Highlights
-
•
A LNA–DNA hybrid oligomer (miravirsen) is targeted to a host micro RNA instead of the pathogen (hepatitis C virus)
-
•
Miravirsen demonstrates broad antiviral activity against drug-resistant hepatitis C virus.
-
•
Development of resistance to miravirsen is relatively low.
Abstract
Antisense antimicrobial therapeutics are synthetic oligomers that silence expression of specific genes. This specificity confers an advantage over broad-spectrum antibiotics by avoiding unintended effects on commensal bacteria. The sequence-specificity and short length of antisense antimicrobials also pose little risk to human gene expression. Because antisense antimicrobials are a platform technology, they can be rapidly designed and synthesized to target almost any microbe. This reduces drug discovery time, and provides flexibility and a rational approach to drug development. Recent work has shown that antisense technology has the potential to address the antibiotic-resistance crisis, since resistance mechanisms for standard antibiotics apparently have no effect on antisense antimicrobials. Here, we describe current reports of antisense antimicrobials targeted against viruses, parasites, and bacteria.
Current Opinion in Microbiology 2016, 33:47–55
This review comes from a themed issue on Antimicrobials
Edited by Michael J Pucci and Thomas J Dougherty
For a complete overview see the Issue and the Editorial
Available online 29th June 2016
http://dx.doi.org/10.1016/j.mib.2016.05.017
1369-5274/© 2016 Elsevier Ltd. All rights reserved.
Introduction
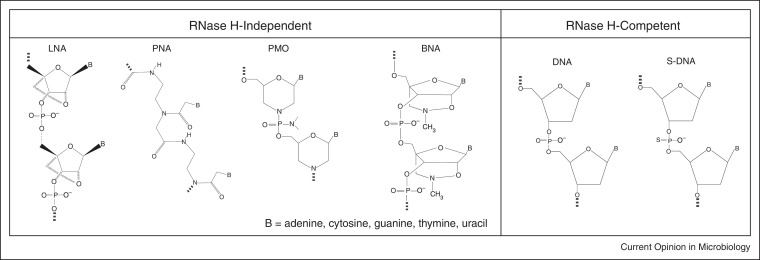
Antisense antimicrobials are short, single-stranded oligomers that mimic the structure of DNA or RNA, and bind to specific, complementary RNA in a target organism. In microorganisms, antisense therapeutics bind to complementary mRNA and inhibit translation or promote degradation of the targeted mRNA [1]. Although there are many chemical structures that have been designed for antisense technology, we will focus on four structural types that have recently gained the most attention (Figure 1 ): phosphorothioates, locked nucleic acids, peptide nucleic acids, and phosphorodiamidate morpholino oligomers, plus a few others with structural modifications of these four.
Figure 1.
Chemical structures of antisense oligomers. Five commonly used antisense oligomers include phosphorothioates (S-DNA), locked nucleic acids (LNA), peptide nucleic acids (PNA), phosphorodiamidate morpholino-oligomers (PMO), and bridged nucleic acids (BNA).
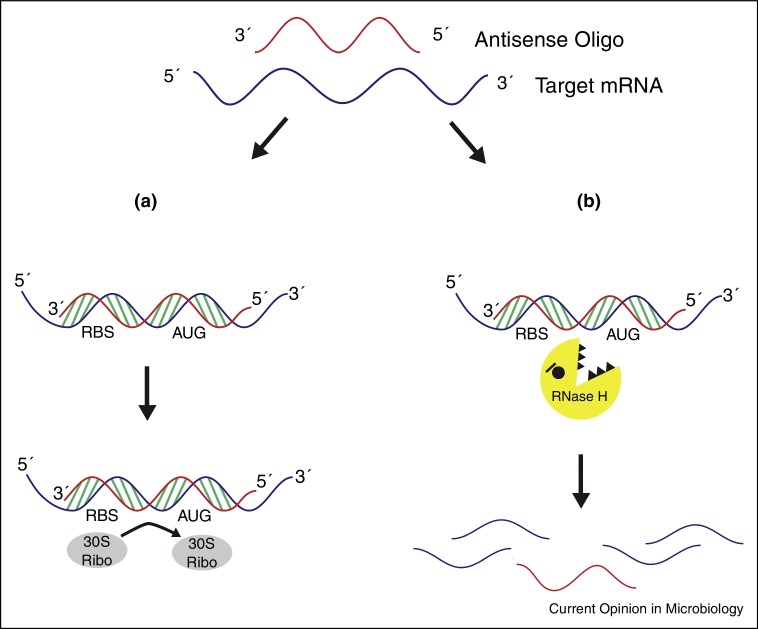
Phosphorothioate oligodeoxynucleotides (S-oligos) are analogues of phosphodiester oligonucleotides with a sulfur atom instead of one of the non-bridging oxygen atoms on the phosphate linkage. This modification increases the stability of the oligonucleotide to nucleases [2, 3, 4]. S-oligos bind to complementary mRNA and activate RNase H degradation of the targeted mRNA [1] (Figure 2 ). An S-oligo, fomivirsen (brand name Vitravene), is the only FDA-approved antisense therapeutic that targets a microorganism. Fomivirsen was approved in 1998 for treatment of cytomegalovirus-induced retinitis [5].
Figure 2.
Mechanisms of antisense oligomer inhibition of gene expression. (a) The antisense oligomer binds to the target complementary mRNA, sterically blocking the 30S ribosomal subunit and initiation of translation. (b) RNase H is activated upon oligomer binding, leading to the degradation of the targeted mRNA.
Locked nucleic acids (LNAs) are oxyphosphorothioate analogues with a 2′-O,4′-C-methylene bridge that locks the ribose ring in the C3′-endo conformation [6]. Bridged nucleic acids (BNA) are analogues of LNA, as shown in Figure 1. LNA and BNA oligomers are stable to nucleases, have very high affinity for DNA and RNA, exhibit low toxicity, and also may act through RNase H degradation of targeted mRNA.
Peptide nucleic acids (PNAs) are constructed by attaching bases to a modified polyamide backbone [7]. They are resistant to nucleases and proteases, and act by blocking translation [8, 9]. PNAs are uncharged, which in part accounts for their high affinity for RNA [8].
Phosphorodiamidate morpholino-oligomers (PMOs) are comprised of the same 4 bases as DNA, but have a modified linkage between bases. A morpholine ring is substituted for the ribose, and a dimethyl amine is substituted for one of the non-bridging oxygen atoms on the phosphate linkage. PMOs are nearly net neutral in charge, water soluble, and resistant to nucleases [10]. PMOs act by sterically blocking initiation of translation and do not activate RNase H degradation [11, 12]. PMOplus are analogues of PMO with positive charged piperazinyl phosphorodiamidate linkages.
The need for new antimicrobials has never been greater due to a limited selection of available therapeutics and the proliferation of multidrug resistant organisms. Recent developments in antisense inhibition of microbial targets has shown great potential for addressing these urgent needs and presents an entirely new and exciting paradigm for drug development. While antisense technology is used as a molecular tool to selectively silence RNA for identification of gene function [13, 14] or as a substitute for knockout mutations [15], this compilation is focused on antisense technology developed as therapies against microbial infections. Here, we will review the most recent uses of antisense technology as antivirals, antiparasitics, and antibacterials, and the future directions of this platform technology.
Antivirals
Antisense technology has been used for many years to combat viral infections, including RNA viruses such as influenza virus [16, 17], dengue virus [18], coronavirus [19], and West Nile virus [20], and DNA viruses such as cytomegalovirus [21, 22], and hepatitis C virus [23••]. Recent results have shown promising efficacy of antisense oligomers against the extremely pathogenic filoviruses, Marburg and Ebola [24, 25].
Early work with antisense therapeutics for Ebola virus (EBOV) showed that therapeutic treatment with a combination of two PMO-based oligomers increased survival up to 90% in infected mice or guinea pigs [26]. Further tests in a rhesus monkey model of infection showed that antisense therapy increased survival up to 62%, depending on the dose, and reduced viremia, inflammation and liver damage. More recently, Warren et al. now report that therapeutic treatment with one oligomer alone, targeting the VP24 EBOV protein, protected 75% of rhesus monkeys against a lethal EBOV infection [27••]. A particularly interesting development is that these antisense oligomers (called PMOplus [28]) did not require a cationic, membrane-penetrating peptide for efficacy. Instead, positive charges are added to the molecular backbone of the molecule. Although the mechanism is unknown by which the positive charges on the backbone enable efficacy, it appears that they enable membrane penetration and provide intracellular access to the replicating virus.
Another related, hemorrhagic virus, Marburg virus (MARV), is also susceptible to antisense inhibition. A PMOplus that targets the MARV nucleoprotein, which is the major nucleoprotein involved in RNA encapsidation and interference with interferon signaling, showed a dose-dependent survival rate up to 100% in a non-human model of infection [29••]. This PMOplus was also tested for safety and pharmacokinetics in humans (intravenous, range of 1–16 mg kg−1 for 14 days), and the results showed no significant effects in any safety endpoint evaluated, although some non-serious adverse events were reported by the participants.
Chikungunya virus (CHIKV) is a mosquito-borne, zoonotic RNA virus that has experienced a resurgence in the last decade, with large outbreaks being seen in Africa and areas of southern Asia [30, 31, 32]. In recent work, two anti-CHIKV phosphorodiamidate morpholino oligomers (CPMOs) were designed to bind to the two open reading frames of the viral genomic RNA [33]. The authors show that CPMO1 (targeted to ORF1) suppressed viral replication in human cells (HeLa), and significantly lowered viral titers in the tissues using a neonatal mouse model of infection. Importantly, no cytotoxicity was seen in HeLa cells nor was there any PMO-induced toxicity in the neonatal mice.
Enterovirus 71 (EV71) is a main causative pathogen of hand, foot, and mouth disease, the impact of which is felt worldwide but primarily in Asia [34]. Recent work describes EV5, an antisense phosphorothioate oligonucleotide which shows a protective effect both in vitro and in vivo [35]. Treatment of infected tissue culture cells with EV5 reduced viral replication, viral VP1 protein expression, and cell death. When administered in vivo using a mouse model of EV71 infection, EV5 protected 70–90% of mice and reduced viral replication in selected organs.
Hepatitis B virus (HBV) is carried by approximately 350 million people worldwide with 0.5–1 million dying annually due to hepatocellular carcinoma or chronic HBV liver failure [36, 37]. The HBV S gene encodes the three envelope proteins which comprise the Hepatitis B surface antigens (HBsAg) [38]. The HBcAg, encoded by the C gene, is found in the core of the nucleocapsid and is a precursor to the secretion of HBeAg [39]. Deng et al. synthesized 3 locked nucleic acids targeted to either the HBV S gene, C gene, or the S/C double gene, encapsulated each in liposomes, and treated HBV transgenic mice [40]. The results show that all three treatments decreased HBV DNA replication, but the dual-target S/C LNA showed the greatest reduction. Furthermore, each of the three HBV LNAs reduced the number of HBsAg-positive or HBcAg-positive liver cells as measured by immunohistochemistry. These results, taken with the findings that there was no obvious toxicity, suggest that these LNAs warrant further consideration as therapies against HBV.
Work by Billioud et al. also targeted HBsAg production, but used a 2′-O-methoxyethyl-modified phosphorothioate to enhance potency and stability [41•]. In a hepatoma culture, the HBsAg oligomer reduced HBV RNA and DNA, as well as HBsAg and HBeAg proteins. When administered to HBV transgenic mice, the HBsAg oligomer decreased HBsAg and HBeAg serum levels within the first days of treatment, which then remained below the original levels for three weeks.
Hepatitis C virus (HCV) affects 170 million people and is a major causative agent of chronic liver diseases such as cirrhosis and hepatocellular carcinoma [42]. Miravirsen is a 15-base β-d-oxy-LNA-modified phosphorothioate antisense oligonucleotide complementary to part of miR-122, a liver-specific miRNA expressed in hepatocytes and important factor for HCV virus replication [43, 44]. Recently, Ottosen et al. showed that treatment with miravirsen has an additive antiviral effect when used in conjunction with inhibitors of nonstructural HCV proteins [23••]. Importantly, miravirsen alone is active against HCV replicons resistant to these inhibitors. Currently, humans who have received miravirsen in Phase 2 clinical trials exhibit a prolonged decrease in plasma miR-122 levels [45]. Although miravirsen appears effective in clinical trials, its commercial fate is unknown, particularly since the introduction of effective small molecule inhibitors of HCV, Sovaldi and Harvoni [46].
Antiparasitics
While parasitic diseases continue to affect hundreds of millions of people worldwide, most current therapies are outdated and have deleterious side effects [47]. Anti-parasitic drug discovery is fraught with difficulties, mainly because of the biological complexity of parasites associated with the physiologic changes during parasitic life cycles. To complicate matters further, resistance is also a major problem [48, 49]. These recent studies with gene-specific antisense therapeutics against parasite infections pave the way for further development.
The malaria-causing parasite Plasmodium falciparum replicates inside red blood cells and infects an estimated 350–600 million people per year [50]. A unique feature of P. falciparum is a genome comprised of 80% AT base pairs [51]. This distinction from the human genome makes the P. falciparum genome a great candidate for targeting by sequence-specific inhibitors. Kolevzon et al. utilized a PNA to alter gene expression in P. falciparum [52••]. The PNA targeted PfSec13, which is an ortholog of Sec13, a conserved nucleoporin that is essential for viability in P. falciparum [53]. As a means to facilitate solubility and permeability through the cell membranes, the PNA was conjugated to a poly-d-lysine. The results show that the Sec13 PNA reached the nucleus, downregulated PfSec13 expression, and decreased viability of the parasite in culture.
A different group targeted essential genes of P. falciparum, involved in apicoplast biogenesis (PfDXR), membrane biosynthesis (PfPMT), and drug/metabolite transport (PfCRT) [54]. Peptide-conjugated PMOs (PPMOs) and octa-guanidinium-conjugated PMOs (VMOs) [55] were constructed and tested in culture. Exposure of the parasite to the conjugates decreased target RNA expression and inhibited parasite viability inside red blood cells. Notably, the authors are able to restore chloroquine sensitivity to a resistant strain of P. falciparum by treating it with PfCRT-VMO.
Chagas’ disease is caused by the parasite Trypanosoma cruzi and is historically found in rural Latin America [56]. The disease affects approximately eight million people, and 25–35% of those infected will develop cardiomyopathy [57, 58]. Hashimoto et al. utilized phosphorothioate antisense oligos (S-oligos) targeted to T. cruzi inositol 1,4,5-triphosphate receptor (TcIP 3 R) mRNA [59]. TcIP3R is an essential protein required for the parasitic invasion into host cells [60]. The results show that the antisense oligomer (antisense 5995) decreased the level of the TcIP3R in T. cruzi trypomastigotes. Moreover, this decrease of TcIP3R corresponded with an inhibition of invasion of T. cruzi into 3T3-Swiss albino cells and HeLa cells. Hashimoto et al. has also tested a morpholino oligomer targeted to a splicing site of pre-TcIP3R mRNA [61]. This oligomer (MAO-1) bound to the spliced leader acceptor region and inhibited the maturation of the target mRNA. Exposure of the parasites to the MAO-1 impaired growth in culture and decreased invasion into host cells. The mechanism of action of the MAO-1 was confirmed to be the inhibition of trans-splicing using real-time RT-PCR.
Toxoplasma gondii is an obligate intracellular, zoonotic parasite that infects one third of the world's human population [62]. There is no effective medicine or safe and effective vaccine for T. gondii. However, the results of a recent report suggest that an antisense PPMO could be developed as a therapeutic [63]. Treatment with a PPMO targeted to the mRNA of GRA10, which is thought to be important for intracellular growth and survival, reduced GRA10 expression, survival and growth of T. gondii in fibroblasts and monocytes.
Antibacterials
In vitro efficacy
Antibiotic resistance is an escalating, world-wide problem. There is an urgent need for new and effective antimicrobials. The usual strategies of screening libraries of compounds or chemically modifying existing antibiotics has produced diminishing returns in recent years, particularly for antibiotics against Gram negative pathogens [64]. An alternative strategy is to design gene-specific oligomers that can specifically target any single pathogen. This approach nearly eliminates or significantly reduces the time required for discovery of a new antimicrobial and broadens the range of potentially available targets to any gene with a known base sequence in any bacterium. Synthetic DNA analogues have been designed and shown to inhibit growth of bacteria since 1981 [65]. Significant improvements have been made along the way, aided by the identification of essential genes and the number of sequenced genomes. But perhaps the most important improvement has been the attachment of cell-penetrating peptides (CPPs) [66]. Because the cell walls of bacteria are nearly impenetrable by high molecular weight oligomers, delivery of synthetic antisense oligomers into the bacterial cytoplasm requires the attachment of another compound that can penetrate the bacterial cell wall. Most of the recent reports have used antisense oligomers coupled to CPP, often with a sequence of alternating cationic and non-polar amino acids.
Wesolowski et al. described a CPP-PMO conjugate that targets Escherichia coli gyrA [67], a highly conserved gene that is found across multiple bacterial species [68, 69]. The authors tested the sensitivity of a variety of both Gram-positive and Gram-negative bacterial strains to the CPP-PMO. Their results show that GyrA CPP-PMO reduced the viability of Enterococcus faecalis and Staphylococcus aureus more than that of Pseudomonas aeruginosa or Streptococcus pneumoniae. Gyrase was also targeted in S. pyogenes, but using a CPP-PNA. The results show a reduction in gyrA mRNA expression and an inhibition of growth [70]. In addition, the CPP-PNA was synergistic with various standard antibiotics.
More recently, Liang et al. described a peptide-conjugated PNA (PPNA) that is targeted to ftsZ in S. aureus, which is required for cell division [71, 72]. Their results show that the FtsZ PPNA inhibited growth in culture in a concentration-dependent manner, and decreased expression of ftsZ mRNA.
Intracellular efficacy
The delivery of antisense oligomers into bacteria is even more challenging when addressing intracellular pathogenic bacteria, because the oligomer may have to translocate through both host and pathogen membranes. Early work targeting intracellular bacteria found that a modified PPMO inhibited growth of intracellular Salmonella [73]. Recent work by Abushahba et al. compared the efficacy of various CPPs to deliver their payload to an intracellular pathogen, Listeria monocytogenes. The authors examined five different CPPs, each conjugated to a PNA targeting RNA polymerase α subunit (rpoA) [74]. The results show that an arginine-rich peptide with an alternating non-polar amino acid, (RXR)4XB, described previously by Mellbye et al. [75], was the most effective at delivering the antisense oligomer and reducing viability of L. monocytogenes. The report also showed that (RXR)4-PNA was the most effective oligomer tested in vivo using a Caenorhabditis elegans model of infection.
CPP-conjugated PNAs were also effective against the facultative intracellular pathogen Brucella suis, the causative agent of brucellosis, which affects swine and can be passed to humans. A report by Rajasekaran et al. showed that a PNA targeted to polA was bactericidal in pure culture [76]. However, in infected macrophages, more potent targets for PNAs were asd and dnaG, which are genes involved in cell wall synthesis and chromosome replication, respectively. The difference in potency of the PNAs in pure culture and in macrophages was attributed to differences in the available nutrients for growth between the two very different environments.
In vivo efficacy
While there have been a considerable number of reports showing in vitro efficacy of synthetic antisense antibacterials over the past 35 years, there are far fewer reports showing efficacy in animal models of infection. The first such report of in vivo efficacy showed that a PMO (without conjugated peptide) targeted to the essential gene acpP reduced viability of E. coli in a mouse model of infection [77]. Subsequent reports have established the in vivo efficacy of most of the structural types of synthetic oligomers shown in Figure 1, using animal models of infection with a variety of pathogens. Some of the most recent reports are described below.
Sawyer et al. recently showed that a CPP-PMO targeted to gyrA (an essential gene required for replication) reduced viability of Staphylococcus aureus in a skin wound model of infection [78•]. In addition, the CPP-PMO also improved healing time and quality. The results suggest that CPP-PMOs may also be an effective treatment for other types of bacterial skin infections. In another report of efficacy against S. aureus, a CPP-LNA targeted to ftsZ, which is required for cell division [71], was tested in a mouse model of sepsis [79]. The results show that a single therapeutic dose of 3 mg/kg FtsZ CPP-LNA reduced bacterial burden by about 4 logs in various tissues and increased survival by 60%.
Geller et al. examined the efficacy of a PPMO targeted to acpP in two different species of Acinetobacter, including the multidrug resistant A. baumannii [80••]. The PPMO was bactericidal in vitro, but more importantly, was also effective in a mouse model of pneumonia. The results show that bacterial burden and pro-inflammatory cytokines in the lungs were significantly reduced, and survival was increased by up to 100%, depending on the PPMO dose and bacterial species. This result suggests that PPMOs may be therapeutically useful for treating pneumonias when administered directly to the lungs.
Targeting non-essential bacterial genes
A newer, alternative strategy for using synthetic, antisense oligomers is to target non-essential genes required for virulence, such as those that confer invasiveness or biofilm formation. Silencing the expression of a virulence gene should make the pathogen less fit for infection. The advantage of targeting a non-essential gene is that it should reduce the risk of resistance [81]. Antibiotic resistance genes are examples of virulence factors, and have been targeted by synthetic antisense oligomers. The strategy of targeting antibiotic resistance genes is to knock down expression of the antibiotic resistance, which would restore susceptibility to an approved antibiotic that would be co-administered with the oligomer.
P. aeruginosa is an opportunistic pathogen that is often multidrug resistant and difficult to treat. The Greenberg lab has recently found that PPMOs targeted to genes required for biofilm synthesis, quorum sensing regulation, or alternative sigma factors inhibited the formation of biofilms and reduced existing biofilms formed by P. aeruginosa [82]. They have also found that targeting antibiotic resistance genes in P. aeruginosa with PPMOs restored susceptibility to standard antibiotics, which were useless without the PPMO.
Campylobacter jejuni is a common foodborne pathogen that expresses a multidrug efflux pump (CmeABC). CmeABC confers resistance to a broad range of antibiotics [83]. Oh et al. tested PNAs targeted to various regions of the cmeABC operon [84]. They identified two PNAs targeted to cmeA or cmeB that restored susceptibility to ciprofloxacin and erythromycin.
Goh et al. tested PNAs targeted to mecA and ftsZ of methicillin-resistant S. aureus and Staphylococcus pseudintermedius to determine if they could increase susceptibility to oxacillin [85]. The results show that each PNA decreased target mRNA levels and increased susceptibility to oxacillin in vitro. Others have also targeted mecA of S. aureus. Meng et al. utilized a phosphorothioate oligodeoxynucleotide (PS-ODN) targeted to mecA and delivered it with an anionic liposome [86•]. They report that the PS-ODN increased susceptibility to oxacillin in vitro. In a mouse model of sepsis, Meng showed co-therapy with oxacillin and the MecA PS-ODN reduced bacterial burden in the blood and improved survival by 30-50%.
Lopez et al. targeted AAC(6′)-Ib, which is an enzyme found in many Gram-negative clinical isolates that confers resistance to aminoglycosides [87]. A branched nucleic acid-DNA hybrid oligomer (BNANC-DNA) covalently bound to a cell penetrating peptide (CPPBD4) inhibited the activity of AAC(6′)-Ib and reduced viability of A. baumannii in cultures containing amikacin. In vivo, CPPBD4 protected infected Galleria mellonella larvae when administered concomitantly with amikacin.
Current work our lab and our collaborators labs is testing a PPMO targeted to blaNDM-1, which produces the NDM-1 carbapenemase. NDM-1 is a particularly dangerous resistance gene because it rapidly spreads in association with other antibiotic resistance genes by horizontal gene transfer to many genera of bacterial pathogens, and renders useless one of our most potent classes of antibiotics [88]. The NDM-1 PPMO restores susceptibility to carbapenems in all three species of Gram-negative pathogens tested: E. coli, A. baumannii, and P. aeruginosa. In the absence of carbapenems, the PPMO has no effect on bacterial growth. Most importantly, the NDM-1 PPMO is effective in vivo. In a mouse model of sepsis, concomitant treatment with the NDM-1 PPMO and meropenem reduced bacteremia and inflammation, and increased survival by 92% compared to treatment with PPMO or meropenem alone.
Concluding remarks
Antisense antimicrobials have come a long way in the past three decades. Potency has been improved to the point where many are now as potent as standard antimicrobials in vitro. Resistance to standard antibiotics seems to have no effect against antisense oligomers. Some reports have shown significant efficacy in animal models of infection using doses in a clinically relevant range. A few have been tested in non-human primate models, and recently at least one more has reached clinical testing. We think the future is bright for this exciting platform technology that has great potential for addressing the urgent need for new strategies of discovering new antimicrobials.
Competing financial interests
BLG is a consultant to Sarepta Therapeutics and an inventor on numerous patents and patent applications involving PPMOs. EKS declares no competing financial interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by US National Institutes of Health grants AI 098724, AI 111753, and AI 105980, and the N.L. Tartar Fund. We thank Jessica Humphrey for the artwork. We thank Drs. David Greenberg and Patrick Iversen for critically reading the manuscript.
References
- 1.Dias N., Stein C.A. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther. 2002;1:347–355. [PubMed] [Google Scholar]
- 2.Brown D.A., Kang S.H., Gryaznov S.M., DeDionisio L., Heidenreich O., Sullivan S., Xu X., Nerenberg M.I. Effect of phosphorothioate modification of oligodeoxynucleotides on specific protein binding. J Biol Chem. 1994;269:26801–26805. [PubMed] [Google Scholar]
- 3.Wagner R.W. The state of the art in antisense research. Nat Med. 1995;1:1116–1118. doi: 10.1038/nm1195-1116. [DOI] [PubMed] [Google Scholar]
- 4.Monia B.P., Johnston J.F., Sasmor H., Cummins L.L. Nuclease resistance and antisense activity of modified oligonucleotides targeted to Ha-ras. J Biol Chem. 1996;271:14533–14540. doi: 10.1074/jbc.271.24.14533. [DOI] [PubMed] [Google Scholar]
- 5.Orr R.M. Technology evaluation: fomivirsen, Isis Pharmaceuticals Inc/CIBA vision. Curr Opin Mol Ther. 2001;3:288–294. [PubMed] [Google Scholar]
- 6.Doessing H., Vester B. Locked and unlocked nucleosides in functional nucleic acids. Molecules. 2011;16:4511–4526. doi: 10.3390/molecules16064511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen P.E., Egholm M., Berg R.H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen P.E., Egholm M. An introduction to peptide nucleic acid. Curr Issues Mol Biol. 1999;1:89–104. [PubMed] [Google Scholar]
- 9.Good L., Nielsen P.E. Antisense inhibition of gene expression in bacteria by PNA targeted to mRNA. Nat Biotechnol. 1998;16:355–358. doi: 10.1038/nbt0498-355. [DOI] [PubMed] [Google Scholar]
- 10.Hudziak R.M., Barofsky E., Barofsky D.F., Weller D.L., Huang S.B., Weller D.D. Resistance of morpholino phosphorodiamidate oligomers to enzymatic degradation. Antisense Nucleic Acid Drug Dev. 1996;6:267–272. doi: 10.1089/oli.1.1996.6.267. [DOI] [PubMed] [Google Scholar]
- 11.Summerton J., Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- 12.Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta (BBA) – Gene Struct Expr. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 13.Hwang D., Lim Y.-H. Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci Rep. 2015;5:10029. doi: 10.1038/srep10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee N., Moss W.N., Yario T.A., Steitz J.A. EBV noncoding RNA binds nascent RNA to drive host PAX5 to viral DNA. Cell. 2015;160:607–618. doi: 10.1016/j.cell.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandolia A., Rathor N., Sharma M., Saini N.K., Sinha R., Malhotra P., Brahmachari V., Bose M. Functional analysis of mce4A gene of Mycobacterium tuberculosis H37Rv using antisense approach. Microbiol Res. 2014;169:780–787. doi: 10.1016/j.micres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Mizuta T., Fujiwara M., Hatta T., Abe T., Miyano-Kurosaki N., Shigeta S., Yokota T., Takaku H. Antisense oligonucleotides directed against the viral RNA polymerase gene enhance survival of mice infected with influenza A. Nat Biotechnol. 1999;17:583–587. doi: 10.1038/9893. [DOI] [PubMed] [Google Scholar]
- 17.Ge Q., Pastey M., Kobasa D., Puthavathana P., Lupfer C., Bestwick R.K., Iversen P.L., Chen J., Stein D.A. Inhibition of multiple subtypes of influenza A virus in cell cultures with morpholino oligomers. Antimicrob Agents Chemother. 2006;50:3724–3733. doi: 10.1128/AAC.00644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holden K.L., Stein D.A., Pierson T.C., Ahmed A.A., Clyde K., Iversen P.L., Harris E. Inhibition of dengue virus translation and RNA synthesis by a morpholino oligomer targeted to the top of the terminal 3′ stem–loop structure. Virology. 2006;344:439–452. doi: 10.1016/j.virol.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 19.Neuman B.W., Stein D.A., Kroeker A.D., Churchill M.J., Kim A.M., Kuhn P., Dawson P., Moulton H.M., Bestwick R.K., Iversen P.L. Inhibition, escape, and attenuated growth of severe acute respiratory syndrome coronavirus treated with antisense morpholino oligomers. J Virol. 2005;79:9665–9676. doi: 10.1128/JVI.79.15.9665-9676.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deas T.S., Binduga-Gajewska I., Tilgner M., Ren P., Stein D.A., Moulton H.M., Iversen P.L., Kauffman E.B., Kramer L.D., Shi P.Y. Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication. J Virol. 2005;79:4599–4609. doi: 10.1128/JVI.79.8.4599-4609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azad R.F., Driver V.B., Tanaka K., Crooke R.M., Anderson K.P. Antiviral activity of a phosphorothioate oligonucleotide complementary to RNA of the human cytomegalovirus major immediate-early region. Antimicrob Agents Chemother. 1993;37:1945–1954. doi: 10.1128/aac.37.9.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margraf S., Bittoova M., Vogel J.U., Kotchekov R., Doerr H.W., Cinatl J., Jr. Antisense oligonucleotide ISIS 2922 targets IE-expression and prevents HCMV-IE-induced suppression of TSP-1 and TSP-2 expression. Nucleos Nucl Nucleic Acids. 2001;20:1425–1428. doi: 10.1081/NCN-100002569. [DOI] [PubMed] [Google Scholar]
- 23••.Ottosen S., Parsley T.B., Yang L., Zeh K., van Doorn L.J., van der Veer E., Raney A.K., Hodges M.R., Patick A.K. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob Agents Chemother. 2015;59:599–608. doi: 10.1128/AAC.04220-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; The importance of this antisense therapeutic (miravirsen) is that it targets a host micro RNA instead of the pathogen. This strategy should reduce the selelction pressure for resistance, which is supported by data in this study.
- 24.Warfield K.L., Swenson D.L., Olinger G.G., Nichols D.K., Pratt W.D., Blouch R., Stein D.A., Aman M.J., Iversen P.L., Bavari S. Gene-specific countermeasures against Ebola virus based on antisense phosphorodiamidate morpholino oligomers. PLoS Pathog. 2006;2:e1. doi: 10.1371/journal.ppat.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler T., Bamberg S., Moller P., Klenk H.D., Meyer T.F., Becker S., Rudel T. Inhibition of Marburg virus protein expression and viral release by RNA interference. J Gen Virol. 2005;86:1181–1188. doi: 10.1099/vir.0.80622-0. [DOI] [PubMed] [Google Scholar]
- 26.Warren T.K., Warfield K.L., Wells J., Swenson D.L., Donner K.S., Van Tongeren S.A., Garza N.L., Dong L., Mourich D.V., Crumley S. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat Med. 2010;16:991–994. doi: 10.1038/nm.2202. [DOI] [PubMed] [Google Scholar]
- 27••.Warren T.K., Whitehouse C.A., Wells J., Welch L., Heald A.E., Charleston J.S., Sazani P., Reid S.P., Iversen P.L., Bavari S. A single phosphorodiamidate morpholino oligomer targeting VP24 protects rhesus monkeys against lethal Ebola virus infection. mBio. 2015;6:e02344–e2414. doi: 10.1128/mBio.02344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study showing that a PMO targeting Ebola virus VP24 protects primates from a lethal Ebola infection.
- 28.Swenson D.L., Warfield K.L., Warren T.K., Lovejoy C., Hassinger J.N., Ruthel G., Blouch R.E., Moulton H.M., Weller D.D., Iversen P.L. Chemical modifications of antisense morpholino oligomers enhance their efficacy against Ebola virus infection. Antimicrob Agents Chemother. 2009;53:2089–2099. doi: 10.1128/AAC.00936-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Heald A.E., Charleston J.S., Iversen P.L., Warren T.K., Saoud J.B., Al-Ibrahim M., Wells J., Warfield K.L., Swenson D.L., Welch L.S. AVI-7288 for marburg virus in nonhuman primates and humans. N Engl J Med. 2015;373:339–348. doi: 10.1056/NEJMoa1410345. [DOI] [PubMed] [Google Scholar]; A study showing that a PMOplus protects primates from a lethal challenge with Marburg virus.
- 30.Burt F.J., Rolph M.S., Rulli N.E., Mahalingam S., Heise M.T. Chikungunya: a re-emerging virus. The Lancet. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 31.Thiberville S.-D., Moyen N., Dupuis-Maguiraga L., Nougairede A., Gould E.A., Roques P., de Lamballerie X. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345–370. doi: 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindsey N.P., Prince H.E., Kosoy O., Laven J., Messenger S., Staples J.E., Fischer M. Chikungunya virus infections among travellers — United States, 2010–2013. Am J Trop Med Hyg. 2015;92:82–87. doi: 10.4269/ajtmh.14-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam S., Chen H., Chen C.K., Min N., Chu J.J.H. Antiviral phosphorodiamidate morpholino oligomers are protective against Chikungunya virus infection on cell-based and murine models. Sci Rep. 2015;5:12727. doi: 10.1038/srep12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chong P., Liu C.C., Chow Y.H., Chou A.H., Klein M. Review of enterovirus 71 vaccines. Clin Infect Dis. 2015;60:797–803. doi: 10.1093/cid/ciu852. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Zhou Z., Li K., Han M., Yang J., Wang S. In vitro and in vivo protection against enterovirus 71 by an antisense phosphorothioate oligonucleotide. Arch Virol. 2014;159:2339–2347. doi: 10.1007/s00705-014-2054-y. [DOI] [PubMed] [Google Scholar]
- 36.Hou J., Liu Z., Gu F. Epidemiology and prevention of hepatitis B virus infection. Int J Med Sci. 2005;2:50–57. doi: 10.7150/ijms.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 38.Yum J.S., Ahn B.C., Jo H.J., Kim D.Y., Kim K.H., Kim H.S., Sung Y.C., Yoon J., Morrey J., Moon H.M. Use of pre-S protein-containing hepatitis B virus surface antigens and a powerful adjuvant to develop an immune therapy for chronic hepatitis B virus infection. Clin Vaccine Immunol. 2012;19:120–127. doi: 10.1128/CVI.05355-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jean-Jean O., Levrero M., Will H., Perricaudet M., Rossignol J.M. Expression mechanism of the hepatitis B virus (HBV) C gene and biosynthesis of HBe antigen. Virology. 1989;170:99–106. doi: 10.1016/0042-6822(89)90356-5. [DOI] [PubMed] [Google Scholar]
- 40.Deng Y.B., Qin H.J., Luo Y.H., Liang Z.R., Zou J.J. Antiviral effect of hepatitis B virus S/C gene loci antisense locked nucleic acid on transgenic mice in vivo. Genet Mol Res. 2015;14:10087–10095. doi: 10.4238/2015.August.21.16. [DOI] [PubMed] [Google Scholar]
- 41•.Billioud G., Kruse R.L., Carrillo M., Whitten-Bauer C., Gao D., Kim A., Chen L., McCaleb M.L., Crosby J.R., Hamatake R. In vivo reduction of hepatitis B virus antigenemia and viremia by antisense oligonucleotides. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.11.032. [DOI] [PubMed] [Google Scholar]; A study showing a phosphorothioate HBsAg oligomer decreases HBsAg and HBeAg serum levels in HBV transgenic mice.
- 42.Modi A., Liang T. Hepatitis C: a clinical review. Oral Dis. 2008;14:10–14. doi: 10.1111/j.1601-0825.2007.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jopling C.L., Yi M., Lancaster A.M., Lemon S.M., Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 44.Jopling C.L., Schutz S., Sarnow P. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe. 2008;4:77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Ree M.H., van der Meer A.J., van Nuenen A.C., de Bruijne J., Ottosen S., Janssen H.L., Kootstra N.A., Reesink H.W. Miravirsen dosing in chronic hepatitis C patients results in decreased microRNA-122 levels without affecting other microRNAs in plasma. Aliment Pharmacol Ther. 2016;43:102–113. doi: 10.1111/apt.13432. [DOI] [PubMed] [Google Scholar]
- 46.McQuaid T., Savini C., Seyedkazemi S. Sofosbuvir, a significant paradigm change in HCV treatment. J Clin Transl Hepatol. 2015;3:27–35. doi: 10.14218/JCTH.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renslo A.R., McKerrow J.H. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 48.Stock R.P., Olvera A., Sanchez R., Saralegui A., Scarfi S., Sanchez-Lopez R., Ramos M.A., Boffa L.C., Benatti U., Alagon A. Inhibition of gene expression in Entamoeba histolytica with antisense peptide nucleic acid oligomers. Nat Biotechnol. 2001;19:231–234. doi: 10.1038/85671. [DOI] [PubMed] [Google Scholar]
- 49.Andrews K.T., Fisher G., Skinner-Adams T.S. Drug repurposing and human parasitic protozoan diseases. Int J Parasitol Drugs Drug Resist. 2014;4:95–111. doi: 10.1016/j.ijpddr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bousema T., Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardner M.J., Hall N., Fung E., White O., Berriman M., Hyman R.W., Carlton J.M., Pain A., Nelson K.E., Bowman S. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Kolevzon N., Nasereddin A., Naik S., Yavin E., Dzikowski R. Use of peptide nucleic acids to manipulate gene expression in the malaria parasite Plasmodium falciparum. PLOS ONE. 2014;9:e86802. doi: 10.1371/journal.pone.0086802. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study that shows that a PNA targeted to PfSec13 significantly reduces Plasmodium falciparum viability in red blood cells.
- 53.Dahan-Pasternak N., Nasereddin A., Kolevzon N., Pe’er M., Wong W., Shinder V., Turnbull L., Whitchurch C.B., Elbaum M., Gilberger T.W. PfSec13 is an unusual chromatin-associated nucleoporin of Plasmodium falciparum that is essential for parasite proliferation in human erythrocytes. J Cell Sci. 2013;126:3055–3069. doi: 10.1242/jcs.122119. [DOI] [PubMed] [Google Scholar]
- 54.Garg A., Wesolowski D., Alonso D., Deitsch K.W., Ben Mamoun C., Altman S. Targeting protein translation, RNA splicing, and degradation by morpholino-based conjugates in Plasmodium falciparum. Proc Natl Acad Sci U S A. 2015;112:11935–11940. doi: 10.1073/pnas.1515864112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morcos P.A., Li Y., Jiang S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. Biotechniques. 2008;45 doi: 10.2144/000113005. 613–614, 616, 618 passim. [DOI] [PubMed] [Google Scholar]
- 56.Bern C., Kjos S., Yabsley M.J., Montgomery S.P. Trypanosoma cruzi and Chagas’ disease in the United States. Clin Microbiol Rev. 2011;24:655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rassi A., Jr., Rassi A., Marin-Neto J.A. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 58.Maya J.D., Orellana M., Ferreira J., Kemmerling U., Lopez-Munoz R., Morello A. Chagas disease: present status of pathogenic mechanisms and chemotherapy. Biol Res. 2010;43:323–331. [PubMed] [Google Scholar]
- 59.Hashimoto M., Nara T., Hirawake H., Morales J., Enomoto M., Mikoshiba K. Antisense oligonucleotides targeting parasite inositol 1,4,5-trisphosphate receptor inhibits mammalian host cell invasion by Trypanosoma cruzi. Sci Rep. 2014;4:4231. doi: 10.1038/srep04231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hashimoto M., Enomoto M., Morales J., Kurebayashi N., Sakurai T., Hashimoto T., Nara T., Mikoshiba K. Inositol 1,4,5-trisphosphate receptor regulates replication, differentiation, infectivity and virulence of the parasitic protist Trypanosoma cruzi. Mol Microbiol. 2013;87:1133–1150. doi: 10.1111/mmi.12155. [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto M., Nara T., Mita T., Mikoshiba K. Morpholino antisense oligo inhibits trans-splicing of pre-inositol 1,4,5-trisphosphate receptor mRNA of Trypanosoma cruzi and suppresses parasite growth and infectivity. Parasitol Int. 2016;65:175–179. doi: 10.1016/j.parint.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 62.Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Witola W.H., Bauman B., McHugh M., Matthews K. Silencing of GRA10 protein expression inhibits Toxoplasma gondii intracellular growth and development. Parasitol Int. 2014;63:651–658. doi: 10.1016/j.parint.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Drawz S.M., Bonomo R.A. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jayaraman K., McParland K., Miller P., Ts’o P.O. Selective inhibition of Escherichia coli protein synthesis and growth by nonionic oligonucleotides complementary to the 3′ end of 16S rRNA. Proc Natl Acad Sci U S A. 1981;78:1537–1541. doi: 10.1073/pnas.78.3.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Good L., Awasthi S.K., Dryselius R., Larsson O., Nielsen P.E. Bactericidal antisense effects of peptide–PNA conjugates. Nat Biotechnol. 2001;19:360–364. doi: 10.1038/86753. [DOI] [PubMed] [Google Scholar]
- 67.Wesolowski D., Alonso D., Altman S. Combined effect of a peptide–morpholino oligonucleotide conjugate and a cell-penetrating peptide as an antibiotic. Proc Natl Acad Sci. 2013;110:8686–8689. doi: 10.1073/pnas.1306911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levine C., Hiasa H., Marians K.J. DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim Biophys Acta (BBA) – Gene Struct Expr. 1998;1400:29–43. doi: 10.1016/s0167-4781(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 69.Weigel L.M., Steward C.D., Tenover F.C. gyrA mutations associated with fluoroquinolone resistance in eight species of Enterobacteriaceae. Antimicrob Agents Chemother. 1998;42:2661–2667. doi: 10.1128/aac.42.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patenge N., Pappesch R., Krawack F., Walda C., Mraheil M.A., Jacob A., Hain T., Kreikemeyer B. Inhibition of growth and gene expression by PNA–peptide conjugates in Streptococcus pyogenes. Mol Ther Nucleic Acids. 2013:2. doi: 10.1038/mtna.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh P., Panda D. FtsZ inhibition: a promising approach for antistaphylococcal therapy. Drug News Perspect. 2010;23:295–304. doi: 10.1358/dnp.2010.23.5.1429489. [DOI] [PubMed] [Google Scholar]
- 72.Liang S., He Y., Xia Y., Wang H., Wang L., Gao R., Zhang M. Inhibiting the growth of methicillin-resistant Staphylococcus aureus in vitro with antisense peptide nucleic acid conjugates targeting the ftsZ gene. Int J Infect Dis. 2015;30:1–6. doi: 10.1016/j.ijid.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 73.Mitev G.M., Mellbye B.L., Iversen P.L., Geller B.L. Inhibition of intracellular growth of Salmonella enterica serovar Typhimurium in tissue culture by antisense peptide-phosphorodiamidate morpholino oligomer. Antimicrob Agents Chemother. 2009;53:3700–3704. doi: 10.1128/AAC.00099-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abushahba M.F.N., Mohammad H., Thangamani S., Hussein A.A.A., Seleem M.N. Impact of different cell penetrating peptides on the efficacy of antisense therapeutics for targeting intracellular pathogens. Sci Rep. 2016;6:20832. doi: 10.1038/srep20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mellbye B.L., Puckett S.E., Tilley L.D., Iversen P.L., Geller B.L. Variations in amino acid composition of antisense peptide-phosphorodiamidate morpholino oligomer affect potency against Escherichia coli in vitro and in vivo. Antimicrob Agents Chemother. 2009;53:525–530. doi: 10.1128/AAC.00917-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajasekaran P., Alexander J.C., Seleem M.N., Jain N., Sriranganathan N., Wattam A.R., Setubal J.C., Boyle S.M. Peptide nucleic acids inhibit growth of Brucella suis in pure culture and in infected murine macrophages. Int J Antimicrob Agents. 2013;41:358–362. doi: 10.1016/j.ijantimicag.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geller B.L., Deere J., Tilley L., Iversen P.L. Antisense phosphorodiamidate morpholino oligomer inhibits viability of Escherichia coli in pure culture and in mouse peritonitis. J Antimicrob Chemother. 2005;55:983–988. doi: 10.1093/jac/dki129. [DOI] [PubMed] [Google Scholar]
- 78•.Sawyer A.J., Wesolowski D., Gandotra N., Stojadinovic A., Izadjoo M., Altman S., Kyriakides T.R. A peptide-morpholino oligomer conjugate targeting Staphylococcus aureus gyrA mRNA improves healing in an infected mouse cutaneous wound model. Int J Pharm. 2013;453:651–655. doi: 10.1016/j.ijpharm.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that infected, cutaneous wounds in mice healed faster when treated with an antisense peptide-conjugated oligomer that targets gyrA of S. aureus.
- 79.Meng J., Da F., Ma X., Wang N., Wang Y., Zhang H., Li M., Zhou Y., Xue X., Hou Z. Antisense growth inhibition of methicillin-resistant Staphylococcus aureus by locked nucleic acid conjugated with cell-penetrating peptide as a novel FtsZ inhibitor. Antimicrob Agents Chemother. 2015;59:914–922. doi: 10.1128/AAC.03781-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80••.Geller B.L., Marshall-Batty K., Schnell F.J., McKnight M.M., Iversen P.L., Greenberg D.E. Gene-silencing antisense oligomers inhibit acinetobacter growth in vitro and in vivo. J Infect Dis. 2013 doi: 10.1093/infdis/jit460. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study showing a PPMO targeted to an essential gene increases survival of mice infected with Acinetobacter. This is also the first pulmonary delivery of an antisense oligomer.
- 81.Rasko D.A., Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 82.Sturge C.R., Howard J., Justice H., Wong M., Geller B.L., Greenberg D.E. Inhibition of biofilm and quorum-sensing pathways in Pseudomonas aeruginosa by peptide-conjugated phosphorodiamidate morpholino oligomers (PPMOs). Poster. ASM Microbe; Boston, MA; 2016. [Google Scholar]
- 83.Yan M., Sahin O., Lin J., Zhang Q. Role of the CmeABC efflux pump in the emergence of fluoroquinolone-resistant Campylobacter under selection pressure. J Antimicrob Chemother. 2006;58:1154–1159. doi: 10.1093/jac/dkl412. [DOI] [PubMed] [Google Scholar]
- 84.Oh E., Zhang Q., Jeon B. Target optimization for peptide nucleic acid (PNA)-mediated antisense inhibition of the CmeABC multidrug efflux pump in Campylobacter jejuni. J Antimicrob Chemother. 2014;69:375–380. doi: 10.1093/jac/dkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goh S., Loeffler A., Lloyd D.H., Nair S.P., Good L. Oxacillin sensitization of methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus pseudintermedius by antisense peptide nucleic acids in vitro. BMC Microbiol. 2015;15:1–10. doi: 10.1186/s12866-015-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86•.Meng J., He G., Wang H., Jia M., Ma X., Da F., Wang N., Hou Z., Xue X., Li M. Reversion of antibiotic resistance by inhibiting mecA in clinical methicillin-resistant Staphylococci by antisense phosphorothioate oligonucleotide. J Antibiot (Tokyo) 2015;68:158–164. doi: 10.1038/ja.2014.132. [DOI] [PubMed] [Google Scholar]; A study using a PS-ODN that restores oxacillin-sensitivity to antibiotic-resistant S. aureus and protects mice from lethal infection when administered with oxacillin.
- 87.Lopez C., Arivett B.A., Actis L.A., Tolmasky M.E. Inhibition of AAC(6′)-Ib-mediated resistance to amikacin in Acinetobacter baumannii by an antisense peptide-conjugated 2′,4′-bridged nucleic acid–NC–DNA hybrid oligomer. Antimicrob Agents Chemother. 2015;59:5798–5803. doi: 10.1128/AAC.01304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sully E., Li L., Greenberg D.E., Bailey S., Moore A., Wong M., Geller B.L. NDM-1 positive Escherichia coli restored to carbapenem susceptibility in vivo by a peptide phosphorodiamidate morpholino oligomer. Poster. ICAAC/ICC; San Diego, CA; 2015. [Google Scholar]