Abstract
A recombinant Newcastle disease virus (rNDV) expressing simian immunodeficiency virus (SIV) Gag protein (rNDV/SIVgag) was generated. The rNDV/SIVgag virus induced Gag-specific cellular immune responses in mice, leading to a specific anti-Gag antiviral immunity. This was evidenced by the inhibition of growth of recombinant vaccinia virus expressing an identical Gag antigen (rVac/SIVgag) but not of wild-type vaccinia virus in rNDV/SIVgag-immunized mice. Among intravenous, intraperitoneal, or intranasal immunization routes, intranasal administration induced the strongest protective response against challenge with rVac/SIVgag. We further demonstrated that these immune responses were greatly enhanced after booster immunization with recombinant influenza viruses expressing immunogenic portions of SIV Gag. The magnitude of the protective immune response correlated with the levels of cellular immune responses to Gag, which were still evident 9 weeks after immunization. These results suggest that rNDV and influenza virus vectors are suitable candidate vaccines against AIDS as well as against other infectious diseases.
Cell-mediated immunity has been shown to play an essential role in fighting many virus infections. Cumulative evidence suggests that this may also be the case in human immunodeficiency virus type 1 (HIV-1)-infected individuals (29, 34, 52). HIV-1-specific cytotoxic T lymphocytes (CTLs) have been detected early in the course of infection before the appearance of humoral responses, and they appear to play an important role in the control of the initial viremia (8, 23, 45). In addition, it has been reported that viral load is inversely related to the level of HIV-1-specific CTLs later in infection (35). Moreover, several reports have shown that long-term nonprogressors have higher levels of HIV-specific CTLs than progressors (15, 16, 22, 42). Therefore, the induction of strong HIV-specific CTL responses is likely to be an important factor for the efficacy of an HIV vaccine.
Many different approaches to induce potent HIV CTL responses are currently under investigation. Among these approaches, the use of attenuated viral vectors expressing selected HIV antigens appears to be a promising strategy, based on the strong and long-lasting immune responses elicited by live attenuated viral vaccines in general (reviewed in references 47 and 50). An important consideration concerns the choice of the viral vector, which ideally should be safe and able to induce effective T-cell responses against the expressed antigen. The generation of five recombinant influenza viruses expressing different portions of SIV Gag (rFlu/SIVgag no. 1 to rFlu/SIVgag no. 5) has been described previously, and it has been demonstrated that rFlu/SIVgag no. 3 and rFlu/SIVgag no. 4 viruses induced strong cellular immune responses in systemic and mucosal tissues of immunized mice (31). It has also been shown that cellular immunity against SIV Gag was enhanced by boosting rFlu/SIVgag-immunized mice with a recombinant vaccinia virus expressing SIV Gag (rVac/SIVgag) (31).
In the present study, we describe the immunogenic properties in mice of a second negative-strand RNA virus vector, Newcastle disease virus (NDV), a member of the Avulavirus genus in the Paramyxovirinae family. Infections by NDV are usually limited to avian species, and this virus is categorized into three pathotypes depending on the severity of the disease it causes in birds: lentogenic, mesogenic, and velogenic (48). NDV replication appears to be attenuated in mammalian species due in part to its inability to counteract innate immune responses in these hosts (37). The lack of NDV preexisting immunity in most humans and the fact that NDV infections in humans are only associated with mild self-limiting conjunctivitis (1) prompted us to investigate its potential as a vaccine vector. We have reported that a recombinant NDV (rNDV) based on the lentogenic avirulent vaccine strain Hitchner B1 expressing the influenza virus hemagglutinin (HA) protein (rNDV/B1-HA) induced a humoral immune response which was able to protect against a lethal influenza virus challenge in mice. These results underscore the potential of NDV as an effective vaccine vector in mammals (30). We now describe the generation of an rNDV expressing the full-length SIV Gag protein (rNDV/SIVgag). rNDV/SIVgag virus induces Gag-specific cellular immunity in mice. In addition, we found that combined immunizations in mice with rNDV/SIVgag and rFlu/SIVgag result in a significant boost of the levels of antiviral protective immune responses induced against a surrogate challenge virus, rVac/SIVgag. Furthermore, cellular immune responses to SIV Gag were maintained in immunized mice for at least 9 weeks. These properties warrant further exploration of rNDV vectors for use in vaccine strategies against HIV.
MATERIALS AND METHODS
Cells, viruses, and animals.
P815 cells were used in enzyme-linked immunospot (ELISPOT) assays. Chicken embryo fibroblasts (CEFs) were prepared from 10-day-old specific-pathogen-free embryonated eggs (Charles River SPAFAS, North Franklin, Conn.). CEFs were maintained in minimal essential medium containing 10% fetal bovine serum (FBS). HeLa, CV-1, HEp-2, and Vero cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% FBS. The rNDV/B1 virus was generated previously (30). Wild-type New York City Board of Health vaccinia virus (Vac/wt) and recombinant vaccinia virus expressing SIVmac239 Gag VabT252-51 (rVac/SIVgag) were kindly provided by Gómez Yafal at Therion Biologicals and were grown in HeLa cells. Madin-Darby bovine kidney cells were used for the generation and growth of the chimeric influenza A/WSN/33 (WSN) viruses expressing SIVmac239 Gag fragments (rFlu/SIVgag no. 3 and rFlu/SIVgag no. 4 viruses) as described previously (31) (Fig. 1A). Six-week-old female BALB/c mice, purchased from Charles River Laboratories, were used in the animal experiments. All animal procedures were in accordance with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.
FIG. 1.
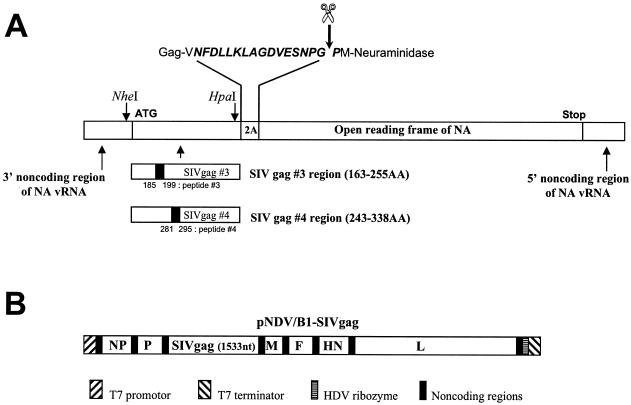
Recombinant viruses used in immunizations. (A) Schematic representation of Flu/SIVgag/2A/NA genes in rFlu/SIVgag viruses. rFlu/SIVgag viruses were constructed as described previously (31). Briefly, the SIV Gag-specific sequences were inserted in negative sense between the 3′ noncoding region of the neuraminidase (NA) gene and the protease recognition sequence 2A (NFDLLKLAGDVESNPGP) derived from foot-and-mouth disease virus by using NheI and HpaI restriction sites (41), and the corresponding genes were rescued into infectious influenza viruses. The expressed Gag-2A-NA polyprotein is cleaved into Gag-2A and NA polypeptides due to the autocatalytic activity of the 2A protease (44). However, it should be noted that recent evidence favors a mechanism of action of 2A due to “ribosomal skip” rather than to proteolytic self-cleavage (12). Peptides 3 and 4 within the Gag antigens used in the ELISPOT assays are also indicated. These two peptides were previously found to contain an epitope recognized by CD8+ T cells in BALB/c mice (31). (B) Schematic representation of pNDV/B1-SIVgag used to rescue rNDV/SIVgag. The pNDV/B1-SIVgag construct was made by inserting the SIV Gag gene into the unique XbaI cloning site (nucleotide 3163) located between the P and M genes of the original pNDV/B1 clone (30). The inserted gene contains the gene end, intergenic, and gene start sequences (5′-TTAGAAAAAATACGGGTAGAA-3′) required for expression of SIV Gag as a new transcriptional unit by the NDV RNA-dependent RNA polymerase. In addition, seven nucleotides (5′-CGCCACC-3′) were inserted upstream of the SIV Gag initiation site to introduce an optimal Kozak sequence (24). The final length of the encoded NDV genome was divisible by six. The indicated plasmid regions are not to scale.
Construction and growth of rNDV/SIVgag.
The full-length SIV Gag cDNA was obtained by PCR by using DNA isolated from rVac/SIVgag virus-infected cells. The Gag cDNA was cloned into a new transcriptional unit inserted between the P and M genes of the previously described rNDV/B1 cDNA (30) (Fig. 1B). A potential gene-end sequence for NDV polymerase (5′-46TTAGAAAAAA55-3′) was present in the original Gag cDNA. This gene-end signal was eliminated by site-directed mutagenesis with the following oligonucleotides: SDM(+), 5′-33GAAAGCAGATGAACTGGAGAAAATTAGGCT62-3′; SDM(−), 5′-62AGCCTAATTTTCTCCAGTTCATCTGCTTTC33-3′. Silent nucleotide changes in the primers are italicized. The obtained plasmid (pNDV/B1-SIVgag) was confirmed by sequencing. The recombinant virus, rNDV/SIVgag, was then rescued from pNDV/B1-SIVgag by using previously described methods (30, 38). Expression of the SIV Gag protein was determined by indirect immunofluorescence of rNDV/SIVgag virus-infected cells by using an anti-SIVmac p27 monoclonal antibody 55-2F12 (17), obtained from Niels Pedersen through the AIDS Research and Reference Reagent Program, AIDS Program, National Institute of Allergy and Infectious Diseases, NIH (undiluted hybridoma culture supernatants at concentrations of 35 μg/ml), followed by fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (IgG) (1:40 dilution; DAKO).
Virulence index of rNDV/SIVgag in embryonated eggs.
In order to determine the virulence index of rNDV/SIVgag virus, mean death time (MDT) in infected embryonated eggs was determined. Serial 10-fold dilutions of infectious allantoic fluid (10−6 to 10−8) were inoculated in each of five embryonated eggs, and the average mean time resulting in embryonic death as a result of the minimal lethal dose was recorded.
Growth kinetics of rNDV/SIVgag in embryonated chicken eggs.
Embryonated chicken eggs were inoculated with wild-type rNDV/B1 or rNDV/SIVgag viruses at 100 PFU per egg. Viral titers (50% tissue culture infective dose [TCID50]) present in the allantoic fluid at different time points postinfection were determined by using a previously described immunofluorescence assay (30).
Prime and boosting immunizations.
rFlu/SIVgag no. 3 or rFlu/SIVgag no. 4 (31) and rNDV/SIVgag viruses were used to immunize mice. In prime and boost immunizations, 5 × 102 PFU of rFlu/SIVgag no. 3 or rFlu/SIVgag no. 4 was administered to mice intranasally, and 5 × 107 PFU of rNDV/SIVgag was administered intranasally, intravenously, or intraperitoneally. Boosters were given 3 weeks after the first immunization.
Preparation of CD8+ lymphocytes.
Spleens and cervical and mediastinal-draining lymph nodes of the respiratory tracts of immunized BALB/c mice were collected at day 5 or 30 following the last immunization and were used for isolating lymphocyte populations. Pooled spleens from three mice were dissociated into single-cell suspensions by grinding. Pooled lymph nodes from three mice were dissociated into single cells by using a mixture of collagenase and dispase (1 mg/ml; Boehringer Mannheim) in DMEM at 37°C for 1 h with shaking. CD8+ T cells present in these preparations were incubated with a rat anti-mouse CD8a monoclonal antibody (5H10-1; Pharmingen, SanDiego, Calif.). The cells were then positively selected with magnetic microbeads conjugated to anti-rat IgG antibody (Polyscience, Warrington, Pa.).
Peptides.
Peptides used in the ELISPOT assay were obtained from the AIDS research and reference reagents program (NIH, Bethesda, Md.). Peptide 3 (DINQMLNCVGDHQAA) and peptide 4 (TNILDVKQGPKEPFQ) correspond to amino acid residues 185 to 199 and 281 to 295 of SIV Gag, respectively. It was previously found that these peptides contain a CTL epitope recognized by SIV Gag-specific CD8+ T cells of BALB/c mice (31).
ELISPOT assay for the detection of IFN-γ-producing cells.
An ELISPOT assay to detect antigen-specific CTLs was performed according to a previously described protocol (31, 51). Briefly, 96-well nitrocellulose plates (Millipore Corp., Bedford, Mass.) were coated with 100 μl of phosphate-buffered saline (PBS) containing 5 μg of anti-mouse gamma interferon (IFN-γ) monoclonal antibody R4 (no. R4-6A2; Pharmingen) per ml. After overnight incubation at 4°C, the wells were washed eight times with DMEM-high glucose medium (Life Technologies, Rockville, Md.) containing 10% FBS (HyClone, Logan, Utah) and incubated for more than 1 h at 37°C. Twofold dilution series of CD8+ spleen or lymph node cells, starting at 5 × 105 cells per well, were placed into the coated wells and cocultured with P815 cells pulsed with 10 μg of the individual peptides. Nonpulsed P815 cells were used as negative controls. The plates were incubated in a 5% CO2 incubator for 30 h. Subsequently, the plates were extensively washed with PBS-Tween 20 (0.05%) and then 0.1 ml of 2.5 mg of biotinylated anti-mouse IFN-γ monoclonal antibody XMG1.2 (XGX1.2; Pharmingen) per ml was added to each well. After overnight incubation at 4°C, the plates were incubated with peroxidase-labeled streptavidin (1: 1,000; Kirkegaard & Perry Laboratories, Gaithersburg, Md.) for 1 h at room temperature. Wells were washed with PBS-Tween 20 and PBS, and substrate (3,3′-diaminobenzidine tetrahydrochloride; Sigma, Saint Louis, Mo.) at a concentration of 1 mg/ml and containing 0.015% hydrogen peroxidase (Sigma) in 50 mM Tris-HCl, pH 7.5, was added. The spots were counted with the help of a microscope.
Challenge infections with rVac/SIVgag.
At 3, 6, and 9 weeks after the last immunization, mice were challenged with 5 × 106 PFU of rVac/SIVgag administered intranasally or intravenously. Vac/wt was used in control experiments. Five days after the challenge infection, the mice were sacrificed, and their lungs were extracted and homogenized for virus titration by using CV-1 cells. At 2 days postinfection, the CV-1 cells were stained with 0.1% crystal violet solution to count the number of vaccinia virus plaques.
RESULTS
Construction of rNDV/SIVgag.
The SIV Gag open reading frame was cloned between the P and M genes of the previously described rNDV/B1 cDNA (Fig. 1B) (30, 38). rNDV/SIVgag was rescued from cDNA by previously described methods (30). The presence of the inserted SIV Gag gene in the viral genome was confirmed by reverse transcription-PCR and sequencing (data not shown). Growth of the Gag-expressing virus in CEF cells was comparable to that of the wild-type virus (rNDV/B1) without the SIV Gag insert (data not shown). SIV Gag expression in rNDV/SIVgag-infected cells was confirmed by immunofluorescence with anti-SIV p27 monoclonal antibody (Fig. 2). In addition, we determined whether the SIV Gag protein was incorporated into rNDV/SIVgag virions. rNDV/SIVgag and wild-type rNDV/B1 viruses were purified and analyzed by a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblotting with anti-SIV Gag monoclonal antibody. We did not detect Gag in purified virions, suggesting that the SIV Gag protein was not incorporated into NDV virions (data not shown). However, we cannot exclude the possibility that SIV Gag is packaged into virions at low levels, undetectable in our assays.
FIG. 2.
Expression of SIV Gag protein in Vero cells infected with rNDV/SIVgag virus. rNDV/SIVgag virus-infected cells were fixed at day 2 postinfection, and permeabilized cells were used for immunofluorescence analysis. SIV Gag protein expression was evidenced by using anti-SIV Gag monoclonal antibody, as described in Material and Methods. Vero cells infected with wild-type rNDV/B1 virus were used as negative controls.
Lethality and replication of rNDV/SIVgag virus in embryonated eggs.
In order to determine the virulence index of rNDV/SIVgag virus, the MDT in eggs was determined. The MDT of rNDV/SIVgag was 146.8 h. In contrast, the MDT of rNDV/B1 was 113 h. Lentogenic strains of NDV are characterized by MDTs of more than 90 h (1). Our results suggest that rNDV/SIVgag is lentogenic and more attenuated in chickens than the parental vaccine strain Hitchner B1. Nevertheless, despite a slightly delayed replication, rNDV/SIVgag viruses reached titers comparable to those of wild-type rNDV/B1 (around 108 TCID50/ml) in embryonated eggs (Fig. 3).
FIG. 3.
Growth kinetics of rNDV/SIVgag in embryonated chicken eggs. Embryonated eggs were inoculated with 100 PFU of rNDV/B1 or rNDV/SIVgag viruses and allantoic fluids were harvested at different time points (24, 48, and 72 h postinoculation). Viral titers at these time points were determined as TCID50 by using an immunofluorescence assay. Briefly, 96-well plates containing 70 to 80% confluent Vero cells were infected with serial 10-fold dilutions of allantoic fluids (4 wells per dilution). Cells were incubated for 2 days and then fixed with 2.5% formaldehyde containing 0.1% Triton X-100. Viral proteins were visualized by using an anti-NDV rabbit serum followed by fluorescein isothiocyanate-conjugated anti-rabbit IgG.
Protection against challenge infection with rVac/SIVgag in mice immunized with rNDV/SIVgag.
In order to evaluate whether rNDV/SIVgag induces an SIV Gag-specific antiviral cellular immune response, we immunized BALB/c mice with this recombinant virus. Since SIV does not replicate in mice, we used vaccinia virus expressing SIV Gag as a surrogate challenge virus. In these assays, it has been previously shown that the inhibition of replication of vaccinia virus expressing a foreign antigen is mediated by CTLs (7). Groups of three mice were immunized with 5 ×107 PFU of rNDV/SIVgag intranasally, intraperitoneally, or intravenously. Immunizations were performed once or twice. As in the case with rNDV/B1, immunized mice did not show a loss of body weight or any other disease signs, indicating that the insertion of the Gag gene did not increase the pathogenicity of NDV in mice (data not shown). Three weeks after single or double immunization, challenge infections with rVac/SIVgag or Vac/wt viruses were performed. In a pilot experiment, naïve mice were infected with vaccinia virus intranasally or intravenously, and vaccinia virus titers were determined 5 days later in lungs, heart, liver, spleen, kidney, pancreas, and ovary. Vaccinia virus grew to titers higher than 104 PFU in lung, ovary, and spleen. In particular, the virus replicated to high titers in lung by both administration routes (data not shown). We then determined vaccinia virus titers in lungs of rNDV/SIV gag-immunized mice at 5 days postchallenge (Fig. 4).
FIG. 4.
Challenge experiments in immunized mice with rNDV/SIVgag virus. rNDV/SIVgag virus (5 × 107 PFU) was inoculated once (single) or twice (double) to groups of three mice intranasally, intraperitoneally, or intravenously. Control mice were inoculated with PBS. Three weeks after the last inoculation, mice were intranasally or intravenously infected with 5 × 106 PFU of Vac/wt or rVac/SIVgag virus. Five days after vaccinia virus challenge, mice were sacrificed, and vaccinia virus titers in lungs were determined. Homogenized lungs in 1 ml of PBS were used to determine vaccinia virus titers in CV-1 cells.
Mice singly immunized by intranasal administration of rNDV/SIVgag, but not by intravenous or intraperitoneal administrations, showed an antiviral response against rVac/SIVgag (Fig. 4A), as evidenced by an approximately 1 log reduction in rVac/SIVgag virus titers in lungs at day 5 after both intranasal (mucosal) and intravenous (systemic) challenges. A second intranasal immunization with rNDV/SIVgag increased the levels of Gag-specific antiviral immunity, resulting in a reduction of approximately 3 logs in rVac/SIVgag virus titers (Fig. 4B). Intraperitoneal immunizations with rNDV/SIVgag were less efficient in mediating a reduction of vaccinia virus titers, especially after mucosal challenge. Finally, intravenous immunization only resulted in a 1 log reduction in rVac/SIVgag virus titers after systemic challenge in doubly immunized mice. Inhibition of rVac/SIVgag replication was dependent on Gag expression, since no reduction in viral titers was observed when animals were challenged with Vac/wt. Moreover, wild-type rNDV/B1 virus did not induce any protection against either rVac/SIVgag challenge or Vac/wt challenge, as shown in Table 1.
TABLE 1.
Vaccinia virus titers in mice intranasally immunized two times with rNDV/SIVgag, wild-type rNDV/B1, or PBS
| Immunizationa | Challengeb | Vaccinia virus titer (log 10 PFU/ml)c
|
||||
|---|---|---|---|---|---|---|
| Mouse 1 | Mouse 2 | Mouse 3 | Average | SD | ||
| rNDV/SIVGag | Vac/wt | 8.2 | 8.6 | 8.7 | 8.5 | 0.264575 |
| rNDV/SIVGag | rVac/SIVGag | 5.4 | 5.85 | 6.1 | 5.78 | 0.360555 |
| rNDV/B1 | Vac/wt | 8.8 | 8.35 | 8.6 | 8.58 | 0.225462 |
| rNDV/B1 | rVac/SIVGag | 8.7 | 8.57 | 8.95 | 8.74 | 0.193132 |
| PBS | Vac/wt | 8.3 | 8.5 | 8.8 | 8.53 | 0.251661 |
| PBS | rVac/SIVGag | 8.2 | 8.6 | 8.55 | 8.45 | 0.217945 |
Mice immunized with virus received 5 × 107 PFU of virus intranasally at week 0 and week 3.
Mice were intranasally challenged at week 6 with 5 × 106 PFU of Vac/wt or rVac/SIVgag virus.
Five days after vaccinia virus challenge, mice were sacrificed, and vaccinia virus titers in lungs were determined. Results from three individual mice are shown.
We thus conclude that of all the protocols of immunization used in the present study, intranasal double immunization with rNDV/SIVgag is the most effective in inducing antiviral Gag-specific immune responses that inhibit replication of rVac/SIVgag virus after mucosal or systemic challenges in mice. Of note, when vaccinia virus titers where measured after intravenous challenge in a distal site of immunization, e.g., in ovaries, intranasal double immunizations with rNDV/SIVgag also resulted in a specific reduction (approximately 1 log) of rVac/SIVgag virus titers (data not shown). These results indicate that intranasal rNDV/SIVgag induces Gag-specific T-cell responses that prevent viral replication not only in lungs but also in distant organs, although at a lower efficiency.
Combined immunizations with rNDV/SIVgag and rFlu/SIVgag no. 3 enhance protective Gag-specific antiviral responses in mice.
It has previously been reported that rFlu/SIVgag no. 3 and rFlu/SIVgag no. 4 viruses induced a strong cellular anti-Gag immune response in mice, and this immune response was further enhanced by a booster with a heterologous virus expressing the same antigen, rVac/SIVgag (31). Therefore, we now determined whether rFlu/SIVgag could enhance the rNDV/SIVgag-induced immune response when both heterologous vectors were used in combined immunizations. For this purpose, rNDV/SIVgag or rFlu/SIVgag (no. 3 or no. 4) virus was used to intranasally immunize mice. Three weeks after priming, the immunized mice were boosted with the corresponding heterologous viruses. Mice were then challenged with rVac/SIVgag or Vac/wt virus 3 weeks after the boosting. Immunized mice showed a reduction in rVac/SIVgag but not in Vac/wt virus replication in lungs (Fig. 5). Interestingly, mice primed with rNDV/SIVgag and boosted with rFlu/SIVgag no. 3 revealed the strongest inhibition of rVac/SIVgag growth, compared to mice receiving homologous prime and boost immunizations or even to mice receiving a reversed order of immunization, namely, immunization with rFlu/SIVgag no. 3, followed by immunization with rNDV/SIVgag. Thus, priming immunization with rNDV/SIVgag followed by a booster with rFlu/SIVgag no. 3 viruses resulted in approximately a 106-fold reduction in rVac/SIVgag titers at day 5 after challenge compared to titers of PBS control mice (average titers in lungs of 102.6 versus 108.6 PFU/ml) (Fig. 5). In contrast, rFlu/SIVgag no. 3 priming followed by rNDV/SIVgag boosting reduced the rVac/SIVgag growth by approximately 100-fold (average titers of 107.1 PFU/ml) (Fig. 5). Similar results were obtained when rFlu/SIVgag no. 4 was used as a booster in rNDV/SIVgag-primed mice, albeit the levels of protection were lower than those in rFlu/SIVgag no. 3-immunized mice (average titers of 104.7 PFU/ml). The differences in efficacy between the rFlu/SIVgag viruses may represent the presence or absence of optimal CTL epitopes in the corresponding Gag sequences. As also shown in Fig. 4, double immunization with rNDV/SIVgag reduced rVac/SIVgag replication more than 1,000-fold (average titers of 105.3 PFU/ml), while double immunization with rFlu/SIVgag only slightly reduced rVac/SIVgag growth (Fig. 5). These results indicate that among the prime-boost vaccination protocols tested, priming with rNDV/SIVgag followed by boosting with rFlu/SIVgag no. 3 was the most efficient vaccine combination, resulting in high levels of induction of protective antiviral immunity against rVac/SIVgag challenge. The booster effects of rFlu/SIVgag were dependent on the expression of the Gag antigen, since there was no unspecific reduction of rVac/SIVgag titers in mice immunized with wild-type influenza virus (31).
FIG. 5.
Challenge experiments of mice immunized with rNDV/SIVgag virus and/or rFlu/SIVgag viruses. rNDV/SIVgag virus (5 × 107 PFU), each of the rFlu/SIVgag viruses (5 × 102 PFU), or a mixture of rFlu/SIVgag no. 3 and rFlu/SIVgag no. 4 viruses (5 × 102 PFU) were intranasally administered to groups of three mice. Control mice were inoculated with PBS. Three weeks after inoculation, mice were intranasally immunized (booster) with rNDV/SIVgag virus (5 × 107 PFU), each of the rFlu/SIVgag viruses (5 × 102 PFU), or a mixture of rFlu/SIVgag viruses (5 × 102 PFU). Three weeks postimmunization, mice were intranasally infected with 5 × 106 PFU of Vac/wt or rVac/SIVgag viruses. Five days after vaccinia virus challenge, mice were sacrificed, and vaccinia virus titers in lungs were determined.
Induction of cellular immune responses against SIV Gag after immunization with rNDV/SIVgag.
To quantify the cellular immune response against SIV Gag induced in mice immunized with rNDV/SIVgag and rFlu/SIVgag viruses, we performed ELISPOT assays. Spleen and cervical and mediastinal lymph nodes were obtained on days 5 and 30 postboosting, and the number of Gag-specific CD8+ IFN-γ-producing cells in each tissue was counted to analyze systemic and local cellular immunity. Cervical and mediastinal lymph nodes belong to the mucosa-associated lymphoid tissues which drain CTLs in the respiratory mucosal tissue (33). CD8+ IFN-γ-producing cells specific to SIV Gag peptides (31) were detected in both spleen and lymph nodes of immunized mice, suggesting that rNDV/SIVgag and/or rFlu/SIVgag immunization induced systemic and local cellular immune responses against the Gag protein (Fig. 6). Immunizations performed by rNDV/SIVgag priming followed by rFlu/SIVgag boosting induced a higher number of IFN-γ-producing cells than the reversed order of vaccination (Fig. 6). These results correlate with the increased inhibition of rVac/SIVgag replication observed in rNDV/SIVgag-primed and rFlu/SIVgag-boosted mice (Fig. 5). Despite a decrease in the number of Gag-specific CD8+ IFN-γ-producing cells from day 5 to day 30 postboosting, significant numbers of these cells were still detectable 30 days postboosting, indicating that the systemic and local cellular immune responses induced by rNDV/SIVgag and rFlu/SIVgag were maintained for at least 1 month.
FIG. 6.
Quantification of SIV Gag peptide-specific IFN-γ-producing CD8+ T cells in mice receiving prime and boost immunizations. Three mice per group were intranasally immunized with rFlu/SIVgag no. 3 (5 × 102 PFU), rFlu/SIVgag no. 4 (5 × 102 PFU), and rNDV/SIVgag (5 × 107 PFU) as indicated. The spleen cells (A) and lymphocytes derived from cervical and mediastinal lymph nodes (B) were obtained 5 or 30 days after boosting. CD8+ T cells were selected and incubated with specific Gag peptide-pulsed P815 cells in an ELISPOT assay. Gag peptides 3 and 4 were used for stimulation of CD8+ T cells derived from rFlu/SIVgag no. 3 and rFlu/SIVgag no. 4 virus-immunized animals, respectively. As controls, CD8+ cells derived from spleen and lymph nodes of mice inoculated with PBS were used. The numbers of IFN-γ-secreting cells relative to the Gag peptides per million cells are represented.
Long-lasting antiviral immunity in mice immunized with rNDV/SIVgag and rFlu/SIVgag viruses.
We next evaluated whether the immune response induced by rNDV/SIVgag and rFlu/SIVgag viruses was able to reduce replication of the surrogate rVac/SIVgag challenge virus for more than 1 month after vaccination. Groups of three mice were immunized with rNDV/SIVgag followed by rFlu/SIVgag according to the protocol established for the earlier experiments. At 3, 6, and 9 weeks after boosting, the immunized mice were challenged with rVac/SIVgag or Vac/wt, and vaccinia virus titers in lungs were determined at day 5 postchallenge. Consistent with results shown in Fig. 5, immunized mice showed a significant inhibition of rVac/SIVgag replication when challenged 3 weeks after immunization. Although the ability to inhibit rVac/SIVgag replication decreased with the time after immunization, a Gag-specific antiviral response was still detectable in immunized mice at 6 and 9 weeks after vaccination (Fig. 7). These results suggest that the cellular immunity against SIV Gag induced by vaccination with rNDV/SIVgag and rFlu/SIVgag is long-lived in mice.
FIG. 7.
Maintenance of antiviral cellular immunity against SIV Gag in mice immunized with rNDV/SIVgag and rFlu/SIVgag viruses. Three mice per group were intranasally immunized with rNDV/SIVgag (5 × 107 PFU) followed by boosting with rFlu/SIVgag (5 × 102 PFU), as indicated. Three weeks (group 1), 6 weeks (group 2), and 9 weeks (group 3) after boosting with rFlu/SIVgag virus, immunized mice were challenged with 5 × 106 PFU of Vac/wt or rVac/SIVgag viruses. Group 4 included control nonimmunized animals. Mice were sacrificed 5 days after vaccinia virus challenge, and vaccinia virus titers in lungs were determined.
DISCUSSION
It has previously been reported that rNDV/B1-HA induced an HA-specific humoral immune response in mice, supporting the possibility of using rNDV as a vaccine vector in mammals (30). In this study we show that rNDV/SIVgag induces Gag-specific cellular immune responses in mice. As far as we know, this is the first report showing that an NDV-based vector induces a cellular immune response in mammals. We also report that mice intranasally immunized with rNDV/SIVgag exhibit a remarkable attenuation of rVac/SIVgag growth in lungs and that this effect correlated with the induction of Gag-specific cellular immune responses. Finally, we demonstrate that the protective antiviral immune response induced by rNDV/SIVgag is enhanced when rFlu/SIVgag is used as a booster immunogen.
Intranasal immunization of mice with rNDV/SIVgag induced stronger antiviral responses against rVac/SIVgag than intraperitoneal or intravenous immunizations (Fig. 4). In the case of rNDV/B1-HA, a protective humoral immune response against influenza virus challenge was induced by intravenous administration of the vector (30). However, the expressed HA protein was efficiently incorporated into rNDV virions, while this was not the case for the Gag antigen (data not shown). These results might indicate that when de novo protein expression is required to induce a cellular immune response, intranasal administration of rNDV vectors might be more efficient than systemic (intravenous) delivery. However, we cannot exclude the possibility that differences in immunogenicity between the influenza virus HA and SIV Gag are responsible for the different optimal routes of immunization of the corresponding rNDV vectors.
Since specific CTL activity against HIV correlates with the clinical stage of disease in infected individuals, as evidenced by a loss of CTL activity with disease progression (18), it might be important for an HIV vaccine to induce potent cellular immune responses. Previously, we have demonstrated that cellular immune responses to SIV Gag were enhanced when a combined prime-boost immunization regimen with rFlu/SIVgag and rVac/SIVgag viruses was used (31). Similar results were obtained when different CTL epitopes derived from HIV or from malaria parasites were expressed in recombinant influenza- and vaccinia virus-based vectors (13, 25, 27). We now show that combined immunization with rNDV/SIVgag and a second heterologous RNA virus vector expressing SIV Gag, namely rFlu/SIVgag no. 3, induces stronger immune responses to SIV Gag in mice than when only the homologous vectors are used. It is not known why the order of prime and boost (first rNDV/SIVgag, followed by rFlu/SIVgag) is critical to induce potent Gag-specific immune responses in this system. One possibility is that rFlu/SIVgag viruses induce a more restricted CTL response because one of the viruses possesses only a portion (approximately 100 residues) of the Gag protein. However, when both rFlu/SIVgag no. 3 and rFlu/SIVgag no. 4 viruses were administered at the same time, we did not find any significant enhancement of the immune response (Fig. 5). The same results were obtained when five different rFlu/SIVgag viruses (rFlu/SIVgag no. 1 to rFlu/SIVgag no. 5) encompassing almost the complete amino acid sequence of SIV Gag (31) were used together (data not shown). Another possibility is that immunization with rFlu/SIVgag might significantly reduce viral gene expression of rNDV/SIVgag in mice, preventing a potent booster response by this virus. However, at this moment we cannot exclude the possibility that the effects observed with respect to the order of immunizations are due to uncharacterized inherent immunogenic properties of these two viruses that affect their priming and boosting abilities. Interestingly, it has previously been reported that the order of prime and boost immunizations was critical to induce potent cellular immune responses by combined immunizations with recombinant influenza and vaccinia virus vectors (27).
Homologous prime and boost immunizations with rNDV/SIVgag were more efficient than those with rFlu/SIVgag (Fig. 4 and 5). This might be due to a lower neutralizing response against the immunization vector induced by single administration of NDV, a replication-impaired virus in mammalian hosts, compared with influenza virus, a replication-competent virus. If this is the case, NDV will still be able to infect host cells when administered for the second time, resulting in the boosting of the T-cell responses.
One important aspect to consider in vaccination protocols is whether the vaccine is able to induce a long-lasting immune response (9). In this study, we found a high degree of reduction of rVac/SIVgag replication in mice immunized with rNDV/SIVgag and rFlu/SIVgag viruses when challenged 3 weeks postimmunization (between 104- and 106-fold reduction at day 5 postchallenge) (Fig. 5 and 7). We also found that antiviral immunity against rVac/SIVgag induced by the combined immunization with rNDV/SIVgag and rFlu/SIVgag was detectable 9 weeks postboosting, albeit to a lower extent. These results suggest that the immunity induced by combined immunizations with NDV- and influenza virus-based vectors could last for a long time in mammals.
A large number of vaccine strategies have been tested in the SIV or simian-human immunodeficiency virus (SHIV) macaque model of AIDS. Of note, some approaches have shown protection from onset of disease after challenge (19). So far, the only effective vaccine to protect rhesus macaques from SIV infection is the use of live, attenuated SIV (21). However, such a live vaccine approach has the risk of inducing disease (3, 53). In more recent studies, vaccination with DNA expression plasmids encoding SIV viral proteins combined with cytokine administration or followed by a recombinant viral vector has been shown to control SHIV replication in the macaque model system (2, 4, 5). However, several administrations are required to induce immunity in the case of DNA vaccination. Shiver et al. systematically compared vaccination strategies by using DNA molecules, modified vaccinia virus- and (replication-incompetent) adenovirus-expressing SIV Gag and demonstrated that the most effective immune responses were elicited by an adenovirus-based vaccine (49). The efficacy of an AIDS vaccine based on attenuated vesicular stomatitis virus vectors expressing the Env and Gag genes has been convincingly demonstrated in rhesus monkeys (43). Poliovirus-based SIV vaccines protected cynomolgus macaques against SIV challenge (10). More recently, the use of a replicating adenovirus vector expressing SIV antigens followed by a boost with a subunit protein vaccine resulted in protection against mucosal SIV challenge in rhesus macaques (39). Despite these encouraging results, further research is needed to improve these AIDS vaccine approaches and to develop novel vectors that might be used in combined immunization strategies.
One such approach might be based on NDV and influenza virus vectors. In the case of NDV, it has been reported that this virus can infect humans without severe adverse effects (11, 32, 46). Clinical trials with NDV used as an antitumor agent have shown encouraging preliminary results in patients with a variety of cancers, and phase III trials are beginning in Europe (32, 40). These results support the use of recombinant NDV as a safe and effective viral vector in humans. In the case of influenza viruses, cold-adapted attenuated influenza viruses have been developed as live, stable, and nonpathogenic vaccines against influenza in humans (6, 20, 26). Interestingly, cold-adapted influenza virus vectors are also able to induce strong cellular immune responses against their expressed antigens (14). Moreover, several attenuated strains of influenza virus have been developed by reverse genetics techniques (36). Therefore, it should be possible to use attenuated influenza virus vectors for vaccine development in humans. In addition to the availability of attenuated strains in humans, other potential advantages of NDV- as well as influenza virus-based vaccine vectors are the following. (i) Immunizations can be performed intranasally, and this results in the induction of mucosal immunity (28). Mucosal immunity might be important as a first line of protection against infection with pathogens, such as HIV, that can enter the host through mucosal surfaces. (ii) Both NDV and influenza virus vectors are easily amplified in embryonated eggs. (iii) Since negative-strand RNA viruses do not go through a DNA phase, these viruses cannot transform cells by integrating their genetic information into the host cell genome. (iv) There is no preexisting immunity against NDV in humans, and immunity against influenza virus in humans could be circumvented through the use of viral glycoprotein genes from specific strains. While our studies show the ability of rNDV/SIVgag and rFlu/SIVgag to induce a potent antiviral Gag-specific antiviral response in mice, future studies in monkeys will be needed to demonstrate whether immunizations with rNDV/SIVgag and rFlu/SIVgag viruses induce efficient immune responses able to prevent AIDS induced by SIV and/or SHIV viruses. Our results thus encourage further investigations towards the development of recombinant NDV and influenza virus vectors as potential AIDS vaccines.
Acknowledgments
We are grateful to Gómez-Yafal at Therion Biologicals for kindly providing Vac/wt and rVAC/SIVgag. We also thank Richard Cadagan and Neva Morales for excellent technical assistance. SIV Gag antibodies and peptides were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This work was partially supported by grants to A.G.-S. and P.P. from the NIH. Y.N. was supported by a Uehara Memorial Bio-Medical Research Foundation fellowship.
REFERENCES
- 1.Alexander, D. J. 2000. Newcastle disease and other avian paramyxoviruses. Rev. Sci. Tech. 19:443-462. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., P. F. McKay, S. M. Sumida, S. Santra, S. S. Jackson, D. A. Gorgone, M. A. Lifton, B. K. Chakrabarti, L. Xu, G. J. Nabel, and N. L. Letvin. 2003. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J. Virol. 77:8729-8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 6.Belshe, R. B., E. M. Swierkosz, E. L. Anderson, F. K. Newman, S. L. Nugent, and H. F. Maassab. 1992. Immunization of infants and young children with live attenuated trivalent cold-recombinant influenza A H1N1, H3N2, and B vaccine. J. Infect. Dis. 165:727-732. [DOI] [PubMed] [Google Scholar]
- 7.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 95:1709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, J. 2001. AIDS research. Debate begins over new vaccine trials. Science 293:1973. [DOI] [PubMed] [Google Scholar]
- 10.Crotty, S., C. J. Miller, B. L. Lohman, M. R. Neagu, L. Compton, D. Lu, F. X. Lu, L. Fritts, J. D. Lifson, and R. Andino. 2001. Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J. Virol. 75:7435-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csatary, L. K., S. Eckhardt, I. Bukosza, F. Czegledi, C. Fenyvesi, P. Gergely, B. Bodey, and C. M. Csatary. 1993. Attenuated veterinary virus vaccine for the treatment of cancer. Cancer Detect. Prev. 17:619-627. [PubMed] [Google Scholar]
- 12.Donnelly, M. L., G. Luke, A. Mehrotra, X. Li, L. E. Hughes, D. Gani, and M. D. Ryan. 2001. Analysis of the aphthovirus 2A/2B polyprotein “cleavage” mechanism indicates not a proteolytic reaction, but a novel translational effect: a putative ribosomal “skip.” J. Gen. Virol. 82:1013-1025. [DOI] [PubMed] [Google Scholar]
- 13.Gherardi, M. M., J. L. Najera, E. Perez-Jimenez, S. Guerra, A. García-Sastre, and M. Esteban. 2003. Prime-boost immunization schedules based on influenza virus and vaccinia virus vectors potentiate cellular immune responses against human immunodeficiency virus Env protein systemically and in the genitorectal draining lymph nodes. J. Virol. 77:7048-7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Aseguinolaza, G., Y. Nakaya, A. Molano, E. Dy, M. Esteban, D. Rodríguez, J. R. Rodríguez, P. Palese, A. García-Sastre, and R. S. Nussenzweig. 2003. Induction of protective immunity against malaria by priming-boosting immunization with recombinant cold-adapted influenza and modified vaccinia Ankara viruses expresing a CD8+-T-cell epitope derived from the circumsporozoite protein of Plasmodium yoelii. J. Virol. 77:11859-11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrer, T., E. Harrer, S. A. Kalams, P. Barbosa, A. Trocha, R. P. Johnson, T. Elbeik, M. B. Feinberg, S. P. Buchbinder, and B. D. Walker. 1996. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. Breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J. Immunol. 156:2616-2623. [PubMed] [Google Scholar]
- 16.Harrer, T., E. Harrer, S. A. Kalams, T. Elbeik, S. I. Staprans, M. B. Feinberg, Y. Cao, D. D. Ho, T. Yilma, A. M. Caliendo, R. P. Johnson, S. P. Buchbinder, and B. D. Walker. 1996. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res. Hum. Retrovir. 12:585-592. [DOI] [PubMed] [Google Scholar]
- 17.Higgins, J. R., S. Sutjipto, P. A. Marx, and N. C. Pedersen. 1992. Shared antigenic epitopes of the major core proteins of human and simian immunodeficiency virus isolates. J. Med. Primatol. 21:265-269. [PubMed] [Google Scholar]
- 18.Hoffenbach, A., P. Langlade-Demoyen, G. Dadaglio, E. Vilmer, F. Michel, C. Mayaud, B. Autran, and F. Plata. 1989. Unusually high frequencies of HIV-specific cytotoxic T lymphocytes in humans. J. Immunol. 142:452-462. [PubMed] [Google Scholar]
- 19.Hulskotte, E. G., A. M. Geretti, and A. D. Osterhaus. 1998. Towards an HIV-1 vaccine: lessons from studies in macaque models. Vaccine 16:904-915. [DOI] [PubMed] [Google Scholar]
- 20.Jin, H., B. Lu, H. Zhou, C. Ma, J. Zhao, C. F. Yang, G. Kemble, and H. Greenberg. 2003. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology 306:18-24. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, R. P., and R. C. Desrosiers. 1998. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 10:436-443. [DOI] [PubMed] [Google Scholar]
- 22.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof Garde, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koup, R. A., and D. D. Ho. 1994. Shutting down HIV. Nature 370:416. [DOI] [PubMed] [Google Scholar]
- 24.Kozak, M. 1987. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J. Mol. Biol. 196:947-950. [DOI] [PubMed] [Google Scholar]
- 25.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, R. S. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. USA 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendelman, P. M., J. Cordova, and I. Cho. 2001. Safety, efficacy and effectiveness of the influenza virus vaccine, trivalent, types A and B, live, cold-adapted (CAIV-T) in healthy children and healthy adults. Vaccine 19:2221-2226. [DOI] [PubMed] [Google Scholar]
- 27.Murata, K., A. García-Sastre, M. Tsuji, M. Rodrigues, D. Rodriguez, J. R. Rodriguez, R. S. Nussenzweig, P. Palese, M. Esteban, and F. Zavala. 1996. Characterization of in vivo primary and secondary CD8+ T cell responses induced by recombinant influenza and vaccinia viruses. Cell. Immunol. 173:96-107. [DOI] [PubMed] [Google Scholar]
- 28.Muster, T., B. Ferko, A. Klima, M. Purtscher, A. Trkola, P. Schulz, A. Grassauer, O. G. Engelhardt, A. García-Sastre, P. Palese, and H. Katinger. 1995. Mucosal model of immunization against human immunodeficiency virus type 1 with a chimeric influenza virus. J. Virol. 69:6678-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabel, G. J. 2003. Vaccine for AIDS and Ebola virus infection. Virus Res. 92:213-217. [DOI] [PubMed] [Google Scholar]
- 30.Nakaya, T., J. Cros, M.-S. Park, Y. Nakaya, H. Zheng, A. Sagrera, E. Villar, A. García-Sastre, and P. Palese. 2001. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 75:11868-11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakaya, Y., H. Zheng, and A. García-Sastre. 2003. Enhanced cellular immune responses to SIV Gag by immunization with influenza and vaccinia virus recombinants. Vaccine 21:2097-2106. [DOI] [PubMed] [Google Scholar]
- 32.Nelson, N. J. 1999. Scientific interest in Newcastle disease virus is reviving. J. Natl. Cancer Inst. 91:1708-1710. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen, H. H., Z. Moldoveanu, M. J. Novak, F. W. van Ginkel, E. Ban, H. Kiyono, J. R. McGhee, and J. Mestecky. 1999. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology 254:50-60. [DOI] [PubMed] [Google Scholar]
- 34.Nixon, D. F., A. R. Townsend, J. G. Elvin, C. R. Rizza, J. Gallwey, and A. J. McMichael. 1988. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature 336:484-487. [DOI] [PubMed] [Google Scholar]
- 35.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 36.Palese, P., and A. García-Sastre. 2002. Influenza vaccines: present and future. J. Clin. Investig. 110:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park, M. S., A. García-Sastre, J. F. Cros, C. F. Basler, and P. Palese. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park, M. S., M. L. Shaw, J. Munoz-Jordan, J. F. Cros, T. Nakaya, N. Bouvier, P. Palese, A. García-Sastre, and C. F. Basler. 2003. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J. Virol. 77:1501-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson, L. J., N. Malkevitch, D. Venzon, J. Pinczewski, V. R. Gomez-Roman, L. Wang, V. S. Kalyanaraman, P. D. Markham, F. A. Robey, and M. Robert-Guroff. 2004. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 78:2212-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pecora, A. L., N. Rizvi, G. I. Cohen, N. J. Meropol, D. Sterman, J. L. Marshall, S. Goldberg, P. Gross, J. D. O'Neil, W. S. Groene, M. S. Roberts, H. Rabin, M. K. Bamat, and R. M. Lorence. 2002. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol. 20:2251-2266. [DOI] [PubMed] [Google Scholar]
- 41.Percy, N., W. S. Barclay, A. García-Sastre, and P. Palese. 1994. Expression of a foreign protein by influenza A virus. J. Virol. 68:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinaldo, C., X. L. Huang, Z. F. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, and M. Cottrill. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69:5838-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 44.Ryan, M. D., and J. Drew. 1994. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 13:928-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safrit, J. T., C. A. Andrews, T. Zhu, D. D. Ho, and R. A. Koup. 1994. Characterization of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte clones isolated during acute seroconversion: recognition of autologous virus sequences within a conserved immunodominant epitope. J. Exp. Med. 179:463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schirrmacher, V., T. Ahlert, T. Probstle, H. H. Steiner, C. Herold-Mende, R. Gerhards, and E. Hagmuller. 1998. Immunization with virus-modified tumor cells. Semin. Oncol. 25:677-696. [PubMed] [Google Scholar]
- 47.Schnell, M. J. 2001. Viral vectors as potential HIV-1 vaccines. FEMS Microbiol. Lett. 200:123-129. [DOI] [PubMed] [Google Scholar]
- 48.Seal, B. S., D. J. King, and H. S. Sellers. 2000. The avian response to Newcastle disease virus. Dev. Comp. Immunol. 24:257-268. [DOI] [PubMed] [Google Scholar]
- 49.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 50.Stratov, I., R. DeRose, D. F. Purcell, and S. J. Kent. 2004. Vaccines and vaccine strategies against HIV. Curr. Drug Targets 5:71-88. [DOI] [PubMed] [Google Scholar]
- 51.Talon, J., M. Salvatore, R. E. O'Neill, Y. Nakaya, H. Zheng, T. Muster, A. García-Sastre, and P. Palese. 2000. Influenza A and B viruses expressing altered NS1 proteins: a vaccine approach. Proc. Natl. Acad. Sci. USA 97:4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker, C. M., G. A. Thomson-Honnebier, F. C. Hsueh, A. L. Erickson, L. Z. Pan, and J. A. Levy. 1991. CD8+ T cells from HIV-1-infected individuals inhibit acute infection by human and primate immunodeficiency viruses. Cell. Immunol. 137:420-428. [DOI] [PubMed] [Google Scholar]
- 53.Wyand, M. S., K. H. Manson, A. A. Lackner, and R. C. Desrosiers. 1997. Resistance of neonatal monkeys to live attenuated vaccine strains of simian immunodeficiency virus. Nat. Med. 3:32-36. [DOI] [PubMed] [Google Scholar]