Abstract
Fear is an important emotional reaction in response to threatening stimuli and is important for survival. However, when fear occurs in inappropriate circumstances then it can lead to pathological conditions including an increased vulnerability for developing anxiety disorders such as post-traumatic stress disorder (PTSD). Patients with PTSD generalize fear to contexts or to environments that are not associated with the trauma. We sought to explore if increasing the level of dissimilarity relative to the context in which mice learn fear results in changes in the level of fear responding to the new context. We also determined with this procedure if the number of cells expressing the immediate early gene cfos changes with the corresponding level of expressed fear within brain regions known to be important in modulating fear, including the basolateral amygdala (BLA) and hippocampus. Our results indicate that mice that were tested in increasingly different contexts showed significantly different levels of fear responses. Freezing level was higher in the context most similar to the acquisition context than the one that was highly different. The level of cfos within the BLA, but not hippocampus was also significantly different between the test contexts, with higher levels in the somewhat similar compared to the most different context. Overall, these results highlight the BLA as a critical region in the node of fear circuitry for modulating fear generalization.
summary
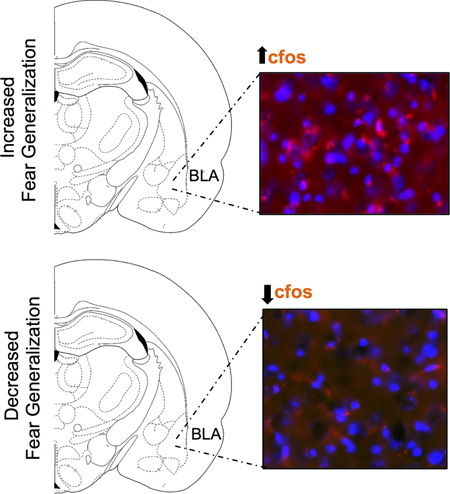
While fear is critical for survival to react of threatening stimuli, inappropriately high levels of fear lead to pathological conditions including post-traumatic stress disorder (PTSD). Using enhanced fear in a rodent model, our findings show that mice that acquire an enhanced level of fear show significantly higher level of freezing in a novel context that most closely resembles the context where fear was originally acquired. In these mice, neuronal activity is also augmented within the basolateral nuclei of the amygdala (BLA). However, freezing and levels of neuronal activity were lower in the context that was most dissimilar to the fear acquisition context. This indicates that BLA is an important region in which enhanced fear generalization leads to increased neuronal activity.
Graphical abstract
INTRODUCTION
Fear is a natural emotional reaction to any threatening stimulus and important for survival and to protect us from imminent danger. Disproportionate levels of fear can however, lead to pathological conditions and increased vulnerability for developing anxiety-related disorders such as post-traumatic stress (PTSD) (Orr et al., 2002; Pitman et al., 2012). Fear generalization, a phenomenon through which fear is transferred from a stimulus associated with an aversive event to a similar stimulus, is a characteristic of many anxiety disorders (Grillon and Morgan, 1999; Rothbaum and Davis, 2003; Milad et al., 2006; Jovanovic et al., 2010; Norrholm et al., 2011; Sijbrandij et al., 2013; Lissek et al., 2014; Bowers and Ressler, 2015). Hypervigilance, re-experiencing and avoidance are relevant to fear generalization especially in PTSD (Kessler et al., 1995; American Psychiatric Association, 2000; Yehuda, 2001; Yehuda and LeDoux, 2007; Lissek and Grillon, 2010; Association, 2013).
Fear becomes associated with stimuli through Pavlovian fear conditioning (FC). To study this associative process in laboratory animals a neutral stimulus (conditional stimulus, CS), such as a tone, is paired with an aversive stimulus (unconditional stimulus, US), such as a footshock. After this occurs the CS elicits defensive responses such as freezing (Fanselow et al., 1988; Rosen and Schulkin, 1998; Fanselow and Wassum, 2016). Therefore, the time spent freezing provides a good indication of fear in rodents (Fanselow, 1980). Inappropriately high levels of freezing suggest levels of fear that could interfere with normal adaptive functions. In mice, fear generalization can be measured by training animals to fear one context and then testing them in a different context by measuring freezing levels in that context. As mice are exploratory by nature in a novel environment, higher level of freezing in the novel context indicates higher fear. Due to similarities in the fear circuity in rodents and humans, investigation of the neural circuitry involved in fear generalization in mice is likely to have translational significance (Fendt and Fanselow, 1999; Milad et al., 2006; Mineka and Oehlberg, 2008).
Fear can trigger multiple cellular and molecular cascades including expression of the immediate early gene, cfos. Changes in levels of cfos are extensively used as a proxy for neuronal activity and analyzing cfos levels within a brain region provides an indirect measure of cellular activity in that region. Processing of context memory requires both emotional and context components, with the amygdala and hippocampus being critical nodes in the respective neural circuits (Davis, 1992; Kim and Fanselow, 1992; Bechara et al., 1995; Maren and Fanselow, 1996; Fanselow and Gale, 2003; LeDoux, 2003; Kim and Jung, 2006; Shin and Liberzon, 2010).
In the present study, we first sought to investigate if mice demonstrate different levels of freezing, as indicative of different level of fear generalization, in contexts that are increasingly different from the one in which they acquired the fear learning. Secondly, we wanted to determine if differences in graded fear generalization lead to changes in expression levels of cfos within the basolateral amygdala (BLA) and hippocampus in a manner that corresponds to the level of freezing. We hypothesized that the level of freezing would be linearly graded across the three increasingly dissimilar contexts and levels of cfos within the BLA and hippocampus would match the level of freezing.
MATERIALS AND METHODS
Subjects
A total of 20 male mice PACAP-EGFP mice (n=20) (3–4 months) expressing enhanced green fluorescent protein in PACAP-containing neurons were housed in plastic clear cages in the vivarium with lights on at 7 AM and lights off at 7 PM (Condro et al., 2016). These mice were backcrossed backcrossed from FVB/NTac to C57BL/6 for at least five generations, leading them to be used like wild-type mice. Previous studies have shown dysregulation in the neuropeptide PACAP in PTSD patients (Ressler et al., 2011). Although it was not a current focus of the experiments presented here, experiments in the future will explore whether there are changes in levels of cfos specifically within PACAP-containing cells in the fear circuitry. Experiments were performed between 10 AM and 3 PM. The mice were kept on ad libitum access to food and water in a light- and temperature-controlled vivarium. All experimental procedures were in accordance with the Animal Research Committee at the University of California, Los Angeles.
Behavioral procedure
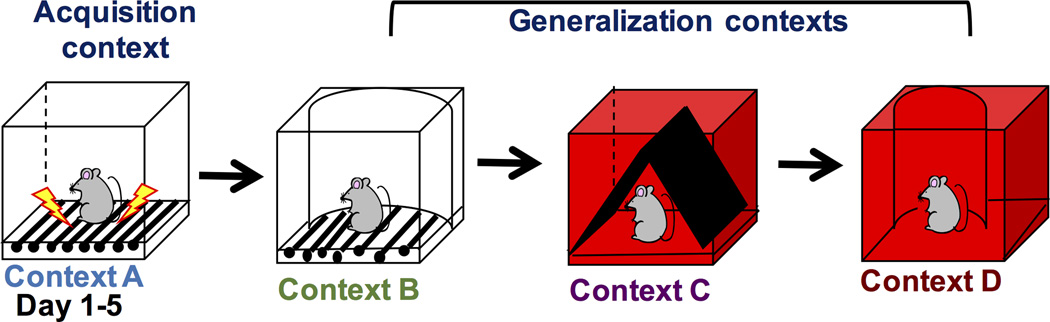
Conditioning Apparatus: The conditioning apparatus consisted of 4 sound and light attenuated conditioning boxes (Med Associates Inc., Georgia, VT) and mice were run individually in the conditioning boxes (Fig. 1). The conditioning boxes were equipped with Near Infra-Red (NIR) Video Fear Conditioning System and could be configured to represent different contexts by changing the internal structure, floor texture, illumination, and odor. We used Context A (28 × 21 × 21 cm) with a clear Plexiglas back wall, ceiling, and front door with aluminum sidewalls. Context A consisted of a grid floor with evenly spaced and stainless steel rods and cleaned and scented with 50% Windex. The floor in context A was connected to a shocking apparatus, which delivered a scrambled foot shock. Context B had a clear Plexiglas back wall, ceiling, and door with aluminum sidewalls. The inner structure of the chamber was altered by adding a white curved sidewall that extended across the back wall. The floors of context B consisted of grid floor with stainless steel rods that were evenly spaced but at alternating heights and cleaned and scented with 1 % acetic acid solution. Context C consisted of triangular opaque black Plexiglas sidewalls at an angle of 60° to the floor, with an acrylic white board floor. The context was cleaned and scented with 50 % Simple Green and illuminated with just red light. Context D consisted of white acrylic flooring, a white curved sidewall that extended to only one side of the wall. This context was cleaned and odored with 70% isopropyl alcohol and illuminated with just red lights.
Figure 1.
Behavioral testing procedures. Three groups of animals went through fear acquisition first in context A, and then were separately tested in increasingly different contexts B, C, or D.
Measure of freezing
Freezing is defined as the lack of movement except for respiration (Fanselow, 1980). The software (VideoFreeze, Med-Associates Inc.) performed real-time video recordings at 30 frames per second using a set threshold level that has been previously validated to match human scored freezing (Anagnostaras et al., 2001). Each frame has an "activity unit" score and based on previously validated hand scoring measures, freezing was defined as subthreshold activity, i.e. when the motion threshold held at 50 activity units for longer than 1 sec. Percentage freezing=Time Freezing/Total time ×100. Data are presented as mean percentages (+/− SEM).
Fear generalization
The behavioral testing procedure was divided into two parts. The experimental design is shown in Figure 1. The first part involved fear acquisition, in which mice (n=20) were placed in context A every day and subjected to a 0.65 mA, 1-second foot shock after 4 minutes. Freezing in this context was measured every day for 5 days until all the mice displayed an asymptotic level of freezing. Post-shock reactivity to shocks or activity bursts was also measured by analyzing activity bursts after the shock (Fanselow, 1982). After subjects acquired fear, they were divided randomly into three groups (First group n=6; Second group n=7; and Third group n=7) and each group went to one of the three-generalization context a day after fear acquisition and their freezing was measured for 8 minutes. After all groups acquired an asymptotic level of fear, 24 hours later each group were tested in a different generalization test context once (Fig. 1). The first group (n=6) went through a fear generalization context, which consisted of a different grid floors, scent used, transport and their freezing was measured for 8 minutes. The second group (n=7) was placed in a context C that was quite different from the acquisition context, with the grid floor covered with a while plastic board. The last group (n=7) was placed in another context D, which was most different. Ninety minutes after the generalization test, all the animals were anesthetized and perfused with phosphate-buffered saline (PBS) containing 4% paraformaldehyde and their brains kept in the paraformaldehyde for a day in 4 degrees Celsius and transferred into 30% sucrose solution before slicing.
Immunohistochemistry
For immunohistochemistry, brains were cryoprotected, and 40-micrometer coronal sections were collected serially containing the hippocampus and the BLA. Positive cfos immunolabeling was analyzed and quantified brain sections containing the BLA and hippocampus. We chose 3 representative slices from the anterior (Bregma +2.46), middle (Bregma +1.94) and posterior (Bregma +1.62) (Paxinos, 1998) regions immunohistochemistry. On day 1, tissue sections were washed in 1×TBS three times for five minutes, then blocked in 1mL of 1×TBS with 5% Normal Donkey Serum, 0.1% BSA and 0.3% Triton-X for 1 hour. Then the tissue sections were incubated overnight at 4 degrees Celsius with the primary goat polyclonal to cfos (1:500, 24 h, abcam; RRID: SCR_012931) primary antibody. According to the manufacturer, this antibody is a ‘synthetic peptide conjugated to Blue Carrier Protein by a Cysteine residue linker corresponding to the internal sequence amino acids 283–295 of Human c-Fos (NP_005243.1)’. On the second day, the sections were washed in 1×TBS three times five minutes each and then incubated in the Alexa 594 donkey anti-goat secondary antibody (1:200, 2 H, Life Technologies) for 2 hours at room temperature. After washing with 1×TBS for 3 times 5 minutes each, tissue sections were mounted on glass slides and cover-slipped using Prolong Gold (Thermo Fisher Scientific) with 4',6-diamidino-2-phenylindole (DAPI) and the edges were sealed with clear nail polish.
The tissue sections were analyzed using Keyence BZ-X700 -All-in-One Fluorescence Microscope. Images were analyzed with Fiji image processing software (NIH, Bethesda, MD; RRID: SCR_002285). Images were converted to binary mode (black and white image). Two experimenters blind to the experimental conditions counted the cells. Number of positively cfos labeled neurons within a defined square region (320 × 320 micrometer) that was held constant within a brain region of interest. The signal density was calculated by setting density threshold to 80 and acceptable particle size was (0.5–80) for immunoreactivity pixel sizes and circularity of the cfos labeled cells at a level that allowed to automatically subtract background. For each animal, cfos cells were counted from both hemispheres and averaged over 3 sections. Consistency in counting was also verified by another investigator.
Statistical Analysis
Separate groups of mice were used for each generalization test and their percentage freezing levels were analyzed with a two-factor analysis of variance (ANOVA) for the acquisition and with a between subjects-ANOVA for the generalization data. Significant effects indicated by the ANOVA were further analyzed with a post-hoc Bonferroni correction. The level of significance used for all analyses was p < .05.
RESULTS
All groups of animals acquired an asymptotic level of freezing
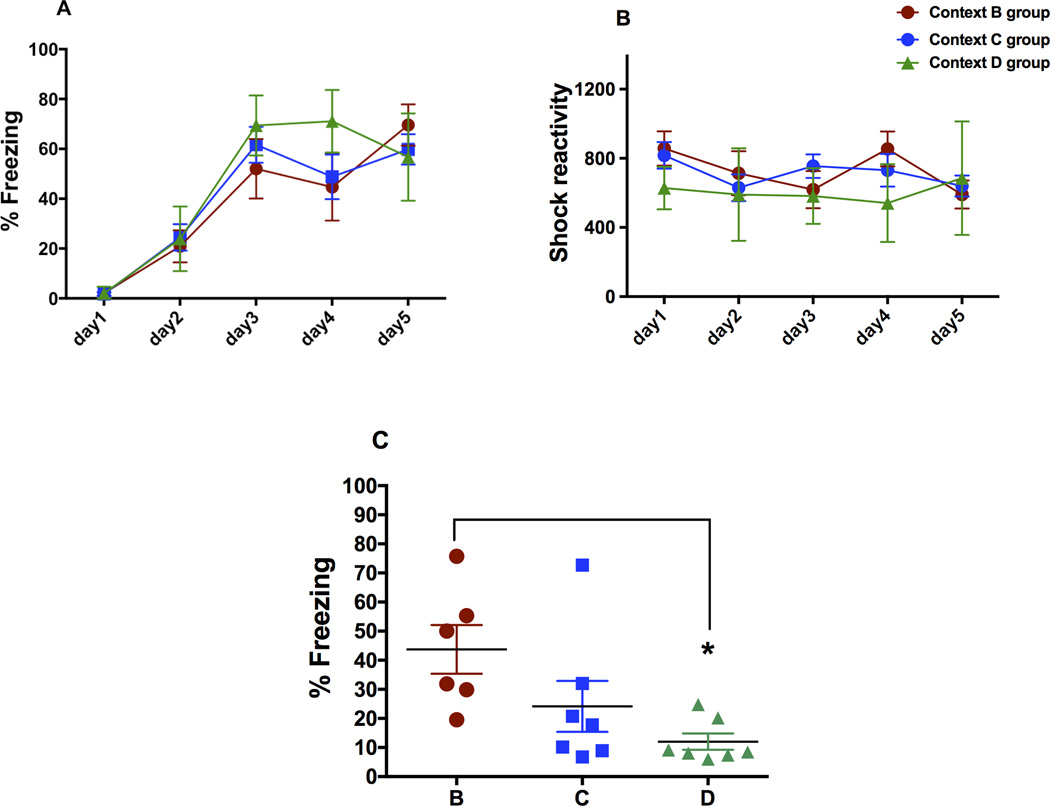
As shown in Fig 2, all three randomly divided groups of animals acquired asymptotic levels of freezing. A two-factor analysis of variance revealed that there was no main effect of group but a main effect of day on the acquired fear in all three groups (P<0.05). Post-hoc analysis revealed that day 1 was significantly different from day 5 freezing in all three groups (P<0.05). We also analyzed post-shock reactivity in all the groups (Fig 2B), which revealed that the animals did not differ from each other in their reactivity to shock.
Figure 2.
Mean percent freezing during acquisition, shock reactivity, and mean percent freezing during generalization tests. A) Mean percent freezing (+/− SEM) during the first 4 minutes of the acquisition before delivery of shock in three groups (context B group, n=6; context C group, n=7 and context D group, n=7). Between subjects-ANOVA revealed that the three groups did not differ from each other in fear acquisition. B) Post-shock reactivity to shock during acquisition in the three groups was not different. C) Mean percent freezing (+/− SEM) of the three groups of mice during the 8-minute context test in three varying contexts B, C and D. *Post-hoc analysis revealed that mean percent freezing in context B was significantly different from context D (P<0.05).
Percent freezing was significantly higher in the group tested in context B versus the group tested in context D
Between subjects-ANOVA revealed that there was a main effect of test context on percent freezing (Fig 2C; F=3.87, P<0.05). Post-hoc test revealed that percent freezing of the group in context B was not significantly different from the group in context C, however, it was significantly different from the group in context D (P<0.05). Groups in context C and D did not differ from each other in their percent freezing.
Increased levels of freezing in the different generalization contexts led to increased number of cfos expressing cells in the BLA but not in the hippocampus
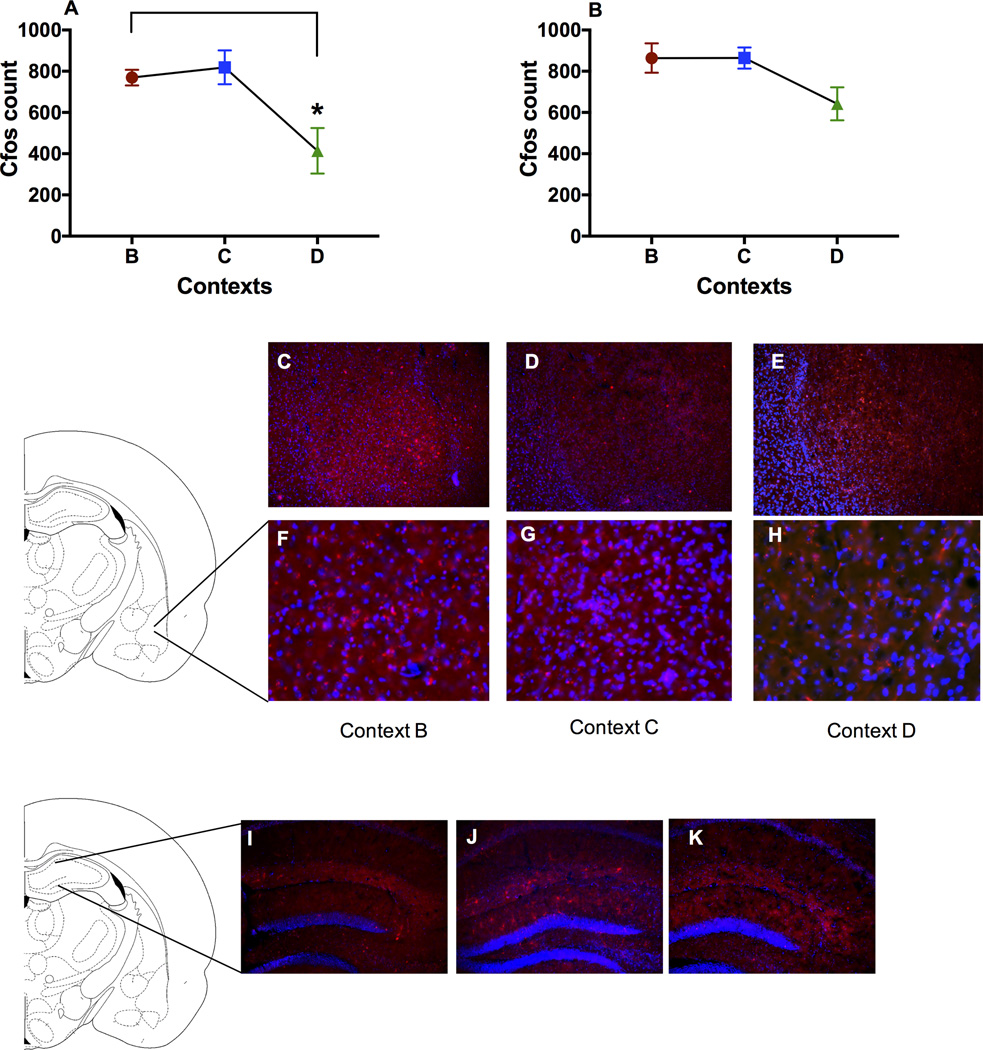
To investigate neuronal activity in the BLA and hippocampus in the fear generalization paradigm, we analyzed number of cfos-expressing cells in these brain regions. Between-subejects-ANOVA revealed that there was a main effect of context in number of cfos positive cells in the BLA (Fig 3A) but not in the hippocampus (Fig 3B). An individual sample T-test revealed that the number of cfos positive cells in the group with highest freezing, that is the group tested in context B, was significantly higher than the group tested in context D, but not different from the group tested in context C. In contrast, ANOVA revealed that there was no main effect of context in the number of cfos positive cells in the hippocampus (Fig 3A, B). T-test revealed cfos expression levels in the BLA were significantly higher than on context D (P<0.05). Again, this was not the case in the hippocampus. The number of cfos positive cells in group in context B (highest freezing, Fig 1C) was significantly higher than the group tested in context D, but not different from the group tested in context C.
Figure 3.
Level of immunoreactivity of cfos positive cells in the BLA and hippocampus of the groups tested in context B, C and D in BLA and hippocampus. A) *Post-hoc analysis revealed that cfos level in context B was significantly higher than in context D in the BLA (P<0.05). B) No difference was found in the level of cfos in the hippocampus. C–H) Representative photomicrographs showing cfos immunoreactivity in the BLA. Top panel (C, D, E) showing BLA at a 10× magnification and bottom insets showing higher magnification images. I–K) Representative photomicrographs showing cfos in the hippocampus.
DISCUSSION
The first goal of the experiments described here was to determine whether level of contextual fear is graded when tested in three contexts that are increasingly dissimilar to the one in which the initial conditioning or learning took place. The second goal of the experiments was to determine whether the level of cfos within the BLA and hippocampus matches the level of expressed fear in the test contexts. Our findings revealed that when mice are conditioned in one context and tested in three increasingly dissimilar contexts than the acquisition context, the level of freezing was significantly higher in the context most similar to the conditioning context compared to the one that was completely dissimilar. Moreover, the level of cfos within the BLA was also significantly different between those two contexts.
Our finding that enhanced fear generalization led to increases in cfos within the BLA fits with the findings that show that heightened activity in the amygdala in anxiety-related disorders including PTSD and in animal models as a result of fear generalization or decreases in fear extinction (Fendt and Fanselow, 1999; Milad et al., 2006; Shin and Liberzon, 2010). Others have shown that this brain region is important for modulating fear and safety signals by working in concert with other regions such as the mPFC (Likhtik and Paz, 2015). BLA is an anatomically situated hub for providing emotional valence to sensory information via its glutamatergic-auditory, taste, visual, and somatosensory inputs/outputs from and to cortical/sub-cortical regions, and expresses some GABAergic interneurons. Fear information is processed by the BLA and sent to the central amygdala, which in turn plays an important role in fear expression via its projections to autonomic and endocrine systems including the hypothalamus, mid-brain and brainstem to elicit behaviors like freezing (LeDoux et al., 1988; LeDoux et al., 1990; Davis, 1992; Fanselow and LeDoux, 1999; LeDoux, 2000; Jovanovic and Ressler, 2010). While studies have shown the importance of the amygdala in fear acquisition, our study adds to our previous findings that showed context fear generalization in rats enhances levels of Arc, another neuronal activity marker within the BLA specific to context fear but unspecifically in the hippocampus (Zelikowsky et al., 2014).
Differential freezing in the different contexts did not lead to a significantly different level of cfos expression in the hippocampus. A plethora of research shows the importance of the dorsal hippocampus (DH) for processing contextual information and the integration of that information into a unified representation of the context that supports differentiation from other contexts (Fanselow, 2000; McHugh et al., 2007; Nakashiba et al., 2012; Krasne et al., 2015). However, this research also shows that the dorsal hippocampus does not create the context-fear association (Fanselow, 2000; Barrientos et al., 2002; Stote and Fanselow, 2004). These data for cfos are consistent with data from another immediate early gene, Activity Regulated Cytoskeletal protein (ARC) within the DH (Zelikowsky et al., 2014), in supporting these theoretical notions. If the dorsal hippocampus supports contextual but not emotional processing, then one would expect similar cfos expression in this region irrespective of what context was being processed. On the other hand, if the BLA is processing the emotional value of the context one would expect, as was found here, that BLA would track emotional responding.
SIGNIFICANCE STATEMENT.
As fear generalization occurs in individuals suffering from anxiety disorders including post-traumatic stress disorder (PTSD), understanding the neurobiology of this phenomenon could help develop effective therapeutics for such disorders. Our results show that basolateral portion of the amygdala is an important region in which enhanced fear generalization leads to increased neuronal activity.
Acknowledgments
This work was supported by National Institute of Mental Health R21MH098506 grant (MSF and JW) and National Research Service Award (AKR) F32 MH10721201A1.
Footnotes
Conflict of Interest Statement
None of the authors of any conflict of interest or financial arrangements pertaining to this work.
Role of Authors
All the authors of this paper had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: MSF, AKR, JW. Acquisition of data: AKR. Analysis and interpretation of data: AKR. Drafting of the manuscript: AKR. Critical revision of the manuscript for important intellectual content: AKR, MSF, JW. Statistical analysis: AKR. Obtained funding: JW, MSF. Administrative, technical, and material support: MSF, JW. Study supervision: JW, MSF
References
- American Psychiatric Association, editor. Washington DC: American Psychiatric Association; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Association AP. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Fifth. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Barrientos RM, O'Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behavioural brain research. 2002;134:299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Ressler KJ. An Overview of Translationally Informed Treatments for Posttraumatic Stress Disorder: Animal Models of Pavlovian Fear Conditioning to Human Clinical Trials. Biol Psychiatry. 2015;78:E15–E27. doi: 10.1016/j.biopsych.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condro MC, Matynia A, Foster NN, Ago Y, Rajbhandari AK, Jayaram B, Parikh S, Diep AL, Nguyen E, May V, Dong HW, Waschek JA. High-resolution characterization of a PACAP-EGFP transgenic mouse model for mapping PACAP-expressing neurons. J Comp Neurol. 2016 doi: 10.1002/cne.24035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Fanselow M. The postshock activity burst. Animal learning and behavior. 1982;10:448–454. [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behavioural brain research. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Gale GD. The amygdala, fear, and memory. Ann N Y Acad Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Wassum KM. The Origins and Organization of Vertebrate Pavlovian Conditioning. Cold Spring Harb Perspect Biol. 2016;8:a021717. doi: 10.1101/cshperspect.a021717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Lester LS, Helmstetter FJ. Changes in feeding and foraging patterns as an antipredator defensive strategy: a laboratory simulation using aversive stimulation in a closed economy. J Exp Anal Behav. 1988;50:361–374. doi: 10.1901/jeab.1988.50-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasne FB, Cushman JD, Fanselow MS. A Bayesian context fear learning algorithm/automaton. Front Behav Neurosci. 2015;9:112. doi: 10.3389/fnbeh.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci. 1990;10:1062–1069. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Paz R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci. 2015;38:158–166. doi: 10.1016/j.tins.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Grillon C. Overgeneralization of conditioned fear in the anxiety disorders. Journal of Psychology. 2010;218:146–148. [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-Sturges T, Reynolds RC, Grillon C. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc Cogn Affect Neurosci. 2014;9:1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Paxinos GF, K BJ. The mouse brain in stereotaxic coordinates. 1998 [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij M, Engelhard IM, Lommen MJ, Leer A, Baas JM. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD) J Psychiatr Res. 2013 doi: 10.1016/j.jpsychires.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Stote DL, Fanselow MS. NMDA receptor modulation of incidental learning in Pavlovian context conditioning. Behav Neurosci. 2004;118:253–257. doi: 10.1037/0735-7044.118.1.253. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62(Suppl 17):41–46. [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci. 2014;34:8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]