Abbreviations
- AFP
alpha‐fetoprotein
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- OPTN
Organ Procurement and Transplantation Network
- TT
transplantable tumor
- UCSF
University of California San Francisco
- UNOS
United Network for Organ Sharing
Training for details produces tunnel vision, and men of broader perspective are required for useful application of scientific progress.
Michael Shimkin
Nearly every day, the issue of liver transplantation (LT) for hepatocellular carcinoma (HCC) is debated worldwide during rounds, publications, meetings, and—more importantly—in front of patients with liver cancer who are seeking their doctors' advice, often after the digital information media have left them and their families empty‐handed.
Physicians have realized how their certainties can weaken and can strongly differ, regardless of whether the prediction of post‐LT outcome is applied to large populations or to single individuals with liver cancer. In addition, liver‐dedicated physicians with nontransplantation expertise may find themselves puzzled when dealing with the existing restrictions on the distribution of the scarce resource of donated organs.1, 2, 3 Allocation rules are in fact continuously released based on adjustments adopted within the transplantation community to maximize patient benefit—defined as an improvement in quantum of life in each patient independent of tumor stage—while avoiding harm to other patients who are waiting for a liver graft. Putting this into practice, the mission of doing justice in transplantation is attempted either through application of the utility principle (i.e., when organs are allocated to patients who have the best post‐LT predicted survival) or in adherence to the mandate to care for the “sickest patient first.”4
In transplantation candidates with HCC, the main obstacle to a smooth organ allocation is the lack of instruments able to determine, with sufficient detail, exactly how sick a patient is, how specific a given tumor presentation is, and how likely the tumor response to various treatments will be. Scores modulated on HCC characteristics have been proposed,4, 5, 6, 7, 8 but the estimation of the risk of pretransplantation dropout or posttransplantation benefit remains suboptimal. What is missing to fully accomplish the “nearly impossible mission” to frame the complex scenario of LT for HCC is the ability to capture, in a weighty manner, the evolution of a given cancer in relation to treatment, as this could be the main driver to predict posttransplantation outcome in patients who have cirrhosis with HCC, similarly to what the Model for End‐Stage Liver Disease (MELD) system does in noncancer candidates. Notably, the lack of precise prognostication tools for transplantation candidates with HCC has been repeatedly reported as causing detrimental effects for non‐HCC patients, who may be disfavored by unbalances in points systems that oversupply cancer patients.4, 9
Looking at the magnitude of the information presented on LT for HCC and at its diffuse interpretation, any further attempt to create new prognostication scores seems inadequate unless a precise description of the objective granularity of tumor presentation and of its spectrum of responses to different treatment options is taken into account. Also, as modern discussions on LT in HCC move into the broaden concept of a medicine made of economic, social and ethical components, a more accountable description of the tumor conditions could be instrumental to move the scale of priorities into a more realistic and treatment‐oriented approach to HCC.
Priority in organ allocation and patient selection are crucial factors that are difficult to merge because they have endpoints that are inherently far from one another. Optimization of a given resource in the case of organ allocation and maximization of outcomes when patient selection is targeted are in fact the driving forces that tend to split LT for HCC apart. Yet the attempt to reconcile these postulates is often referred to as an effort to “square the circle.”
In this article, the impossible task to square the closed circle of patient selection and graft allocation in LT for HCC is approached as it would be during a math class, solving this same problem by multiplying the square of the radius (r2) by π: an irrational number (i.e., a number that never ends) used to approximate a solution that otherwise would be to the infinitum. To do this, a comprehensive assessment of HCCs examined for transplantation is proposed.
In the proposed model, tumor presentation and response to therapy are used as a “π”: a sort of rectifying factor to be used within the challenging contexts of listing and prioritization, with the aim of improving their mutual efficiency in optimizing patient outcome and resource allocation in the field of LT for HCC.
Background: The Fruits of Long Endeavors
The likelihood of patient survival after transplantation remains an essential criterion when deciding on LT for HCC and represents the most important factor for indicating such a demanding therapeutic option.1, 3, 4, 10 About two decades ago, the Milan criteria defined the benchmark for achieving the best post‐LT survival in HCC.11 Since then, these restricted criteria (single nodule ≤5 cm or multiple [≤3] nodules ≤3 cm in size) have become the best predictor of excellent post‐LT outcome and cost‐effective transplantation. This result strongly influenced staging systems for HCC, guidelines, recommedations, and allocation policies for deceased donor liver grafts.12, 13, 14
Starting with the University of California San Francisco (UCSF) criteria (single nodule ≤6.5 cm; 2 to 3 lesions each ≤5 cm or 4 to 5 lesions each ≤3 cm, with the maximum sum of diameters ≤8 cm in all cases)15 multiple other metrics have been established over time in an attempt to predict the results of LT for HCC.16 Most of these metrics have shown the same good survival results achieved when restricted indications were met, even though subsequent observations revealed a progressively increased rate of cancer recurrence in tumors transplanted beyond conventional limits.
Clearly, the expanded criteria mechanism built on pure morphologic tumor indexes (i.e., largest diameter and number of tumor nodules) did not help in defining which patients with cirrhosis and HCC beyond the Milan criteria should be offered LT first, but did unravel the negative prognostic influence of biological and pathological features rarely observed in patients meeting conventional criteria (such as high alpha‐fetoprotein [AFP] serum level, presence of microvascular invasion, and poorly differentiated [G3] tumors).4, 16
Conventional selection criteria have persisted, however, in guidelines and organ procurement organization policies, while the “transplantable HCC” category (i.e., curable with transplantation only) has been enriched over time, with cases at worse prognosis (i.e., T3 stage according to the United Network for Organ Sharing [UNOS] system) defined as transplantable on the basis of local dynamics of the waiting list that did not prejudice other noncancer recipients with a better prognosis.17 It is conceivable that 25%‐40% of the current HCC patients listed for LT belong to such a T3 subgroup receiving exception points and presumably some form of tumor downstaging.4, 18
The introduction of direct antiviral agents in daily practice19 will further increase organ availability for patients with HCC in the near future, as a significant number of patients with decompensated cancer‐free hepatitis C virus (HCV) cirrhosis will likely be inactivated and delisted within 1 year just by the introduction of second‐generation direct antiviral agents. In a recent multicenter European study, it was estimated that 33% and 25% of HCV cancer‐free listed cirrhosis will be inactivated or delisted, respectively.20 In parallel, the practice of downstaging tumors in patients who were originally thought to be ineligible for transplantation21 will increase the number of borderline HCC cases presented to liver transplantation boards for decision.
Emboldened by its own success, transplantation for HCC—a neglected indication just 20 years ago—is likely to become the leading indication for liver replacement in the near future.
The Inverse Perspective of Case Selection: From Tumor Presentation to Response to Therapy
Tumor subclasses correlating with diverse molecular assays and clinicopathologic behavior have been discovered progressively,22, 23 with gene signatures also playing a role in the prognostication of LT patients beyond the Milan criteria.24 However, the extreme molecular heterogeneity of HCC still represents a significant limitation to the full introduction of precision medicine in patients within the transplantation landscape.23 Despite the absence of reliable biomarkers or genetic alterations influencing clinical decisions, a different kind of individualized medicine has progressively gained credit from multidisciplinary tumor board discussions in which all tumor and individual characteristics of each patient are weighed by different specialties and routed to variegated therapeutic alternatives. Perhaps the less known but most relevant result of this approach is the inverse perspective that has emerged in centers with a large referral of HCC patients regardless of their indication for transplantation.
In practice, rather than considering up front patients with HCC as being eligible for LT according to disease presentation, most patients with HCC remain within the spectrum of eligibility for LT—the exclusions determined only by macrovascular invasion, extrahepatic spread, comorbidities, and age beyond limits—and are assigned to different forms of combined therapy that, if sufficiently effective within a certain time, may allow liver transplantation listing.
A flexible approach aimed at merging tumor stage and results of treatment is going to be adopted in a large European region25 and is based on the observations that post‐LT survival outcomes in HCC beyond Milan criteria with objective and sustained response to pre‐LT therapy are not significantly different compared with those patients who meet conventional criteria at presentation.17 In order to avoid the risks of uncontrolled expansion of HCC criteria, such an “inverse selection approach” based on response to therapy requires a few restrictions:
All suitable patients with cirrhosis who have treatable HCC by nontransplantation means should be treated, regardless of whether LT is in their therapeutic future. The best available option (i.e., monotherapy or combination therapy) should be determined after thorough multidisciplinary discussion.
A minimal observation period after the conclusion of a given (combination) treatment is mandatory, because time is a surrogate of tumor aggressiveness and therefore an additional factor in the selection process.17, 21, 23 Time as a covariate is also required to assess tumor response and evolutionary posttreatment outcome.23
All possible information on tumor biology should be collected and discussed before the board. With this particular aim, absolute values and variations of AFP serum levels over time, as well as tissue biopsies (obtained during percutaneous ablation, laparoscopic staging, or resection), should be collected.26
The minimal expected survival for patients undergoing LT under this conditions should be increased from the conventional limit of 50% at 5 years to 60% or higher. In doing so, the benefit achievable in HCC beyond conventional criteria could be adjusted to acceptable levels of posttransplantation utility4, 7, 25 while avoiding any harm to patients who remain on waiting lists.17, 27, 28
Transplantable Tumors
Based on these and other observations, a possible reappraisal of patients with HCC who are eligible for and curable with transplantation could be attempted within a comprehensive frame able to capture the large majority of tumor presentations and describe in a simplified manner the granularity of possible responses to therapy. Figure 1 presents a scale of HCC disease severity that is designed to prioritize classes capable of routing organ allocation by means of points systems determined according to national and regional scenarios. Accordingly, transplantable tumors (TTs) will be defined following the Milan, UCSF, or expanded criteria together with the donor rate in each allocation area, the proportion of enlisted HCC/non‐HCC patients and the dynamic of the waiting list.
Figure 1.
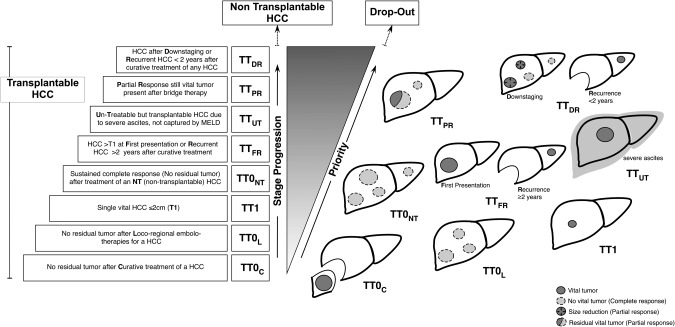
Staging and allocation for HCC within the spectrum of LT eligibility. Classes of progression and allocation priority within the TT stages identified for HCC in well‐compensated cirrhosis. LT eligibility and priority are not determined completely up front, but they both come into focus after the best available therapy has been applied. Details on application rules are given in Table 1.
Classes of progression within TTs range from patients with “zeroed” HCC (i.e., disease completely removed by surgery, ablation, or embolo‐therapies) to patients carrying conventional criteria tumors either at diagnosis or as late recurrence (after >2 years from a previous curative treatment) considered as de novo cancers on cirrhotic oncogenic livers rather than intrahepatic metastases.23, 29 Finally, patients in whom transplantable criteria are still met—whether at inception or after complete or near‐complete response to downstaging treatment—should be ranked as the highest priority. These are patients who have achieved a suboptimal response (i.e., a partial response) despite adequate locoregional treatment or patients presenting with early cancer recurrence (≤2 years from a previous curative treatment) and whose cumulative tumor staging still correspond to a potentially transplantable tumor. The main principles governing what can be called an adaptive approach are summarized in Table 1, which should be consulted in conjunction with Figure 1.
Table 1.
Application Rules for Staging and Allocation
| 1 | The system applies only to early and intermediate stage HCC presenting in compensated cirrhosis/chronic liver diseases (stages BCLC‐A and BCLC‐B). Exclusion criteria are vascular invasion, extrahepatic spread and comorbidities. HCC arising in decompensated (Child‐Pugh class C) cirrhosis is determined by laboratory MELD score and receives priority accordingly. |
| 2 | In principle, any HCC arising in compensated cirrhosis is considered as TT once inclusion/exclusion criteria are satisfied. Morphology criteria (i.e., tumor size and number) used for transplantation eligibility should be defined a priori at a regional level depending on the dynamics of the waiting list, proportion of enlisted HCC versus non‐HCC patients, harm to patients who remain on the waiting list, donor availability, etc., and should not be modified at any time during patient follow‐up (i.e., up to LT, dropout, or death). Morphology criteria reported objectively should be integrated with pathologic/biologic information (e.g., tumor biopsy, AFP levels) when available, and all information should be discussed before the tumor transplantation board (see points 3 and 4 below). AFP cutoff levels able to exclude transplantation eligibility even in presence of permissive tumor morphology conditions should be defined a priori as well, as that limit currently ranges from 200 to 1000 ng/mL21, 32, 39, 41, 42, 43 or according to steady increase over time.32 |
| 3 | All TT should be treated with the single/combined best available treatment according to internal protocols and/or accepted guidelines and should be reconsidered for class assignment at the end of each treatment course. Accordingly, any decision regarding treatment of a TT should take into account the transplantation implications before and after therapy courses. |
| 4 | Reproducible criteria for imaging, diagnosis, classification, and reporting in HCC before and after treatment should follow common accepted standards determined a priori 30, 31 and should also consider the contribution of tumor growth rate49 and patterns of residual disease determination.50 Digital imaging should be accessible for internal or external audits. |
| 5 | If TT are not treatable due to technical or medical reasons not captured by MELD score (i.e., ascites), the patient should be classified as having untreatable HCC (TTUT) and prioritized accordingly. |
| 6 |
Point assignment and priority class should be managed dynamically, because disease status may change over time depending on biology and therapy. Stepwise assessments should be undertaken at a minimum of four possible time points: a) at tumor presentation (baseline assessment), if TT meets points n.1 and 2 above; b) in stabilized tumor conditions (i.e., stable disease for a sufficient period of time [at least 3 months])7, 17, 21; c) in case of tumor progression during treatment;a d) at the end of each treatment course. |
| 7 | Patients included in downstaging protocols should be considered as TT0NT (intermediate priority) in case of complete response at the end of treatment—due to the initial tumor stage exceeding conventional criteria—or as TTDR (high priority) in case of suboptimal downsizing and/or residual tumor remaining reasonably stable over time in patients still meeting transplantation criteria. For patients included in “extended limits for downstaging” protocols, LT listing could be considered only after complete response and if part of prospective investigations. |
| 8 | Because changes that occur in serum AFP levels while patients are on the waiting list correspond closely to changes in posttransplantation mortality,51 AFP trends should parallel radiologic tumor response (or progression) of a transplantable tumor during treatment and/or follow‐up. In principle, patients who have a major drop in AFP level after treatment should be considered at a more significant level than those who do not. In patients included in downstaging protocols (see point 7 above), differential drop and absolute APF level could help in discriminating various levels of response—and priority‐among different patients with similar radiology‐assessed posttreatment response. |
| 9 |
Recurrent HCC should be approached similarly to naïve HCC, with identical treatment aims and general requirements as listed above in points 1‐5. Recurrent HCC may be classified as TTFR or TTDR according to the time of recurrence, whether this is ≤2 years (i.e., early recurrence) or >2 years (i.e., late recurrence) from the original curative treatment. This yields different priorities because of the higher risk of dropout in early recurring tumors. • Early recurrences should be listed only if the tumor meets transplantable criteria both at the time of original treatment and after cumulative staging, which is calculated at the time of transplantation consideration. The cumulative stage of an early recurring HCC considers one tumor entity as the sum of the first presenting HCC + recurrent tumor. • Late recurrences should be listed if meeting transplantation criteria at the time of transplantation consideration, as they could be rated as TTFR regardless of the stage of the first‐presenting HCC curatively removed >2 years ago. |
| 10 | Exceptions to the general frame of stage progression and priorities are allowed with approval from a regional reviewer board. In the current scenario, exceptions may be related to: experimental de principe transplantation strategies applied to resected tumors (TT0c); observational strategies pausing treatment for <2 cm lesions (TT1); downgrade in priority for recurrent although nontreatable HCCs; complete posttreatment responses of segmental portal vein thrombosis; and salvage surgery to achieve complete response in TTPR tumors (and consequent reduction in TTNT stage, etc.). |
Rules apply to the system shown in Fig. 1.
HCC progression should be rated in order of severity as (A) progression of the treated tumor; (B) appearance of an additional nodule; (C) evidence of vascular invasion; or (D) extrahepatic tumor spread. In this model, tumor progression types A and B may indicate further treatment, upgrade in priority, or dropout depending on whether the detected progression still meets transplantation criteria; progression types C and D exclude the patient from transplantation consideration (i.e., dropout from the waiting list).
Following the line of reasoning proposed in this approach, the heterogeneities of HCC's presentations and treatments are routed into different subclasses that include current standards, variations in local capabilities and resource allocation, and evolutionary conditions related to treatment response, as well as HCC tumor recurrence, AFP fluctuations, and objective tumor assessment with up‐to‐date radiology criteria.30, 31, 32 Although with different relative weight, all these conditions play a role in the current decision‐making on LT for HCC and are incorporated in a system in which transplantation eligibility and priority are not completely determined up front but they both come into focus after all relevant components have been considered and therapy has been applied.
Such a definition of a “transplantable tumor” is inherently open to more specific contribution of staging and allocation updates and is in line with recent UNOS policies of HCC exceptions, in which inclusive tumor subclasses of “growing,” “treated,” and “exceeding T2” have been identified to increase convergence among tumor staging parameters and points in priority (i.e., classes 5A‐g, 5T, and 5X in the Organ Procurement and Transplantation Network [OPTN] Policy 2015) for liver allocation in HCC1 in which standardized radiology criteria for imaging, diagnosis and classification are incorporated.31
Priority as a Function of Allocation Principles
Questions may arise regarding the scale of priority in the proposed approach. Indeed, priority itself is a concept with several practical applications, depending on which allocation principle prevails. In addition, the priority endpoints may be weighted differently, ranging from the minimization of pre‐LT risk to drop out (or death) when the urgency principle is adopted to the maximization of post‐LT outcome in case the utility is targeted, particularly for the transplantation benefit— namely, the net survival obtained by subtracting the survival achieved with LT by the survival obtainable with nontransplantation options.23, 33, 34
In addition, patients, physicians, and society at large significantly influence the perception of priority as well as decision‐making with subjective convictions. Complex statistical models have been advocated to balance all of these components; however, controversies persist, because within this context even a very light shift in the design of surveys or variation in predetermined assumptions lead to quite different conclusions.33, 34, 35
In the proposed concept of TTs, a series of relatively straightforward observations support the existence of a continuity in HCC subgroups whose severity—and consequently priority—evolves, not only as a consequence of tumor biology, but also as a product of medical and surgical interventions. The list of premises upon which each priority class is identified is summarized in Table 2.
Table 2.
Staging and Priority Classification of HCC in the LT Setting: Patient Stratification According to Allocation Principles
| TT Categories | Priority According to HCC Dropout Models | Priority According to Transplantation Benefit | Priority Perception of Patient and Societal Expectations |
|---|---|---|---|
| TT0C | Very Low | Low | Low |
| No residual tumor after curative treatment of HCC | Very low risk of dropout in cured HCC | Transplantation benefit depends on MELD score only | The patient should not undergo transplantation |
| TT0L | Low‐Intermediate | Low | Intermediate |
| No residual tumor after locoregional embolo‐therapies for transplantable HCC | Low risk of dropout in cured HCC | Transplantation benefit depends on HCC‐MELD | The patient was eligible for transplantation but can be placed on hold because the tumor seems to be cured |
| TT1 | Low | Low | Low |
| Single HCC ≤2 cm | Low risk of dropout in very early HCC | Low benefit in presence of alternative nontransplantation treatments | The patient should not undergo transplantation if there are other treatment options |
| TT0NT | Not Applicable | Low | Low |
| No residual tumor after treatment of a nontransplantable HCC (successful downstaging) | NT HCC should not be listed up front, similarly to non‐HCC in patients with low MELD scores | Transplantation benefit depends on MELD score only | The patient was not eligible for transplantation and has been cured by other means |
| TTFR | Intermediate | Intermediate | High |
| Transplantable HCC > T1 at first presentation or recurrent HCC >2 years after curative treatment | Demonstrated increase of dropout risk over time for both size and number parameters | Benefit depends on true applicability of alternative treatments | This patient has the best posttransplantation survival (utility) |
| TTUT | Intermediate | High | High |
| Transplantable HCC judged untreatable for reasons not captured by MELD (i.e., ascites) | Increased dropout risk; short time to liver decompensation | There is no therapeutic alternative for HCC | The patient is expected to have good utility posttransplantation |
| TTPR | Intermediate/High | High | High |
| Partial response after complete bridge therapy in a transplantable tumor | Risk of selection of biologically aggressive clones with increased proliferative activity | Failure of a bridge therapy with no residual therapeutic alternative | The patient is expected to have good utility posttransplantation |
| TTDR | Intermediate/High | High | High |
| Transplant eligibility after downstaging (sustained partial response) or recurrent HCC <2 years after curative treatment of any HCC | High dropout risk over time for both size and number parameters | Benefit depends on absence of true alternative treatments | Transplantation should be offered in relatively stable patients before it is too late |
After all, the apparent distance between the assumptions defining each priority class can be normalized through composite evaluations of the need of transplantation that correspond to a stepwise increment of a numeric point scale. Obviously, the entity of progression into the priority scale has to be determined at a regional and/or national level.
Selection and Allocation Principles Reconciled: Has the Circle Been Squared?
Over the years, the growing number of analyses advocating adjustments not just in selection but also in allocations rules for LT in HCC, has elicited the current effort to place transplantation decisions for patients with HCC within a modern perspective. The assumption is that transplantation eligibility and allocation in HCC could be moderately loosened without undue prejudice to other recipients; this is very likely to occur, as we will soon witness a net decrease in transplantation indication for HCV‐related cirrhosis and an increase in the practice of downstaging HCC. The proposed system might be capable of transforming downstaged tumor responses from exceptions to drivers for both the selection and allocation processes (Table 1, points 7 and 8).
The attempt to square the circles and reconcile tumor stage, effects of treatment, and priority in allocation is by definition imperfect, and there is an actual risk that several factors may render this proposal into wishful thinking. In order to consolidate the model, the following important areas will need to be implemented.
System Solidity and Flexibility Should Be Reinforced
Although inclusive by all means, the application of the proposed model of priority leans slightly toward transplantation benefit (i.e., utility‐based) endpoints.4, 23, 33, 34 This may be criticized by those who consider crude long‐term survival as a more important target to be achieved compared with life‐years gained.17, 36 However, the redefinition of transplantable HCC by way of pretransplantation treatment, rather than precluding ideal transplantation candidates from such a curative option simply delays their priority in favor of patients who are still within transplantation criteria but are at a higher risk of dropout due to tumor progression or incomplete response at the end of successful treatment courses (see also Table 1). In fact, a benefit‐oriented approach combines both pre‐ and posttransplantation outcomes, integrates the results of alternative treatment strategies, makes obvious the unacceptable survival targets for transplantation, and allows a comparison between patients with and without HCC.7, 23, 29 Following the same line of reasoning, a variable age threshold beyond which the net benefit for HCC patients does not justify LT over resection or ablation seems questionable, at least until a transplantation‐ versus nontransplantation‐related benefit comparison is adjusted on reference cohorts derived from the general population matched by sex, age, and years of diagnosis. It is worth noting that within the context of patient benefit, both the absolute number of years of life gained and years of life lost with respect to life span (i.e., relative survival) should be considered when assessing the efficacy of surgical therapies offered to older patients.37
Conversely, a more realistic wait time and priority score based on assumptions shown in Table 2 could silence both the utilitarian and urgency aims while including patients and societal perspectives—a process that seems essential to improve the flexibility of the transplantation system in HCC with respect to noncancer indications.
Common Criteria for Staging, Treatment, and Response Assessment Should Be Adopted
In the proposed system, each patient with HCC who has reasonably stable cirrhosis stays within the heterogeneous group of TTs while staging and priority is determined according to the results of the applied therapies. This means that the only condition required to obtain an optimal result is the management of patients with similar pretransplantation locoregional treatments, response, monitoring, and delisting criteria.17, 25, 30, 31, 38 Such practices should be enforced within each liver allocation system and among HCC referral centers, as they are essential to help the implementation of the models detailed above.
Transplantation Criteria and Minimum Accepted Survival Should Be Defined
A precise definition of transplantation criteria should be determined a priori within large geographic regions according to the principles detailed above (Table 1, point 2). This definition should incorporate a minimum expected survival (which should target at least 50% at 10 years, rather than 5 years) and also the likelihood of complete/partial response achievable with nontransplantation treatment strategies.
The use of treatment response as a selection tool to complement other prognostic pathologic/biologic covariates in patients exceeding Milan and/or UNOS criteria appears crucial to promote the use of expanded HCC criteria on a routine basis. Considering the unfeasibility of randomized trials in this field, the search for a credible hierarchy within the expanded criteria (i.e., up to seven, total tumor volume, morphology adjusted on AFP7, 23, 39, 40) could be aided by comparing of prospective cohorts generated by variegated applications of these criteria to the presented model.
Limits for Downstaging Strategies Should Be Agreed Upon
Because downstaging is a strategy aimed at selecting more favorable tumor biology, the downstaged HCC population must be a fraction of the total amount of patients with HCC who have a potential indication to transplantation. By definition, downstaged patients are at higher risk of dropout, and opposite success rates are observed depending on whether more restrictive or more relaxed tumor burdens are targeted as endpoints.
Restrictive criteria for downstaging eligibility should be agreed upon within the framework of the adopted transplantation criteria. Evidence supporting this statement has come from the most recent update of the UCSF downstaging protocol, in which the upper limit to indicate downstaging was determined up front (see “Background: The Fruits of Long Endeavors” section). In this experience, 65% of patients with HCC were converted to Milan criteria, whereas only 7.5% of patients had posttransplantation tumor recurrence.21
Whatever strategy of downstaging will be determined, AFP trends over time should parallel radiologic tumor response (or progression) during treatment and at the time of the final prelisting assessment. AFP cutoff levels able to exclude transplantation eligibility even in the presence of permissive tumor morphology conditions should be defined a priori as well, as that limit currently ranges from 200 to 1000 ng/mL21, 32, 39, 41, 42, 43 or according to steady increase over time.32
Waiting Time Duration and Posttransplantation Tumor Recurrences Should Be Monitored
It is well known that HCC variables predicting dropout from the waiting list are also associated with poorer posttransplantation survival and a higher tumor recurrence rate,44, 45 with dropout largely depending on waiting time length. Therefore, the dynamic shifts of transplantation candidates with HCC within the proposed frame implies an even closer monitoring of waiting times and posttransplantation outcomes. Optimization of waiting list time and post‐LT results will minimize tumor recurrence, even though a minimal observation time for disease stabilization after treatment is highly suggested, to decrease the risk of selecting patients with rapidly progressing lesions. The length of the “no transplantation” observation period should be determined on a regional basis and should consider the current standard of 3 months.3, 46
Envisioned Future and Conclusion
To some extent, the history of disease comprises a metamorphosis in treatments and paradigm shifts anticipating by far the conclusions of large and structured clinical investigations. For transplantation in the particular setting of HCC, this model proposed herein is consistent with previous reports47, 48 and will surely undergo validation studies in a large European region according to a strategy that is in line with modern perspectives of HCC management. Figure 2 summarizes such a change in perspective as it relates to the two most paradigmatic conditions in which patients are within or beyond predetermined transplantation criteria. Even at first glance, the envisioned future of these two conditions appears to be enriched by expansions of therapeutic options, transplantation selection, listing, and priority (Tables 1 and 2 provide additional details in this respect). Future studies are warranted to ascertain whether this perspective may develop into an implementable system capable of incorporating the current complexities of HCC management into LT mechanisms.
Figure 2.
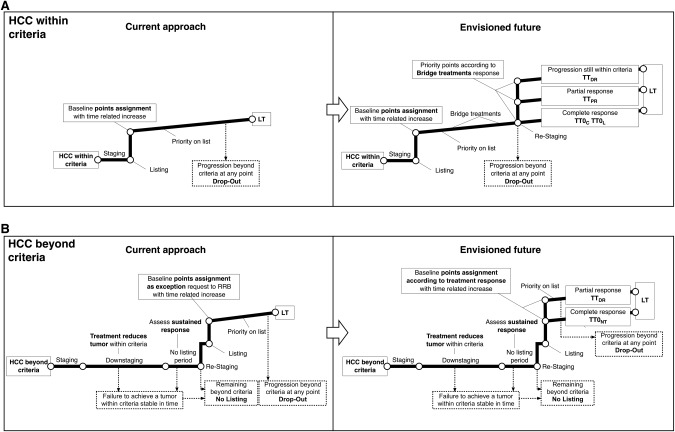
Paradigm shift in the management of LT in patients with HCC. The multistep process covers the diagnosis of HCC to LT in patients who are within (A) and beyond (B) the criteria. With respect to the current rules, the proposed system uses tumor response to bridging or downstaging as the main drivers for patient selection and priority allocation.
Over the last two decades, tangible advancement in survival and in knowledge of HCC have been fueled not only by major scientific achievements but also by changes in the way physicians have dealt with the role of LT in liver cancer therapy. The shift in perspective explained in Figs. 1 and 2 is geared toward maximizing all tumor and therapy heterogeneities in a model that utilizes variations in HCC presentation and response to treatment as adjusting factors to reconcile selection and allocation logistics, with the ultimate aim of increasing the benefit, effectiveness, and justice of transplantation for cancer. As new factors emerge and show significant impacts on HCC, treatment strategies with an even more pronounced passion for details should be employed. It is difficult to think of an investment in transplantation that would have a greater impact than one that creates robust frames within which to improve the quality of medical indications and resource allocation for patients with liver cancer.
Acknowledgment
I would like to thank many people for their contributions to the concepts reported in this article, in particular Sherrie Bhoori, Marco Bongini, and Carlo Sposito, who collaborated on constructive thoughts and early reads. The opinions reported in this article have been discussed with members of the Italian Board of Experts in the field of Liver Transplantation, in particular Luca S. Belli, Patrizia Burra, Lucio Caccamo, Matteo Cescon, Alessandro Cucchetti, Umberto Cillo, Luciano De Carlis, Michele Colledan, Tullia De Feo, Alessandro Nanni Costa, Antonio D. Pinna, Enrico Regalia, Renato Romagnoli, Giorgio Rossi, Massimo Rossi, Marco Spada, and Pierluigi Toniutto. I also thank Massimo Colombo, Richard B. Freeman, Gianluca Grazi, Pietro Majno, Sasan Roayaie, Riad Salem, Mario Strazzabosco, Juan C. Garcia‐Valdecasas, and Francis Y. Yao for productive discussions.
Potential conflict of interest: Dr. Mazzaferro is on the speakers' bureau for Bayer and BTG.
This study was supported in part by the Italian Association for Cancer Research, INT‐Milan, and the Italian Ministry of Health.
REFERENCES
- 1. Organ Procurement and Transplantation Network . Policy 9: allocation of livers and liver‐intestines. http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf#nameddest=Policy_09. Accessed December 7, 2015.
- 2. Organ Procurement and Transplantation Network . Guidance on MELD PELD exception review. http://optn.transplant.hrsa.gov/resources/by‐organ/liver‐intestine/guidance‐on‐meld‐peld‐exception‐review/. Accessed December 7, 2015.
- 3. Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 2010;16:262‐278. [DOI] [PubMed] [Google Scholar]
- 4. Toso C, Mazzaferro V, Bruix J, Freeman RB, Mentha G, Majno PE. Toward a better liver graft allocation that accounts for candidates with and without hepatocellular carcinoma. Am J Transplant 2014;14:2221‐2227. [DOI] [PubMed] [Google Scholar]
- 5. Freeman RB. Transplantation for hepatocellular carcinoma: the Milan criteria and beyond. 2006;12:S8‐S13. [DOI] [PubMed] [Google Scholar]
- 6. Piscaglia F, Camaggi V, Ravaioli M, Grazi GL, Zanello M, Leoni S, et al. A new priority policy for patients with hepatocellular carcinoma awaiting liver transplantation within the model for end‐stage liver disease system. Liver Transpl 2007;13:857‐866. [DOI] [PubMed] [Google Scholar]
- 7. Toso C, Dupuis‐Lozeron E, Majno PE, Berney T, Kneteman NM, Perneger T, et al. A model for dropout assessment of candidates with or without hepatocellular carcinoma on a common liver transplant waiting list. Hepatology 2012;56:149‐156. [DOI] [PubMed] [Google Scholar]
- 8. Vitale A, Volk ML, De Feo TM, Burra P, Frigo AC, Ramirez Morales R, et al. A method for establishing allocation equity among patients with and without hepatocellular carcinoma on a common liver transplant waiting list. J Hepatol 2014;60:290‐297. [DOI] [PubMed] [Google Scholar]
- 9. Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end‐stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl 2012;18:434‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gentry SE, Chow EKH, Massie A, Luo X, Shteyn E, Pyke J, et al. Liver sharing and organ procurement organization performance under redistricted allocation. Liver Transpl 2015;21:1031‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693‐699. [DOI] [PubMed] [Google Scholar]
- 12. Omata M, Lesmana LA, Tateishi R, Chen P‐J, Lin S‐M, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bruix J, Sherman M, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. European Association for the Study of the Liver , European Organisation for Research and Treatment of Cancer . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908‐943. [DOI] [PubMed] [Google Scholar]
- 15. Yao FY. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394‐1403. [DOI] [PubMed] [Google Scholar]
- 16. Mazzaferro V, Bhoori S, Sposito C, Bongini MA, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence‐based analysis of 15 years of experience. 2011;(17 Suppl. 2):S44‐S57. [DOI] [PubMed]
- 17. Clavien P‐A, Lesurtel M, Bossuyt PMM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:E11‐E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halazun KJ, Patzer RE, Rana AA, Verna EC, Griesemer AD, Parsons RF, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology 2014;60:1957‐1962. [DOI] [PubMed] [Google Scholar]
- 19. Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model‐based predictions. Ann Intern Med 2014;161:170‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belli LS, Berenguer M, Cortesi P, Strazzabosco M, Rockenshaub S‐R, Martini S. Impact of direct anti‐viral agents on inactivation/de‐listing of liver transplant candidates listed for decompensated C cirrhosis: a European study. J Hepatol. In press. [Google Scholar]
- 21. Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, et al. Downstaging of hepatocellular cancer before liver transplant: long‐term outcome compared to tumors within Milan criteria. Hepatology 2015;61:1968‐1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet 2015;47:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014;63:844‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miltiadous O, Sia D, Hoshida Y, Fiel MI, Harrington AN, Thung SN, et al. Progenitor cell markers predict outcome of patients with Hepatocellular Carcinoma beyond Milan criteria undergoing liver transplantation. J Hepatol 2015;63:1368‐1377. [DOI] [PubMed] [Google Scholar]
- 25. Cillo U, Burra P, Mazzaferro V, Belli L, Pinna AD, Spada M, et al. A multistep, consensus‐based approach to organ allocation in liver transplantation: toward a “blended principle model”. Am J Transplant 2015;15:2552‐2561. [DOI] [PubMed] [Google Scholar]
- 26. Torbenson M, Schirmacher P. Liver cancer biopsy—back to the future?! Hepatology 2015;61:431‐433. [DOI] [PubMed] [Google Scholar]
- 27. Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant 2008;8:839‐846. [DOI] [PubMed] [Google Scholar]
- 28. Samuel D, Colombo M, El‐Serag H, Sobesky R, Heaton N. Toward optimizing the indications for orthotopic liver transplantation in hepatocellular carcinoma. Liver Transpl 2011;(17 Suppl. 2):S6‐S13. [DOI] [PubMed] [Google Scholar]
- 29. Mazzaferro V, Lencioni RA, Majno PE. Early hepatocellular carcinoma on the procrustean bed of ablation, resection, and transplantation. Semin Liver Dis 2014;34:415‐426. [DOI] [PubMed] [Google Scholar]
- 30. Purysko AS, Remer EM, Coppa CP, Leão Filho HM, Thupili CR, Veniero JC. LI‐RADS: a case‐based review of the new categorization of liver findings in patients with end‐stage liver disease. Radiographics 2012;32:1977‐1995. [DOI] [PubMed] [Google Scholar]
- 31. Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 2013;266:376‐382. [DOI] [PubMed] [Google Scholar]
- 32. Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant 2010;10:129‐137. [DOI] [PubMed] [Google Scholar]
- 33. Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant 2005;5:307‐313. [DOI] [PubMed] [Google Scholar]
- 34. Schaubel DE, Sima CS, Goodrich NP, Feng S. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant 2008;8:419‐425. [DOI] [PubMed] [Google Scholar]
- 35. Cholongitas E, Germani G, Burroughs AK. Prioritization for liver transplantation. Nat Rev Gastroenterol Hepatol 2010;7:659‐668. [DOI] [PubMed] [Google Scholar]
- 36. Schiano TD, Bourgoise T, Rhodes R. High‐risk liver transplant candidates: an ethical proposal on where to draw the line. Liver Transpl 2015;21:607‐611. [DOI] [PubMed] [Google Scholar]
- 37. Cucchetti A, Sposito C, Pinna AD, Citterio D, Ercolani G, Flores M, et al. Effect of age on survival in patients undergoing resection of hepatocellular carcinoma.. Br J Surg 2016;103(2):e93‐99. doi:10.1002/bjs.10056. [DOI] [PubMed] [Google Scholar]
- 38. Agopian VG, Morshedi MM, McWilliams J, Harlander‐Locke MP, Markovic D, Zarrinpar A, et al. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg 2015;262:536‐545. [DOI] [PubMed] [Google Scholar]
- 39. Duvoux C, Roudot‐Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including α‐fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986‐994. [DOI] [PubMed] [Google Scholar]
- 40. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35‐43. [DOI] [PubMed] [Google Scholar]
- 41. Toso C, Meeberg G, Hernandez‐Alejandro R, Dufour J‐F, Marotta P, Kneteman NM. Total tumor volume and alpha‐fetoprotein for selection of transplant candidates with hepatocellular carcinoma: a prospective validation. Hepatology 2015;62:158‐165. [DOI] [PubMed] [Google Scholar]
- 42. Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha‐fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mehta N, Dodge JL, Goel A, Roberts JP, Hirose R, Yao FY. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19:1343‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cucchetti A, Cescon M, Bigonzi E, Piscaglia F, Golfieri R, Ercolani G, et al. Priority of candidates with hepatocellular carcinoma awaiting liver transplantation can be reduced after successful bridge therapy. Liver Transpl 2011;17:1344‐1354. [DOI] [PubMed] [Google Scholar]
- 45. Cescon M, Cucchetti A, Ravaioli M, Pinna AD. Hepatocellular carcinoma locoregional therapies for patients in the waiting list. Impact on transplantability and recurrence rate. J Hepatol 2013;58:609‐618. [DOI] [PubMed] [Google Scholar]
- 46. Roberts JP, Venook A, Kerlan RK Jr, Yao FY. Hepatocellular carcinoma: ablate and wait versus rapid transplantation. Liver Transpl 2010;16:925‐929. [DOI] [PubMed] [Google Scholar]
- 47. De Giorgio M, Vezzoli S, Cohen E, Armellini E, Lucà MG, Verga G, et al. Prediction of progression‐free survival in patients presenting with hepatocellular carcinoma within the Milan criteria. Liver Transpl 2010;16:503‐512. [DOI] [PubMed] [Google Scholar]
- 48. Sala M, Llovet JM, Vilana R, Bianchi LS, Sol M, Ayuso C, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004;40:1352‐1360. [DOI] [PubMed] [Google Scholar]
- 49. Kappeler C, Healy DP, Baumer C, Meinhardt G, Elisei R, Schlumberger M, et al. Analysis of tumor growth rate for radioiodine (RAI)‐refractory differentiated thyroid cancer patients receiving placebo and/or sorafenib in the phase III DECISION study. ASCO Meeting Abstracts 2015;33:6015. [Google Scholar]
- 50. Riaz A, Memon K, Miller FH, Nikolaidis P, Kulik LM, Lewandowski RJ, et al. Role of the EASL, RECIST, and WHO response guidelines alone or in combination for hepatocellular carcinoma: radiologic‐pathologic correlation. J Hepatol 2011;54:695‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berry K, Ioannou GN. Serum alpha‐fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl 2013;19:634‐645. [DOI] [PubMed] [Google Scholar]


