Abstract
A third of the world’s population uses solid fuel derived from plant material (biomass) or coal for cooking, heating, or lighting. These fuels are smoky, often used in an open fire or simple stove with incomplete combustion, and result in a large amount of household air pollution when smoke is poorly vented. Air pollution is the biggest environmental cause of death worldwide, with household air pollution accounting for about 3·5–4 million deaths every year. Women and children living in severe poverty have the greatest exposures to household air pollution. In this Commission, we review evidence for the association between household air pollution and respiratory infections, respiratory tract cancers, and chronic lung diseases. Respiratory infections (comprising both upper and lower respiratory tract infections with viruses, bacteria, and mycobacteria) have all been associated with exposure to household air pollution. Respiratory tract cancers, including both nasopharyngeal cancer and lung cancer, are strongly associated with pollution from coal burning and further data are needed about other solid fuels. Chronic lung diseases, including chronic obstructive pulmonary disease and bronchiectasis in women, are associated with solid fuel use for cooking, and the damaging effects of exposure to household air pollution in early life on lung development are yet to be fully described. We also review appropriate ways to measure exposure to household air pollution, as well as study design issues and potential effective interventions to prevent these disease burdens. Measurement of household air pollution needs individual, rather than fixed in place, monitoring because exposure varies by age, gender, location, and household role. Women and children are particularly susceptible to the toxic effects of pollution and are exposed to the highest concentrations. Interventions should target these high-risk groups and be of sufficient quality to make the air clean. To make clean energy available to all people is the long-term goal, with an intermediate solution being to make available energy that is clean enough to have a health impact.
Introduction
Definition of household air pollution
Household air pollution (HAP) is usually measured indoors, and arises from domestic activities of cooking, heating, and lighting, particularly in low and middle income countries (LMICs). 3 billion people worldwide are exposed to toxic amounts of HAP every day because they use solid fuels, a term that includes biomass fuels (derived from plant sources) or coal for combustion resulting in the release of products of incomplete combustion such as carbon monoxide and particulate matter (PM). Furthermore, solid fuel is commonly used in homes with poor or absent chimney ventilation of smoke.
Cooking is the energy requirement that consumes most solid fuel worldwide. The sources of fuel vary considerably, with coal use being predominant in China, described in the later section on lung cancer, but wood and charcoal being more common in Africa and India. Animal dung is used among pastoralist communities, particularly those at high altitude (eg, Nepal, Afghanistan) or in savannahs where wood is rare (eg, Kenya, Ethiopia). Fuel-deprived communities often burn domestic rubbish and plant residues (eg, straw, maize husks); whereas urban communities commonly burn kerosene or charcoal. The toxic content of smoke from all of these fuels differs widely and has overlap with the known toxicity of traffic, industrial, and tobacco smoke. Lighting can also result in substantial HAP. Smoky unvented wicks in simple lamps that burn kerosene and in candles can result in substantial black carbon smoke. The increasing availability of light emitting diode (LED) lamps has reduced this form of pollution, but it remains a major problem. Heating needs are highly variable by latitude, altitude, and season. In extreme climates (eg, Nepal, north India), ventilation is deliberately minimised to conserve energy, resulting in extremely toxic amounts of HAP for a substantial proportion of the year. Urban poor people in Africa often bring a simple cooking stove indoors to keep their sleeping area warm at night.
The behavioural context of HAP
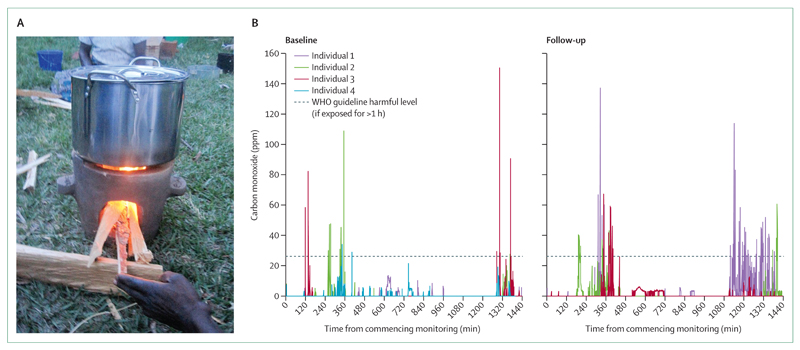
Household behaviour for cooking, lighting, and heating varies by culture, gender, age, and socioeconomic status. These behavioural norms determine both exposure and resulting health risks for women, children, and men. Cultural differences define a fascinating range of cooking methods, from roasting over flame or in an open earthen oven, to boiling, broiling, steaming, and stewing, found almost everywhere. In most cultures, women have a leading role in domestic cooking, with men cooking when at work or away from home. In the typical domestic context, therefore, women have several periods of intense cooking smoke exposure per day. Young children and infants, typically carried on the back or placed near their mother to sleep, are also exposed to these short, very high level, exposures to smoke (figure 1). There is particular concern when young children are exposed to smoke because data suggest that smoke exposure during the window of developmental susceptibility in early life is particularly detrimental. Men in most cultures have greater exposure to occupational, industrial, or agricultural smoke, and higher consumption of tobacco, which is outside the scope of our Commission; however, we note the confounding effect of these exposures on studies of HAP.
Figure 1. Exposure of children to household air pollution at home and at school.
(A) An infant in Malawi is exposed to very high levels of cooking smoke. Both mother and child had evidence of eye irritation. (B) Household air pollution exposure continues at school. Used with permission of CAPS/Handstand productions.
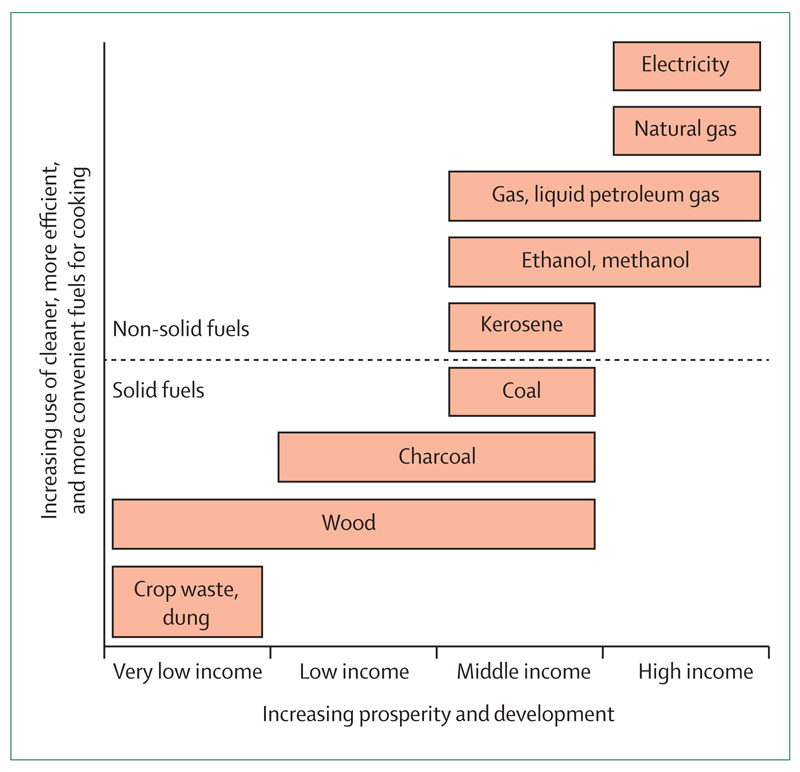
Socioeconomic status is a major predictor of exposure to HAP in most cultures. The less expensive fuel options in any context are generally less efficient fuels, produce more smoke, and are used by people with the most poorly designed homes. For example, propane, liquid petroleum gas (LPG), or ethanol often burn very cleanly, but remain too expensive for many households. Electricity is the least polluting form of domestic energy (assuming that households are geographically separated from power stations), but is not affordable to most people. As we descend the energy ladder1 to cheaper forms of fuel (such as charcoal, wood and dung or crop residues), there are polluting fuels with both poor combustibility and highly toxic emissions. Simple homes built with mud, thatch, and animal skins rarely have a chimney and, when present, the chimney is usually a simple vent with no air-drawing flue. In addition, correctly installed flues must be maintained with regular inspections and cleanings, an activity that, when not done, can result in marked increases in HAP.
The global burden of disease attributed to HAP
Poverty, disease, and the use of solid fuel are inextricably linked because poverty is a risk factor for disease in all communities (figure 2). The attribution of disease burden to HAP exposure or other risk factors is complex and needs systemic analysis from multiple perspectives. The 2012 report of the comparative risk assessment2 for the Global Burden of Disease study (GBD) 2010 is the gold standard for such analysis, and its findings attributed nearly 3·5 million deaths to direct exposure to HAP. HAP is also an important contributor to ambient air pollution (estimated to contribute 16% of the global disease burden from ambient air pollution), as detailed in the methods of the report.2 Deaths from air pollution, including from HAP and ambient air pollution, far exceed deaths attributed to other environmental factors (table 1). The great increase in the disease attribution between previous comparative risk assessments and the GBD in 2010 resulted partly from inclusion of cardiovascular and cerebrovascular deaths associated with HAP exposure. The WHO Global Health Observatory report3 updated the estimates and noted that HAP caused 4·3 million deaths worldwide in 2012, and ambient air pollution caused a further 3·7 million deaths.
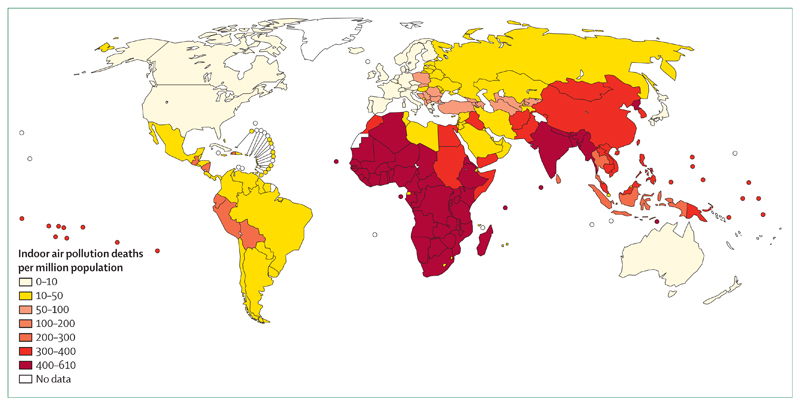
Figure 2. WHO map of household air pollution and mortality.
World map of poverty (not shown) shows nearly identical geographical distribution. © WHO 2005. All rights reserved.
Table 1. Deaths attributable to environmental risks worldwide.
| Deaths in 2010 (95% CI) | |
|---|---|
| Household air pollution* | 3·55 million (2·68 million to 3·62 million) |
| Ambient pollution | 3·22 million (2·82 million to 3·62 million) |
| Occupational risk factors† | 0·85 million (0·66 million to 1·06 million) |
| Lead exposure | 0·67 million (0·58 million to 0·78 million) |
| Second-hand smoke | 0·60 million (0·45 million to 0·52 million) |
| Unimproved sanitation | 0·24 million (0·01 million to 0·48 million) |
| Unimproved water source | 0·12 million (0·01 million to 0·23 million) |
| Residential radon | 0·10 million (0·01 million to 0·22 million) |
HAP is associated with many health effects, including both acute and chronic disorders, pulmonary and systemic. Respiratory risks are the focus of this review and so we devote full sections to respiratory infections, chronic lung diseases, and cancers. It is important, however, not to neglect the cardiovascular risks associated with HAP, which have been reviewed elsewhere.4 Data support a role for HAP in the pathogenesis of both myocardial infarction and hypertension-related stroke.5 There are other factors that are also beyond the scope of our Commission. For example, the burning of solid fuel in either open fires or simple stoves results in frequent burns to adults and particularly to children. Burns in children are often severe. There are also indirect health risks to women and children gathering fuel including trauma, assault, and injury.
Interventions to reduce respiratory and other health risks from HAP
HAP is now recognised to be a modifiable exposure against which specific interventions such as the use of improved fuels, cookstoves, or heaters, and improved ventilation using improved cooking technology, can improve human health. In practice, culturally acceptable and context-specific solutions involve consideration of many factors including combinations of interventions. In 2011, RESPIRE6 showed, for the first time using a randomised controlled trial (RCT), that a reduction in disease is possible—in this case a reduction in severe pneumonia in children after a chimney stove intervention to reduce HAP. Similar RCTs are continuing elsewhere in the world, from Nepal to Ghana to Malawi, that use different technologies, both to reduce exposure to HAP and to determine the exposure–response. Such data provide the evidence base to understand how much exposure levels need to be reduced to improve health worldwide. Now that such information is becoming available, it will drive commitments by governments, industries, and non-governmental organisations (NGOs) to find culturally sensitive, affordable, and sustainable technologies that households can use to reduce the burden of preventable death.
Of many fuels used worldwide, the most popular are electricity and gas; indeed most people living in high-income countries use a combination of electricity for oven use and gas for pot cooking. Both are clean fuels and, although electricity is easily and safely delivered throughout cities and rural areas, the greater immediate control when cooking with gas makes it an attractive option. There are, however, alternative fuel solutions with intermediate cost that have widespread applicability in middle-income regions that retain some of the features of gas cooking. Pellets made of wood or crop residues offer much better burning, particularly in combination with an advanced cookstove, than does wood. Clean liquid fuels such as propane, LPG, and biofuels such as ethanol are all increasingly available, but are not often used because of costs, for cooking needs such as boiling water for tea or similar items. Biogas for villages is another clean fuel possibility that could be an inexpensive and clean energy source for a household, but needs a substantial initial investment, which might well prove cost-effective in time. Ultimately, ideal clean fuels could be made available throughout the world, but they are not presently realistic for most households in LMICs.
Improved cookstove technology for households living in poverty in LMICs has been available for 40 years, initially driven by the need to reduce fuel use that caused deforestation and, more recently, by health risks from HAP. This historical imperative to reduce wood use by improved cooking efficiency led to the invention of many stoves that burn less fuel (usually wood), but, in the absence of known health effects, less consideration has been given to the concentration and toxicity of emissions. Improvements in cookstove design often come as a result of controlling entrainment of air during combustion or with assisted ventilation such as a fan that might use a battery, thermoelectric generator, or another source of electric assistance. Insulated heat direction, correct use of a pot appropriate for the stove, and careful fuel selection (at least dry wood) are other important improvements in design. There are a wide variety of cookstoves available worldwide (figure 3), with equally variable levels of efficiencies that can reduce fuel use or emissions.7 The kitchen performance test that is used to compare alternative technologies typically measures weight of fuel used to heat a defined volume of water to boiling point; improved stoves can improve the percentage of energy transferred from fuel to water from less than 20% (three-stone fire) to more than 80% (advanced cookstoves; figure 4). Unfortunately, available kitchen performance tests do not include a measure of toxicity. Certain innovative and attractive solutions involving renewable energy (eg, solar cookers) can be very effective at reducing cooking-related exposures to zero. However solar cookers have limited use because the cooking is not easily controlled, the time and location of cooking needs access to the sun, and it is often viewed as not being culturally suitable (eg, when staples need to be cooked at night, etc). Many new technologies are being developed that will enhance the efficiencies of various cooking and heating solutions, with particular attention to products that effectively reduce exposures to HAP, improve safety to avoid burns and scalds, and enhance multiple household uses of energy from solid and liquid fuel combustion. Such progress is needed to increase market demand for such improved cooking and heating solutions.
Figure 3. Cookstove types used around the world.
(A) Three-stone, minimally tended, wood fuel. (B) Berkeley–Darfur, wood fuel. (C) Envirofit G-3300, wood fuel. (D) Onil, wood fuel. (E) Philips HD4008, wood fuel. (F) Philips HD4012, wood fuel. (G) Sampada, wood fuel. (H) StoveTec GreenFire, wood fuel. (I) Upesi Portable, wood fuel. (J) GERES, charcoal fuel. (K) Gyapa, charcoal fuel. (L) Jiko, ceramic, charcoal fuel. (M) Jiko, metal, charcoal fuel. (N) KCJ Standard, charcoal fuel. (O) Kenya Uhai, charcoal fuel. (P) StoveTec prototype, charcoal fuel. (Q) Belonio Rice Husk Gasifier, rice hull fuel. (R) Mayon Turbo, rice hull fuel. (S) Oorja, biomass pellet fuel. (T) StoveTec TLUD prototype, wood pellet fuel. (U) Jinqilin CKQ-80I, corn cob fuel. (V) Protos, plant oil fuel. Photo is courtesy of James Jetter, US Environmental Protection Agency, NC, USA.
Figure 4. Improved cookstoves offer greatly reduced household air pollution exposure.
(A) A woman in Malawi using the Philips improved cookstove as part of the Cooking and Pneumonia Study (CAPS) funded by the Joint Global Health Trials of the Medical Research Council, Wellcome Trust, and Department for International Development. (B) Taken at the same location in Malawi, non-invasive monitoring, in this case od carboxyhaemoglobin, can be used to objectively assess cookstove effect. Used with permission of CAPS/Handstand productions.
House design is shaped by people developing pragmatic solutions to deal with climate and security with use of materials available locally. Chimneys are far from being universally available and, even when built, chimneys must be correctly designed and installed and have regular maintenance to be effective. In many cultures, the solution to not having smoke from open fires or non-ventilated stoves is cooking outdoors or on simple verandas. This seems like a simple and direct solution, but the exposure to the smoke might remain unacceptably high, even outdoors, and it is dependent on weather and season. Simple ventilation solutions, including air bricks and holes in the roof or eaves, can greatly reduce levels of HAP. Adapted ovens such as the plancha stove in Guatemala or Afghanistan that pipes smoke from a central oven out of the house are also effective interventions to reduce HAP. These interventions need behavioural changes in activities that are held very precious in life, and so there can be immediate resistance to implementation of ventilation.
Even the most effcient technology fails if adoption is only for a brief period after introduction, or if the new technology is used concurrently with traditional methods. This latter behaviour, known as stacking, is common in western kitchens, in which use of an oven, cooker, toaster, and microwave is now usual. Combined use of new and old technologies in households in LMICs at risk from HAP negates any reduction in health risks by new efficient cooking or heating technologies. To reduce HAP, households and communities must share a substantial will to change behaviour at both the household and the community level. This effort involves not only an affordable effective technology but also an appreciation of the benefits of change. In fact, it is unusual for communities to change behaviour to achieve a health-related benefit. More attractive benefits include the convenience in cooking, economy of fuel use, time savings for gathering fuel and cooking, and the value of it being more modern. Modern co-benefits include stoves that also charge a mobile phone or battery light with use of thermoelectric coupling.
Global efforts to reduce HAP
Climate change and the increasing demand for energy, along with an increasing awareness of pollution having an adverse health effect on women and children, have driven a global interest to reduce HAP. Starting with the US Environmental Protection Agency’s Partnership for Clean Indoor Air (PCIA), hundreds of motivated NGOs have joined with government and international organisations to design innovative and regionally specific solutions. In 2010, the US Government, together with the UN Foundation, created a public–private partnership that incorporated the PCIA into the Global Alliance for Clean Cookstoves, enhancing development and implementation of clean cooking solutions for millions of households, to reduce the effect of deforestation and climate change, and to empower women. In addition, the UN launched the Initiative for Sustainable Energy for All in 2011 as a set of plans to provide clean cooking energy for people at the bottom of the world’s energy ladder through the advancement of cleaner technologies, such as LPG by the year 2030. Such efforts offer interdisciplinary platforms to promote global change in energy use for poor people that can result in major economic and social improvement, and ultimately, reduce the global burden of disease, especially diseases related to HAP.
Summary
Air pollution is the number one environmental cause of death in the world, with HAP being a major contributor to this burden. In this Commission, we discuss evidence to link HAP with respiratory infections, chronic lung diseases, and respiratory tract cancers. We then review issues with quantifying the exposure. Finally, we discuss available interventions and those in development to reduce HAP.
Respiratory infections
Introduction
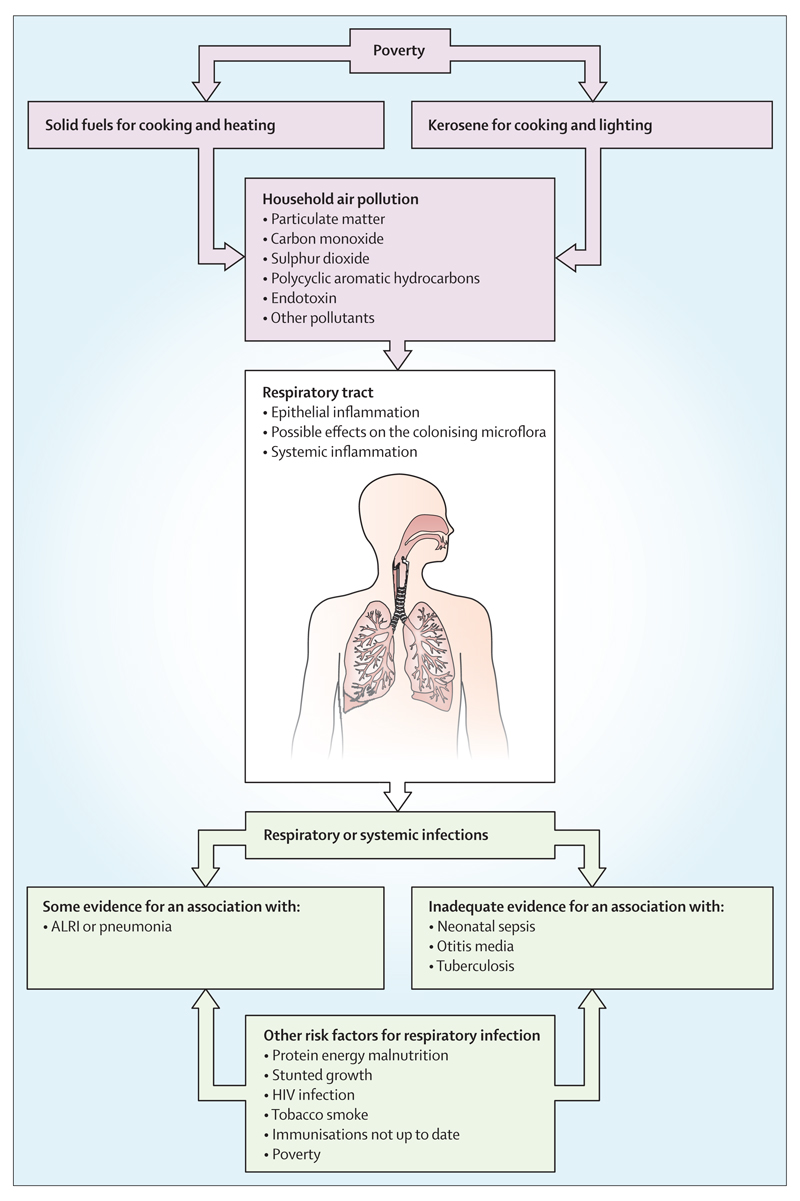
Respiratory infections are a leading cause of morbidity and mortality across all ages, particularly in children younger than 5 years.8 Most of this burden occurs in countries where solid fuels are the primary source of household energy. In this section, we discuss what is known about the contribution of HAP to respiratory infection. We assess the biological plausibility of a causal role of HAP on respiratory infections, partly drawing from evidence about the role of cigarette smoking (direct exposure in adults and second-hand exposure in children) in infections of the lower respiratory tract.9–12 In addition, we examine the relation of HAP exposure to other risk factors for respiratory infection in LMICs, particularly HIV infection and malnutrition. The burden of respiratory infection will be relieved only when all risk factors are addressed (figure 5).
Figure 5. Household air pollution and other risk factors for respiratory infections.
ALRI=acute lower respiratory infection.
Is HAP a risk factor for respiratory tract infections in infants and children?
About 700 000 of the 3 million neonatal deaths that occur every year in low-resource regions are due to serious infection.13 Most serious neonatal infections are bacterial,14 half of which are due to neonatal pneumonia. Signs of sepsis and pneumonia in young babies are notoriously subtle and difficult to recognise,15 and so many studies of HAP exposure in neonates have focused on mortality rather than infection. With use of results from two studies, Bruce and colleagues16 calculated a pooled odds ratio (OR) of 1·14 (95% CI 0·87–1·48) for neonatal mortality in households using solid fuels;17,18 both studies included kerosene in the reference (clean fuel) group. Since then, Epstein and colleagues19 reported that neonatal death in India was strongly associated with household use of coal (18·54, 6·31–54·45) and might be associated with kerosene, but the OR for kerosene crosses the null, so the risk is not clear (2·30, 0·95–5·55). Solid fuel use was associated with increased risk of neonatal death in infants born to women with no further than primary school education (7·56, 2·40–23·80). Although a relationship is plausible, the evidence for whether HAP has a role in neonatal sepsis and pneumonia, as well as death, is weak and additional studies are needed to clarify.
Acute lower respiratory infections (ALRIs) such as pneumonia or bronchiolitis are the leading cause of mortality in children aged 2 months to 5 years worldwide, and infections occur mostly in LMICs. In 2010, about 1·3 million children aged younger than 5 years died of pneumonia.20 Several meta-analyses and systematic reviews have summarised the relationship between HAP from solid fuels and risk of acute respiratory infection (upper and lower respiratory infections) in children. Diagnosis of acute respiratory infection is based mostly on parent-reported symptoms and lacks diagnostic and aetiological specificity. In a meta-analysis of 27 studies for morbidity endpoints in children from households using solid fuel, Dherani and colleagues21 reported a summary OR of HAP and pneumonia (ALRI) of 1·78 (95% CI 1·45–2·18), whereas a meta-analysis of eight studies from Po and colleagues22 produced a summary risk ratio for acute respiratory infections of 3·53 (1·93–6·43). The differing effect size estimates might be due to heterogeneous definitions of exposure (HAP) and outcome (pneumonia, acute respiratory infection). Both meta-analyses included studies in which kerosene was classed as a clean fuel, possibly resulting in a bias towards a lesser effect. A 2013 case-control study of clinically diagnosed ALRI in children younger than 36 months in Nepal compared use of kerosene or solid fuel for cooking to electric stoves.23 Compared with electric stoves, kerosene (OR 1·87, 95% CI 1·24–2·83) and solid fuel use (1·93, 1·24–2·98) were both significantly associated with ALRI. A recent estimate of the global disease burden of HAP suggests that every year, household solid fuel use causes 455 000 ALRI deaths, the loss of 39 100 000 disability-adjusted life-years, and an ALRI population attributable fraction of 52%.24
Recurrent acute otitis media can lead to chronic suppurative otitis media. Chronic suppurative otitis media is a frequent childhood infectious disease and the most common cause of hearing impairment in children, occurring mostly in resource-poor populations. It results in speech delay and educational difficulties, with subsequent reduction in long-term societal economic productivity.25 Second-hand smoke exposure, particularly from household tobacco smoke, is an established risk factor for acute otitis media.26 HAP might also be a risk factor for acute otitis media and chronic suppurative otitis media, but only two studies, both from Nigeria, investigated this possibility. One study showed that compared with controls, children with chronic suppurative otitis media were more likely to be exposed to indoor rather than outdoor cooking (adjusted OR 2·34, 95% CI 1·18–4·66), but stove and fuel type were not reported.27 A cross-sectional study of 600 children aged 0–12 years showed an association (p<0·05) between chronic suppurative otitis media and exposure to wood smoke from household cooking, but few details were reported.26 So far, there is inadequate evidence for a causal role of HAP in otitis media.
Few studies have examined the association between tuberculosis in children and combustion of solid fuel, probably because of the challenges of tuberculosis diagnosis in children. We identified two studies from India, a country with one of the highest tuberculosis burdens. One study showed no association between tuberculosis and use of solid fuel relative to LPG use in children aged 0–14 years (crude OR 1·32, 95% CI 0·86–2·01);28 the other reported an adjusted OR of 2·67 (1·02–6·97) for tuberculosis and exposure to HAP in children younger than 5 years.29 The reason for the discrepancy is not clear, but the first study included both pulmonary and extrapulmonary tuberculosis, whereas the second focused on pulmonary tuberculosis. With these sparse and conflicting results, the role of HAP in pulmonary and extrapulmonary tuberculosis in children remains unclear.
Is HAP a risk factor for respiratory tract infections in older children and adults?
Most published studies have focused on ALRI in children or acute respiratory infections in women because of their risk of exposure to HAP during cooking. Acute respiratory infection includes upper respiratory tract infection and lower respiratory tract infection (ALRI), with ALRI being the more serious. Unfortunately, the literature is not always clear about whether the acute respiratory infection is upper respiratory tract infection or ALRI. As with children, acute respiratory infection is usually diagnosed on the basis of self-reported symptoms (cough and difficult or rapid breathing) and is likely to be predominantly upper (less invasive) respiratory infections, not ALRI. We identified four reports on associations between HAP and acute respiratory infections in adults. Two of these studies reported a risk of association between HAP exposure and acute respiratory infections but did not take into account possible confounders.30,31 Taylor and Nakai32 examined acute respiratory infections in 520 women aged 15–45 years in Sierra Leone who used wood or charcoal for cooking. The OR of acute respiratory infections for cooking with wood compared with charcoal was 1·14 (95% CI 0·71–1·82), but since charcoal is not a clean-burning fuel, the results of this study are difficult to interpret. Use of charcoal has itself been associated with paediatric respiratory infections.33 The fourth study7 reported acute respiratory infections and ALRI and measured PM10 (PM up to 10 μm in size) in village huts in a rural Kenyan population. For both acute respiratory infections and ALRI, there was an increasing exposure–response relationship between measured exposure to PM in the 229 participants, both men and women, aged 5–49 years. This study presents the most convincing available evidence that HAP is associated with acute respiratory infections and ALRI in adults, but overall, the evidence is weak.
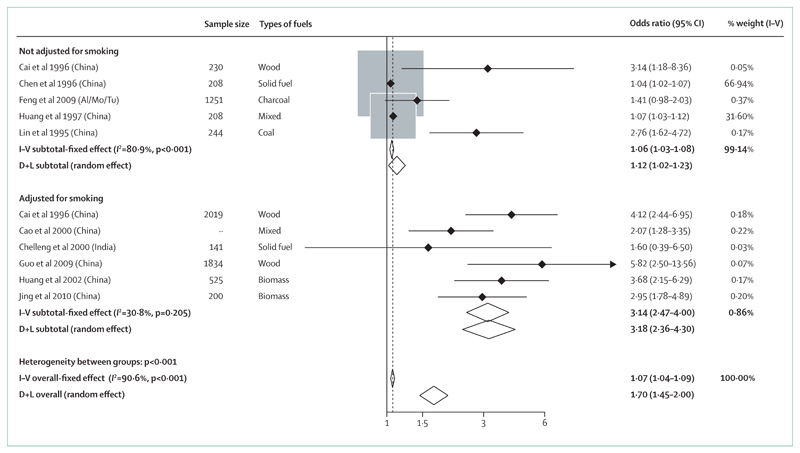
Since exposure to tobacco smoking is a risk factor for pulmonary tuberculosis,11,12 HAP might also increase the risk of pulmonary tuberculosis. Slama and colleagues34 reviewed six epidemiological studies on the possible relationship between use of polluting fuels and pulmonary tuberculosis, mostly in women. They concluded that there was insufficient evidence for an association between pulmonary tuberculosis and HAP. Consequently, the GBD 2010 study did not include a potential role of HAP in pulmonary tuberculosis. After the Slama review, Sumpter and Chandramohan35 re-examined the relationship between tuberculosis and HAP in a meta-analysis of seven studies.36–42 They reported a summary OR for all participants of 1·30 (95% CI 1·04–1·62) and 1·70 for women (1·10–8·20), although there was heterogeneity among study results. A study by Woldesemayat and colleagues,43 published after the meta-analysis, showed no association between tuberculosis and use of solid fuels for cooking, but nearly all participants used solid fuels, so there was insufficient exposure variation for useful analysis.43
A case-control study from Nepal showed an OR of 3·45 (95% CI 1·44–8·27) for risk of tuberculosis with use of solid fuel for heating, but use of solid fuel for cooking had an OR of 1·21 (0·48–3·05).36 The authors suggested the difference might be attributable to higher exposures from the reduced ventilation and proximity to the fire during heating of the house. The same study reported associations between tuberculosis and use of a kerosene cooking stove (3·36, 1·01–11·22) and kerosene lamps for lighting (9·43, 1·45–61·32). The high relative risk estimate associated with lighting could have been due to prolonged proximity to the lamps. One limitation of this study was selection of tuberculosis cases from a regional tuberculosis centre, whereas controls were recruited from a nearby hospital. By contrast, Lakshmi and colleagues42 reported no association between tuberculosis and kerosene stove use in a study in India, and Woldesemayat and colleagues43 also showed no association between tuberculosis and kerosene lamp use in Ethiopia. In summary, the relationship between HAP and tuberculosis in adults is not established. Additional studies that measure emissions and document the diagnosis of tuberculosis are needed.
Effects of HAP on mechanisms of defence against respiratory tract infection
Before consideration of cofactors that change the interaction of HAP and infection epidemiology, or of mitigating strategies to reduce the adverse effect of HAP on pulmonary infection, we briefly review the effect of HAP on pulmonary defence against infection. HAP includes both PM and pollutants that induce specific responses at multiple levels of the respiratory tract.44 Deposition of constituents of HAP begins in the nasopharynx and continues throughout the respiratory pathway to the alveolus, with each level filtering and protecting the lower airways. Particles of 2·5 μm or less (PM2·5) can reach the alveolus, and ultrafine particles (<100 nm) might translocate to the systemic circulation.45
HAP-induced epithelial inflammation46 might change the integrity of the epithelial barrier and increase the risk of bacterial invasion.47 Wood smoke has adverse effects on surfactant48 and cilial function.49 To offset particle oxidative effects, epithelial lining fluid contains high levels of the antioxidant glutathione,50 which is up-regulated after exposure to wood smoke.51 Although incompletely studied, such redox changes will probably alter host response to infection52,53 through effects on inflammatory signalling54 and recognition of apoptotic cells.55 Streptococcus pneumoniae relies on extracellular glutathione to survive oxidative insults,56 and changes in the epithelial lining fluid might also alter the bacterial responses.
HAP induces acute effects on lung cells, chronic adaptive responses in lung biochemistry, and altered resolution of infection responses in the lung. Different fuels result in many different particle sizes and also very different toxicities, with HAP from burned animal dung being particularly toxic.57,58 Pro-inflammatory responses are seen in firefighters acutely exposed to wood smoke, with increased oxidative stress resulting in systemic interleukin-8 rises and neutrophilia.59,60 Experimental acute exposure of human beings to wood smoke also causes pulmonary inflammation and oxidative stress, as measured by increases in exhaled nitric oxide and malondialdehyde.61 Inflammatory responses depend on particle source, size, composition, and adsorbed molecules,62 particularly the organic fractions.63 However, there are some potential differences between smoke from wild fires and HAP,57 which limits the generalisability of data from firefighters. Specifically, smoke from wild fires is more potent than conventionally collected ambient particles,64 and whereas ultrafine particles (<100 nm) have greater oxidative and inflammatory potential due to their large surface areas, in HAP, larger particle effects (PM2·5–10) might be more important because of adsorbed endotoxin,66 which is particularly prominent in sources of HAP.67
Non-opsonised particles that reach the alveolus might interact with macrophage scavenger receptors, such as the scavenger receptor A group of surface proteins, including MARCO,68 allowing their phagocytic clearance. The same receptors define the inflammatory state of alveolar macrophages,69 and participate in uptake of bacteria,70 providing a potential for interaction between infective and inert inhaled particles, although the relevance of this finding has not been fully explored. Indeed, exposure to inhaled ultrafine carbon in mice enhances survival from subsequent pneumococcal infection.71 This counterintuitive finding is associated with increased early neutrophil influx and suggests that the timing of inflammation is important—ie, early and focused responses are advantageous.
The little evidence available for subacute and chronic HAP exposure (in vivo) suggests that compensatory changes limit inflammatory responses in mice, with lower interferon-γ response in T-cell co-culture 7 days after exposure to wood smoke.72 In rats, the lung shows minor pulmonary inflammation and reduced interleukin-1β after 70 days of in-vivo HAP exposure.73 Similar findings of reduced release of interleukin-8 by ex-vivo alveolar macrophages at baseline, and after further challenge with wood smoke particles, have been seen in human beings exposed to HAP.72,74 Adequate cytokine and chemokine responses are important for neutrophil recruitment.75 A dampened inflammatory response mediated by both altered glutathione metabolism and regulatory T cells could explain why HAP-exposed individuals show increased susceptibility to respiratory infections and pneumonia.76
HAP exposure might also affect the clinical course of respiratory infections. During established infection, alveolar macrophages act to contain pathogens and limit the inflammatory milieu. This response improves bacterial clearance77 and reduces mortality78 in mice with pneu-mococcal pneumonia. Alveolar macrophages exposed to urban and ultrafine carbon particles have diminished capacity for phagocytosis of S pneumoniae, and show evidence of oxidative stress,79,80 which might adversely affect the inflammatory balance and bacterial containment.80 Later, phagocytosis of apoptotic neutrophils (efferocytosis)81 and timely alveolar macrophage apoptosis82 seem key for survival. Cigarette smoke is known to reduce alveolar macrophage efferocytosis,83 but data for HAP, particularly for the containment of respiratory pathogens including pneumococcus and Mycobacterium tuberculosis, are not available.
The lung microbiome
Recent studies indicate that the lung is not sterile.84 HAP changes mechanisms of defence against infection, and so it is plausible (but not yet proven) that HAP modifies bacterial populations throughout the respiratory tract; these changes in the native flora might alter the risk of respiratory infection. Receptor-dependent adhesion to respiratory epithelium of S pneumoniae increases in cigarette smoke exposure,85 and there is increased ribosomal RNA from Streptococcus genus in the bronchoalveolar lavage fluid of individuals who are highly exposed to particulates (Rylance J; unpublished).
HAP and other important risk factors for respiratory infection
Risk of ALRI from HAP must be considered in the context of other important risk factors that enhance susceptibility to respiratory infection such as under-nutrition and HIV infection. These risk factors might relate to shared underlying mechanisms of disease as noted above, or might be crucial to develop an integrated approach to reduce the global burden of disease from pneumonia and other life-threatening respiratory infections. Briefly, we review these other risks and the opportunities to seek a more integrated approach to global ALRI prevention.
Undernutrition includes a range of diseases, from severe protein energy malnutrition including stunting and severe wasting, to micronutrient deficiencies, to suboptimal breastfeeding. Undernutrition is reported to be causative in a third of deaths of children younger than 5 years, of which many are due to ALRI.86 Severe protein energy malnutrition, particularly severe wasting (low weight for height: weight-for-height Z score <–3), is an important cause of secondary immunodeficiency worldwide, resulting in diminished cell-mediated (T-cell) immunity, immunoglobulin A in secretions, complement concentrations, and phagocytosis.87 Children with severe protein energy malnutrition are at increased risk of infection, including ALRI, and of mortality from ALRI.88–90 Severe protein energy malnutrition also includes severe stunting (low height for age) and reflects chronic malnutrition. Stunting affects 162 million children worldwide, 92% of whom live in Asia or Africa.91 Risk factors for stunting include lack of appropriate breastfeeding, chronic infection, or inflammation, and recurrent diarrhoea, and exposure to HAP. Bruce and colleagues16 reported that HAP exposure was associated with both moderate stunting (pooled OR 1·27, 95% CI 1·12–1·43) and severe stunting (1·55, 1·04–2·30). Although it has not been specifically studied, the combined insults to the immune system in malnourished children exposed to HAP probably work synergistically to increase the risk of ALRI and adverse outcomes.
Exposure to HAP is associated with a depletion of antioxidants and an altered balance between oxidant and antioxidant compounds,92 and a similar association has been reported in children exposed to second-hand smoke.93,94 Although there is no direct evidence that nutritional factors modify the effects of HAP on risk of respiratory tract infection, several studies raise the possibility that good nutrition could mitigate the harmful proinflammatory effects of HAP.95 Pregnant women exposed to PM2·5 who consumed higher amounts of fish had a lower risk of having a low birthweight baby,96 and in mice models investigating the combined effects of malnutrition and air pollution, zinc and vitamin E supplementation mitigated the harmful effects of air pollution.97,98 Conversely, an in-vitro study showed that high fructose and LDL increased the oxidative damage caused by ultrafine carbon particles.99 Trials of micronutrient supplementation for the prevention or treatment of ALRI have had mixed results.100–103 Although zinc,104,105 vitamin D,106,107 and multivitamins108,109 seem to be the most promising, the effect of giving micronutrients to women and children exposed to HAP has not been studied directly. Thus, animal and human studies indicate that nutrition might modulate the immune response to respiratory infection, and might be an effect modifier for the relationship between HAP and its harmful effects. Although the benefits of improved nutrition are self-evident by themselves, the primary approach to mitigation of the effect of environmental exposures such as HAP or tobacco use should not be through nutrition or other modifiers, but through reduction of exposures.
Tobacco-smoking patients with HIV often have severe emphysema, but this presentation is uncommon in LMICs. Individuals with HIV in LMICs are at higher risk of infection from many respiratory pathogens, therefore, they might be disproportionately affected by HAP. Although there is little direct evidence for the interaction between HIV infection and HAP, a 2013 meta-analysis of 14 observational studies showed that tobacco smoking increases the risk of bacterial pneumonia in patients with HIV, and that smoking cessation is effective to decrease that risk.110 Two studies of the effects of outdoor air pollution on pneumocystis pneumonia showed that exposure to higher temperatures and sulphur dioxide were associated with increased risk of hospital admissions due to pneumocystis pneumonia,111 and that the serological immune response to pneumocystis pneumonia was attenuated by both ambient PM (PM10) and cigarette smoking.112
HAP reduction and vaccination as related strategies to reduce respiratory infections
Pneumonia vaccines are very important for ALRI prevention. Pneumococcal conjugate vaccines have resulted in dramatic and sustained reductions in invasive pneumococcal disease in resource-rich and resource-poor countries; however, their effect on radiologically confirmed pneumonia has been slight and effects on incidence of clinical pneumonia are poor, both in RCTs113–118 and observational studies.119–123 The small effect is predictable because pneumonia might be caused by a wide range of pathogens, including vaccine-type pneumococci, and pneumococcal serotypes not included in current conjugate vaccines (ten-valent and 13-valent pneumococcal conjugate vaccines).124–127 Although pneumococcus is a major cause of pneumonia, reductions in clinical pneumonia will need approaches that work broadly across a range of respiratory infections, including mixed infection, with many different causative pathogens.
Despite their small direct effectiveness, programmes for pneumococcal conjugate vaccine have resulted in large absolute reductions in pneumonia hospitalisation in wealthy countries,119 although this outcome was not observed in all settings,120 and evidence from LMICs is sparse.128,129 The longevity of population level benefits might be shortened by serotype replacement occurring rapidly after vaccine introduction (as seen with seven-valent pneumococcal conjugate vaccines),130–132 and by subsequent increases in non-vaccine-serotype pneumonia. H influenzae type b vaccines reduce pneumonia burden in low-income countries,133 and some evidence also supports a herd effect.134 However, vaccination will not affect the burden of colonisation with non-typable (not type b) H influenzae, which is substantially more common in developing countries than is H influenzae type b135 and has long been recognised as an important cause of pneumonia.127,136 Vaccines are very important weapons in the battle against pneumonia, but will only ever be partially effective. Complementary strategies to reduce the burden of pneumonia-associated morbidity and mortality remain necessary.
Effectiveness of vaccine against pneumonia might be further impaired by HAP exposure through two potential mechanisms: first, directly reduced vaccine immuno-genicity; or second, an increase in bacterial colonisation of the nasopharynx—a necessary prerequisite for subsequent pneumonia (table 2). The little evidence available argues against directly reduced vaccine immunogenicity. In a study of seven-valent pneumococcal conjugate vaccines in adults, people who smoked had lower immune responses to vaccination with seven-valent pneumococcal conjugate vaccines for two reported serotypes (6B and 23F) than did people who did not smoke.137 But another study in adults showed no association between pneumococcal disease serotype and being a current tobacco smoker.138 A study of the effectiveness of H influenzae type b vaccine against radiological pneumonia in children showed no evidence of effect modification by smoke exposure.139 Studies of pneumococcal polysaccharide vaccines140,141 and influenza vaccines142 in adults also adjusted for smoking status, but none reported whether smoking was a significant predictor of vaccine response.
Table 2. Causal relationships: household air pollution and the introduction of pneumococcal conjugate and Haemophilus influenzae type b vaccines.
| Mechanism | Affects | |
|---|---|---|
| HAP affecting vaccine effectiveness or vaccine programme impact | ||
| HAP increases risk of pneumonia in a vaccinated child (eg, through impaired ciliary clearance) | Causal | Vaccine effectiveness |
| HAP associated with high risk of pneumonia in a vaccinated child through confounding associations (eg, poverty, overcrowding) | Confounding | Apparent vaccine effectiveness |
| HAP associated with lower rates of vaccine uptake through confounding associations | Confounding | Programme impact |
| HAP increases risk of pneumonia in an unvaccinated child who is in contact with a vaccinated child | Causal | Programme impact through indirect effects |
| Vaccine affecting impact of HAP | ||
| Vaccine reduces the effect of smoke exposure on carriage | Effect modification | Programme impact through direct and indirect effects |
| Vaccine and HAP as independent factors | ||
| Vaccine reduces the rate of pneumonia independently of smoke exposure | Independent protective effect | Vaccine effectiveness and programme impact |
The evidence that smoke exposure increases bacterial colonisation arises from studies of tobacco smoke. Maternal tobacco smoking was associated with earlier acquisition of pneumococcal colonisation in infants in a study from the Thai–Myanmar (Burma) border,143 whereas a study in Perth reported no such association.144 Neither study addressed exposure to cooking smoke or household use of open fires. A study in Kenya showed that there was earlier pneumococcal carriage in infants exposed to tobacco smoke and to cooking smoke.145 A cross-sectional study from Taiwan reported an association between tobacco smoke exposure and pneumococcal colonisation only in unvaccinated children (and not in vaccinated children).146
For adults with HIV who were vaccinated with the pneumococcal conjugate vaccine, smoking tobacco remained a significant risk factor for subsequent pneumococcal colonisation.147 Investigators did a prevalence survey of pneumococcal carriage in a high-burden indigenous population in the Northern Territory of Australia after widespread uptake of seven-valent pneumococcal conjugate vaccine and polysaccharide vaccine booster.148 Pneumococcal carriage was associated with exposure to open fires in adults, but surprisingly, they reported no association with pneumococcal carriage in children. The absence of data for HAP and colonisation in children is a knowledge gap that needs to be addressed by future studies.
Long-term view of HAP reduction and vaccination interaction
Vaccination against respiratory pathogens reduces the burden of ALRI, and widespread implementation programmes are in place. The longevity of population benefits from vaccination will be limited by replacement disease due to pathogens not covered by vaccines. Additionally, there is a significant burden of ALRI in the first 6 months of life, during the time that immunisations are being given and before fully protective immunity is achieved; and so complementary strategies to reduce ALRIs are important even in the era of new-generation conjugate vaccines.
Summary
Despite many reports on HAP and respiratory infections, there are numerous knowledge gaps and concern about the quality of available data. Considerable progress could be made by adding household and personal measures of exposure to HAP and tobacco smoke and improved diagnosis of respiratory infections to epidemiological studies, particularly if useful biomarkers or surrogate markers were also available.149 Future vaccine trials or effectiveness studies should include measures of exposure to HAP and tobacco smoke and effects on bacterial and viral carriage in the nasopharynx.
Since HAP affects mainly children and adults living in poverty, the additional risk factors of undernutrition, co-infection, and poor growth (all highly prevalent in low-resource settings) augment the effects of HAP. In populations for which HAP cannot readily be eliminated by provision of reliable electricity, combating its effects will need a multipronged approach that addresses each contributor to the causal pathway of HAP-related respiratory infection. Reduction of ALRI or pneumonia is a global priority,150 and will need the study of multimodal and environmental interventions and investment of appropriate resources. Furthermore, respiratory infections and HAP exposure both contribute to risk for development and exacerbation of chronic lung diseases. Thus, reductions in the risk of respiratory infections from HAP might also positively impact on efforts to reduce the risk of death and disability from chronic lung disorders.
Obstructive lung disease
Chronic obstructive pulmonary disease and asthma
Chronic obstructive pulmonary disease (COPD) and asthma are two of the most common chronic diseases worldwide;151 about 80 million people have COPD and 235 million people have asthma.152 In 2005, 3 million people died from COPD, making it the fifth leading cause of mortality. According to GBD 2012, COPD is now the third leading cause of death worldwide, something that WHO had not predicted to occur until 2030.153 COPD and asthma are major causes of morbidity due to persistent symptoms, reduced lung function, and intermittent exacerbations that adversely affect functional status and quality of life.
Although most (90%) people with COPD and asthma live and die in lower-income regions much of the research for these diseases has been done in high-income countries. International research programmes such as the Burden of Obstructive Lung Disease Initiative and the International Study of Asthma and Allergies in Childhood have made important steps towards addressing this knowledge gap, but some areas of the world, notably sub-Saharan Africa, have been substantially under-represented. Both disorders benefit from simple technologies such as spirometry for diagnosis, but these methods are frequently unavailable, unaffordable, or unreliable, forcing pragmatic, but probably, inaccurate diagnostic and management decision making.154
Good evidence is available that exposure to HAP is associated with an increased risk of developing COPD.155–158 Since tobacco is biomass and inhalation of the smoke from the combustion of tobacco is an established driver of COPD development, this association is unsurprising. Tobacco and biomass smoke are both generated from the combustion of plant material, which generates complex carbon-based particles coated with organic compounds such as polycyclic hydrocarbons and irritant gases such as formaldehyde and acrolein. Mechanistically, biomass smoke increases the expression of some of the same matrix metalloproteinases that are increased by tobacco smoke.159,160 The question is more whether there are differences in COPD phenotype and treatment responsiveness depending on the type of biomass exposure (tobacco vs other types) causing the disease rather than whether or not there is an association. The risk of individuals developing COPD from exposure to HAP seems to be about double that of those with no exposure and probably is between the risks of passive and active tobacco smoking. In communities who are heavily exposed to solid fuel smoke and have low rates of tobacco smoking, exposure to solid fuel smoke is probably the leading cause of COPD. Confirmation of this from high-quality studies that include exposure measurements of HAP is continuing. The health risks associated with COPD, whether due to tobacco smoking or solid fuel exposure, do not differ much, although there seem to be more prominent airway disease manifestations and less emphysema with COPD associated with solid fuel smoke versus that associated with tobacco smoke exposure.161–163 Whether this difference is due to the magnitude of exposure, to differential smoke components, or to genetic background is unknown. In terms of survival, a 7 year follow-up study showed that women with biomass-induced COPD have similar survival to men with tobacco-related COPD.164
Unlike the very strong evidence that smoking cessation has beneficial health effects and can reduce the rate of decline in forced expiratory volume in 1 s (FEV1) in patients with COPD, there is little evidence that cessation of HAP exposure does the same. Romieu and colleagues165 did a trial in Mexico to evaluate the effect of a chimney wood stove (patsari) intervention versus the traditional open fire stove on respiratory symptoms and lung function in 552 women. Although adherence to the intervention was poor (50%), use of the chimney stoves reduced respiratory symptoms (rate ratio 0·29, 95% CI 0·11–0·77; for wheeze) and declines in lung function (31 mL vs 62 mL over 1 year, p=0·01) over a 12 month period compared with those using the open fire. Smith-Sivertsen and colleagues166 did a study in Guatemala to explore the effect of another chimney wood stove (plancha) on pneumonia in young children and, as a secondary outcome, assessed the effects on respiratory symptoms and lung function in the mothers of the included children. Although they noted a reduction in respiratory symptoms (OR 0·7, 95% CI 0·50–0·97), there was no effect on lung function over 12–18 months of follow-up.166 Both of these studies were done in the context of an RCT, but have limitations of a relatively young cohort and short follow-up time. Investigators from China that followed participants for up to 9 years reported that using biogas instead of biomass for cooking reduced the annual decline of FEV1 by 12 mL per year and improved kitchen ventilation reduced the decline by 13 mL per year, compared with those who took up neither intervention.155 Although this study addresses the limitations of the Romieu and colleagues and Smith-Sivertsen and colleagues studies,165,166 patients were not randomly allocated to intervention groups and the prevalence of tobacco smoke exposure was high. Taken together, these studies give evidence that reductions in exposure to HAP will reduce chronic respiratory symptoms and likely the risk of COPD development and progression, but more robust evidence is needed from studies of appropriate patient groups (ideally middle-aged and older men and women with minimal exposure to tobacco smoke) over sufficiently long periods of follow-up.
Irritant smoke from solid fuel combustion is a potential trigger for asthma exacerbations, but not much evidence exists that the risk of asthma or exacerbations of pre-existing asthma is associated with exposure to HAP. For the association between solid fuel exposure and asthma, a 2011 systematic review and meta-analysis reported a pooled OR of 0·50 (95% CI 0·12–1·98) in children and 1·34 (0·93–1·93) in women.22 This review was limited by a small number of studies of sufficient quality and size to be included. The most robust evidence supporting an increased risk of asthma due to cooking with solid fuels was reported by the International Study of Asthma and Allergies in Childhood, which surveyed almost 513 000 children in 1999–2004.167 The sole use of an open fire for cooking (assessed by the questionnaire) was associated with an increased risk of wheeze in the past year in both young children (ages 6–7 years; OR 2·17, 95% CI 1·64–2·87), and in older children (ages 13–14 years; 1·35, 1·11–1·64).
Bronchiectasis
Some cylindrical bronchiectasis has been described in individuals with COPD due to tobacco smoke or smoke exposure from solid fuels.161 Severe cystic bronchiectasis is uncommon in women exposed to solid fuel smoke and no evidence is available either to link its presence to HAP exposure or that would indicate whether these exposures affect the development or clinical course of the disorder. Nevertheless, chronic cough is a common problem in populations in LMICs in which HAP exposures are high and the underlying causative factors and pathology of chronic cough in these settings have not been characterised because CT scanning and epidemiological studies are not available. Bronchiectasis, or a syndrome that could perhaps more pragmatically be described as complex airways disease, which overlaps with other diagnoses such as chronic bronchitis, is a probable contributor to this burden of chronic cough, as has been shown in indigenous children in developed countries.168,169 This disorder is likely to be the result of many respiratory insults across the lifespan including in-utero and early life malnutrition, predisposition to and repeated episodes of acute lower respiratory tract infections, poor access to prompt effective treatment for ALRI, HIV infection, and complications after pulmonary tuberculosis. Many of these insults are linked to poverty in the same way that exposure to HAP is and so teasing out the relative contributions of these different factors will be challenging. It seems most likely that these other factors will dominate the picture, with HAP acting as a cofactor that increases the risk of ALRI.
Diagnosis and management of HAP-induced obstructive lung disease
Diagnostic facilities for obstructive lung disease in LMICs are poor154 and so diagnoses are often made on the basis of clinical features alone, which is a distinct limitation for a set of diseases that is so dependent on the quantifiable assessment of airways obstruction. Use of peak flow meters (Burden of Obstructive Lung Disease Initiative, PLATINO study) or simplified spirometers could improve screening for COPD because their low cost would allow rollout in developing countries. Interventions to reduce HAP exposure are discussed in the section on interventions, although these might have an insufficient effect on exposures to reduce health effects of HAP-induced lung disease. There is a small evidence base overall, particularly in relation to the effect of HAP reduction on decline in lung function.156
Poor access to basic effective treatments for obstructive lung diseases in settings where exposure to HAP (and therefore poverty) is common is the major factor that limits the management of HAP-induced obstructive lung disease. The lack of data for the efficacy of these treatments (particularly for non-smoking related COPD) is another limitation, although it is probably reasonable to extrapolate largely from the studies published in tobacco (ie, nicotine-containing biomass). Improved diagnosis, appropriate treatment, and the prevention of underlying causes will all be needed to control HAP-related obstructive lung diseases, but these have not yet been identified as priorities in most LMICs.
Research recommendations
Accurate burden of disease estimates for asthma, COPD, and bronchiectasis in populations exposed to HAP are needed, with careful characterisation of exposures, disease outcomes, and an evaluation of the contribution of HAP to disease development and progression. Intervention studies to assess the efficacy and effectiveness of improved stove and ventilation interventions are also needed because they are able to separate exposure from poverty—a severe limitation of many observational studies on health effects of indoor pollution. To achieve these research objectives, there needs to be investment in research capacity building and development of clinical and health systems capacity. The American Thoracic Society and Pan African Thoracic Society Methods in Epidemiologic, Clinical and Operations Research (MECOR) has been leading the way in this regard.
Summary
Obstructive lung diseases are major global health problems that cause substantial morbidity and mortality. Strong evidence links HAP to the risk of COPD, but less conclusive evidence exists in relation to the risk of asthma and bronchiectasis. On the basis of the published work about tobacco, HAP probably has a role in asthma development and increases the likelihood of exacerbations of COPD and asthma. There are important gaps in the evidence base that links exposures, their health effects, and which interventions will make a clinically relevant impact. WHO have identified reductions in these exposures as a priority, but poverty impedes the use of cleaner fuels and clean-burning cookstoves and there remains uncertainty about how clean the air in the cooking environment needs to be for health risks to be reduced. Research and health system capacity building is needed to fill these gaps and for effective health care to be offered to those with chronic respiratory symptoms in LMICs.
Lung cancer and upper airway cancers
Introduction
Cancer is a major and growing global public health problem that is not only present in high-income countries. Although estimates suggest that overall death rates from cancer are higher in the high-income countries,153 with their decline in infant and child mortality and the spread of tobacco use, the cancer burden in LMICs will increase. It is projected that by 2030, there will be an 81–100% increase in cancer incidence in LMICs compared with 2008.170 Apart from tobacco use (active or passive), HAP poses a related major threat to health in LMICs.171 The total proportion of households using solid fuels is decreasing continuously from 62% in 1980, to 53% in 1990, to 46% in 2005, and to the latest estimate of 41% in 2010, but the absolute number of people at risk has remained stable for the past 3 decades at about 2·8 billion people.171 Solid fuels are usually burnt in stoves with very low energy conversion efficiency because partial combustion often takes place, leading to the production of carbon (the particulate fraction of smoke) and a range of toxic inorganic and organic compounds such as carbon monoxide, polycyclic aromatic hydrocarbons, aldehydes, and free radicals. Long-term exposure to HAP has been associated with increased risks of lung cancer and other cancers (table 3).12,21,156,172–174 In this section, we focus on cancers of the respiratory system.
Table 3. Compounds present in emissions from combustion of wood or coal.
| Assessment of carcinogenicity |
Source of smoke | Available evidence for types of wood or coal | |||
|---|---|---|---|---|---|
| Animals | Human beings | Group* | |||
| Polycyclic aromatic hydrocarbons | |||||
| Benz(a)anthracene | Sufficient | Inadequate | 2B | Wood and coal | Hardwood, Petocarpus indicus, eucalyptus chip, oak, firewood (not specified), coal briquette |
| Benzo(b)fluoranthene | Sufficient | Inadequate | 2B | Coal | ·· |
| Benzo(k)fluoranthene | Sufficient | Inadequate | 2B | Coal | ·· |
| Benzo(a)pyrene | Sufficient | Inadequate | 1 | Wood and coal | Hardwood, Petocarpus indicus, eucalyptus chip, oak, firewood (not specified), coal briquette |
| Dibenzo(a,h)anthracene | Sufficient | Inadequate | 2A | Wood and coal | Hardwood, Petocarpus indicus, eucalyptus chip, oak, firewood (not specified), coal briquette |
| Chrysene | Sufficient | Inadequate | 2B | Coal | ·· |
| Cyclopenta(c,d)pyrene | Sufficient | Inadequate | 2A | Coal | ·· |
| Indeno(1,2,3-c,d)pyrene | Sufficient | Inadequate | 2B | Coal | ·· |
| Naphthalene | Sufficient | Inadequate | 2B | Wood and coal | Hardwood, Petocarpus indicus, eucalyptus chip, oak, firewood (not specified), coal briquette |
| Volatile organic compounds | |||||
| Acetaldehyde | Sufficient | Inadequate | 2B | Wood and coal | Hardwood, firewood (not specified), coal (three types) |
| Benzene | Sufficient | Sufficient | 1 | Wood and coal | Hardwood, firewood (not specified), coal (four types) |
| 1,3-Butadiene | Sufficient | Little | 2A | Wood and coal | Hardwood, firewood (not specified), coal (four types) |
| Formaldehyde | Sufficient | Sufficient | 1 | Wood and coal | Hardwood, firewood (not specified), coal (three types) |
| Styrene | Limited | Inadequate | 2B | Wood and coal | Hardwood, firewood (not specified), coal (four types) |
| Metal and metal compounds | |||||
| Arsenic | Sufficient | Sufficient | 1 | Wood and coal | ·· |
| Nickel | Sufficient | Sufficient | 1 | Wood and coal | ·· |
International Agency for Research on Cancer carcinogenicity group: 2A=probably carcinogenic to human beings; 2B=possibly carcinogenic to human beings; 1=carcinogenic to human beings. Adapted with permission from Hosgood and colleagues.172
Indoor burning of coal and wood as important carcinogens
The International Agency for Research in Cancer has classified emissions from burning coal as known (group 1) carcinogens and those from solid fuels as probable (group 2A) carcinogens.175 WHO’s Global Comparative Risk Assessment Project estimated that, in 2000, about 200 million people used coal for household cooking in east Asia (most from China)176 and about 25 million from south Asia.177 The proportion of people using solid fuel in China has decreased significantly from 64% in 1990, to 46% in 2010.171 However, in rural China, about two-thirds of people still use solid fuels, particularly coal, as their main source of energy for cooking and heating.172,178 In China, people use mainly two types of coal, predominantly smoky coal (bituminous coal), but also non-smoky or smokeless coal (anthracite). Anthracite is low in sulphur but high in carbon compared with bituminous coal.172 Use of solid fuel, particularly wood, is more common in south Asian countries (particularly India, Pakistan, Bangladesh, and Nepal) and sub-Saharan African countries. India uses about 30% of the total solid fuel worldwide with huge differences among states–eg, 85% of households in Odisha rely on solid fuel compared with 40% in Punjab.179 It is plausible that people who are exposed to higher doses of smoke exposure from coal or wood burning for prolonged duration are at greater risk of developing cancer than are those using other fuels.178
Carcinogenic constituents of coal and wood smoke
About 8–10% of solid fuels undergo partial combustion during cooking depending on the type of cooking stoves and supply of oxygen.180 Incomplete combustion of wood and coal releases large amounts of inorganic compounds and inorganic and organic hydrocarbons into the air, along with metals and non-metals. The individual components of emissions released in the atmosphere after combustion depend on several factors such as types and subtypes of fuels (coal vs types of solid fuel; types of wood, etc), types of stoves used for burning the fuels (improved stoves or traditional stoves), and the burn rate (smouldering will produce more emission products compared with a hot fire). There is a major overlap of emission products from incomplete combustion of coal and wood.175 Several studies have reported differences in types of coal on the basis of their geographical location (where mined) in China and also differences in the types of emission products such as the volatility levels of benzene and formaldehyde, which vary the carcinogenicity.172,178,181 For example, smoky coal is predominantly used in the southern regions of China and tends to have increased polycyclic aromatic hydrocarbons, silica, nickel, and arsenic contents and thus has higher carcinogenic potential than do different types of coal such as anthracite.172
Exposure of toxicity to coal and wood smoke in animal studies
Most toxicity studies on coal smoke in China are recent but toxicity has been studied in experimental animal models for some years.182,183 This toxicity of coal smoke has been studied in animals (predominantly rats and mice) by exposing them in four different ways: inhalation and whole-body exposure, intra-tracheal administration, dermal exposure, and subcutaneous injection.175 The studies report a higher incidence of adenocarcinoma in mice and squamous-cell carcinoma in rats with clear dose–response relationships, irrespective of the methods of exposure.182–188 Compared with studies on coal smoke, only a few studies have assessed the toxicity of exposure to wood smoke in animals and these studies have generally failed to find any positive association,185,187,189–194 except in one study, which reported that extracts of smoke from softwood were more tumorigenic than were those of smoke from hardwood.195,196
Mechanism of carcinogenesis for coal and wood smoke
The main components released from solid fuel because of incomplete combustion that are thought to have a role in the mechanism of carcinogenesis are polycyclic aromatic hydrocarbons with inhalable particles, volatile organic compounds, and some metals. Insoluble particles deposited in the extra-thoracic or trachea-thoracic regions are cleared, either by exhalation or mucociliary clearance; those in alveolar regions can potentially undergo a cascade of events leading to tumour formation after uptake of particles by phagocytes and other cells. Particles deposited at crucial target cells or tissues of the lung might initiate a number of biological processes such as sustained inflammation, cell injury, cell proliferation, depletion of antioxidants or impairment of other defence mechanisms, production of reactive oxygen species, and gene mutation.175
Most of the evidence for the association between lung cancer and coal comes from studies of polycyclic aromatic hydrocarbons, particularly benzo(a)pyrene present in the emission products of coal or cigarette smoke. Polycyclic aromatic hydrocarbons absorbed through the respiratory tract, gastrointestinal tract, and skin are widely distributed to most organs and tissues where they are metabolised rapidly to release several soluble metabolites such as epoxides, phenols, dihydrodiols, phenol dihydrodiol epoxides, quinines, and tetrols. A working group of the International Agency for Research in Cancer, established to explain the carcinogenesis of polycyclic aromatic hydrocarbons, has proposed two major mechanisms—mono-oxygenation to yield diol-epoxides and one-electron oxidation to form radical cations.175,197 In the diol-epoxides mechanism, the epoxide reactive intermediate binds with DNA to form stable and unstable adducts at adenine and guanine sites, leading to mutations in proto-oncogenes (ras genes) and tumour-suppressor genes (TP53), resulting in tumour formation. In the radical cation mechanism, the cation acts as a reactive intermediate to bind with DNA to generate unstable adducts at adenine and guanine sites, leading to apurinic sites and mutations in HRAS gene.198,199 Polycyclic aromatic hydrocarbons such as 5-methyl-chrysene, and benzo(c)phenanthrene are activated to exclusively diol-epoxides intermediates whereas benzo(a)pyrene, dibenzo(a,l)pyrene, 7,12-dimethyl-benz(a)anthracene and 3-methylcholanthrene, are activated by formation of diol epoxides and radical cations.175,197
Lung cancer
Lung cancer causes more deaths worldwide than any other cancer, with 1·8 million new cases and 1·5 million deaths in 2012.200 It is the most diagnosed cancer in men and third most common in women after breast and colorectal cancers.200 There is also substantial geographical variation in the incidence of lung cancer. Age-standardised incidence is higher in high-income countries (30·8 per 100 000 person-years) compared with low-income countries (20·0 per 100 000), although individual middle-income countries, such as China (36·1 per 100 000), have high incidence rates.200 However, it should be noted that the disparity in health-care provisions and quality in LMICs, among many other factors, might have contributed to underdiagnosis or misdiagnosis of lung cancer, which could potentially lead to substantial underestimation of the cancer burden in LMICs.
Tobacco smoking is a major cause of lung cancer, accounting for most cases of lung cancer in high-income countries such as the USA and UK. In many LMICs, where the epidemic in tobacco use has only recently begun and use of solid fuels is widespread, the situation is quite different. In LMICs, emissions from solid fuel combustion were estimated to account for about 17% of all lung cancer deaths in men and 22% in women.24 This might partly explain the high proportion of non-smoking women with lung cancer in east and south Asia (83% of all cases) compared with 15% in the USA (three times higher lung cancer mortality in Chinese non-smoking women than in US non-smoking women).176,201–203
Several studies, mostly case-control studies, have examined the relationship between household emissions and lung cancer risk. Most studies on coal burning were done in China where coal is commonly used for cooking and heating. A meta-analysis identified 28 case-control studies (17 from China, three from Taiwan, two from India, and one study each from Japan, Mexico, USA, Canada, and Europe) investigating solid fuel use in patients with lung cancer.172 Individuals exposed to coal smoke had a greater risk of lung cancer (pooled OR 1·82, 95% CI 1·60–2·06) compared with those exposed to wood (1·50, 1·17–1·94) and mixed solid fuels (1·13, 0·52–2·46).
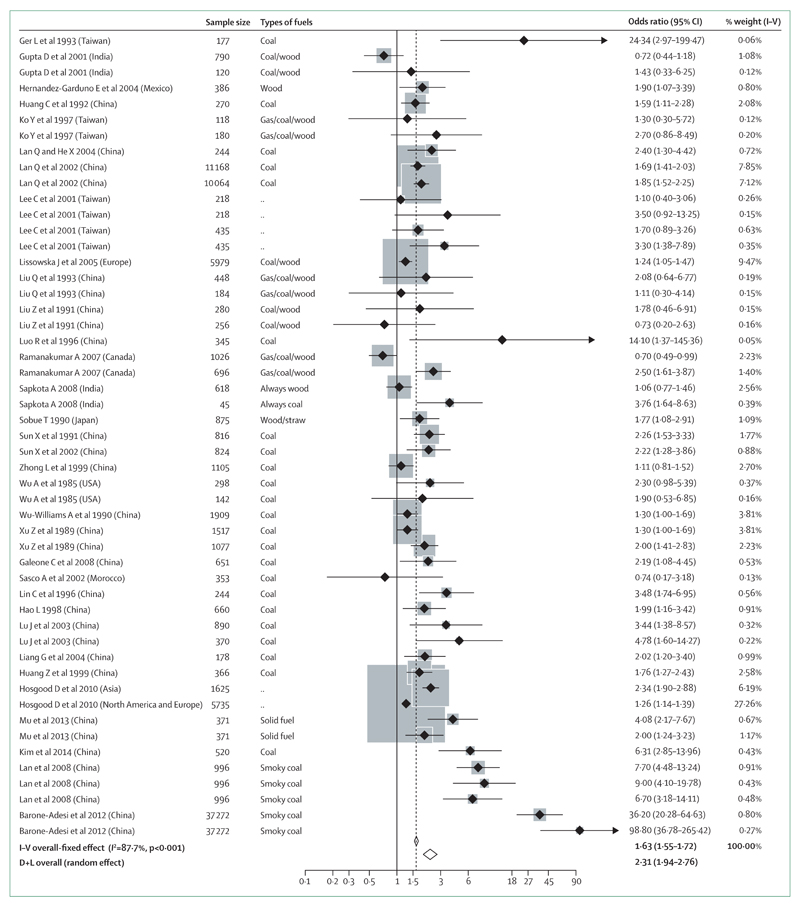
The effects of exposure to HAP on lung cancer also tended to differ by histological subtype. In eight studies in which the lung cancer histological subtype was available, the pooled effect size for HAP exposure was greater, although not statistically significantly, for squamous-cell carcinoma (OR 3·58, 95% CI 1·58–8·12) compared with for adenocarcinoma (2·33, 1·72–3·17). However, findings of the recent European Study of Cohorts for Air Pollution Effects (ESCAPE) study204 have shown adenocarcinoma (HR 1·51, 1·10–2·08), but not squamous-cell carcinoma (0·84, 0·50–1·40), to be significantly associated with ambient particulate air pollution, particularly PM10. Although most studies on HAP adjusted for tobacco use or used a non-smoking sample, only half of the studies included in the meta-analysis collected information on passive smoking exposure, and of those with such data, only 25% of the studies presented data with adjustment for passive smoking. The pooled effect estimate for exposure to HAP with adjustment for passive smoking (OR 1·47, 95% CI 1·13–1·91) was lower than the effect without any adjustment (1·74, 1·60–1·89), suggesting residual confounding was probably present in most of the studies. The quality of exposure assessment was not without concern. Most studies relied on questionnaires that were often based on surrogates (such as frequency of cooking or whether kitchen was ventilated) or the use of a specific type of fuel (such as whether an individual has ever used coal or solid fuel) rather than on direct exposure measurement. The duration of exposure in most of the studies was also not clearly defined. As a result, it is difficult to derive exposure–response risk for lung cancer caused by solid fuel use. When this meta-analysis was updated recently,173 the new pooled effect estimate (figure 6) increased to 2·31 (1·94–2·76) with significant heterogeneity in effect sizes (I2=87·7%) across different studies and major publication bias (coefficient=1·91, p<0·001). Very few cohort studies have assessed the effects of exposure to HAP on lung cancer. A large retrospective cohort study followed up more than 20 000 residents from Xuanwei county in south-western China for 20 years (1976–96) and compared lung cancer mortality between lifelong users of either bituminous coal and anthracite.205 The study showed that bituminous coal increased lung cancer mortality by 36-fold in men and 99-fold in women compared with anthracite coal users. This suggests that the carcinogenicity of different types of coal could vary significantly. Together with the findings from other studies, there is now sufficient evidence to suggest an association between HAP from household fuel combustion and lung cancer.173
Figure 6. Forest plot of studies to assess the link between lung cancer and exposure to household air pollution173.
Upper airway cancers
Upper airway cancers are less common than lung cancer, but the burden is significantly higher in LMICs, where about 70% of all cases are diagnosed worldwide.200 Worldwide, the International Agency for Research on Cancer estimated 386 000 new cases of, and 230 000 deaths from, upper airway cancers in 2012.200 Tumours of the larynx and nasopharynx were the most common types, accounting for 63% of all incident cases and 58% of deaths. According to the GBD 2010, between 1990 and 2010, the incidence of larynx cancer increased by 20% and nasopharynx cancer increased by 44%.153 The use of tobacco and alcohol are reported as the main risk factors for upper airway cancers.206,207 Genetic susceptibility208 and infections209 have also been implicated, perhaps explaining the remarkable geographical variation of upper airway cancers, particularly nasopharyngeal carcinoma. Other relevant factors include diet and nutrition,210 and exposure to certain chemicals in the workplace (eg, asbestos211 and wood dust212).
Nasopharyngeal cancer has poor survival because it is usually not detected until at an advanced stage.213 Most cases are squamous-cell carcinoma arising in the epithelial lining of the nasopharynx. Tobacco use, diet, and Epstein– Barr virus infection are the purported risk factors of nasopharyngeal cancer,213 but only a few studies (mainly done in China in the 1990s or earlier) have examined the possible link with the burning of coal or wood for domestic purposes. A 2005 meta-analysis by Han and colleagues214 based on published Chinese studies reported six risk factors: family history of nasopharyngeal cancer, consumption of pickled or cured food, smoky household or HAP, lack of consumption of fresh fruit and vegetables, previous diseases of the nasopharynx, and tobacco smoking. For exposure to HAP, which included seven case-control studies, the pooled OR estimate was 1·27 (95% CI 1·11–1·46).214 A 2014 systematic review24 of HAP and nasopharyngeal cancer included case-control studies done in China and elsewhere in Asia (India, Hong Kong, Singapore, and Malaysia). We did a meta-analysis by combining studies from the two previous reports,24,214 plus three additional studies215–217 that we retrieved through cross-referencing. Most of the studies were hospital-based with small sample sizes (roughly 100 cases). The level of heterogeneity in the exposure measure was high—all studies relied on self-reported ever (or the number of years of) use of solid fuel or the presence (or intensity) of smoke during cooking or heating. The pooled random effects OR derived from studies that had adjusted for smoking (n=6; OR 3·18; 95% CI 2·36–4·30) was much greater than for those without adjustment for smoking (n=5; 1·12; 1·02–1·23; figure 7). When all 11 studies were combined, the overall OR was 1·70 (1·45–2·00), which was greater than either previous meta-analysis.24,214 However, caution must be taken when interpreting the pooled estimates. Most of the included Chinese studies tended to show a statistically significant association, with an OR greater than 2, whereas a non-significant association with an OR closer to 1 was noted in two studies (one from India218 and the other from north Africa219). In summary, although there is some evidence to suggest household solid fuel use might be associated with increased risk of nasopharyngeal cancer, future research should include better measures of exposure and of confounding factors.
Figure 7. Forest plot of studies to assess the link between nasopharyngeal cancer and exposure to household air pollution stratified by adjustment for tobacco smoking status.
Al/Mo/Tu=North Africa (Algeria, Morocco, Tunisia).
Interaction with tobacco smoking
Benzo(a)pyrene and polycyclic aromatic hydrocarbons are two of the major genotoxic components of tobacco smoke and smoke from burning biomass and solid fuels. Given the similarity of the route of entry to the lungs (ie, via inhalation), and the similarities in the associated pathophysiological changes, it is impossible to distinguish between lung and upper airway cancers caused by emissions from burning solid fuels and those due to smoking in people exposed to both risks. The carcinogenic effects of household indoor emissions are relatively weak compared with those of tobacco smoking and so the signal from HAP might be masked in populations with a high prevalence of smokers.2 In many LMICs, where smoking rates are only now starting to rise and where a substantial proportion of women do not smoke,219 the risk assessment for cancer due to household indoor emissions can be made in many cases without the confounding effect of tobacco use. For this reason, a number of studies have restricted or stratified their study populations to either non-smoking women or women in LMICs.173
There are some epidemiological data to suggest a different pattern of lung cancer risk in users of solid fuels in relation to tobacco smoking compared with nonsolid-fuel users. People who smoke tobacco might be more susceptible than are non-smokers to lung cancer when exposed to smoke from burning solid fuels. For example, a large pooled analysis of seven studies from the Lung Cancer Consortium (5105 cases and 6535 controls) reported a larger lung cancer risk for those who use wood as fuel and who have smoked (OR 1·22, 95% CI 1·05–1·42) than for people who have never smoked (1·01, 0·74–1·37).220 A case-control study221 in Singapore examined the risk of lung cancer in Chinese women (703 cases and 1578 controls) who used wood-burning or charcoal-burning stoves.221 Although the authors reported an increased lung cancer risk in current or ex-smokers who cooked every day compared with those who cooked less than daily (1·61, 1·01–2·56), no excess risk was found in non-smokers (0·89, 0·68–1·16). A similar pattern was seen in people who used charcoal or wood stoves every day, although unlike many previous studies, the relationship was not statistically significant in either smokers (1·25, 0·74–2·12) or non-smokers (0·81, 0·56–1·17). Tang and colleagues221 did not show evidence of interaction between tobacco smoking and daily use of either charcoal or wood stoves. These mixed results suggest a possible synergism between tobacco smoke and household indoor emissions and more research is needed.
Such synergism has not always been noted in other health effects of HAP. A study in China investigated the risk of cardiovascular disease in residents in Shanghai and reported that the association between household solid fuel use and hypertension (OR 1·84, 95% CI 1·45–2·33) and coronary heart disease (3·65, 1·85–7·22) was stronger for never smokers (1·39, 0·99–1·94) than for previous or current smokers (1·10, 0·49–2·47).222 The authors postulated a pressure effect, in which a stronger environmental-pollutants–cardiovascular association is seen among never smokers compared with current smokers,223 although it is possible that smokers exposed to HAP might have had a higher mortality, leading to non-recruitment and a failure of the cross-sectional study design.
Other than tobacco smoking, studies from Taiwan and Hong Kong have suggested that cooking emissions from oils, particularly in wok cooking, might also increase the risk of lung cancer in women.224–226 The stir frying process of cooking can aerosolise toxic and carcinogenic products from hot oils and food ingredients, resulting in emissions of PM,227 volatile organic compounds,228 and other organic compounds, including polycyclic aromatic hydrocarbons229 and heterocyclic amines.230 Thus, the process of cooking in south Asia might also increase cancer risk, in addition to the emissions from solid fuels. The interaction between emissions from solid fuel burning and cooking methods should be investigated further in future studies of cancer risk.
Summary
There is strong evidence for a causal relationship between exposure to coal smoke and lung cancer, but the association between different types of solid fuel smoke and lung cancer shows probable but not conclusive association. Most studies on solid fuels are based only on exposure to wood smoke and there should be other studies using other types of solid fuel smoke to assess their association with lung cancer risk.
Exposure and biomarkers
Introduction
The previous sections provide some evidence to link HAP exposure and risks of acute and chronic respiratory diseases. However, reduction of these risks and, ultimately, prevention of respiratory diseases, needs a clear understanding of exposure assessment to inform the most effective intervention strategies (see section on interventions). HAP, although mostly the result of domestic cooking, heating, and lighting, both indoors and outdoors, also includes a contribution from ambient outdoor air pollution and smoke from tobacco or other habitual smoking. Exposed individuals vary in their behaviour, respiratory volumes, and vulnerability to inhaled pollutants. Therefore, measurement of exposure and biomarkers is complex, both for sample acquisition and data interpretation.
Complexity of exposure
The movement of pollutants between indoors and outdoors is governed by house air exchange rates. In high-income countries, most concern is focused on the movement of pollutants from outdoors, where pollution concentrations are typically higher, to indoors (infiltration) to apportion exposures between indoor and outdoor sources.231–235 However, in LMICs, burning of solid fuel creates extremely high concentrations of indoor pollutants such that exfiltration can result in homes being a large contributor to outdoor air pollution. This contribution is especially problematic in larger cities where a substantial fraction of homes might use solid fuels for heating or cooking. As a result, homes that do not burn solid fuels might still have high burdens of indoor pollution due to infiltration of pollution from neighbouring solid-fuel-burning homes.
Salje and colleagues236 documented high indoor PM pollution in non-solid-fuel burning homes in Dhaka, Bangladesh. By examining long-term and short-term temporal patterns of indoor PM pollution, the investigators noted that non-solid-fuel burning homes had indoor pollution concentrations well above WHO guidelines and that pollution peaked during cooking times. These findings strongly suggest that PM exfiltration from solid-fuel-burning homes created high ambient PM concentrations that substantially affected non-solid-fuel-burning homes. These results have large implications for the design of intervention efforts. Intervention projects to promote improved cookstoves that target individual households might not observe expected reductions in PM exposures due to the pollution from neighbours who continue to burn solid fuels. Conversely, interventions that target all the solid-fuel-burning homes in the community can potentially affect both individual targeted households and households using clean fuels. Furthermore, stove interventions that include a chimney to vent the combustion products outside directly can exacerbate ambient air pollution, resulting in an increased exposure risk to the wider population. The authors of the RESPIRE interventional trial6 noted this point and concluded that their observed small reduction in exposure might have been due to the fact that chimney stoves vent smoke to the outdoors, some of which re-enters the homes and generally contaminates both outdoor and indoor environments where children spend their time.
Second-hand tobacco smoke continues to be a source of HAP and, subsequent health risk in LMICs. Although tobacco use is declining in high-income countries, it is increasing in LMICs, which contain nearly 80% of the more than 1 billion people who smoke worldwide.207 The Global Adult Tobacco Survey237 data showed that smoking rates were high in most LMICs and were highest in Asian countries (54·9% in Bangladesh, 67·3% in China, 40·0% in India, 54·4% in the Philippines, 33·2% in Thailand, and 73·1% in Vietnam). Smoking in the home and other indoor environments is an established source of HAP, including PM, nicotine, carbon monoxide, benzene, tobacco-specific nitrosamines, and many other toxic compounds.238 Established methods to assess exposure to second-hand smoke have been developed239 and have been used extensively to assess exposure in public places in LMICs, but few studies have included the home environment. Wipfli and colleagues240 did a 2008 in-home exposure study in 31 countries, including some in Asia and Latin America, and showed that smoking at home remains a major source of second-hand smoke exposure in non-smoking adults and children worldwide. Analysis of nicotine in hair identified non-smoking women and children in LMICs as being at the greatest risk of in-home health effects related to second-hand smoke.241 Although there are few data for in-home second-hand smoke exposure from LMICs, smoking has been identified as an important source of indoor PM in high-income countries, where homes of people who smoke have been found to have a two-to-three-fold increase in PM compared with homes without a smoker.242,243
Exposure assessment
Most research on air pollution has focused on the health effects linked to exposure to air pollution outdoors in high-income countries, with much effort placed on measuring of concentrations of pollutants at fixed sites for regulatory purposes. Although ambient levels of pollution clearly affect the exposure of an individual over the course of the day as they move from one microenvironment to another,244 evidence suggests that, in LMICs, exposure to emissions from indoor sources such as stoves and lamps probably dominates the total daily intake for many pollutant types.245 It is probably unhelpful and unrealistic to consider exposure within defined silos of the community, the home, and the workplace, but instead investigators should take a broader approach exemplified by the exposome in which the totality of exposure is considered.246 For this reason, personal exposure assessment is likely to be key to make the link between air pollution and respiratory ill health. Incomplete combustion of carbon-based fuel material produces two main pollutants that are likely to contribute most to mortality and morbidity. These are fine PM and carbon monoxide. Use of plastics, diesel, and other materials to assist with lighting stoves is common and these materials might produce many other copollutants including endocrine-disrupting chemicals, heavy metals, polyaromatic hydrocarbons, and endotoxin, depending on the accelerant used and fuel storage or contamination.67,247,248
Epidemiological evidence for cardiopulmonary health effects suggests that increase in exposure to fine PM produces an increase in risk for many adverse outcomes.156,249–251 There are four main issues that present particular problems for investigators trying to generate comparable exposure data for indoor PM and health in LMICs. These can be broadly defined as equipment size or noise, battery life, air sample averaging times (eg, 24 h), and differences in exposure metric. The first three of these are interlinked, with short averaging times being a result of using pump-based methods with equipment designed for shift-work (8 h) use or with a mains-electricity supply (typically unreliable in LMICs). Data for household PM concentrations measured from 0800 h to 1600 h might markedly underestimate daily exposure by not capturing cooking events that take place in the early morning or evening, with broader questions about the comparability of similar-duration samples collected at differing times of the day or night. The size and noise generated from these devices also makes them difficult to use as personal samplers within home settings. Given the heterogeneity of pollutant concentrations in homes, fixed-site monitoring can lead to exposure misclassification depending on the placement of the device in relation to the person under study. A device placed on a living room shelf can underestimate exposure for a woman who spends a lot of time tending a stove, while a device placed in the kitchen near the stove will generally overestimate the women’s exposure in this setting. The fourth problem refers to the metric used to quantify exposure to PM. Methods to measure PM concentrations have developed either from occupational health (which uses size-selective techniques to sample respirable or inhalable PM of workers involved in extractive industries)252 or from environmental sciences (which use PM2·5 and PM10 as markers of ambient air pollution levels to compare to national and international standards75,253). Research on exposure to solid fuel smoke in LMICs has used a mixture of these differing methods with data often being difficult to compare because of poor calibration or placement of instruments.254 This problem goes further in that studies tend to report an exposure averaged over the sampling time, yet the health effect of interest might be linked to peaks of exposure or the amount of time that people are exposed to concentrations higher than a certain biological threshold. For example, Gurley and colleagues254 identified a health risk associated with amount of time exposed to PM2·5 concentrations higher than 100 μg/m3.
Carbon monoxide is simpler to measure and avoids many of the size-selection and battery-life issues and problems with averaging time.255 New low-cost devices have the added advantage of being lightweight, noiseless, and are much more amenable to being worn by study participants, and can therefore provide personal exposure data. Although carbon monoxide concentrations might be of interest to avoid acute effects of exposure to solid fuel smoke, relating this carbon monoxide exposure to respiratory effects, which are most likely to be produced by PM, is difficult (figure 8). To use carbon monoxide as a marker for PM exposure is difficult and depends on investigators establishing a valid relationship between the two measures—something that might differ between populations, fuel types, stove and fire-lighting behaviours, and many other variables.257 Portable measurement of carboxyhaemoglobin using finger-clip devices and other biomarkers offers another way to triangulate these data.
Figure 8. Exposure monitoring is essential to properly assess the effect of an intervention.
(A) A simple clay stove implemented in community studies to reduce fuel consumption and hence improve livelihoods for women. (B) The effect of stacking (use of several different energy sources in cooking) on air monitored in four households before (left) and after (right) the introduction of a simple clay stove to reduce fuel consumption. Households have used both the stove and fire, resulting in increased household air pollution being measured after the intervention. Reprinted with permission of the International Union Against Tuberculosis and Lung Disease.256
Biomarkers of exposure
Personal monitors for PM are expensive and their bulk and need for recharging means that they can be used only for short-term exposures. Furthermore, external monitoring does not capture differences in lung deposition. A putative direct biomarker of the dose of chronic particulate inhalation can be the capacity of lower airway macrophages to phagocytose and retain PM in a dose-dependent manner. Airway macrophages are obtained either non-invasively with use of sputum induction or by bronchoalveolar lavage, and the area of black material in airway macrophages (showing inhaled carbonaceous PM) is determined by image analysis. Most studies have assessed airway macrophage carbon in individuals exposed to fossil-fuel emissions.258–260 However, three studies have recruited individuals exposed to PM from HAP. First, Kulkarni and colleagues261 reported higher airway macrophage carbon in Ethiopian children and women exposed to solid fuel smoke PM compared with individuals in the UK exposed only to traffic smoke. Second, Fullerton and colleagues262 reported that, in healthy people in Malawi who attended for research bronchoscopy, those exposed to smoke from wood or wood and charcoal during cooking had a significantly higher airway macrophage carbon than had those with homes using electric cookers. Third, Kalappanavar and colleagues263 reported that a higher proportion of Indian children living in areas polluted by smoke from puffed rice units (which burn rice husks, wood, and agricultural residues) had airway macrophages that were heavily laden with carbon compared with children living in areas away from this industry (8·3% of children vs 0·7% of children), but fuels used in the home were not recorded in this study. The questions that remain to be addressed include the intraindividual variability of airway macrophage carbon, the contribution of PM peaks to airway macrophage carbon, and the association between airway macrophage carbon and health outcomes. Airway macrophage carbon is probably not a valid marker of internal dose in individuals with reduced airway macrophage phagocytic function associated with severe asthma259 and COPD.264 Since assessment of airway macrophage carbon is time consuming and therefore limited to relatively few patients, it will probably be most useful to determine the association between internal (by airway macrophage carbon) and external dose (by monitoring). When combined with personal monitoring, airway macrophage carbon might also validate findings from experimental inhalation and modelling data suggesting increased inhaled dose in children and women.
Some biomarkers have been measured in urine, and these can so far be classed into three main groups: hydroxylated polycyclic aromatic hydrocarbons (OH-PAHs), methoxyphenols, and levoglucosan. OH-PAHs are the most used and seem to show good responses in exposure settings relevant to residential combustion of biomass fuel in developing countries. However, confounding exposures might bias results since biomass smoke is not the only source of the parent compounds of these biomarkers. The other classes of biomarkers, methoxyphenols, and levoglucosan, are also not unique to biomass smoke and might be consumed in foods. Nevertheless, they might be more unique to biomass smoke, particularly that of wood, than are OH-PAHs.265–268 Migliaccio and colleagues269 reported that mean urinary levoglucosan, a major organic component of PM emitted from solid fuel combustion, was increased in children living in homes with woodstoves. However, the difference was not significant and further studies are needed to validate this biomarker in wood-smoke-exposed populations. Beyond OH-PAHs, methoxyphenols, and levoglucosan, there are a few other biomarkers that warrant mention. Since tobacco smoke is an important confounding variable, urinary cotinine is a useful marker of active and passive exposure. Urinary and salivary cotinine are non-invasive and validated markers of acute exposure.270 Disadvantages of urinary biomarkers including cotinine, OH-PAHs, methoxyphenols, and levoglucosan include the need for privacy during collection, difficulty in coordinating collection and storage in population-based or child studies, and a need to adjust for creatinine clearance.270 Variability in cotinine conversion factor is a disadvantage of urinary biomarkers specific to cotinine. Hair nicotine has been used as a biomarker of long-term smoking exposure.241 Carboxyhaemoglobin gives a stable measure of carbon monoxide exposure—HAP contains a variable carbon monoxide to PM ratio depending on fuel and combustion method. There is still a need to research and develop new biomarkers for exposure to biomass smoke. Ideally, these new biomarkers would be unique to biomass smoke and be relatively easy to measure. The development of such a biomarker faces many challenges, not least the fact that composition of biomass fuels varies and so the composition of biomass smoke is not consistent, even within the same fuel type such as wood.
Risks across the lifecycle
For the fetus, developmental processes are most vulnerable to air pollutants inhaled by the mother. The mechanisms whereby pollutants deposited in the maternal lung affect the fetus remain unclear, but strong epidemiological evidence from smoking271 and fossil fuel emissions272 supports associations between inhalation of indoor pollutants by the mother and changes in fetal development. In-utero exposure to PM increases the risk of preterm birth and can perhaps lead to low birthweight.273–275
Children are at increased risk from HAP owing to impaired lung growth and changed pulmonary physiology, particularly because children inhale more pollutants for the same external dose. Sturm276 recently modelled the complex interactions between deposition and breathing patterns (eg, infants have low tidal volumes and shallow breathing); compared with adults, an increased number of particles smaller than 10 μm in aerodynamic diameter were deposited in the larger airways of children and were therefore cleared more rapidly than are particles reaching more distal airways. However, particle dose per area of lung tissue reaches much higher concentrations in young children.276 There is epidemiological evidence of differing associations between air pollution and respiratory health for women and men, but meta-analysis of the effect of sex on the association between air pollution and respiratory health has been difficult. For example, in a systematic review, Clougherty277 concluded that the broad difference in exposure mixes, outcome, and analytical techniques precluded meta-analysis of findings. Despite differences between studies of cooking and housing conditions, type of fuel, ventilation, and the time taken for kitchen work, evidence suggests stronger effects in women.277 Major determinants of vulnerability of women to HAP are the social, economic, and political structures within society that lead to women being nearer to sources of HAP than are men. Indeed in LMICs, cooking is done mainly by women, who can spend up to 7 h per day by the fire. Thus, exposure to toxic components such as PM in some women is much higher than for many industrial workers in extremely polluted environments. Vulnerability to HAP is also determined by existing health disorders, which might differentially affect women. For example, anaemia interacts with air pollution to increase vulnerability to infection,278 and worldwide, anaemia affects 42% of pregnant women (it only affects 13% of men).279 Although the biological mechanisms for differential responses between men and women remain unclear, hormonal status might be important since Zeka and colleagues280 reported that premenopausal and postmenopausal women in the USA had a different level of association between PM and heart disease mortality. Other putative biological mechanisms include differences in PM deposition due to higher inspiratory flow rates in women281 and differences in epithelial responses. For example, in occupational settings, women report more skin disease than do men.282
Summary
Exposure measurements to determine the health risks associated with HAP are complex and related to age, gender and sex, role in the household and at work, and surrounding environment. Technology developed for occupational and environmental monitoring is being developed to measure individual exposures. Further work is needed to identify useful biomarkers of exposure, particularly for young children and women.
Interventions and proposed experimental approaches
Introduction
A wide range of interventions have the potential to reduce HAP and hence risk of respiratory and other disease (panel 1). Households generally use many devices and fuels to meet energy needs, and it is helpful to see interventions in terms of those technologies (stoves, lamps, etc), fuels, and policies, which can allow a transition towards more exclusive use of cleaner and more efficient energy. The concept of the energy ladder (figure 9) shows this transition, although it is important to keep in mind that multiple use is very common (if not the norm), and that households can move up or down the ladder depending on economic and other circumstances.1 In practice, a combination of all of the interventions listed in panel 1 is likely to be needed, together with supportive policy, which is discussed below.
Panel 1. Interventions to reduce exposure to household airway pollution.
Technologies
Improved solid fuel stoves, including conventional and advanced combustion designs using fans or gasification technology.
Fuels
These can be unprocessed or processed, including pellets for biomass, briquettes for coal, etc. Cleaner fuels include liquid petroleum gas, natural gas, biogas, electricity, solar lamps, and stoves. Kerosene, although a liquid fuel, might have large health risks, especially when used in simple stove and lamp devices.
Ventilation
Most importantly flues and chimneys, but other methods, including larger eaves spaces, can contribute.
Behaviour
User factors that affect how well and exclusively the cleaner technologies and fuels are used—eg, dryness of fuel.
Figure 9. The energy ladder.
Adapted from Rehfuess.1 © WHO. All rights reserved.
The goal for household energy interventions must be to deliver clean air that meets WHO air quality guidelines,283,284 by reducing emissions of health-damaging pollutants to low levels. There has been much debate about how low exposure needs to be to deliver substantive health benefits, given that the transition from the use of open fires in households that collect all or most of their fuel to near exclusive use of clean technologies and fuels incurring higher costs will be neither quick nor straightforward, particularly for low-income and rural homes.285 This issue is one of three key questions that should be considered during development of an intervention strategy, along with the performance of currently available interventions and the factors that can help to ensure adoption and sustained use (panel 2). We review available evidence that addresses these questions, including from recent work to model exposure and risk functions and from several systematic reviews. These considerations have been central to WHO’s development of new indoor air quality guidelines for household fuel combustion, due to be published later in 2014 (expected in October).286 An assessment of the evidence relating to these questions can also identify important gaps that new research will need to fill, and the methods that can provide the best interventions to reduce HAP exposure.
Panel 2. Key questions for intervention strategies.
How much does exposure need to change to substantially reduce risk, noting that this might vary by disease outcome?
What is known about the performance of various interventions, both in laboratory testing (ie, emissions testing in ideal circumstances) and in everyday use in homes?
What factors can help to ensure that households get the best performance and results from available interventions?
Integrated exposure–response relationships for health effects of HAP
Development
As reported in earlier sections of this Commission, most epidemiological studies of risks associated with solid fuel use do not have adequate data for exposure. Exposure–response findings have only been reported by Ezzati and Kammen7 for child and adult ALRI, and Smith and colleagues6 for child ALRI. Recent work carried out for the GBD 2010 study,2 which built on work by Pope and others,287–289 developed integrated exposure–response functions for five disease outcomes (panel 3) by modelling risk estimates for four sources of combustion-derived PM2·5, namely outdoor air pollution, second-hand smoking, HAP, and active smoking.290 These integrated exposure–response functions, or indeed other exposure-response evidence, are not currently available for all important respiratory health outcomes, including asthma, tuberculosis, or other cancers. But the integrated exposure–response relationship can provide important insights into the relationships between exposure and risk, which allow conclusions to be drawn on the expected effects of various interventions. Here, we consider these relationships for the three respiratory disorders for which integrated exposure–response data are available—child ALRI, lung cancer, and COPD. Burnett and colleagues290 provide full details of the methods, assumptions, and findings of this work.
Panel 3. Disease outcomes with integrated exposure–response relationships.
Child acute lower respiratory infections
Ischaemic heart disease
Stroke
Lung cancer
Chronic obstructive pulmonary disease
Child ALRI
Because young children do not smoke, data for integrated exposure–response relationships rely on studies of outdoor (ambient) air pollution, second-hand smoke, and HAP. This is the only exposure–response with datapoints based on direct HAP exposure measurement, although carbon monoxide was used as a proxy for PM2·5, with PM2·5 and CO in the kitchen in some studies being calculated using colocated measurements,291,292 and only available from one study (figure 10A).The shape of the integrated exposure–response model for child ALRI is relatively steep at lower levels of exposure and tends to flatten off around 300 μg/m3 of PM2·5.290 Thus, for example, a solid fuel (or any) intervention that reduces exposure in a child by 50% from 300 μg/m3 to 150 μg/m3 would result in only a small reduction in relative risk from around 2·9 (95% CI 2·0–3·8) to about 2·4(1·7–3·2). At lower levels of exposure, a similar percentage reduction would have a larger effect on risk, but it is not until exposure reaches a level at or below the WHO Intermediate Target One (IT-1) level of 35 μg/m3 of PM2·5 that risk is substantially reduced to a predicted relative risk of 1·3 (1·2–1·4).
Figure 10. Integrated exposure–response models for acute lower respiratory infection, lung cancer, and COPD.
Predicted values of integrated exposure–response model for acute lower respiratory infection incidence in infants (A), lung cancer mortality in adults (B), and COPD mortality in adults (C). Shaded boxes for COPD mortality and lung cancer mortality represent uncertainty (height) and exposure contrast (width) of RR for HAP estimates for men (smaller darker boxes) and women (larger lighter boxes). HAP=household air pollution. RR=relative risk. PM2·5=particulate matter smaller than 2·5 μm. COPD=chronic obstructive pulmonary disease. Adapted from Burnett and colleagues.290
Lung cancer
None of the epidemiological studies of solid fuel use and lung cancer included direct exposure measurement, and therefore these studies did not contribute datapoints directly to the integrated exposure–response model, which was based on studies of outdoor air pollution, second-hand smoke, and active smoking (figure 10B). The function shows a more or less linear relationship between log exposure and log risk, at least for levels higher than 50–100 μg/m3. For HAP, in the epidemiological studies contributing to the systematic reviews and meta-analyses used for the GBD 2010, the PM2·5 values for exposed and non-exposed groups were estimated to be 300 versus 70 μg/m3 for women, and 200 versus 45 μg/m3 for men, with pooled ORs of 1·81 (95% CI 1·07–3·06) for women and 1·26 (95% CI 1·04–1·52) for men.24,290 These values were consistent with the integrated exposure–response function based on the other sources of PM2·5. By contrast with the findings for child ALRI, the linear function for lung cancer implies a more proportionate relationship between the effect of an intervention on exposure and the resulting reduction in risk. A time lag of 10–20 years can be expected before such benefits are seen because of the long latent period for lung cancer.
COPD
As with lung cancer, epidemiological studies of solid fuel use and COPD do not have much exposure data, and the integrated exposure–response model is also based on outdoor atmospheric pollution, second-hand smoking, and active smoking risk estimates (figure 10C). For HAP, the systematic reviews and meta-analyses provided ORs of 2·70 (1·95–3·75) for women with exposure contrasts of 300 versus 100 μg/m3 and 1·90 (1·56–2·32) for men with exposure contrasts of 200 versus 65 μg/m3 PM2·5.290 Unlike for lung cancer, however, these risk estimates do not fit the integrated exposure–response model, being somewhat higher than would be predicted. The shape of the function is therefore uncertain across the range of exposure concentrations associated with HAP exposure. But the curve (based on outdoor air pollution, second-hand smoking, and active smoking) suggests a steadily rising function, from which similar conclusions can be drawn about the effect of interventions on risk, as for lung cancer. However, if the relative risks from the systematic review and meta-analysis could be incorporated into the model (and assuming the estimated exposure levels were confirmed empirically), then the shape of the curve would be closer to that seen for child ALRI.
HAP-reducing intervention performance and performance testing
Although a range of actions can reduce exposure to HAP (panel 1), reduction of emissions at source is the most effective intervention. An important consideration is that, although ventilation (eg, chimneys) can remove some of the smoke from the kitchen, the pollution still enters the ambient environment—eg, there is evidence that, in Indian villages, average levels of ambient PM2·5 can exceed 100 μg/m3, most of which arises from exfiltration of household smoke (London street air has an average value of 30 μg/m3).293 This pollution not only exposes people when outside, but also re-enters the home. The effects of different technologies and fuels on levels of emission and the resulting air quality in the home is also the intervention most extensively studied, and is the main focus of our discussion.
Two main sources of evidence are available for the effects of interventions on HAP—laboratory and inhome (field) studies. Most laboratory studies report emission rates for PM, carbon monoxide, and some other pollutants, whereas there are relatively fewer field studies and most report concentrations of PM and carbon monoxide in the kitchen, with few providing data for concentrations in other areas and outside of the home. Some field studies also provide data for personal exposure to PM and carbon monoxide.
The most comprehensive and standardised set of laboratory emissions data have been compiled by the US Environmental Protection Agency and reported by Jetter and colleagues.294 These data provide comparisons between the traditional three-stone fire and a wide range of improved solid fuel stoves, including some advanced stoves that use fan-assisted combustion or gasification. These studies show that stoves using conventional (unassisted) combustion typically reduce emission rates (often expressed as concentration per unit of energy delivered to the cooking pot) by 40–50%, and the more advanced technologies can reduce emissions by 80% or more. It must be appreciated, however, that these tests are carried out in ideal circumstances, and although very important to show the potential of the technology, there is growing evidence that these emission reductions might not be achieved in everyday use in homes. These studies have not included liquid and gaseous fuels (eg, LPG, ethanol) for comparison.
An increasing number of studies, many using experimental (mainly before-and-after) designs, have reported levels of PM2·5 (or PM4·0) in kitchens (and some personal exposure) associated with several solid fuel improved stoves. A number of studies are included in a recent review on exposure to HAP by Clark and colleagues.295 This review, together with three studies on liquid and gaseous fuels,296–298 and one on electricity,299 provide some important insights into the effects of solid and clean fuel interventions in everyday use. Although these studies might not be entirely representative of use at scale because of the project-based nature of many, they do provide a more realistic assessment of performance than that implied by findings from laboratory testing.
In summary, these studies report very high baseline average kitchen levels of PM2·5, ranging from several hundred to several thousand μg per m3, and substantial relative (50% or more) and absolute reductions in PM2·5 of several hundred μg per m3. These findings were seen for all intervention types, although with larger reductions for the chimney compared with non-chimney stoves, and for clean fuels. Of concern, however, was the finding that despite large reductions, average postintervention PM2·5 concentrations remained very high, at levels of up to several hundred μg per m3 for the solid fuel stoves. Vented stoves achieved lower postintervention levels than did non-chimney stoves, and some examples of vented stoves tested in Central America (chimneys) and Nepal (hood venting to exterior) achieved average 24 h kitchen PM2·5 concentrations in the range of 50 μg/m3 to 60 μg/m3.300,301 The lowest group average postintervention concentrations were seen for clean fuels (ethanol, gas, electricity), although studies were few in number and levels were still well above the WHO IT-1 of 35 μg/m3—eg, 100 μg/m3 or higher for ethanol (eg, Practical Action Consulting 2011297) and 80 μg/m3 for the one study of electrification.299 Very few studies were available for advanced solid fuel stoves using fans and gasification, and to our knowledge, none have been reported for biogas stoves or solar cookers. The reasons why postintervention concentrations of PM2·5 remain high, particularly for users of clean fuels, are discussed further below.
Kerosene remains a widely used fuel, particularly for lighting and cooking in LMICs. Lam and colleagues’302 systematic review provided a comprehensive overview of pollutants and emission levels from kerosene and the health risks in epidemiological studies. Although fuel grade and contaminants (eg, sulphur), combustion source and type (eg, lamp or stove), and operator conditions all affect emissions, there is ample evidence that use of household kerosene devices can lead to PM concentrations that exceed WHO guidelines, substantially so in homes in LMICs. Levels of carbon monoxide, polycyclic aromatic hydrocarbons, nitrogen dioxide, and sulphur dioxide might also exceed guideline levels. More than 20 epidemiological studies have reported on risks of a range of cancer and non-malignant respiratory, allergic, and ocular outcomes, but Lam and colleagues302 concluded that these studies do not yet allow strong conclusions nor reliably quantified risk estimates. Available data for the role of HAP on respiratory infections are often from studies comparing self-reported infections in households using solid fuel for cooking with those in households using cleaner-burning fuels. Recently, evidence has implicated kerosene use as a risk factor for tuberculosis and respiratory infections, with relative risk estimates comparable to those for solid fuels.19,23,36 Many older studies included kerosene as a clean cooking fuel in a reference category with LPG, biogas, and electricity and so the emissions from kerosene stoves and lamps used widely for lighting in LMICs have been ignored. Potentially, this grouping has resulted in underestimation of the health effects from HAP. However, the combination of widespread use, high levels of exposure to PM and other health damaging pollutants, and tentative epidemiological evidence, suggests that there should be strong concern about the possible or probable health effects of kerosene combustion.
Gas is one of the most widely used household fuels, and is one of the most important clean fuel options for replacement of solid fuels. A systematic review by Lin and colleagues303 noted that there is good evidence that use of gas for cooking and heating can result in levels of pollutants including nitrogen dioxide, carbon monoxide, and PM2·5 that exceed WHO indoor air quality guidelines, but these emissions seem to mainly be the result of equipment that is poorly fitted or maintained and with inadequate ventilation. A previous review reported an increased risk of lower respiratory illness in children exposed to increased levels of nitrogen dioxide.304 The review by Lin and colleagues303 provides evidence of increased risks of asthma with gas cooking compared with cooking by electricity and wheeze with increased levels of nitrogen dioxide, although residual confounding might be playing some part.305 We cannot determine from these studies the extent to which the observed risks are the result of technical issues (poor equipment, maintenance, and ventilation) or other sources of pollution, but related evidence does suggest that these technical factors are likely to be important. Although, as a source of energy in the home, gas might not be as clean as electricity (at the point of use), it seems to have a very low excess risk of adverse health outcomes when used optimally. When promoting gas as a household fuel, efforts should be made so that gas cookers and heaters function correctly and are adequately ventilated.
The focus of our discussion is stoves and fuels because these are seen as the most crucial factor to achieve low emissions. Several other structural and behavioural interventions have been proposed to contribute to reductions in HAP and personal exposure.306 Although most programmes introducing new stoves and fuels include some measure of behavioural change (eg, education about the risks of smoke exposure, suggestions for how to reduce exposure by changing the location of cooking or where small babies sleep, and particularly the use of dry fuel) through user training and advice, identification of the contribution of these components to changes in air quality is rarely possible. A few studies have investigated the potential of behavioural change, including those by Barnes and colleagues307 in South Africa, but these did not include data for HAP or exposure. A study carried out in China investigated a behavioural intervention (health education programme to promote smoke avoidance) in conjunction with an improved stove,308 but interpretation was made difficult by large discrepancies between numbers at baseline and follow-up, including for the comparisons between behavioural interventions and the improved cooking stoves.
Factors that affect intervention adoption and use
No matter how clean a stove or fuel is for emissions, if the household does not use it more or less exclusively to displace more polluting methods, and maintain these practices over time, then health benefits will be suboptimal and might not be realised at all. Two recent systematic reviews have reported on factors that affect adoption and sustained use of solid fuel improved stoves,309,310 and on four types of clean fuel (LPG, biogas, alcohol fuels, and solar cookers).311 These reviews show that a wide range of factors affect whether a household will adopt the new technology or fuel, the extent to which it displaces the existing arrangements, and whether use is maintained and equipment replaced when required. The authors of one of the reviews, Puzzolo and colleagues,311 describe seven key domains that affect this process, each incorporating a range of factors identified through their review of more than 100 qualitative, quantitative, and case studies, covering characteristics of the technology and fuel, household and community factors, and a set of programmatic and societal factors (figure 11). An important finding from this and other work, as previously discussed, is that multiple device and fuel use within the home is more or less the norm, and that complete transition from one type to another should not be expected, at least not in the short term.
Figure 11. Seven key domains (D) of factors affecting adoption and sustained use of household energy interventions.
Adapted from Puzzolo and colleagues.311
Experimental and other research priorities
This section has identified several specific research priorities that complement those identified through a workshop by thr National Institutes of Health and reported by Martin and colleagues (panel 4).285 As noted by the investigators, these research gaps need a mix of experimental and other study designs and approaches that are discussed below. Intervention-based studies, whether using RCTs or other designs, have a very important part to play in strengthening evidence for respiratory disease outcomes and in the evaluation of intervention effect. We identify four types of intervention study, each with different contributions and methodological aspects and challenges.
Panel 4. Major gaps and research needed for health outcomes.
Cancer
Determine the risk from coal-related HAP exposure on cancer of organ systems other than the lung.
Assess the risk from biomass-related HAP exposure for cancer of the lung, upper airway, and other organ systems.
Investigate whether risk is mediated via germline, somatic, or epigenetic changes and whether there is a developmental window of susceptibility.
Infections
Carry out population-based studies to determine the impact on important infectious diseases, including tuberculosis and malaria (the latter via effects of smoke on biting and disease transmission), and the impacts of interventions.
Extend the experience of the RESPIRE study on acute childhood pneumonia to other populations and cultures and determine cause (pathogens) and exposure–response relationships more precisely.
Leverage existing epidemiological studies investigating pneumonia and the impacts of new vaccines by adding HAP exposure assessment.
Cardiovascular disease
Use short-term and longer-term observational studies (including those leveraging existing cohorts) and intervention studies to determine the risk of completed cardiovascular outcomes, indicators of disease process (eg, ECG findings), and risk (eg, blood pressure, lipid concentrations, inflammatory biomarkers).
Determine the role of HAP in the developmental origins of cardiovascular disease through long-term cohort studies.
Maternal, neonatal, and child health
Strengthen existing evidence on pregnancy outcomes (pre-term birth, intrauterine growth restriction, stillbirth), with assessment of gestational age and vulnerable periods of exposure during pregnancy.
Investigate the risk of severe infection in neonates and young infants.
Strengthen emerging evidence on child growth and cognitive development to age 5–7 years.
Determine the risk of HAP exposure for the main causes of maternal mortality and morbidity.
Establish long-term cohorts to study the role of early HAP exposure and associated mechanisms (including epigenetic) in the developmental origins of later childhood and adult disease.
Respiratory disease
Use cohort studies and clinical trials to determine roles of HAP in causation and exacerbation of asthma in children.
Assess the impacts of HAP exposure reduction on the rate of lung function decline over the medium term (eg, 5 years) in young and middle-aged women.
Describe the risks of HAP exposure in pregnancy and early life for lung development, asthma, and COPD.
Burns
Enhance surveillance and population-based evidence on the causes and incidence, and mortality, disability, and longer-term social impacts of burn injuries.
Assess the impact of safety testing of new stoves.
Determine the value of prevention strategies on morbidity and mortality related to burn injuries or accidental poisoning (eg, with kerosene) from cooking, heating, and lighting.
Ocular disorders
Extend the evidence on cataracts in men and in exposed populations outside of India.
Ensure better control of potentially serious confounding in studies of cataract (eg, smoking, ultraviolet light exposure, nutrition).
Strengthen tentative evidence on risk for other important ocular disorders, such as trachoma.
Investigate the motivational potential of reduced eye symptoms (tearing, irritation) for intervention programmes.
HAP=household air pollution. Adapted from Martin and colleagues.285
Laboratory testing, which seeks to compare the emissions and efficiency of alternative intervention technologies and fuels during standard cooking tasks with use of consistent protocols, can provide valuable information about potential device and fuel performance. In future, it will be important to include cleaner liquid and gaseous fuels and electricity in these comparisons along with the associated devices for which efficiency could be improved.
Only two RCTs have been completed in this field,6,165 although four are currently underway (table 4). Depending on the results of these studies, further RCTs could be useful to strengthen evidence on the effect of HAP reduction on key respiratory outcomes, although RCT design is most suited to short-term, acute outcomes such as child ALRI (including severe pneumonia). Since an RCT requires the deliberate allocation of interventions, even with a cluster design, this type of study will tend to provide evidence on efficacy (in trials) rather than effectiveness in the real world. As has been emphasised by Martin and colleagues,285 it is vital to establish the acceptability and effect of the planned intervention on HAP and exposure before starting the trial, and it is recommended that clean fuels be included to ensure low exposures. Trials should also include thorough exposure assessment since effectiveness in any given situation cannot be assumed based on laboratory performance.
Table 4. Continuing randomised trials to assess effect of household air pollution exposure by location.
| Institution of principal investigator | Main interventions | Primary outcomes | Trial registration | |
|---|---|---|---|---|
| Ghana | Columbia University, New York, NY, USA (Pat Kinney and Darby Jack) | Biolite fan stove; liquefied petroleum gas | Incidence of physician-assessed acute lower respiratory infections in children younger than 12 months; birthweight (as a continuous variable; all livebirths) | NCT01335490 |
| Nepal | Johns Hopkins, Baltimore, MA, USA (James Tielsch) | Envirofit rocket stove; liquefied petroleum gas | Incidence of acute lower respiratory infections in children younger than 36 months; incidence of low birthweight (all livebirths) | NCT00786877 |
| Malawi | Liverpool School of Tropical Medicine, Liverpool, UK (Kevin Mortimer) | Philips fan stove | Incidence of pneumonia in children younger than 5 years | ISRCTN59448623 |
| Nigeria | University of Chicago, Chicago, IL, USA (Christopher Olopade) | Ethanol clean cookstove | Incidence of adverse pregnancy outcomes | GACC RCP 12-1 award |
NCT=National Clinical Trial. ISRCTN=International Standard Randomised Controlled Trial Number. GACC=Global Alliance for Clean Cookstoves.
Quasi-experimental studies, including before-and-after studies, with and without control groups, can have an important role in initial field evaluation of the acceptance and effects of interventions on HAP and exposure, and have been widely used for this purpose. The use of a house as its own control helps to limit confounding, but attention should be paid to factors that can change over time (eg, seasonal practices, numbers of people cooked for, etc), especially for studies with longer periods of follow-up. The use of parallel control groups adds strength to this type of study design.296
Finally, the evaluation of the effects of intervention at scale (including on health outcomes) is important to understand effectiveness. This type of assessment presents the greatest design challenges since (by contrast with the RCT) adoption at scale will probably involve a major component of market-based approaches, and patterns of adoption over time and across settings will complicate comparisons because earlier adopters will differ in many respects from later or non-adopters. This type of programmatic-effect evidence has been lacking, and where confidence about intervention efficacy, acceptability, and affordability is established, such studies should be a priority. Some opportunities might already be available—eg, the national kerosene to LPG conversion programme in Indonesia, which has involved more than 40 million homes.312
Cohort and case-control studies can make an important contribution to the evaluation of interventions, particularly for chronic diseases with long latent periods and when these can be done retrospectively, as for lung cancer and COPD in China.313,314 Reliable assessment of exposure, including historical, is a challenge for all studies of chronic disease, and is one aspect of such studies that needs more attention. Observational study designs also have an important role to strengthen evidence for important respiratory disease outcomes such as tuberculosis, for which the current body of evidence is still inconsistent and of low quality.315 Opportunities should be taken to build HAP assessment into existing or planned large-scale observational studies.
Qualitative research methods, particularly when combined with quantitative assessment of HAP, efficiency, and other aspects of intervention use and performance, have a very important role in finding out the reasons for which households use interventions in the way they do, including fuel and stove stacking, maintenance and replacement, and other aspects of adoption that ultimately determine whether or not exposure is reduced sufficiently and whether respiratory and other health benefits are obtained. Such investigations should not be limited to the household and should include factors contributing to all of the domains presented in figure 11.
Summary
The evidence we present can make an important contribution to understanding what types of intervention have the potential to deliver substantive benefits to respiratory health, and how policy can help to ensure these benefits are realised in practice and at scale. Much of this evidence is new, and we provide further interpretation below, and approaches and a summary of the findings. In view of the paucity of empirical data on exposure and risk in epidemiological studies of HAP, recently developed integrated exposure–response functions have been used to assess the expected health effects of differing levels of HAP exposure. These functions are innovative, and involve a number of assumptions as discussed by Burnett and colleagues.290 Only one of the functions (child ALRI) has direct exposure assessment, and this is from only one study. Given the potential value of these exposure–response functions in disease burden calculation and other applications,24 research to strengthen the HAP component through standardised exposure assessment should be a priority.
For lung cancer, the well-established evidence from smoking helps to define a more linear function with greater confidence, and consequently, risk reduction can be expected to be more proportionate to exposure reduction, albeit with a 10–20 year time-lag consistent with the latent period for this disease. A cohort study from the coal-using area of Xuanwei, China,313 which showed statistically significant reductions in risk of lung cancer of 41% in men and 46% in women with long-term use of chimney stoves, lends empirical support to the conclusion from the exposure–response model, because exposure reductions with these stoves are likely to be moderate rather than large.316,317
The integrated exposure–response for COPD is less certain because of the poorer fit between the epidemiological evidence and the model based on outdoor air pollution, second-hand smoking, and active smoking. The reasons for this finding are unclear, but might be because HAP exposure begins very much earlier in life (in utero and from the neonatal period) than does active smoking (typically in the teenage years), thereby increasing the risk for COPD in relative terms. At this stage, it is perhaps safest to assume that the integrated exposure–response relationship is intermediate in shape between that for ALRI (steep at low exposures and then flattening off) and that for lung cancer (more or less linear, rising to high levels of risk at very high exposure). A second cohort study from Xuanwei studied the effect of long-term use of chimney stoves on risk of COPD and reported statistically significant reductions of 42% in men and 25% in women,314 consistent at least with the conclusion above.
Although we acknowledge the limitations of the work on integrated exposure–responses, this evidence points towards the conclusion that concentrations of PM2·5 exposure at or below the WHO IT-1 of 35 μg/m3 are needed to prevent most cases of child ALRI attributable to HAP, and such benefits should be realised quickly. More proportionate risk reductions are expected for lung cancer after 10–20 years, whereas a pattern of risk reduction intermediate between that for ALRI and lung cancer might be seen for new cases of COPD, also with a lag of 10–20 years. Reductions in exacerbations of COPD might be seen more quickly.
For laboratory-based emissions studies, the availability of standardised comparative testing raises confidence in the relative performance of different solid fuel stoves. These studies have not so far included clean fuels. For in-home assessments, a growing number of studies have used standard (comparable) pollution assessment methods, which contribute to the overall quality of the available information, although more rigorous study designs with a control group have rarely been used. However, very few studies were available for some intervention categories, notably advanced solid fuels stoves and all of the clean fuels. There were also relatively few studies for personal exposure, especially for PM2·5, showing the historical lack of convenient and lightweight technology to measure personal PM exposure.295 Many of the studies also had short follow-up periods, some of only a few weeks, so they might not reflect true longer-term performance. Nevertheless, the postintervention averages for PM2·5 make clear that most solid fuel stove interventions are not delivering results that are even close to the levels needed. Results for advanced solid fuel stoves and clean fuels can be expected to be less reliable due to the paucity of studies.
The explanations for these high postintervention levels, particularly for clean fuels that have very low emissions, are important for policy decisions, and are due to a combination of factors. One important reason is continued use of the traditional stove.309 Other contributing sources of pollution within homes include kerosene lamps, which emit high levels of PM.302 Outside of homes under study, emissions from solid fuel stoves in neighbouring houses, along with other sources of outdoor air pollution, can be expected to contribute to increased levels in all but the most sparsely populated areas. For example, in Indian villages with average ambient levels of PM2·5 of 100 μg/m3 or higher,293 even in the absence of any household combustion source, it would not be possible to achieve a level lower than 100 μg/m3 within homes. This influence from ambient pollution is expected to be less in more sparsely populated rural areas. The two main implications of these findings are that improved stoves that burn solid fuel alone are not able to deliver the required level of air quality, and near exclusive use of clean fuels across communities with policy to control other ambient pollution sources, is needed. Future research priorities include comparative laboratory studies of clean and solid fuels, more field-based assessments of advanced solid fuel combustion stoves and clean fuels, and more comprehensive characterisation of multiple use of devices and fuels, and other sources in and around the home.
Several limitations exist in the evidence used in reviews of factors that affect adoption, which constrained inferring a causal relationship between any given factor and the adoption outcome, and also limited prioritisation of factors. Although some factors, such as meeting a household’s needs or affordability (albeit taking account of financial assistance options), can be considered necessary, on their own they are not sufficient to ensure substantive and longer-term use. Only a few studies included sufficiently long periods of time to study factors that affect sustained use, as opposed to initial adoption. Puzzolo and colleagues311 conclude that all factors can be important, but these are also setting, technology, and fuel dependent, and require assessment in the relevant context. More work is needed on the development of policy for effective and equitable adoption and more exclusive use of interventions that would deliver low emission rates, including clean fuels. This work should include research on sustained use, and use mixed methods combining quantitative and qualitative studies that have considerable value to quantify and explain the outcomes observed.
We have identified a number of priority areas for research, including to strengthen the HAP component of exposure–response functions, additional field-based evaluation of more advanced solid fuel stoves and clean fuels, and evaluation of policy to ensure adoption and sustained use of the most effective interventions. A mix of study designs and research situations are needed, including intervention-based studies that include laboratory testing, RCTs and other experimental designs, and programme-level evaluation.
Conclusion
Worldwide, respiratory health effects account for nearly a half of the overall deaths and disabilities from HAP. In each section of this Commission, we have focused on how the available published research can inform on specific respiratory disease risk and the complexities that underpin this risk. As is clear from this Commission, severe poverty and fuel use at the bottom of the world’s energy ladder are the main risk factors for HAP-related respiratory disease. But this is not simply a story about poverty and energy access. Risks of death and disability from HAP-related respiratory disease are not shared equally in the household. As noted in each section of this Commission, complex social and cultural factors affect the origins of disease and all proposed solutions. HAP is by definition a domestic exposure, and due to their domestic roles and activities, women and children have especially high HAP exposures. As for all environmental exposures, the initial primary effort to protect a household or a population from adverse health effects is primary prevention—ie, removal of the risk as early in life as possible, before there is any evidence of respiratory disease or start of disease mechanisms that predispose to eventual expression of respiratory disease.
The solution to death and disability from HAP is a classic example of a global health problem that needs public health prevention strategies that include many stakeholders such as households, communities, NGOs, businesses, health systems, governments, and global agencies. The challenge of changing how the world cooks is enormous, with about 600–800 million households at risk from HAP worldwide. Because cooking is by far the greatest source of polluting emissions to HAP, interventions with cleaner cooking solutions have been the primary prevention strategy. However, the solutions will need to be sustainable and so stoves should be durable and capable of lasting several years not several months. Most importantly, there must be a market demand for such stoves based on them being affordable, having improved cooking functionalities for the family, and being culturally suitable. There is an even larger issue at stake which is the means by which clean fuel can be provided at an affordable cost and in a manner that is both ecologically and economically sustainable for the poorest 1 billion of the world’s population. Successful businesses rely on responding to the voice of the customer and understanding how to address these needs and to create the supply chains needed to bring the solutions to a global scale. NGOs familiar with the traditions and cultures of communities will be essential partners with businesses and governments when large-scale implementation programmes are planned. NGOs working within local communities are invaluable as a means to build both trust in the community and the appropriate solutions for local needs. Only in this context can outside organisations contribute to improved, sustainable, household energy solutions that are acceptable to communities.
But where do health-care providers and scientists fit with this overarching prevention strategy? To begin with, most physicians and other health-care providers become aware of respiratory symptoms or diseases as individuals seek care. Individuals from LMICs who now reside in high-income countries might seek care and be diagnosed with respiratory diseases, which need careful assessment of a past medical history for environmental exposures such as HAP. This Commission will hopefully inform health-care providers of this risk and to be vigilant for its relevance when appropriate. For physicians and healthcare providers in LMICs, early-life and lifelong exposures to HAP should always be noted as part of a medical evaluation for the many associated disease risks.
Scientists and public health leaders at universities within LMICs can be instrumental to inform and move this discussion from the clinical setting to the community to the country level, provided that governmental priorities are made clear to the scientific community. Clearly, this is a dialogue that must occur to develop effective policies. There is an opportunity and perhaps a responsibility for health-care providers and scientists to work with governments and other stakeholders to develop policies and approaches that could reduce adverse health effects from HAP.
Solutions to HAP must use multiple and integrated strategies that include improvement of economic conditions for poor people worldwide, increased access to cleaner and more affordable household energy, improved access to health care, and advancement of technologies that are appropriate and adaptable to many different cultures and settings. Governments, societies, NGOs, businesses, and health systems are needed to develop such integrated strategies that can be sustained, evaluated, and improved over decades. Such efforts are underway with the recent establishment of the Global Alliance for Clean Cookstoves. But its success and that of other organisations who share this mission needs continuing involvement and commitment from health-care providers, scientists, and public health leaders, a group that has been under-represented. Hillary Rodham Clinton who, as US Secretary of State, promoted global awareness of the urgent need to reduce the global burden of HAP and its effects on women and children, once famously said that to solve complex problems “it takes a village”. We hope that this Commission begins a journey for readers, which engages themselves, their colleagues, and their scientific and professional societies in a global discussion to consider elimination of this major, preventable, cause of death in the world.318,319
Acknowledgments
We thank Jane Ardrey for collating, editing, and managing the production of this report, and we thank Shaun Pennington for technical assistance with figures and references. We thank Han Duijvendak and his team at HANDSTAND productions for photography. We acknowledge funding from the Medical Research Council (BREATHE Partnership grant) and input to the discussion of this Commission at the Collaboration for Applied Health Research and Delivery (CAHRD) meeting at the Liverpool School of Tropical Medicine, Liverpool, UK, on June 12–13, 2014. We thank James Jetter (US Environmental Protection Agency, NC, USA) for permission to use figure 3.
Footnotes
Contributors
SBG and WJM both wrote the outline of the Commission and the text for the introduction and conclusion sections. SBG and WJM reviewed and edited the entire Commission as it was developed by the other authors. SJ contributed to the introduction. PLH was the lead author for the section on respiratory infections. MNB wrote the text related to infants and children and older children and adults. JR wrote the text on mechanisms of defence, PM wrote the text on other risk factors and NB-Z wrote the text on vaccinations. PLH structured and contributed text to all parts in this section. KM was the lead author for the section on obstructive lung diseases. KM wrote the first draft for this section, with JB, SB, DH, RP-P, and KM contributing equally to reviewing and revising. OPK and K-bHL were the lead authors for the section on lung cancer and upper airway cancers, and were responsible for the concept and writing. HK and ZC contributed to reviewing and revising this section. JG was the lead author for the section on exposure and biomarkers. JG wrote the parts about direct biomarkers of exposure, risks across the lifecycle, and gender differences. PNB wrote the parts about indoor and outdoor air pollution, and second-hand smoking, SS wrote the part about exposure assessment and additional respiratory risks, and LN wrote the part about indirect biomarkers of exposure. AP contributed to the part about gender differences. NGB was lead author for the section about interventions, with KPA, KB, SM, DP, and DJ contributing equally to reviewing and revising.
Declaration of interests
JG reports personal fees from GlaxoSmithKline and personal fees from Novartis. He is a member of the UK Government’s Committee on the medical effects of air pollution and is co-chair of the Royal College of Physicians working party on the long-term effects of air pollution. SBG and KM received grants from Joint Global Health Trials to carry out an interventional trial in Malawi. The other authors report no competing interests.
Contributor Information
Prof Stephen B Gordon, Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, UK.
Prof Nigel G Bruce, Department of Public Health and Policy, University of Liverpool, Liverpool, UK.
Prof Jonathan Grigg, Centre for Paediatrics, Blizard Institute, Queen Mary, University of London, London, UK.
Prof Patricia L Hibberd, Division of Global Health, Department of Pediatrics, Massachusetts General Hospital, and Harvard Medical School, Boston, MA, USA.
Om P Kurmi, Clinical Trials Service Unit and Epidemiological Studies Unit, Nuffeld Department of Population Health, University of Oxford, Oxford, UK.
Kin-bong Hubert Lam, Institute of Occupational and Environmental Medicine, School of Health and Population Sciences, University of Birmingham, Birmingham, UK.
Kevin Mortimer, Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, UK.
Kwaku Poku Asante, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, NY, USA.
Prof Kalpana Balakrishnan, Department of Environmental Health Engineering, Sri Ramachandra University, Chennai, India.
Prof John Balmes, Department of Medicine, University of California San Francisco, San Francisco, CA, USA; Environmental Health Sciences, School of Public Health, University of California, Berkeley, CA, USA.
Naor Bar-Zeev, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, College of Medicine, University of Malawi, Blantyre, Malawi; Institute of Infection and Global Health, University of Liverpool, Liverpool, UK.
Prof Michael N Bates, Divisions of Epidemiology and Environmental Health Sciences, School of Public Health, University of California, Berkeley, CA, USA.
Prof Patrick N Breysse, Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Sonia Buist, Oregon Health and Science University, Portland, OR, USA.
Prof Zhengming Chen, Clinical Trials Service Unit and Epidemiological Studies Unit, Nuffeld Department of Population Health, University of Oxford, Oxford, UK.
Deborah Havens, Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, UK.
Darby Jack, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, New York, NY, USA.
Surinder Jindal, Jindal Clinics, Chandigarh, India.
Prof Haidong Kan, School of Public Health, Fudan University, Shanghai, China.
Sumi Mehta, Health Effects Institute, Boston, MA, USA.
Peter Moschovis, Division of Global Health, Department of Pediatrics, Massachusetts General Hospital, and Harvard Medical School, Boston, MA, USA.
Luke Naeher, The University of Georgia, College of Public Health, Department of Environmental Health Science, Athens, GA, USA.
Prof Archana Patel, Lata Medical Research Foundation, Nagpur, India.
Rogelio Perez-Padilla, Instituto Nacional de Enfermedades Respiratorias, Mexico City, Mexico.
Daniel Pope, Department of Public Health and Policy, University of Liverpool, Liverpool, UK.
Jamie Rylance, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, College of Medicine, University of Malawi, Blantyre, Malawi.
Prof Sean Semple, University of Aberdeen, Scottish Centre for Indoor Air, Division of Applied Health Sciences, Royal Aberdeen Children’s Hospital, Aberdeen, UK.
Prof William J Martin, II, Division of Environmental Health Sciences, College of Public Health, The Ohio State University, Columbus, OH, USA.
References
- 1.Rehfuess E. Fuel for Life: Household Energy and Health. Geneva: World Health Organization; 2006. [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. [accessed Aug 18, 2014];The Global Health Observatory. 2014 http://apps.who.int/gho/data/node.main.135?lang=en.
- 4.Rajagopalan S, Brook RD. The indoor-outdoor air-pollution continuum and the burden of cardiovascular disease: an opportunity for improving global health. Glob Heart. 2012;7:207–13. doi: 10.1016/j.gheart.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloomfield GS, Lagat DK, Akwanalo OC, et al. Waiting to inhale: an exploratory review of conditions that may predispose to pulmonary hypertension and right heart failure in persons exposed to household air pollution in low- and middle-income countries. Glob Heart. 2012;7:249–59. doi: 10.1016/j.gheart.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet. 2011;378:1717–26. doi: 10.1016/S0140-6736(11)60921-5. [DOI] [PubMed] [Google Scholar]
- 7.Ezzati M, Kammen DM. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: an exposure-response study. Lancet. 2001;358:619–24. doi: 10.1016/s0140-6736(01)05777-4. [DOI] [PubMed] [Google Scholar]
- 8.WHO. The top 10 causes of death in the world. Fact sheet number 310. Geneva: World Health Organization; 2014. [Google Scholar]
- 9.Hwang SH, Hwang JH, Moon JS, Lee DH. Environmental tobacco smoke and children’s health. Korean J Pediatr. 2012;55:35–41. doi: 10.3345/kjp.2012.55.2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4:12. doi: 10.1186/1617-9625-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167:335–42. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 12.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4:e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 14.Downie L, Armiento R, Subhi R, Kelly J, Clifford V, Duke T. Community-acquired neonatal and infant sepsis in developing countries: efficacy of WHO’s currently recommended antibiotics–systematic review and meta-analysis. Arch Dis Child. 2013;98:146–54. doi: 10.1136/archdischild-2012-302033. [DOI] [PubMed] [Google Scholar]
- 15.Edmond K, Zaidi A. New approaches to preventing, diagnosing, and treating neonatal sepsis. PLoS Med. 2010;7:e1000213. doi: 10.1371/journal.pmed.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce NG, Dherani MK, Das JK, et al. Control of household air pollution for child survival: estimates for intervention impacts. BMC Public Health. 2013;13(suppl 3):S8. doi: 10.1186/1471-2458-13-S3-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashima S, Yorifuji T, Tsuda T, Ibrahim J, Doi H. Effects of traffic-related outdoor air pollution on respiratory illness and mortality in children, taking into account indoor air pollution, in Indonesia. J Occup Environ Med. 2010;52:340–45. doi: 10.1097/JOM.0b013e3181d44e3f. [DOI] [PubMed] [Google Scholar]
- 18.Tielsch JM, Katz J, Thulasiraj RD, et al. Exposure to indoor biomass fuel and tobacco smoke and risk of adverse reproductive outcomes, mortality, respiratory morbidity and growth among newborn infants in south India. Int J Epidemiol. 2009;38:1351–63. doi: 10.1093/ije/dyp286. [DOI] [PubMed] [Google Scholar]
- 19.Epstein MB, Bates MN, Arora NK, Balakrishnan K, Jack DW, Smith KR. Household fuels, low birth weight, and neonatal death in India: the separate impacts of biomass, kerosene, and coal. Int J Hyg Environ Health. 2013;216:523–32. doi: 10.1016/j.ijheh.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–16. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86:390–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232–39. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 23.Bates MN, Chandyo RK, Valentiner-Branth P, et al. Acute lower respiratory infection in childhood and household fuel use in Bhaktapur, Nepal. Environ Health Perspect. 2013;121:637–42. doi: 10.1289/ehp.1205491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith KR, Bruce N, Balakrishnan K, et al. HAP CRA Risk Expert Group Millions dead: how do we know and what does it mean? Methods used in the comparative risk assessment of household air pollution. Annu Rev Public Health. 2014;35:185–206. doi: 10.1146/annurev-publhealth-032013-182356. [DOI] [PubMed] [Google Scholar]
- 25.Adoga A, Nimkur T, Silas O. Chronic suppurative otitis media: socio-economic implications in a tertiary hospital in Northern Nigeria. Pan Afr Med J. 2010;4:3. doi: 10.4314/pamj.v4i1.53613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones LL, Hassanien A, Cook DG, Britton J, Leonardi-Bee J. Parental smoking and the risk of middle ear disease in children: a systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:18–27. doi: 10.1001/archpediatrics.2011.158. [DOI] [PubMed] [Google Scholar]
- 27.Lasisi AO, Olaniyan FA, Muibi SA, et al. Clinical and demographic risk factors associated with chronic suppurative otitis media. Int J Pediatr Otorhinolaryngol. 2007;71:1549–54. doi: 10.1016/j.ijporl.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Patra S, Sharma S, Behera D. Passive smoking, indoor air pollution and childhood tuberculosis: a case control study. Indian J Tuberc. 2012;59:151–55. [PubMed] [Google Scholar]
- 29.Jubulis J, Kinikar A, Ithape M, et al. Modifiable risk factors associated with tuberculosis disease in children in Pune, India. Int J Tuberc Lung Dis. 2014;18:198–204. doi: 10.5588/ijtld.13.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilabuko JH, Matsuki H, Nakai S. Air quality and acute respiratory illness in biomass fuel using homes in Bagamoyo, Tanzania. Int J Environ Res Public Health. 2007;4:39–44. doi: 10.3390/ijerph2007010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrestha IL, Shrestha SL. Indoor air pollution from biomass fuels and respiratory health of the exposed population in Nepalese households. Int J Occup Environ Health. 2005;11:150–60. doi: 10.1179/oeh.2005.11.2.150. [DOI] [PubMed] [Google Scholar]
- 32.Taylor ET, Nakai S. Prevalence of acute respiratory infections in women and children in western Sierra Leone due to smoke from wood and charcoal stoves. Int J Environ Res Public Health. 2012;9:2252–65. doi: 10.3390/ijerph9062252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bautista LE, Correa A, Baumgartner J, Breysse P, Matanoski GM. Indoor charcoal smoke and acute respiratory infections in young children in the Dominican Republic. Am J Epidemiol. 2009;169:572–80. doi: 10.1093/aje/kwn372. [DOI] [PubMed] [Google Scholar]
- 34.Slama K, Chiang CY, Hinderaker SG, Bruce N, Vedal S, Enarson DA. Indoor solid fuel combustion and tuberculosis: is there an association? Int J Tuberc Lung Dis. 2010;14:6–14. [PubMed] [Google Scholar]
- 35.Sumpter C, Chandramohan D. Systematic review and meta-analysis of the associations between indoor air pollution and tuberculosis. Trop Med Int Health. 2013;18:101–08. doi: 10.1111/tmi.12013. [DOI] [PubMed] [Google Scholar]
- 36.Pokhrel AK, Bates MN, Verma SC, Joshi HS, Sreeramareddy CT, Smith KR. Tuberculosis and indoor biomass and kerosene use in Nepal: a case-control study. Environ Health Perspect. 2010;118:558–64. doi: 10.1289/ehp.0901032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behera D, Aggarwal G. Domestic cooking fuel exposure and tuberculosis in Indian women. Indian J Chest Dis Allied Sci. 2010;52:139–43. [PubMed] [Google Scholar]
- 38.García-Sancho MC, García-García L, Báez-Saldaña R, et al. Indoor pollution as an occupational risk factor for tuberculosis among women: a population-based, gender oriented, case-control study in Southern Mexico. Rev Invest Clin. 2009;61:392–98. [PubMed] [Google Scholar]
- 39.Gninafon M, Ade G, Aït-Khaled N, Enarson DA, Chiang CY. Exposure to combustion of solid fuel and tuberculosis: a matched case-control study. Eur Respir J. 2011;38:132–38. doi: 10.1183/09031936.00104610. [DOI] [PubMed] [Google Scholar]
- 40.Kan X, Chiang CY, Enarson DA, Chen W, Yang J, Chen G. Indoor solid fuel use and tuberculosis in China: a matched case-control study. BMC Public Health. 2011;11:498. doi: 10.1186/1471-2458-11-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolappan C, Subramani R. Association between biomass fuel and pulmonary tuberculosis: a nested case-control study. Thorax. 2009;64:705–08. doi: 10.1136/thx.2008.109405. [DOI] [PubMed] [Google Scholar]
- 42.Lakshmi PV, Virdi NK, Thakur JS, Smith KR, Bates MN, Kumar R. Biomass fuel and risk of tuberculosis: a case-control study from Northern India. J Epidemiol Community Health. 2012;66:457–61. doi: 10.1136/jech.2010.115840. [DOI] [PubMed] [Google Scholar]
- 43.Woldesemayat EM, Datiko DG, Lindtjørn B. Use of biomass fuel in households is not a risk factor for pulmonary tuberculosis in South Ethiopia. Int J Tuberc Lung Dis. 2014;18:67–72. doi: 10.5588/ijtld.12.0980. [DOI] [PubMed] [Google Scholar]
- 44.Sussan TE, Ingole V, Kim JH, et al. Source of biomass cooking fuel determines pulmonary response to household air pollution. Am J Respir Cell Mol Biol. 2014;50:538–48. doi: 10.1165/rcmb.2013-0201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemmar A, Hoet PH, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–14. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 46.Hawley B, Volckens J. Proinflammatory effects of cookstove emissions on human bronchial epithelial cells. Indoor Air. 2013;23:4–13. doi: 10.1111/j.1600-0668.2012.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laffon M, Pittet JF, Modelska K, Matthay MA, Young DM. Interleukin-8 mediates injury from smoke inhalation to both the lung endothelial and the alveolar epithelial barriers in rabbits. Am J Respir Crit Care Med. 1999;160:1443–49. doi: 10.1164/ajrccm.160.5.9901097. [DOI] [PubMed] [Google Scholar]
- 48.Nieman GF, Clark WR., Jr Effects of wood and cotton smoke on the surface properties of pulmonary surfactant. Respir Physiol. 1994;97:1–12. doi: 10.1016/0034-5687(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharyya SN, Dubick MA, Yantis LD, et al. In vivo effect of wood smoke on the expression of two mucin genes in rat airways. Inflammation. 2004;28:67–76. doi: 10.1023/b:ifla.0000033022.66289.04. [DOI] [PubMed] [Google Scholar]
- 50.Chuang HC, Cheng YL, Lei YC, Chang HH, Cheng TJ. Protective effects of pulmonary epithelial lining fluid on oxidative stress and DNA single-strand breaks caused by ultrafine carbon black, ferrous sulphate and organic extract of diesel exhaust particles. Toxicol Appl Pharmacol. 2013;266:329–34. doi: 10.1016/j.taap.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Seagrave J, McDonald JD, Reed MD, Seilkop SK, Mauderly JL. Responses to subchronic inhalation of low concentrations of diesel exhaust and hardwood smoke measured in rat bronchoalveolar lavage fluid. Inhal Toxicol. 2005;17:657–70. doi: 10.1080/08958370500189529. [DOI] [PubMed] [Google Scholar]
- 52.Dutta A, Roychoudhury S, Chowdhury S, Ray MR. Changes in sputum cytology, airway inflammation and oxidative stress due to chronic inhalation of biomass smoke during cooking in premenopausal rural Indian women. Int J Hyg Environ Health. 2013;216:301–08. doi: 10.1016/j.ijheh.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–42. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 54.Ndengele MM, Muscoli C, Wang ZQ, Doyle TM, Matuschak GM, Salvemini D. Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23:186–93. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- 55.Kagan VE, Borisenko GG, Serinkan BF, et al. Appetizing rancidity of apoptotic cells for macrophages: oxidation, externalization, and recognition of phosphatidylserine. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1–17. doi: 10.1152/ajplung.00365.2002. [DOI] [PubMed] [Google Scholar]
- 56.Potter AJ, Trappetti C, Paton JC. Streptococcus pneumoniae uses glutathione to defend against oxidative stress and metal ion toxicity. J Bacteriol. 2012;194:6248–54. doi: 10.1128/JB.01393-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurmi OP, Dunster C, Ayres JG, Kelly FJ. Oxidative potential of smoke from burning wood and mixed biomass fuels. Free Radic Res. 2013;47:829–35. doi: 10.3109/10715762.2013.832831. [DOI] [PubMed] [Google Scholar]
- 58.Mudway IS, Duggan ST, Venkataraman C, Habib G, Kelly FJ, Grigg J. Combustion of dried animal dung as biofuel results in the generation of highly redox active fine particulates. Part Fibre Toxicol. 2005;2:6. doi: 10.1186/1743-8977-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swiston JR, Davidson W, Attridge S, Li GT, Brauer M, van Eeden SF. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J. 2008;32:129–38. doi: 10.1183/09031936.00097707. [DOI] [PubMed] [Google Scholar]
- 60.van Eeden SF, Tan WC, Suwa T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164:826–30. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- 61.Barregard L, Sällsten G, Andersson L, et al. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med. 2008;65:319–24. doi: 10.1136/oem.2006.032458. [DOI] [PubMed] [Google Scholar]
- 62.Schins RP, Lightbody JH, Borm PJ, Shi T, Donaldson K, Stone V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195:1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Kocbach Bølling A, Pagels J, Yttri KE, et al. Health effects of residential wood smoke particles: the importance of combustion conditions and physicochemical particle properties. Part Fibre Toxicol. 2009;6:29. doi: 10.1186/1743-8977-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wegesser TC, Last JA. Lung response to coarse PM: bioassay in mice. Toxicol Appl Pharmacol. 2008;230:159–66. doi: 10.1016/j.taap.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker S, Soukup JM, Gallagher JE. Differential particulate air pollution induced oxidant stress in human granulocytes, monocytes and alveolar macrophages. Toxicol In Vitro. 2002;16:209–18. doi: 10.1016/s0887-2333(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 66.Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171:20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- 67.Semple S, Devakumar D, Fullerton DG, et al. Airborne endotoxin concentrations in homes burning biomass fuel. Environ Health Perspect. 2010;118:988–91. doi: 10.1289/ehp.0901605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobzik L. Lung macrophage uptake of unopsonized environmental particulates. Role of scavenger-type receptors. J Immunol. 1995;155:367–76. [PubMed] [Google Scholar]
- 69.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–34. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 70.Palecanda A, Paulauskis J, Al-Mutairi E, et al. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp Med. 1999;189:1497–506. doi: 10.1084/jem.189.9.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tellabati A, Fernandes VE, Teichert F, et al. Acute exposure of mice to high-dose ultrafine carbon black decreases susceptibility to pneumococcal pneumonia. Part Fibre Toxicol. 2010;7:30. doi: 10.1186/1743-8977-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Migliaccio CT, Kobos E, King QO, Porter V, Jessop F, Ward T. Adverse effects of wood smoke PM(2.5) exposure on macrophage functions. Inhal Toxicol. 2013;25:67–76. doi: 10.3109/08958378.2012.756086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tesfaigzi Y, McDonald JD, Reed MD, et al. Low-level subchronic exposure to wood smoke exacerbates inflammatory responses in allergic rats. Toxicol Sci. 2005;88:505–13. doi: 10.1093/toxsci/kfi317. [DOI] [PubMed] [Google Scholar]
- 74.Dutta A, Ray MR, Banerjee A. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol Appl Pharmacol. 2012;261:255–62. doi: 10.1016/j.taap.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 75.Lee YJ, Kim HT, Lee KW. Development of monitoring technology for airborne particulate matter. Environ Monit Assess. 2001;70:3–20. doi: 10.1023/a:1010679128979. [DOI] [PubMed] [Google Scholar]
- 76.Dutta A, Bhattacharya P, Lahiri T, Ray MR. Immune cells and cardiovascular health in premenopausal women of rural India chronically exposed to biomass smoke during daily household cooking. Sci Total Environ. 2012;438:293–98. doi: 10.1016/j.scitotenv.2012.08.065. [DOI] [PubMed] [Google Scholar]
- 77.Arredouani M, Yang Z, Ning Y, et al. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med. 2004;200:267–72. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marriott HM, Hellewell PG, Cross SS, Ince PG, Whyte MK, Dockrell DH. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J Immunol. 2006;177:6480–88. doi: 10.4049/jimmunol.177.9.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lundborg M, Bouhafs R, Gerde P, et al. Aggregates of ultrafine particles modulate lipid peroxidation and bacterial killing by alveolar macrophages. Environ Res. 2007;104:250–57. doi: 10.1016/j.envres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Sigaud S, Goldsmith CA, Zhou H, et al. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol Appl Pharmacol. 2007;223:1–9. doi: 10.1016/j.taap.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knapp S, Leemans JC, Florquin S, et al. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med. 2003;167:171–79. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 82.Marriott HM, Bingle CD, Read RC, et al. Dynamic changes in Mcl-1 expression regulate macrophage viability or commitment to apoptosis during bacterial clearance. J Clin Invest. 2005;115:359–68. doi: 10.1172/JCI21766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richens TR, Linderman DJ, Horstmann SA, et al. Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am J Respir Crit Care Med. 2009;179:1011–21. doi: 10.1164/rccm.200807-1148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Erb-Downward JR, Thompson DL, Han MK, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One. 2011;6:e16384. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grigg J, Walters H, Sohal SS, et al. Cigarette smoke and platelet-activating factor receptor dependent adhesion of Streptococcus pneumoniae to lower airway cells. Thorax. 2012;67:908–13. doi: 10.1136/thoraxjnl-2011-200835. [DOI] [PubMed] [Google Scholar]
- 86.Black RE, Allen LH, Bhutta ZA, et al. Maternal and Child Undernutrition Study Group Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 87.Krawinkel MB. Interaction of nutrition and infections globally: an overview. Ann Nutr Metab. 2012;61(suppl 1):39–45. doi: 10.1159/000345162. [DOI] [PubMed] [Google Scholar]
- 88.Kaushik PV, Singh JV, Bhatnagar M, Garg SK, Chopra H. Nutritional correlates of acute respiratory infections. Indian J Matern Child Health. 1995;6:71–72. [PubMed] [Google Scholar]
- 89.Rice AL, Sacco L, Hyder A, Black RE. Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull World Health Organ. 2000;78:1207–21. [PMC free article] [PubMed] [Google Scholar]
- 90.Chisti MJ, Salam MA, Ashraf H, et al. Predictors and outcome of hypoxemia in severely malnourished children under five with pneumonia: a case control design. PLoS One. 2013;8:e51376. doi: 10.1371/journal.pone.0051376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.UNICEF, WHO, The World Bank. [accessed Aug 18, 2014];UNICEF-WHO-World Bank Joint Child Malnutrition Estimates. 2012 [Google Scholar]
- 92.Oluwole O, Arinola GO, Ana GR, et al. Relationship between household air pollution from biomass smoke exposure, and pulmonary dysfunction, oxidant-antioxidant imbalance and systemic inflammation in rural women and children in Nigeria. Glob J Health Sci. 2013;5:28–38. doi: 10.5539/gjhs.v5n4p28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brady H, Lamb MM, Sokol RJ, et al. Plasma micronutrients are associated with dietary intake and environmental tobacco smoke exposure in a paediatric population. Public Health Nutr. 2007;10:712–18. doi: 10.1017/S1368980007662296. [DOI] [PubMed] [Google Scholar]
- 94.Wilson KM, Finkelstein JN, Blumkin AK, Best D, Klein JD. Micronutrient levels in children exposed to secondhand tobacco smoke. Nicotine Tob Res. 2011;13:800–08. doi: 10.1093/ntr/ntr076. [DOI] [PubMed] [Google Scholar]
- 95.Fernández-Fernández FJ, Pía G, Sesma P. Respiratory health effects of indoor air pollution: potential chemoprophylactic strategies. Int J Tuberc Lung Dis. 2011;15:424. [PubMed] [Google Scholar]
- 96.Jedrychowski W, Perera F, Mrozek-Budzyn D, et al. Higher fish consumption in pregnancy may confer protection against the harmful effect of prenatal exposure to fine particulate matter. Ann Nutr Metab. 2010;56:119–26. doi: 10.1159/000275918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neggers YH, Singh J. Zinc supplementation to protein-deficient diet in CO-exposed mice decreased fetal mortality and malformation. Biol Trace Elem Res. 2006;114:269–79. doi: 10.1385/bter:114:1:269. [DOI] [PubMed] [Google Scholar]
- 98.Wang S, Sun NN, Zhang J, Watson RR, Witten ML. Immunomodulatory effects of high-dose alpha-tocopherol acetate on mice subjected to sidestream cigarette smoke. Toxicology. 2002;175:235–45. doi: 10.1016/s0300-483x(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 99.Büchner N, Ale-Agha N, Jakob S, et al. Unhealthy diet and ultrafine carbon black particles induce senescence and disease associated phenotypic changes. Exp Gerontol. 2013;48:8–16. doi: 10.1016/j.exger.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 100.Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;8:CD005532. doi: 10.1002/14651858.CD005532.pub3. [DOI] [PubMed] [Google Scholar]
- 101.Taylor CE, Camargo CA., Jr Impact of micronutrients on respiratory infections. Nutr Rev. 2011;69:259–69. doi: 10.1111/j.1753-4887.2011.00386.x. [DOI] [PubMed] [Google Scholar]
- 102.Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol. 2013;136:321–29. doi: 10.1016/j.jsbmb.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 103.Singh M, Das RR. Zinc for the common cold. Cochrane Database Syst Rev. 2013;6:CD001364. doi: 10.1002/14651858.CD001364.pub4. [DOI] [PubMed] [Google Scholar]
- 104.Yakoob MY, Theodoratou E, Jabeen A, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health. 2011;11(suppl 3):S23. doi: 10.1186/1471-2458-11-S3-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Srinivasan MG, Ndeezi G, Mboijana CK, et al. Zinc adjunct therapy reduces case fatality in severe childhood pneumonia: a randomized double blind placebo-controlled trial. BMC Med. 2012;10:14. doi: 10.1186/1741-7015-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Camargo CA, Jr, Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130:e561–67. doi: 10.1542/peds.2011-3029. [DOI] [PubMed] [Google Scholar]
- 107.Charan J, Goyal JP, Saxena D, Yadav P. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J Pharmacol Pharmacother. 2012;3:300–03. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mda S, van Raaij JM, de Villiers FP, MacIntyre UE, Kok FJ. Short-term micronutrient supplementation reduces the duration of pneumonia and diarrheal episodes in HIV-infected children. J Nutr. 2010;140:969–74. doi: 10.3945/jn.109.110312. [DOI] [PubMed] [Google Scholar]
- 109.Wahed MA, Islam MA, Khondakar P, Haque MA. Effect of micronutrients on morbidity and duration of hospital stay in childhood pneumonia. Mymensingh Med J. 2008;17(suppl):S77–83. [PubMed] [Google Scholar]
- 110.De P, Farley A, Lindson N, Aveyard P. Systematic review and meta-analysis: influence of smoking cessation on incidence of pneumonia in HIV. BMC Med. 2013;11:15. doi: 10.1186/1741-7015-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Djawe K, Levin L, Swartzman A, et al. Environmental risk factors for Pneumocystis pneumonia hospitalizations in HIV patients. Clin Infect Dis. 2013;56:74–81. doi: 10.1093/cid/cis841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blount RJ, Djawe K, Daly KR, et al. International HIV-associated Opportunistic Pneumonias Study Ambient air pollution associated with suppressed serologic responses to Pneumocystis jirovecii in a prospective cohort of HIV-infected patients with Pneumocystis pneumonia. PLoS ONE. 2013;8:e80795. doi: 10.1371/journal.pone.0080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lucero MG, Dulalia VE, Nillos LT, et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev. 2009;4:CD004977. doi: 10.1002/14651858.CD004977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo controlled trial. Pediatr Infect Dis J. 2009;28:455–62. doi: 10.1097/INF.0b013e31819637af. [DOI] [PubMed] [Google Scholar]
- 115.Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25:779–81. doi: 10.1097/01.inf.0000232706.35674.2f. [DOI] [PubMed] [Google Scholar]
- 116.Mackenzie GA, Bottomley C, van Hoek AJ, et al. Efficacy of different pneumococcal conjugate vaccine schedules against pneumonia, hospitalisation, and mortality: re-analysis of a randomised trial in The Gambia. Vaccine. 2014;32:2493–500. doi: 10.1016/j.vaccine.2014.02.081. [DOI] [PubMed] [Google Scholar]
- 117.Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–46. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 118.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–48. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 119.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–63. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Grady KF, Carlin JB, Chang AB, et al. Effectiveness of 7-valent pneumococcal conjugate vaccine against radiologically diagnosed pneumonia in indigenous infants in Australia. Bull World Health Organ. 2010;88:139–46. doi: 10.2471/BLT.09.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Afonso ET, Minamisava R, Bierrenbach AL, et al. Effect of 10-valent pneumococcal vaccine on pneumonia among children, Brazil. Emerg Infect Dis. 2013;19:589–97. doi: 10.3201/eid1904.121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Adam D, Fehnle K. Safety and effectiveness against respiratory tract infections for pneumococcal conjugate vaccine co-administered with routine vaccine combinations. Vaccine. 2008;26:5944–51. doi: 10.1016/j.vaccine.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 123.Ansaldi F, Sticchi L, Durando P, et al. Decline in pneumonia and acute otitis media after the introduction of childhood pneumococcal vaccination in Liguria, Italy. J Int Med Res. 2008;36:1255–60. doi: 10.1177/147323000803600612. [DOI] [PubMed] [Google Scholar]
- 124.McNally LM, Jeena PM, Gajee K, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet. 2007;369:1440–51. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 125.Cherian T, Mulholland EK, Carlin JB, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–59. [PMC free article] [PubMed] [Google Scholar]
- 126.Madhi SA, Whitney CG, Nohynek H. Lessons learned from clinical trials evaluating pneumococcal conjugate vaccine efficacy against pneumonia and invasive disease. Vaccine. 2008;26(suppl 2):B9–B15. doi: 10.1016/j.vaccine.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 127.Adegbola RA, Falade AG, Sam BE, et al. The etiology of pneumonia in malnourished and well-nourished Gambian children. Pediatr Infect Dis J. 1994;13:975–82. doi: 10.1097/00006454-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 128.Hortal M, Estevan M, Meny M, Iraola I, Laurani H. Impact of pneumococcal conjugate vaccines on the incidence of pneumonia in hospitalized children after five years of its introduction in Uruguay. PLoS One. 2014;9:e98567. doi: 10.1371/journal.pone.0098567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pírez MC, Algorta G, Chamorro F, et al. Changes in hospitalizations for pneumonia after universal vaccination with pneumococcal conjugate vaccines 7/13 valent and Haemophilus influenzae type b conjugate vaccine in a pediatric referral hospital in Uruguay. Pediatr Infect Dis J. 2014;33:753–59. doi: 10.1097/INF.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 130.Roca A, Dione MM, Bojang A, et al. Nasopharyngeal carriage of pneumococci four years after community-wide vaccination with PCV-7 in The Gambia: long-term evaluation of a cluster randomized trial. Plos One. 2013;8:e72198. doi: 10.1371/journal.pone.0072198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rodrigues F, Foster D, Caramelo F, et al. Progressive changes in pneumococcal carriage in children attending daycare in Portugal after 6 years of gradual conjugate vaccine introduction show falls in most residual vaccine serotypes but no net replacement or trends in diversity. Vaccine. 2012;30:3951–56. doi: 10.1016/j.vaccine.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 133.Khowaja AR, Mohiuddin S, Cohen AL, et al. Pakistan Hib Vaccine Study Group Effectiveness of Haemophilus influenzae type b conjugate vaccine on radiologically-confirmed pneumonia in young children in Pakistan. J Pediatr. 2013;163(1 suppl):S79–S85.e1. doi: 10.1016/j.jpeds.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen WJ, Moulton LH, Saha SK, Mahmud AA, Arifeen SE, Baqui AH. Estimation of the herd protection of Haemophilus influenzae type b conjugate vaccine against radiologically confirmed pneumonia in children under 2 years old in Dhaka, Bangladesh. Vaccine. 2014;32:944–48. doi: 10.1016/j.vaccine.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 135.Yoshida LM, Nguyen HA, Watanabe K, et al. Incidence of radiologically-confirmed pneumonia and Haemophilus influenzae type b carriage before Haemophilus influenzae type b conjugate vaccine introduction in Central Vietnam. J Pediatr. 2013;163(1 suppl):S38–43. doi: 10.1016/j.jpeds.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 136.Pilishvili T, Chernyshova L, Bondarenko A, et al. Evaluation of the effectiveness of Haemophilus influenzae type b conjugate vaccine introduction against radiologically-confirmed hospitalized pneumonia in young children in Ukraine. J Pediatr. 2013;163(1 suppl):S12–18. doi: 10.1016/j.jpeds.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 137.Roseman C, Truedsson L, Kapetanovic MC. The effect of smoking and alcohol consumption on markers of systemic inflammation, immunoglobulin levels and immune response following pneumococcal vaccination in patients with arthritis. Arthritis Res Ther. 2012;14:R170. doi: 10.1186/ar3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lujan M, Burgos J, Gallego M, et al. Effects of immunocompromise and comorbidities on pneumococcal serotypes causing invasive respiratory infection in adults: implications for vaccine strategies. Clin Infect Dis. 2013;57:1722–30. doi: 10.1093/cid/cit640. [DOI] [PubMed] [Google Scholar]
- 139.de Andrade AL, de Andrade JG, Martelli CM, et al. Effectiveness of Haemophilus influenzae B conjugate vaccine on childhood pneumonia: a case-control study in Brazil. Int J Epidemiol. 2004;33:173–81. doi: 10.1093/ije/dyh025. [DOI] [PubMed] [Google Scholar]
- 140.Singleton RJ, Butler JC, Bulkow LR, et al. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska native adults. Vaccine. 2007;25:2288–95. doi: 10.1016/j.vaccine.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 141.Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–55. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 142.Suzuki M, Yoshimine H, Harada Y, et al. Estimating the influenza vaccine effectiveness against medically attended influenza in clinical settings: a hospital-based case-control study with a rapid diagnostic test in Japan. PLoS One. 2013;8:e52103. doi: 10.1371/journal.pone.0052103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Turner P, Turner C, Jankhot A, et al. A longitudinal study of Streptococcus pneumoniae carriage in a cohort of infants and their mothers on the Thailand-Myanmar border. PLoS One. 2012;7:e38271. doi: 10.1371/journal.pone.0038271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun W, Jacoby P, Riley TV, et al. Association between early bacterial carriage and otitis media in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia: a cohort study. BMC Infect Dis. 2012;12:366. doi: 10.1186/1471-2334-12-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tigoi CC, Gatakaa H, Karani A, et al. Rates of acquisition of pneumococcal colonization and transmission probabilities, by serotype, among newborn infants in Kilifi District, Kenya. Clin Infect Dis. 2012;55:180–88. doi: 10.1093/cid/cis371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hsieh YC, Chiu CH, Chang KY, et al. The impact of the heptavalent pneumococcal conjugate vaccine on risk factors for Streptococcus pneumoniae carriage in children. Pediatr Infect Dis J. 2012;31:e163–68. doi: 10.1097/INF.0b013e31825cb9f9. [DOI] [PubMed] [Google Scholar]
- 147.Obrink-Hansen K, Sogaard OS, Harboe ZB, Schonheyder HC. Risk factors for pneumococcal nasopharyngeal colonization before and after pneumococcal conjugate vaccination in persons with HIV: brief report. Curr HIV Res. 2012;10:252–55. doi: 10.2174/157016212800618101. [DOI] [PubMed] [Google Scholar]
- 148.Mackenzie GA, Leach AJ, Carapetis JR, Fisher J, Morris PS. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC Infect Dis. 2010;10:304. doi: 10.1186/1471-2334-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rylance J, Gordon SB, Naeher LP, et al. Household air pollution: a call for studies into biomarkers of exposure and predictors of respiratory disease. Am J Physiol Lung Cell Mol Physiol. 2013;304:L571–78. doi: 10.1152/ajplung.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rudan I, Nair H, Marušić A, Campbell H. Reducing mortality from childhood pneumonia and diarrhoea: the leading priority is also the greatest opportunity. J Glob Health. 2013;3:010101. doi: 10.7189/jogh.03.010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Salvi S, Barnes PJ. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest. 2010;138:3–6. doi: 10.1378/chest.10-0645. [DOI] [PubMed] [Google Scholar]
- 152.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–73. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 153.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mehrotra A, Oluwole AM, Gordon SB. The burden of COPD in Africa: a literature review and prospective survey of the availability of spirometry for COPD diagnosis in Africa. Trop Med Int Health. 2009;14:840–48. doi: 10.1111/j.1365-3156.2009.02308.x. [DOI] [PubMed] [Google Scholar]
- 155.Zhou Y, Zou Y, Li X, et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med. 2014;11:e1001621. doi: 10.1371/journal.pmed.1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kurmi OP, Semple S, Simkhada P, Smith WC, Ayres JG. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax. 2010;65:221–28. doi: 10.1136/thx.2009.124644. [DOI] [PubMed] [Google Scholar]
- 157.Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138:20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 158.Eisner MD, Anthonisen N, Coultas D, et al. and the Committee on Nonsmoking COPD, Environmental and Occupational Health Assembly. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 159.Montaño M, Sansores RH, Becerril C, et al. FEV1 inversely correlates with metalloproteinases 1, 7, 9 and CRP in COPD by biomass smoke exposure. Respir Res. 2014;15:74. doi: 10.1186/1465-9921-15-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guarnieri MJ, Diaz JV, Basu C, et al. Effects of woodsmoke exposure on airway inflammation in rural Guatemalan women. PLoS One. 2014;9:e88455. doi: 10.1371/journal.pone.0088455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rivera RM, Cosio MG, Ghezzo H, Salazar M, Pérez-Padilla R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis. 2008;12:972–77. [PubMed] [Google Scholar]
- 162.Moran-Mendoza O, Pérez-Padilla JR, Salazar-Flores M, Vazquez-Alfaro F. Wood smoke-associated lung disease: a clinical, functional, radiological and pathological description. Int J Tuberc Lung Dis. 2008;12:1092–98. [PubMed] [Google Scholar]
- 163.González-García M, Maldonado Gomez D, Torres-Duque CA, et al. Tomographic and functional findings in severe COPD: comparison between the wood smoke-related and smoking-related disease. J Bras Pneumol. 2013;39:147–54. doi: 10.1590/S1806-37132013000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med. 2006;173:393–97. doi: 10.1164/rccm.200504-568OC. [DOI] [PubMed] [Google Scholar]
- 165.Romieu I, Riojas-Rodríguez H, Marrón-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med. 2009;180:649–56. doi: 10.1164/rccm.200810-1556OC. [DOI] [PubMed] [Google Scholar]
- 166.Smith-Sivertsen T, Díaz E, Pope D, et al. Effect of reducing indoor air pollution on women’s respiratory symptoms and lung function: the RESPIRE Randomized Trial, Guatemala. Am J Epidemiol. 2009;170:211–20. doi: 10.1093/aje/kwp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wong GW, Brunekreef B, Ellwood P, et al. and the ISAAC Phase Three Study Group. Cooking fuels and prevalence of asthma: a global analysis of phase three of the International Study of Asthma and Allergies in Childhood (ISAAC) Lancet Respir Med. 2013;1:386–94. doi: 10.1016/S2213-2600(13)70073-0. [DOI] [PubMed] [Google Scholar]
- 168.Redding GJ, Singleton RJ, Valery PC, et al. Respiratory exacerbations in indigenous children from two countries with non-cystic fibrosis chronic suppurative lung disease/bronchiectasis. Chest. 2014 doi: 10.1378/chest.14-0126. published online May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singleton RJ, Valery PC, Morris P, et al. Indigenous children from three countries with non-cystic fibrosis chronic suppurative lung disease/bronchiectasis. Pediatr Pulmonol. 2014;49:189–200. doi: 10.1002/ppul.22763. [DOI] [PubMed] [Google Scholar]
- 170.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 171.Bonjour S, Adair-Rohani H, Wolf J, et al. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ Health Perspect. 2013;121:784–90. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hosgood HD, 3rd, Wei H, Sapkota A, et al. Household coal use and lung cancer: systematic review and meta-analysis of case-control studies, with an emphasis on geographic variation. Int J Epidemiol. 2011;40:719–28. doi: 10.1093/ije/dyq259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kurmi OP, Arya PH, Lam KB, Sorahan T, Ayres JG. Lung cancer risk and solid fuel smoke exposure: a systematic review and meta-analysis. Eur Respir J. 2012;40:1228–37. doi: 10.1183/09031936.00099511. [DOI] [PubMed] [Google Scholar]
- 174.Pokhrel AK, Smith KR, Khalakdina A, Deuja A, Bates MN. Case-control study of indoor cooking smoke exposure and cataract in Nepal and India. Int J Epidemiol. 2005;34:702–08. doi: 10.1093/ije/dyi015. [DOI] [PubMed] [Google Scholar]
- 175.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Household use of solid fuels and high-temperature frying. IARC Monogr Eval Carcinog Risks Hum. 2010;95:1–430. [PMC free article] [PubMed] [Google Scholar]
- 176.Liu BQ, Peto R, Chen ZM, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–22. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Smith KR, Mehta S, Feuz M. Indoor smoke from household solid fuels. In: Ezzati M, Rodgers AD, Lopez AD, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease due to selected major risk factors. Geneva: World Health Organization; 2004. [Google Scholar]
- 178.Hosgood HD, 3rd, Chapman RS, Wei H, et al. Coal mining is associated with lung cancer risk in Xuanwei, China. Am J Ind Med. 2012;55:5–10. doi: 10.1002/ajim.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.International Energy Agency. [accessed Aug 18, 2014];World Energy Outlook 2012. 2012 http://www.iea.org/publications/freepublications/publication/WEO2012_free.pdf.
- 180.Naeher LP, Brauer M, Lipsett M, et al. Woodsmoke health effects: a review. Inhal Toxicol. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- 181.Mumford JL, Li X, Hu F, Lu XB, Chuang JC. Human exposure and dosimetry of polycyclic aromatic hydrocarbons in urine from Xuan Wei, China with high lung cancer mortality associated with exposure to unvented coal smoke. Carcinogenesis. 1995;16:3031–36. doi: 10.1093/carcin/16.12.3031. [DOI] [PubMed] [Google Scholar]
- 182.Campbell JA. Carcinogenic agents present in the atmosphere and incidence of primary tumors in mice. Br J Exp Pathol. 1939;20:122–32. [Google Scholar]
- 183.Seelig MG, Benignus EL. Coal smoke soot and tumors of the lung in mice. Am J Cancer. 1936;28:96–111. [Google Scholar]
- 184.Passey RD, Carter-Braine J. Experimental soot cancer. J Pathol Bacteriol. 1925;28:133–44. [Google Scholar]
- 185.Liang CK, Guan NY, Ma F, Zhang Y, Wang EM, Yin XR. Carcinogenicity of extract of soot from xuan wei county after administering subcutaneously to mice. In: Chu EY, Generoso W, editors. Mutation, cancer, and malformation. New York: Springer; 1984. pp. 826–27. [Google Scholar]
- 186.Mumford JL, He XZ, Chapman RS, et al. Lung cancer and indoor air pollution in Xuan Wei, China. Science. 1987;235:217–20. doi: 10.1126/science.3798109. [DOI] [PubMed] [Google Scholar]
- 187.Liang CK, Quan NY, Cao SR, He XZ, Ma F. Natural inhalation exposure to coal smoke and wood smoke induces lung cancer in mice and rats. Biomed Environ Sci. 1988;1:42–50. [PubMed] [Google Scholar]
- 188.Lin C, Dai X, Sun X. Expression of oncogene and anti-oncogene in mouse lung cancer induced by coal-burning smoke. Zhonghua Zhong Liu Za Zhi. 1995;17:432–34. (in Chinese) [PubMed] [Google Scholar]
- 189.Sulman E, Sulman F. The carcinogenicity of wood soot from the chimney of a smoked sausage factory. Cancer Res. 1946;6:366. [PubMed] [Google Scholar]
- 190.Khesina AI, Gaevaia TI, Linnik AB. Polycyclic aromatic hydrocarbon content and carcinogenic activity of soot extracts from the heating systems. Gig Sanit. 1977;8:107–09. (in Chinese) [PubMed] [Google Scholar]
- 191.Mumford JL, He XZ, Chapman RS. Human lung cancer risks due to complex organic mixtures of combustion emissions. Recent Results Cancer Res. 1990;120:181–89. doi: 10.1007/978-3-642-84068-5_13. [DOI] [PubMed] [Google Scholar]
- 192.Mumford JL, Helmes CT, Lee XM, Seidenberg J, Nesnow S. Mouse skin tumorigenicity studies of indoor coal and wood combustion emissions from homes of residents in Xuan Wei, China with high lung cancer mortality. Carcinogenesis. 1990;11:397–403. doi: 10.1093/carcin/11.3.397. [DOI] [PubMed] [Google Scholar]
- 193.McDonald JD, White RK, Barr EB, Zielinska B, Chow JC, Grosjean E. Generation and characterization of hardwood smoke inhalation exposure atmospheres. Aerosol Sci Technol. 2006;40:573–84. [Google Scholar]
- 194.Reed MD, Campen MJ, Gigliotti AP, et al. Health effects of subchronic exposure to environmental levels of hardwood smoke. Inhal Toxicol. 2006;18:523–39. doi: 10.1080/08958370600685707. [DOI] [PubMed] [Google Scholar]
- 195.Lewtas J. Complex mixtures of air pollutants: characterizing the cancer risk of polycyclic organic matter. Environ Health Perspect. 1993;100:211–18. doi: 10.1289/ehp.93100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lewtas J, Mumford J, Everson RB, et al. Comparison of DNA adducts from exposure to complex mixtures in various human tissues and experimental systems. Environ Health Perspect. 1993;99:89–97. doi: 10.1289/ehp.939989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group Carcinogenicity of household solid fuel combustion and of high-temperature frying. Lancet Oncol. 2006;7:977–78. doi: 10.1016/s1470-2045(06)70969-x. [DOI] [PubMed] [Google Scholar]
- 198.Ross JA, Nesnow S. Polycyclic aromatic hydrocarbons: correlations between DNA adducts and ras oncogene mutations. Mutat Res. 1999;424:155–66. doi: 10.1016/s0027-5107(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 199.Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 200.Ferlay J, Soerjomataram I, Ervik M, et al. Globocan 2012 v1.0, cancer incidence and mortality worldwide. [accessed Aug 18, 2014];IARC CancerBase No. 11. 2013 http://www.iarc.fr/en/publications/eresources/cancerbases/ [Google Scholar]
- 201.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–90. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 202.Smith KR, Mehta S, Maeusezahl-Feuz M. Indoor smoke from household solid fuels. In: Ezzati M, Rogers AD, Lopez DA, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease due to selected major risk factors. Geneva: World Health Organization; 2004. pp. 1435–93. [Google Scholar]
- 203.Niu SR, Yang GH, Chen ZM, et al. Emerging tobacco hazards in China: 2. Early mortality results from a prospective study. BMJ. 1998;317:1423–24. doi: 10.1136/bmj.317.7170.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts; prospective analyses from the ESCAPE. Lancet Oncol. 2013;14:813–22. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 205.Barone-Adesi F, Chapman RS, Silverman DT, et al. Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ. 2012;345:e5414. doi: 10.1136/bmj.e5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Maier H, Dietz A, Gewelke U, Heller WD, Weidauer H. Tobacco and alcohol and the risk of head and neck cancer. Clin Investig. 1992;70:320–27. doi: 10.1007/BF00184668. [DOI] [PubMed] [Google Scholar]
- 207.Macfarlane TV, Macfarlane GJ, Oliver RJ, et al. The aetiology of upper aerodigestive tract cancers among young adults in Europe: the ARCAGE study. Cancer Causes Control. 2010;21:2213–21. doi: 10.1007/s10552-010-9641-3. [DOI] [PubMed] [Google Scholar]
- 208.Canova C, Hashibe M, Simonato L, et al. Genetic associations of 115 polymorphisms with cancers of the upper aerodigestive tract across 10 European countries: the ARCAGE project. Cancer Res. 2009;69:2956–65. doi: 10.1158/0008-5472.CAN-08-2604. [DOI] [PubMed] [Google Scholar]
- 209.Rees L, Birchall M, Bailey M, Thomas S. A systematic review of case-control studies of human papillomavirus infection in laryngeal squamous cell carcinoma. Clin Otolaryngol Allied Sci. 2004;29:301–06. doi: 10.1111/j.1365-2273.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 210.Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(suppl):559S–69S. doi: 10.1093/ajcn/78.3.559S. [DOI] [PubMed] [Google Scholar]
- 211.Goodman M, Morgan RW, Ray R, Malloy CD, Zhao K. Cancer in asbestos-exposed occupational cohorts: a meta-analysis. Cancer Causes Control. 1999;10:453–65. doi: 10.1023/a:1008980927434. [DOI] [PubMed] [Google Scholar]
- 212.Berrino F, Richiardi L, Boffetta P, et al. Milan JEM Working Group. Occupation and larynx and hypopharynx cancer: a job-exposure matrix approach in an international case-control study in France, Italy, Spain and Switzerland. Cancer Causes Control. 2003;14:213–23. doi: 10.1023/a:1023661206177. [DOI] [PubMed] [Google Scholar]
- 213.Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104:272–78. doi: 10.1016/j.radonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 214.Han MY, Yin GJ, Chen WQ. Meta-analysis of risk factors associated with nasopharyngeal carcinoma in China. China Trop Med (Chinese) 2005;5:1442–43. [Google Scholar]
- 215.Cai YL, Zheng YM, Tang MZ. Analysis of non-viral risk factors of nasopharyngeal carcinoma using logistic regression improved by principal component. Modern Prev Med (Chinese) 2009;36:2416–19. [Google Scholar]
- 216.Feng BJ, Khyatti M, Ben-Ayoub W, et al. Cannabis, tobacco and domestic fumes intake are associated with nasopharyngeal carcinoma in North Africa. Br J Cancer. 2009;101:1207–12. doi: 10.1038/sj.bjc.6605281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Jing SL, Zhu YM, Zeng SK. Risk factors for nasopharyngeal carcinoma in Yao nationally area in northen Guangdong: a case-control study. Contemporary Med (Chinese) 2010;16:158–59. [Google Scholar]
- 218.Chelleng PK, Narain K, Das HK, Chetia M, Mahanta J. Risk factors for cancer nasopharynx: a case-control study from Nagaland, India. Natl Med J India. 2000;13:6–8. [PubMed] [Google Scholar]
- 219.Mackay J, Amos A. Women and tobacco. Respirology. 2003;8:123–30. doi: 10.1046/j.1440-1843.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 220.Hosgood HD, 3rd, Boffetta P, Greenland S, et al. In-home coal and wood use and lung cancer risk: a pooled analysis of the International Lung Cancer Consortium. Environ Health Perspect. 2010;118:1743–47. doi: 10.1289/ehp.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tang L, Lim WY, Eng P, et al. Lung cancer in Chinese women: evidence for an interaction between tobacco smoking and exposure to inhalants in the indoor environment. Environ Health Perspect. 2010;118:1257–60. doi: 10.1289/ehp.0901587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lee MS, Hang JQ, Zhang FY, Dai HL, Su L, Christiani DC. In-home solid fuel use and cardiovascular disease: a cross-sectional analysis of the Shanghai Putuo study. Environ Health. 2012;11:18. doi: 10.1186/1476-069X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect. 2010;118:182–90. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mu L, Liu L, Niu R, et al. Indoor air pollution and risk of lung cancer among Chinese female non-smokers. Cancer Causes Control. 2013;24:439–50. doi: 10.1007/s10552-012-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yu IT, Chiu YL, Au JS, Wong TW, Tang JL. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006;66:4961–67. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- 226.Wang XR, Chiu YL, Qiu H, Au JS, Yu IT. The roles of smoking and cooking emissions in lung cancer risk among Chinese women in Hong Kong. Ann Oncol. 2009;20:746–51. doi: 10.1093/annonc/mdn699. [DOI] [PubMed] [Google Scholar]
- 227.Zhang Q, Gangupomu RH, Ramirez D, Zhu Y. Measurement of ultrafine particles and other air pollutants emitted by cooking activities. Int J Environ Res Public Health. 2010;7:1744–59. doi: 10.3390/ijerph7041744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Huang Y, Ho SS, Ho KF, Lee SC, Yu JZ, Louie PK. Characteristics and health impacts of VOCs and carbonyls associated with residential cooking activities in Hong Kong. J Hazard Mater. 2011;186:344–51. doi: 10.1016/j.jhazmat.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 229.Chen BH, Chen YC. Formation of polycyclic aromatic hydrocarbons in the smoke from heated model lipids and food lipids. J Agric Food Chem. 2001;49:5238–43. doi: 10.1021/jf0106906. [DOI] [PubMed] [Google Scholar]
- 230.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 231.Bennett DH, Koutrakis P. Determining the infiltration of outdoor particles in the indoor environment using a dynamic model. J Aerosol Sci. 2006;37:766–85. [Google Scholar]
- 232.Abt E, Suh HH, Catalano P, Koutrakis P. Relative contribution of outdoor and indoor particle sources to indoor concentrations. Environ Sci Technol. 2000;34:3579–87. [Google Scholar]
- 233.Allen R, Larson T, Sheppard L, Wallace L, Liu LJS. Use of real-time light scattering data to estimate the contribution of infiltrated and indoor-generated particles to indoor air. Environ Sci Technol. 2003;37:3484–92. doi: 10.1021/es021007e. [DOI] [PubMed] [Google Scholar]
- 234.Geller MD, Chang MH, Sioutas C, Ostro BD, Lipsett MJ. Indoor/outdoor relationship and chemical composition of fine and coarse particles in the southern California deserts. Atmos Environ. 2002;36:1099–110. [Google Scholar]
- 235.Sarnat SE, Coull BA, Ruiz PA, Koutrakis P, Suh HH. The influences of ambient particle composition and size on particle infiltration in Los Angeles, CA, residences. J Air Waste Manag Assoc. 2006;56:186–96. doi: 10.1080/10473289.2006.10464449. [DOI] [PubMed] [Google Scholar]
- 236.Salje H, Gurley ES, Homaira N, et al. Impact of neighborhood biomass cooking patterns on episodic high indoor particulate matter concentrations in clean fuel homes in Dhaka, Bangladesh. Indoor Air. 2014;24:213–20. doi: 10.1111/ina.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.King BA, Mirza SA, Babb SD GATS Collaborating Group. A cross-country comparison of secondhand smoke exposure among adults: findings from the Global Adult Tobacco Survey (GATS) Tob Control. 2013;22:e5. doi: 10.1136/tobaccocontrol-2012-050582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.DHEW U. [accessed Aug 18, 2014];The Health Consequences of Smoking. Reports of the Surgeon General 1964 Report on smoking and health: secondary smoking, individual rights, and public space. http://profiles.nlm.nih.gov/ps/retrieve/Narrative/NN/p-nid/60.
- 239.Apelberg BJ, Hepp LM, Avila-Tang E, et al. Environmental monitoring of secondhand smoke exposure. Tob Control. 2013;22:147–55. doi: 10.1136/tobaccocontrol-2011-050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wipfli H, Avila-Tang E, Navas-Acien A, et al. Famri Homes Study Investigators. Secondhand smoke exposure among women and children: evidence from 31 countries. Am J Public Health. 2008;98:672–79. doi: 10.2105/AJPH.2007.126631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kim S, Wipfli H, Navas-Acien A, et al. FAMRI Homes Study Investigators. Determinants of hair nicotine concentrations in nonsmoking women and children: a multicountry study of secondhand smoke exposure in homes. Cancer Epidemiol Biomarkers Prev. 2009;18:3407–14. doi: 10.1158/1055-9965.EPI-09-0337. [DOI] [PubMed] [Google Scholar]
- 242.Leaderer BP, Hammond SK. Evaluation of vapor-phase nicotine and respirable suspended particle mass as markers for environmental tobacco-smoke. Environ Sci Technol. 1991;25:770–77. [Google Scholar]
- 243.McCormack MC, Breysse PN, Hansel NN, et al. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ Res. 2008;106:148–55. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Johnson T, Long T, Ollison W. Prediction of hourly microenvironmental concentrations of fine particles based on measurements obtained from the Baltimore scripted activity study. J Expo Anal Environ Epidemiol. 2000;10:403–11. doi: 10.1038/sj.jea.7500093. [DOI] [PubMed] [Google Scholar]
- 245.Pollard SL, Williams DL, Breysse PN, et al. CRONICAS Cohort Study Group. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ Health. 2014;13:21. doi: 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–50. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 247.Wu WZ, Wang JX, Zhao GF, You L. The emission soot of biomass fuels combustion as a source of endocrine disrupters. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2002;37:579–600. doi: 10.1081/ese-120003239. [DOI] [PubMed] [Google Scholar]
- 248.Shen G, Xue M, Wei S, et al. Influence of fuel moisture, charge size, feeding rate and air ventilation conditions on the emissions of PM, OC, EC, parent PAHs, and their derivatives from residential wood combustion. J Environ Sci (China) 2013;25:1808–16. doi: 10.1016/s1001-0742(12)60258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shah ASV, Langrish JP, Nair H, et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382:1039–48. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Painschab MS, Davila-Roman VG, Gilman RH, et al. CRONICAS Cohort Study Group. Chronic exposure to biomass fuel is associated with increased carotid artery intima-media thickness and a higher prevalence of atherosclerotic plaque. Heart. 2013;99:984–91. doi: 10.1136/heartjnl-2012-303440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect. 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sherwood RJ, Greenhalgh DM. A personal air sampler. Ann Occup Hyg. 1960;2:127–32. [PubMed] [Google Scholar]
- 253.Delgado-Saborit JM. Use of real-time sensors to characterise human exposures to combustion related pollutants. J Environ Monit. 2012;14:1824–37. doi: 10.1039/c2em10996d. [DOI] [PubMed] [Google Scholar]
- 254.Gurley ES, Homaira N, Salje H, et al. Indoor exposure to particulate matter and the incidence of acute lower respiratory infections among children: a birth cohort study in urban Bangladesh. Indoor Air. 2013;23:379–86. doi: 10.1111/ina.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dionisio KL, Howie SRC, Dominici F, et al. The exposure of infants and children to carbon monoxide from biomass fuels in The Gambia: a measurement and modeling study. J Expo Sci Environ Epidemiol. 2012;22:173–81. doi: 10.1038/jes.2011.47. [DOI] [PubMed] [Google Scholar]
- 256.Jary HR, Kachidiku J, Banda H, et al. Feasibility of conducting a randomised controlled trial of a cookstove intervention in rural Malawi. Int J Tuberc Lung Dis. 2014;18:240–47. doi: 10.5588/ijtld.13.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Commodore AA, Hartinger SM, Lanata CF, et al. A pilot study characterizing real time exposures to particulate matter and carbon monoxide from cookstove related woodsmoke in rural Peru. Atmos Environ. 1994;2013:380–84. doi: 10.1016/j.atmosenv.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kulkarni N, Pierse N, Rushton L, Grigg J. Carbon in airway macrophages and lung function in children. N Engl J Med. 2006;355:21–30. doi: 10.1056/NEJMoa052972. [DOI] [PubMed] [Google Scholar]
- 259.Brugha RE, Mushtaq N, Round T, et al. Carbon in airway macrophages from children with asthma. Thorax. 2014;69:654–59. doi: 10.1136/thoraxjnl-2013-204734. [DOI] [PubMed] [Google Scholar]
- 260.Nwokoro C, Ewin C, Harrison C, et al. Cycling to work in London and inhaled dose of black carbon. Eur Respir J. 2012;40:1091–97. doi: 10.1183/09031936.00195711. [DOI] [PubMed] [Google Scholar]
- 261.Kulkarni NS, Prudon B, Panditi SL, Abebe Y, Grigg J. Carbon loading of alveolar macrophages in adults and children exposed to biomass smoke particles. Sci Total Environ. 2005;345:23–30. doi: 10.1016/j.scitotenv.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 262.Fullerton DG, Jere K, Jambo K, et al. Domestic smoke exposure is associated with alveolar macrophage particulate load. Trop Med Int Health. 2009;14:349–54. doi: 10.1111/j.1365-3156.2009.02230.x. [DOI] [PubMed] [Google Scholar]
- 263.Kalappanavar NK, Vinodkumar CS, Gouli C, et al. Carbon particles in airway macrophage as a surrogate marker in the early detection of lung diseases. Int J Occup Environ Med. 2012;3:68–75. [PubMed] [Google Scholar]
- 264.Berenson CS, Kruzel RL, Eberhardt E, Sethi S. Phagocytic dysfunction of human alveolar macrophages and severity of chronic obstructive pulmonary disease. J Infect Dis. 2013;208:2036–45. doi: 10.1093/infdis/jit400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Torres-Dosal A, Pérez-Maldonado IN, Jasso-Pineda Y, Martínez Salinas RI, Alegría-Torres JA, Díaz-Barriga F. Indoor air pollution in a Mexican indigenous community: evaluation of risk reduction program using biomarkers of exposure and effect. Sci Total Environ. 2008;390:362–68. doi: 10.1016/j.scitotenv.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 266.Riojas-Rodriguez H, Schilmann A, Marron-Mares AT, et al. Impact of the improved patsari biomass stove on urinary polycyclic aromatic hydrocarbon biomarkers and carbon monoxide exposures in rural Mexican women. Environ Health Perspect. 2011;119:1301–07. doi: 10.1289/ehp.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Li Z, Sjödin A, Romanoff LC, et al. Evaluation of exposure reduction to indoor air pollution in stove intervention projects in Peru by urinary biomonitoring of polycyclic aromatic hydrocarbon metabolites. Environ Int. 2011;37:1157–63. doi: 10.1016/j.envint.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 268.Clark M, Paulsen M, Smith KR, Canuz E, Simpson CD. Urinary methoxyphenol biomarkers and woodsmoke exposure: comparisons in rural Guatemala with personal CO and kitchen CO, levoglucosan, and PM2.5. Environ Sci Technol. 2007;410:3481–87. doi: 10.1021/es061524n. [DOI] [PubMed] [Google Scholar]
- 269.Migliaccio CT, Bergauff MA, Palmer CP, Jessop F, Noonan CW, Ward TJ. Urinary levoglucosan as a biomarker of wood smoke exposure: observations in a mouse model and in children. Environ Health Perspect. 2009;117:74–79. doi: 10.1289/ehp.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Avila-Tang E, Al-Delaimy WK, Ashley DL, et al. Assessing secondhand smoke using biological markers. Tob Control. 2013;22:164–71. doi: 10.1136/tobaccocontrol-2011-050298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000;11:427–33. doi: 10.1097/00001648-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 272.Elm C, Rohde M, Vaerman JP, Chhatwal GS, Hammerschmidt S. Characterization of the interaction of the pneumococcal surface protein SpsA with the human polymeric immunoglobulin receptor (hpIgR) Indian J Med Res. 2004;119(suppl):61–65. [PubMed] [Google Scholar]
- 273.Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A. Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. Lancet. 2014;383:1549–60. doi: 10.1016/S0140-6736(14)60082-9. [DOI] [PubMed] [Google Scholar]
- 274.Thompson LM, Bruce N, Eskenazi B, Diaz A, Pope D, Smith KR. Impact of reduced maternal exposures to wood smoke from an introduced chimney stove on newborn birth weight in rural Guatemala. Environ Health Perspect. 2011;119:1489–94. doi: 10.1289/ehp.1002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wylie BJ, Coull BA, Hamer DH, et al. Impact of biomass fuels on pregnancy outcomes in central East India. Environ Health. 2014;13:1. doi: 10.1186/1476-069X-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis. 2012;4:368–76. doi: 10.3978/j.issn.2072-1439.2012.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect. 2010;118:167–76. doi: 10.1289/ehp.0900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Harris AM, Sempértegui F, Estrella B, et al. Air pollution and anemia as risk factors for pneumonia in Ecuadorian children: a retrospective cohort analysis. Environ Health. 2011;10:93. doi: 10.1186/1476-069X-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.de Benoist Bruno, McLean Erin, Egli Ines, Cogswell Mary., editors. WHO. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. 2008. [accessed Aug 18, 2014]. http://www.who.int/vmnis/publications/anaemia_prevalence/en/ [Google Scholar]
- 280.Zeka A, Zanobetti A, Schwartz J. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006;163:849–59. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]
- 281.Kim CS, Hu SC. Regional deposition of inhaled particles in human lungs: comparison between men and women. J Appl Physiol. 1985;1998:1834–44. doi: 10.1152/jappl.1998.84.6.1834. [DOI] [PubMed] [Google Scholar]
- 282.Meding B. Differences between the sexes with regard to work-related skin disease. Contact Dermat. 2000;43:65–71. doi: 10.1034/j.1600-0536.2000.043002065.x. [DOI] [PubMed] [Google Scholar]
- 283.WHO. WHO Air quality guidelines global update 2005. Copenhagen: World Health Organization; 2006. [Google Scholar]
- 284.WHO. WHO guidelines for indoor air quality: selected pollutants. Bonn: World Health Organization; 2010. [PubMed] [Google Scholar]
- 285.Martin WJ, 2nd, Glass RI, Araj H, et al. Household air pollution in low- and middle-income countries: health risks and research priorities. PLoS Med. 2013;10:e1001455. doi: 10.1371/journal.pmed.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bruce N, Dora C, Krzyzanowski M, Adair-Rohani H, Wangchuk T, Morawska L. Tackling the health burden from household air pollution (HAP): development and implementation of new WHO Guidelines. Air Quality and Climate Change. 2013;47:32–38. [Google Scholar]
- 287.Pope CA, 3rd, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–48. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 288.Pope CA, 3rd, Burnett RT, Turner MC, et al. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119:1616–21. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Smith KR, Peel JL. Mind the gap. Environ Health Perspect. 2010;118:1643–45. doi: 10.1289/ehp.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Burnett RT, Pope CA, 3rd, Ezzati M, et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ Health Perspect. 2014;122:397–403. doi: 10.1289/ehp.1307049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Smith KR, McCracken JP, Thompson L, et al. Personal child and mother carbon monoxide exposures and kitchen levels: methods and results from a randomized trial of woodfired chimney cookstoves in Guatemala (RESPIRE) J Expo Sci Environ Epidemiol. 2010;20:406–16. doi: 10.1038/jes.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.McCracken JPSJ, Schwartz J, Diaz A, Bruce N, Smith KR. Longitudinal relationship between personal CO and personal PM2.5 among women cooking with woodfired cookstoves in Guatemala. PLoS One. 2013;8:e55670. doi: 10.1371/journal.pone.0055670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Balakrishnan K, Ghosh S, Ganguli B, et al. State and national household concentrations of PM2.5 from solid cookfuel use: results from measurements and modeling in India for estimation of the global burden of disease. Environ Health. 2013;12:77. doi: 10.1186/1476-069X-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Jetter J, Zhao Y, Smith KR, et al. Pollutant emissions and energy efficiency under controlled conditions for household biomass cookstoves and implications for metrics useful in setting international test standards. Environ Sci Technol. 2012;46:10827–34. doi: 10.1021/es301693f. [DOI] [PubMed] [Google Scholar]
- 295.Clark ML, Peel JL, Balakrishnan K, et al. Health and household air pollution from solid fuel use: the need for improved exposure assessment. Environ Health Perspect. 2013;121:1120–28. doi: 10.1289/ehp.1206429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.CEIHD. Indoor air pollution monitoring summary for Gaia association-Ethiopiaís clean cook stove tests in the Bonga refugee camp Gambella regional state Ethiopia. Berkeley: Center for Entrepreneurship in International Health and Development; 2007. [Google Scholar]
- 297.Practical Action Consulting PAC. [accessed Aug 18, 2014];Ethanol as a household fuel in Madagascar: health benefits, economic assessment and review of African lessons for scaling up. Component A - analysis of household air pollution interventions in Madagascar. 2011 http://practicalaction.org/consulting-madagascar-ethanol-household-fuel.
- 298.Bates E, Bruce N. Cooking interventions to reduce household air pollution in Sudan. Intermediate Technology Development Group; 2005. [Google Scholar]
- 299.Röllin HB, Mathee A, Bruce N, Levin J, von Schirnding YE. Comparison of indoor air quality in electrified and un-electrified dwellings in rural South African villages. Indoor Air. 2004;14:208–16. doi: 10.1111/j.1600-0668.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 300.Terrado EN, Eitel B. Pilot commercialization of improved cookstoves in Nicaragua. Washington DC: Energy Sector Management Assistance Program; 2005. [Google Scholar]
- 301.Bates E, Pope D, Bruce N. Smoke, health and household energy Volume 1: Participatory methods for design, installation, monitoring and assessment of smoke alleviation technologies. Rugby: Intermediate Technology Development Group; 2005. [Google Scholar]
- 302.Lam NL, Smith KR, Gauthier A, Bates MN. Kerosene: a review of household uses and their hazards in low- and middle-income countries. J Toxicol Environ Health B Crit Rev. 2012;15:396–432. doi: 10.1080/10937404.2012.710134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lin W, Brunekreef B, Gehring U. Meta-analysis of the effects of indoor nitrogen dioxide and gas cooking on asthma and wheeze in children. Int J Epidemiol. 2013;42:1724–37. doi: 10.1093/ije/dyt150. [DOI] [PubMed] [Google Scholar]
- 304.Hasselblad V, Eddy DM, Kotchmar DJ. Synthesis of environmental evidence: nitrogen dioxide epidemiology studies. J Air Waste Manage Assoc. 1992;42:662–71. doi: 10.1080/10473289.1992.10467018. [DOI] [PubMed] [Google Scholar]
- 305.Vrijheid M. Commentary: gas cooking and child respiratory health–time to identify the culprits? Int J Epidemiol. 2013;42:1737–39. doi: 10.1093/ije/dyt189. [DOI] [PubMed] [Google Scholar]
- 306.Ballard-Tremeer G, Mathee A. Review of interventions to reduce the exposure of women and young children to indoor air pollution in developing countries. USAID/WHO International Consultation on Household Energy. Indoor Air Pollution and Health; Washington, DC: 2000. pp. 4–6. Background paper for. [Google Scholar]
- 307.Barnes BR, Mathee A, Shafritz LB, Krieger L, Zimicki S. A behavioral intervention to reduce child exposure to indoor air pollution: identifying possible target behaviors. Health Educ Behav. 2004;31:306–17. doi: 10.1177/1090198103260630. [DOI] [PubMed] [Google Scholar]
- 308.Zhou Z, Jin Y, Liu F, et al. Community effectiveness of stove and health education interventions for reducing exposure to indoor air pollution from solid fuels in four Chinese provinces. Environ Res Lett. 2006;1:014010. [Google Scholar]
- 309.Rehfuess EA, Puzzolo E, Stanistreet D, Pope D, Bruce NG. Enablers and barriers to large-scale uptake of improved solid fuel stoves: a systematic review. Environ Health Perspect. 2014;122:120–30. doi: 10.1289/ehp.1306639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lewis JJ, Pattanayak SK. Who adopts improved fuels and cookstoves? A systematic review. Environ Health Perspect. 2012;120:637–45. doi: 10.1289/ehp.1104194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Puzzolo E, Stanistreet D, Pope D, Bruce N, Rehfuess E. What are the factors influencing the large-scale uptake by households of cleaner and more efficient household energy technologies? London: Evidence for Policy and Practice Information and Co-ordinating Centre; 2013. [Google Scholar]
- 312.Budya H, Arofat M. Providing cleaner energy access in Indonesia through the megaproject of kerosene conversion to LPG. Energy Policy. 2011;39:7575–86. [Google Scholar]
- 313.Lan Q, Chapman RS, Schreinemachers DM, Tian L, He X. Household stove improvement and risk of lung cancer in Xuanwei, China. J Natl Cancer Inst. 2002;94:826–35. doi: 10.1093/jnci/94.11.826. [DOI] [PubMed] [Google Scholar]
- 314.Chapman RS, He X, Blair AE, Lan Q. Improvement in household stoves and risk of chronic obstructive pulmonary disease in Xuanwei, China: retrospective cohort study. BMJ. 2005;331:1050–55. doi: 10.1136/bmj.38628.676088.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Lin HH, Suk CW, Lo HL, Huang RY, Enarson DA, Chiang CY. Indoor air pollution from solid fuel and tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2014;18:613–21. doi: 10.5588/ijtld.13.0765. [DOI] [PubMed] [Google Scholar]
- 316.Edwards RD, Liu Y, He G, et al. Household CO and PM measured as part of a review of China’s National Improved Stove Program. Indoor Air. 2007;17:189–203. doi: 10.1111/j.1600-0668.2007.00465.x. [DOI] [PubMed] [Google Scholar]
- 317.Sinton JE, Smith KR, Peabody JW, et al. An assessment of programs to promote improved household stoves in China. Energy Sustain Dev. 2004;8:33–52. [Google Scholar]
- 318.ISO. IWA 11: 2012 Guidelines for evaluating cookstove performance. Geneva: International Organisation on Standardisation; 2012. [Google Scholar]
- 319.ISO. [accessed March 28, 2014];ISO/TC 285 Clean cookstoves and clean cooking solutions. 2014 http://www.iso.org/iso/home/standards_development/list_of_iso_technical_committees/iso_technical_committee.htm?commid=4857971.