Abstract
Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus-68 (MHV-68) establish latent infections and are associated with various types of malignancies. They are members of the gamma-2 herpesvirus subfamily and encode a replication and transcriptional activator, RTA, which is necessary and sufficient to disrupt latency and initiate the viral lytic cycle in vitro. We have constructed a recombinant MHV-68 virus that overexpresses RTA. This virus has faster replication kinetics in vitro and in vivo, is deficient in establishing latency, exhibits a reduction in the development of a mononucleosis-like disease in mice, and can protect mice against challenge by wild-type MHV-68. The present study, by using MHV-68 as an in vivo model system, demonstrated that RTA plays a critical role in the control of viral latency and suggests that latency is a determinant of viral pathogenesis in vivo.
Kaposi's sarcoma-associated herpesvirus (KSHV, or HHV-8)and murine gammaherpesvirus-68 (MHV-68) are members of the gamma-2 subfamily of herpesviruses (rhadinoviruses), which have the ability to establish latent infections and are associated with various types of malignancies, such as Kaposi's sarcoma and B-cell lymphomas (2, 4, 5, 22). Due to the difficulty in culturing KSHV in vitro and the lack of an in vivo system to directly study KSHV, MHV-68 has been used as an in vitro and in vivo model for gammaherpesvirus infection (15, 16). Mice infected with MHV-68 develop a latent infection in B cells, macrophages, and dendritic cells (5, 22, 27). At the peak of latent infection, mice develop a mononucleosis-like disease known as splenomegaly due to the increase in spleen size and cell number (24). A small percentage of mice also develop B-cell lymphomas (20). Thus, MHV-68 can be used to study latency and the pathogenesis of gammaherpesviruses in vivo.
A viral replication and transcription activator (RTA) is conserved among the gammaherpesviruses (7, 19, 30, 31). Both KSHV RTA and MHV-68 RTA are known to be sufficient and necessary to reactivate their respective viruses from latently infected cells (6, 12, 19, 29, 30). RTA is also necessary for MHV-68 de novo infection in vitro (14, 29). Thus, RTA functions as a key regulator of the gamma-2 herpesvirus subfamily life cycle in vitro. However, the question of whether RTA controls viral latency in vivo has not been addressed. To address this, we have constructed a recombinant MHV-68 virus that constitutively overexpresses RTA (C-RTA/MHV-68). We have characterized the in vitro and in vivo replication kinetics of the virus and determined its ability to establish latency and to induce latency-associated pathogenesis in vivo. We have also tested its ability to protect mice from subsequent infection by wild-type (WT) MHV-68.
(Preliminary data were presented at the 2002 International Workshop on Kaposi Sarcoma-Associated Herpesvirus and Related Agents, the 2003 International Herpesvirus Workshop, and the 2003 International Workshop on Kaposi Sarcoma-Associated Herpesvirus and Related Agents.)
MATERIALS AND METHODS
Viruses, cells, and plaque assays.
MHV-68 virus was originally obtained from the American Type Culture Collection (VR1465). C-RTA/MHV-68 was constructed by traditional homologous recombination by using tw25 (GFP/MHV-68) as the parental virus (29). The RTA gene contained only 150 bp of the open reading frame 49 (ORF49) region with a stop codon inserted in the center. This insert was generated by PCR by using the pCMVFLAG/Rta construct as the template (29) and the following sets of primers (stop codon in boldface): pFLAG/FLAG (5′-TCTCATGCATTTGATCTACCATGGACTACA-3′) and TMR6(−49)R (5′-GAACATTGATTGATGAAAT ACTGATCTGTC-3′); TMR6(−49)F (5′-TTTCATCAATCAATGTTCCCTAGTATC TATGAC-3′) and pFLAG/polyA (5′-TCTCGGTACCGATATCGTACCCAATTCAACAG-3′). These products were the template in a third PCR with the primers R3TR/NotI (5′-TCTCGGTACCGCGGCCGCGACAGCGATGGCCTCTGAC-3′) and R4 (30) to generate the insert that was cloned into the Not1 and XbaI sites of pFLAG-CMV2 to generate pFLAG/MRTA(−49). The cytomegalovirus (CMV) promoter, RTA gene cassette, and poly(A) signal from pFLAG/MRTA(−49) were cloned into tw76 for homologous recombination. Virus infection, viral growth, and plaque assays were performed as previously described (30).
Northern and Southern blot analysis.
RNA and DNA extraction, blotting, and probe synthesis were performed (30). For the Northern blot the probe was made from DNA fragments generated by PCR of viral DNA or cellular DNA. For the Southern blot the DNA was digested overnight with SmaI, and the probe DNA was generated by PCR from viral DNA and the following pair of primers: tRNA1 (5′-CCGACCATTCGATGCAAATGTT-3′) and tRNA2 (5′-CTACACATGAAAATCCTGTGAG-3′). Hybridization, washes, and detection of radioactivity were done as previously described (30). Quantification of the RNA was done by using ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.).
Growth curves.
BHK-21 cells (baby hamster kidney cells) were seeded at 2 × 105 cells per well for the single-step (multiplicity of infection, 5) and 1 × 105 cells per well for the multiple-step growth curve (multiplicity of infection, 0.05). The cells and supernatant were harvested, frozen and thawed three times, and subjected to plaque assays in triplicate.
Transient transfections.
293 T cells were seeded in a 24-well plate (105 cells per well) and a total of 600 ng of DNA, including 5 ng of a Renilla luciferase construct as a control, was transfected per well by the calcium phosphate method. The cells were transfected with 10 ng of 57pLuc (11) and 4 ng of RTA or 10 ng of M3Luc (13) and 5 ng RTA. A plasmid expressing the p65 subunit of NF-κb (1) was transfected into the indicated samples. Cell extracts were harvested 24 h after transfection and analyzed as previously described (1).
Real-time PCR.
The DNeasy Tissue Kit (QIAGEN, Valencia, Calif.) was used according to manufacturer's instructions to extract genomic DNA from splenocytes. The following primers and probe for the MHV-68 M9 gene were used: M9-160F primer (5′-GTCAGGGCCCAGTCCGTA-3′), M9-226R primer (5′-TGGCCCTCT ACCTTCTGTTGA-3′), and M9-179T probe 5′-[6-FAM]CACAGGCCTCCCTCCCTTTGAGGAA[Tamra∼Q]-3′ (QIAGEN). Reaction volumes were 20 μl, with 900 nM concentrations of each primer, 250 nM of the probe, and Taqman Universal PCR Master Mix (Applied Biosystems, Calif.). Amplification and detection were performed by using a Bio-Rad iCycler. No template controls were included for each reaction, and 300 ng of DNA was analyzed in duplicate for each sample. A standard curve was obtained by measuring 1 to 106 copies of a bacterial artificial chromosome containing the MHV-68 genome on a background of 300 ng of uninfected splenocyte DNA. The detection limit was 10 copies/300 ng of genomic DNA.
Mice, lung titer, and reactivation assays.
Female BALB/c mice (Charles River Laboratories, Wilmington, Mass.), 3 to 4 weeks of age, were infected intranasally with 500 PFU of each virus or mock infected with Dulbecco's modified Eagle's medium in a total volume of 20 μl. We determined that 500 PFU is a substantial amount of virus since we have successfully infected mice with 1 PFU of MHV-68, confirming previous observations (23). The viral titer in the lung was determined by homogenizing lungs in 1 ml of Dulbecco's modified Eagle's medium and performing three independent plaque assays. For the ex vivo limiting dilution assay, 103 BHK-21 cells were seeded per well in a 96-well plate. Splenocytes were harvested (21), and serial twofold dilutions of the splenocytes were plated, starting with 106 splenocytes, with 24 wells per dilution. After 7 days, each well was assessed for cytopathic effects (CPE), and the percentage of CPE per 24 wells was determined per dilution. The infectious center assay, including the assessment of preformed virus, was performed as previously described (21). A majority of the samples in the assay for preformed virus resulted in 0 infectious centers, with a minority of samples displaying 1 to 2 infectious centers per ∼107 splenocytes.
Statistical analysis.
Limiting dilution analyses were analyzed by multiple regression of the percentage of wells showing CPE on log10 cell counts/well, with experimental effects defined by the interaction of cell number and experimental group. All other experiments were analyzed by using a two-sample unequal variance t test.
RESULTS
RTA overexpression resulted in faster MHV-68 replication kinetics in vitro.
The genomic structure of the RTA gene (ORF50) contains an intron which includes ORF49 (30). We constructed the RTA expression cassette by deleting the majority of the intron to preserve RTA expression but prevent any expression of ORF49 (Fig. 1A). In transcriptional activation and reactivation assays, RTA expressed from this cassette was as active as RTA expressed from a full-length genomic clone and more active than that from the cDNA clone (data not shown). This RTA expression cassette, the CMV promoter, and polyadenylation signal, were cloned into tw76. The tw76 plasmid contains a sequence from the left end of the MHV-68 genome and was used for homologous recombination.
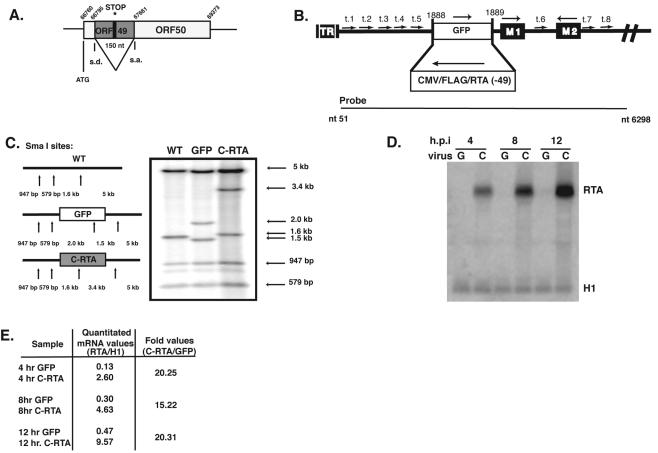
FIG. 1.
Construction and confirmation of the C-RTA/MHV-68 virus. (A) The diagram represents the construction of MRTA(−49). The asterisk notes where the stop codon was inserted into ORF49, and the splice donor (s.d.) and splice acceptor (s.a.) sites that are used to form the mRNA of RTA (ORF50) are shown. (B) The diagram represents the structure of the left end of the unique region of the MHV-68 viral genome. The arrows represent eight tRNA-like sequences (t.1 to t.8). The solid black boxes represent two ORFs in this region, M1 and M2, with orientations indicated above. The striated box represents the terminal repeat (TR) region. The open boxes represent the expression cassettes of either enhanced GFP driven by the human CMV promoter (GFP/MHV-68) or FLAG/RTA driven by the human CMV promoter (C-RTA/MHV-68), with orientations indicated above. The nucleotide region (nucleotides 51 to 6298) of MHV-68 used to probe the Southern blot (shown in C) is indicated under the diagram. (C) Confirmation of C-RTA/MHV-68 by Southern blotting. SmaI sites on the left end of the viral genome are indicated by arrows. (D) Northern blotting by using a probe against full-length endogenous RTA to determine overexpression of RTA. A probe against the H1 cellular mRNA was used to ensure equal loading of RNA. (E) Quantitation of mRNA levels was measured from the Northern blot in panel D by using ImageQuant. The values obtained were normalized to the H1 band for each sample.
The tw76/RTA plasmid was linearized and cotransfected into BHK-21 cells with viral DNA from GFP/MHV-68 (Fig. 1B). GFP/MHV-68 virus contains a green fluorescent protein (GFP) expression cassette in the left end of the viral genome (29). Viral progeny were screened for loss of GFP expression. After three rounds of plaque purification, the correct insertion of the RTA expression cassette was confirmed by Southern blotting with a probe that hybridizes to the left end of the viral genome (Fig. 1C). There did not appear to be any other mutations in that region. We next confirmed by Northern blotting that the RTA transcript was being overexpressed by C-RTA/MHV-68 (Fig. 1D). Both recombinant viruses retain the endogenous RTA gene, which is tightly regulated and expressed at low levels during lytic replication (13). RTA transcript was detected at 4 h postinfection and, at each time point, the level of RTA transcript was 15- to 20-fold higher in C-RTA/MHV-68-infected cells than in GFP/MHV-68-infected cells (Fig. 1E).
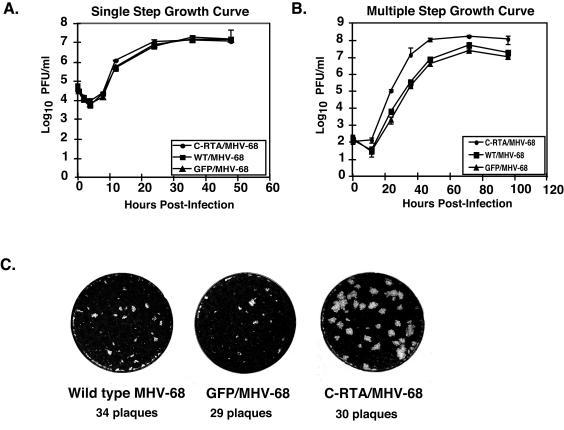
We next measured the ability of C-RTA/MHV-68 to replicate in vitro in comparison to WT MHV-68 (WT/MHV-68) virus and GFP/MHV-68. C-RTA/MHV-68 replicated to similar levels as WT/MHV-68 and GFP/MHV-68 at each time point postinfection in the single-step growth curve (Fig. 2A). In contrast, in the multiple-step growth curve C-RTA/MHV-68 exhibited faster kinetics and reached a 40- to 66-fold higher titer than WT/MHV-68 or GFP/MHV-68 at 36 h postinfection (Fig. 2B). We also observed that the plaques formed by C-RTA/MHV-68 were significantly larger than those formed by WT/MHV-68 or GFP/MHV-68 (Fig. 2C). Viral gene arrays were used to examine the gene expression pattern of C-RTA/MHV-68 in comparison to that of GFP/MHV-68 virus. At all of the time points examined, the majority of the 85 viral genes represented on the array were significantly upregulated by C-RTA/MHV-68 in comparison to GFP/MHV-68 (13). Thus, the overexpression of RTA by C-RTA/MHV-68 increases viral gene expression and accelerates the lytic cycle of the virus, resulting in faster replication kinetics and larger plaque size.
FIG. 2.
In vitro kinetics of C-RTA/MHV-68. (A) Single-step growth curve of C-RTA/MHV-68, WT/MHV-68, and GFP/MHV-68. (B) Multiple-step growth curve of C-RTA/MHV-68, WT/MHV-68, and GFP/MHV-68. (C) Plaque morphology of the three viruses. Each well pictured represents one well of a plaque assay performed on BHK-21 cells with similar titers of each virus.
RTA overexpression results in faster replication kinetics and latency-deficiency in vivo.
Intranasal infection of MHV-68 in mice results in an acute infection in lung epithelial cells, which is cleared by the immune system by 13 days postinfection (28). At 14 days postinfection, the virus has established a significant latent infection in splenic B cells, macrophages, dendritic cells, and lung epithelium (5, 18, 22). A significant increase in the spleen weight (splenomegaly) is observed and is caused by an amplification of lymphocytes in the spleen and is similar to mononucleosis (24). Based on these observations, we performed an in vivo characterization of C-RTA/MHV-68 virus. BALB/c mice were infected intranasally with 500 PFU of each virus, and viral titers were determined from homogenized lungs at various times postinfection. Three days postinfection the C-RTA/MHV-68 titer was 18-fold higher than that of WT/MHV-68 (Fig. 3A). The C-RTA/MHV-68 viral titer also decreased more rapidly than that of WT/MHV-68, indicating a more rapid clearance of C-RTA/MHV-68. Therefore, although both viruses reached a similar titer, C-RTA/MHV-68 virus has faster overall kinetics than WT/MHV-68 virus during acute infection in vivo.
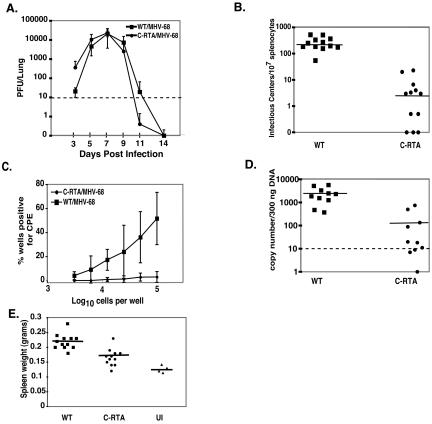
FIG. 3.
In vivo characterization of C-RTA/MHV-68. (A) In vivo replication kinetics of C-RTA/MHV-68 and WT/MHV-68. The data are compiled from six mice per time point. Dotted line, detection limit of the assay. (B) Infectious center assay comparing C-RTA/MHV-68-infected mice and WT/MHV-68-infected mice. Solid line, average values of the mice in each group. (C) Ex vivo limiting dilution reactivation assay comparing C-RTA/MHV-68 infected mice and WT/MHV-68 infected mice. (D) Real-time PCR quantitation of viral genomes in C-RTA/MHV-68-infected mice and WT/MHV-68-infected mice. Dotted line, detection limit of the assay; solid line, average values of the mice in each group. (E) Quantitation of spleen size in C-RTA/MHV-68-infected mice, WT/MHV-68-infected mice, and uninfected (UI) mice. Solid line, average values of the mice in each group. The statistical difference between the values for C-RTA/MHV-68-infected mice and WT/MHV-68 infected mice in the assays in panels B to E was P < 0.0001. All of the data in panels B to E were compiled from two independent experiments consisting of a total of 10 to 12 mice per virus.
To assess the level of latency in C-RTA/MHV-68-infected mice, splenocytes were harvested at 20 days postinfection. We assayed latency by using an infectious center assay which measures the amount of latent virus that is able to reactivate per population of B cells (22). On average, the mice infected with C-RTA/MHV-68 had ∼68-fold fewer infectious centers than those infected with WT/MHV-68 (Fig. 3B). Three of the C-RTA/MHV-68-infected mice had no evidence of infectious centers, indicating that there was no detectable latent virus. To confirm this data, we performed an ex vivo limiting dilution reactivation assay on each sample (Fig. 3C). In general, the results supported the infectious center assay. Four of the 12 C-RTA/MHV-68-infected mice did not show any evidence of reactivating virus in this assay. It has been reported recently that the left end of MHV-68 is genetically unstable, which could result in the loss of the C-RTA gene cassette (3). We therefore recovered the reactivated virus from the other eight C-RTA/MHV-68-infected mice and assayed for the C-RTA overexpression cassette by Southern blotting. We determined that three of the C-RTA/MHV-68-infected mice were latently infected with C-RTA/MHV-68 that retained the cassette, four were latently infected with a mixture of C-RTA/MHV-68 and a recombinant virus with the C-RTA cassette deleted, and one was latently infected with only a recombinant virus that had deleted the C-RTA cassette. This result indicates that there is a strong selection pressure to delete the RTA overexpression cassette so that the virus can establish latency and further supports the role of RTA in regulating the balance between the viral life cycles.
To determine if the significant lack of reactivation from C-RTA/MHV-68-infected splenocytes is due to a lack of latent virus infection or an inability of the latent virus itself to reactivate, we quantitated the amount of viral genomes per splenocyte sample by real-time PCR (Fig. 3D). The limit of detection of the real-time PCR assay was 10 copies per 300 ng of splenocyte DNA (∼105 splenocytes). Overall, the mice infected with C-RTA/MHV-68 had ∼20-fold fewer latent viral genomes than those infected with WT/MHV-68. The C-RTA/MHV-68-infected mice that exhibited the highest number of genome copies were the individuals that were either latently infected with a recombinant virus that deleted the C-RTA overexpression cassette or had a mixed population of full-length and deleted recombinant viruses. By real-time PCR, the mice that harbored only C-RTA/MHV-68 had an undetectable amount of viral genome. This suggests that the higher genome copy numbers in the mice with mixed virus can be attributed to the recombinants that have deleted the C-RTA overexpression cassette. We also performed real-time PCR on splenocyte DNA from earlier time points postinfection. The amount of latent viral genome in C-RTA-infected mice was undetectable or consistently lower than that in WT/MHV-68-infected mice (data not shown). This confirmed that C-RTA/MHV-68 is deficient in establishing latency and is not establishing latency at an earlier time point. Thus, overexpression of RTA significantly reduced the capacity of MHV-68 to establish latency.
Since splenomegaly is a prominent pathological event associated with MHV-68 infection, we examined whether the infected mice had developed this condition. In comparison to WT/MHV-68-infected mice, there was a significant reduction in splenomegaly in the C-RTA/MHV-68-infected mice (Fig. 3E). As the establishment of latency is linked to splenomegaly, these results correlate with those from the reactivation assays. Thus, C-RTA/MHV-68 virus is significantly less pathogenic than WT/MHV-68 virus, and the establishment of latency is necessary for the development of splenomegaly.
A latency-deficient MHV-68 protects mice against challenge by WT/MHV-68.
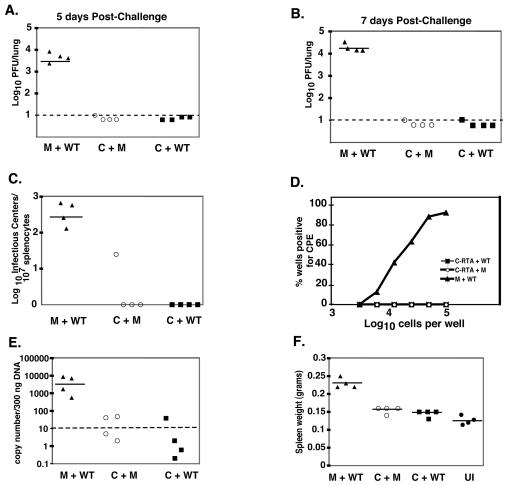
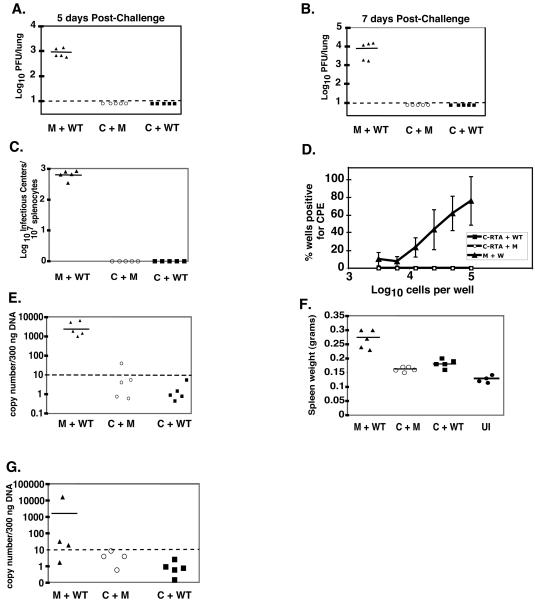
Gammaherpesvirus latency is associated with the development of various malignancies. Therefore, the goal of a gammaherpesvirus vaccine is to fully protect against exposure without generating a latent infection. Previous attempts to protect against MHV-68 infection by using vaccines against individual lytic or latent viral epitopes reduce the amount of early latency but have not been successful at preventing the establishment of latency (10, 17, 25, 28). However, a virus that is protective without establishing latency itself may be a promising vaccine candidate. Hence, we tested whether C-RTA/MHV-68 can protect against subsequent infection by WT/MHV-68. Mice were either infected with 500 PFU of C-RTA/MHV-68 or mock infected. At 30 days postinfection the mice were either challenged intranasally with 500 PFU of WT/MHV-68 or mock challenged, and viral titers in the lung were measured at 5 and 7 days postchallenge (Fig. 4A and B). Seven days postinfection is the peak time for acute viral replication in the lung when mice are infected with 500 PFU of WT/MHV-68 (Fig. 3A). An earlier time point (day 5) was taken since the immune system may be able to clear the virus more quickly in a challenge infection. At both time points, mice that were previously infected with C-RTA/MHV-68 had viral titers that were at or below the detection of the assay (Fig. 4A and B). We also performed infectious center assays on splenocytes 20 days postchallenge and found that C-RTA/MHV-68-infected mice that were challenged with WT/MHV-68 had no evidence of infectious centers (Fig. 4C). In contrast, mock-infected mice that had been challenged had an average of 409 infectious centers per 107 splenocytes. We also performed an ex vivo limiting dilution assay. C-RTA/MHV-68-infected mice that had been challenged with WT/MHV-68 had no evidence of reactivating virus. In contrast, mock-infected mice that had been challenged had a reactivation frequency of about 1 in 2 × 105 cells (Fig. 4D). The splenocytes were then analyzed by real-time PCR (Fig. 4E). C-RTA/MHV-68-infected mice that had been challenged had significantly fewer latent viral genome copies than mice that were mock infected and challenged. Three of the four C-RTA/MHV-68-infected mice that were challenged had viral genome copy numbers that were below detection, and all four of these mice had amounts of latent viral genome that were similar to the amount in infected mice that were mock challenged. Also, mice that were mock infected and challenged had significantly more splenomegaly than C-RTA/MHV-68-infected mice that were challenged (Fig. 4F). Thus, infection with C-RTA/MHV-68 was able to protect mice from acute viral infection, the establishment of latency by WT/MHV-68, and the development of latency-associated pathogenesis 30 days postvaccination.
FIG. 4.
Vaccination with C-RTA/MHV-68 prevents acute viral infection and establishment of latency. Viral titer in the lungs of C-RTA/MHV-68-infected mice or mock-infected mice at 5 (A) and 7 (B) days postchallenge. The statistical difference between values for mock-infected and C-RTA/MHV-68-infected mice that were challenged was P < 0.0001 for both time points. Dotted line, limit of detection of the assay. (C) Infectious center assay comparing C-RTA/MHV-68-infected mice and mock-infected mice 20 days postchallenge. The statistical difference between values for mock-infected and C-RTA/MHV-68-infected mice that were challenged was P = 0.0003. (D) Ex vivo limiting dilution assay performed on the splenocytes 20 days postchallenge. The statistical difference between mock-infected and C-RTA/MHV-68-infected mice that were challenged was P = 0.0003. (E) Real-time PCR quantitation of viral genomes in the splenocytes. Solid line, the average of the values of the mice in each group. The statistical difference between the values for the mock-infected and C-RTA/MHV-68-infected mice that were challenged was P = 0.0001. (F) Quantitation of spleen size in the mice 20 days postchallenge and in uninfected mice. The statistical difference between the values for the mock-infected and C-RTA/MHV-68-infected mice that were challenged was P < 0.0001. For panels A to F, there were four mice per group, and the solid lines indicate the averages of the values of the mice in each group. M+WT, mock-infected mice challenged with WT/MHV-68; C+M, C-RTA/MHV-68-infected mice and mock challenge; C+WT, C-RTA/MHV-68-infected mice challenged with WT/MHV-68; UI, uninfected mice.
We then determined the duration of the protection by challenging and mock challenging mice at 90 days postinfection with C-RTA/MHV-68. As before, at 5 and 7 days postchallenge, the C-RTA/MHV-68-infected mice were protected against acute viral infection (Fig. 5A and B). We did not detect any reactivating virus in the C-RTA/MHV-68-infected mice that were challenged when we performed an infectious center assay or limiting dilution assay (Fig. 5C and D). By real-time PCR, all of the samples from C-RTA/MHV-68-infected mice that were challenged were below detection, and these mice had significantly less splenomegaly than mock-infected mice that were challenged (Fig. 5E and F). We analyzed mice 2 months postchallenge, and the C-RTA/MHV-68-infected mice that were challenged had undetectable latent viral genome, whereas the mock-infected mice that were challenged had a significant level of latent viral genome (Fig. 5G). Therefore, C-RTA/MHV-68 can offer long-term protection against acute viral replication, the establishment of latency, and the development of splenomegaly by WT/MHV-68.
FIG. 5.
WT/MHV-68 challenge 90 days post-primary infection. Viral titer in lungs of C-RTA/MHV-68-infected mice or mock-infected mice at 5 (A) and 7 (B) days postchallenge. The statistical difference between the values for mock-infected and C-RTA/MHV-68-infected mice that were challenged was P < 0.0001 for both time points. Dotted line, the limit of detection of the assay. (C) Infectious center assay comparing C-RTA/MHV-68-infected mice and mock-infected mice 20 days postchallenge. The statistical difference between the values for mock-infected and C-RTA/MHV-68-infected mice that were challenged was P < 0.0001. (D) Ex vivo limiting dilution assay performed on the splenocytes 20 days postchallenge. The statistical difference between the values for mock-infected and C-RTA/MHV-68-infected mice that were challenged was P < 0.0001. (E) Real-time PCR quantitation of viral genomes in the splenocytes. The statistical difference between the values for the mock-infected and C-RTA/MHV-68-infected mice that were challenged was P < 0.0001. (F) Quantitation of spleen size in the mice 20 days postchallenge and in uninfected mice. The statistical difference between the values for mock-infected and C-RTA/MHV-68-infected mice that were challenged was P = 0.001. For panels A to F, there were five mice per group, and the solid lines indicate the averages of the values of the mice in each group. (G) Real-time PCR quantitation of viral genomes in splenocytes of mice 2 months postchallenge. The statistical difference between the values for the mock-infected and C-RTA/MHV-68-infected mice that were challenged was P = 0.05. M+WT, mock-infected mice challenged with WT/MHV-68; C+M, C-RTA/MHV-68-infected mice and mock challenge; C+WT, C-RTA/MHV-68-infected mice challenged with WT/MHV-68; UI, uninfected mice.
Overexpression of MHV-68 RTA overcomes repression by NF-κB on RTA-responsive promoters.
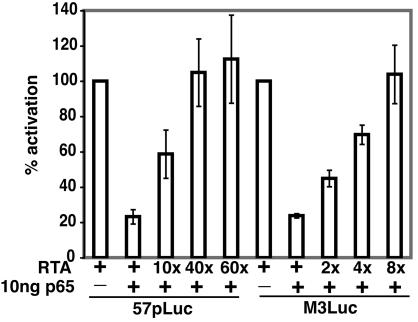
We next explored the mechanism behind the inability of C-RTA/MHV-68 to establish latency. It has been shown that the p65 subunit of NF-κB plays a role in controlling the expression and function of RTA in KSHV- and Epstein-Barr virus-infected B-cell lines in vitro (1). Inhibition of NF-κB leads to lytic gene expression in B cells. Conversely, overexpression of NF-κB in permissive cells inhibits MHV-68 replication and transcriptional activation by RTA. It has also been shown that overexpression of RTA can overcome the suppression of p65 on KSHV and Epstein-Barr virus RTA-responsive promoters in vitro (1). We determined if this is also true for MHV-68 RTA-responsive promoters. We transfected 293 T cells with either the MHV-68 ORF57 promoter (57pLuc) or the MHV-68 M3 promoter (M3Luc) and various amounts of plasmids expressing MHV-68 RTA or p65. Both promoters were activated by RTA and this activation is inhibited by p65 (Fig. 6). However, this repression can be overcome by overexpression of RTA (Fig. 6). Only 10-fold more RTA was needed to release over half of the repression by p65 on the ORF57 promoter and only 4-fold more RTA was needed to overcome a similar amount of repression on the M3 promoter. Thus, our model is that overexpression of RTA by C-RTA/MHV-68 allows the virus to overcome cellular repression factors and circumvent the establishment of latency.
FIG. 6.
Overexpression of RTA can overcome suppression of RTA promoters by NF-κB. 293T cells were transfected with promoter constructs 57pLuc or M3Luc. Various amounts of RTA and a plasmid expressing the p65 subunit of NF-κb were cotransfected with the promoter constructs into the indicated samples.
DISCUSSION
The ability to establish latency is a hallmark of herpesviruses. It has been shown in previous studies that RTA of KSHV and MHV-68 plays an important role in the switch from latency to lytic replication and is essential for viral lytic replication in vitro (6, 11, 12, 14, 19, 29, 30). This study provides the first evidence of RTA's ability to regulate the balance between latency and lytic replication in vivo. Here we have shown that overexpression of RTA in a recombinant MHV-68 is sufficient to enhance the kinetics of viral replication, to severely compromise the ability of the recombinant virus to establish latency, and to reduce pathogenicity associated with latency in vivo. We have also demonstrated that a virus that is deficient in the ability to establish latency is able to protect mice against subsequent challenge by WT/MHV-68.
We have shown that overexpression of RTA can severely affect the ability of MHV-68 to establish latency in the splenocytes of infected mice. This is the first time a herpesvirus that is constitutively lytic has been constructed. The failure to establish latency is not a result of the RTA expression cassette residing in the left end of the viral genome, as we have constructed several recombinant MHV-68 viruses with gene expression cassettes in the same location that are not impaired in their ability to establish latency (T.-T. Wu, L. Tong, E. Crabb-Breen, J. Gage, O. Martinez-Maza, and Ren Sun, unpublished data). Although there were some C-RTA/MHV-68 mice that exhibited a latent infection, we were able to determine that some of these viruses had undergone recombination and deleted the C-RTA overexpression cassette. These revertant MHV-68 viruses that occurred naturally in the mice further support the association between RTA expression and the failure to establish latency. In the small number of mice where the RTA cassette was retained, there is the possibility either that the CMV promoter of C-RTA/MHV-68 is transcriptionally inactive in some cells or that there are small mutations in the C-RTA gene cassette that are affecting RTA expression. We then determined if a latency-deficient MHV-68 virus was protective against WT/MHV-68 challenge. At 30 and 90 days postinfection, mice that were infected with C-RTA/MHV-68 and challenged with WT/MHV-68 were protected against acute viral replication in the lung, the establishment of any detectable latency in the spleen, and the development of latency-associated splenomegaly. Thus, C-RTA/MHV-68 was sufficient to elicit protection against exposure to WT/MHV-68.
It has been shown that a recombinant MHV-68 with a deletion in the v-cyclin gene is deficient in reactivation from latency and is effective as a vaccine against WT challenge (23). The advantage to our strategy is that the initial vaccinating virus strain is deficient in establishing latency, is cleared from the host, and is still protective. Thus, the host would not be subject to complications that can arise from the presence of a latent gammaherpesvirus infection, such as B-cell lymphomas. To address the issue of possible recombination mutants, we would make mutations in latency-associated genes to ensure that the virus will not be able to establish latency, even if the RTA cassette is deleted from the virus. Additional alterations to the MHV-68 viral genome, such as attenuation of viral replication, would be carried out to ensure the safety of the virus used for vaccination.
Since there is a significant amount of homology between gammaherpesviruses (26), this vaccine strategy may also be applied to vaccines that target human gammaherpesviruses such as KSHV. In Africa, Kaposi's sarcoma is an endemic disease and affects more individuals than anywhere else in the world (8, 9). An effective vaccine against KSHV would be extremely beneficial for lowering the prevalence of this disease.
We have provided evidence that RTA is a key regulator of MHV-68 viral life cycles in vivo. We have also demonstrated that a virus that is deficient in the establishment of latency can serve as a protective vaccine against MHV-68 infection. Due to its deficiency in establishing latency in vivo, the C-RTA/MHV-68 virus will also be an invaluable tool to study the role of latency in gammaherpesvirus-associated pathogenesis.
Acknowledgments
This work was supported by NIH grants CA83525, CA91791, and DE14153; the Stop Cancer Foundation (R.S.); the UCLA AIDS Institute; the USPHS National Research Service Award GM07185 (T.M.R.); and the Leukemia and Lymphoma Society Special Fellow Award (T.-T.W.).
REFERENCES
- 1.Brown, H. J., M. J. Song, H. Deng, T. T. Wu, G. Cheng, and R. Sun. 2003. NF-κB inhibits gammaherpesvirus lytic replication. J. Virol. 77:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 3.Clambey, E. T., H. W. Virgin IV, and S. H. Speck. 2002. Characterization of a spontaneous 9.5-kilobase-deletion mutant of murine gammaherpesvirus 68 reveals tissue-specific genetic requirements for latency. J. Virol. 76:6532-6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 6.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hengge, U. R., T. Ruzicka, S. K. Tyring, M. Stuschke, M. Roggendorf, R. A. Schwartz, and S. Seeber. 2002. Update on Kaposi's sarcoma and other HHV8 associated diseases. Part 2: pathogenesis, Castleman's disease, and pleural effusion lymphoma. Lancet Infect. Dis. 2:344-352. [DOI] [PubMed] [Google Scholar]
- 9.Kasolo, F. C., E. Mpabalwani, and U. A. Gompels. 1997. Infection with AIDS-related herpesviruses in human immunodeficiency virus-negative infants and endemic childhood Kaposi's sarcoma in Africa. J. Gen. Virol. 78:847-855. [DOI] [PubMed] [Google Scholar]
- 10.Liu, L., E. J. Usherwood, M. A. Blackman, and D. L. Woodland. 1999. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 73:9849-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, S., I. V. Pavlova, H. W. Virgin IV, and S. H. Speck. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J. Virol. 74:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Guzman, D., T. Rickabaugh, T. T. Wu, H. Brown, S. Cole, M. J. Song, L. Tong, and R. Sun. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 77:10488-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavlova, I. V., H. W. Virgin IV, and S. H. Speck. 2003. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J. Virol. 77:5731-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simas, J. P., and S. Efstathiou. 1998. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 6:276-282. [DOI] [PubMed] [Google Scholar]
- 16.Speck, S. H., and H. W. Virgin. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr. Opin. Microbiol. 2:403-409. [DOI] [PubMed] [Google Scholar]
- 17.Stewart, J. P., N. Micali, E. J. Usherwood, L. Bonina, and A. A. Nash. 1999. Murine gamma-herpesvirus 68 glycoprotein 150 protects against virus-induced mononucleosis: a model system for gamma-herpesvirus vaccination. Vaccine 17:152-157. [DOI] [PubMed] [Google Scholar]
- 18.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 21.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 22.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 23.Tibbetts, S. A., J. S. McClellan, S. Gangappa, S. H. Speck, and H. W. Virgin IV. 2003. Effective vaccination against long-term gammaherpesvirus latency. J. Virol. 77:2522-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usherwood, E. J., A. J. Ross, D. J. Allen, and A. A. Nash. 1996. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J. Gen. Virol. 77:627-630. [DOI] [PubMed] [Google Scholar]
- 25.Usherwood, E. J., K. A. Ward, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2001. Latent antigen vaccination in a model gammaherpesvirus infection. J. Virol. 75:8283-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodland, D. L., E. J. Usherwood, L. Liu, E. Flano, I. J. Kim, and M. A. Blackman. 2001. Vaccination against murine gamma-herpesvirus infection. Viral Immunol. 14:217-226. [DOI] [PubMed] [Google Scholar]
- 29.Wu, T.-T., L. Tong, T. Rickabaugh, S. Speck, and R. Sun. 2001. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J. Virol. 75:9262-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu, T.-T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]