Abstract
The retrograde response signals mitochondrial status to the nucleus, compensating for accumulating mitochondrial dysfunction during Saccharomyces cerevisiae aging and extending replicative lifespan. The histone acetylase Gcn5 is required for activation of nuclear genes and lifespan extension in the retrograde response. It is part of the transcriptional coactivators SAGA and SLIK, but it is not known which of these complexes is involved. Genetic manipulation showed that these complexes perform interchangeably in the retrograde response. These results, along with the finding that the histone deacetylase Sir2 was required for a robust retrograde response informed a bioinformatics screen that reduced to four the candidate genes causal for longevity of the 410 retrograde response target genes. Of the four, only deletion of PHO84 suppressed lifespan extension. Retrograde-response activation of PHO84 displayed some preference for SAGA. Increased PHO84 messenger RNA levels from a second copy of the gene in cells in which the retrograde response is not activated achieved >80% of the lifespan extension observed in the retrograde response. Our studies resolve questions involving the roles of SLIK and SAGA in the retrograde response, pointing to the cooperation of these complexes in gene activation. They also finally pinpoint the gene that is both necessary and sufficient to extend replicative lifespan in the retrograde response. The finding that this gene is PHO84 opens up a new set of questions about the mechanisms involved, as this gene is known to have pleiotropic effects.
Keywords: mitochondria, transcriptional coactivation, SAGA/SLIK, Sir2, Pho84
THE retrograde response is an intracellular signaling pathway that communicates mitochondrial dysfunction to the nucleus, resulting in myriad changes in nuclear gene expression (Parikh et al. 1987; Liu and Butow 2006). These changes portend major metabolic adjustments and enhanced resistance to stress. Among those that stand out are the up-regulation of anaplerotic pathways, the generation of α-ketoglutarate for biosynthetic processes, and the ability to mobilize acetate both for energy and for net production of macromolecules in the absence of a fully functional, mitochondrial electron transport chain (Jazwinski 1999). The retrograde response was first identified in Saccharomyces cerevisiae (budding yeast), but it has since been demonstrated in Caenorhabditis elegans, Drosophila melanogaster, Mus musculus, and in human cells in tissue culture (Dell’agnello et al. 2007; Passos et al. 2007; Caldeira da Silva et al. 2008; Lapointe and Hekimi 2008; Copeland et al. 2009; Lee et al. 2010; Yang and Hekimi 2010; Durieux et al. 2011; Liu et al. 2011; Walter et al. 2011; Liu et al. 2012; Jazwinski 2015; Mishur et al. 2016). The retrograde response is a compensatory pathway (Jazwinski 2014) whose activation extends yeast replicative lifespan (RLS) (Kirchman et al. 1999). Similarly, it has a life extending effect in the other aging models listed above (Jazwinski 2015). Interestingly, the metabolic gene expression changes found under conditions of nutrient limitation that extend yeast RLS resemble those shown in the retrograde response (Jiang et al. 2000, 2002; Wang et al. 2010). The primary signal that triggers the yeast retrograde response is the drop in mitochondrial membrane potential (Miceli et al. 2011), although reduced ATP levels may play a secondary role (Zhang et al. 2013). Reactive oxygen species (ROS) signaling is not involved; however, mitochondrial ROS are a signal that is relevant for chronological lifespan extension in yeast (Pan et al. 2011).
One of the multitude of changes in nuclear gene expression is an increase in CIT2 messenger RNA (mRNA) levels, which is most frequently used as a diagnostic for activation of the retrograde response (Liao et al. 1991). However, induction of this gene is not necessary for RLS extension upon retrograde-response activation (Kirchman et al. 1999). Given the large number of genes activated (Epstein et al. 2001; Traven et al. 2001), the task of identifying the one gene or the many genes that act together to affect longevity is a daunting task that has not been completed up until now.
Our interest in the role of chromatin-dependent gene regulation in yeast longevity (Jazwinski 1999, 2005) led us to examine the impact of transcriptional coactivator and corepressor complexes on RLS. We determined that the histone deacetylases Rpd3 and Sir2 both have large effects on RLS but in opposite directions (Kim et al. 1999). Deletion of RPD3 extends, while deletion of SIR2 curtails it. In fact, Sir2 was shown to function in yeast longevity by two mechanisms (Kaeberlein et al. 1999). Rpd3 is part of the Rpd3L transcriptional corepressor complex with Sin3 that typically possesses the subunits Ume1, Ume6, Sds3, Sap30, and Pho23 (Yang and Seto 2008; Lardenois et al. 2015) and opposes the Gcn5-containing coactivator complex at many sites throughout the genome (Huisinga and Pugh 2004; Lardenois et al. 2015). This implies that activation of transcription of certain genes is important for longevity. This conclusion was supported by our observation made concurrently with our work on Rpd3 that deletion of GCN5 shortens RLS. This analysis was expanded to examine the potential function of Gcn5 in the retrograde response, and we determined that deletion of GCN5 prevents activation of CIT2 and the extension of RLS caused by retrograde-response activation (Kim et al. 2004).
The histone acetyltransferase Gcn5 is part of the large transcriptional coactivator complex SAGA, which is conserved throughout phylogeny (Sterner et al. 1999; Srivastava et al. 2015). SAGA dominates over another transcriptional coactivator TFIID at ∼10% of yeast genes, which contain TATA boxes and are primarily involved in stress responses (Huisinga and Pugh 2004). However, SAGA can be found throughout the yeast genome, as shown recently (Bonnet et al. 2014). SAGA is also responsible for the retention of DNA circles in mother cells during the yeast RLS (Denoth-Lippuner et al. 2014).
More recently, a transcriptional coactivator complex called SLIK/SALSA, closely related to SAGA, was identified (Pray-Grant et al. 2002; Sterner et al. 2002). It is distinguished from SAGA by the absence of Spt8 and the presence of Rtg2 protein in its place. The conversion of SAGA to SLIK requires the specific, proteolytic truncation of another SAGA component, Spt7 (Wu and Winston 2002; Mischerikow et al. 2009; Spedale et al. 2010). SAGA with full-length Spt7 contains Spt8, while in SLIK with the truncated Spt7, the Spt8 is substituted by Rtg2 (Pray-Grant et al. 2002). SLIK was claimed to be the coactivator involved in the retrograde response by virtue of the detection of Rtg2 at the promoter of CIT2 and by an increase in CIT2 mRNA levels (Pray-Grant et al. 2002). However, these events were induced by growth on acetate in this study, which requires a fully active mitochondrial electron transport chain, unlike the retrograde response described earlier. Induction of CIT2 expression in this retrograde response is activated by mitochondrial dysfunction and requires RTG2 (Liao and Butow 1993). Although Rtg2 appears to function only in the cytoplasm to support the translocation of the transcription factor Rtg1–Rtg3 into the nucleus, which is required for induction of retrograde-response target genes (Sekito et al. 2000), we documented a separate role for Rtg2 in the nucleus in promoting genome stability (Borghouts et al. 2004). Thus, the function of the SLIK complex in retrograde signaling is not entirely clear.
Clarification of the role of SLIK compared to SAGA in the retrograde response triggered by mitochondrial dysfunction from the perspective of both gene activation and RLS extension remains to be accomplished. Here, we have separated the activities of these closely related complexes by gene manipulation, and we have determined the phenotypic effects of each complex. This analysis shows that they function largely interchangeably. We have used this information in conjunction with other gene expression studies to narrow the focus to 4 genes of the 410 retrograde-response target genes. Testing these 4 genes individually has allowed us for the first time to identify the gene whose activation is both necessary and sufficient to result in the RLS extension afforded by the retrograde response. As part of these analyses, we have also uncovered a new role of Sir2 in retrograde signaling and RLS extension.
Materials and Methods
Yeast strains and growth conditions
All strains used originated from S. cerevisiae strain YPK9 (MATa, ade2-101ochre his3-Δ200 leu2-Δ1 lys2-801amber trp1-Δ63 ura3-52 [rho+]) and its rho0 derivative YJR2 (Kirchman et al. 1999; Miceli et al. 2011).
SPT7 mutants were created as follows: SPT7 was amplified from yeast genomic DNA by PCR and cloned into pRS406 (Sikorski and Hieter 1989) between the EcoRI and XhoI sites. The SPT7 sequence was verified by DNA sequencing. Site-directed mutagenesis was performed to create the L1141V, L1142V, and S200 (Spt7 without amino acids 1125–1150) mutants. Site-directed mutagenesis was performed using the Quick Change Site-Directed Mutagenesis protocol (Stratagene, La Jolla, CA) and further modified (Wang and Malcolm 2002). The primers used for mutagenesis were: 5′-ATC TGT TCC ATT ACA G GTA CTG ACT ACT CAG TTT C-3′ (SPT7 L1141V forward), 5′-G AAA CTG AGT AGT CAG TAC C TGT AAT GGA ACA GAT-3′ (SPT7 L1141V reverse), 5′-CT GTT CCA TTA CAG TTA GTG ACT ACT CAG TTT CAA AC-3′ (SPT7 L1142V forward), 5′-GT TTG AAA CTG AGT AGT CAC TAA CTG TAA TGG AAC AG-3′ (SPT7 L1142V reverse), 5′-TTT GGT TTT AGA GAG CTT GGG GAA ACC AAA GTG CAG G-3′ (SPT7 S200 forward), 5′-C CTG CAC TTT GGT TTC CCC AAG CTC TCT AAA ACC AAA-3′ (SPT7 S200 reverse). (Sequence changes for single amino acid substitutions are italicized.) Mutants were confirmed by DNA sequencing. The pRS406 plasmids with the mutants were digested with NruI and individually transformed into the yeast strain by selecting for uracil prototrophy. Positive strains were grown and counterselected using fluorouracil, to evict the copy of URA3. Mutant strains were confirmed by sequencing of genomic DNA.
The ADA2, SPT7, SPT8, MDH1, and PHO84 deletion strains were all constructed similarly. Deletion strains were purchased from Open Biosystems. The respective deletion cassettes containing the kanMX marker were amplified from genomic DNA using PCR primers containing sequences flanking the deleted genes. These were purified and used in a one-step gene replacement, by selecting for G418 resistance. All strains were verified by PCR using primers flanking the gene and an internal primer specific for kanMX. The BAP2 deletion strain was constructed differently. First, the natMX4 module in plasmid pAG25 (Goldstein and McCusker 1999) was amplified by PCR using primers containing BAP2 upstream and downstream sequences. The purified DNA was then transformed into the relevant yeast strain by selection for resistance to nourseothricin. Strains were confirmed by PCR using primers flanking the gene and an internal primer specific for natMX4.
The 2XPHO84 strain was created by first replacing the ura3-52 mutant in YPK9 by the URA3 gene PCR amplified from plasmid pRS406 and selection for uracil prototrophy. Then, PHO84 was PCR amplified from genomic DNA with its promoter and 3′-UTR, and this DNA was cloned into pRS406. PHO84, flanked by URA3 sequences, was released from the plasmid by restriction enzyme digestion and gel purified. This fragment was then transformed into the YPK9 URA3 strain. Growth on 5-fluorouracil was used to select for YPK9 ura3-52::PHO84. This strain was confirmed by DNA sequencing.
Yeast strains were grown in YPD (2% peptone, 1% yeast extract, 2% glucose) or for selection of strains for prototrophy in SC medium lacking the relevant nutrient (Sherman 1991). For extrachromosomal ribosomal DNA circle (ERC) determinations, adenine sulfate was added to YPD medium to a final concentration of 50 μg/ml (YPAD). Solid medium contained 2% agar. Incubations were at 30°. YPK9 and its derivatives were cultured overnight in YPG (2% peptone, 1% yeast extract, 3% glycerol) prior to spotting on YPD plates for initiation of RLS determinations.
RNA isolation and reverse transcription, real-time quantitative PCR
One to 5 ml of yeast culture was harvested in midexponential growth phase. Cells were washed, resuspended in 400 µl TES buffer (10 mM Tris-HCl, 10 mM EDTA, 0.5% SDS, pH 7.5), and incubated for 1 hr at 65° with 400 µl of acidic phenol (Sigma, St. Louis, MO; pH 4.3) with mixing every 15 min. The samples were placed on ice for 5 min and centrifuged. The aqueous phase was removed and extracted with chloroform. RNA was precipitated at −20° by adding 1/10 volume of 3.0 M sodium acetate (pH 5.3) and 2.5 volume of 100% ethanol. Precipitates were centrifuged, washed with 70% ethanol, and dissolved in 80 µl of sterile, deionized water. RNA solutions were frozen and stored at −80°. Up to 15 µg of RNA was mixed with buffer RDD and DNase I (30 Kunitz units, QIAGEN RNase-Free DNase Kit) and incubated at 25° for 30 min. Subsequently, the RNA was cleaned using the QIAGEN RNeasy Mini Kit.
Up to 2 µg of RNA was mixed with TaqMan Reverse Transcription buffer, 25 µM MgCl2, 2.5 mM (each) dNTP mix, 50 µM random hexamers, 40 units RNase inhibitor and 125 units reverse transcriptase (Applied Biosystems Reverse Transcription kit). Incubation was for 10 min at 25°, followed by 45 min at 48°, and finally 5 min at 95°. The resulting complementary DNA (cDNA) was directly used for quantitative PCR (qPCR). For qPCR, 5 µl of this cDNA was mixed with 20 µl BioRad iTaq Universal SYBR Green mix and the respective primers. The PCR program consisted of one incubation of 5 min at 50° and 40 cycles of 1 min at 95° and 1 min at 60°, using an Applied Biosystems 7300 Real-Time PCR system. The primers were: 5′-CGA AAT CTA CCC CAT CCA TGC-3′ (CIT2 forward) and 5′-TCC CAT ACG CTC CCT GGA ATA-3′ (CIT2 reverse), 5′-ACG GTT TGG AAA GAG CTT CT-3′ (PHO84 forward) and 5′-TCA GAT TAC CGA CAG CAG TAT CA-3′ (PHO84 reverse), and 5′-TTC CAT CCA AGC CGT TTT GT-3′ (ACT1 forward) and 5′-CAG CGT AAA TTG GAA CGA CGT-3′ (ACT1 reverse).
A standard curve was generated using serial dilutions for ACT1. The expression of CIT2 and PHO84 was normalized to that of ACT1, as a constitutive control, in each sample, and the relative levels of the CIT2 and PHO84 mRNA were calculated from the standard curve. The resident Applied Biosystems 7300 system software was used. Results are expressed as mRNA levels relative to the ACT1 mRNA levels in the sample. Assays were in at least three replicates, and error bars are the SE.
RLS determination
A 1-µl drop of an overnight yeast culture was spotted on an agar plate. Budding cells were micromanipulated to an isolated spot on the plate. The buds were retained to initiate the experiment. Plates were incubated at 30°. Each time the retained cell budded, the resulting bud was removed, and the cell (now a mother cell) was counted one generation older. This was continued until the mother cell stopped dividing and lost refractility. The total number of buds produced by the cell is the age in generations. There were 40 cells for each strain in each determination, except that there were 80 cells each for YPK9 and YPK9 ura3::PHO84 in Figure 7B. RLS of each strain within an experiment was compared using the Mann–Whitney test. Two-tailed P-values are shown. Lifespans of at least two separate clones of each mutant strain were tested to ascertain reproducibility.
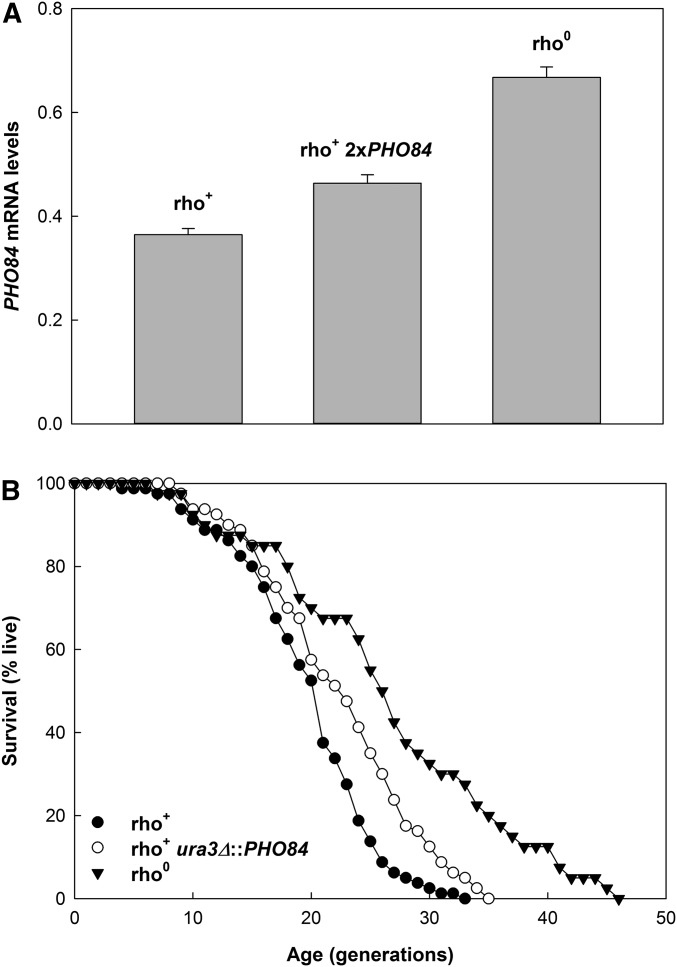
Figure 7.
Effect of PHO84 overexpression on the RLS of the rho+ strain. (A) The effect of an additional copy of the gene on PHO84 mRNA levels (relative to ACT1 mRNA) determined by RT-qPCR. The rho+ 2xPHO84 strain possessed a second copy of the gene at the ura3 locus (rho+ ura3Δ::PHO84). (B) The effect of an additional copy of PHO84 in the rho+ strain YPK9 (rho+ ura3Δ::PHO84) compared to both the rho+ (YPK9) and its rho0 derivative strain (YJR2). Mean RLS are 18.8, 21.4, and 25.6 for rho+, rho+ ura3Δ::PHO84, and rho0 strains, respectively. Lifespans for the first and second strain and for the first and third strain are significantly different (P = 0.014 and P < 0.001, respectively). They also differ significantly for the second and third strain (P = 0.021).
ERC determination
The protocol used has been described (Borghouts et al. 2004), and the modifications are presented here. Cells were collected by centrifugation from 5-ml overnight cultures in YPAD medium, to a concentration of 0.8–1.0 × 108/ml. The cells were washed with sterile, deionized water, and the pellets were resuspended in 240 µl of YD Digestion buffer (Zymo Research). To the suspension, 10 μl of R-Zymolyase (Zymo Research) were added, and the suspension was incubated at 37° for 1 hr. After addition of 240 μl of YD Lysis Buffer (Zymo Research, Irvine, CA) the suspension was mixed well by gently rolling the tube. Chloroform (500 µl) was added. The lysate was mixed thoroughly for 1 min and centrifuged for 2 min at >10,000 rpm. The upper phase was aspirated to a clean tube and two volumes of ethanol were added to precipitate yeast DNA (Kim et al. 2004). The DNA pellet was washed with 70% ethanol and resuspended in sterile, deionized water. DNA (10 µg) was digested with SpeI (New England BioLabs, Ipswich, MA) for at least 6 hr at 37°, which releases a 4.1-kb DNA fragment containing the ACT1 gene. Digests were loaded into lanes of a 1% agarose gel containing TAE (40 mM Tris-acetate, 1 mM EDTA, pH 8.0) and electrophoresed for 24 hr at 1.8 V/cm. A parallel lane of the gel was loaded with DNA-size markers. Gels were blotted onto Hybond-N+ nylon membranes (Amersham, Piscataway, NJ) according to the protocol provided by the manufacturer. 35S ribosomal DNA (rDNA) and ACT1 coding (401–1233 bp of the ORF) (Borghouts et al. 2004) fragments were employed as templates to produce DIG-labeled probes to detect ERC and the housekeeping gene ACT1. After probing for ERC, the blots were stripped and reprobed for ACT1. Detailed procedures are described in the DIG High Primer DNA Labeling and Detection Starter Kit II (Roche, Indianapolis, IN). Chemiluminescence detection and quantitation was carried out on the BioRAD ChemiDocTM XRs+ Imaging System, and the resident Quantity One software was used for quantitation. ERC levels from each strain were normalized to the housekeeping gene ACT1.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Strains are available upon request.
Results
Either SLIK or SAGA can support activation of CIT2 in the retrograde response
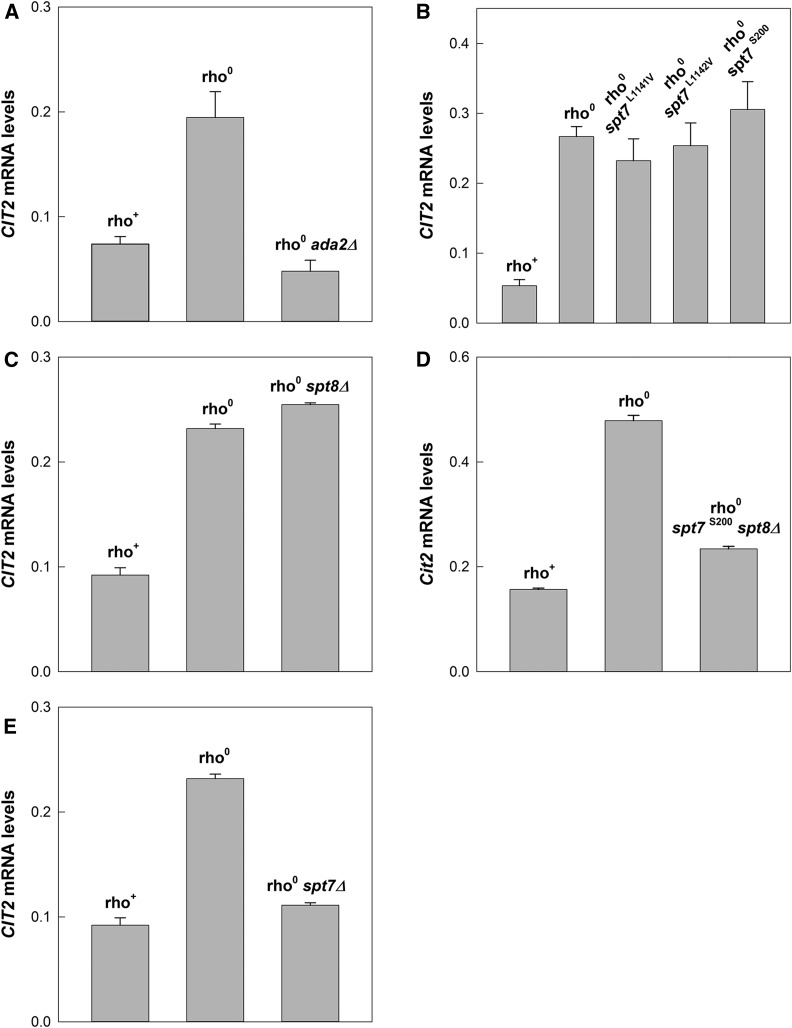
Previously, we showed that deletion of GCN5 abrogated the increase in CIT2 mRNA levels in rho0 cells compared to the rho+ cells from which they were derived (Kim et al. 2004). Gcn5 can function in a complex smaller than SAGA that contains Ada2 (Grant et al. 1997). To ascertain whether this complex is responsible for the role of Gcn5 in CIT2 expression, we examined the effect of deletion of ADA2 on CIT2 mRNA levels. As shown in Figure 1A, deletion of the gene prevented the increase in CIT2 mRNA in rho0 cells. Thus, Gcn5 does not function alone in the activation of the retrograde response. However, Gcn5, along with Ada2 is also part of the large SAGA and SLIK complexes (Pray-Grant et al. 2002).
Figure 1.
Activation of the retrograde response in rho0 strains lacking SAGA or SLIK. Activation of the retrograde response was assessed by comparing the CIT2 mRNA levels (relative to ACT1) by RT-qPCR in the rho+ strain YPK9 and its rho0 derivative YJR2. (A) Deletion of ADA2, a core component of SAGA/SLIK in rho0 cells. (B) Elimination of SLIK in rho0 cells by the S200 mutation in SPT7, which prevents the processing of this protein to allow SLIK formation and comparison to point mutations in the specific Pep4 cleavage site encompassed by S200. (C) Elimination of SAGA in rho0 cells by deletion of SPT8, which is a component of SAGA but not SLIK. (D) Elimination of both SLIK and SAGA in rho0 cells by the S200 mutation in SPT7 and the deletion of SPT8, respectively. (E) Elimination of both SLIK and SAGA in rho0 cells by removal of the common component Spt7.
To distinguish the potential function of SAGA and SLIK in activation of the retrograde response, it was necessary to eliminate them in turn. Rtg2 replaces Spt8 in SLIK. This requires truncation of Spt7 at a specific site (Pray-Grant et al. 2002; Wu and Winston 2002; Mischerikow et al. 2009; Spedale et al. 2010). Therefore, we subjected SPT7 to site-directed mutagenesis to eliminate this specific Pep4 protease cleavage site. Three separate mutants were generated: L1141V, L1142V, and S200. The first two are point mutants in the Pep4 cleavage site. The last one is a short deletion that eliminates 25 amino acids spanning it, and it is known to leave the uncleaved Spt7 available for participation in SAGA complex activity while preventing SLIK formation (Wu and Winston 2002; Mischerikow et al. 2009; Spedale et al. 2010). None of these mutations eliminated induction of CIT2 mRNA in rho0 cells (Figure 1B). This result indicates that SLIK is not necessary for activation of the retrograde response.
Elimination of SAGA was readily accomplished by deletion of SPT8, which is unique to this complex (Pray-Grant et al. 2002). In the absence of Spt8, Spt7 undergoes truncation to allow Rtg2 to become part of the complex, resulting in SLIK formation. As shown in Figure 1C, deletion of SPT8 did not prevent the induction of CIT2 mRNA in rho0 cells. Thus, SAGA is not necessary for activation of the retrograde response.
SLIK and SAGA can readily be eliminated in tandem by combining the S200 mutation in SPT7, which eliminates the proteolytic cleavage site in the protein with the deletion of SPT8. As shown in Figure 1D, this genetic manipulation virtually abolished CIT2 induction, suggesting that either SLIK or SAGA is necessary for transcriptional coactivation of this gene. This was confirmed by deletion of SPT7, as Spt7 is an essential component of both SLIK and SAGA (Pray-Grant et al. 2002). Deletion of SPT7 prevented the increase in CIT2 mRNA levels in rho0 compared to rho+ cells (Figure 1E). We conclude that either SLIK or SAGA can facilitate the activation of the retrograde response, acting interchangeably. The results also confirm that the SPT7 mutants that prevent truncation of the protein do not disturb its function in the SAGA complex (Wu and Winston 2002). In addition, they indicate that smaller Gcn5-containing complexes cannot replace SLIK or SAGA in the retrograde response.
Either SLIK or SAGA can support RLS extension in the retrograde response
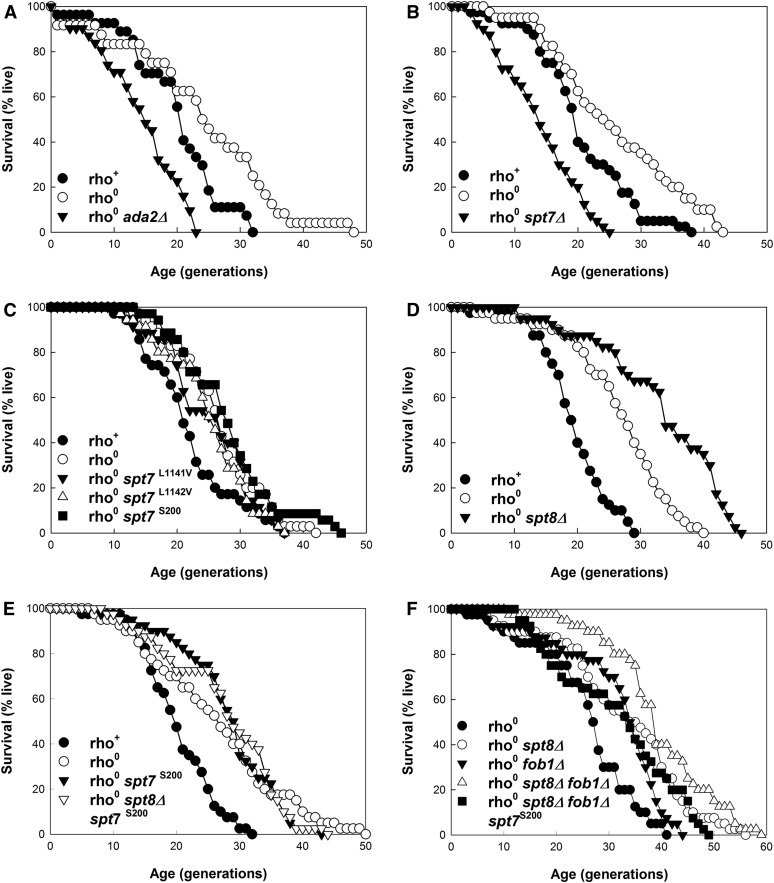
Because the retrograde-response target gene or genes that are responsible for extension of yeast longevity are not known, induction of CIT2 is not sufficient as a readout for lifespan extension. The RLS of rho0 cells is significantly longer than that of rho+ cells (Kirchman et al. 1999), and this extension is prevented by deletion of GCN5 (Kim et al. 2004). This shows that the Gcn5 histone acetyltransferase is essential for RLS extension in the retrograde response; however, it does not indicate whether it acts alone or in a complex. To assess the possibility that the Gcn5–Ada2 complex is involved, we deleted ADA2. This deletion suppressed the RLS extension observed in rho0 cells (Figure 2A), indicating that Gcn5 does not act alone.
Figure 2.
RLS extension in rho0 strains lacking SAGA or SLIK. (A) Deletion of ADA2, a core component of SAGA/SLIK. Mean RLS are 19.9, 25.6, and 13.8, for rho+, rho0, and rho0 ada2Δ strains, respectively. Lifespans for the first and second strain and for the second and third strain are significantly different (P = 0.009 and P < 0.001, respectively). (B) Elimination of both SLIK and SAGA in rho0 cells by removal of the common component Spt7. Mean RLS are 19.5, 24.7, and 12.7, for rho+, rho0, and rho0 spt7Δ strains, respectively. Lifespans for the first and second strain and for the second and third strain are significantly different (P = 0.022 and P < 0.001, respectively). (C) Elimination of SLIK in rho0 cells by the S200 mutation in SPT7, which prevents the processing of this protein to allow SLIK formation and comparison to point mutations in the specific Pep4 cleavage site encompassed by S200. Mean RLS are 20.9, 25.8, 24.3, 24.1, and 27.2, for rho+, rho0, rho0 spt7 L1141V, rho0 spt7 L1142V, and rho0 spt7 S200 strains, respectively. Lifespans for the first and second strain are significantly different (P = 0.003). There is no significant difference between the lifespans of the second and the third, fourth, and fifth strains (P-values of 0.502, 0.43, and 0.466, respectively). (D) Elimination of SAGA by deletion of SPT8, which is a component of SAGA but not SLIK. Mean RLS are 18.5, 25.8, and 32.6, for rho+, rho0, and rho0 spt8Δ strains, respectively. Lifespans for the first and second strain and for the second and third strain are significantly different (each P < 0.001). (E) Combined elimination of SLIK and SAGA by the S200 mutation in SPT7 and the deletion of SPT8. The mean RLS for the rho+, rho0, rho0 spt7 S200, and rho0 spt8Δ spt7 S200 strains were 19.4, 25.5, 27.4, and 27.0, respectively. Lifespans for the first and second strains are significantly different (P = 0.006), but those for the second and third and second and fourth are not (P = 0.345 and P = 0.373, respectively). (F) Combined prevention of ERC accumulation by FOB1 deletion and elimination of SAGA and SLIK by SPT8 deletion and the spt7 S200 mutation, respectively. The mean RLS for the rho0, rho0 spt8Δ, rho0 fob1Δ, rho0 spt8Δ fob1Δ, and rho0 spt8Δ fob1Δ spt7 S200 strains are 25.0, 32.1, 30.6, 38.5, and 30.7, respectively. The differences in lifespan between the rho0 strain and all of the others were significant. Except for the triple mutant, the P-values are 0.005 or lower, while for the triple mutant, P = 0.026. There are no significant differences between the rho0 spt8Δ strain and the rho0 fob1Δ or the triple mutant (P-values of ≥0.419). However, the differences between the rho0 spt8Δ fob1Δ and the rho0 spt8Δ (P = 0.034) and the rho0 fob1Δ (P < 0.001) strains are significant, while those for the rho0 fob1Δ and the triple mutant are not (P = 0.862). YPK9 is the rho+ strain and YJR2 is its rho0 derivative.
Gcn5 and Ada2 not only act as a complex, but they also are components of larger protein complexes. Therefore, it was necessary to determine the effects of SAGA and SLIK on RLS, again notwithstanding the effects on CIT2 induction. Elimination of Spt7 by deleting the gene suppressed the RLS extension in rho0 cells (Figure 2B), indicating that either or both SAGA and SLIK are required.
To distinguish between the possibilities, the three SPT7 mutants in the Pep4 cleavage site were tested. None of the mutations prevented RLS extension (Figure 2C), indicating that SLIK was not essential and leaving SAGA as the player. To verify that SAGA is the responsible transcriptional coactivator complex that can facilitate RLS extension in rho0 cells, SPT8 was deleted because its product is unique to SAGA. However, this did not prevent the increase in lifespan (Figure 2D), showing that either SLIK or SAGA can perform as the transcriptional coactivator in the retrograde response. Surprisingly, deletion of SPT8 resulted in a further increase in RLS in rho0 cells (Figure 2D). Thus, elimination of Spt8 likely affects additional cellular processes, potentially masking effects of the gene deletion. Mutation of SPT7, blocking the proteolytic processing of the protein that allows SLIK formation suppressed the additional RLS extension seen in the rho0 spt8Δ strain (compare Figure 2D with Figure 2E), apparently leaving this additional cellular process intact while eliminating CIT2 induction (Figure 1D).
Activation of the retrograde response results in a progressive and extensive accumulation of ERCs during the RLS in both rho+ and rho0 cells, although much more extensive in the latter (Borghouts et al. 2004), as a result of recombination within the 100–200 tandem repeats of rDNA on chromosome 12 (Szostak and Wu 1979; Clark-Walker and Azad 1980; Larionov et al. 1980; Park et al. 1999; Johzuka and Horiuchi 2002). Such a burden of ERC normally curtails yeast RLS (Sinclair and Guarente 1997). However, rho0 cells can tolerate as much as five times the number of ERCs about the midpoint of their RLS as rho+ cells at a point when the latter have nearly exhausted their life expectancy, and this appears due to the partial compensation of the negative effects of the ERC by an active retrograde response (Borghouts et al. 2004). ERC production is inhibited by deletion of FOB1, and this increases RLS in rho+ cells (Defossez et al. 1999; Johzuka and Horiuchi 2002). Interestingly, elimination of ERC by deletion of this gene results in a synergistic increase in RLS when combined with the rho0 genotype (Borghouts et al. 2004) (Figure 2F), reflecting the deleterious effects on RLS of ERC in rho0 just as in rho+ cells. It has been suggested that ERC accumulation curtails yeast RLS by titration of replication proteins by these episomes (Sinclair and Guarente 1997).
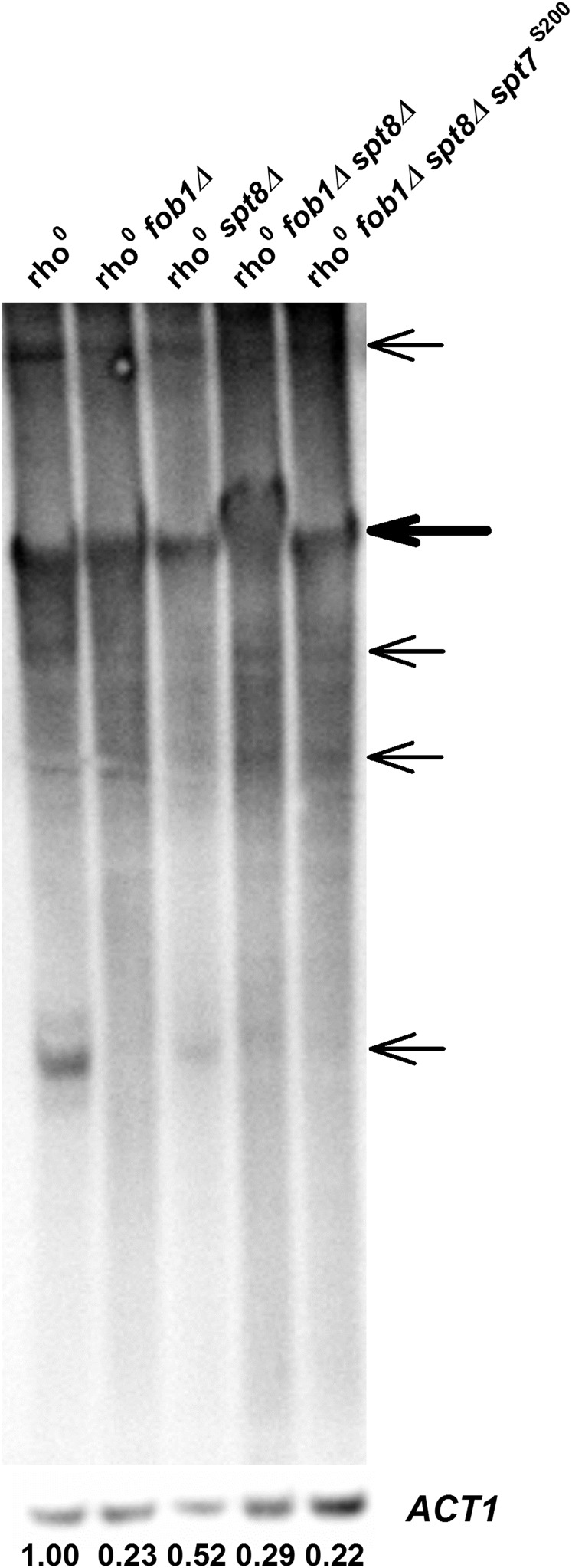
Previously, we found that GCN5 deletion markedly reduced ERC levels in rho0 cells, in which Rtg2 protein plays a part (Kim et al. 2004). We wondered whether the increase in RLS by elimination of SAGA resulting from SPT8 deletion in rho0 cells could be due to an effect on ERC, as well. As expected (Borghouts et al. 2004), the deletion of FOB1 reduced ERC levels in rho0 cells (Figure 3). Deletion of SPT8 had a similar effect, although to a lesser extent (Figure 3). When both genes were deleted, ERCs were reduced to the level seen in the FOB1 deletion. Next, we examined the effects of these gene deletions on RLS. As seen in Figure 2F, both FOB1 and SPT8 deletion extended RLS in rho0 cells, and combining them yielded a further increase. When the SPT7 S200 mutation that prevents SLIK formation was combined with the deletions in FOB1 and SPT8, this further increase in RLS was suppressed to the level seen in the presence of either deletion alone (Figure 2F). ERC levels remained low in the triple mutant (Figure 3). We interpret these complex results as follows: Elimination of SAGA and SLIK by deletion of SPT8 and the S200 mutation in SPT7 prevents the induction of CIT2 and extension of RLS in the retrograde response. However, the SPT8 deletion affects another cellular process, which may be involved in ERC maintenance. The difference between Gcn5 and Spt8 is likely due to the fact that deletion of GCN5 eliminates both SLIK and SAGA, while deletion of SPT8 only prevents SAGA formation. This is addressed further in the Discussion.
Figure 3.
Analysis of ERCs in rho0 strains. DNA prepared from the indicated rho0 strains was electrophoresed and Southern blots were probed for rDNA and ACT1 (see Materials and Methods). Bands representing the hybridization signal for genomic rDNA are indicated by the thick arrow, while the ERC bands are indicated by the thin ones. For quantification of ERCs, the sums of the signal intensities for the ERC bands in each lane were normalized against the hybridization signals of ACT1 in those lanes, and the results are shown at the bottom of each lane with the intensity of the parental rho0 strain set to 1.00. Similar results were obtained in three separate experiments.
Search for retrograde-response target genes that potentially affect RLS extension
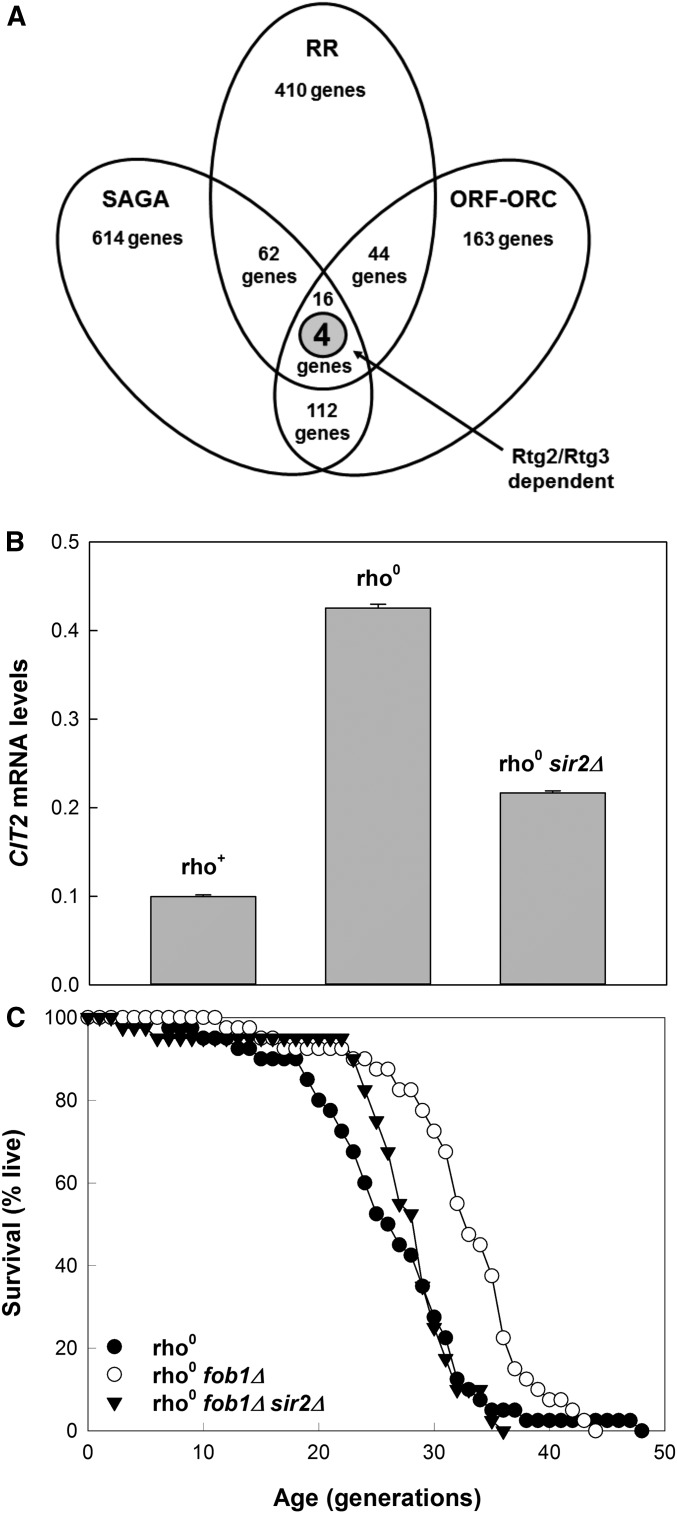
There are 410 gene expression changes that are found in rho0 cells compared to rho+ cells (Epstein et al. 2001; Traven et al. 2001). One or more of these are the likely effector of the increase in RLS in the retrograde response. The involvement of SAGA/SLIK in induction of the retrograde-response diagnostic gene CIT2 and especially in the RLS extension afforded by activation of the response indicates that the effector(s) of longevity should be found at the intersection of the 614 yeast genes at which SAGA dominates and these 410 genes (Figure 4A).
Figure 4.
Identification of the genes that are dependent on the retrograde response, SAGA, and ORF-ORC. (A) The dependent genes in each of these three cases were identified in published sources (Epstein et al. 2001; Huisinga and Pugh 2004; Shor et al. 2009). The three databases were merged to find shared sets of genes. This identified four genes common to all three that are also Rtg2 and Rtg3 dependent in the retrograde response. (B) Effect of SIR2 deletion on the induction of CIT2 expression in the retrograde response. (C) SIR2 deletion suppresses RLS in rho0 cells independently of effects on ERCs, which are eliminated by FOB1 deletion. Mean RLS are 25.1, 31.4, and 26.4, for rho0, rho0 fob1Δ, and rho0 fob1Δ sir2Δ strains, respectively. Lifespans for the first and second strain and for the second and third strain are significantly different (each P < 0.001). As discussed in the text, the results in B and C provide additional rationale for inclusion of ORF-ORC genes in the analysis shown in A. RR, retrograde response.
We also considered another set of genomic sites, called ORF-origin recognition complexes (ORCs), in this bioinformatics analysis. The ORC that is important in DNA replication (Bell 2002) also binds to silencers in the yeast genome where it recruits silent information regulator (Sir) proteins (Bose et al. 2004; Fox and McConnell 2005). These silent genomic sites bind ORCs tightly, as compared to many active replication origins (Palacios DeBeer et al. 2003). A subset of the ORC-binding sites outside silenced genomic regions can also bind ORCs tightly, but they do not function as origins of replication. They are generally found in close proximity to ORFs of highly transcribed, metabolic genes (Shor et al. 2009). The 163 sites with these characteristics have been named ORF-ORCs. The consideration of ORF-ORC genes was motivated by the fact that the retrograde response involves a major metabolic adaptation (Epstein et al. 2001; Traven et al. 2001). Based on the known interactions of ORC and Sir complexes in silent and transcribed regions of the yeast genome (Shor et al. 2009), we reasoned that these ORF-ORCs are potential sites for Sir2 recruitment in the retrograde response. Indeed, Sir2 has been shown to associate with 24% of the most frequently transcribed genes (Tsankov et al. 2006). We therefore examined the effect of SIR2 deletion on expression of CIT2. As shown in Figure 4B, deletion of SIR2 decreased induction of CIT2 by ∼50% in rho0 cells in which the retrograde response is activated.
This raises the question of whether SIR2 deletion compromises the RLS extension afforded by activation of the retrograde response. However, deletion of SIR2 enhances ERC production and this suppresses RLS (Kaeberlein et al. 1999), confounding interpretation of its effect in rho0 cells. Deletion of FOB1 eliminates this confounder by suppressing ERC formation (Kaeberlein et al. 1999). Figure 4C demonstrates that deletion of SIR2 in rho0 cells lacking Fob1 resulted in suppression of RLS. This supports the role of Sir2 in the retrograde response, independent of its effects on ERC production. It also provides additional rationale for inclusion of ORF-ORC genes in our bioinformatics analysis.
Not all genes induced in rho0 cells require the retrograde regulators Rtg2 and Rtg3 (Epstein et al. 2001). Both regulators are required for RLS extension in rho0 cells (Kirchman et al. 1999; Borghouts et al. 2004). Of the 16 genes at the intersection of rho0 dependence, SAGA dominance, and ORF-ORC association, only 4 genes require Rtg2 and Rtg3 for activation in the retrograde response (Figure 4A). These genes are MDH1, BAP2, PHO84, and CIT2.
PHO84 is necessary for RLS extension in the retrograde response
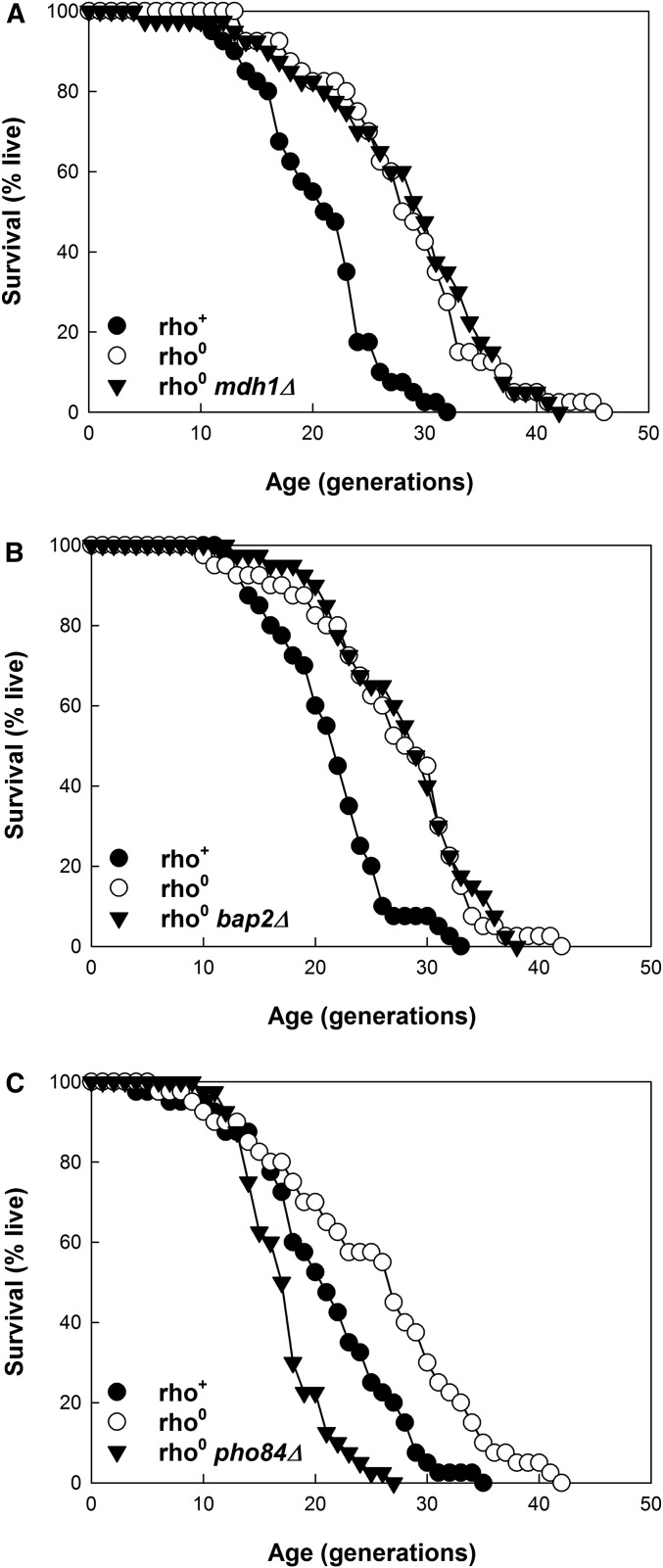
If our bioinformatics approach was successful, the effector(s) of RLS extension would be among the four genes identified above. The effector could be a single gene or combination of the four genes, which involves a total of 15 potential combinations. We decided to first delete each of the genes individually in rho0 cells to determine the effect on RLS. We already had the answer for CIT2. Deletion of this gene does not curtail the RLS of the cells (Kirchman et al. 1999). Deletion of MDH1 and BAP2, individually, also did not affect the RLS extension in rho0 cells (Figure 5, A and B, respectively). However, deletion of PHO84 completely abrogated RLS extension (Figure 5C). This indicates that PHO84 is necessary for the RLS extension caused by activation of the retrograde response. Thus, this is at least one of the retrograde-response effectors of longevity.
Figure 5.
Effect of deletion of the genes at the intersection of the retrograde response, SAGA, and ORF-ORC on RLS extension in rho0 strains. (A) Deletion of MDH1. Mean RLS are 19.7, 27.3, and 27.3, for rho+, rho0, and rho0 mdh1Δ strains, respectively. Lifespans for the first and second strain are significantly different (P < 0.001). They are not significantly different for the second and third strain (P = 0.736). (B) Deletion of BAP2. Mean RLS are 20.5, 26.2, and 27.0, for rho+, rho0, and rho0 bap2Δ strains, respectively. Lifespans for the first and second strain are significantly different (P < 0.001). They are not significantly different for the second and third strain (P = 0.765). (C) Deletion of PHO84. Mean RLS are 19.9, 24.3, and 16.4 for rho+, rho0, and rho0 pho84Δ strains, respectively. Lifespans for the first and second strain and for the second and third strain are significantly different (P = 0.019 and P < 0.001, respectively).
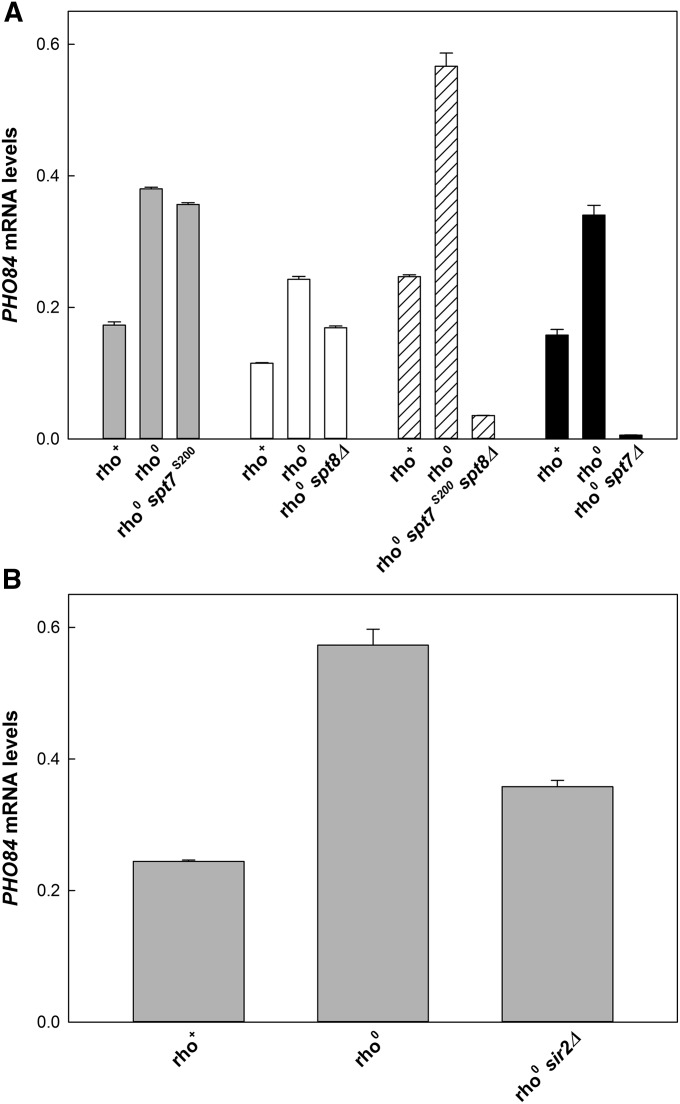
It has been shown previously that CIT2, MDH1, BAP2, and PHO84 are among the retrograde-response target genes (Epstein et al. 2001). As discussed above, activation of CIT2 requires either SAGA or SLIK (Figure 1). We wanted to verify that this is also the case for PHO84, the gene whose activation is necessary for the RLS extension in the retrograde response. Previously, it was shown that expression of PHO84 is decreased in spt7 null mutants (Nishimura et al. 1999), implicating SLIK and/or SAGA. Therefore, we examined the effects of the various mutants that affect SLIK and SAGA differentially (Figure 6A). The S200 mutant of SPT7, which prevents SLIK assembly, caused only a small (6%) reduction in the activation of PHO84 in rho0 cells. On the other hand, deletion of SPT8, which prevents SAGA formation, lowered PHO84 mRNA levels by ∼28%. This suggests that SAGA plays a greater role in expression of this gene than does SLIK, although the transcription of PHO84 supported by SLIK appears sufficient for effective RLS extension (Figure 2D). However, combination of the S200 mutation in SPT7 with the SPT8 deletion virtually abolished PHO84 expression in rho0 cells (Figure 6A). This was also the case when SPT7 was deleted. These results demonstrate that either SLIK or SAGA function as transcriptional coactivators of PHO84 in the retrograde response. Thus, retrograde-response activation of PHO84 can utilize either SLIK or SAGA interchangeably, with a preference for the latter. Nevertheless, one or the other must be available for expression of this gene and extension of RLS.
Figure 6.
Effects of elimination of SLIK and SAGA and SIR2 on activation of PHO84 in rho0 cells. (A) SLIK was eliminated by the S200 mutation in SPT7. SAGA was eliminated by deletion of SPT8. SLIK and SAGA were eliminated together by combining the S200 mutant with the SPT8 deletion or by the deletion of SPT7. (B) Effect of SIR2 deletion on expression of PHO84 in rho0 cells. YPK9 is the rho+ strain and YJR2 is its rho0 derivative.
The requirement for Sir2 for RLS extension in the retrograde response implies that deletion of SIR2 would suppress induction of PHO84. This was indeed the case (Figure 6B), providing further support for the essential role of SIR2 in gene induction and RLS extension in rho0 cells.
PHO84 is sufficient for RLS extension in the retrograde response
The results above raise the question of whether PHO84 is sufficient for RLS extension in the retrograde response. To answer this, we inserted a second copy of PHO84 into the genome of our rho+ strain at the ura3-52 locus. As shown in Figure 7A, the extra copy of PHO84 resulted in a 27% increase in PHO84 mRNA levels. This compares to the 83% increase observed in the rho0 derivative of the parental strain. Thus, the extra copy of the gene provides an increase in the expression of the gene that is 33% of that observed when the retrograde response is induced in rho0 cells. We then determined the RLS of the rho+ strain with and without the extra copy of PHO84 (Figure 7B). The mean RLS of the strain with the extra copy was 14% greater. This compares to the 37% increase observed in the rho0 strain (Figure 7B), which corresponds to ∼86% of the increase in RLS that would be expected if RLS was a linear function of PHO84 mRNA levels. These results indicate that PHO84 is sufficient for RLS extension in the retrograde response, in addition to being necessary. However, it is possible that another gene or genes can augment the effect of PHO84 even further, as there is still a small increment of longevity that appears to remain to reach the RLS of the rho0 strain.
Discussion
Our initial goal was to unravel the contributions of SAGA and SLIK in retrograde signaling of gene expression changes and RLS extension. We have found that these two, large transcriptional coactivator complexes act interchangeably in the induction of the retrograde-response diagnostic gene CIT2 (Figure 1). This behavior of these complexes was paralleled in their effects on RLS extension in the retrograde response (Figure 2). The implication of SAGA and SLIK in the retrograde response provided an opportunity to winnow the potential retrograde-response target genes responsible for longevity extension (Figure 4A). This effort was aided by considering ORF-ORC genes in tandem. The rationale behind their inclusion stems from their association with metabolic gene expression. It is also supported by the effect of SIR2 deletion on CIT2 induction and by the ERC-independent effect of SIR2 deletion on RLS extension in the retrograde response (Figure 4, B and C).
The juxtaposition of retrograde-response target genes, SAGA dominated genes, and ORF-ORC genes resulted in only four genes to test for a role in RLS extension. Only deletion of one, PHO84, suppressed RLS extension (Figure 5). Thus, this gene is necessary for RLS extension in the retrograde response. PHO84 expression is regulated by SAGA and SLIK interchangeably, just like CIT2 (Figure 6A). In addition, SIR2 was required for robust expression of PHO84 in rho0 cells (Figure 6B). Activation of PHO84 expression is also sufficient for RLS extension. An extra copy of the gene in the rho+ cells extended RLS to at least 86% of that expected, based on the increase in PHO84 mRNA levels (Figure 7). This potentially leaves some space for an additional retrograde-response target gene or genes to affect RLS. These additional genes may reside at the intersection of the Rtg2/Rtg3-dependent retrograde response and SAGA (Figure 4A), as our justification to include ORF-ORC genes was to some extent theoretical compared to the experimental data forming the basis for inclusion of the other sets of genes.
Our interest in SLIK was engendered by the discovery that it contains Rtg2 protein as an essential component in place of Spt8 and that Rtg2 can be found at the CIT2 promoter where SLIK appears to regulate transcription (Pray-Grant et al. 2002). These properties of SLIK were described in terms of the retrograde response. However, growth on acetate as a carbon source was used in that study. The retrograde response was originally defined as a phenomenon that occurs when mitochondria are dysfunctional, a condition under which yeast cells cannot grow on acetate. However, our current study demonstrates that SLIK does function in the bona fide retrograde response along with SAGA.
Deletion of SPT8 did not suppress RLS extension in rho0 cells (Figure 2D). In fact, it resulted in a further increase. One possibility is that elimination of SAGA leaves SLIK as the coactivator and a more efficient one at that. This is not likely because there is a preference for SAGA in PHO84 activation (Figure 6A). It is also possible that Spt8 and SAGA play additional roles in RLS. Support for one such alternative role is provided by the decrease in ERC levels on GCN5 deletion in rho0 cells (Kim et al. 2004). Deletion of SPT8 had a similar effect on ERC levels (Figure 3). Although this effect was not as great as that of a FOB1 deletion, it yielded the same effect on RLS (Figure 2F), which may reflect the high threshold for ERC tolerance in rho0 cells as they progress through their RLS (Borghouts et al. 2004). The fact that the SPT7 S200 mutation suppressed the excess RLS extension in the presence of the SPT8 deletion, whether or not FOB1 is present (Figure 2, D–F), while the SPT7 S200 mutation on its own did not affect the RLS of rho0 cells (Figure 2C) suggests that elimination of both SLIK and SAGA is required to prevent RLS extension in the retrograde response.
SLIK and SAGA can be distinguished chromatographically. The SPT7 S200 mutation prevents the formation of SLIK by blocking specific cleavage of Spt7, which in turn prevents Rtg2 from replacing Spt8 in SAGA (Pray-Grant et al. 2002; Wu and Winston 2002). SAGA still forms and contains all its subunits (Wu and Winston 2002). SAGA partial complexes can form that are missing certain subunits. Deletion of GCN5 or of SPT8 eliminates SAGA activity, but all the remaining subunits associate in the respective SAGA partial complexes (Wu and Winston 2002). This includes the specifically truncated Spt7, at least in the case of the SPT8 deletion (Wu and Winston 2002). Spt7 appears to perform a scaffolding role for several SAGA components. Its absence eliminates SAGA as a complex (Grant et al. 1997). These properties of the SAGA/SLIK complex likely contribute to the results obtained here. The effect of SPT7 deletion (Figure 2B) or GCN5 deletion (Kim et al. 2004) on RLS in rho0 cells was very similar. RLS was shorter than in rho+ cells. Thus, the histone acetylase activity of Gcn5 within an intact SAGA/SLIK appears to be essential for maintaining RLS even at rho+ cell levels. The effects on CIT2 expression of GCN5 or SPT7 deletion were not that severe (Kim et al. 2004) (Figure 1D), suggesting that other mechanisms that maintain these rho+ cell levels of RLS are in play. However, drastic effects of SPT7 deletion on PHO84 expression were seen (Figure 6A), reflecting its effect on RLS (Figure 2B). As shown in Figure 5C and Figure 7, PHO84 is necessary and sufficient for RLS extension in the retrograde response.
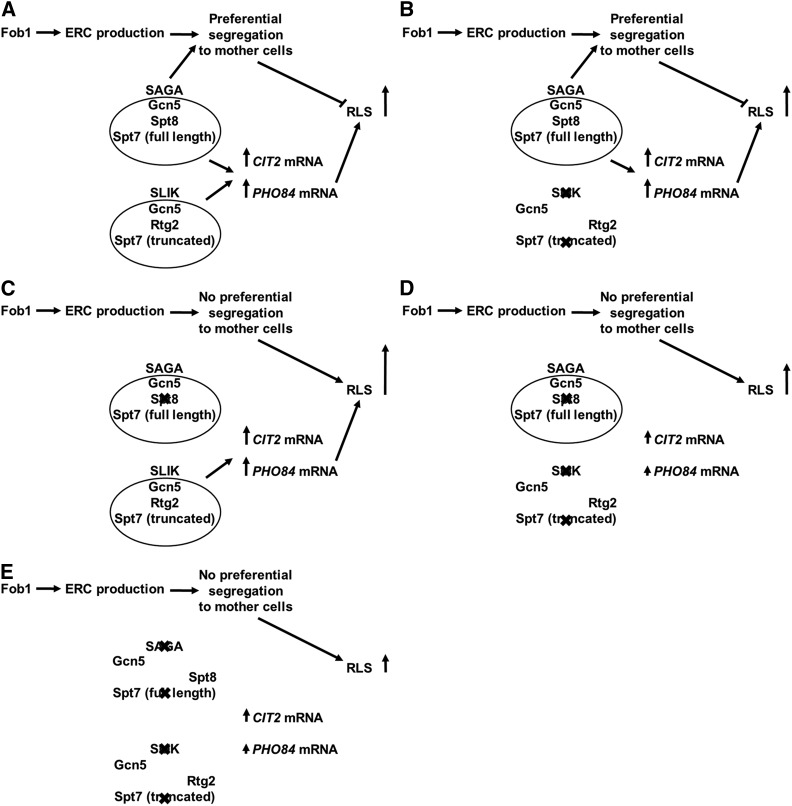
The results presented here are consistent with the recent observation that SAGA plays a role in the asymmetric segregation of DNA circles, including ERCs, to mother cells, by their anchorage to nuclear pore complexes by SAGA (Denoth-Lippuner et al. 2014). These authors showed that this anchorage required SAGA components such as Gcn5, Spt3, and Sgf73. Deletion of these genes markedly reduced ERC content in aging mother cells. Denoth-Lippuner et al. (2014) also found that deletion of the SAGA component gene SGF73 extended RLS, while the elimination of GCN5 did not. They ascribed this difference to the multiple pathways on which SAGA impinges through its role as a transcriptional coactivator. Our present study identifies one such pathway. Disruption of SAGA by SPT8 deletion here would relieve some of the ERC burden in mother cells to enhance their RLS. This effect would be particularly noticeable in rho0 cells with their large accumulation of ERCs as they progress through their RLS (Borghouts et al. 2004). The additional increase in RLS of rho0 cells possessing both SPT8 and FOB1 deletions (Figure 2F) is not directly reflected in their ERC content (Figure 3). However, ERC levels were determined in batch cultures that are primarily composed of young cells. The loss of asymmetric segregation of ERCs when SPT8 is deleted would manifest itself to a greater extent as the yeasts progress through their RLS. Although deletion of GCN5 abolishes asymmetric segregation of ERCs (Denoth-Lippuner et al. 2014), it also eliminates both SLIK and SAGA, masking any beneficial effect of ERC reduction on RLS of rho0 cells, as observed earlier (Kim et al. 2004). It is noteworthy that elimination of SLIK by SPT7 deletion or S200 mutation did not cause an increase in RLS (Figure 2, B and C), while deletion of SPT8 did (Figure 2D). Thus, various components of SAGA and SLIK have divergent effects on different aspects of these transcriptional coactivators’ functions, probably including retention of ERCs in mother cells as well. Our model for the role of SAGA/SLIK in the RLS of rho0 cells summarizing this discussion is shown in Figure 8. It is certainly possible that there are yet other cellular functions of SAGA. Recently, another study showed a role for SAGA in RLS (McCormick et al. 2014). This study implicated the deubiquitinase module of the transcriptional coactivator complex in rho+ cells. Our parallel study in contrast, addresses the roles in RLS of both the SLIK and the SAGA core complexes in the retrograde response in rho0 cells. Nevertheless, a connection between the two studies may exist, and this will be a subject for future study.
Figure 8.
Schematic summarizing the discussion of the role of SAGA/SLIK in the RLS of rho0 cells. (A) Depiction of the situation in wild-type cells. (B–E) The effects of elimination of SAGA/SLIK components on expression of retrograde-response target genes, segregation of ERCs to mother cells, and RLS. In B, there is no effect on RLS because preventing Spt7 cleavage leaves SAGA activity unaltered to support a wild-type retrograde transcriptional response, and eliminating the intact SLIK complex has no impact on ERCs. In C, RLS increases because eliminating Spt8 from SAGA reduces ERC accumulation in mother cells but leaves SLIK activity unaltered to support a wild-type retrograde response. In D, there is no effect on RLS, despite the loss of both an intact SLIK complex and SAGA complex activity and the attendant retrograde response, because the elimination of Spt8 from SAGA reduces ERC accumulation in mother cells. In E, RLS decreases by elimination of Spt7 and the resulting disruption of both SAGA and SLIK complexes even though the impairment of the retrograde response and reduction in ERC accumulation in mother cells is indistinguishable from that given by the situation in D. The different outcomes in D and E imply that SAGA has an additional unknown function(s) in promoting RLS that can be executed by the partial SAGA complex lacking only Spt8. Other possibilities include additional functions in the cell of SAGA components, such as Spt8, that have a negative effect on RLS when not assembled into intact SAGA complexes.
Our efforts to identify the effectors of the RLS extension in the retrograde response prompted examination of a potential role for SIR2. Deletion of this gene markedly suppressed induction of both CIT2 (Figure 4B) and PHO84 (Figure 6B) in rho0 cells. It also abolished RLS extension in the retrograde response independently of the effects of SIR2 on ERC production (Figure 4C). These results indicate a hitherto unappreciated role of SIR2.
PHO84 encodes a high-affinity inorganic phosphate transporter in the plasma membrane (Bun-Ya et al. 1991; Wykoff and O’Shea 2001). How does activation of this gene, which would result in an increase in phosphate import into the cell extend RLS? PHO84 is pleiotropic, affecting several cellular processes. For example, overexpression of this gene triggers the endoplasmic reticulum unfolded protein response (ER-UPR) (Ofiteru et al. 2012). It has been shown that activation of this response in yeasts can extend RLS (Cui et al. 2015). Although the key ER-UPR genes IRE1 and HAC1 are not induced in rho0 cells (Epstein et al. 2001), triggering of the ER-UPR at the level of Ire1 and/or Hac1 protein is an attractive possibility.
This study resolves the questions surrounding the roles of SAGA and SLIK in the retrograde response. In the process, we have bolstered the role of SAGA in ERC-mediated effects on yeast longevity, and we have uncovered a role for SIR2 in the retrograde response. Importantly, we have succeeded in identifying the retrograde-response target gene responsible for RLS extension as PHO84. This finding opens up new avenues of research regarding the role of the retrograde response in yeast aging. Future efforts will be directed toward an elucidation of the proximal effector(s) of longevity downstream of PHO84 triggered by mitochondrial dysfunction.
Acknowledgments
We thank Jennifer Wyckoff for DNA sequencing, which was carried out in the Genomics and Biostatistics Core, supported by grant GM103629 from the National Institutes of Health (NIH). This research was supported by grant AG006168 from the NIH.
Footnotes
Communicating editor: A. Hinnebusch
Literature Cited
- Bell S. P., 2002. The origin recognition complex: from simple origins to complex functions. Genes Dev. 16: 659–672. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Wang C. Y., Baptista T., Vincent S. D., Hsiao W. C., et al. , 2014. The SAGA coactivator complex acts on the whole transcribed genome and is required for RNA polymerase II transcription. Genes Dev. 28: 1999–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghouts C., Benguria A., Wawryn J., Jazwinski S. M., 2004. Rtg2 protein links metabolism and genome stability in yeast longevity. Genetics 166: 765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose M. E., McConnell K. H., Gardner-Aukema K. A., Muller U., Weinreich M., et al. , 2004. The origin recognition complex and Sir4 protein recruit Sir1p to yeast silent chromatin through independent interactions requiring a common Sir1p domain. Mol. Cell. Biol. 24: 774–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun-Ya M., Nishimura M., Harashima S., Oshima Y., 1991. The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol. Cell. Biol. 11: 3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira da Silva C. C., Cerqueira F. M., Barbosa L. F., Medeiros M. H., Kowaltowski A. J., 2008. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell 7: 552–560. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Azad A. A., 1980. Hybridizable sequences between cytoplasmic ribosomal RNAs and 3 micron circular DNAs of Saccharomyces cerevisiae and Torulopsis glabrata. Nucleic Acids Res. 8: 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J. M., Cho J., Lo T., Jr, Hur J. H., Bahadorani S., et al. , 2009. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr. Biol. 19: 1591–1598. [DOI] [PubMed] [Google Scholar]
- Cui H. J., Liu X. G., McCormick M., Wasko B. M., Zhao W., et al. , 2015. PMT1 deficiency enhances basal UPR activity and extends replicative lifespan of Saccharomyces cerevisiae. Age (Dordr.) 37: 9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defossez P. A., Prusty R., Kaeberlein M., Lin S. J., Ferrigno P., et al. , 1999. Elimination of replication block protein Fob1 extends the life span of yeast mother cells. Mol. Cell 3: 447–455. [DOI] [PubMed] [Google Scholar]
- Dell’agnello C., Leo S., Agostino A., Szabadkai G., Tiveron C., et al. , 2007. Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Hum. Mol. Genet. 16: 431–444. [DOI] [PubMed] [Google Scholar]
- Denoth-Lippuner A., Krzyzanowski M. K., Stober C., Barral Y., 2014. Role of SAGA in the asymmetric segregation of DNA circles during yeast ageing. eLife 3: 03790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J., Wolff S., Dillin A., 2011. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. B., Waddle J. A., Hale W. t., Dave V., Thornton J., et al. , 2001. Genome-wide responses to mitochondrial dysfunction. Mol. Biol. Cell 12: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. A., McConnell K. H., 2005. Toward biochemical understanding of a transcriptionally silenced chromosomal domain in Saccharomyces cerevisiae. J. Biol. Chem. 280: 8629–8632. [DOI] [PubMed] [Google Scholar]
- Goldstein A. L., McCusker J. H., 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Grant P. A., Duggan L., Cote J., Roberts S. M., Brownell J. E., et al. , 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11: 1640–1650. [DOI] [PubMed] [Google Scholar]
- Huisinga K. L., Pugh B. F., 2004. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol. Cell 13: 573–585. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., 1999. Molecular mechanisms of yeast longevity. Trends Microbiol. 7: 247–252. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., 2005. The retrograde response links metabolism with stress responses, chromatin-dependent gene activation, and genome stability in yeast aging. Gene 354: 22–27. [DOI] [PubMed] [Google Scholar]
- Jazwinski S. M., 2014. The retrograde response: a conserved compensatory reaction to damage from within and from without. Prog. Mol. Biol. Transl. Sci. 127: 133–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., 2015. Mitochondria to nucleus signaling and the role of ceramide in its integration into the suite of cell quality control processes during aging. Ageing Res. Rev. 23: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. C., Jaruga E., Repnevskaya M. V., Jazwinski S. M., 2000. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 14: 2135–2137. [DOI] [PubMed] [Google Scholar]
- Jiang J. C., Wawryn J., Shantha Kumara H. M., Jazwinski S. M., 2002. Distinct roles of processes modulated by histone deacetylases Rpd3p, Hda1p, and Sir2p in life extension by caloric restriction in yeast. Exp. Gerontol. 37: 1023–1030. [DOI] [PubMed] [Google Scholar]
- Johzuka K., Horiuchi T., 2002. Replication fork block protein, Fob1, acts as an rDNA region specific recombinator in S. cerevisiae. Genes Cells 7: 99–113. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M., McVey M., Guarente L., 1999. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13: 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Benguria A., Lai C. Y., Jazwinski S. M., 1999. Modulation of life-span by histone deacetylase genes in Saccharomyces cerevisiae. Mol. Biol. Cell 10: 3125–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Ohkuni K., Couplan E., Jazwinski S. M., 2004. The histone acetyltransferase GCN5 modulates the retrograde response and genome stability determining yeast longevity. Biogerontology 5: 305–316. [DOI] [PubMed] [Google Scholar]
- Kirchman P. A., Kim S., Lai C. Y., Jazwinski S. M., 1999. Interorganelle signaling is a determinant of longevity in Saccharomyces cerevisiae. Genetics 152: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe J., Hekimi S., 2008. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J. Biol. Chem. 283: 26217–26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenois A., Stuparevic I., Liu Y., Law M. J., Becker E., et al. , 2015. The conserved histone deacetylase Rpd3 and its DNA binding subunit Ume6 control dynamic transcript architecture during mitotic growth and meiotic development. Nucleic Acids Res. 43: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larionov V. L., Grishin A. V., Smirnov M. N., 1980. 3 micron DNA: an extrachromosomal ribosomal DNA in the yeast Saccharomyces cerevisiae. Gene 12: 41–49. [DOI] [PubMed] [Google Scholar]
- Lee S. J., Hwang A. B., Kenyon C., 2010. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr. Biol. 20: 2131–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Butow R. A., 1993. RTG1 and RTG2: two yeast genes required for a novel path of communication from mitochondria to the nucleus. Cell 72: 61–71. [DOI] [PubMed] [Google Scholar]
- Liao X. S., Small W. C., Srere P. A., Butow R. A., 1991. Intramitochondrial functions regulate nonmitochondrial citrate synthase (CIT2) expression in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Wu Q., He D., Ma T., Du L., et al. , 2011. Drosophila sbo regulates lifespan through its function in the synthesis of coenzyme Q in vivo. J. Genet. Genomics 38: 225–234. [DOI] [PubMed] [Google Scholar]
- Liu J. L., Desjardins D., Branicky R., Agellon L. B., Hekimi S., 2012. Mitochondrial oxidative stress alters a pathway in Caenorhabditis elegans strongly resembling that of bile acid biosynthesis and secretion in vertebrates. PLoS Genet. 8: e1002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Butow R. A., 2006. Mitochondrial retrograde signaling. Annu. Rev. Genet. 40: 159–185. [DOI] [PubMed] [Google Scholar]
- McCormick M. A., Mason A. G., Guyenet S. J., Dang W., Garza R. M., et al. , 2014. The SAGA histone deubiquitinase module controls yeast replicative lifespan via Sir2 interaction. Cell Reports 8: 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli M. V., Jiang J. C., Tiwari A., Rodriguez-Quinones J. F., Jazwinski S. M., 2011. Loss of mitochondrial membrane potential triggers the retrograde response extending yeast replicative lifespan. Front. Genet. 2: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischerikow N., Spedale G., Altelaar A. F., Timmers H. T., Pijnappel W. W., et al. , 2009. In-depth profiling of post-translational modifications on the related transcription factor complexes TFIID and SAGA. J. Proteome Res. 8: 5020–5030. [DOI] [PubMed] [Google Scholar]
- Mishur R. J., Khan M., Munkacsy E., Sharma L., Bokov A., et al. , 2016. Mitochondrial metabolites extend lifespan. Aging Cell 15: 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K., Yasumura K., Igarashi K., Harashima S., Kakinuma Y., 1999. Transcription of some PHO genes in Saccharomyces cerevisiae is regulated by spt7p. Yeast 15: 1711–1717. [DOI] [PubMed] [Google Scholar]
- Ofiteru A. M., Ruta L. L., Rotaru C., Dumitru I., Ene C. D., et al. , 2012. Overexpression of the PHO84 gene causes heavy metal accumulation and induces Ire1p-dependent unfolded protein response in Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 94: 425–435. [DOI] [PubMed] [Google Scholar]
- Palacios DeBeer M. A., Muller U., Fox C. A., 2003. Differential DNA affinity specifies roles for the origin recognition complex in budding yeast heterochromatin. Genes Dev. 17: 1817–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Schroeder E. A., Ocampo A., Barrientos A., Shadel G. S., 2011. Regulation of yeast chronological life span by TORC1 via adaptive mitochondrial ROS signaling. Cell Metab. 13: 668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V. S., Morgan M. M., Scott R., Clements L. S., Butow R. A., 1987. The mitochondrial genotype can influence nuclear gene expression in yeast. Science 235: 576–580. [DOI] [PubMed] [Google Scholar]
- Park P. U., Defossez P. A., Guarente L., 1999. Effects of mutations in DNA repair genes on formation of ribosomal DNA circles and life span in Saccharomyces cerevisiae. Mol. Cell. Biol. 19: 3848–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos J. F., Saretzki G., Ahmed S., Nelson G., Richter T., et al. , 2007. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 5: e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pray-Grant M. G., Schieltz D., McMahon S. J., Wood J. M., Kennedy E. L., et al. , 2002. The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol. Cell. Biol. 22: 8774–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekito T., Thornton J., Butow R. A., 2000. Mitochondria-to-nuclear signaling is regulated by the subcellular localization of the transcription factors Rtg1p and Rtg3p. Mol. Biol. Cell 11: 2103–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., 1991. Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- Shor E., Warren C. L., Tietjen J., Hou Z., Muller U., et al. , 2009. The origin recognition complex interacts with a subset of metabolic genes tightly linked to origins of replication. PLoS Genet. 5: e1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P., 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D. A., Guarente L., 1997. Extrachromosomal rDNA circles: a cause of aging in yeast. Cell 91: 1033–1042. [DOI] [PubMed] [Google Scholar]
- Spedale G., Mischerikow N., Heck A. J., Timmers H. T., Pijnappel W. W., 2010. Identification of Pep4p as the protease responsible for formation of the SAGA-related SLIK protein complex. J. Biol. Chem. 285: 22793–22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Rai K. M., Pandey B., Singh S. P., Sawant S. V., 2015. Spt-Ada-Gcn5-Acetyltransferase (SAGA) complex in plants: genome wide identification, evolutionary conservation and functional determination. PLoS One 10: e0134709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner D. E., Grant P. A., Roberts S. M., Duggan L. J., Belotserkovskaya R., et al. , 1999. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol. Cell. Biol. 19: 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner D. E., Belotserkovskaya R., Berger S. L., 2002. SALSA, a variant of yeast SAGA, contains truncated Spt7, which correlates with activated transcription. Proc. Natl. Acad. Sci. USA 99: 11622–11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Wu R., 1979. Insertion of a genetic marker into the ribosomal DNA of yeast. Plasmid 2: 536–554. [DOI] [PubMed] [Google Scholar]
- Traven A., Wong J. M., Xu D., Sopta M., Ingles C. J., 2001. Interorganellar communication. Altered nuclear gene expression profiles in a yeast mitochondrial dna mutant. J. Biol. Chem. 276: 4020–4027. [DOI] [PubMed] [Google Scholar]
- Tsankov A. M., Brown C. R., Yu M. C., Win M. Z., Silver P. A., et al. , 2006. Communication between levels of transcriptional control improves robustness and adaptivity. Mol. Syst. Biol. 2: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L., Baruah A., Chang H. W., Pace H. M., Lee S. S., 2011. The homeobox protein CEH-23 mediates prolonged longevity in response to impaired mitochondrial electron transport chain in C. elegans. PLoS Biol. 9: e1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Jiang J. C., Jazwinski S. M., 2010. Gene regulatory changes in yeast during life extension by nutrient limitation. Exp. Gerontol. 45: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Malcolm B. A., 2002. Two-stage polymerase chain reaction protocol allowing introduction of multiple mutations, deletions, and insertions, using QuikChange site-directed mutagenesis. Methods Mol. Biol. 182: 37–43. [DOI] [PubMed] [Google Scholar]
- Wu P. Y., Winston F., 2002. Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex. Mol. Cell. Biol. 22: 5367–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff D. D., O’Shea E. K., 2001. Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159: 1491–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Hekimi S., 2010. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 8: e1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. J., Seto E., 2008. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat. Rev. Mol. Cell Biol. 9: 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Pracheil T., Thornton J., Liu Z., 2013. Adenosine triphosphate (ATP) is a candidate signaling molecule in the mitochondria-to-nucleus retrograde response pathway. Genes (Basel) 4: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Strains are available upon request.