Abstract
To function normally, all cells must maintain ion homeostasis, establish a membrane potential, and regulate water content. These actions require active Na-K transport provided by Na,K-ATPase. The lens, however, is made up almost entirely of fiber cells that have little or no Na,K-ATPase activity. Lens ion and water homeostasis rely on Na,K-ATPase activity in a small number of cells at the periphery of epithelium monolayer. Therefore, the function of the epithelium must be integrated with the needs of the fiber mass. This suggests that a remote control mechanism may adjust Na,K-ATPase activity to match increases or decreases of ion leakage, which may occur a considerable distance away. Here, we review evidence that TRPV4 channels in the epithelium become activated when the lens is subjected to osmotic- or damage-induced swelling. This triggers a chain of events in the lens epithelium that opens connexin hemichannels, allowing ATP release that stimulates purinergic receptors, activates Src family tyrosine kinases, and increases Na,K-ATPase activity. Recent studies also revealed functional connexin hemichannels along with TRPV4 channels in nonpigmented ciliary epithelial (NPE) cells that secrete aqueous humor into the eye. Because TRPV4 channels are mechanosensitive, we speculate they might enable the NPE to respond to stimuli such as mechanical distortion associated with volume homeostasis during fluid transfer across the ciliary epithelium or changes in intraocular pressure.
Keywords: : lens epithelium; ciliary epithelium; Na,K-ATPase activity; remote sensing; TRPV4; hemichannels
Lens
Pathophysiology stems from physiology, and physiology is largely a product of specialization at the level of cells. The lens has been described as biological glass1 and its transparency is the result of precisely integrated function of highly specialized living cells. The bulk of the lens consists of fiber cells that lack mitochondria, endoplasmic reticulum, and nuclei. A gradient of water content in the fiber mass (low in the center) gives rise to a gradient of refractive index that adds focusing power and reduces spherical aberration. The unusual cellular specializations mean lens fibers are unable to function in isolation. Fiber cell homeostasis relies on the anterior monolayer of epithelial cells.
Lens epithelial cells have a high Na,K-ATPase activity.2 In contrast, mature fiber cells that account for the bulk of the lens structure have negligible Na,K-ATPase activity.3 Sodium and potassium homeostasis of the fiber mass, and thus water homeostasis and ultimately transparency, all are supported by Na,K-ATPase activity in epithelial cells.4 Therefore, the function of the epithelium must be integrated with the needs of the fiber mass. We envision there to be a remote control mechanism that adjusts Na,K-ATPase activity to match increases or decreases of ion leakage that may occur a considerable distance away.
TRPV4 Sensory Mechanism
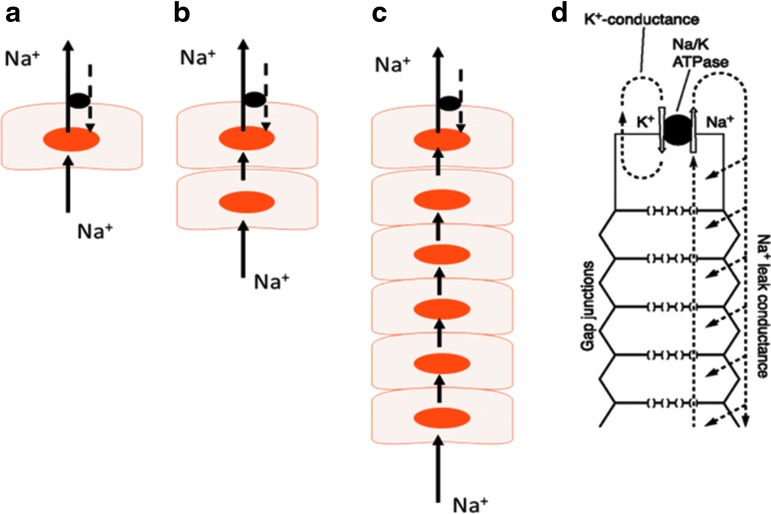
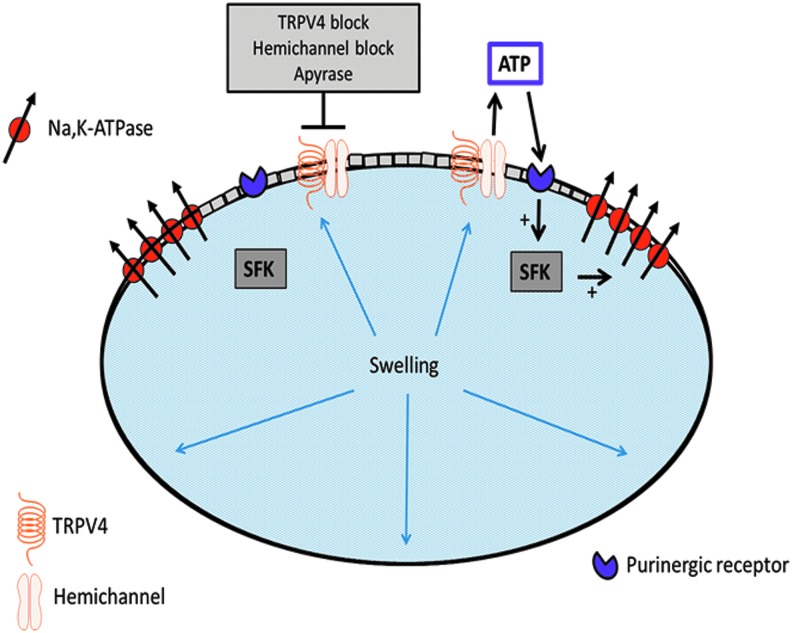
Because of their high protein content, cells must continuously compensate for osmotic swelling. With few exceptions, cells require metabolism and Na,K-ATPase to regulate cytosolic Na+ and K+ concentration and this, in turn, maintains water homeostasis. Without active Na-K transport, a cell gains Na+ and loses K+, which impairs osmotic balance, causing it to gain water, swell, burst, and die. Under normal circumstances, Na,K-ATPase compensates for Na+ and K+ leaks. This works well in a single cell but not in a syncytium of many coupled lens cells, most of which have no Na,K-ATPase (Fig. 1). Na,K-ATPase in the epithelium is not in contact with Na+ concentration in remotely located fibers. We propose instead that the Na,K-ATPase activity is regulated by a remote control mechanism that utilizes TRPV4 channels as sensors. Our studies suggest that when the lens is subjected to osmotic- or damage-induced swelling, TRPV4 channels in the epithelium become activated. This permits Ca2+ entry and triggers a chain of events that open connexin hemichannels and possibly pannexin channels. Open hemichannels are conduits for relatively large molecules, up to 1 kDa,5 that otherwise are poorly able to exit or enter a cell. Opening hemichannels in the lens epithelium allows ATP release and then subsequent activation of purinergic receptors in the epithelium and certain Src family tyrosine kinases (SFKs) (Fig. 2). This, in the end, stimulates Na,K-ATPase activity.6 The ATP release step is critical. It has been known for some time that purinergic agonists ATP and uridine 5′-triphosphate activate SFKs and so cause the intrinsic activity (Vmax) of Na,K-ATPase in the lens epithelium to increase.7 We now know that the source of the ATP is the lens itself when TRPV4 causes hemichannels to open.8 If, as the evidence suggests, the response is activated when TRPV4 channels are stimulated, then the mechanism is likely to be mechanosensitive. This means feedback responses could occur quickly. Indeed, in a study on remote damage in the fiber mass, we found that a TRPV4-dependent mechanism activates SFKs in the epithelium within 1 min and increases Na,K-ATPase activity.9 TRPV4 channels are mechanosensitive10 and we reason they are opened by stretching forces from swelling of the lens structure caused by damage to the fibers.
FIG. 1.
In a single cell (a), Na-K homeostasis is achieved automatically because Na,K-ATPase activity increases or decreases in response to fluctuations in cytoplasmic sodium concentration at the Na+ binding site on the cytoplasmic face. Na-K homeostasis is more complicated when the cell is coupled to one (b) or more cells that lack Na,K-ATPase (c). It must export Na+ ions that enter at a distant site. This is the case in the lens (d) where Na,K-ATPase is located in the epithelium but not fibers. (d) Is adapted from Mathias et al.16 It depicts how the arrangement of Na,K-ATPase, K channels, and gap junctions at the lens surface creates a circulating flow of current/ions through coupled cells of the fiber mass. In an intact lens, the flow exits at the equator and enters at the anterior and posterior poles.
FIG. 2.
Simplified model showing key elements of the swelling response in which activation of TRPV4 channels causes connexin hemichannels in the epithelium to open. This allows release of ATP that stimulates P2Y purinergic receptors. Then, SFKs are activated and Na,K-ATPase activity increases. The hemichannel opening—ATP release step, is a must. The response is abolished by hydrolysis of released ATP or blockade of either TRPV4 or hemichannels. SFKs, Src family tyrosine kinases.
Feedback Mechanism
If we are correct in thinking a TRPV4 hemichannel mechanism operates a feedback pathway that stimulates Na,K-ATPase activity in the porcine lens epithelium, how might reduction of Na,K-ATPase activity occur? On this question we do not have a great deal of information. However, recent studies on hydrostatic pressure in mouse lens suggest the existence of a feedback mechanism to compensate for lens shrinkage.11 It is possible that to achieve steady state under normal conditions, the lens may oscillate between stimulation and inhibition of Na,K-ATPase activity (Fig. 2). We know little about the shrinkage response, but Western blot preliminary studies show that the epithelium expresses TRPV1, better known as a “pain receptor channel” and the receptor for capsaicin, the heat molecule in peppers (Fig. 3). This fits with a hydrostatic pressure response detected in lenses exposed to capsaicin.11 Interestingly, TRPV1 in neurons is activated by compressive forces caused by cell shrinkage.12
FIG. 3.
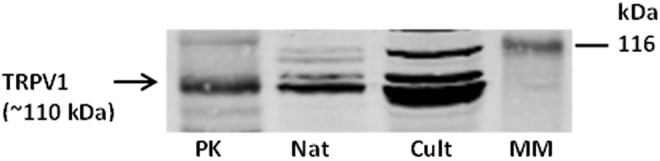
Western blot showing TRPV1 expression in native (Nat) and cultured (Cult) porcine lens epithelium. Pig kidney (PK) was used as a positive control. MM, molecular marker.
The unique structure of the lens and specialization of its cells give rise to a curious link between spatial distribution of Na,K-ATPase activity and refractive index. Because refractive index of the fiber mass is dependent on water content, water concentration arguably determines lens optical function. Donaldson emphasizes the point that a lower water concentration in the lens center establishes a refractive index gradient that adjusts for spherical aberration.13–15 In the mammalian lens, the low water content at the lens center is made possible by a circulatory flow that carries water outward at the equator.16 High Na,K-ATPase activity in the equatorial epithelium provides the driving force for the circulatory ion flow necessary for lens water homeostasis.17 To quote the authors, “the circulation of Na+ creates a circulation of solute that is coupled through local osmosis to fluid movement following the same pattern.” The circulatory ion flow, part of which is depicted in Fig. 1, requires high Na,K-ATPase activity in the correct place, in surface cells at the equator of the lens.16 On this basis, feedback regulation of Na,K-ATPase activity by a mechanism that responds to mechanical distortion caused by remote stimuli in the fiber cell mass is potentially important.
Ciliary Epithelium
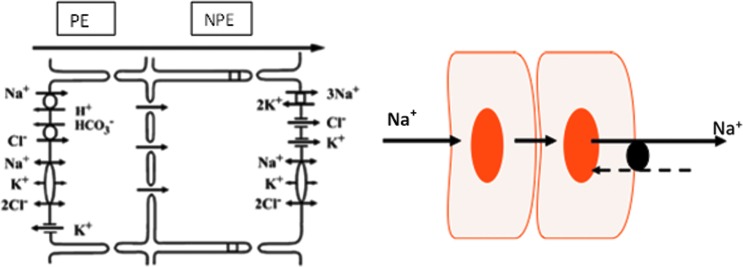
Studies on TRPV4 and hemichannels in the lens led us to consider the possibility of an autocrine control mechanism triggered by TRPV4 ion channels in the ciliary epithelium bilayer. The 2 layers of cells, pigmented ciliary epithelium (PE) and nonpigmented ciliary epithelium (NPE), are developmentally quite distinct in their origin. The apical surfaces of the PE and NPE face each other and the 2 layers are coupled by gap junctions. While both cell types express Na,K-ATPase, the abundance of Na,K-ATPase activity is localized to the basolateral surface of the NPE. Thus, in respect to coupling and distribution of Na,K-ATPase activity (Fig. 4), the tissue resembles the 2-cell model shown in Fig. 1. It was interesting, therefore, to discover TRPV4 channels as well as unpaired connexin-50 on the surface of NPE cells that faces the aqueous humor. Instead of forming a gap junction with an adjacent cell, the unpaired connexin-50 is a sign that hemichannels connect NPE cells with aqueous humor.
FIG. 4.
The ciliary epithelial bilayer. The scheme on the left depicts the localization of transport mechanisms and is adapted from McLaughlin et al.31 The model is a simplification since transport activity is not uniform over the ciliary epithelial surface. Electron microprobe analysis of rabbit ciliary epithelium indicates enhanced secretion posteriorly and enhanced absorption anteriorly.32 Aqueous humor flow occurs in the PE-to-NPE direction. The PE and NPE are coupled by gap junctions and function in the manner of a syncytium. Na,K-ATPase activity and protein expression are highest at the basolateral, aqueous humor-facing surface of the NPE. In respect to coupling and Na,K-ATPase distribution, the tissue is similar to the 2-cell model shown in Fig. 1. NPE, nonpigmented ciliary epithelium; PE, pigmented ciliary epithelium.
The presence of mechanosensitive TRPV4 channels in the ciliary epithelium was not anticipated but perhaps it should have been. In vivo, NPE cells are subjected to swelling or shrinkage whenever there is a temporary mismatch between entry and exit of water that passes vectorially through them. In 1 min, the water throughput is estimated as an amount equivalent to 30% of the NPE cell's own volume.18 Generally speaking, epithelial fluid transport may involve cell volume oscillation as water enters on one side and exits the other in a pulsatile manner.19 The NPE is specialized to swell and then respond with a regulatory volume decrease (RVD)20 that shifts water outward on the aqueous side, so contributing to aqueous humor formation. TRPV4 activation is thought to be critical for RVD as is Na,K-ATPase activity reduction. In addition, the simple presence of mechanosensitive channels raises the interesting notion that the ciliary epithelium might be able to sense and respond to distortion of the ciliary process caused by intraocular pressure (IOP) elevation.
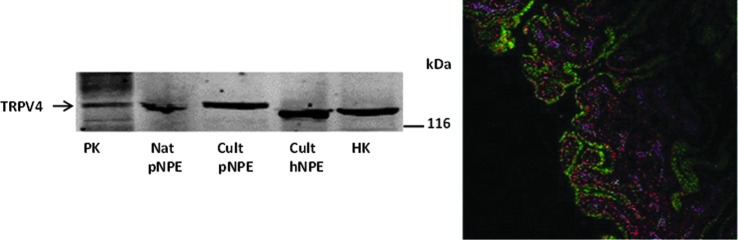
Immunolocalization experiments show expression of TRPV4 at the aqueous humor-facing basolateral surface of the NPE in porcine ciliary processes (Fig. 5). Immunolocalization studies described elsewhere also show unpaired connexin-50 (Cx50) at the same aqueous humor-facing NPE surface.21 A different connexin, Cx43, appears mainly at the interface between the apical surfaces of the NPE and PE,21 which is consistent with the notion that Cx43 forms gap junctions between the 2-cell layers.22 Pannexin-1, which forms channels similar in some respects to connexin hemichannels, also is expressed at the basolateral surface of the NPE.21 Because connexin hemichannels are known to open in low calcium conditions,23 evidence for functionality of NPE hemichannels was obtained by measuring entry of a large solute, propidium iodide (PI) (MW 668 Da), when a calcium-free solution containing PI was introduced into the aqueous humor compartment of arterially perfused intact porcine eyes. Confocal microscopy revealed PI in the NPE cell layer of calcium-free treated eyes.21 PI entry into the NPE was inhibited by calcium and by the connexin antagonist 18α-glycyrrhetinic acid (18-AGA).
FIG. 5.
Western blot (left panel) study shows the presence of TRPV4 in porcine NPE and immunolocalization (right panel) study shows its location on the basolateral membrane.
In keeping with the notion that TRPV4 is functionally important, the TRPV4 agonist GSK1016790A reduces IOP and TRPV4 knockout mice have elevated IOP.24 Studies on primary cultured NPE grown on permeable supports indicate that ATP release via hemichannels is possible (data not shown). ATP may play a crucial role in the autocrine responses of the NPE. Early on, Civan, Mitchell and coworkers recognized ATP release by the ciliary epithelium as an “enabling step in purinergic regulation of aqueous humor formation,”25 but certain mechanistic questions were left unanswered. We hope to explore the possibility that ATP release occurs from the NPE via the TRPV4-hemichannel mechanism. Because hemichannels are poorly selective,26 when they open transiently to release ATP, they likely also release other signaling molecules such as cyclic nucleotides into the aqueous humor. ATP has been shown previously to reduce the rate of aqueous humor formation in the isolated arterially perfused porcine eye.27 Acting as an extracellular signaling molecule, we envision that ATP released by the NPE might elicit autocrine functional changes in the NPE itself. Preliminary studies on NPE indicate that extracellular ATP may cause reduction of Na,K-ATPase activity, which is the opposite of the Na,K-ATPase response in lens epithelium. NPE hemichannels may also have indirect outflow effects because of their impact on the concentration of adenosine in aqueous humor. ATP and cAMP released by hemichannels would likely be metabolized to adenosine.28 In effect, hemichannels could add adenosine to the aqueous humor. It is well established that adenosine in aqueous humor keeps outflow resistance low.29,30
Acknowledgments
This review article is based on a presentation by one of the authors (N.A.D.) at a March 2015 symposium at the University of Pennsylvania. The symposium was held to honor Mortimer M. Civan, a leader in the field of aqueous humor physiology and a cherished colleague, friend, and mentor to authors N.A.D. and M.S. Generous support for the symposium was provided by the Department of Physiology at the University of Pennsylvania. Much of the research reported here was made possible by Grants EY009532 and EY06915 from the National Eye Institute.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bassnett S., Shi Y., and Vrensen G.F. Biological glass: structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:1250–1264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamiya S., Dean W.L., Paterson C.A., and Delamere N.A. Regional distribution of Na,K-ATPase activity in porcine lens epithelium. Invest. Ophthalmol. Vis. Sci. 44:4395–4399, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Delamere N.A., and Dean W.L. Distribution of lens sodium-potassium-adenosine triphosphatase. Invest. Ophthalmol. Vis. Sci. 34:2159–2163, 1993 [PubMed] [Google Scholar]
- 4.Gao J., Sun X., Yatsula V., Wymore R.S., and Mathias R.T. Isoform-specific function and distribution of Na/K pumps in the frog lens epithelium. J. Membr. Biol. 178:89–101, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Giaume C., Leybaert L, Naus CC, and Sáez JC. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front. Pharmacol. 4:88, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahidullah M., Mandal A., Beimgraben C., and Delamere N.A. Hyposmotic stress causes ATP release and stimulates Na,K-ATPase activity in porcine lens. J. Cell. Physiol. 227:1428–1437, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Tamiya S., Okafor M.C., and Delamere N.A. Purinergic agonists stimulate lens Na-K-ATPase-mediated transport via a Src tyrosine kinase-dependent pathway. Am. J. Physiol. Cell Physiol. 293:C790–C796, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Shahidullah M., Mandal A., and Delamere N.A. TRPV4 in porcine lens epithelium regulates hemichannel-mediated ATP release and Na-K-ATPase activity. Am. J. Physiol. Cell Physiol. 302:C1751–C1761, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahidullah A., Mandal A., and Delamere N.A. Damage to lens fiber cells causes TRPV4-dependent Src family kinase activation in the epithelium. Exp. Eye Res. 140:85–93, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Conor C.J., Leddy H.A., Benefield H.C., Liedtke W.B., and Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc. Natl. Acad. Sci. U S A. 111:1316–1321, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J., Sun X., White T.W., Delamere N.A., and Mathias R.T. Feedback regulation of intracellular hydrostatic pressure in surface cells of the lens. Biophys. J. 109:1830–1839, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prager-Khoutorsky M., Khoutorsky A., Bourque and Charles W. Unique interweaved microtubule scaffold mediates osmosensory transduction via physical interaction with TRPV1. Neuron. 83:866–878, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Vaghefi E., Pontre B.P., Jacobs M.D., and Donaldson P.J. Visualizing ocular lens fluid dynamics using MRI: manipulation of steady state water content and water fluxes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301:R335–R342, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Smith G., Cox M.J., Calver R., and Garner L.F. The spherical aberration of the crystalline lens of the human eye. Vision Res. 41:235–243, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Artal P., and Guirao A. Contributions of the cornea and the lens to the aberrations of the human eye. Opt. Lett. 23:1713–1715, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Mathias R.T., Kistler J., and Donaldson P. The lens circulation. J. Membr. Biol. 216:1–16, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Donaldson P., Kistler J., and Mathias R.T. Molecular solutions to mammalian lens transparency. News Physiol. Sci. 16:118–123, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Schtte M., Chen S., Buku A., and Wolosin J.M. Connexin50, a gap junction protein of macroglia in the mammalian retina and visual pathway. Exp. Eye Res. 66:605–613, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Fischbarg J. Mechanism of fluid transport across corneal endothelium and other epithelial layers: a possible explanation based on cyclic cell volume regulatory changes. Br. J. Ophthalmol. 81:85–89, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolosin J.M., Schutte M., and Chen S. Connexin distribution in the rabbit and rat ciliary body. A case for heterotypic epithelial gap junctions. Invest. Ophthalmol. Vis. Sci. 38:341–348, 1997 [PubMed] [Google Scholar]
- 21.Shahidullah M., and Delamere N.A. Connexins form functional hemichannels in porcine ciliary epithelium. Exp. Eye Res. 118:20–29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Do C.W., Valiunas V., Leung C.T., Cheng A.K.W., Clark A.F., Wax M.B., Chatterton J.E., and Civan M.M. Regulation of gap junction coupling in bovine ciliary epithelium. Am. J. Physiol. Cell Physiol. 298:C798–C806, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W., Hui H., Pelegrin P., and Surprenant A. Pharmacological characterization of pannexin-1 currents expressed in mammalian cells. J. Pharmacol. Exp. Ther. 328:409–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo N., Conwell M.D., Chen X., Kettenhofen C.I., Westlake C.J., Cantor L.B., Wells C.D., Weinreb R.N., Corson T.W., Spandau D.F., Joos K.M., Iomini C., Obukhov A.G., and Sun Y. Primary cilia signaling mediates intraocular pressure sensation. Proc. Natl. Acad. Sci. U S A. 111:12871–12876, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li A., Leung C.T., Peterson-Yantorno K., Mitchell C.H., and Civan M.M. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am. J. Physiol. Cell Physiol. 299:C1308–C1317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans W.H., De vuyst E., and Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem. J. 397:1–14, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahidullah M., and Wilson W.S. Atriopeptin, sodium azide and cyclic GMP reduce secretion of aqueous humour and inhibit intracellular calcium release in bovine cultured ciliary epithelium. Br. J. Pharmacol. 127:1438–1446, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farahbakhsh N.A. Ectonucleotidases of the rabbit ciliary body nonpigmented epithelium. Invest. Ophthalmol. Vis. Sci. 44:3952–3960, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Shearer T.W., and Crosson C.E. Adenosine A1 receptor modulation of MMP-2 secretion by trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 43:3016–3020, 2002 [PubMed] [Google Scholar]
- 30.Zhong Y., Yang Z., Huang W.-C., and Luo X. Adenosine, adenosine receptors and glaucoma: an updated overview. Biochim. Biophys. Acta. 1830:2882–2890, 2013 [DOI] [PubMed] [Google Scholar]
- 31.McLaughlin C.W., Zellhuber-McMillan S., Macknight A.D., and Civan M.M. Electron microprobe analysis of ouabain-exposed ciliary epithelium: PE-NPE cell couplets form the functional units. Am. J. Phys. Cell Physiol. 286:C1376–C1389, 2004 [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin C.W., Zellhuber-McMillan S., Macknight A.D.C., and Civan M.M. Electron microprobe analysis of rabbit ciliary epithelium indicates enhanced secretion posteriorly and enhanced absorption anteriorly. Am. J. Physiol. Cell Physiol. 293:C1455–C1466, 2007 [DOI] [PubMed] [Google Scholar]