a unique challenge in this regard due to their characteristic outer membrane (OM)—an asymmetric barrier with an inner leaflet primarily composed of glycerophospholipids and an outer leaflet composed of lipopolysaccharide (LPS). In addition to its structural roles in decreasing permeability and increasing the rigidity of the bacterial cell, LPS is a potent activator of the innate immune response and is recognized at picomolar levels by the Toll-like receptor 4 (TLR4) (1). For these reasons, it is critically important to develop a deep understanding of the pathways involved in LPS biogenesis and modification.
The lipid anchor of LPS, lipid A, forms the outer leaflet of the OM and is the epitope of LPS that is recognized by TLR4. This complex lipid is produced in a nine-step conserved pathway known as the Raetz pathway (2), of which the first six steps are essential in most Gram-negative bacteria, including in clinically relevant species such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and most strains of Acinetobacter baumannii. The enzymes in this pathway first convert UDP-GlcNAc, acyl-acyl carrier protein (acyl-ACP), and ATP into lipid IVA, to which secondary acyl chains and 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) sugars are added to produce lipid A.
The final steps in the Raetz pathway involve the secondary acylation of the R-3-hydroxyacyl chains at positions 2′ and 3′ of Kdo2-lipid IVA to produce lipid A, catalyzed by the LpxL and LpxM enzymes, respectively. These enzymes belong to a large and diverse superfamily of proteins known as the lysophospholipid acyltransferases (LPLATs), which exist within all domains of life. Within this family, LpxL and LpxM belong to a group of proteins known as lipid A biosynthesis lauryl/myristyl acyltransferases (LABLATs). Other representative clades in the LPLAT family include glycerol-3-phosphate acyltransferases (GPATs), monoacylglycerol acyltransferases (MGATs), and acylglycerolphosphate acyltransferases (AGPATs), which are each necessary for the early steps in triglyceride biosynthesis (3, 4).
To date, the structure of only one member of this superfamily has been reported: a stromal GPAT protein from Cucurbita moschata (the squash plant) (5). The squash protein is only distantly related to the bacterial LPLAT family (with some similarity to acyltransferases in Chlamydiae species, but not to proteobacterial LPLATs). Furthermore, the C. moschata GPAT has not been cocrystallized with substrates or substrate analogs. Thus, despite careful biochemical analyses of these proteins (6), there is currently no direct structural evidence published that identifies a mechanism of action, nor have binding sites for acyl-ACP and/or lipid substrate been reported. Elucidation of bacterial LABLAT structures may enable the discovery of compounds that perturb lipid A biosynthesis, resulting in leaky membranes that may decrease virulence and potentiate the uptake of bactericidal compounds.
Whereas the LABLAT proteins are not essential for bacterial growth under typical laboratory conditions, their genetic deletions result in a wide variety of phenotypes among different bacterial species. In particular, disruption of lpxM can have a profound impact on the integrity of the OM, which in turn can have important effects on cell survival in certain environments. In K. pneumoniae, deletion of lpxM results in increased permeability in the OM and subsequent sensitivity to cationic antimicrobial peptides (CAMPs) (7). A. baumannii (8), E. coli (9), and Salmonella typhimurium (9) also require functional LpxM for CAMP resistance, possibly due to increased OM permeability in its absence. In Vibrio cholerae, the LpxM ortholog LpxN transfers a 3-hydroxylaurate to the 3′-linked acyl chain on its lipid A precursor, which is then modified with glycine to confer resistance to CAMP (10, 11). Thus, LpxM, and other LpxM orthologs, may contribute both directly and indirectly to CAMP resistance.
Deletion of lpxM has also been shown to have important effects on virulence in many human and animal pathogens. In the nosocomial pathogen A. baumannii, LpxM is required for virulence in the Galleria mellonella (greater wax moth) infection model, and for protection from desiccation, which increases infection and transmission rates (8). Additionally, in E. coli clinical isolate H16, deletion of chromosomal lpxM results in a drastic decrease in virulence after i.p. injection into BALB/c mice (12), and deletion of the gene in the E058 strain of avian pathogenic E. coli (APEC) results in defects in avian macrophage invasion and decreased bacterial loads in several organs following inoculation into the left thoracic air sac in chickens (13). Salmonella enterica serovar Typhimurium infection of ligated bovine ileal loops is also attenuated in lpxM deletion mutants, demonstrating reduced inflammatory response and correlating to a decrease in secretion of virulence factors through the type III secretion system (14). Interestingly, all documented pathogenic strains of Shigella flexneri, another Gram-negative that causes disease in humans, carry a virulence plasmid that includes a second copy of lpxM (15). This second lpxM (msbB2) is regulated in response to magnesium by the two-component PhoP/PhoQ regulatory system, perhaps as a mechanism to decrease the permeability of the OM and to enhance S. flexneri survival in an intracellular environment (16). Deletion of either or both lpxM paralogs in S. flexneri results in decreased host immune response, as well as defects in bacterial invasion and replication in epithelial cells (15, 17). In virulent strains of Yersinia pestis, deletion of lpxM does not appear to affect the LD50 after s.c. injection in mice (18). However, deletion of lpxM in the vaccine strain EV results in a significant increase in protective immunity, while decreasing endotoxicity (18, 19). Thus, whereas not essential for viability of these pathogens, LpxM is still relevant with respect to virulence and perhaps to the permeability of antibiotic compounds.
In this work, we present the X-ray crystal structure of a bacterial LPLAT protein, the LABLAT LpxM from the pathogenic bacterium A. baumannii (AbLpxM), which possesses two known acyltransferase activities (8) (Fig. 1). Further, we report a high-throughput label-free mass spectrometric assay for rapid and quantitative measurement of acyltransferase activity, and we use this assay in conjunction with the structure to devise a model for the mechanism of action of this important class of enzymes.
Fig. 1.
Reaction scheme for AbLpxM. The two acyl chains transferred by AbLpxM are highlighted in green. The Kdo sugars (added by KdtA) and the secondary acylation on the R-3-hydroxylaurate at position 2′ (added by LpxL) may or may not be present in the AbLpxM lipid substrate and are highlighted in yellow. Acyl chain lengths shown are representative of A. baumannii strain ATCC 17978 (33). Note that the acyl chain lengths and compositions differ among other bacterial species and strains.
Results
The Structure of AbLpxM Reveals a Deep Hydrophobic Binding Pocket.
To begin to build a structure–function relationship for LABLAT proteins, we solved the structure of the A. baumannii LpxM homolog using single wavelength anomalous dispersion to 1.99-Å resolution (Fig. 2 and SI Appendix, Table S1). AbLpxM adopts a globular structure bisected by a large seven-stranded β-sheet. The predicted transmembrane domain is composed of a single α-helix protruding from the globular domain and forms a substantial crystal contact with AbLpxM in a neighboring asymmetric unit.
Fig. 2.
The structure of AbLpxM. (A) Cartoon schematic of AbLpxM. From the N terminus to C terminus, the color shifts from blue to red. Two angles are shown. TM, predicted transmembrane helix. (B) Predicted surface electrostatics map generated by PyMol. The putative binding cleft is indicated with a dashed line. (C) Cutaway diagram showing deep pockets within the putative binding cleft (pockets indicated with red arrows; cutaway in black to show the depth of the binding cleft).
Electrostatic surface visualization of the AbLpxM structure revealed a very large hydrophobic pocket, which bound a copurifying n-dodecyl-β-d-maltoside (DDM) molecule (Fig. 2B and SI Appendix, Fig. S3C). Due to the hydrophobicity and size of the lipid substrate of AbLpxM, and given the lack of any other large hydrophobic surface, this pocket likely represents the binding site of the acyl chain acceptor. Intriguingly, this large pocket also contains several deep hydrophobic channels, which may serve as hydrocarbon “rulers,” and which may determine specificity for acyl chain length on either acyl-ACP or on the lipid A precursors (Fig. 2C, red arrows). Supporting a potential role as a hydrocarbon ruler, one of these pockets, in the structure of the catalytic mutant AbLpxME127A, contains electron density consistent with the presence of an acyl chain (SI Appendix, Fig. S3D).
As there is no discernible primary sequence identity between AbLpxM and the squash GPAT, or between AbLpxM and any other structures deposited in the Protein Data Bank (PDB), we looked for structural homologs using the programs PDBeFold (20) and DaliLite (21). Although there were no high-confidence hits, both PDBeFold and DaliLite identified the squash GPAT as the closest structural homolog to AbLpxM—albeit with less than half of the secondary structural elements aligned. The residues with the most similar secondary structure comprised the region surrounding the active site, suggesting that the positioning of the catalytic residues in the GPATs and LABLATs rely on conserved secondary structural elements. Overall, however, the secondary structure differs greatly between LpxM and the squash GPAT (Q score <0.1, rmsd = 5.80 Å, SI Appendix, Fig. S1), suggesting that bacterial LABLATs represent a distinct fold compared with other LPLAT proteins.
AbLpxM Possesses Acyltransferase and Acylprotein Thioesterase Activities.
To measure activity of AbLpxM, we measured the formation of holo-ACP by high-throughput solid-phase-extraction triple quadrupole mass spectrometry (SPE-MS) on an Agilent RapidFire 300 and AB SCIEX 5500 mass spectrometer (SI Appendix, Fig. S2 and detailed methods in SI Materials and Methods). Previously reported assays used to track the activity of LABLAT enzymes require the production of radiolabeled lipids and use of TLC and therefore are traditionally performed in a low-throughput manner. The phosphopantetheine ejection ions, representing preferential gas-phase cleavage of the prosthetic group and any attached thioester, were used for quantitation from the intact ACP (SI Appendix, Fig. S2A) (22). We were able to measure holo-ACP concentrations at a rate of one sample every 7 s, enabling high-throughput data collection with good signal-to-noise separation (>3 dB) and linearity down to ∼100 nM of holo-ACP product (SI Appendix, Fig. S2 B and C) without any additional sample preparation.
With this assay, we measured acyl-transferase activity for AbLpxM using lauryl-ACP as an acyl chain donor and titrating lipid IVA as the acyl chain acceptor. As reported for E. coli LpxL (6), we found that AbLpxM was capable of using lipid IVA as a substrate, even lacking the Kdo moieties, with an apparent KM of 1.7 ± 0.6 μM (Fig. 3A). Indeed, AbLpxM produced both penta- and hexa-acylated lipid A species when incubated with lauryl-ACP and lipid IVA as detected by quadrupole (Q)-TOF liquid chromatography (LC)-MS and TLC (SI Appendix, SI Results and Figs. S7C, S10, and S11).
Fig. 3.
Kinetic analysis of AbLpxM activity. (A) Specific activity of AbLpxM as a function of lipid IVA concentration for wild-type AbLpxM, AbLpxME127A, and AbLpxMR159A. (B) Close-up of the kinetics of AbLpxME127A and AbLpxMR159A. (C) Production of lauric acid by AbLpxM in the presence of lauryl-ACP after 2 h. (D) Close-up of the AbLpxM putative active site with conserved residues and their interaction distances shown. DDM is shown in purple. (E) Logo showing conservation of residues shown in D. All error bars represent the SEM. All experiments were repeated in triplicate. Specific activity represents the production of holo-ACP.
Interestingly, we found that AbLpxM could produce holo-ACP in the absence of any acyl chain acceptor, suggesting a previously unreported acyl-protein thioesterase activity (Fig. 3A). Supporting this hypothesis, we found that AbLpxM produced free lauric acid when incubated with lauryl-ACP, as determined by accurate-mass Q-TOF LC-MS (Fig. 3C). Indeed, this enzyme-dependent hydrolysis was ∼60-fold greater than the spontaneous rate of production without protein in our assay conditions. We note that this activity would be observed only by direct measurement of holo-ACP production, and not by tracking radiolabeled lipid A precursors as has previously been done (6, 9).
The AbLpxM Active Site Resides Within the Deep Hydrophobic Cleft.
Acyltransferase proteins typically contain an invariant histidine residue in their active sites, followed by four nonconserved residues, and then a catalytic acidic residue (HX4D/E) (23). This catalytic dyad is thought to create a charge relay system, wherein the histidine abstracts the proton from the hydroxyl group and activates the oxygen for nucleophilic attack on the acyl thioester carbon (23, 24). LpxM also contains a conserved HX4D/E motif (using glutamate in the A. baumannii ortholog), which is present within the deep hydrophobic cleft (Fig. 3D). The positioning of these residues within the cleft supports our hypothesis that this is the location of the active site. Aside from the invariant His122, an alignment of 27 LpxM and LpxL orthologs revealed three other totally conserved residues: Asn51, Arg159, and Asp192 (Fig. 3E). Interestingly, Arg159 also resides within the cleft and is positioned very close to the putative catalytic histidine (His122) and glutamate (Glu127). Analysis of the crystal structure suggests that this residue interacts with the C6-hydroxyl on the bound DDM molecule. If the diglucosamine head group of lipid IVA binds similarly to the maltose head group of DDM, this interaction would correspond to the C1 phosphate on lipid IVA, suggesting that Arg159 may play a role in substrate binding.
We wished to examine the possible role(s) of Arg159 for both acyltransferase and acyl-protein thioesterase activities. We therefore expressed and purified AbLpxMR159A and measured its activity with increasing concentrations of lipid IVA (Fig. 3A). We also produced and assayed AbLpxME127A, which was expected to eliminate activity through substitution of the putative catalytic glutamate. We found that both AbLpxMR159A and AbLpxME127A substitutions greatly abrogated activity of the enzyme (Fig. 3A). However, a closer examination of the kinetics of both variants revealed residual activity that differed greatly between the two (Fig. 3B). Whereas the AbLpxME127A variant strongly responded to increasing lipid IVA substrate, the AbLpxMR159A variant displayed a much more attenuated response (Fig. 3B). These data are consistent with the hypothesis that the conserved Arg159 residue contributes to either substrate recognition and binding or to the proper positioning of substrates before the acyl chain transfer.
To confirm proper folding of the active site in the catalytic variant, we solved the structure of AbLpxME127A to 1.9-Å resolution (SI Appendix, Fig. S3A and Table S1). AbLpxME127A adopted an almost identical conformation as the wild-type protein, except for two notable differences in the rotomeric orientation of His98 and Trp126 (which defines one end of the active site; SI Appendix, Fig. S3B). Like the wild-type protein, the catalytic variant also copurified and cocrystallized with DDM (SI Appendix, Fig. S3C).
AbLpxM Demonstrates Acyl Chain Length Selectivity and Substrate Inhibition.
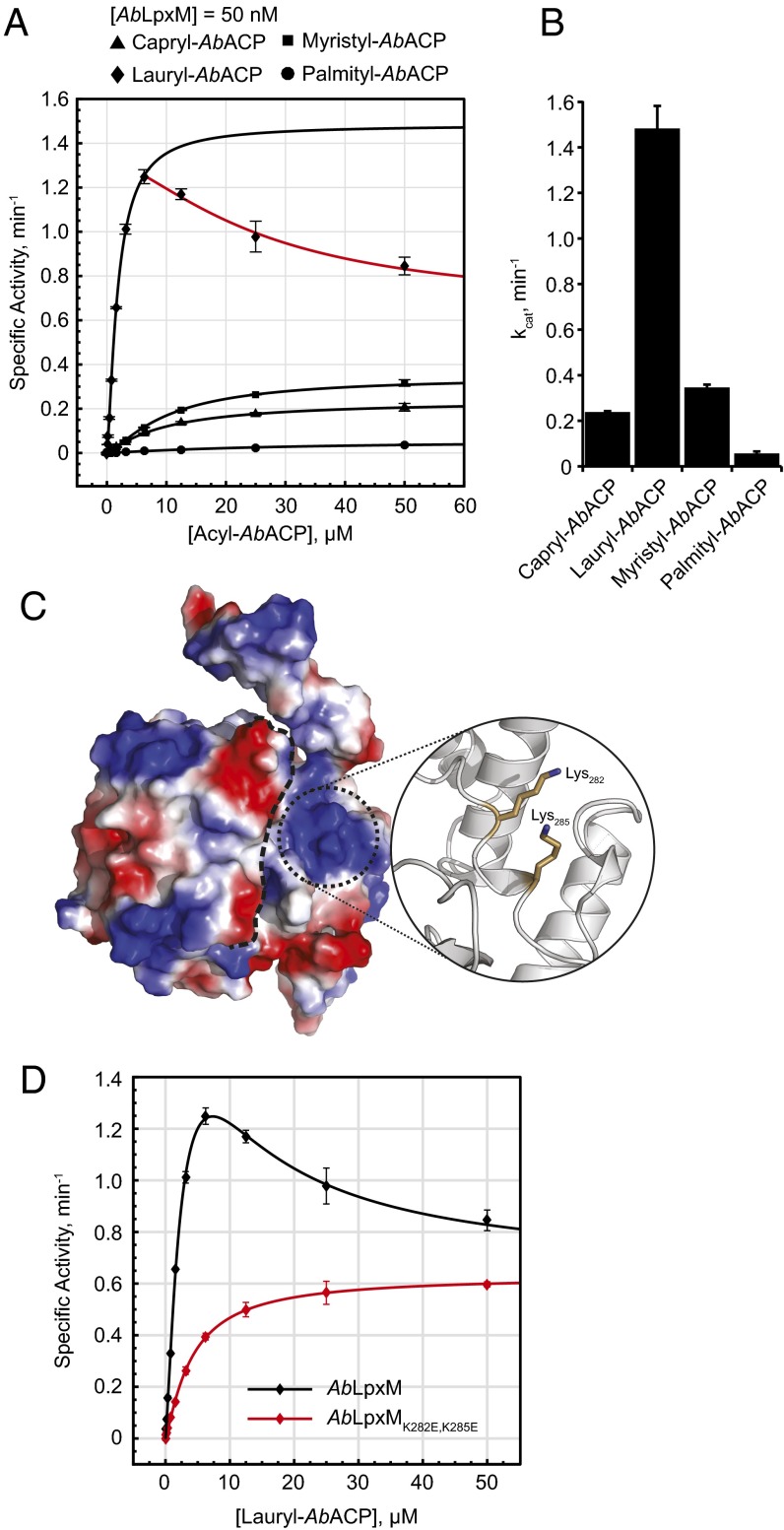
We hypothesized that the deep channels in the putative active site (Fig. 2C) may act as rulers to gauge acyl chain length of either acyl chains on the acceptor lipid IVA or the donor acyl-ACP. To test whether AbLpxM displays a preference for acyl chain length on acyl-ACP, we measured AbLpxM activity using either capryl-, lauryl-, myristyl-, or palmityl-ACP. We found that AbLpxM displayed a strong preference for lauryl-ACP over the other acyl chain lengths tested (Fig. 4 A and B and Table 1), with a measured apparent KM of 1.8 ± 0.2 μM. As the acyl chain length deviated from 12 carbons in either direction, the KM increased and the specific activity decreased. A strong preference by AbLpxM for a lauryl-ACP donor is also consistent with careful measurements of the mass of lipid A produced by A. baumannii with and without LpxM (8).
Fig. 4.
Analysis of AbLpxM activity with acyl-ACP. (A) Specific activity of AbLpxM with increasing concentrations of acyl-ACP with carbon lengths 10 (capryl-), 12 (lauryl-), 14 (myristyl-), and 16 (palmityl-) as determined by high-throughput mass spectrometry. Inhibited region for lauryl-ACP shown in red. Error bars represent the SEM. (B) Calculated apparent kcat for AbLpxM with each acyl-ACP. Error bars represent 95% confidence interval. (C) Identification of possible ACP-binding site near the putative active site. Inset shows residues that were predicted to drive the interaction and were substituted. (D) Data shown in A for lauryl-ACP fit to new kinetic model (black), as well as the kinetics of AbLpxMK282E,K285E with increasing lauryl-ACP (red). All experiments were repeated in triplicate. Specific activity represents the production of holo-ACP.
Table 1.
Analysis of acyl-ACP acyl chain length specificity on AbLpxM thioesterase activity as determined by high-throughput mass spectrometry
| Acyl chain donor | KMApp, μM | Specific activity, min−1 | Hill coefficient |
| Capryl-ACP | 9.887 ± 0.481 | 0.238 ± 0.004 | 1.134 ± 0.041 |
| Lauryl-ACP* | 1.845 ± 0.238 | 1.483 ± 0.099 | 1.392 ± 0.134 |
| Myristyl-ACP | 10.35 ± 0.943 | 0.346 ± 0.013 | 1.328 ± 0.115 |
| Palmityl-ACP | 30.47 ± 10.3 | 0.057 ± 0.009 | 1.128 ± 0.196 |
Values were calculated from data shown in Fig. 4A using Matlab; error shown as 95% confidence interval. All experiments were repeated in triplicate.
Parameters for lauryl-ACP were generated using data in the noninhibited regime only.
We also observed that AbLpxM displays substrate inhibition with respect to lauryl-ACP, with a steady decrease in activity at lauryl-ACP concentrations above 7 μM. This substrate inhibition was observed in both the presence and absence of lipid IVA (SI Appendix, Fig. S4A), and is consistent with an ordered binding mechanism. We hypothesize that premature binding of acyl-ACP to AbLpxM may sterically occlude the binding site and prevent proper binding of lipid IVA. Indeed, our observed biochemical inhibition may be physiologically relevant as reported cellular ACP concentrations in E. coli are on the order of 40–100 μM (25). Whereas the exact distribution of the bound acyl species is unknown, it is reasonable to propose that the concentration of lauryl-ACP falls at or near the inhibited regime observed.
Kinetic Analyses Suggest AbLpxM Possesses Multiple ACP-Binding Sites.
Contrary to our initial hypothesis that AbLpxM has one acyl-ACP binding site that is substrate inhibited (SI Appendix, Fig. S4C, orange curve), we observed that as lauryl-ACP concentration was increased, the substrate inhibition decreased AbLpxM activity only by approximately twofold. Classical substrate inhibition kinetics, assuming a single ACP-binding site, would predict that as the concentration of substrate approaches infinity, the velocity should asymptotically approach zero (SI Appendix, Fig. S4C, orange curve, and SI Results for equations).
This prediction was not observed in the case of AbLpxM substrate inhibition with lauryl-ACP. We therefore attempted to model our kinetic data with the assumption that AbLpxM contains at least two ACP-binding sites, one of which follows standard nonsubstrate-inhibited kinetics. We found that assuming the presence of two ACP-binding sites, we could accurately model the behavior of the enzyme (Fig. 4D and SI Appendix, Fig. S4C, purple curve, and SI Appendix, SI Results for equations), supporting the hypothesis that AbLpxM indeed possesses multiple ACP-binding sites. Because AbLpxM acylates lipid IVA at two distinct sites, the hypothesis of multiple ACP-binding sites is reasonable.
We hypothesized that the observed substrate inhibition resulted from premature ACP binding sterically occluding the active site cleft and preventing the second substrate (lipid IVA or H2O in the case of the thioesterase activity) from binding and/or properly positioning itself with respect to the AbLpxM catalytic residues. These observations led us to reexamine the AbLpxM structures for putative ACP-binding sites within or near the active site cleft. By looking for large patches of predicted electropositive surface charge, we identified a putative ACP-binding site (Fig. 4C and SI Appendix, Fig. S4D), which would position ACP directly over the entrance to the active site. To test our hypothesis, we substituted two residues (K282E/K285E) within the putative binding site to invert the surface charge and abrogate ACP binding (Fig. 4C, Inset, and SI Appendix, Fig. S4D). That ACP interaction with proteins is primarily driven by electrostatics is well established (26, 27). Indeed, we found that by substituting these residues, the observed substrate inhibition was attenuated, and the remaining AbLpxM activity exhibited a Vmax of ∼0.6⋅min−1, directly supporting our model (SI Appendix, Fig. S4C, blue curve, and Fig. 4D, red curve). These data suggest that: (i) the electropositive patch near the active site represents an ACP-binding site; (ii) ACP binding to this site is responsible for the substrate inhibition; and (iii) there is at least one other ACP-binding site located elsewhere on the protein that is responsible for the residual, noninhibited activity.
Discussion
A Proposed Model for LpxM Activity.
Based on the available data, we propose the following model for an ordered-binding and “reset” mechanism for LpxM (Fig. 5). We hypothesize that under ideal conditions, LpxM first binds to either lipid IVA, Kdo2-lipid IVA, or Kdo2-(lauryl)-lipid IVA (the preferred substrate is not clear), followed by lauryl-ACP. Proper positioning of the acceptor lipid substrate is facilitated by interactions with Arg159, possibly at either the 1- or 4′-phosphate on the diglucosamine head group, whereas the acyl chain lengths on either (or both) substrates are regulated by deep hydrophobic channels within the putative binding cleft. Upon proper positioning of the substrate, coordinating the hydroxyl of the R-3-hydroxyacyl chain at position 3′ (and position 2, for the A. baumannii ortholog) near the catalytic dyad, the acyl chain transfer is catalyzed and holo-ACP and lipid A products are generated and released. However, if lauryl-ACP binds first, then the lipid acceptor substrate is sterically occluded from binding to the cleft, and the enzyme is trapped in a nonproductive state. In this case, the LpxM thioesterase activity ensures that the lauryl-ACP is eventually hydrolyzed to holo-ACP and laurate, thereby regenerating active LpxM enzyme at an energy cost of just one ATP (one ATP is required to regenerate the lauryl-ACP), as opposed to, in the extreme example, synthesizing another LpxM molecule at a much higher energetic cost.
Fig. 5.
Model for AbLpxM mechanism. (Step 1) AbLpxM binds to its lipid substrate (shown here as lipid IVA). (Step 2) Following binding of the lipid substrate, lauryl-ACP binds at two sites, one of which is over the active site cleft. (Step 3) The acyl chain transfer is catalyzed by the HX4D/E catalytic dyad. (Step 4) The holo-ACP and acylated lipid products dissociate from AbLpxM, regenerating the enzyme. (Step 5) If lauryl-ACP concentrations are too high, or lipid substrate concentrations are too low, then lauryl-ACP can bind AbLpxM first. (Step 6) When this happens, the lipid substrate cannot fit into the active site. (Step 7) AbLpxM then cleaves the thioester linkage between the lauric acid and the phosphopantetheine on the lauryl-ACP. (Step 8) This regenerates the enzyme, which may otherwise be trapped in a nonproductive state. (Step 9) Each lauryl-ACP can be regenerated with one ATP by acyl-ACP synthase (AasS).
The reset mechanism may also prevent acyl-ACP from being sequestered by LpxM during conditions when its lipid acceptor substrates are scarce (such as when cells slow their biosynthesis of lipid A precursors and of other OM substituents). Rather than being an aberrant or promiscuous activity, we hypothesize that this release allows bacteria to efficiently conserve resources as the supply of lipid A precursors fluctuates, which is in turn reflective of the cells’ energy status.
In addition to this reset mechanism, we propose that the A. baumannii LpxM ortholog binds to two acyl-ACP molecules on separate and distinct binding surfaces, one of which is partially regulatory.
Substrate Specificity of LpxM.
Whereas LpxM is typically depicted as accepting Kdo2-lauryl-lipid IVA as its substrate, making it the terminal enzyme in the Raetz pathway, many LpxM orthologs exhibit substantial substrate promiscuity. For example, Neisseria meningitides produces fully acylated lipid A even when the Kdo transferase, kdtA, is knocked out, suggesting LpxL and LpxM can use lipid IVA in this species (28). Similarly, P. aeruginosa can produce fully acylated lipid A even when CMP-Kdo synthase (KdsB) is inhibited or when acting upon purified lipid IVA (29, 30), suggesting that the presence of the Kdo moieties on the lipid acceptor is dispensable for some LpxL/LpxM orthologs. In A. baumannii, LpxM adds lauryl groups to the R-3-hydroxyacyl chains at both the 3′- and 2-positions of lipid A precursors, producing hepta-acylated lipid A (8). This secondary activity may necessitate flexibility in the active site to accommodate multiple conformations of lipid A, so as to position the substrate properly. Our data, taken together with previous studies (8), suggest that the A. baumannii LABLAT proteins likely lack any defined order of operations, as both AbLpxL and AbLpxM produce lipid products in the absence of the other. Interestingly, when an alignment of LpxM orthologs is examined, those enzymes with predicted substrate promiscuity (Neisseria, Pseudomonas, and Acinetobacter) align more closely together, compared with orthologs that are reported to be relatively specific in their acyl transfer activities (SI Appendix, Fig. S5). This alignment suggests that there may exist a common set of mutations that relaxes ligand specificity. Close analysis of the sequence differences between these and other LpxM orthologs may reveal more information about substrate specificity.
Concluding Remarks.
LPLAT proteins are key enzymes in many important biological processes in humans and may represent useful targets for pharmacological intervention—especially in the treatment of diabetes and obesity (31). To date, several MGAT inhibitors have been developed and patented for this purpose (32). Whereas there is still no published structure of a mammalian LPLAT protein, let alone representative structures from each family within the LPLAT superfamily, comparisons between the structures of A. baumannii LpxM and C. moschata GPAT may enable researchers to make inferences about which structural elements are broadly conserved and likely found among mammalian LPLAT homologs and which may be more specific to certain subfamilies.
Our detailed characterization of A. baumannii LpxM may be relevant to the discovery of inhibitors of lipid A maturation in A. baumannii and in other Gram-negative pathogens, as our work may enable both high-throughput biochemical screening and structure-aided inhibitor discovery. We hypothesize that it may be advantageous to select for inhibitors of the LpxM thioester hydrolase activity or of its acyl-ACP binding, rather than for inhibitors that compete with the lipid IVA acceptor substrate (as such lipid IVA-competitive inhibitors might tend to possess unfavorable properties such as high molecular mass and lipophilicity). Our high-throughput MS assay system may allow for the discovery of such inhibitors in the absence of lipid IVA. We hypothesize that molecules that stabilize the LpxM–acyl-ACP complex (Fig. 5, step 5) might have the dual benefit of preventing lipid A maturation and of sequestering acyl-ACP, thus depleting ACP pools and possibly causing broad metabolic disruption in the bacteria.
Our findings may facilitate several interesting areas of inquiry in the acyltransferase field. We have discovered that at least one LPLAT protein contains a thioesterase activity in addition to its known acyltransferase activity. It is likely that other LPLAT proteins, even ones that have been studied extensively using radio-labeled lipid analysis, also contain secondary activities that may be regulatory in nature. Further examination using our assay platform may identify opportunities for both direct and allosteric inhibition of targeted enzymes relevant to human disease. Overall, our structural and mechanistic characterizations, coupled with a robust and target-adaptable biochemical MS assay platform, set the stage for a broad characterization of LPLAT structure–function relationships.
Materials and Methods
Native and Se Met-derivatized N-terminally polyhistidine-tagged A. baumannii LpxM (AbLpxM) were heterologously overexpressed in E. coli membranes. These membranes were detergent solubilized, and AbLpxM was purified by immobilized metal affinity and ion exchange chromatographic methods. Crystals of AbLpxM were grown by sitting-drop vapor diffusion at room temperature in 200 mM NaBr, 2.2 M (NH4)2SO4, at a protein:mother liquor ratio of 70:30 and an AbLpxM concentration of 21 mg/mL (SI Appendix, Fig. S2). The crystals were cryoprotected with the addition of 20% glycerol to the mother liquor solution. Data were collected for native and Se-Met AbLpxM crystals at the Advanced Light Source beamline 5.0.2 at wavelengths of 1.000 Å and 0.9797 Å, respectively; these data were used to solve the structure using single anomalous dispersion methods. Enzyme assay data were collected by high-throughput automated SPE-MS using an Agilent RapidFire 300 front-end and an AB SCIEX 5500 mass spectrometer. The phosphopantetheine ejection assay, which monitors a diagnostic fragment ion formed from the preferential release of the serine-bound prosthetic group, was used to monitor the depletion of the acyl-ACP substrates and the generation of holo-ACP products of the reaction (SI Appendix, Fig. S2). Full methods are available in the SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank David A. Six for helpful discussions, Jennifer Leeds and David Carpenter for critical reading of the manuscript, and members of the Emeryville Protein Sciences, Structural Chemistry, Bacteriology New Technologies, Global Discovery Chemistry, and legal groups at Novartis Institutes for BioMedical Research for help and support. We acknowledge the legacy of Christian R. H. Raetz (1946–2011), discoverer of the Raetz pathway of lipid A biosynthesis and mentor to L.E.M.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. W.D. is a Guest Editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5KN7 and 5KNK).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610746113/-/DCSupplemental.
References
- 1.Zughaier SM, Zimmer SM, Datta A, Carlson RW, Stephens DS. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun. 2005;73(5):2940–2950. doi: 10.1128/IAI.73.5.2940-2950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitfield C, Trent MS. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem. 2014;83(1):99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, Cheng D. Beyond triglyceride synthesis: The dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am J Physiol Endocrinol Metab. 2009;297(1):E10–E18. doi: 10.1152/ajpendo.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. 2009;296(6):E1195–E1209. doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull AP, et al. Analysis of the structure, substrate specificity, and mechanism of squash glycerol-3-phosphate (1)-acyltransferase. Structure. 2001;9(5):347–353. doi: 10.1016/s0969-2126(01)00595-0. [DOI] [PubMed] [Google Scholar]
- 6.Six DA, Carty SM, Guan Z, Raetz CRH. Purification and mutagenesis of LpxL, the lauroyltransferase of Escherichia coli lipid A biosynthesis. Biochemistry. 2008;47(33):8623–8637. doi: 10.1021/bi800873n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements A, et al. Secondary acylation of Klebsiella pneumoniae lipopolysaccharide contributes to sensitivity to antibacterial peptides. J Biol Chem. 2007;282(21):15569–15577. doi: 10.1074/jbc.M701454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boll JM, et al. Reinforcing lipid A acylation on the cell surface of Acinetobacter baumannii promotes cationic antimicrobial peptide resistance and desiccation survival. MBio. 2015;6(3):e00478-15. doi: 10.1128/mBio.00478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran AX, et al. Resistance to the antimicrobial peptide polymyxin requires myristoylation of Escherichia coli and Salmonella typhimurium lipid A. J Biol Chem. 2005;280(31):28186–28194. doi: 10.1074/jbc.M505020200. [DOI] [PubMed] [Google Scholar]
- 10.Hankins JV, et al. Elucidation of a novel Vibrio cholerae lipid A secondary hydroxy-acyltransferase and its role in innate immune recognition. Mol Microbiol. 2011;81(5):1313–1329. doi: 10.1111/j.1365-2958.2011.07765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hankins JV, Madsen JA, Giles DK, Brodbelt JS, Trent MS. Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in gram-positive and gram-negative bacteria. Proc Natl Acad Sci USA. 2012;109(22):8722–8727. doi: 10.1073/pnas.1201313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somerville JE, Jr, Cassiano L, Darveau RP. Escherichia coli msbB gene as a virulence factor and a therapeutic target. Infect Immun. 1999;67(12):6583–6590. doi: 10.1128/iai.67.12.6583-6590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, et al. Role of the lpxM lipid A biosynthesis pathway gene in pathogenicity of avian pathogenic Escherichia coli strain E058 in a chicken infection model. Vet Microbiol. 2013;166(3–4):516–526. doi: 10.1016/j.vetmic.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 14.Watson PR, et al. Mutation of waaN reduces Salmonella enterica serovar Typhimurium-induced enteritis and net secretion of type III secretion system 1-dependent proteins. Infect Immun. 2000;68(6):3768–3771. doi: 10.1128/iai.68.6.3768-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Hauteville H, et al. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J Immunol. 2002;168(10):5240–5251. doi: 10.4049/jimmunol.168.10.5240. [DOI] [PubMed] [Google Scholar]
- 16.Goldman SR, Tu Y, Goldberg MB. Differential regulation by magnesium of the two MsbB paralogs of Shigella flexneri. J Bacteriol. 2008;190(10):3526–3537. doi: 10.1128/JB.00151-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranallo RT, et al. Virulence, inflammatory potential, and adaptive immunity induced by Shigella flexneri msbB mutants. Infect Immun. 2010;78(1):400–412. doi: 10.1128/IAI.00533-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anisimov AP, et al. Effect of deletion of the lpxM gene on virulence and vaccine potential of Yersinia pestis in mice. J Med Microbiol. 2007;56(Pt 4):443–453. doi: 10.1099/jmm.0.46880-0. [DOI] [PubMed] [Google Scholar]
- 19.Feodorova VA, et al. Pleiotropic effects of the lpxM mutation in Yersinia pestis resulting in modification of the biosynthesis of major immunoreactive antigens. Vaccine. 2009;27(16):2240–2250. doi: 10.1016/j.vaccine.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krissinel E, Henrick K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2256–2268. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 21.Holm L, Rosenstrom P. Dali server: Conservation mapping in 3D. Nucleic Acids Res. 2010;38(Web Server issue):W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meluzzi D, Zheng WH, Hensler M, Nizet V, Dorrestein PC. Top-down mass spectrometry on low-resolution instruments: Characterization of phosphopantetheinylated carrier domains in polyketide and non-ribosomal biosynthetic pathways. Bioorg Med Chem Lett. 2008;18(10):3107–3111. doi: 10.1016/j.bmcl.2007.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heath RJ, Rock CO. A conserved histidine is essential for glycerolipid acyltransferase catalysis. J Bacteriol. 1998;180(6):1425–1430. doi: 10.1128/jb.180.6.1425-1430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Röttig A, Steinbüchel A. Acyltransferases in bacteria. Microbiol Mol Biol Rev. 2013;77(2):277–321. doi: 10.1128/MMBR.00010-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu P, Vogel C, Wang R, Yao X, Marcotte EM. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat Biotechnol. 2007;25(1):117–124. doi: 10.1038/nbt1270. [DOI] [PubMed] [Google Scholar]
- 26.Masoudi A, Raetz CRH, Zhou P, Pemble CW., 4th Chasing acyl carrier protein through a catalytic cycle of lipid A production. Nature. 2014;505(7483):422–426. doi: 10.1038/nature12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y-M, Wu B, Zheng J, Rock CO. Key residues responsible for acyl carrier protein and beta-ketoacyl-acyl carrier protein reductase (FabG) interaction. J Biol Chem. 2003;278(52):52935–52943. doi: 10.1074/jbc.M309874200. [DOI] [PubMed] [Google Scholar]
- 28.Tzeng YL, Datta A, Kolli VK, Carlson RW, Stephens DS. Endotoxin of Neisseria meningitidis composed only of intact lipid A: Inactivation of the meningococcal 3-deoxy-D-manno-octulosonic acid transferase. J Bacteriol. 2002;184(9):2379–2388. doi: 10.1128/JB.184.9.2379-2388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman RC, Doran CC, Kadam SK, Capobianco JO. Lipid A precursor from Pseudomonas aeruginosa is completely acylated prior to addition of 3-deoxy-D-manno-octulosonate. J Biol Chem. 1988;263(11):5217–5223. [PubMed] [Google Scholar]
- 30.Mohan S, Raetz CR. Endotoxin biosynthesis in Pseudomonas aeruginosa: Enzymatic incorporation of laurate before 3-deoxy-D-manno-octulosonate. J Bacteriol. 1994;176(22):6944–6951. doi: 10.1128/jb.176.22.6944-6951.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson DW, Gao Y, Yen M-I, Yen C-LE. Intestine-specific deletion of acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J Biol Chem. 2014;289(25):17338–17349. doi: 10.1074/jbc.M114.555961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohshiro T, Tomoda H. Acyltransferase inhibitors: A patent review (2010-present) Expert Opin Ther Pat. 2015;25(2):145–158. doi: 10.1517/13543776.2014.989833. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier MR, et al. Unique structural modifications are present in the lipopolysaccharide from colistin-resistant strains of Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57(10):4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.