Review on alcohol induction of neuroinflammation and the potential for nuclear receptor agonist in prevention or treatment of FASD and AUD.
Keywords: fetal alcohol spectrum disorders, alcohol use disorders, neuroinflammation, peroxisome proliferator-activated receptor, microglia
Abstract
Fetal alcohol spectrum disorder (FASD), which results from ethanol exposure during pregnancy, and alcohol use disorder (AUD), which includes both binge and chronic alcohol abuse, are strikingly common and costly at personal and societal levels. These disorders are associated with significant pathology, including that observed in the CNS. It is now appreciated in both humans and animal models that ethanol can induce inflammation in the CNS. Neuroinflammation is hypothesized to contribute to the neuropathologic and behavioral consequences in FASD and AUD. In this review, we: 1) summarize the evidence of alcohol-induced CNS inflammation, 2) outline cellular and molecular mechanisms that may underlie alcohol induction of CNS inflammation, and 3) discuss the potential of nuclear receptor agonists for prevention or treatment of neuropathologies associated with FASD and AUD.
AUD AND FASD
AUD, which is common in adolescents and adults, and FASD, which results from ethanol exposure during pregnancy, produce major sequelae, including those observed in the CNS. These disorders are alarmingly common, have dramatic impact on affected individuals, and are costly at a societal level.
Recent estimates of the lifetime prevalence of AUD, as defined in the Diagnostic and Statistical Manual–V and based on results of the National Epidemiologic Survey on Alcohol and Related Conditions–III, are 29% in the United States [1]. In adults, AUD occurs in 36% of men and 23% of women [1]. Earlier studies reveal that binge drinking occurs in at least 25% and 13% of adult men and women, respectively [2, 3], and 1 in 6 adults binge drinks 4 times/mo [2–4]. Both binge and chronic drinking produce extensive CNS pathology and behavioral dysfunction [5].
Despite public health warnings, over 12% of pregnant women in the United States drink alcohol [6], exposing the developing fetal brain to alcohol. Two to 5% of U.S. children are born with FASD, which is the leading cause of mental retardation [7]. Fetal alcohol exposure can result in significant neuropathology and associated cognitive and behavioral impairments that persist throughout life, including increased risk of AUD [8, 9].
CNS development continues in adolescence and young adulthood. Heightened vulnerability of adolescents and young adults to long-term effects of alcohol in the CNS has been recently appreciated. Neurogenesis, dendritic growth, synaptic remodeling, and development of critical cognitive functions are dynamic at these ages and subject to alcohol disruption [10–12]. Concern about alcohol consumption at these ages is amplified by the binge pattern of alcohol drinking in almost 40% of young adults [3]. Among adolescents who drink, binge drinking is most common, and 3.4% of adolescents already, at this young age, have an AUD [3]. Among college students, 20% binge drink and 20% have an AUD [13]. These age groups are relatively resistant to the sedative and motor-impairing effects of alcohol, resulting in the possibility of dangerously high blood alcohol concentrations. Alcohol consumption in these groups produces significant long-term neuropathological and behavioral consequences, including increased risk of AUD [14].
Investigations focusing on the molecular and cellular mechanisms of neuropathology and the consequences of alcohol consumption on the fetus during pregnancy and in adolescents and adults are being performed in animal models. Important to this goal, studies in binge drinking or chronic drinking adult animal models demonstrate the CNS pathology and behavioral abnormalities observed in humans with AUD [15, 16]. These models are providing new insights into alcohol effects in the developing fetal and adolescent brains, as well as the mature adult brain.
Recent investigations in humans and animal models have revealed that alcohol exposure of the CNS at any stage of life, from fetal development into adulthood, generates neuroimmune responses, including neuroinflammation [17]. Furthermore, these responses are believed to contribute to ethanol-induced neuropathology and behavioral deficits, including cognitive dysfunction and increased alcohol drinking. This suggests that anti-inflammatory drugs may be effective in the treatment of FASD and AUD. This review will consider this important emerging area of research.
NEUROIMMUNE ACTIVITY IN THE CNS
Glia (microglia, astrocytes, and oligodendrocytes) and neurons are the principal cells of the CNS. Glia normally maintain homeostasis in the CNS and respond to CNS insults for the protection of neurons and CNS function. Collectively, glia maintain energetic balance, remove toxic molecules, provide neurotrophic factors, phagocytose cellular debris, promote neurotransmission, and modulate synaptic plasticity [18]. In addition, microglia and astrocytes function as immune cells in the CNS and mediate innate immune responses in the parenchyma. Glia represent the first line of defense against CNS insult, as the blood-brain barrier restricts access of peripheral immune cells to the CNS parenchyma.
Microglia constitute 5–10% of the cells in the CNS parenchyma, with the density varying somewhat in different regions. Microglia in the unperturbed, mature CNS have classically been designated as “resting” or “quiescent” cells. Although commonly used, these descriptors are gross misnomers, as these cells in the healthy CNS are far from resting or quiescent, being surveillant, homeostatic, and neuroprotective, as noted above. In fact, microglia are highly motile in the mature CNS, as evidenced in multiphoton in vivo imaging [19, 20]. Microglia express many neurotransmitter receptors and actively sense neural activity with direct contact with synapses and spines. Several lines of evidence demonstrate they constitutively modulate synaptic plasticity by functional interaction with synapses [19].
It is well characterized that microglia change to an activated state and exhibit neuroimmune activity in association with CNS insult, neuropathology, neuroinflammation, aging, or neurodegenerative disease [21–25]. Under these situations that activate microglia, their morphology changes. Microglia in the unperturbed CNS exhibit a ramified morphology characterized by the presence of a relatively small cell body and long, branched processes. Activated microglia are characterized by hypertrophy of the cell body with shorter and thicker processes, a bushy appearance, or an ameboid appearance. Microglial activation with morphologic change is a hallmark of a spectrum of microglial neuroimmune responses to CNS insult [21–25]. In fact, activated microglia can assume an array of functional and morphologic phenotypes, which vary with the type, severity, duration, and region of insult, for the purposes of neuroprotection, tissue repair, or neurodegeneration. Attempts to categorize changes in microglial function with activation include the definition of phenotypes of activation. Such categorization systems include designation of the following: 1) "intermediate" and "reactive" phenotypes of activation between the unperturbed homeostatic, ramified phenotype and a phagocytic, ameboid phenotype [26]; 2) stages 0–3b, with "resting," "alert," "homing," "phagocytic," and "bystander activation" phenotypes [27]; and 3) "M1 classically activated" and "M2a–d alternatively activated" phenotypes [28]. The M1/M2a–d system is based on an extensive array of cell-surface and secreted molecules that form a biomarker gene signature of functional changes in the cells [28, 29]. M1/M2a–d categorization has garnered much recent attention because it not only reflects changes in microglial function but also, importantly, is contributing to design of therapeutic interventions in neuroinflammatory and neurodegenerative disease conditions [28–30].
Neuroimmune responses of microglia include increased production and secretion of neuroinflammatory molecules including chemokines and cytokines, and molecules toxic to CNS cells, including cytokines, reactive nitrogen species, and reactive oxygen species. Activated microglia can express MHC proteins required for antigen presentation to T cells of the adaptive immune system. In this manner, activated microglia play an important role in removal of pathogens from the CNS. However, with prolonged or high levels of activation, microglia produce molecules that may cause death of neurons [28–31]. This suggests microglia may contribute to neurodegenerative diseases [28–32]. In the absence of activating stimuli, microglia can revert to an apparently nonactivated phenotype. However, the reverted cells are not truly naïve and may be primed for subsequent amplified activation by a second inflammatory stimulus [25, 33].
Microglia are of hematopoietic origin, in contrast to astrocytes, oligodendrocytes, and neurons that are derived from neuroectoderm [23, 34]. Microglia are believed to share a common lineage with peripheral monocytes and macrophages [35]. They perform similar functions and share the requirement for the growth factor CSF-1 and the transcription factor PU.1 to mediate their differentiation. Microglia appear to be long-lived cells that as evidenced by genetic fate-mapping studies, undergo constant self-renewal from CNS microglial progenitors [36] or proliferation under conditions of CNS pathology [37, 38]. Parabiosis studies involving connection of donor and recipient blood supplies further indicate that few peripheral monocytes enter the CNS following formation of the blood-brain barrier and peripheral monocytes do not differentiate into microglia [39].
Microglia colonize the developing CNS via migration from the vasculature before the formation of the blood-brain barrier from their site of origin in the primitive yolk sac [34, 40]. The first microglia take up residence in the CNS before differentiation of other neural cell types. Microglia increase rapidly in number during fetal life by migration and proliferation, followed by loss of cells to achieve the size of the adult microglial population. Initially ameboid as they enter the CNS, microglial morphology transitions to a ramified state as the CNS matures in fetal and postnatal life.
The scope and impact of microglial function in the developing CNS are becoming increasingly impressive [34, 40]. In the normally developing CNS, they present an activated phenotype, proliferate, and become phagocytic in response to neuronal apoptosis and to remove excess neural precursor cells in neurogenic niches. At the same time, they exhibit trophic activity for the processes of neurogenesis, including proliferation of neural progenitor cells and neuron differentiation, neuronal survival, astrogenesis, and oligodendrogenesis. Microglia are important for development and maintenance of neural circuits, as clearly demonstrated in the developing CNS in studies using genetic or pharmacologic depletion of microglia. They strongly influence migration and spatial patterning of interneurons, as well as axon development and remodeling. In their dynamic physical contacts with neurons, they phagocytose dendritic spines and axon terminal fragments. They have a striking effect on structural and functional synapse development, synapse pruning, and synaptic plasticity. Studies demonstrate that even a transient decrease in microglia at critical stages of CNS development results in altered synaptic plasticity [41]. Normal molecular interactions between microglia and neurons in the developing CNS are necessary for adult cognitive function, and deletion of key microglia-neuron signaling mechanisms leads to abnormal behavior associated with neuropsychiatric disorders [34, 40, 41].
ALCOHOL INDUCES NEUROINFLAMMATION THROUGHOUT THE LIFESPAN
Developing CNS
It is well understood that ethanol has detrimental effects in the developing CNS. The effect of ethanol on neuroimmune responses in the fetal CNS is understudied and has only recently begun to be evaluated. We have used a rodent model of FASD involving early neonatal ethanol exposure, a time that is developmentally equivalent to third-trimester ethanol exposure in humans. With the use of this model, we [42] and others [43–45] have demonstrated that ethanol decreases the viability of cerebellar Purkinje and granule cell neurons. We [42] also have shown that ethanol is toxic to cerebellar microglia. Microglia in the cerebellum that survived ethanol toxicity have been shown by us [42] and others [46] to exhibit morphologically an activated phenotype. We [47] and others [48] have further demonstrated that the effects of neonatal ethanol exposure on microglial activation extends beyond the cerebellum to the hippocampus and cerebral cortex. In addition to microglial activation, we have shown that ethanol induces other neuroimmune responses in the developing CNS with increased expression of proinflammatory cytokines and chemokines, including IL-1β, TNF-α, and CCL2 in the cerebellum, hippocampus, and cerebral cortex [47]. Our findings are supported by studies from other laboratories further demonstrating ethanol induction of IL-1β, TNF-α, CCL4, and NF-κB in these regions [46, 48, 49]. The finding that ethanol induces neuroinflammatory processes in FASD models is important, as even transient microglial activation or neuroinflammation during CNS development can cause cognitive and psychiatric disorders in adults [50, 51]. The mechanisms by which ethanol induces neuroinflammation in the developing CNS are not well understood. Changes in homeostatic interactions between microglia and neurons that mediate synaptic plasticity or phagocytosis of neuronal debris during development are likely involved. We hypothesize that ethanol-induced neuroinflammation and neurodegeneration in each of these brain regions during early development may be associated with specific long-term behavioral deficits common in FASD [52, 53].
Adolescent CNS
The adolescent CNS is highly vulnerable to ethanol-induced neuropathology and behavioral deficits, as dynamic neuronal development continues into this period. The role of neuroimmune responses in these ethanol effects is under investigation. Studies concerning the effects of ethanol on microglial activation and neuroinflammation in the adolescent CNS have provided mixed results. Administration of ethanol to adolescent rats using a 4 d binge-drinking model resulted in changes in hippocampal microglial morphology in the absence of changes in expression of the microglial activation markers ED1 and MHC class II, suggesting partial microglial activation [54]. Ethanol administration to adolescent mice using a 10 d drinking model did not increase expression of IL-6, TNF-α, or CCL2 in the cerebral cortex, cerebellum, or hippocampus [55]. These studies did, however, demonstrate increased glial fibrillary acidic protein expression, characteristic of astrogliosis [55]. Neuroimmune signaling pathways were not observed to be significantly activated in the adolescent CNS in other studies, as determined by global gene-expression analysis [56]. In contrast, IL-1β, TNF-α, TLR2, and TLR4 expression was increased in the prefrontal cortex region of adolescent mice using a binge-drinking model [57]. Ethanol treatment resulted in increased alcohol preference in wild-type but not TLR4 knockout adolescent mice, suggesting a role of neuroinflammation in modulating this behavior [58]. In addition, intermittent binge ethanol exposure of adolescent rats resulted in increased expression of TLR4, HMGB1, cytokines, and chemokines in the hippocampus [59]. Collectively, these studies suggest that ethanol may induce glial activation and neuroinflammation in the adolescent CNS but that ethanol-induced neuroinflammation may be more modest in adolescents than at other life stages, and the features of neuroinflammation may vary depending on the paradigm of ethanol treatment and possibly other factors. Interestingly, prenatal ethanol exposure resulted in increased alcohol drinking in adolescent offspring, and neuroinflammation was suggested to play a role in this process [60]. Additional studies concerning ethanol-induced neuroinflammation in adolescence are warranted due to the detrimental effect of adolescent alcohol consumption on long-term behavioral deficits and addiction.
Adult CNS
Chronic alcohol abuse is common in adults and can result in significant neuropathology and cognitive deficits. In adult alcoholics, the expression of a variety of inflammatory molecules is increased in the CNS [61, 62]. This includes the transcription factor NF-κB, which plays a critical role in activating multiple genes encoding proinflammatory molecules, such as cytokines and chemokines. Studies using multiple ethanol exposure paradigms in animal models have demonstrated increased expression of proinflammatory molecules in distinct brain regions. For example, in a 10 d ethanol treatment paradigm, cytokines and chemokines, including CCL2, IL-1β, and TNF-α, as well as TLR4 and the TLR4 agonist HMGB1, were elevated in adult mice [55, 63, 64]. These molecules were generally elevated in the cerebral cortex, cerebellum, and hippocampus, but all of these proinflammatory molecules were not induced in every brain region investigated. In this 10 d drinking model, aged mice also presented a neuroinflammatory response, albeit more limited, that was also region specific [65]. With the use of an acute model of ethanol treatment, in which tissue was isolated 3 h following ethanol administration to adult rats, IL-6 and IκBα were increased and TNF-α was decreased in multiple brain regions, indicating ethanol-altered expression of immune molecules in this experimental paradigm [66]. It should be noted that neuroinflammation was not observed in adult rats in a 4 d binge-drinking model [67]. Overexpression of the chemokine CCL2 is interesting, in particular, because CCL2-overexpressing mice exhibit altered hippocampal synaptic transmission [68], suggesting that increased CCL2 expression in human alcoholics may alter synaptic transmission and cognition. It is important now to determine whether the neuropathologic and behavioral consequences of adult alcohol consumption are linked to alcohol induction of neuroinflammation.
ETHANOL-INDUCED INFLAMMATORY SIGNALING PATHWAYS
TLR4 signaling
Glia react to pathogens in the CNS, principally through TLRs present on their surface. Recent studies have begun to evaluate the role of TLR4 signaling in ethanol-induced neuroinflammation. These studies suggest that endogenous danger signals, including the TLR4 ligand HMGB1, are released following ethanol insult to the CNS and trigger TLR4 signaling in a manner similar to that observed in response to pathogens [69]. Ethanol activates TLR4-dependent downstream transcription factors, such as AP-1 and NF-κB, resulting in transcription of genes encoding a variety of proinflammatory cytokines, chemokines, COX-2, and iNOS [70]. Ethanol triggers the movement of TLR4 and IL-1R to lipid rafts in glia [71]. TLR4- and IL-1R-neutralizing antibodies suppress the production of proinflammatory molecules in response to ethanol [70]. Collectively, these studies support a role of TLR4 in ethanol-induced neuroinflammation through mechanisms involving movement of these receptors to lipid rafts.
Studies using TLR4-deficient mice further support the role of this receptor in mediating ethanol-induced neuroinflammation. For example, ethanol-activated JNK, ERK, and p38 MAPK and induced expression of COX-2 and iNOS in microglia derived from wild-type but not TLR4-deficient mice. Conditioned media from ethanol-treated wild-type but not TLR4-deficient microglia was toxic to cortical neurons, suggesting that ethanol triggered microglial production of neurotoxic molecules in a TLR4-dependent manner [72]. Additional studies indicated that ethanol increased the expression of the cytokines TNF-α and IL-6, the transcription factor NF-κB, and the apoptotic marker caspase-3 in a TLR4-dependent manner [73]. TLR4-dependent signaling was also shown to be critical in ethanol-induced changes in histone acetylation, suggesting ethanol-induced neuroinflammation may involve epigenetic changes in chromatin configuration [74]. Collectively, these studies demonstrate that TLR4 is critical in ethanol-induced neuroinflammation and neurodegeneration.
Neuroinflammation is associated with increased alcohol consumption in rodents [75, 76]. TLR4 signaling may play a critical role in this process. This concept is supported by studies demonstrating that TLR4-deficient mice consume less ethanol than wild-type control mice [74]. Furthermore, TLR4-deficient mice exhibit less sedation in response to ethanol than control mice, in spite of similar alcohol pharmacodynamics [77]. These studies also demonstrated that infusion of TLR4 small interfering RNA into the amygdala of alcohol-preferring rats suppressed binge-like drinking behavior [77]. As the neurotransmitter GABA is known to modulate alcohol consumption, the observation that GABAA α2 mediates TLR4-dependent changes in ethanol consumption [78] suggests a mechanism by which TLR4 modulates drinking behavior. Collectively, these studies suggest ethanol-induced neuroinflammation triggers increased alcohol consumption, and this occurs through TLR4-dependent mechanisms.
Although it is clear that TLR4 signaling modulates ethanol-induced neuroinflammation and alcohol consumption, the signaling pathways downstream of TLR4 that are involved in these processes have not been adequately investigated. TLR4 signaling can result in downstream activation of MyD88-dependent or alternatively, MyD88-independent (TRIF-dependent) signaling pathways [79]. The MyD88-dependent signaling pathway involves TLR4 interaction with MyD88, resulting in the activation of the transcription factor NF-κB. The MyD88-independent pathway involves interaction of TLR4 with the adaptor protein TRIF, leading to activation of the transcription factors NF-κB and IFN regulatory factor 3. Interestingly, chronic ethanol treatment potentiated the induction of proinflammatory cytokines and chemokines in the brain by the TLR3 agonist polyinosinic:polycytidylic acid [80]. As TLR3 acts through a MyD88-independent pathway, these studies suggest that ethanol may act, at least in part, through this pathway. Determination of the signaling pathways involved in ethanol-induced neuroinflammation is critical, as blocking these signaling pathways may prove effective in the treatment of FASD and AUD.
Inflammasome activation
Inflammasomes are multiprotein complexes that respond to pathogens, as well as endogenous danger signals, and function as part of the innate immune system. Caspase-1-activating inflammasomes function to process inactive pro-caspase-1 to active caspase-1. In turn, caspase-1 processes pro-IL-1β to mature IL-1β. Ethanol insult results in release of the danger signal HMGB1, which contributes to increased expression of caspase-1 and IL-1β in the CNS [49, 73, 81]. This suggests a role for caspase-1 inflammasomes in ethanol-induced neuroinflammation. The NLRP3 inflammasome is a caspase-1-activating inflammasome composed of multiple proteins, including NLRP3 and the adaptor molecule ASC. Studies that indicate ethanol increased caspase-1 and IL-1β expression in the CNS of wild-type but not NLRP3- or ASC-deficient mice support a role of the NLRP3 inflammasome in modulating ethanol-induced neuroinflammation [81].
Fractalkine signaling
Fractalkine–fractalkine receptor (CX3CL1–CX3CR1) signaling plays a critical role in suppressing the activation of microglia, as established in studies using CX3CL1- or CX3CR1-deficient mice [82]. CX3CR1 is expressed exclusively on microglia, and CX3CL1 is expressed exclusively on neurons in the CNS. CX3CL1–CX3CR1 signaling regulates the balance between neuron death and survival following CNS insult [83]. CX3CR1-deficient mice exhibit increased microglial activation and neurotoxicity [82]. Treatment of neuron-glia cocultures with CX3CL1 suppresses the expression of proinflammatory cytokines and protects neurons [84, 85]. We have demonstrated in recent studies that ethanol suppresses expression of CX3CL1 in a neonatal mouse model of FASD [unpublished results]. This suggests that ethanol may induce neuroinflammation and neurodegeneration through a mechanism involving alterations in CX3CL1–CX3CR1 signaling.
Summary
TLR4 signaling, inflammasome activation, and fractalkine signaling each represent a possible mechanistic pathway through which ethanol induces neuroinflammation, which is believed to contribute to FASD and AUD. Further defining the exact mechanisms by which ethanol modulates these inflammatory processes is critical to the development of therapies for FASD and AUD.
NUCLEAR RECEPTORS AS POTENTIAL THERAPEUTICS FOR FASD AND AUD
Nuclear receptors comprise a large group of transcription factors that are activated in response to a diverse array of molecules, including steroid hormones and lipid-soluble signals. They play critical roles in development, metabolism, and reproduction [86]. Type II nuclear receptors include PPAR-α, -β/δ, and -γ and LXRs. PPARs play essential roles in lipid and fatty acid metabolism. LXRs are essential to cholesterol homeostasis. These type II nuclear receptors function as heterodimers with retinoid X receptors. In the absence of bound ligand, PPARs and LXRs are unable to activate transcription, as a result of receptor interaction with corepressor complexes in the nucleus. Upon ligand binding, corepressors are released and coactivators bind the receptor. The receptor-coactivator complex then activates transcription of target genes by binding to specific response elements in the promoters of these genes [87]. In this manner, PPARs and LXRs regulate cellular energy and metabolic demands.
PPARs and LXRs also play a central role in regulation of inflammatory responses through a receptor-dependent transrepression mechanism [88]. In this case, ligand binding results in sumoylation of PPAR or LXR, allowing interaction with transcription factors, including NF-κB and AP-1, which play a critical role in activation of genes encoding proinflammatory mediators. The receptor–transcription factor complex then interacts with a corepressor that blocks binding of NF-κB to target genes.
Nuclear receptor agonists regulate ethanol-induced neuroinflammation and protect against ethanol-induced neuropathology in animal models of FASD [42, 47]. These agonists have also been demonstrated to protect against AUD in animal models, including suppression of ethanol consumption [89–91]. Additional studies are needed to determine the exact mechanisms by which nuclear receptor agonists protect against the damaging effects of ethanol in the CNS. It will be interesting to compare the efficacy of agonists specific for nuclear receptor subtypes for protection against ethanol and determine if receptor subtypes act through similar or distinct mechanisms. It is hoped anti-inflammatory pharmaceuticals, including nuclear receptor regulators, prove to be effective in the treatment of FASD and AUD.
PPAR-γ agonists
The PPAR-γ agonist pioglitazone, a member of the thiazolidinedione class of medications, is commonly used for the treatment of type II diabetes. PPAR-γ agonists can also suppress inflammatory responses, including those that occur in the CNS. For example, PPAR-γ agonists are potent suppressors of microglial and astrocyte activation in vitro [92]. They also suppress disease in a variety of animal models characterized by neuroinflammation and neurodegeneration, including multiple sclerosis, Alzheimer’s disease, spinal cord injury, amyotrophic lateral sclerosis, and stroke [93–97]. However, the effect of PPAR-γ agonists on neuroinflammation in animal models of FASD and AUD have only begun to be evaluated. We demonstrated recently that microglia and cerebellar granule cell neurons in culture were protected from the toxic effects of alcohol by the PPAR-γ agonists pioglitazone and 15-deoxy-Δ12,14 PGJ2. These agonists also protected against ethanol-induced loss of microglia and Purkinje cell neurons in an animal model of FASD [42]. We demonstrated that pioglitazone and 15-deoxy-Δ12,14 PGJ2 blocked ethanol-induced morphologic microglial activation [42]. We further demonstrated that pioglitazone suppressed ethanol induction of proinflammatory cytokines and chemokines in the cerebellum, hippocampus, and cortex in the FASD model [47]. We demonstrated that the PPAR-γ agonist DHA [98] also has potent anti-inflammatory activity in the FASD model [unpublished results]. DHA is an ω-3 polyunsaturated fatty acid that plays a critical role in normal neuronal development and synaptic function [99]. DHA is safe in the human fetus, and supplementation of maternal diet or infant formula with DHA increases the problem-solving ability in infants [100, 101]. Exposure to prenatal ethanol reduced DHA concentrations in the brain [102, 103]. Addition of DHA suppressed ethanol-induced neuroinflammation and neurodegeneration in adult rat hippocampal-entorhinocortical slices in vitro [104]. Treatment with a DHA metabolite ameliorated ethanol-induced impairment of neural stem cell differentiation in culture [105]. In particular, DHA supplementation ameliorated deficits in somatosensory system-dependent behaviors in rats exposed prenatally to ethanol [106]. This opens the possibility that the PPAR-γ agonist DHA may be safe and effective in the treatment of FASD.
In humans, polymorphisms in PPAR-γ and PGC-1α, a coactivator of PPAR-γ, are associated with alcohol withdrawal and dependence [91]. PPAR-γ agonist treatment decreased alcohol consumption and alcohol-seeking behavior in rodents [90, 91]. The ability of PPAR-γ agonists to suppress ethanol consumption was blocked by PPAR-γ antagonist injection into the lateral cerebroventricle. This finding suggests that PPAR-γ agonists have direct effects against ethanol in the brain [90], and PPAR-γ agonists may be effective in the treatment of AUD. A link between neuroinflammation and increased alcohol consumption is supported by studies indicating treatment of rodents with LPS resulted in a prolonged increase in voluntary ethanol consumption [107] and mice deficient in a variety of genes encoding inflammatory molecules consumed less ethanol [75, 76]. It may be important that naltrexone, commonly used in the treatment of AUD, suppresses ethanol-induced microglial activation [80]. This opens the possibility that inhibition of microglial activation may play a primary role in naltrexone suppression of alcohol consumption. In support of this hypothesis, (+) naltrexone and the related (+) naloxone are TLR4 antagonists that block microglial activation [108] and alcohol induced sedation and motor impairment [77], in spite of the fact that these isomers are opioid receptor inactive. Interestingly, pioglitazone and naltrexone acted cooperatively in suppressing ethanol consumption, perhaps suggesting distinct mechanisms of action of these agents [109]. This may suggest that a combination of anti-inflammatory agents acting through distinct mechanisms may provide the most effective treatment of AUD and FASD.
PPAR-α agonists
Fibrates are PPAR-α agonists that are commonly used in the treatment of dyslipidemia. The fibrates gemfibrozol and fenofibrate [89, 91, 110, 111] were previously determined to suppress ethanol consumption in rodent models. The effect of fenofibrate on suppression of ethanol consumption was shown to be PPAR-α dependent, as this fibrate did not suppress ethanol consumption in PPAR-α-deficient mice [89]. Fenofibrate also more strongly suppressed ethanol consumption in male than female mice [89]. PPAR-α agonists likely suppress ethanol consumption, at least in part, by suppressing inflammatory responses in glia. Interestingly, PPAR-α agonists produced a robust neuronal signature in the mouse brain, as determined by unbiased genomic profiling. The results suggested that these agonists may act through neuropeptide and dopaminergic signaling pathways in the amygdala, a brain region known to be important in alcohol addiction [110]. Additional studies demonstrated that oleoylethanolamide also reduced ethanol consumption in rodents [112]. Oleoylethanolamide is an endocannabinoid-like compound that does not bind to cannabinoid receptors but instead, acts through PPAR-α [113]. The effect of PPAR-α agonists on ethanol-induced neuroinflammation and associated neuropathology in animal models of FASD and adolescent and adult AUD models has not been investigated. However, studies indicating that PPAR-α agonists suppress the production of proinflammatory molecules in glia and are effective in the treatment of animal models of neuroinflammatory and neurodegenerative disorders [114, 115] suggest that this may be a productive avenue of investigation.
Other type II nuclear receptors
Other type II nuclear receptors, including PPAR-β/δ and LXR, and their agonists have not been adequately investigated for their role in mediating ethanol effects. Studies have indicated that PPAR-β/δ agonists did not suppress ethanol consumption in rodents, so these receptors may not be viable therapeutic targets for ethanol-induced neuroinflammation and associated neuropathologies. Previous studies have clearly indicated that LXR agonists suppress glial activation and are protective in animal models of neuroinflammatory and neurodegenerative disorders [116–118]. However, the possibility that LXR agonists may be therapeutic for ethanol-induced neuroinflammation and associated neuropathologies has not been investigated.
CONCLUDING REMARKS
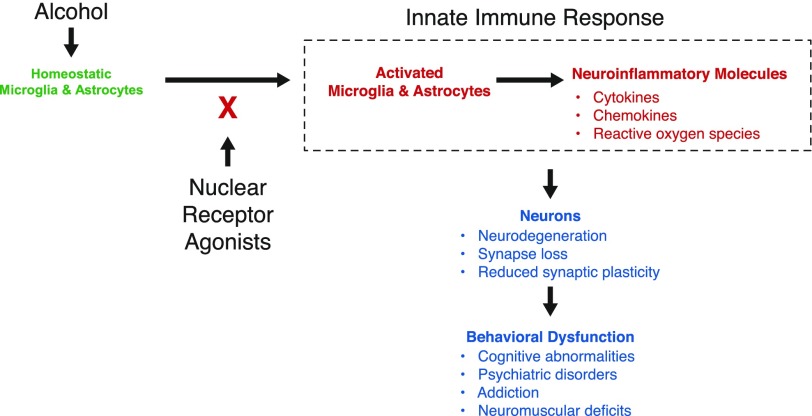
The CNS consequences of alcohol consumption are extensive and produce major health concerns. There are no effective treatments. Thus, there is a major push to understand the mechanisms underlying alcohol-induced pathologies in the CNS. The resident CNS immune cells, microglia and astrocytes, appear to be central players in ethanol induction of neuroinflammatory processes in FASD and AUD. Recent studies into the neuroimmune response to alcohol reveal that alcohol exposure generates inflammatory events in the CNS at any stage of life from fetal development into adulthood. Animal models provide a resource for preclinical testing of anti-inflammatory strategies for prevention of and therapy for FASD and AUD. Recent studies provide exciting insights that suggest nuclear receptors may be targets for intervention in these disorders (Fig. 1). Based on new and existing knowledge of immune responses in the CNS, including the finding that alcohol induces inflammatory events in the CNS, this review summarizes foundational studies for the promise that anti-inflammatory pharmaceuticals, including PPAR agonists, hold as effective strategies for prevention or treatment of FASD and AUD.
Figure 1. Nuclear receptor agonists can block innate immune responses to alcohol in the CNS.
Alcohol induces activation of glia (microglial and astrocytes) with expression of neuroinflammatory molecules. This, in turn, may lead to neuron death, impaired neuron function, and long-term behavioral dysfunction associated with FASD and AUD. We hypothesize that nuclear receptor agonists can inhibit alcohol-induced glial activation and neuroinflammation and protect against FASD and AUD.
AUTHORSHIP
C.J.M.K. and P.D.D. wrote and edited the manuscript.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (Grants AA018834 and AA023723) and National Institute of General Medical Sciences Institutional Development Award (P30 GM110702).
Glossary
- ASC
apoptosis-associated speck-like protein
- AUD
alcohol use disorders
- COX
cyclooxygenase
- DHA
docosahexaenoic acid
- FASD
fetal alcohol spectrum disorders
- HMGB
high-mobility group box
- LXR
liver X receptor
- NLRP3
NACHT, LRR, and PYD domain-containing protein 3
- PPAR
peroxisome proliferator-activated receptor
- TRIF
Toll/IL-1R domain-containing adapter-inducing IFN-β
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Grant B. F., Goldstein R. B., Saha T. D., Chou S. P., Jung J., Zhang H., Pickering R. P., Ruan W. J., Smith S. M., Huang B., Hasin D. S. (2015) Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (2013) Binge Drinking - United States, 2011. Morb. Mortal. Wkly. Rep. 62, 77–80. [Google Scholar]
- 3. Substance Abuse and Mental Health Services Administration. (2014) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD.
- 4.Centers for Disease Control and Prevention (CDC) (2012) Vital signs: binge drinking prevalence, frequency, and intensity among adults—United States, 2010. Morb. Mortal. Wkly. Rep. 61, 14–19. [PubMed] [Google Scholar]
- 5.Sullivan E. V., Harris R. A., Pfefferbaum A. (2010) Alcohol’s effects on brain and behavior. Alcohol Res. Health 33, 127–143. [PMC free article] [PubMed] [Google Scholar]
- 6.Floyd R. L., Weber M. K., Denny C., O’Connor M. J. (2009) Prevention of fetal alcohol spectrum disorders. Dev. Disabil. Res. Rev. 15, 193–199. [DOI] [PubMed] [Google Scholar]
- 7.May P. A., Gossage J. P., Kalberg W. O., Robinson L. K., Buckley D., Manning M., Hoyme H. E. (2009) Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 15, 176–192. [DOI] [PubMed] [Google Scholar]
- 8.Lebel C., Roussotte F., Sowell E. R. (2011) Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol. Rev. 21, 102–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattson, S. N., Roesch, S. C., Glass, L., Deweese, B. N., Coles, C. D., Kable, J. A., May, P. A., Kalberg, W. O., Sowell, E. R., Adnams, C. M., Jones, K. L., Riley, E. P., Collaborative Initiative on Fetal Alcohol Spectrum Disorders (2013) Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 37, 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerri C., Pascual M. (2010) Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 44, 15–26. [DOI] [PubMed] [Google Scholar]
- 11.Broadwater M. A., Liu W., Crews F. T., Spear L. P. (2014) Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev. Neurosci. 36, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClain J. A., Hayes D. M., Morris S. A., Nixon K. (2011) Adolescent binge alcohol exposure alters hippocampal progenitor cell proliferation in rats: effects on cell cycle kinetics. J. Comp. Neurol. 519, 2697–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco C., Okuda M., Wright C., Hasin D. S., Grant B. F., Liu S. M., Olfson M. (2008) Mental health of college students and their non-college-attending peers: results from the National Epidemiologic Study on Alcohol and Related Conditions. Arch. Gen. Psychiatry 65, 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Squeglia L. M., Boissoneault J., Van Skike C. E., Nixon S. J., Matthews D. B. (2014) Age-related effects of alcohol from adolescent, adult, and aged populations using human and animal models. Alcohol. Clin. Exp. Res. 38, 2509–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfonso-Loeches S., Guerri C. (2011) Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit. Rev. Clin. Lab. Sci. 48, 19–47. [DOI] [PubMed] [Google Scholar]
- 16.Patten A. R., Fontaine C. J., Christie B. R. (2014) A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr. 2, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drew P. D., Kane C. J. M. (2013) Neuroimmune mechanisms of glia and their interplay with alcohol exposure across the lifespan. In Neural-Immune Interactions in Brain Function and Alcohol Related Disorders (Cui C., Grandison L., Noronha A., eds.), Springer, New York, NY, 359–386. [Google Scholar]
- 18.Ransohoff R. M., Brown M. A. (2012) Innate immunity in the central nervous system. J. Clin. Invest. 122, 1164–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nimmerjahn A., Kirchhoff F., Helmchen F. (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- 20.Tremblay M. E., Lowery R. L., Majewska A. K. (2010) Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch A. M., Murphy K. J., Deighan B. F., O’Reilly J. A., Gun’ko Y. K., Cowley T. R., Gonzalez-Reyes R. E., Lynch M. A. (2010) The impact of glial activation in the aging brain. Aging Dis. 1, 262–278. [PMC free article] [PubMed] [Google Scholar]
- 22.Kettenmann H., Hanisch U. K., Noda M., Verkhratsky A. (2011) Physiology of microglia. Physiol. Rev. 91, 461–553. [DOI] [PubMed] [Google Scholar]
- 23.Saijo K., Glass C. K. (2011) Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 11, 775–787. [DOI] [PubMed] [Google Scholar]
- 24.Crotti A., Ransohoff R. M. (2016) Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity 44, 505–515. [DOI] [PubMed] [Google Scholar]
- 25.Matt S. M., Johnson R. W. (2016) Neuro-immune dysfunction during brain aging: new insights in microglial cell regulation. Curr. Opin. Pharmacol. 26, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streit W. J., Walter S. A., Pennell N. A. (1999) Reactive microgliosis. Prog. Neurobiol. 57, 563–581. [DOI] [PubMed] [Google Scholar]
- 27.Raivich G., Bohatschek M., Kloss C. U., Werner A., Jones L. L., Kreutzberg G. W. (1999) Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. Brain Res. Rev. 30, 77–105. [DOI] [PubMed] [Google Scholar]
- 28.Franco R., Fernández-Suárez D. (2015) Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 131, 65–86. [DOI] [PubMed] [Google Scholar]
- 29.Jha M. K., Lee W. H., Suk K. (2016) Functional polarization of neuroglia: implications in neuroinflammation and neurological disorders. Biochem. Pharmacol. 103, 1–16. [DOI] [PubMed] [Google Scholar]
- 30.Hu X., Leak R. K., Shi Y., Suenaga J., Gao Y., Zheng P., Chen J. (2015) Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 11, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown G. C., Vilalta A. (2015) How microglia kill neurons. Brain Res. 1628, 288–297. [DOI] [PubMed] [Google Scholar]
- 32.Doty K. R., Guillot-Sestier M. V., Town T. (2015) The role of the immune system in neurodegenerative disorders: adaptive or maladaptive? Brain Res. 1617, 155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry V. H., Holmes C. (2014) Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10, 217–224. [DOI] [PubMed] [Google Scholar]
- 34.Tay T. L., Hagemeyer N., Prinz M. (2016) The force awakens: insights into the origin and formation of microglia. Curr. Opin. Neurobiol. 39, 30–37. [DOI] [PubMed] [Google Scholar]
- 35.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M. F., Conway S. J., Ng L. G., Stanley E. R., Samokhvalov I. M., Merad M. (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M. B., Leboeuf M., Becker C. D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S. W., Forsberg E. C., Tanaka M., van Rooijen N., García-Sastre A., Stanley E. R., Ginhoux F., Frenette P. S., Merad M. (2013) Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gómez-Nicola D., Fransen N. L., Suzzi S., Perry V. H. (2013) Regulation of microglial proliferation during chronic neurodegeneration. J. Neurosci. 33, 2481–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajami B., Bennett J. L., Krieger C., Tetzlaff W., Rossi F. M. (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538–1543. [DOI] [PubMed] [Google Scholar]
- 40.Frost J. L., Schafer D. P. (2016) Microglia: architects of the developing nervous system. Trends Cell Biol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paolicelli R. C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T. A., Guiducci E., Dumas L., Ragozzino D., Gross C. T. (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- 42.Kane C. J., Phelan K. D., Han L., Smith R. R., Xie J., Douglas J. C., Drew P. D. (2011) Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisome proliferator-activated receptor-γ agonists. Brain Behav. Immun. 25 (Suppl 1), S137–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonthius D. J., Bonthius N. E., Napper R. M., Astley S. J., Clarren S. K., West J. R. (1996) Purkinje cell deficits in nonhuman primates following weekly exposure to ethanol during gestation. Teratology 53, 230–236. [DOI] [PubMed] [Google Scholar]
- 44.Pierce D. R., Serbus D. C., Light K. E. (1993) Intragastric intubation of alcohol during postnatal development of rats results in selective cell loss in the cerebellum. Alcohol. Clin. Exp. Res. 17, 1275–1280. [DOI] [PubMed] [Google Scholar]
- 45.Bonthius D. J., West J. R. (1991) Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology 44, 147–163. [DOI] [PubMed] [Google Scholar]
- 46.Topper L. A., Baculis B. C., Valenzuela C. F. (2015) Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J. Neuroinflammation 12, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drew P. D., Johnson J. W., Douglas J. C., Phelan K. D., Kane C. J. (2015) Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 39, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boschen K. E., Ruggiero M. J., Klintsova A. Y. (2016) Neonatal binge alcohol exposure increases microglial activation in the developing rat hippocampus. Neuroscience 324, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiwari V., Chopra K. (2011) Resveratrol prevents alcohol-induced cognitive deficits and brain damage by blocking inflammatory signaling and cell death cascade in neonatal rat brain. J. Neurochem. 117, 678–690. [DOI] [PubMed] [Google Scholar]
- 50.Green H. F., Nolan Y. M. (2014) Inflammation and the developing brain: consequences for hippocampal neurogenesis and behavior. Neurosci. Biobehav. Rev. 40, 20–34. [DOI] [PubMed] [Google Scholar]
- 51.Schwarz J. M., Bilbo S. D. (2012) The immune system and the developing brain. In Colloquium Series on the Developing Brain (McCarthy M. M., ed.), Morgan & Claypool Life Sciences, San Rafael, CA, 1–118. [Google Scholar]
- 52.Drew P. D., Kane C. J. (2014) Fetal alcohol spectrum disorders and neuroimmune changes. Int. Rev. Neurobiol. 118, 41–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kane C. J., Phelan K. D., Drew P. D. (2012) Neuroimmune mechanisms in fetal alcohol spectrum disorder. Dev. Neurobiol. 72, 1302–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClain J. A., Morris S. A., Deeny M. A., Marshall S. A., Hayes D. M., Kiser Z. M., Nixon K. (2011) Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav. Immun. 25 (Suppl 1), S120–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kane C. J. M., Phelan K. D., Douglas J. C., Wagoner G., Johnson J. W., Xu J., Phelan P. S., Drew P. D. (2014) Effects of ethanol on immune response in the brain: region-specific changes in adolescent versus adult mice. Alcohol. Clin. Exp. Res. 38, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agrawal R. G., Owen J. A., Levin P. S., Hewetson A., Berman A. E., Franklin S. R., Hogue R. J., Chen Y., Walz C., Colvard B. D., Nguyen J., Velasquez O., Al-Hasan Y., Blednov Y. A., Fowler A. K., Syapin P. J., Bergeson S. E. (2014) Bioinformatics analyses reveal age-specific neuroimmune modulation as a target for treatment of high ethanol drinking. Alcohol. Clin. Exp. Res. 38, 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pascual M., Pla A., Miñarro J., Guerri C. (2014) Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol Alcohol. 49, 187–192. [DOI] [PubMed] [Google Scholar]
- 58.Montesinos J., Pascual M., Rodríguez-Arias M., Miñarro J., Guerri C. (2016) Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav. Immun. 53, 159–171. [DOI] [PubMed] [Google Scholar]
- 59.Vetreno R. P., Crews F. T. (2015) Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front. Neurosci. 9, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang G. Q., Karatayev O., Leibowitz S. F. (2015) Prenatal exposure to ethanol stimulates hypothalamic CCR2 chemokine receptor system: Possible relation to increased density of orexigenic peptide neurons and ethanol drinking in adolescent offspring. Neuroscience 310, 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J., Lewohl J. M., Harris R. A., Iyer V. R., Dodd P. R., Randall P. K., Mayfield R. D. (2006) Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology 31, 1574–1582. [DOI] [PubMed] [Google Scholar]
- 62.He J., Crews F. T. (2008) Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 210, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin L., He J., Hanes R. N., Pluzarev O., Hong J. S., Crews F. T. (2008) Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crews F. T., Qin L., Sheedy D., Vetreno R. P., Zou J. (2013) High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol. Psychiatry 73, 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kane C. J., Phelan K. D., Douglas J. C., Wagoner G., Johnson J. W., Xu J., Drew P. D. (2013) Effects of ethanol on immune response in the brain: region-specific changes in aged mice. J. Neuroinflammation 10, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doremus-Fitzwater T. L., Gano A., Paniccia J. E., Deak T. (2015) Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiol. Behav. 148, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall S. A., McClain J. A., Kelso M. L., Hopkins D. M., Pauly J. R., Nixon K. (2013) Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: the importance of microglia phenotype. Neurobiol. Dis. 54, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson T. E., Hao C., Manos J., Ransohoff R. M., Gruol D. L. (2011) Altered hippocampal synaptic transmission in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Brain Behav. Immun. 25 (Suppl 1), S106–S119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vetreno R. P., Qin L., Crews F. T. (2013) Increased receptor for advanced glycation end product expression in the human alcoholic prefrontal cortex is linked to adolescent drinking. Neurobiol. Dis. 59, 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blanco A. M., Vallés S. L., Pascual M., Guerri C. (2005) Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J. Immunol. 175, 6893–6899. [DOI] [PubMed] [Google Scholar]
- 71.Blanco A. M., Perez-Arago A., Fernandez-Lizarbe S., Guerri C. (2008) Ethanol mimics ligand-mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J. Neurochem. 106, 625–639. [DOI] [PubMed] [Google Scholar]
- 72.Fernandez-Lizarbe S., Pascual M., Guerri C. (2009) Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 183, 4733–4744. [DOI] [PubMed] [Google Scholar]
- 73.Alfonso-Loeches S., Pascual-Lucas M., Blanco A. M., Sanchez-Vera I., Guerri C. (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 30, 8285–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pascual M., Baliño P., Alfonso-Loeches S., Aragón C. M., Guerri C. (2011) Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav. Immun. 25 (Suppl 1), S80–S91. [DOI] [PubMed] [Google Scholar]
- 75.Blednov Y. A., Bergeson S. E., Walker D., Ferreira V. M., Kuziel W. A., Harris R. A. (2005) Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav. Brain Res. 165, 110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blednov Y. A., Ponomarev I., Geil C., Bergeson S., Koob G. F., Harris R. A. (2012) Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict. Biol. 17, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y., Lousberg E. L., Moldenhauer L. M., Hayball J. D., Coller J. K., Rice K. C., Watkins L. R., Somogyi A. A., Hutchinson M. R. (2012) Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br. J. Pharmacol. 165, 1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J., Yang A. R., Kelly T., Puche A., Esoga C., June H. L. Jr.,Elnabawi A., Merchenthaler I., Sieghart W., June H. L. Sr.,Aurelian L. (2011) Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc. Natl. Acad. Sci. USA 108, 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Płóciennikowska A., Hromada-Judycka A., Borzęcka K., Kwiatkowska K. (2015) Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 72, 557–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qin L., Crews F. T. (2012) Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J. Neuroinflammation 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lippai D., Bala S., Petrasek J., Csak T., Levin I., Kurt-Jones E. A., Szabo G. (2013) Alcohol-induced IL-1β in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J. Leukoc. Biol. 94, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cardona A. E., Pioro E. P., Sasse M. E., Kostenko V., Cardona S. M., Dijkstra I. M., Huang D., Kidd G., Dombrowski S., Dutta R., Lee J. C., Cook D. N., Jung S., Lira S. A., Littman D. R., Ransohoff R. M. (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–924. [DOI] [PubMed] [Google Scholar]
- 83.Limatola C., Ransohoff R. M. (2014) Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front. Cell. Neurosci. 8, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mizuno T., Kawanokuchi J., Numata K., Suzumura A. (2003) Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 979, 65–70. [DOI] [PubMed] [Google Scholar]
- 85.Zujovic V., Benavides J., Vigé X., Carter C., Taupin V. (2000) Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia 29, 305–315. [PubMed] [Google Scholar]
- 86.Sever R., Glass C. K. (2013) Signaling by nuclear receptors. Cold Spring Harb. Perspect. Biol. 5, a016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saijo K., Crotti A., Glass C. K. (2013) Regulation of microglia activation and deactivation by nuclear receptors. Glia 61, 104–111. [DOI] [PubMed] [Google Scholar]
- 88.Glass C. K., Saijo K. (2010) Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 10, 365–376. [DOI] [PubMed] [Google Scholar]
- 89.Blednov Y. A., Black M., Benavidez J. M., Stamatakis E. E., Harris R. A. (2016) PPAR agonists: I. role of receptor subunits in alcohol consumption in male and female mice. Alcohol. Clin. Exp. Res. 40, 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stopponi S., Somaini L., Cippitelli A., Cannella N., Braconi S., Kallupi M., Ruggeri B., Heilig M., Demopulos G., Gaitanaris G., Massi M., Ciccocioppo R. (2011) Activation of nuclear PPARγ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol. Psychiatry 69, 642–649. [DOI] [PubMed] [Google Scholar]
- 91.Blednov Y. A., Benavidez J. M., Black M., Ferguson L. B., Schoenhard G. L., Goate A. M., Edenberg H. J., Wetherill L., Hesselbrock V., Foroud T., Harris R. A. (2015) Peroxisome proliferator-activated receptors α and γ are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol. Clin. Exp. Res. 39, 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Storer P. D., Xu J., Chavis J., Drew P. D. (2005) Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J. Neuroimmunol. 161, 113–122. [DOI] [PubMed] [Google Scholar]
- 93.Diab A., Deng C., Smith J. D., Hussain R. Z., Phanavanh B., Lovett-Racke A. E., Drew P. D., Racke M. K. (2002) Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 168, 2508–2515. [DOI] [PubMed] [Google Scholar]
- 94.Heneka M. T., Landreth G. E., Feinstein D. L. (2001) Role for peroxisome proliferator-activated receptor-gamma in Alzheimer’s disease. Ann. Neurol. 49, 276. [DOI] [PubMed] [Google Scholar]
- 95.Kiaei M., Kipiani K., Chen J., Calingasan N. Y., Beal M. F. (2005) Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 191, 331–336. [DOI] [PubMed] [Google Scholar]
- 96.McTigue D. M., Tripathi R., Wei P., Lash A. T. (2007) The PPAR gamma agonist pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp. Neurol. 205, 396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tureyen K., Kapadia R., Bowen K. K., Satriotomo I., Liang J., Feinstein D. L., Vemuganti R. (2007) Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J. Neurochem. 101, 41–56. [DOI] [PubMed] [Google Scholar]
- 98.Calder P. C. (2015) Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 1851, 469–484. [DOI] [PubMed] [Google Scholar]
- 99.Patten A. R., Sickmann H. M., Dyer R. A., Innis S. M., Christie B. R. (2013) Omega-3 fatty acids can reverse the long-term deficits in hippocampal synaptic plasticity caused by prenatal ethanol exposure. Neurosci. Lett. 551, 7–11. [DOI] [PubMed] [Google Scholar]
- 100.Helland I. B., Smith L., Saarem K., Saugstad O. D., Drevon C. A. (2003) Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 111, e39–e44. [DOI] [PubMed] [Google Scholar]
- 101.Willatts P., Forsyth J. S., DiModugno M. K., Varma S., Colvin M. (1998) Effect of long-chain polyunsaturated fatty acids in infant formula on problem solving at 10 months of age. Lancet 352, 688–691. [DOI] [PubMed] [Google Scholar]
- 102.Burdge G. C., Postle A. D. (1995) Effect of maternal ethanol consumption during pregnancy on the phospholipid molecular species composition of fetal guinea-pig brain, liver and plasma. Biochim. Biophys. Acta 1256, 346–352. [DOI] [PubMed] [Google Scholar]
- 103.Wen Z., Kim H. Y. (2004) Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J. Neurochem. 89, 1368–1377. [DOI] [PubMed] [Google Scholar]
- 104.Tajuddin N., Moon K. H., Marshall S. A., Nixon K., Neafsey E. J., Kim H. Y., Collins M. A. (2014) Neuroinflammation and neurodegeneration in adult rat brain from binge ethanol exposure: abrogation by docosahexaenoic acid. PLoS One 9, e101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rashid M. A., Kim H. Y. (2016) N-Docosahexaenoylethanolamine ameliorates ethanol-induced impairment of neural stem cell neurogenic differentiation. Neuropharmacology 102, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wellmann K. A., George F., Brnouti F., Mooney S. M. (2015) Docosahexaenoic acid partially ameliorates deficits in social behavior and ultrasonic vocalizations caused by prenatal ethanol exposure. Behav. Brain Res. 286, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blednov Y. A., Benavidez J. M., Geil C., Perra S., Morikawa H., Harris R. A. (2011) Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav. Immun. 25 (Suppl 1), S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang X., Zhang Y., Peng Y., Hutchinson M. R., Rice K. C., Yin H., Watkins L. R. (2016) Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of Toll-like receptor 4. Br. J. Pharmacol. 173, 856–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stopponi S., de Guglielmo G., Somaini L., Cippitelli A., Cannella N., Kallupi M., Ubaldi M., Heilig M., Demopulos G., Gaitanaris G., Ciccocioppo R. (2013) Activation of PPARγ by pioglitazone potentiates the effects of naltrexone on alcohol drinking and relapse in msP rats. Alcohol. Clin. Exp. Res. 37, 1351–1360. [DOI] [PubMed] [Google Scholar]
- 110.Ferguson L. B., Most D., Blednov Y. A., Harris R. A. (2014) PPAR agonists regulate brain gene expression: relationship to their effects on ethanol consumption. Neuropharmacology 86, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barson J. R., Karatayev O., Chang G. Q., Johnson D. F., Bocarsly M. E., Hoebel B. G., Leibowitz S. F. (2009) Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol 43, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bilbao A., Serrano A., Cippitelli A., Pavon F. J., Giuffrida A., Suarez J., Garcia-Marchena N., Baixeras E., Gomez de Heras R., Orio L., Alen F., Ciccocioppo R., Cravatt B. F., Parsons L. H., Piomelli D., Rodriguez de Fonseca F. (2016) Role of the satiety factor oleoylethanolamide in alcoholism. Addict. Biol. 21, 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kleberg K., Hassing H. A., Hansen H. S. (2014) Classical endocannabinoid-like compounds and their regulation by nutrients. Biofactors 40, 363–372. [DOI] [PubMed] [Google Scholar]
- 114.Barbiero J. K., Santiago R., Tonin F. S., Boschen S., da Silva L. M., Werner M. F., da Cunha C., Lima M. M., Vital M. A. (2014) PPAR-α agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 53, 35–44. [DOI] [PubMed] [Google Scholar]
- 115.Lovett-Racke A. E., Hussain R. Z., Northrop S., Choy J., Rocchini A., Matthes L., Chavis J. A., Diab A., Drew P. D., Racke M. K. (2004) Peroxisome proliferator-activated receptor alpha agonists as therapy for autoimmune disease. J. Immunol. 172, 5790–5798. [DOI] [PubMed] [Google Scholar]
- 116.Skerrett R., Pellegrino M. P., Casali B. T., Taraboanta L., Landreth G. E. (2015) Combined liver X receptor/peroxisome proliferator-activated receptor γ agonist treatment reduces amyloid β levels and improves behavior in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 290, 21591–21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu J., Wagoner G., Douglas J. C., Drew P. D. (2009) Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J. Leukoc. Biol. 86, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang-Gandhi C. X., Drew P. D. (2007) Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J. Neuroimmunol. 183, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]