Abstract
Background: Intimate partner violence (IPV) is a public health concern, affecting one-third of US women. Prior research suggests an association between exposure to IPV and poor maternal perinatal health, but the underlying biological correlates are not well understood. This study examined the relationship between exposure to IPV and proinflammatory cytokine levels, a candidate mechanism accounting for poor psychiatric and obstetric outcomes, across the perinatal period.
Materials and Methods: Data were obtained from a prospective, longitudinal cohort study of 171 women receiving obstetrical care from a hospital-based practice serving a predominantly low-income minority population. Participants completed questionnaires on IPV exposure, psychiatric symptoms, and psychosocial and obstetric factors and provided blood samples at 18 and 32 weeks of gestation and 6 weeks and 6 months postpartum. Serum levels of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) were assayed via enzyme-linked immunosorbent assay.
Results: Thirty-five (20.5%) women reported lifetime exposure to IPV and 7 (4.1%) reported being physically hurt in the preceding 12 months (4 while pregnant). Lifetime exposure to IPV was associated with increased likelihood of experiencing perinatal depression and smoking during pregnancy. Women with a history of IPV had significantly higher levels of TNF-α at 18 weeks (z = −2.29, p < 0.05), but significantly smaller changes in levels of IL-6 (β = −0.36, p = 0.04) across time.
Conclusion: Lifetime exposure to IPV was associated with a range of adverse mental health outcomes and may affect proinflammatory cytokine levels in pregnancy.
Introduction
Violence against women is a leading public health concern1,2 with one in three women (35.6%) in the United States reporting experiencing rape, physical violence, and/or stalking by a partner in their lifetime.3 There is conflicting evidence as to whether or not pregnancy increases the risk of experiencing violence compared with other periods in the life cycle, with studies showing increased,4 decreased,5 and similar6 rates. However, it is clear that violence during pregnancy is commonplace, affecting 4%–8% of women in the United States.7
Exposure to intimate partner violence (IPV) before and during pregnancy is associated with a range of adverse pregnancy outcomes, including obstetric complications,7–10 maternal psychiatric illness,10,11 and greater risk of preterm birth, lower birth weight, and infant mortality and morbidity.7–10,12–14 The biological correlates of exposure to violence for pregnant women that might account for these effects are not well understood, and studies to date have produced equivocal results.8 Dysregulation in several biological systems may explain the association between exposure to violence, psychological stress, and adverse perinatal health outcomes; two leading candidates are the hypothalamic–pituitary–adrenal (HPA) axis and immune system.15,16 In this study, we focus on the immune system and specifically inflammatory cytokines, markers of immune function that have been linked with the obstetric and psychiatric outcomes noted above15,17 and undergo considerable (normative) changes in the perinatal period.18–20
For many years, the prevailing model was that pregnancy was an immunosuppressive state. The Th1/Th2 model proposed that successful pregnancy was dependent on a shift to a Th2 (or anti-inflammatory) response17,20,21 that protected the fetus, but left the mother vulnerable to pathogenic attack.18,19 However, data to support this hypothesis were contradictory and it was felt to be an oversimplification of the complex and different immune responses that occurred across the trimesters of pregnancy.17,19–21
Recent data and theories suggest that pregnancy is a unique immune condition that is modulated rather than suppressed.17,20,21 Mor et al.21 propose that pregnancy should not be viewed as a single event, but rather in distinct phases. To summarize briefly, the first trimester is a proinflammatory phase, the second trimester is an anti-inflammatory state, and the late third trimester and initiation of parturition are proinflammatory states.21 Pregnancy is, therefore, both a proinflammatory and anti-inflammatory condition, with differential responses, depending on the stage of gestation.22,23
Empirical evidence on the exact changes in pro- and anti-inflammatory profiles throughout pregnancy is limited and, at times, equivocal,18–20 particularly in relation to stress exposure and psychiatric symptoms. Although there are dynamic changes between pro- and anti-inflammatory cytokines, much of the research on stress, trauma, and psychiatric symptoms in pregnancy has focused on proinflammatory markers.
Interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) were chosen as the focus of the current research because elevated levels of both of these proinflammatory cytokines have been linked with a range of perinatal health conditions. These include adverse obstetric and birth outcomes, including preeclampsia,24 spontaneous preterm birth,25,26 and gestational diabetes.27 IL-6 and TNF-α are among the cytokines produced by activated macrophages, which play a key role in pregnancy maintenance as well as containing receptors for both estrogen and serotonin.17,19,21 Elevated IL-6 and TNF-α have also been linked with antenatal mood symptoms, including stress, depression, and anxiety,28–30 postpartum mood, including blues, anxiety, and depression,31–35 and exposure to childhood trauma.36,37 There are several potential mechanisms through which cytokines may contribute to depressive symptoms, including cytokine-induced changes in 5-HT receptors and cytokine-induced activation of indoleamine 2,3-dioxygenase (IDO) (see Leonard and Maes38 for a review).
Several indirect lines of evidence support the hypothesis that IPV would be associated with an altered proinflammatory state in pregnant women. Nonpregnant women exposed to IPV are at increased risk of chronic health conditions, such as cancer and cardiovascular disease, which are characterized by increased inflammation.36–41 These changes appear to be persistent; that is, despite no longer being in abusive relationships, women exposed to IPV showed persistently increased C-reactive protein, IL-6,39,41 and increased interferon-γ production.39–42 However, these studies were conducted on nonpregnant and/or postmenopausal women and, as such, it is unclear whether the results are relevant to the perinatal period.
The current analyses add to the small but growing literature on the behavioral and biological correlates of exposure to violence in the prenatal period. Specifically, in a prospective longitudinal study, we examined the association between exposure to IPV and IL-6 and TNF-α. Changes in levels of IL-6 and TNF-α were examined and compared between women with and without a history of IPV on four occasions: at 18 and 32 weeks of gestation and at 6 weeks and 6 months postpartum.
Materials and Methods
Sample
Data were obtained from a prospective, longitudinal cohort study of pregnant women receiving obstetrical care from a hospital-based practice serving a predominantly low-income minority population.43 Because we were interested in the associations between IPV, cytokines, and psychiatric symptoms across the perinatal period in a normal (i.e., nondiseased) population, we excluded women with medical conditions that could themselves alter cytokine levels and potentially confound the relationship being tested, for example, HIV, cancer, type 1 diabetes, rheumatoid arthritis, multiple sclerosis, asthma, and lupus. For the same reason, we excluded women who were on steroids to treat/manage a medical condition. All potential exclusions were reviewed by three members of the study team, including a board-certified specialist in Maternal-Fetal Medicine and an immunologist (J.A.M.) following a review of medical notes. In addition, because high medical risk (e.g., prenatal drug use) and adverse clinical obstetric outcomes (e.g., premature labor and delivery, premature rupture of membranes, preeclampsia, stillbirth) may confound any association between IPV and cytokines, these women were also excluded. In practice, however, very few women were excluded for these medical conditions, and the results were not substantially different according to whether or not we excluded the few women with abnormal obstetric outcomes. We also excluded women with a history of psychotic disorder as determined from case notes or clinical interview. Therefore, women included in analyses were considered low to medium obstetric risk by the medical team using standardized definitions (NIH, ACOG), aged 19–34 years, had a confirmed singleton pregnancy, less than 18 weeks of gestation at enrollment, were fluent in English, and able to provide informed consent.
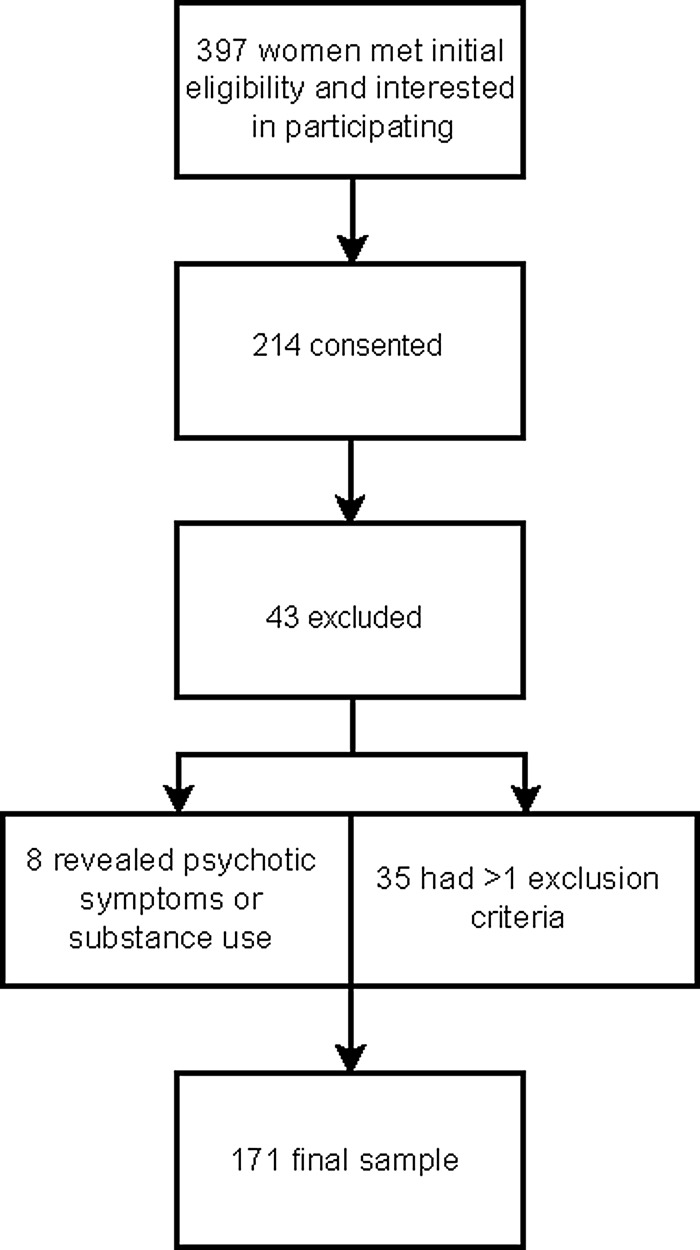
Nursing staff in the obstetric clinic provided an overview of the study to all clinic attendees who met initial inclusion criteria and asked if they were interested in learning more about the study. Study team members met with 397 pregnant women who met initial eligibility criteria and expressed an interest in participating in a research study. After receiving a full explanation of the study procedures, 214/397 (53.9%) women consented to participate in this study. Thirty-five women (35/214, 16.4%) were excluded after the first interview because they had at least one exclusion criterion that was not previously detected, or suffered a perinatal loss, or changed the obstetric provider, or were lost to contact before the clinical interview assessment. Additionally, eight women were excluded following subsequent interviews that revealed the presence of psychotic features or drug abuse that had not been previously disclosed or identified. The final sample included 171 women (Fig. 1). All procedures were approved by the University of Rochester Institutional Review Board.
FIG. 1.
Flowchart depicting enrollment.
Procedures and measures
The women were interviewed on four separate occasions: twice in pregnancy at 18 and 32 weeks of gestation (±1 week) and twice in the postpartum period at 6 weeks and 6 months (±1 week). Assessments were conducted by highly trained clinical interviewers, and participants completed the same series of psychosocial questionnaires and a semistructured diagnostic clinical interview (Structured Clinical Interview for DSM [SCID])44 at each assessment.
Clinical diagnoses of current and past history of depression (major, minor, or not otherwise specified) and generalized anxiety disorder (GAD) were made according to DSM-IV-TR from the mood disorders section of the SCID. Exposure to traumatic events was elicited through the post-traumatic stress disorder (PTSD) section of the SCID. A description of each event that a woman had experienced and the age at which it occurred were recorded. We defined a traumatic event as meeting criterion A1 of the diagnostic criteria for PTSD (309.81). This criterion includes exposure to an extreme traumatic stressor involving direct personal experience of an event that involves actual or threatened death or serious injury or other threat to one's physical integrity; witnessing an event that involves death, injury, or a threat to the physical integrity of another person; or learning about an unexpected or violent death, serious harm, or threat of death or injury experienced by a family member or other close associate (pp. 428–429). For the purposes of these analyses, we created a separate variable that included traumatic exposures other than IPV.
Depressive symptoms at 32 weeks of gestation were assessed using the Edinburgh Postnatal Depression Scale (EPDS).45 A 10-item self-report questionnaire, including an anxiety subscale, the EPDS assesses symptoms in the preceding 7 days and is the most widely used and validated screening tool for depressive symptoms in pregnant and postpartum women.
Interviews took place in the Clinical Research Center (CRC) located within the same hospital in which they received their obstetric care; blood samples were taken by CRC nursing staff during these assessments. Detailed clinical, medical, and sociodemographic data were obtained from interview and medical notes.
Exposure to IPV
At each assessment, women were asked five questions about their history of partner violence. Lifetime exposure to IPV was ascertained through the question “Have you ever been emotionally or physically abused by your partner?” Based on their response (yes/no), participants were divided into two groups, those exposed and not exposed to IPV. Recent exposure to physical violence from their partner was ascertained through the question “Have you been hit, slapped, kicked, or otherwise physically hurt?” If the women gave an affirmative response, they were asked if this had occurred (1) within the past year and (2) during this pregnancy. Seven women (7/171, 4.1%) reported being physically hurt in the past year by either their current boyfriend or the baby's father: three women were hurt before pregnancy and four were abused while pregnant. Two questions were asked about safety, “Do you feel safe in your current relationship?” (three women reported feeling unsafe) and “Is there a person from a previous relationship who is making you feel unsafe now?” (six women endorsed this). Given the relatively low frequencies of physical and emotional violence, all subsequent analyses focused on lifetime exposure to violence more broadly. All comparisons were made between these two groups (e.g., exposed or not exposed to lifetime IPV).
Proinflammatory cytokines
Blood was collected into vacutainer tubes (BD Diagnostics, Franklin Lakes, NJ) via conventional venipuncture. Blood was centrifuged at 1000 g for 10 minutes within 15 minutes of collection, and serum was stored at −80°C until assayed. To ensure that elevated cytokine levels were not due to an underlying medical condition or infection, women were asked before the blood draw if they had been ill recently, the nature of any illness, and any medications that they had taken. Women who had been febrile, experienced a cold or flu, or were taking medications for these were asked to return a week later or, when asymptomatic, to provide blood and to complete assessments (n = 2); medical chart data were also reviewed for evidence of illness. Most blood samples were obtained between 8:00 am and 2:00 pm, although 14 samples were collected between 2:00 pm and 4:00 pm. Those women providing blood samples later in the afternoon did so at each time point. Analyses with and without these 14 samples showed similar results and therefore data are reported on the whole sample.
Serum levels of IL-6 and TNF-α were assayed by enzyme-linked immunosorbent assay using high-sensitivity kits purchased from R&D Systems, Inc. (Minneapolis, MN). The kits were used according to the manufacturer's standard protocols with the standard curve run on each 96-well assay plate. Samples were run in duplicate. Absorbance was read at 490 nm with 650 nm wavelength correction within 30 minutes after development using an automated Opsys MR Microplate Reader (Thermo Labsystems, Chantilly, VA). The minimum detectable limit for IL-6 is 0.039 and 0.106 pg/mL for TNF-α. The intra-assay variability for both cytokines was 4% and the interassay coefficient of variation was <5%.
Data analyses
All data were analyzed using SAS 9.3. Due to the skewness of the data, IL-6 and TNF-α values were log transformed. The IL-6 and TNF-α changes between 32- and 18-week gestation, 6 weeks postpartum and 32-week gestation, and 6 months and 6 weeks postpartum were computed for each participant. Bivariate analyses were performed to compare changes in the cytokines between the two groups. Two-sample independent t-tests, Mann–Whitney, chi-square tests, and logistic regression analyses were used as appropriate. For multivariable analysis, a mixed model of IL-6 and TNF-α change46,47 was performed against exposure to violence and time effect, adjusting for participants' age, race, marital status, parity (coded first child or not), body–mass index (BMI) in early pregnancy, self-reported smoking and drinking status (yes/no) during pregnancy, depressive symptoms (measured by the EPDS) at 32 weeks in pregnancy, and exposure to a traumatic event (other than IPV) (yes/no) (Table 4). In these models, we assumed a compound symmetry correlation structure to control for within-participant clustering effect. The mixed model was applied due to its ability to deal with missing data under the missing at random condition. The overall changes among consecutive follow-ups, and change between each of the consecutive follow-ups, were compared between the two groups at the 0.05 significance level. The study was designed to have 80% power to detect a mean difference of half of a standard deviation (two-tailed) or an effect size of 0.5 based on a sample of 128 women.
Table 4.
Multivariable Analysis of IL-6 and Tumor Necrosis Factor Alpha Change and Lifetime Exposure to Intimate Partner Violence
| IL-6 | TNF-α | |||
|---|---|---|---|---|
| Effect | Coefficient | p | Coefficient | p |
| Intercept | 0.14 | 0.61 | −0.72 | 0.34 |
| Time effect | ||||
| 32 weeks of gestation–18 weeks of gestation | 0.21 | 0.04 | 0.43 | 0.12 |
| 32 weeks of gestation–6 weeks postpartum | −0.03 | 0.80 | 1.54 | <0.01 |
| 6 months postpartum–6 weeks postpartuma | — | — | — | — |
| Lifetime exposure to IPV | ||||
| Yes | −0.36 | 0.04 | −0.13 | 0.79 |
| Noa | — | — | — | — |
| Timea lifetime exposure to IPV | ||||
| 32 weeks of gestation–18 weeks of gestation, IPV history | 0.27 | 0.20 | 0.01 | 0.98 |
| 6 weeks postpartum–32 weeks of gestation, IPV history | 0.48 | 0.04 | 0.24 | 0.70 |
| BMI at 18 weeks of gestation | 0.01 | 0.21 | 0.01 | 0.37 |
| Age at first interview | 0.01 | 0.75 | 0.02 | 0.43 |
| Primiparous | −0.09 | 0.25 | −0.25 | 0.23 |
| Married or cohabiting with partner | −0.09 | 0.23 | −0.20 | 0.38 |
| Race | ||||
| Black | −0.18 | 0.07 | 0.05 | 0.86 |
| Other | −0.09 | 0.39 | 0.03 | 0.93 |
| Whiteb | — | — | — | — |
| Any smoking reported in pregnancy | −0.01 | 0.88 | 0.03 | 0.91 |
| Any alcohol use reported in pregnancy | −0.12 | 0.68 | −0.51 | 0.44 |
| Depressive symptoms at 32 weeks of gestation | −0.03 | 0.73 | 0.12 | 0.61 |
| History of trauma exposure | 0.09 | 0.24 | −0.02 | 0.92 |
| Lifetime IPV history vs. no history of IPV | ||||
| 32 weeks of gestation–18 weeks of gestation | 0.09 | 0.47 | 0.12 | 0.73 |
| 6 weeks postpartum–32 weeks of gestation | −0.12 | 0.44 | −0.12 | 0.78 |
| 6 months postpartum–6 weeks postpartum | 0.36 | 0.04 | 0.13 | 0.79 |
IL-6 and TNF-α were log transformed.
Reference groups.
Results
As shown in Table 1, the overall sample was predominantly single, African American, received Medicaid, and had a high school education or less. Almost half (48.5%, n = 83) reported a history of depression, and 57.3% (n = 98) were classified as overweight or obese according to BMI in early pregnancy. Retention within the study was relatively high for a sample at elevated psychosocial risk, with 91.2% of women completing both pregnancy assessments and 58.2% completing both postpartum assessments.
Table 1.
Sociodemographic, Clinical, and Obstetric Characteristics of the Sample and Grouped by Exposure to Intimate Partner Violence Status
| Characteristic | Overall sample (N = 171) | Exposed to IPV (n = 35) | Not exposed to IPV (n = 136) | Statistic and p-values |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age (years) | 24.5 (3.6) 19–34 | 24.9 (4.2) 20–33 | 24.4 (3.4) 19–34 | t = 0.72 and 0.47 |
| Ethnicity | ||||
| African American | 81 (47.4) | 14 (40.0) | 67 (49.3) | |
| White | 51 (29.8) | 7 (20.0) | 44 (32.4) | |
| Hispanic/Latina | 12 (7.1) | 4 (11.4) | 17 (12.5) | |
| Other | 27 (15.8) | 10 (28.6) | 8 (5.9) | |
| African American vs. others | χ = 0.96 and 0.33 | |||
| Education (years) | 12.8 (2.2) 8–26 | 12.4 (1.6) 10–16 | 12.9 (2.4) 8–26 | t = −1.19 and 0.24 |
| Marital status | χ = 0.02 and 0.89 | |||
| Single | 91 (53.2) | 19 (54.3) | 72 (52.9) | |
| Cohabiting/married | 80 (46.8) | 16 (45.7) | 64 (47.1) | |
| Received Medicaid | 118 (69.0) | 26 (74.3) | 92 (67.6) | χ = 0.57 and 0.45 |
| Clinical | ||||
| First trimester BMI (kg/m2) | 28.1 (7.5) 13–49.4 | 28.3 (7.1) 18.0–43.9 | 28.0 (7.6) 13.0–49.4 | t = 0.19 and 0.85 |
| Obstetrics | ||||
| Age at first pregnancy (years) | 19.4 (3.9) 11–34 | 18.5 (4.1) 13–31 | 19.7 (3.9) 11–34 | t = −1.48 and 0.14 |
| Primigravid | 47 (27.5) | 7 (20.0) | 40 (29.4) | χ = 1.24 and 0.27 |
| Self-reported smoking during pregnancy | 33 (19.3) | 11 (31.4) | 22 (16.2) | χ = 4.16 and 0.04 |
| Self-reported alcohol use during pregnancy | 4/170 (2.4) | 2 (5.7) | 2 (1.5) | 0.18a |
| Self-reported history of miscarriage | 39 (22.8) | 9 (25.7) | 30 (22.1) | χ = 0.21 and 0.65 |
| LBW <2500 g | 13/161 (8.1) | 2 (6.3) | 11 (8.1) | χ = 0.18 and 0.67 |
| Baby birth weight (in grams) | 3292.0 (599.8) 1110–4735.0 | 3262.8 (474.1) 2240–3995.0 | 3299.2 (628.5) 1110–4735.0 | t = −0.31 and 0.76 |
| Premature birth <37 weeks of gestation | 21/161 (13.0) | 7 (21.9) | 14 (10.9) | χ = 2.75 and 0.09 |
| Gestational age (weeks) | 38.8 (2.3) 25–42 | 38.8 (1.7) 33–41 | 38.8 (2.4) 25–42 | t = 0.10 and 0.92 |
| Cesarean section was performed | 55/161 (34.2) | 10/55 (31.3) | 45/55 (34.9) | χ = 0.15 and 0.70 |
| Had a previous history of depression | 83 (48.5) | 25 (71.4) | 58 (42.6) | χ = 9.23 and <0.002 |
| Lifetime history of generalized anxiety disorder (including not otherwise specified) | 64 (37.4) | 21 (60.0) | 43 (31.6) | χ = 9.58 and <0.002 |
| Lifetime diagnosis of PTSD met | 17 (9.9) | 9 (25.7) | 8 (5.9) | χ = 12.23 and <0.002a |
| Experienced depression during the pregnancy studied | 44 (25.7) | 14 (40.0) | 30 (22.1) | χ = 4.69 and <0.03 |
| Experienced depression in the postpartumb | 26/107 (24.3) | 9/22 (40.9) | 17/85 (20.0) | χ = 4.15 and <0.04 |
The values are expressed in mean (SD) range or number (%).
Fisher's exact test.
Sample size was 107 due to missing data.
BMI, body–mass index; IPV, intimate partner violence; LBW, low birth weight baby; PTSD, post-traumatic stress disorder; SD, standard deviation.
Sociodemographic and obstetric correlates of lifetime exposure to IPV
Of the 171 women, 35 (20.5%) reported lifetime exposure to IPV. As shown in Table 1, there were no significant differences between the groups on the majority of demographic or obstetric variables. Lifetime exposure to IPV was significantly associated with smoking during pregnancy (odds ratio [OR] 2.38, 95% confidence interval [CI] 1.01–5.54); there was a nonsignificant trend for violence exposure and premature birth (21.9% vs. 10.9%) (OR 1.03, 95% CI 0.41–2.60).
Lifetime exposure to IPV and psychiatric diagnoses
As shown in Table 2, exposure to IPV during the life course was significantly associated with experiencing a range of psychiatric disorders, including GAD, PTSD, and depression. Furthermore, there was a significant association with experiencing depression during both the pregnancy (antenatal depression) and postpartum periods (postpartum depression) studied.
Table 2.
Logistic Regression Analysis of Lifetime Exposure to Intimate Partner Violence and Experiencing Psychiatric Diagnoses
| Psychiatric diagnosis (DSM-IV TR) | OR (95% CI) |
|---|---|
| History of major depressive episode | 3.36 (1.50–7.54) |
| Lifetime diagnosis of general anxiety disorder | 3.24 (1.51–6.98) |
| Lifetime diagnosis of PTSD | 5.54 (1.96–15.69) |
| Antenatal depressiona | 2.36 (1.07–5.18) |
| Postpartum depressionb | 2.77 (1.01–7.55) |
Experienced major or minor episode of depression according to DSM-IV TR criteria during the pregnancy studied.
Experienced major or minor episode of depression according to DSM-IV TR criteria within 6 months of childbirth.
CI, confidence interval; OR, odds ratio.
IPV status and cytokine levels
Table 3 presents the mean levels of each cytokine at the four assessment points by lifetime IPV status; for descriptive purposes, the raw data are shown. Mann–Whitney analyses compared levels at each assessment using raw and log-transformed data and identical results were obtained. At baseline (18 weeks of gestation), one significant effect emerged: women with a history of IPV had significantly higher levels of TNF-α (z = −2.29, p < 0.05) compared with women who were not IPV exposed.
Table 3.
Mean Levels of Interleukin-6 and Tumor Necrosis Factor Alpha (Raw Data) by Lifetime Intimate Partner Violence Exposure Status at the Four Assessments
| IL-6, pg/mL (mean, SD) | TNF-α, pg/mL (mean, SD) | |||
|---|---|---|---|---|
| Assessment | IPV positive | IPV negative | IPV positive | IPV negative |
| 18 weeks of gestation | 1.98 (1.4) | 2.17 (1.7) | 1.63 (2.8) | 0.93 (1.5) p < 0.05* |
| 32 weeks of gestation | 2.25 (1.4) | 2.60 (1.8) | 1.30 (1.5) | 1.20 (1.6) |
| 6 weeks postpartum | 2.85 (2.2) | 2.31 (2.1) | 3.76 (6.7) | 2.04 (2.9) |
| 6 months postpartum | 1.85 (1.3) | 2.35 (2.1) | 1.71 (1.6) | 1.68 (1.7) |
IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha.
Statistically significant at p < 0.05.
Change model analysis
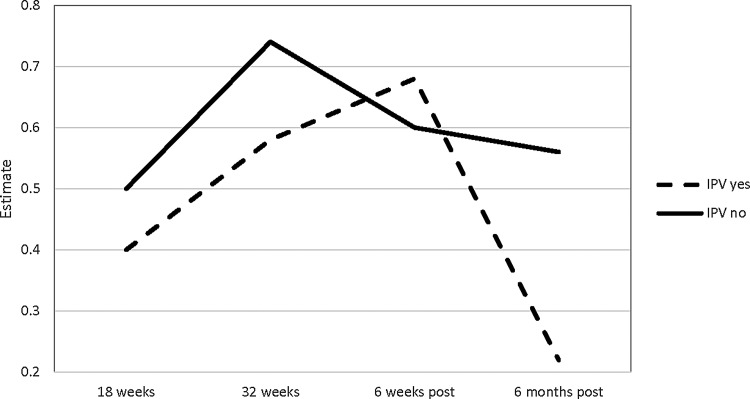
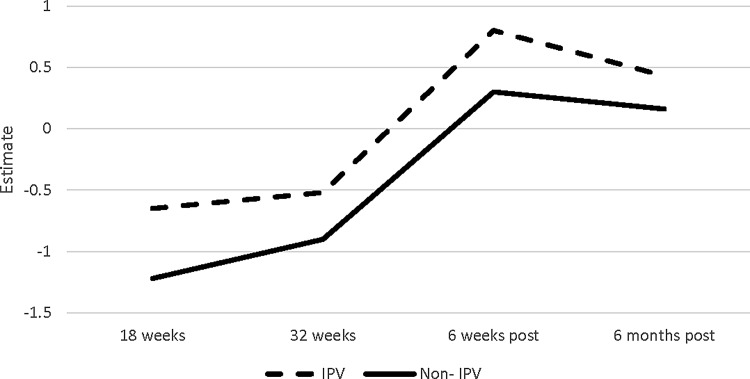
Table 4 shows the multivariable analysis results of IL-6 and TNF-α change model for lifetime exposure to violence, time effect, their interaction, depressive symptoms (measured by the EPDS) at 32 weeks of gestation, lifetime history of trauma exposure (other than IPV), and participants' characteristics (age, race, marital status, primiparity, BMI, and self-reported smoking and drinking status [yes/no] during pregnancy). Within the entire sample, and after controlling for participants' characteristics, there was a significantly greater change in the levels of IL-6 during pregnancy compared with the postpartum period (β = 0.21, p = 0.04). That is, the amount of increase in IL-6 during pregnancy was significantly greater than the amount of decrease during the postpartum period. This general trend was different according to IPV status. Women who experienced violence had significantly smaller changes in IL-6 across the different time points than those not exposed to violence (β = −0.36, p = 0.04) (Fig. 2). In addition, from 6 weeks to 6 months postpartum, the women exposed to violence showed a greater decrease of IL-6 compared with those without exposure to violence (β = 0.36, p = 0.04). The association between IPV exposure and change in TNF-α over time was not statistically significant (β = −0.13, p = 0.79) (Fig. 3). However, the change in TNF-α levels at 32 weeks of gestation to 6 weeks postpartum (for the entire sample) was significantly greater than the change from 6 weeks to 6 months postpartum (β = 1.54, p < 0.01).
FIG. 2.
IL-6 change across time by IPV status. IL-6, interleukin-6; IPV, intimate partner violence.
FIG. 3.
TNF-α change across time by IPV status. TNF-α, tumor necrosis factor alpha.
Discussion
Lifetime IPV and psychiatric and obstetric outcomes
Interpersonal violence is a major public health concern and is associated with a range of adverse maternal and child outcomes. Understanding the biological pathways that link pregnancy and women's history and experiences of violence is a critical area of inquiry. This study adds to a small but growing literature examining the biological correlates of exposure to relationship violence and its effects on perinatal outcomes.48,49 A fifth of women (20.5%) in this study reported experiencing emotional or physical abuse in their lifetime, which is lower than current national and international estimates1–3; this may be due to the use of a single-item measure to assess lifetime exposure of the two subtypes of IPV, physical and emotional abuse. Close to 4% of the study participants reported experiencing physical violence in the year before or during pregnancy; these rates are similar in magnitude to those reported in previous studies using similar measures to assess women's experiences of recent physical violence.4–6
Our results provide further evidence for the link between IPV exposure and increased risk of experiencing psychiatric illness, particularly during the perinatal period.10,11 Maternal mental health in pregnancy and postpartum has been shown to independently predict poorer child behavioral, cognitive, and psychological outcomes50; therefore, early identification and treatment of these conditions are needed to mitigate long-term adverse outcomes. The perinatal period offers an important opportunity to screen for history of and current exposure to IPV and psychological symptoms as these women interact regularly and frequently with healthcare providers.
Although women who experienced lifetime IPV were more likely to smoke during pregnancy, a major risk factor for preterm birth and low birth weight, we did not find direct links between IPV history and adverse birth outcomes. Recent evidence showed that severity of IPV in pregnancy predicted small-for-gestational-age or low birth weight babies8 in a high-risk sample. We report a nonsignificant association between IPV exposure and premature birth; this may be due to lack of power in the exposed group (n = 35) to detect small differences in birth weight and gestational age. Furthermore, we were not able to assess the duration or severity of IPV and had a small number of women who experienced current and perinatal IPV, all of which may strongly predict these outcomes.
Lifetime IPV and cytokine changes
Several studies have longitudinal data on cytokine changes across pregnancy and the postpartum20,26,30,51–53 period with conflicting patterns reported. Overall, IL-6 significantly increased during pregnancy, which has been reported in some,30,51,53 but not all, studies.20,26 Levels of TNF-α were elevated in the IPV group at 18 weeks, but remained relatively stable over time, supporting previous studies,26,30,51,53 but contradicting others.18,20 Notably, our findings are very similar to those of Coussons-Read et al.30 who recruited samples of women with psychosocial risk factors for elevated psychological stress. It may be that observed differences between studies reflect varying sample characteristics in terms of psychological and medical risk factors, timing of assessments, or other methodological factors.
Based on studies of nonpregnant women, one might expect that women with a history of IPV would have elevated levels of proinflammatory cytokines early in pregnancy because a history of stress exposure may be associated with greater inflammation. This was partially supported at the first assessment in the second trimester of pregnancy; women exposed to IPV had significantly higher levels of TNF-α. However, over time, we found differences in levels of IL-6 according to IPV exposure. A lower level of initial IL-6 and a reduced increase over pregnancy may suggest that women exposed to IPV may be hyporesponsive during pregnancy. Importantly, we obtained this effect even after controlling for depression and trauma exposure. In this regard, it is interesting that Shelton et al.52 found that pregnant women with depressive symptoms had lower levels of cytokines in the second trimester compared with nondepressed women and postulated that this may be an exaggeration of the normal immunomodulation of pregnancy. Stress exposure or affective symptoms trigger HPA axis activation that results in downregulation of the normal cytokine balance and hence lower cytokine levels.52
Hyporesponsiveness during pregnancy and the early postpartum period, assessed via HPA axis response and lack of normal postpartum T-cell rebound, has been reported in women exposed to a range of acute and chronic stressors53 and in women with severe affective postpartum mood disorders.54 Future work is needed to consider the complex interplay between behavioral phenotypes and the immune, neuroendocrine, and nervous systems within the perinatal period. In addition, further work is needed that examines a broader range of cytokines, including anti-inflammatory markers. More refined assessments that assess the timing, duration, and severity of IPV are needed. Studies that concurrently assess IPV exposure during the perinatal period, cytokine levels, and obstetric outcomes are also required.
The study had several limitations. We used a single-item measure of lifetime IPV, which did not assess the severity, duration, or timing of abuse. It is possible that for women with chronic exposure to IPV, or those experiencing IPV in the perinatal period, the relationship among cytokines, IPV, and birth outcomes may be clearer. Participants may have underreported experiences of IPV, perhaps due to feelings of fear or shame, and it is possible that women in currently abusive relationships were not willing to participate in research. Future studies are needed that consider subtypes, timing, and duration of IPV. Studies should actively attempt to recruit women who are currently being abused and are at even higher risk of poor maternal and infant outcomes. However, these limitations were offset by several strengths, including a prospective longitudinal design, recruitment of a psychosocial high-risk and ethnically diverse sample, and multiple blood draws during pregnancy and the postpartum period. In addition, the study is one of the few to examine changes in proinflammatory cytokines across the perinatal period26,52,55 rather than cross-sectionally.
Conclusions
Pregnancy and the postpartum period offer a unique opportunity for healthcare providers to screen for current or past exposure to IPV. This study supports the clinical implications of the U.S. Preventive Services Task Force (USPSTF) that call for early intervention, screening, and referral to address IPV among women of childbearing age.56,57 Multicomponent interventions comprising routine annual monitoring of IPV using validated screening instruments and provider engagement in prenatal care settings, as well as targeted home-based and brief interventions, hold promise to reduce adverse outcomes in maternal and child health and to decrease delivery-related healthcare costs.57,58 Furthermore, this study showed that women with a history of IPV had significant and widespread psychiatric morbidity, despite not being in currently abusive relationships. The results also suggest that IPV exposure may affect the levels of proinflammatory cytokines across the perinatal period; these alterations in pregnancy may affect the health of both the mother and child.
Acknowledgments
Dr. Robertson Blackmore was supported by a Young Investigator Award from the Brain & Behavior Foundation (ERB), Grant Number K23MH080290 from the National Institute of Mental Health, and the University of Rochester CTSA award number UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. The funding bodies had no involvement in the study design, collection, analysis and interpretation of the data, or in the writing of the report.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Centers for Disease Control. Understanding intimate partner violence factsheet, 2012. Available at: www.cdc.gov/violenceprevention/pdf/ipv_factsheet-a.pdf Accessed December9, 2014
- 2.World Health Organization. Responding to intimate partner violence and sexual violence against women: WHO clinical and policy guidelines, 2013. Available at: http://apps.who.int/iris/bitstream/10665/85240/1/9789241548595_eng.pdf?ua=1 Accessed December9, 2014 [PubMed]
- 3.Black MC, Basile KC, Breiding MJ, et al. . The National Intimate Partner and Sexual Violence Survey (NISVS): 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 4.Devries KM, Kishor S, Johnson H, et al. . Intimate partner violence during pregnancy: Analysis of prevalence data from 19 countries. Reprod Health Matters 2010;18:158–170 [DOI] [PubMed] [Google Scholar]
- 5.Chu SY, Goodwin MM, D'Angelo DV. Physical violence against U.S. women around the time of pregnancy, 2004–2007. Am J Prev Med 2010;38:317–322 [DOI] [PubMed] [Google Scholar]
- 6.Saltzman LE, Johnson CH, Gilbert BC, Goodwin MM. Physical abuse around the time of pregnancy: An examination of prevalence and risk factors in 16 states. Matern Child Health J 2003;7:31–43 [DOI] [PubMed] [Google Scholar]
- 7.Sharps PW, Laughon K, Giangrande SK. Intimate partner violence and the childbearing year: Maternal and infant health consequences. Trauma Violence Abuse 2007;8:105–116 [DOI] [PubMed] [Google Scholar]
- 8.Alhusen JL, Bullock L, Sharps P, Schminkey D, Comstock E, Campbell J. Intimate Partner violence during pregnancy and adverse neonatal outcomes in low-income women. J Womens Health (Larchmt) 2014;23:920–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambliss LR. Intimate partner violence and its implication for pregnancy. Clin Obstet Gynecol 2008;51:385–397 [DOI] [PubMed] [Google Scholar]
- 10.Silverman JG, Decker MR, Reed E, Raj A. Intimate partner violence victimization prior to and during pregnancy among women residing in 26 U.S. states: Associations with maternal and neonatal health. Am J Obstet Gynecol 2006;195:140–148 [DOI] [PubMed] [Google Scholar]
- 11.Ludermir AB, Lewis G, Valongueiro SA, de Araujo TVB, Araya AR. Violence against women by their intimate partner during pregnancy and postnatal depression: A prospective cohort study. Lancet 2010;376:903–910 [DOI] [PubMed] [Google Scholar]
- 12.Shah PS, Shah J; on behalf of the Knowledge Synthesis Group on Determinants of Preterm = LBW Births. Maternal exposure to domestic violence and pregnancy and birth outcomes: A systematic review and meta-analyses. J Womens Health (Larchmt) 2010;19:2017–2031 [DOI] [PubMed] [Google Scholar]
- 13.Ackerson LK, Subramanian SV. Intimate partner violence and death among infants and children in India. Pediatrics 2009;124:e878–e889 [DOI] [PubMed] [Google Scholar]
- 14.Ahmed S, Koenig MA, Stephenson R. Effects of domestic violence on perinatal and early-childhood mortality: Evidence from north India. Am J Public Health 2006;96:1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunkel Schetter C. Psychological science on pregnancy: Stress processes, biopsychosocial models and emerging research issues. Annu Rev Psychol 2011;62:531–558 [DOI] [PubMed] [Google Scholar]
- 16.Blackburn S. Cytokines in the perinatal and neonatal periods: Selected aspects. J Perinat Neonatal Nurs 2008;22:187–190 [DOI] [PubMed] [Google Scholar]
- 17.Osborne LM, Monk C. Perinatal depression—The fourth inflammatory morbidity of pregnancy? Psychoneuroendocrinology 2013;38:1929–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus TA, Sperling RS, Engel SM, et al. . Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol 2010;64:411–426 [DOI] [PubMed] [Google Scholar]
- 19.Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunol Res 2012;54:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luppi P, Haluszczak C, Betters D, Richard CA, Trucco M, DeLoia JA. Monocytes are progressively activated in the circulation of pregnant women. J Leukoc Biol 2002;72:874–884 [PubMed] [Google Scholar]
- 21.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann N Y Acad Sci 2011;1221:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero R. Novel aspects of neutrophil biology in human pregnancy. Am J Reprod Immunol 2005;53:275 [Google Scholar]
- 23.Mor G. Inflammation and pregnancy: The role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci 2008;1127:121–128 [DOI] [PubMed] [Google Scholar]
- 24.Ahn H, Park J, Gilman-Sachs A, Kwak-Kim J. Immunologic characteristics of preeclampsia, a comprehensive review. Am J Reprod Immunol 2011;65:377–394 [DOI] [PubMed] [Google Scholar]
- 25.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: A systematic review. Obstet Gynecol 2010;116(Pt 1):393–401 [DOI] [PubMed] [Google Scholar]
- 26.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. Am J Reprod Immunol 2014;72:326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe LP, Metzger BE, Lowe WL Jr., et al. . Inflammatory mediators and glucose in pregnancy: Results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab 2010;95:5427–5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain Behav Immun 2010;24:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J Reprod Immunol 2012;94:202–209 [DOI] [PubMed] [Google Scholar]
- 30.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun 2007;21:343–350 [DOI] [PubMed] [Google Scholar]
- 31.Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1b during the first month postpartum. Biol Res Nur 2008;10:128–133 [DOI] [PubMed] [Google Scholar]
- 32.Fransson E, Dubicke A, Bystrom B, Ekman-Ordeberg G, Hjelmstedt A, Lekander M. Negative emotions and cytokines in maternal and cord serum at preterm birth. Am J Reprod Immunol 2012;67:506–514 [DOI] [PubMed] [Google Scholar]
- 33.Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology 2007;32:133–139 [DOI] [PubMed] [Google Scholar]
- 34.Maes M, Lin AH, Ombelet W, et al. . Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology 2000;25:121–137 [DOI] [PubMed] [Google Scholar]
- 35.Skalkidou A, Sylven SM, Papadopoulos FC, Olovsson M, Larsson A, Sundstrom-Poromaa I. Risk of postpartum depression in association with serum leptin and interleukin-6 levels at delivery: A nested case-control study within the UPPSAT cohort. Psychoneuroendocrinology 2009;34:1329–1337 [DOI] [PubMed] [Google Scholar]
- 36.Wieck A, Grassi-Oliveira R, Hartmann do Prado C, Teixeira AL, Bauer ME. Neuroimmunoendocrine interactions in post-traumatic stress disorder: Focus on long-term implications of childhood maltreatment. Neuroimmunomodulation 2014;21:145–151 [DOI] [PubMed] [Google Scholar]
- 37.Kiecolt-Glaser JK, Gouin JP, Hantsoo LV. Close relationships, inflammation, and health. Neurosci Biobehav Rev 2010;35:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012;36:764–785 [DOI] [PubMed] [Google Scholar]
- 39.Newton TL, Fernandez-Botran R, Miller JJ, Lorenz DJ, Ellison Burns V, Fleming KN. Markers of inflammation in midlife women with intimate partner violence histories. J Womens Health (Larchmt) 2011;20:1871–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breiding MJ, Black MC, Ryan GW. Chronic disease and health risk behaviors associated with intimate partner violence—18 U.S. states/territories, 2005. Ann Epidemiol 2008;18:538–544 [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-Botran R, Miller JJ, Burns VE, Newton TL. Correlations among inflammatory markers in plasma, saliva and oral mucosal transudate in post-menopausal women with past intimate partner violence. Brain Behav Immun 2011;25:314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woods AB, Page GG, O'Campo P, Pugh LC, Ford D, Campbell JC. The mediation effect of posttraumatic stress disorder symptoms on the relationship of intimate partner violence and IFN-gamma levels. Am J Community Psychol 2005;36:159–175 [DOI] [PubMed] [Google Scholar]
- 43.Robertson Blackmore E, Putnam FW, Rubinow DR, et al. . Antecedent trauma exposure and risk of depression in the perinatal period. J Clin Psychiatry 2013;74:e942–e948 [DOI] [PubMed] [Google Scholar]
- 44.First M. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition with psychotic screen (SCID-I/P W/PSY SCREEN). New York: Biometrics Research. New York State Psychiatric Institute, 2002 [Google Scholar]
- 45.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782–786 [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson R, Pickett K. The spirit level: Why equality is better for everyone. London: Penguin, 2010 [Google Scholar]
- 47.Joffe M, Mindell J. A framework for the evidence base to support Health Impact Assessment. J Epidemiol Community Health 2002;56:132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feinberg ME, Jones DE, Granger DA, Bontempo D. Relation of intimate partner violence to salivary cortisol among couples expecting a first child. Aggress Behav 2011;37:492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talley P, Heitkemper M, Chicz-Demet A, Sandman CA. Male violence, stress, and neuroendocrine parameters in pregnancy: A pilot study. Biol Res Nurs 2006;7:222–233 [DOI] [PubMed] [Google Scholar]
- 50.Murray L, Arteche A, Fearon P, et al. . The effects of maternal postnatal depression and child sex on academic performance at age 16 years: A developmental approach. J Child Psychol Psychiatry 2010;51:1150–1159 [DOI] [PubMed] [Google Scholar]
- 51.Curry AE, Vogel I, Drews C, et al. . Mid-pregnancy maternal plasma levels of interleukin 2, 6, and 12, tumor necrosis factor-alpha, interferon-gamma, and granulocyte-macrophage colony-stimulating factor and spontaneous preterm delivery. Acta Obstet Gynecol Scand 2007;86:1103–1110 [DOI] [PubMed] [Google Scholar]
- 52.Shelton MM, Schminkey DL, Groer MW. Relationships among prenatal depression, plasma cortisol, and inflammatory cytokines. Biol Res Nurs 2015;17:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: Influences on maternal and fetal outcomes. Neuroendocrinology 2013;98:106–115 [DOI] [PubMed] [Google Scholar]
- 54.Bergink V, Burgerhout KM, Weigelt K, et al. . Immune system dysregulation in first-onset postpartum psychosis. Biol Psychiatry 2013;73:1000–1007 [DOI] [PubMed] [Google Scholar]
- 55.Dibble S, Andersen A, Lassen MR, Cunanan J, Hoppensteadt D, Fareed J. Inflammatory and procoagulant cytokine levels during pregnancy as predictors of adverse obstetrical complications. Clin Appl Thromb Hemost 2014;20:152–158 [DOI] [PubMed] [Google Scholar]
- 56.Nelson HD, Bougatsos C, Blazina I. Screening women for intimate partner violence: A systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med 2012;156:796–808 [DOI] [PubMed] [Google Scholar]
- 57.Miller E, McCaw B, Humphreys BL, Mitchell C. Integrating intimate partner violence assessment and intervention into healthcare in the United States: A systems approach. J Womens Health (Larchmt) 2015;24:92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mogos MF, Araya WN, Masho SW, Salemi JL, Shieh C, Salihu HM. The feto-maternal health cost of intimate partner violence among delivery-related discharges in the United States, 2002–2009. J Interpers Violence 2016;31:444–64 [DOI] [PubMed] [Google Scholar]