Abstract
Purpose
To demonstrate the feasibility of four-dimensional (4D)– flow magnetic resonance (MR) imaging for noninvasive longitudinal hemodynamic monitoring of hepatic blood flow before and after transjugular intrahepatic portosystemic shunt (TIPS) placement.
Materials and Methods
The institutional review board approved this prospective Health Insurance Portability and Accountability Act compliant study with written informed consent. Four-dimensional–flow MR imaging was performed in seven patients with portal hypertension and refractory ascites before and 2 and 12 weeks after TIPS placement by using a time-resolved three-dimensional radial phase-contrast acquisition. Flow and peak velocity measurements were obtained in the superior mesenteric vein (SMV), splenic vein (SV), portal vein (PV), and the TIPS. Flow volumes and peak velocities in each vessel, as well as the ratio of in-stent to PV flow, were compared before and after TIPS placement by using analysis of variance.
Results
Flow volumes significantly increased in the SMV (0.24 L/ min; 95% confidence interval [CI]: 0.07, 0.41), SV (0.31 L/min; 95% CI: 0.07, 0.54), and PV (0.88 L/min; 95% CI: 0.06, 1.70) after TIPS placement (all P < .05), with no significant difference between the first and second post-TIPS placement acquisitions (all P > .11). Ascites resolved in six of seven patients. In those with resolved ascites, the TIPS-to-PV flow ratio was 0.8 ± 6 0.2 and 0.9 ± 0.2 at the two post-TIPS time points, respectively, while the observed ratios were 4.6 and 4.3 in the patient with refractory ascites at the two post-TIPS time points, respectively. In this patient, 4D-flow MR imaging demonstrated arterio-portal-venous shunting, with draining into the TIPS.
Conclusion
Four-dimensional–flow MR imaging is feasible for noninvasive longitudinal hemodynamic monitoring of hepatic blood flow before and after TIPS placement.
Portal hypertension is a well-known and potentially fatal complication of end-stage liver disease (cirrhosis) (1). It is defined as an elevated portosystemic pressure gradient greater than 5 mm Hg. When the portosystemic pressure gradient exceeds 12 mm Hg, patients can develop refractory ascites and variceal bleeding (1,2). When medical therapies are no longer effective, portal hypertension can be treated through placement of a transjugular intrahepatic portosystemic shunt (TIPS) (3). This shunt diverts blood flow from the portal system directly into the systemic circulation, reducing portal pressure and thereby helping resolve ascites and reduce the risk for variceal hemorrhage (2,4).
Unfortunately, excessive shunting of portal blood into the systemic circulation is associated with the risk for development of post-TIPS hepatic encephalopathy (5). Over 30% of patients experience post-TIPS hepatic encephalopathy due to increased levels of circulating ammonia and other metabolites from the gut; 70% of these patients experience hepatic encephalopathy within 3 months after TIPS placement (6). The second major complication is recurrent portal hypertension secondary to development of in-stent stenosis, despite improved patency with the advent of covered stents (7,8). Therefore, comprehensive and longitudinal hemodynamic monitoring of the portal system and the TIPS is needed to monitor stent patency and the fraction of blood flow that is diverted from the liver (9,10).
Currently, two-dimensional Doppler ultrasonography (US) plays a central role in monitoring patients before and after TIPS placement (10). Two-dimensional Doppler US is limited by operator dependence (11). Further, a nonlinear association exists between flow velocity and shunt patency, with a poor correlation between flow velocity and the portosystemic pressure gradient (12,13). Therefore, a noninvasive and operator-independent technique for monitoring the hemodynamic changes after TIPS placement is desirable.
Volumetric blood flow is an alternative measure that can be used to assess TIPS function (14). A useful metric is shunt fraction, the volume of blood flow shunted through the TIPS and normalized by the flow in the main portal vein (PV) (ie, TIPS-to-PV flow ratio). The shunt fraction may be used to predict the risk for hepatic encephalopathy, refractory ascites, or variceal bleeding. Indeed, shunt volume flow measurements made at four-dimensional (4D) Doppler US have shown promise as indicators of shunt function (15). However, 4D Doppler US is also limited by operator dependency similar to that of conventional two-dimensional Doppler US (15).
Four-dimensional–flow magnetic resonance (MR) imaging has been successfully used to monitor hepatic blood flow in patients with portal hypertension (16,17). However, the use of 4D-flow MR imaging has only been reported at a single time point (4 weeks) after TIPS placement, with no long-term follow-up or calculation of TIPS-to-PV flow ratios over time (18). The purpose of this study was to demonstrate the feasibility of 4D-flow MR imaging for longitudinal hemodynamic monitoring of hepatic blood flow before and after TIPS placement.
Materials and Methods
Patients
This prospective study was institutional review board approved and Health Insurance Portability and Accountability Act compliant. Written informed consent was obtained.
Patients who were referred for TIPS placement between December 2013 and November 2014 because of portal hypertension and refractory ascites were eligible for inclusion in the study. Patients were consecutively and prospectively enrolled if none of the following exclusion criteria were present: Acute variceal bleeding and emergency TIPS placement (n = 14), model for end-stage liver disease score greater than 18, encephalopathy that was refractory to medical management, estimated glomerular filtration rate less than 30 mL/min (n = 1), inability to give consent (n = 1), inability to undergo MR imaging (eg, due to a device not compatible with 3T imaging, n = 1), previous liver surgery or TIPS placement, not interested in participating in the study (n = 4), and unstable medical condition (n = 1).
During the recruitment period, a total of 32 TIPS were placed. We were able to include 10 consecutive patients (of 32 [31.2%]) with portal hypertension and refractory ascites who met the inclusion criteria after excluding the 22 patients listed previously.
All patients underwent implantation of an 80 × 10-mm covered stent graft (Viatorr TIPS Endoprothesis; W.L. Gore and Associates, Flagstaff, Ariz). TIPS indication, portosystemic pressure gradients, Child-Pugh score, model for end-stage liver disease score, ascites grade, the need for paracentesis, and the frequency and severity of episodes of hepatic encephalopathy were recorded at each of the three imaging time points (19,20).
Three patients were excluded from the final analyses because they did not complete the three examinations. One patient died after the second MR imaging study, while the other two patients withdrew from the study after the first and second MR imaging studies, respectively.
MR Imaging
MR imaging was performed as a stand-alone research examination after at least 3 hours of fasting within 24 hours before and 2 and 12 weeks after TIPS placement by using a clinical 3-T imager (Discovery MR750; GE Healthcare, Waukesha, Wis) with a 32-channel phased-array body coil (NeoCoil, Pewaukee, Wis). Gadofosveset trisodium (Lantheus, N Billerica, Mass) was intravenously administered at a rate of 0.03 mmol/kg before all 4D-flow MR imaging to increase signal-to-noise ratio. Contrast material–enhanced three-dimensional MR angiography was performed with breath holding before 4D-flow MR imaging during injection of contrast material.
Four-dimensional velocity mapping was achieved by using a respiratory- and cardiac-gated time-resolved three-dimensional radial phase-contrast acquisition with increased velocity sensitivity performance and full coverage of the upper abdomen (21). The following 4D-flow MR imaging acquisition parameters were included: imaging volume, 32 × 32 × 24 cm3; acquired isotropic spatial resolution, 1.25 mm; repetition time msec/echo time msec, 6.4/2.2; flip angle, 16°. Imaging time for each acquisition was approximately 12 minutes, depending on the respiratory pattern. Velocity encoding was adjusted for pre-TIPS imaging at 60 cm/ sec for optimal mapping of slow flow in the hypertensive portal circulation. For post-TIPS imaging, 4D-flow MR imaging was repeated back-to-back with velocity encoding of 80 and 120 cm/sec for optimal imaging of increased flow in the portal vein and TIPS, respectively.
4D-Flow MR Imaging Data Analysis
All data sets were automatically reconstructed to 14 time frames per cardiac cycle. Phase offsets for Maxwell terms and eddy currents were automatically corrected during reconstruction (22,23). Background phase correction was performed by using semiautomatic segmentation of the static tissue, which is fit to a third-order three-dimensional polynomial. This fitting was performed after correction of phase offsets from concomitant gradients by using a vendor-supplied algorithm. Background phase corrections were verified by inspecting the derived angiogram for areas of visual static tissue and through flow measurements of static tissue (liver parenchyma). Velocity-weighted angiograms were automatically calculated from the final velocity and magnitude data for all 14 time frames (24). One radiologist with 7 years of experience in abdominal imaging (P.B.) performed manual vessel segmentation in MIMICs (Materialize, Leuven, Belgium) from the phase-corrected angiograms. Masks resulting from segmentation were used to postprocess velocity data for flow analysis.
The same radiologist visually assessed the patency of the TIPS and manually placed cut-planes in the vessel of interest and TIPS for flow quantification and visualization in EnSight (CEI, Apex, NC). Flow volumes and peak velocities were measured in the central section of PV, superior mesenteric vein (SMV), splenic vein (SV), and TIPS. The ratio of blood flow in the TIPS versus the PV was calculated. Postprocessing of the velocity data sets required 2.0–2.5 hours of intense computer interaction. The radiologist was trained by an MR physicist with 7 years of experience (A.R.A.) by using 10 former 4D-flow data sets of the portal system.
Statistical Analyses
Peak velocities and flow volumes were compared across the three imaging times with repeated measures analyses of variance. Bonferroni correction for three independent t tests was applied for P values and 95% confidence intervals (CIs) of calculated effect sizes. Reported P values were adjusted for three independent t tests, and P < .05 indicated a significant difference. Statistical computations were performed with MedCalc version 12.7.5 (MedCalc Software, Ostend, Belgium).
Results
Radial 4D-flow MR imaging at all three imaging time points was successfully completed in the seven subjects (six men and one woman). The mean age was 52 years ± 10. Before TIPS, the mean portosystemic gradient was 15.7 mm Hg ± 7.4 and was reduced to 8.1 mm Hg ± 5.0 (P < .001) after TIPS placement. Two of the seven patients developed hepatic encephalopathy after TIPS placement. Ascites successfully resolved in six of the seven patients. One patient had refractory ascites and required continued periodic paracentesis after TIPS placement (Table 1).
Table 1.
Patient Characteristics before and 2 and 12 Weeks after TIPS Placement
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age (y) | 63 | 34 | 59 | 54 | 46 | 54 | 54 |
| Sex | Male | Male | Male | Female | Male | Male | Male |
| Disease | Alcoholic cirrhosis | Nodular regenerative hyperplasia | Alcoholic cirrhosis, hepatitis C | Alcoholic cirrhosis, hepatitis C | Idiopathic cirrhosis | Cirrhosis, NASH | Cirrhosis, NASH |
| TIPS Indication | Refractory ascites | Refractory ascites | Refractory ascites | Refractory ascites | Refractory ascites | Refractory ascites | Refractory ascites |
| TIPS device | 10 mm × 8 cm | 10 mm × 8 cm | 10 mm × 8 cm | 10 mm × 8 cm | 10 mm × 8 cm | 10 mm × 7 cm | 10 mm × 8 cm |
| Before TIPS | |||||||
| Child-Pugh score | 9 | 7 | 8 | 9 | 6 | 7 | 7 |
| MELD score | 15 | 9 | 14 | 8 | 13 | 15 | 7 |
| Ascites grade | Large | Large | Large | Large | Large | Large | Large |
| HE present | No | No | No | No | No | No | No |
| Pressure gradient* (mm Hg) | 18 (7) | 14 (9) | 20 (10) | 14 (6) | 28 (18) | 12 (4) | 8 (3) |
| 2 weeks after TIPS | |||||||
| Child-Pugh score | 12 | 9 | 7 | 8 | 6 | 7 | 6 |
| MELD score | 16 | 9 | 12 | 8 | 13 | 15 | 10 |
| Ascites grade | Large | Moderate | Moderate | Mild | Large | Moderate | Mild |
| HE present | No | No | Severe | No | No | Severe | No |
| 12 weeks after TIPS | |||||||
| Child-Pugh score | 9 | 8 | 8 | 7 | 7 | 10 | 6 |
| MELD score | 16 | 12 | 12 | 10 | 12 | 20 | 7 |
| Ascites grade | Moderate | Mild | Mild | Mild | Large | Mild | Mild |
| HE present | No | No | Mild | No | No | Mild | No |
Note.—Patient 5 had persistent post TIPS high portosystemic pressure gradient and refractory ascites. All TIPS were Viatorr stent grafts. HE = hepatic encephalopathy, MELD = Model for End-Stage Liver Disease, NASH = nonalcoholic steatohepatitis.
Data are before TIPS placement, and data in parentheses are after TIPS placement.
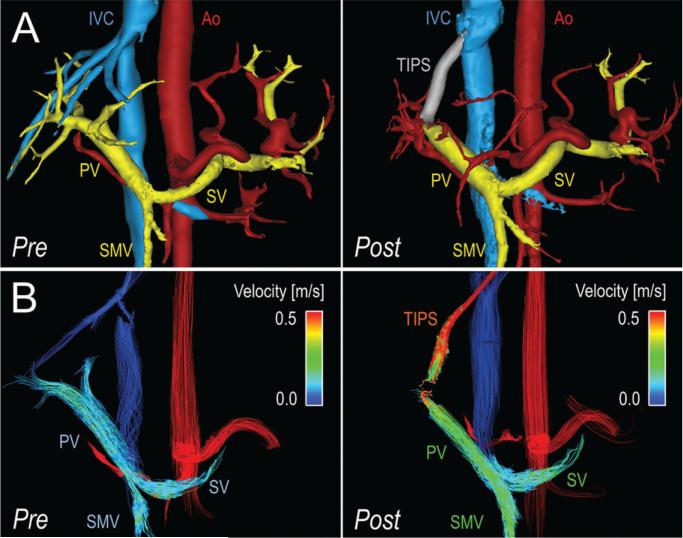
Radial 4D-flow MR imaging allowed visualization of the hepatic vasculature before and after TIPS placement. The TIPS lumen could also be successfully visualized wtih 4D-flow MR imaging. All stent grafts were patent at all imaging time points. Figure 1 shows segmented angiograms in a patient with slow hepatopetal flow before TIPS placement and increased hepatopetal flow 2 and 12 weeks after TIPS placement.
Figure 1.
Four-dimensional–flow MR imaging– based visualization and quantification of hemodynamics in the portal system before and after TIPS placement in a 54-year-old man with portal hypertension and refractory ascites. A, Segmentation of 4D-flow angiograms obtained before (pre) and 2 weeks after (post) TIPS placement show arteries (red), veins (blue), portal vasculature (yellow), and TIPS (gray). B, Velocity-coded 4D-flow MR images obtained before (pre) and 2 weeks after (post) TIPS placement show velocity distribution in the portal circulation, which is indicated by color-coded streamlines that show increased blood flow in the SMV, SV, and PV in response to TIPS placement. Note the high velocity in the TIPS, with a signal dropout at the proximal end of the TIPS due to disordered flow. In this patient (subject 6 in Table 1), TIPS placement successfully reduced ascites but induced hepatic encephalopathy. Ao = aorta, IVC = Inferior vena cava.
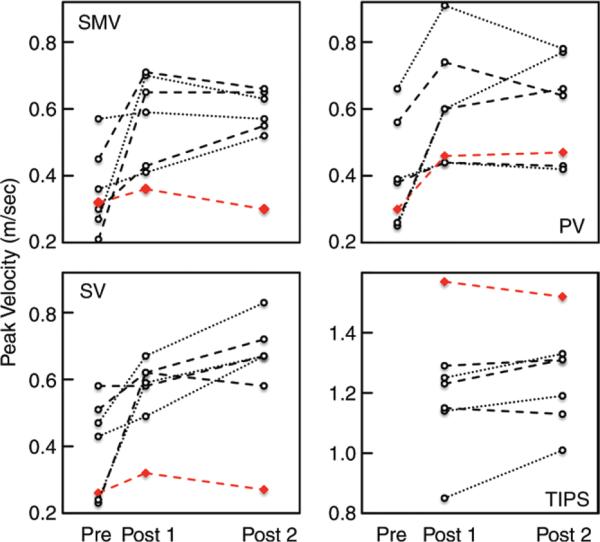
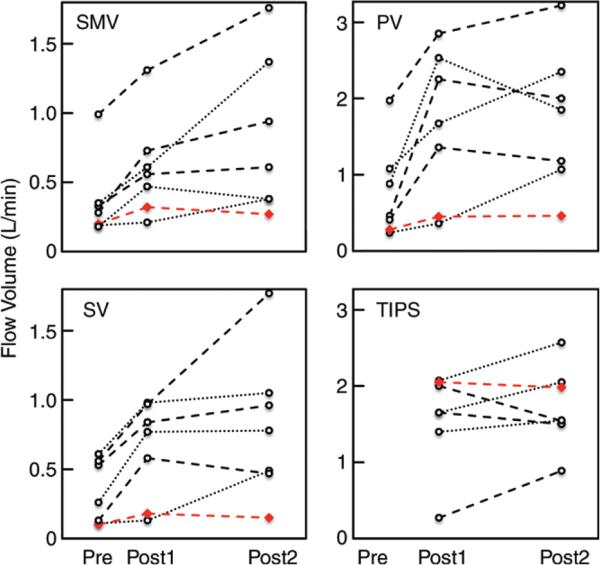
Peak velocity and blood flow volume increased in all patients after TIPS placement (Fig 1B, Movie 1 [online]). The increase in flow volume was significant in the SMV (+0.24 L/min; 95% CI: 0.07, 0.41), SV (+0.31 L/min; 95% CI: 0.07, 0.54), and PV (+0.88 L/min; 95% CI: 0.06, 1.70; all P < .05) 2 weeks after TIPS placement compared with pre-TIPS 4D-flow MR imaging (Table 2). Compared with pre-TIPS 4D-flow MR imaging, the increase in flow volume also remained significant in the SMV (+0.46 L/min; 95% CI: 0.01, 0.90), SV (+0.48 L/min; 95% CI: 0.04, 0.92), and PV (+0.97 L/min; 95% CI: 0.42, 1.52; all P < .05) 12 weeks after TIPS placement. There were no significant differences in peak velocity or flow volume measurements made from the two post-TIPS 4D-flow MR imaging acquisitions (all P > .11). However, the change in peak velocities and blood flow volumes was not uniform in individual patients (Figs 2, 3).
Table 2.
Comparison of Peak Velocities and Flow Volumes before and 2 and 12 Weeks after TIPS Placement
| Time Point | SMV | SV | PV | TIPS |
|---|---|---|---|---|
| Peak Velocity (m/sec) |
||||
| Pre | 0.35 ± 0.12 | 0.39 ± 0.14 | 0.40 ± 0.16 | ... |
| Post 1 | 0.55 ± 0.15 | 0.56 ± 0.12 | 0.60 ± 0.18 | 1.21 ± 0.21 |
| Post 2 | 0.55 ± 0.12 | 0.63 ± 0.18 | 0.60 ± 0.16 | 1.26 ± 0.16 |
| Post 1 to pre* | 0.20 (−0.03, 0.42) | 0.17 (−0.02, 0.36) | 0.20 (0.05, 0.35) | ... |
| P value | .089 | .081 | .015† | ... |
| Post 2 to pre* | 0.20 (−0.01, 0.41) | 0.24 (0.01, 0.48) | 0.20 (−0.04, 0.43) | ... |
| P value | .064 | .046† | .102 | ... |
| Post 2 to post 1* | 0.01 (−0.09, 0.10) | 0.07 (−0.04, 0.19) | −0.01 (−0.13, 0.12) | 0.05 (−0.02-0.11) |
| P value | 1.00 | .211 | 1.00 | .136 |
|
Flow Volume (L/min) |
||||
| Pre | 0.36 ± 0.29 | 0.33 ± 0.23 | 0.76 ± 0.62 | ... |
| Post 1 | 0.60 ± 0.36 | 0.64 ± 0.36 | 1.64 ± 0.98 | 1.58 ± 0.63 |
| Post 2 | 0.82 ± 0.57 | 0.81 ± 0.53 | 1.73 ± 0.92 | 1.73 ± 0.53 |
| Post 1 to pre* | 0.24 (0.07, 0.41) | 0.31 (0.07, 0.54) | 0.88 (0.06-1.70) | |
| P value | .011† | .015† | .037† | ... |
| Post 2 to pre* | 0.46 (0.01, 0.90) | 0.48 (0.04, 0.92) | 0.97 (0.42-1.52) | ... |
| P value | .046† | .034† | .003† | ... |
| Post 2 to post 1* | 0.21 (−0.15, 0.59) | 0.17 (−0.22, 0.56) | 0.09 (−0.55-0.74) | 0.14 (−0.22, 0.50) |
| P value | .327 | .565 | 1.00 | .375 |
Note.—Unless otherwise indicated, data are mean plus or minus standard deviation. Reported P values are adjusted for three independent t tests. P < .05 indicates a significant difference. Pre = before TIPS placement, Post 1 = 2 weeks after TIPS placement, Post 2 = 12 weeks after TIPS placement.
Data are mean differences, and data in parentheses are 95% CIs.
P < .05.
Figure 2.
Graphs show quantitative analysis of blood flow peak velocities before (pre), 2 weeks after (post 1), and 12 weeks after (post 2) TIPS placement. Peak velocities were determined in the SMV (top left), PV (top right), SV (bottom left), and TIPS stent (bottom right). Red line = patient with refractory ascites after TIPS placement.
Figure 3.
Graphs show quantitative analysis of blood flow volumes before (pre), 2 weeks after (post 1), and 12 weeks after (post 2) TIPS placement. Flow volumes were quantified in the SMV (top left), PV (top right), SV (bottom left), and TIPS stent (bottom right). Red line = patient with refractory ascites after TIPS placement.
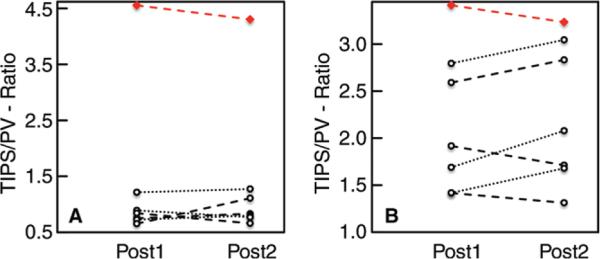
The highest observed TIPS-to-PV peak velocity ratio (first post-TIPS examination, 3.4; second post-TIPS examination, 3.2) was measured in the patient with refractory ascites, although the difference of this TIPS-to-PV peak velocity ratio (2.0 ± 0.5; range, 0.5–2.8) compared with the average in the six remaining patients (2.1 ± 0.6; range, 0.6–3.0) was not striking (Fig 4A). However, the TIPS-to-PV flow volume ratio in patients with successfully resolved ascites was substantially lower (for the first post-TIPS examination, 0.8 ± 0.2 and range of 0.7–1.2; for the second post-TIPS examination, 0.9 ± 02 and range of 0.7–1.3) than that in the patient with refractory ascites (4.6 and 4.3, respectively) (Fig 4B). In this patient, it is notable that 4D-flow MR imaging depicted arterio-portal-venous shunting in the left liver lobe draining into the portal circulation (Fig 5, Movie 2 [online]).
Figure 4.
Ratio of peak velocity and flow volume in TIPS stent versus the PV. A, Graph shows the TIPS-to-PV ratio of peak velocity, which varied among the seven included patients. The highest ratio was observed in the patient with refractory ascites after TIPS placement (red line). B, Graph shows the TIPS-to-PV-ratio of flow volume in six of seven patients 2 (post 1; average, 0.8 ± 0.2) and 12 (post 2; average, 0.9 ± 0.2) weeks after TIPS placement. A higher flow volume ratio (4.6:4.3) was detected in the patient with refractory ascites after TIPS placement (red line).
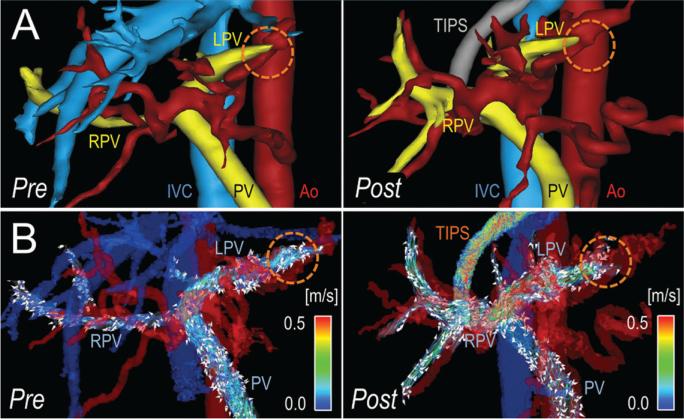
Figure 5.
Idiopathic liver cirrhosis and refractory ascites after TIPS placement in a 46-year-old man. A, Segmentation of 4D-flow angiograms obtained before (pre) and 2 weeks after (post) TIPS placement show the PV, right portal vein (RPV), left portal vein (LPV), inferior vena cava (IVC), and aorta (Ao). B, Velocity-coded 4D-flow MR images show velocity distribution in the portal system, which is indicated by color-coded streamlines that show slow flow in the PV and right portal vein (RPV) and high flow in the left portal vein (LPV). This flow pattern was caused by arterio-portal-venous shunting (dotted orange circle) that drained from a peripheral branch of the left hepatic artery and into a branch of the LPV and induced the highest measured portosystemic gradient of 28 mmHg (patient 5 in Table 1). Because of the shunt, TIPS placement further increased flow in the LPV, resulting in the fastest observed flow in the TIPS, with only a slight reduction of the portosystemic gradient to 18 mmHg. As a result, this patient had refractory ascites, even after TIPS placement.
Discussion
We demonstrated the feasibility of using 4D-flow MR imaging for longitudinal noninvasive monitoring of hepatic blood flow before and after TIPS placement. The use of 4D-flow MR angiograms and the ability to quantify flow measurements in the TIPS and hepatic vasculature allowed for both qualitative and quantitative assessment of the hemodynamic response to TIPS placement. Further, in one patient, arterio-portal-venous shunting was identified with 4D-flow MR imaging, explaining the patient's refractory ascites despite TIPS placement.
As expected, 4D-flow MR imaging depicted increased peak velocities and flow volumes in the PV, SMV, and SV after TIPS placement. Moreover, radial 4D-flow MR imaging allowed visualization of the TIPS lumen and monitoring of TIPS function, confirming the recent results of Stankovic et al (18), who used a Cartesian 4D-flow MR imaging technique. Stankovic et al performed 4D-flow MR imaging at a single time point 4 weeks after TIPS placement. However, complications, such as hepatic encephalopathy, may occur as late as 3 months after TIPS placement (6). Therefore, we compared results of 4D-flow MR imaging not only before and after TIPS placement, but also at two time points after TIPS placement to allow for longitudinal monitoring of hepatic blood flow.
We further extended the findings of Stankovic et al (18) by comparing the total flow volume in the TIPS relative to the flow volume in the portal vein, allowing calculation of the TIPS-to-PV flow ratio, which may serve as a metric of shunt fraction and the hemodynamic effects of the TIPS. The TIPS-to-PV flow ratio may also be useful to evaluate the risk for hepatic encephalopathy, refractory ascites, and future variceal bleeding. The TIPS-to-PV flow ratio was 0.8–0.9 in most cases, indicating that 80%–90% of the portal flow is diverted through the TIPS and 10%–20% is diverted toward the liver.
The calculated TIPS-to-PV flow ratio was markedly different in one patient with arterio-portal shunting (4.3–4.6) relative to those of the other six patients. This patient had refractory ascites despite having undergone TIPS placement. Instead, arterial blood shunting into the left PV rapidly shunted through the TIPS and into the systemic venous system. Portal pressure remained high, and the patient's ascites did not resolve. Future studies are needed to determine cutoff points in the TIPS-to-PV flow ratio to help predict encephalopathy, variceal bleeding, or refractory ascites.
Our observations on the use of 4D-flow MR imaging are concordant with those from a recent study on 4D Doppler US (15). These authors concluded that shunt volume measurements provide an alternative metric to quantify shunt function and that an increased shunt fraction corresponds to a decreased pressure gradient (15). Future and larger studies are needed to determine whether the TIPS-to-PV flow ratio measured with 4D-flow MR imaging may be useful for predicting the risk for hepatic encephalopathy and refractory ascites and whether TIPS revision is needed due to in-stent stenosis or thrombosis. Ideally, these studies should include both 4D Doppler US and 4D-flow MR imaging and compare the quantitative measurement results of both techniques.
This pilot study has important clinical implications. If the TIPS-to-PV ratio determined with 4D-flow MR imaging will allow for the detection, or even prediction, of TIPS-related complications, it may be used as a valuable adjunct to Doppler US. The large volumetric coverage of 4D-flow MR imaging overcomes known limitations of Doppler US, such as a limited acoustic window and a limited capability to resolve complex vascular structures, such as convoluted varices (11).
Prior to TIPS placement, 4D-flow MR imaging allows for comprehensive assessment of the pathologic changes induced by portal hypertension and, thereby, may be used to guide treatment planning for TIPS placement (16,17). After TIPS placement, 4D-flow MR imaging holds promise for monitoring TIPS function and altered complex hemodynamics. Further validation is warranted to fully assess the utility of quantitative 4D-flow MR imaging and its role relative to Doppler US and trans jugular venography with invasive pressure measurements.
This study has several limitations. First, it was a small pilot study in a limited number of patients. Second, it was necessary to repeat 4D-flow MR imaging back-to-back with two different velocity encoding settings to allow for optimal assessment of both slow flow in the portal vein and high flow velocities within the TIPS. The application of recently developed dual-velocity encoding strategies to improve the range of velocity encoding should be used in future studies (25). Four-dimensional– flow MR imaging was also performed at different times of the day, a factor that is known to influence portal flow.
Further, semiautomatic segmentation, as was used here, is subjective and may result in nonvisualization of some small vessels. Another limitation is the manual placement of cut-planes, which may affect the quantitative results of flow measurements. Finally, a reference standard was not available for quantification of blood flow in the portal circulation. However, 4D-flow MR imaging flow measurements were previously and extensively validated in flow phantoms and in vivo compared with two-dimensional phase-contrast MR imaging, Doppler US, and animal studies (25–31). In the human abdomen, 4D-flow MR imaging was validated on the basis of conservation of mass principles (16,27). It should also be noted that invasive pressure measurements were not performed, a limitation that should be addressed in future studies.
Further, we did not assess the potential of the stent mesh to interfere with the accuracy of 4D-flow MR imaging measurements. A recent phantom study showed that in-stent flow quantification varies depending on the type of stent used; however, a covered TIPS stent was not assessed (32). Future phantom studies are needed to assess this issue. Lastly, we did not encounter any patient with a TIPS stenosis or occlusion, which may be the biggest complication of TIPS. Future studies with a longer follow-up period and invasive angiography as a reference standard are needed to address the assessment of TIPS stenosis or occlusion with 4D-flow MR imaging. General limitations of 4D-flow MR imaging are its long imaging times, expense, motion artifacts, long postprocessing times, and operator-dependent manual placement of cut planes.
In summary, 4D-flow MR imaging can provide comprehensive noninvasive longitudinal monitoring of hepatic blood flow before and after TIPS placement. Wide anatomic coverage and comprehensive visualization of abdominal vasculature make 4D-flow MR imaging a potential alternative to current techniques for monitoring the complex hemodynamics of the liver, including TIPS function and patency. Further prospective studies are warranted to determine whether 4D-flow MR imaging can play an important clinical role, such as predicting the risk for TIPS-related complications, and whether it can provide clinically relevant information beyond that available with US.
Supplementary Material
Advances in Knowledge.
■ The use of four-dimensional (4D)–flow MR imaging is feasible for longitudinal noninvasive monitoring of hepatic blood flow before and after transjugular portosystemic shunt (TIPS) placement.
■ Four-dimensional–flow MR imaging angiograms and flow measurements provide both angiographic and quantitative flow assessment of the hepatic hemodynamic response to TIPS implantation.
■ Four-dimensional–flow MR imaging not only provides assessment of peak velocity, but also volumetric flow quantification through the TIPS, providing a measurement of the ratio of TIPS to portal venous flow.
Implications for Patient Care.
■ Prior to TIPS placement, 4D-flow MR imaging may provide comprehensive assessment of flow alterations induced by portal hypertension and has the potential to guide treatment decisions regarding TIPS placement.
■ Volumetric flow measurements obtained with 4D-flow MR imaging provide a potential alternative to standard pulsed-wave Doppler velocity measurements for evaluating TIPS patency and identifying cases that require revision.
Acknowledgments
Supported by the University of Wisconsin-Madison Research and Development Fund (1201-001).
Abbreviations
- CI
confidence interval
- 4D
four-dimensional
- PV
portal vein
- SMV
superior mesenteric vein
- SV
splenic vein
- TIPS
transjugular intrahepatic portosystemic shunt
Footnotes
Online supplemental material is available for this article.
Author contributions:
Guarantors of integrity of entire study, P.B., O.O., S.B.R.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; approval of final version of submitted manuscript, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, P.B., A.R.A., M.A.W., U.M., O.W., S.B.R., H.K.; clinical studies, P.B., A.R.A., K.M.J., M.A.W., O.O., U.M., S.B.R., H.K.; experimental studies, A.R.A., K.M.J., M.A.W., O.W., H.K.; statistical analysis, P.B., U.M.; and manuscript editing, all authors
Disclosures of Conflicts of Interest: P.B. disclosed no relevant relationships. A.R.-A. disclosed no relevant relationships. K.M.J. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: University of Madison receives research support from GE Healthcare. M.A.W. disclosed no relevant relationships. O.O. disclosed no relevant relationships. U.M. disclosed no relevant relationships. O.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: research agreement with GE Healthcare. Other relationships: disclosed no relevant relationships. S.B.R. Activities related to the present article: grant from Bracco Diagnostics and research support for the University of Wisconsin from GE Healthcare. Activities not related to the present article: stock ownership in Neuwave Medical and Cellectar Biosciences and consultant for Parexel International. Other relationships: disclosed no relevant relationships. H.K. disclosed no relevant relationships.
References
- 1.Bosch J, García-Pagán JC. Complications of cirrhosis. I. Portal hypertension. J Hepatol. 2000;32(1,Suppl):141–156. doi: 10.1016/s0168-8278(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362(9):823–832. doi: 10.1056/NEJMra0901512. [Published correction appears in N Engl J Med 2011;364(5):490. Dosage error in article text.] [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G. The transjugular intrahepatic portosystemic shunt for the management of cirrhotic refractory ascites. Nat Clin Pract Gastroenterol Hepatol. 2006;3(7):380–389. doi: 10.1038/ncpgasthep0523. [DOI] [PubMed] [Google Scholar]
- 4.Boyer TD, Haskal ZJ. American Association for the Study of Liver Diseases. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51(1):306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 5.Riggio O, Nardelli S, Moscucci F, Pasquale C, Ridola L, Merli M. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2012;16(1):133–146. doi: 10.1016/j.cld.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Bai M, Qi X, Yang Z, et al. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systematic review. J Gastroenterol Hepatol. 2011;26(6):943–951. doi: 10.1111/j.1440-1746.2011.06663.x. [DOI] [PubMed] [Google Scholar]
- 7.Perarnau JM, Le Gouge A, Nicolas C, et al. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014;60(5):962–968. doi: 10.1016/j.jhep.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Li X, Wei B, et al. Recurrent variceal bleeding and shunt patency: prospective randomized controlled trial of transjugular intrahepatic portosystemic shunt alone or combined with coronary vein embolization. Radiology. 2013;268(3):900–906. doi: 10.1148/radiol.13120800. [DOI] [PubMed] [Google Scholar]
- 9.Berlioux P, Robic MA, Poirson H, et al. Pre-transjugular intrahepatic portosystemic shunts (TIPS) prediction of post-TIPS overt hepatic encephalopathy: the critical flicker frequency is more accurate than psychometric tests. Hepatology. 2014;59(2):622–629. doi: 10.1002/hep.26684. [DOI] [PubMed] [Google Scholar]
- 10.Thabut D, Moreau R, Lebrec D. Noninvasive assessment of portal hypertension in patients with cirrhosis. Hepatology. 2011;53(2):683–694. doi: 10.1002/hep.24129. [DOI] [PubMed] [Google Scholar]
- 11.Owens CA, Bartolone C, Warner DL, et al. The inaccuracy of duplex ultrasonography in predicting patency of transjugular intrahepatic portosystemic shunts. Gastroenterology. 1998;114(5):975–980. doi: 10.1016/s0016-5085(98)70317-8. [DOI] [PubMed] [Google Scholar]
- 12.Feldstein VA, Patel MD, LaBerge JM. Transjugular intrahepatic portosystemic shunts: accuracy of Doppler US in determination of patency and detection of stenoses. Radiology. 1996;201(1):141–147. doi: 10.1148/radiology.201.1.8816535. [DOI] [PubMed] [Google Scholar]
- 13.Murphy TP, Beecham RP, Kim HM, Webb MS, Scola F. Long-term follow-up after TIPS: use of Doppler velocity criteria for detecting elevation of the portosystemic gradient. J Vasc Interv Radiol. 1998;9(2):275–281. doi: 10.1016/s1051-0443(98)70269-6. [DOI] [PubMed] [Google Scholar]
- 14.Itkin M, Trerotola SO, Stavropoulos SW, et al. Portal flow and arterioportal shunting after transjugular intrahepatic portosystemic shunt creation. J Vasc Interv Radiol. 2006;17(1):55–62. doi: 10.1097/01.RVI.0000191362.75969.F6. [DOI] [PubMed] [Google Scholar]
- 15.Pinter SZ, Rubin JM, Kripfgans OD, et al. Volumetric blood flow in transjugular intrahepatic portosystemic shunt revision using 3-dimensional Doppler sonography. J Ultra-sound Med. 2015;34(2):257–266. doi: 10.7863/ultra.34.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roldán-Alzate A, Frydrychowicz A, Niespodzany E, et al. In vivo validation of 4D flow MRI for assessing the hemodynamics of portal hypertension. J Magn Reson Imaging. 2013;37(5):1100–1108. doi: 10.1002/jmri.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stankovic Z, Csatari Z, Deibert P, et al. Normal and altered three-dimensional portal venous hemodynamics in patients with liver cirrhosis. Radiology. 2012;262(3):862–873. doi: 10.1148/radiol.11110127. [DOI] [PubMed] [Google Scholar]
- 18.Stankovic Z, Rössle M, Euringer W, et al. Effect of TIPS placement on portal and splanchnic arterial blood flow in 4-dimensional flow MRI. Eur Radiol. 2015;25(9):2634–2640. doi: 10.1007/s00330-015-3663-x. [DOI] [PubMed] [Google Scholar]
- 19.Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 20.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55(Suppl 6):vi1–vi12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson KM, Markl M. Improved SNR in phase contrast velocimetry with five-point balanced flow encoding. Magn Reson Med. 2010;63(2):349–355. doi: 10.1002/mrm.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein MA, Zhou XJ, Polzin JA, et al. Concomitant gradient terms in phase contrast MR: analysis and correction. Magn Reson Med. 1998;39(2):300–308. doi: 10.1002/mrm.1910390218. [DOI] [PubMed] [Google Scholar]
- 23.Walker PG, Cranney GB, Scheidegger MB, Waseleski G, Pohost GM, Yoganathan AP. Semiautomated method for noise reduction and background phase error correction in MR phase velocity data. J Magn Reson Imaging. 1993;3(3):521–530. doi: 10.1002/jmri.1880030315. [DOI] [PubMed] [Google Scholar]
- 24.Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med. 2008;60(6):1329–1336. doi: 10.1002/mrm.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nett EJ, Johnson KM, Frydrychowicz A, et al. Four-dimensional phase contrast MRI with accelerated dual velocity encoding. J Magn Reson Imaging. 2012;35(6):1462–1471. doi: 10.1002/jmri.23588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frydrychowicz A, Wieben O, Niespodzany E, Reeder SB, Johnson KM, François CJ. Quantification of thoracic blood flow using volumetric magnetic resonance imaging with radial velocity encoding: in vivo validation. Invest Radiol. 2013;48(12):819–825. doi: 10.1097/RLI.0b013e31829a4f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wentland AL, Grist TM, Wieben O. Repeatability and internal consistency of abdominal 2D and 4D phase contrast MR flow measurements. Acad Radiol. 2013;20(6):699–704. doi: 10.1016/j.acra.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frydrychowicz A. Proceedings of the Nineteenth Meeting of the International Society for Magnetic Resonance in Medicine. International Society for Magnetic Resonance in Medicine; Berkeley, Calif: 2011. In-vivo validation of 5-point PC-VIPR for hemodynamic assessment of the hepatic and splanchnic hemodynamics in swine [abstr]. [Google Scholar]
- 29.Chang W, Landgraf B, Johnson KM, et al. Velocity measurements in the middle cerebral arteries of healthy volunteers using 3D radial phase-contrast HYPRFlow: comparison with transcranial Doppler sonography and 2D phase-contrast MR imaging. AJNR Am J Neuroradiol. 2011;32(1):54–59. doi: 10.3174/ajnr.A2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang J, Strother C, Johnson K, et al. Comparison of blood velocity measurements between ultrasound Doppler and accelerated phase-contrast MR angiography in small arteries with disturbed flow. Phys Med Biol. 2011;56(6):1755–1773. doi: 10.1088/0031-9155/56/6/015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bley TA, Johnson KM, François CJ, et al. Noninvasive assessment of transstenotic pressure gradients in porcine renal artery stenoses by using vastly undersampled phase-contrast MR angiography. Radiology. 2011;261(1):266–273. doi: 10.1148/radiol.11101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunck AC, Jüttner A, Kröger JR, et al. 4D phase contrast flow imaging for in-stent flow visualization and assessment of stent patency in peripheral vascular stents--a phantom study. Eur J Radiol. 2012;81(9):e929–e937. doi: 10.1016/j.ejrad.2012.05.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.