Abstract
Insulin regulates glucose homeostasis by binding and activating the insulin receptor, and defects in insulin responses (insulin resistance) induce type 2 diabetes. SH2-B, an Src homology 2 (SH2) and pleckstrin homology domain-containing adaptor protein, binds via its SH2 domain to insulin receptor in response to insulin; however, its physiological role remains unclear. Here we show that SH2-B was expressed in the liver, skeletal muscle, and fat. Systemic deletion of SH2-B impaired insulin receptor activation and signaling in the liver, skeletal muscle, and fat, including tyrosine phosphorylation of insulin receptor substrate 1 (IRS1) and IRS2 and activation of the phosphatidylinositol 3-kinase/Akt and the Erk1/2 pathways. Consequently, SH2-B−/− knockout mice developed age-dependent hyperinsulinemia, hyperglycemia, and glucose intolerance. Moreover, SH2-B directly enhanced autophosphorylation of insulin receptor and tyrosine phosphorylation of IRS1 and IRS2 in an SH2 domain-dependent manner in cultured cells. Our data suggest that SH2-B is a physiological enhancer of insulin receptor activation and is required for maintaining normal insulin sensitivity and glucose homeostasis during aging.
Insulin is secreted from pancreatic β cells in response to blood glucose, which maintains glucose homeostasis by promoting glucose utilization in skeletal muscle and fat and inhibiting hepatic gluconeogenesis. Defects of either insulin secretion or insulin action cause disorders of glucose and lipid metabolism, resulting in metabolic disregulation that progresses to type 2 diabetes. Insulin receptor (IR) contains intrinsic tyrosine kinase activity, and insulin stimulates IR autophosphorylation, which is required for the recruitment and phosphorylation of cellular substrates (15). Upon activation, IR tyrosyl phosphorylates and activates multiple signaling molecules, including IR substrate 1 (IRS1), IRS2, IRS3, IRS4, Shc, APS, and SH2-B (4, 27, 30, 40, 41, 43). IRS1 and IRS2 are critical activators of multiple downstream pathways, including the phosphoinositide (PI) 3-kinase/Akt and the mitogen-activated protein kinase (MAPK) pathways (45). Deletion of either IRS1 or IRS2 causes insulin resistance; however, only IRS2−/− mice progress to diabetes because of β cell dysfunction (6, 42, 46). The PI 3-kinase/Akt pathway is required for glucose uptake in skeletal muscle and fat and suppression of hepatic glucose production in response to insulin (9, 11, 38, 39, 47). The essential role of Akt is revealed in mice deficient in Akt2 that develop severe peripheral insulin resistance and type 2 diabetes (11).
Recent structural studies suggest that dimeric APS binds to phosphorylated tyrosines within the activation loop of IR, contributing to IR activation (14). APS is a member of the SH2-B family of Src homology 2 (SH2) domain-containing proteins that includes SH2-B and Lnk. SH2-Bβ was originally identified through its association with JAK2, a cytoplasmic tyrosine kinase that mediates cytokine actions (36). SH2-Bβ is composed of a pleckstrin homology domain, an SH2 domain, and multiple phosphorylation sites. It binds to JAK2 via its SH2 domain, resulting in potentiation of JAK2 activation in response to growth hormone in cultured cells (32). SH2-Bβ also binds via its SH2 domain to multiple receptor tyrosine kinases including receptors for insulin, insulin-like growth factor 1, platelet-derived growth factor, fibroblast growth factor, and nerve growth factor receptor TrkA (17, 18, 29, 30, 33, 35, 36). Alternative splicing of the SH2-B mRNA produces at least four isoforms (α, β, γ, and δ) that differ in their C termini after the SH2 domain (25, 48); therefore, all isoforms are expected to bind to similar tyrosine kinases via their SH2 domains.
SH2-B binds via its SH2 domain to the activation loop of IR (18, 23, 25). Stable overexpression of SH2-B enhances insulin-stimulated activation of both Erk1/2 and Akt in Chinese hamster ovary cells (3). These observations raise the possibility that SH2-B may play a positive regulatory role during insulin action. In this work, we demonstrate that systemic deletion of the SH2-B gene severely impaired insulin signaling in skeletal muscle, the liver, and fat. Consequently, SH2-B−/− knockout mice progressively developed hyperinsulinemia, hyperglycemia, and glucose intolerance. Our results suggest that SH2-B is a physiological enhancer of insulin action and is required for glucose homeostasis during aging.
MATERIALS AND METHODS
Generation of SH2-B-deficient mice.
The SH2-B gene was obtained by screening a genomic DNA library derived from mouse 129/Sv embryonic stem (ES) cells. A DNA fragment (−5.2 to −1.2 kb upstream of the SH2-B gene) was ligated to the pPNT vector 5′ to the neo cassette. A 6.5-kb DNA sequence, encoding the C-terminal half of the SH2 domain and 3′ untranslated region, was ligated to the same pPNT vector 3′ to the neo cassette (Fig. 1B). The SH2-B targeting vector was introduced into R1 ES cells by electroporation. The transfectants were selected with neomycin (G418) and ganciclovir. SH2-B+/− heterozygous ES cells were injected into C57BL/6 blastocysts to generate SH2-B-deficient mice. Animals (on a 129/Sv and C57BL/6 mixed genetic background) were housed on a 12-h-light and 12-h-dark cycle in the Unit for Laboratory Animal Medicine at the University of Michigan, with free access to water and a standard mouse diet (9% fat content) unless indicated otherwise. Animal experiments were conducted by following the protocols approved by the University Committee on the Use and Care of Animals.
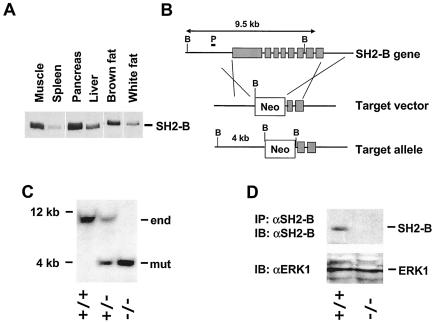
FIG. 1.
Disruption of the SH2-B gene in mice. (A) Tissue distributions of SH2-B protein. Tissue extracts were prepared from a wild-type male (5 weeks old; 129/Sv and C57BL/6 mixed background), immunoprecipitated, and immunoblotted with anti-SH2-B. Proteins (2 mg for spleen, pancreas, and liver or 1 mg for muscle and fat) were used for immunoprecipitation. (B) Schematic representation of target vector and homologous recombination. The positions of the probe for Southern blotting (P) and BamHI sites (B) were marked. (C) Southern blots for the SH2-B gene. Genomic DNAs were digested with BamHI and subjected to Southern blot analysis. (D) Liver extracts were prepared from wild-type or SH2-B−/− males. SH2-B was immunoprecipitated (IP) and immunoblotted (IB) with anti-SH2-B (αSH2-B) (upper panel). Liver extracts were immunoblotted with anti-Erk1 (αERK1) (lower panel).
Blood glucose, plasma insulin, ITT, and GTT.
Blood samples collected from the tail vein were used to measure blood glucose by the glucose oxidase method (Glucometer Elite; Bayer Corp., Tarrytown, N.Y.) and plasma insulin with a rat insulin enzyme-linked immunosorbent assay kit (Crystal Chem, Inc., Chicago, Ill.). For insulin tolerance tests (ITT), mice were subjected to fasting for 6 h and human insulin (1 IU/kg of body weight) was injected intraperitoneally. Blood glucose was monitored at 0, 15, 30, and 60 min after glucose injection. For glucose tolerance tests (GTT), mice were subjected to fasting overnight (∼16 h) and d-glucose (2 g/kg of body weight) was injected intraperitoneally. Blood glucose was monitored at 0, 15, 30, 60, 90, 120, and 150 min after glucose injection.
Immunoprecipitation and immunoblotting.
Animals were anesthetized with Avertin (0.5 g of tribromoethanol and 0.25 g of tert-amyl alcohol in 39.5 ml of water; 0.02 ml/g of body weight) and treated for 5 min with either phosphate-buffered saline (PBS) or human insulin (3 U per mouse) via inferior vena cava injection. Liver, gastrocnemius muscle, and epididymal fat were isolated 5 min after stimulation and homogenized in lysis buffer (50 mM Tris [pH 7.5], 1% Nonidet P-40, 150 mM NaCl, 2 mM EGTA, 1 mM Na3VO4, 100 mM NaF, 10 mM Na4P2O7, 50 nM okadaic acid, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml). Tissue extracts were incubated with indicated antibodies on ice for 2 h. The immune complexes were collected on protein A-agarose during a 1-h incubation at 4°C. The beads were washed three times with washing buffer (50 mM Tris [pH 7.5], 1% Nonidet P-40, 150 mM NaCl, 2 mM EGTA) and boiled for 5 min in a mixture (80:20) of lysis buffer and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (250 mM Tris-HCl [pH 6.8], 10% SDS, 10% β-mercaptoethanol, 40% glycerol, 0.01% bromophenol blue). The solubilized proteins were separated by SDS-PAGE. Proteins on the gel were transferred to nitrocellulose membranes and detected by immunoblotting with the indicated antibodies by enhanced chemiluminescence. Some membranes were subsequently incubated at 55°C for 30 min in stripping buffer (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl [pH 6.7]) to prepare them for reprobing.
Immunostaining.
Mice were sacrificed by administering an overdose of sodium amino barbital (200 mg/kg of body weight). The pancreas was removed, cleared of fat and lymph nodes, fixed in Bouin's solution, and embedded in paraffin. Pancreatic sections (5 μm thick) were prepared, stained with hematoxylin and eosin or antibodies against insulin or glucagon, and photographed.
In vitro kinase assays.
Mice were treated with insulin (3 U) for 5 min as described above. For the IR kinase assay, the liver was isolated and homogenized in lysis buffer. The extracts were immunoprecipitated with anti-IR antibodies. After extensive washing, IR immunoprecipitates were incubated with [γ-32P]ATP (15 μCi) for 30 min at room temperature in reaction buffer (20 mM HEPES [pH 7.6], 5 mM MnCl2, 100 mM NaCl, 0.5 mM dithiothreitol, 1 mM Na3VO4, 5 mM MgCl2, 20 μM cold ATP, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml). The reaction was stopped by adding 10 mM EDTA. The reaction mixture was resolved by SDS-PAGE and subsequently subjected to autoradiography. For the PI 3-kinase assay, gastrocnemius muscles were isolated and homogenized in lysis buffer. IRS1 was immunoprecipitated with anti-IRS1 and subjected to an in vitro kinase assay as described previously (24). Briefly, after extensive washing, IRS1 immunoprecipitates were incubated with l-α-phosphatidylinositol (2 μg) and [γ-32P]ATP (5 μCi) for exactly 15 min at room temperature in kinase reaction buffer (10 mM Tris-HCl [pH 7.5], 100 mM NaCl, 14 mM MgCl2, 1.4 mM cold ATP, 1 mM EDTA, 100 μM NaVO4). The reaction was stopped by sequentially adding HCl (8 M) and CHCl3-MeOH (1:1). The lipid products were extracted by centrifugation, spotted on a thin-layer chromatography plate, separated by running buffer (CHCl3-MeOH-H2O-NH4OH at a ratio of 120:94:23.2:4), and visualized by autoradiography.
Transfection.
HEK293 cells were transfected with the indicated plasmids by using Lipofectamine 2000 reagents (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. Cells were deprived of serum overnight 24 h after transfection and then treated with 100 nM insulin for 5 or 30 min. Cell extracts were prepared and subjected to immunoprecipitation and immunoblotting with the indicated antibodies as described above.
RESULTS
Disruption of the SH2-B gene causes age-dependent hyperglycemia, hyperinsulinemia, and glucose intolerance in mice.
To examine whether SH2-B is involved in insulin action in vivo, we determined the expression of SH2-B in insulin target tissues. SH2-B protein was abundant in the skeletal muscle, liver, fat, and pancreas as measured by immunoblotting with anti-SH2-B antibodies (Fig. 1A). To determine the role of SH2-B in vivo, we generated SH2-B-deficient mice by replacing most of the SH2-B gene from its promoter region (−1.2 kb) through the majority of the coding sequence with a neo cassette (Fig. 1B). All isoforms of SH2-B are predicted to be eliminated in SH2-B−/− knockout mice. Southern blot analysis revealed complete disruption of the SH2-B gene in homozygotes (Fig. 1C). The SH2-B protein was detected by immunoblotting in liver extracts from wild-type mice but not from SH2-B−/− knockout mice, confirming that the gene was successfully deleted (Fig. 1D). Deletion of the SH2-B gene did not affect the expression of other signaling molecules, including Erk1, Akt, and p85 (Fig. 1D and data not shown).
SH2-B−/− mice were born at the Mendelian frequency (1:2:1 for SH2-B+/+, SH2-B+/−, and SH2-B−/−), suggesting that SH2-B was not required for embryonic development. However, SH2-B−/− homozygotes were smaller than their wild-type littermates during their first 5 weeks of life; the fertility of SH2-B−/− mice was impaired as previously described (26).
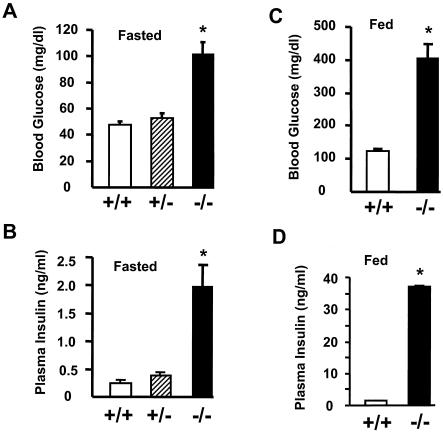
Because SH2-B binds to IR (18), we examined whether disruption of SH2-B affects insulin-regulated glucose metabolism. Blood glucose and plasma insulin were continuously monitored in SH2-B knockout and wild-type control littermates. SH2-B−/− knockout mice exhibited normal levels of blood glucose and plasma insulin during their first 8 weeks of life (data not shown); however, as male SH2-B−/− mice aged, they developed hyperglycemia. At 11 weeks of age, blood glucose levels following an overnight 16-h fast increased by ∼1.56 times in SH2-B−/− males (SH2-B−/−, 102.8 ± 8.9 mg/dl, n = 10; wild type, 65.8 ± 3.9 mg/dl, n = 12). However, SH2-B−/− mice responded normally to intraperitoneally injected glucose during GTT, suggesting that the insulin resistance was relatively mild at this age (data not shown).
Insulin resistance was more severe in older SH2-B−/− males. Blood glucose was elevated more than twofold, and plasma insulin levels were elevated more than eightfold in SH2-B−/− males at an age of 6 to 7 months after a 16-h fast (Fig. 2A and B). Moreover, during a random feeding, insulin levels were 20-fold higher than normal while blood glucose levels were 3.3-fold above those of age-matched wild-type controls (Fig. 2C and D). Female SH2-B−/− mice also developed hyperinsulinemia, but to a lesser extent, and their blood glucose was slightly increased (H. Yang and L. Rui, unpublished data), consistent with various other mouse models in which males are more susceptible to glucose intolerance and type 2 diabetes than females (8, 46).
FIG. 2.
SH2-B−/− mice develop hyperglycemia and hyperinsulinemia. Wild-type (n = 12) and SH2-B−/− (n = 10) males were fed a standard mouse diet (9% fat content) for 6 to 7 months. (A) Blood glucose from fasting mice; (B) plasma insulin from fasting mice; (C) blood glucose from randomly fed mice; (D) plasma insulin from randomly fed mice. *, P < 0.05 compared with wild-type controls.
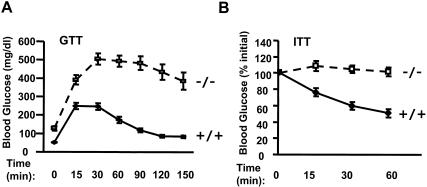
To validate the role of SH2-B on glucose homeostasis, GTT and ITT were conducted on 6- to 7-month-old male mice. Intraperitoneal injection of exogenous glucose elevated blood glucose levels in both wild-type and SH2-B−/− mice; however, the magnitude and duration of glucose elevation were much larger in SH2-B−/− males than in age-matched wild-type controls (Fig. 3A), suggesting that SH2-B−/− mice are intolerant to exogenous glucose. Exogenous insulin lowered blood glucose in wild-type mice as expected; however, the ability of insulin to reduce blood glucose was dramatically impaired in SH2-B−/− mice (Fig. 3B). Together, these data indicated that SH2-B−/− mice are insulin resistant and progress toward type 2 diabetes with increasing age.
FIG. 3.
SH2-B−/− mice develop glucose intolerance and reduced response to exogenous insulin. Wild-type (n = 12) and SH2-B−/− (n = 10) males were a fed standard mouse diet (9% fat content) for 6 to 7 months. (A) GTT. Mice were injected intraperitoneally with d-glucose (2 mg/kg of body weight), and blood glucose was monitored at 0, 15, 30, 60, 90, 120, and 150 min after injection. (B) ITT. Mice were injected intraperitoneally with human insulin (1 IU/kg of body weight), and blood glucose was monitored at 0, 15, 30, and 60 min after injection.
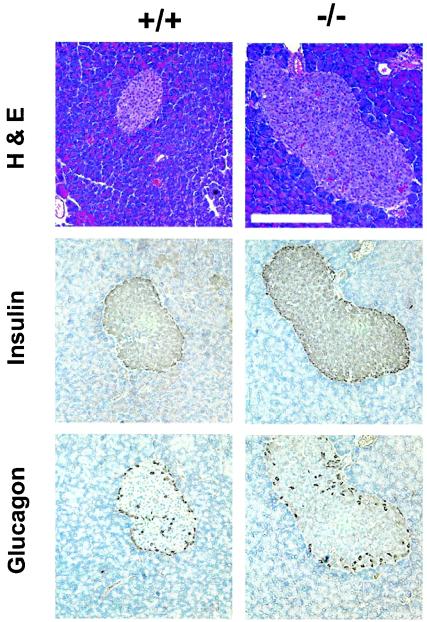
Type 2 diabetes develops as a result of a combination of peripheral insulin resistance and dysfunction of pancreatic β cells that fail to secrete sufficient insulin in response to elevated blood glucose. To determine whether islets contribute to hyperglycemia and glucose intolerance in SH2-B−/− mice, the pancreas was isolated from wild-type or SH2-B−/− males at 6 months and islets were visualized by hematoxylin and eosin staining or immunostaining with antibodies against glucagon or insulin. Islet size was increased significantly in SH2-B−/− mice, consistent with profound peripheral insulin resistance (Fig. 4). The large islets retained normal architecture with glucagon-producing α cells at the periphery and insulin-producing β cells at the center, suggesting that SH2-B may not be required for β cell growth and insulin production (Fig. 4). Hyperplasia of β cells is a common compensatory mechanism in response to peripheral insulin resistance.
FIG. 4.
Disruption of the SH2-B gene causes hyperplasia of islets. Pancreatic sections (5 μm thick) from wild-type or SH2-B−/− knockout males (6 months old) were stained with hematoxylin and eosin (H&E) or antibodies against insulin or glucagon as indicated. Bar, 2.5 μm.
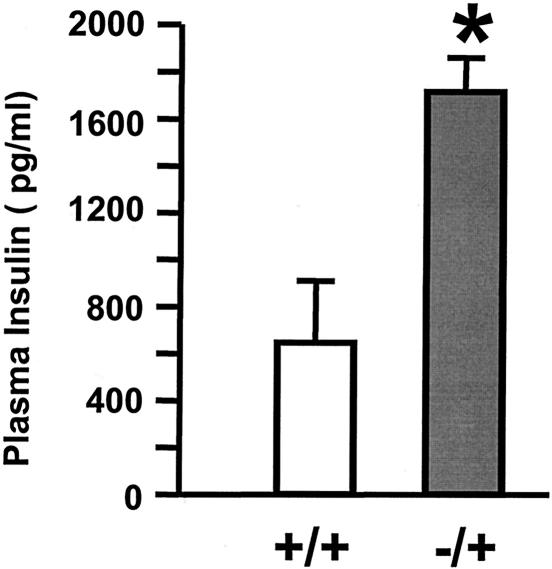
Blood glucose and plasma insulin levels were similar between wild-type mice and SH2-B+/− heterozygotes (Fig. 2A and B); however, SH2-B+/− heterozygous males developed hyperinsulinemia on high-fat diets (45% fat content) to a much greater extent than age-matched wild-type control males (Fig. 5). Haploinsufficiency of SH2-B may predispose the mutants to insulin resistance and type 2 diabetes.
FIG. 5.
Heterozygous deletion of SH2-B enhances high-fat-diet-induced insulin resistance. Wild-type or heterozygous SH2-B+/− mice (5-month-old males, n = 5) were fed a high-fat diet (45% fat, D12451; Research Diets, Inc.) for 4 weeks. Animals were subjected to fasting overnight (16 h), and plasma insulin was measured by using rat insulin enzyme-linked immunosorbent assay kits. *, P < 0.05 compared with wild-type controls.
Deletion of SH2-B impairs insulin signaling in vivo.
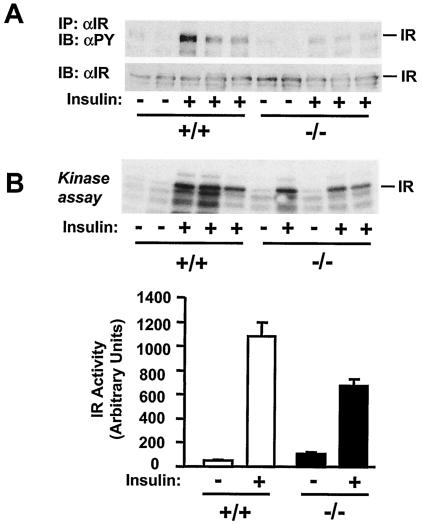
To gain insight into potential mechanisms of SH2-B action, we examined insulin signaling in tissues of SH2-B-deficient mice. Mice (7 months) were subjected to fasting overnight and then treated for 5 min with human insulin (3 U per mouse) or PBS as a control. The skeletal muscle and liver were removed and prepared for specific immunoprecipitation, immunoblot, and in vitro kinase analysis. The autophosphorylation of IR immunoprecipitates was measured by immunoblotting with antiphosphotyrosine antibodies. Insulin rapidly stimulated tyrosine phosphorylation of IR in wild-type mice; however, IR autophosphorylation was significantly reduced in SH2-B−/− mice even though IR protein levels were similar between SH2-B−/− mice and age-matched wild-type controls (Fig. 6A). IR activity was also measured directly by an in vitro kinase assay. Hepatic IR was immunoprecipitated from SH2-B−/− or age-matched wild-type control mice and subjected to an in vitro kinase assay in the presence of [γ-32P]ATP. In PBS-treated control mice, basal IR activity was similar between SH2-B−/− and wild-type control mice. Insulin strongly stimulated IR kinase activity in wild-type mice; in contrast, insulin-stimulated IR activity was reduced in SH2-B−/− mice by approximately 38% (Fig. 6B). These results suggest that SH2-B may be a physiological enhancer of IR activation in animals.
FIG. 6.
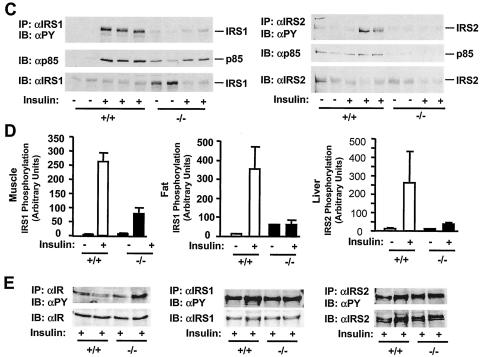
Disruption of the SH2-B gene inhibits IR activation, tyrosine phosphorylation of IRS1 and IRS2, and interaction of IRS proteins with p85. Wild-type or SH2-B−/− males (7 months old) were subjected to fasting overnight (∼18 h) and administered human insulin (3 U per mouse) or PBS as a control via the inferior vena cava. The liver, gastrocnemius muscle, and epididymal fat were isolated 5 min after stimulation and homogenized. (A) Muscle extracts were immunoprecipitated (IP) with anti-IR (αIR) and immunoblotted (IB) with antiphosphotyrosine (αPY, top panel) or anti-IR (bottom panel). Each lane represents an individual mouse. (B) Immunopurified hepatic IR was subjected to in vitro kinase assays in the presence of [γ-32P]ATP with (+) and without (−) insulin. (C) IRS1 was immunoprecipitated with anti-IRS1 (αIRS1) from muscle extracts and separated by SDS-PAGE. The blot was cut into two pieces along the molecular standard of 117 kDa. The upper piece was immunoblotted with antiphosphotyrosine and subsequently reprobed with anti-IRS1. The bottom piece was immunoblotted with the anti-p85 regulatory subunit of PI 3-kinase (αp85). Similarly, hepatic IRS2 was immunoprecipitated with anti-IRS2 (αIRS2) and immunoblotted with anti-phosphotyrosine, anti-p85, or anti-IRS2 as indicated. (D) Tyrosine phosphorylation of IRS1 or IRS2 was quantified and normalized to total IRS1 or IRS2, respectively. Each bar represents the average of the results from two to three mice. (E) SH2-B−/− and wild-type littermates (7-week-old males) were subjected to fasting overnight (∼18 h) and treated with human insulin (3 U per mouse) for 5 min. Liver extracts were immunoprecipitated with anti-IR, anti-IRS1, or anti-IRS2 and immunoblotted with antiphosphotyrosine. The blots were reprobed with anti-IR, anti-IRS1, or anti-IRS2 as indicated.
To examine whether deletion of SH2-B impairs tyrosine phosphorylation of IRS1 and IRS2, old wild-type and SH2-B−/− mice (7 months) were subjected to fasting overnight (∼18 h) and then treated with PBS or human insulin. Immunopurified IRS1 or IRS2 was immunoblotted with antiphosphotyrosine antibodies. Insulin-stimulated tyrosine phosphorylation of IRS1 was significantly reduced in muscle from SH2-B−/− mice (Fig. 6C). Quantitative analysis revealed a 70% reduction in skeletal muscle and an 80% reduction in adipose tissues (Fig. 6D). Similarly, insulin-stimulated tyrosine phosphorylation of hepatic IRS2 was reduced by 85% in SH2-B−/− mice (Fig. 6C and D). Interestingly, insulin-stimulated tyrosine phosphorylation of IR, IRS1, and IRS2 was similar between young SH2-B−/− and wild-type littermates at an age of 7 weeks (Fig. 6E), consistent with normal glucose metabolism in young SH2-B knockout mice less than 8 weeks old.
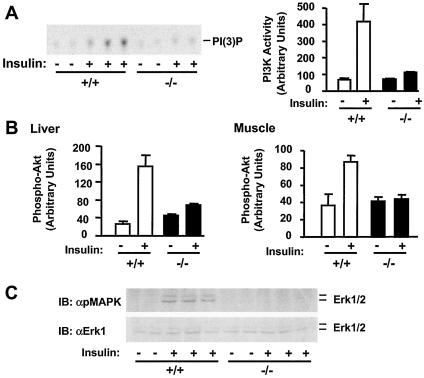
Activation of the PI 3-kinase → Akt cascade plays an important role in insulin action. The cascade is initiated through the interaction of IRS1 or IRS2 with the p85 regulatory subunit of the PI 3-kinse (44, 45). To determine the interaction of p85 with IRS proteins, mice (7 months) were subjected to fasting overnight and treated with insulin or PBS as a control. IRS1 and IRS2 were immunoprecipitated with anti-IRS1 and anti-IRS2 antibodies, respectively, and immunoblotted with anti-p85 antibody. Insulin promoted coimmunoprecipitation of p85 with both IRS1 and IRS2 in wild-type control mice, whereas these interactions were significantly reduced in SH2-B−/− mice (Fig. 6C). Moreover, IRS1-associated PI 3-kinase activity was measured by in vitro kinase assays. Insulin stimulated the IRS1-associated PI 3-kinase activity by 6-fold in the skeletal muscle from wild-type control animals, whereas PI 3-kinase activity increased only 1.5-fold in response to insulin in SH2-B−/− knockout mice (Fig. 7A). Akt activation was estimated by immunoblotting with anti-phospho-Akt that specifically recognizes phosphorylated Akt at Thr308 or Ser473. Insulin stimulated the phosphorylation of Akt at Thr308 by 5-fold in the liver and 1.5-fold in muscle from wild-type mice, whereas the same treatment increased Thr308 phosphorylation by only 0.6-fold in the liver and 0.1-fold in muscle from SH2-B−/− mice (Fig. 7B). The insulin-stimulated phosphorylation of Akt at Ser473 was also reduced significantly in SH2-B−/− mice (C. Duan and L. Rui, unpublished data). Moreover, the activation of Erk1 and Erk2 was examined by immunoblotting with anti-phospho-MAPK that specifically recognizes phosphorylated and activated Erk1 and Erk2. Insulin rapidly stimulated the activation of Erk1/2 in wild-type control mice, whereas Erk1/2 activation was dramatically reduced in SH2-B−/− mice, although the protein levels of Erk1 and Erk2 were similar between SH2-B−/− and wild-type control mice (Fig. 7C). These data suggest that inhibition of insulin signaling in the liver, muscle, and fat from SH2-B−/− mice, including the activation of IR, IRS1, IRS2, and the PI 3-kinase pathways, may contribute to hyperglycemia, hyperinsulinemia, and glucose intolerance.
FIG. 7.
Deletion of SH2-B impairs insulin-induced activation of the PI 3-kinase/Akt and the MAPK pathways. Wild-type or SH2-B−/− males (7 months old) were subjected to fasting overnight (∼18 h) and administered human insulin (3 U per mouse) (+) or PBS (−) as a control via the inferior vena cava. Tissues were isolated 5 min after stimulation and homogenized. (A) IRS1 proteins were immunoprecipitated from the gastrocnemius muscle with anti-IRS1, and IRS1-associated PI 3-kinase was subjected to in vitro kinase assays. (B) Tissue extracts were immunoblotted with anti-phospho-Akt that recognizes Akt phosphorylated specifically on Thr308. The same blots were stripped and reprobed with anti-Akt. Akt phosphorylation was normalized to the total amounts of Akt proteins. (C) Interscapular brown fat extracts were immunoblotted (IB) with anti-phospho-MAPK (αpMAPK) that specifically recognizes phosphorylated and activated Erk1/2. The same blots were stripped and reprobed with anti-Erk1 (αErk1) that recognizes both Erk1 and Erk2. Each lane represents the results from an individual mouse.
SH2-B enhances IR activation.
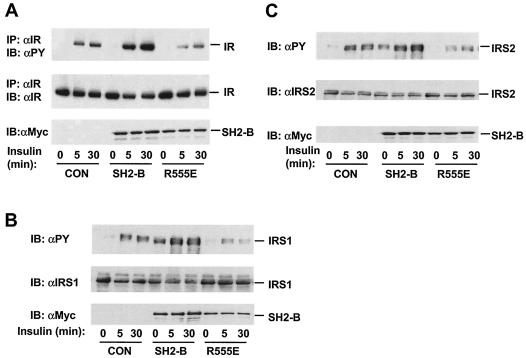
It has been shown previously that SH2-B binds directly to JAK2 via its SH2 domain, resulting in potent activation of JAK2 (32). To determine whether SH2-B promotes IR activation in a similar fashion, HEK293 cells were transiently cotransfected with plasmids encoding Myc-tagged SH2-Bβ and IR and then treated with insulin. Insulin stimulated the autophosphorylation of IR in cells transfected with control empty vector (Fig. 8A). Coexpression of SH2-Bβ greatly increased IR autophosphorylation, although IR protein levels were not changed (Fig. 8A). In contrast, SH2-B (R555E), which contains a point mutation (Arg555 to Glu) within the SH2 domain and is unable to bind to IR, failed to stimulate IR activation even though it was expressed at a similar level (Fig. 8A).
FIG. 8.
The SH2 domain is required for SH2-B stimulation of IR. (A) HEK293 cells were transiently cotransfected with plasmids encoding IR and Myc-tagged SH2-Bβ or SH2-B (R555E). IR was immunoprecipitated (IP) with anti-IR (αIR) and immunoblotted (IB) with antiphosphotyrosine (αPY). The same blots were reprobed with anti-IR. SH2-B in cell extracts was immunoblotted with anti-Myc (αMyc). (B) HEK293 cells were transiently cotransfected with plasmids encoding IRS1 and Myc-tagged SH2-Bβ or SH2-B (R555E) in the presence of IR. Proteins in cell extracts were immunoblotted with antiphosphotyrosine, anti-IRS1, or anti-Myc. (C) HEK293 cells were transiently cotransfected with plasmids encoding IRS2 and Myc-tagged SH2-Bβ or SH2-B (R555E) in the presence of IR. Proteins in cell extracts were immunoblotted with antiphosphotyrosine, anti-IRS2 (αIRS2), or anti-Myc. CON, control.
The potentiation of IR activation by SH2-B was further confirmed by examining the IR-mediated phosphorylation of IRS1 and IRS2 in the presence or absence of SH2-B. IRS1 or IRS2 was coexpressed in HEK293 cells with Myc-tagged SH2-Bβ or SH2-B (R555E) in the presence of IR. Insulin stimulated the tyrosine phosphorylation of IRS1 and IRS2 by IR in control cells; SH2-B increased both the basal and insulin-stimulated tyrosine phosphorylation of IRS1 and IRS2 without a change in protein levels (Fig. 8B and C). In contrast, SH2-B (R555E), as a dominant-negative mutant, inhibited the tyrosine phosphorylation of IRS1 and IRS2 (Fig. 8B and C). Together, SH2-B binds via its SH2 domain to the activation loop of IR, resulting in enhancement of IR activation.
DISCUSSION
SH2-B was reported previously to directly bind to IR in cultured cells (18, 25, 30). In this study, we demonstrated that SH2-B was expressed in insulin target tissues including the liver, muscle, and fat. Deletion of SH2-B inhibited IR activation and signaling in the liver, muscle, and fat, including tyrosine phosphorylation of IRS1 and IRS2 and activation of the PI 3-kinase/Akt and Erk1/2 pathways. Consequently, SH2-B knockout mice developed age-dependent hyperglycemia, hyperinsulinemia, and glucose intolerance. These findings indicate that SH2-B is required for maintaining normal glucose homeostasis in vivo, presumably by enhancing IR activation and signaling in the liver, muscle, and/or fat. Consistent with this idea, SH2-B binds via its SH2 domain directly to the activation loop of IR (18, 25, 30). This interaction promotes insulin signaling, as SH2-B, but not SH2-B (R555E) lacking the functional SH2 domain, enhanced IR autophosphorylation and tyrosine phosphorylation of IRS1 and IRS2 in cultured cells.
SH2-B could promote IR activation in several ways. Structure data revealed that the SH2 domain of APS, which is 80% identical in amino acid sequence to the SH2 domain of SH2-B, binds as a dimer to the activation loops of both β subunits of IR (14). SH2-B forms multimers through an N-terminal association domain, suggesting that SH2B may interact with the IR in a similar way (28). Multimeric SH2-B may not only increase its affinity for IR but also stabilize IR in an active state. Alternatively, the binding of SH2-B may protect the activated IR from dephosphorylation by protein tyrosine phosphatases (e.g., PTP1B), prolonging IR activity and signaling.
The late onset of hyperglycemia, hyperinsulinemia, and glucose intolerance in SH2-B knockout mice is intriguing. Increasing evidence suggests that chronic cellular stress, which is associated with aging, impairs insulin signaling, contributing to type 2 diabetes. Stress activates various Ser/Thr kinases including JNK, which may phosphorylate IRS1 and IRS2 at inhibitory serines or threonines (1, 2, 12, 13, 16, 19, 31, 49). In addition, cellular stress also induces a reduction of IRS1 and IRS2 via the ubiquitin/proteasome-mediated degradation, further decreasing insulin sensitivity (34, 37). SH2-B may provide a mechanism to antagonize stress-induced insulin resistance by enhancing IR activation, thus maintaining relatively normal insulin sensitivity during aging and environmental stress. Interestingly, SH2-B is widely expressed in multiple tissues in addition to the liver, muscle, and fat. It has been shown previously that SH2-B binds directly to JAK2 and serves as a substrate of JAK2. JAK2 is a cytoplasmic tyrosine kinase that mediates cell signaling by a verity of cytokines including growth hormone, prolactin, leptin, interleukin-6, and gamma interferon. Cytokines are well characterized to modulate insulin sensitivity. Deletion of SH2-B may also alter the actions of some cytokines, resulting in the inhibition of insulin responses. Therefore, SH2-B may enhance insulin sensitivity both directly by binding to IR and indirectly via modulation of JAK2-mediated cytokine responses.
SH2-B shares homology with APS, especially within the pleckstrin homology and SH2 domains. Both APS and SH2-B bind to the same site in IR and enhance insulin-stimulated activation of Akt1 and Erk1/2 in cultured cells (3, 4, 18, 23, 25, 30). APS binds to Cbl and recruits the Cbl/TC10 pathway in response to insulin (5, 7, 10, 20, 21). The Cbl/TC10 pathway has been reported to be required for insulin-stimulated glucose uptake in adipocytes (7, 10). Surprisingly, disruption of the APS gene increases insulin sensitivity in mice (22). In contrast, deletion of SH2-B induces severe insulin resistance and glucose intolerance, as demonstrated in this study. This striking phenotypic difference indicates that SH2-B cannot be functionally replaced by APS in vivo. A simple explanation would be that SH2-B and APS perform distinct functions in vivo. Alternatively, APS may not be expressed in SH2-B target tissues, or APS expression may not be sufficient to compensate for SH2-B action in SH2-B knockout mice. In contrast, SH2-B may be highly expressed in APS target tissues and may mediate insulin action even better than APS. APS competes for the same binding site in IR with SH2-B. APS also heteromultimerizes with SH2-B (28) and may inhibit SH2-B action. Therefore, deletion of the APS gene may relieve inhibition of SH2-B from APS, resulting in increased insulin sensitivity in APS knockout mice.
In summary, we have shown that disruption of the SH2-B gene impaired insulin signaling in the liver, skeletal muscle, and fat. Consequently, SH2-B knockout mice developed hyperglycemia, hyperinsulinemia, and glucose intolerance during aging. Moreover, SH2-B directly enhanced IR activation and subsequent tyrosine phosphorylation of IRS1 and IRS2 in cultured cells, suggesting that SH2-B is a physiological enhancer of IR activation. SH2-B expression and its regulated signaling events may serve as potential drug targets for therapeutic intervention of insulin resistance and type 2 diabetes.
Acknowledgments
This study was supported by an American Diabetes Association Career Development Award (no. 7-03-CD-77) and NIH grant no. RO1 DK 065122 (both to L.R.).
We thank Decheng Ren and David Morris for assistance.
REFERENCES
- 1.Aguirre, V., T. Uchida, L. Yenush, R. Davis, and M. F. White. 2000. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 275:9047-9054. [DOI] [PubMed] [Google Scholar]
- 2.Aguirre, V., E. D. Werner, J. Giraud, Y. H. Lee, S. E. Shoelson, and M. F. White. 2002. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 277:1531-1537. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, Z., and T. S. Pillay. 2003. Adapter protein with a pleckstrin homology (PH) and an Src homology 2 (SH2) domain (APS) and SH2-B enhance insulin-receptor autophosphorylation, extracellular-signal-regulated kinase and phosphoinositide 3-kinase-dependent signalling. Biochem. J. 371:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed, Z., B. J. Smith, K. Kotani, P. Wilden, and T. S. Pillay. 1999. APS, an adapter protein with a PH and SH2 domain, is a substrate for the insulin receptor kinase. Biochem. J. 341(Pt 3):665-668. [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed, Z., B. J. Smith, and T. S. Pillay. 2000. The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett. 475:31-34. [DOI] [PubMed] [Google Scholar]
- 6.Araki, E., M. A. Lipes, M. E. Patti, J. C. Bruning, B. Haag III, R. S. Johnson, and C. R. Kahn. 1994. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 372:186-190. [DOI] [PubMed] [Google Scholar]
- 7.Baumann, C. A., V. Ribon, M. Kanzaki, D. C. Thurmond, S. Mora, S. Shigematsu, P. E. Bickel, J. E. Pessin, and A. R. Saltiel. 2000. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature 407:202-207. [DOI] [PubMed] [Google Scholar]
- 8.Burks, D. J., J. F. de Mora, M. Schubert, D. J. Withers, M. G. Myers, H. H. Towery, S. L. Altamuro, C. L. Flint, and M. F. White. 2000. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407:377-382. [DOI] [PubMed] [Google Scholar]
- 9.Cheatham, B., C. J. Vlahos, L. Cheatham, L. Wang, J. Blenis, and C. R. Kahn. 1994. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol. Cell. Biol. 14:4902-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang, S. H., C. A. Baumann, M. Kanzaki, D. C. Thurmond, R. T. Watson, C. L. Neudauer, I. G. Macara, J. E. Pessin, and A. R. Saltiel. 2001. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410:944-948. [DOI] [PubMed] [Google Scholar]
- 11.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolomei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 12.Gao, Z., A. Zuberi, M. J. Quon, Z. Dong, and J. Ye. 2003. Aspirin inhibits serine phosphorylation of insulin receptor substrate 1 in tumor necrosis factor-treated cells through targeting multiple serine kinases. J. Biol. Chem. 278:24944-24950. [DOI] [PubMed] [Google Scholar]
- 13.Hirosumi, J., G. Tuncman, L. Chang, C. Z. Gorgun, K. T. Uysal, K. Maeda, M. Karin, and G. S. Hotamisligil. 2002. A central role for JNK in obesity and insulin resistance. Nature 420:333-336. [DOI] [PubMed] [Google Scholar]
- 14.Hu, J., J. Liu, R. Ghirlando, A. R. Saltiel, and S. R. Hubbard. 2003. Structural basis for recruitment of the adaptor protein APS to the activated insulin receptor. Mol. Cell 12:1379-1389. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard, S. R. 1997. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 16:5572-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, G., Q. Dallas-Yang, F. Liu, D. E. Moller, and B. B. Zhang. 2003. Salicylic acid reverses phorbol 12-myristate-13-acetate (PMA)- and tumor necrosis factor alpha (TNFalpha)-induced insulin receptor substrate 1 (IRS1) serine 307 phosphorylation and insulin resistance in human embryonic kidney 293 (HEK293) cells. J. Biol. Chem. 278:180-186. [DOI] [PubMed] [Google Scholar]
- 17.Kong, M., C. S. Wang, and D. J. Donoghue. 2002. Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B. A role in STAT5 activation. J. Biol. Chem. 277:15962-15970. [DOI] [PubMed] [Google Scholar]
- 18.Kotani, K., P. Wilden, and T. S. Pillay. 1998. SH2-Balpha is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem. J. 335(Pt 1):103-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, Y. H., J. Giraud, R. J. Davis, and M. F. White. 2003. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 278:2896-2902. [DOI] [PubMed] [Google Scholar]
- 20.Liu, J., S. M. DeYoung, J. B. Hwang, E. E. O'Leary, and A. R. Saltiel. 2003. The roles of Cbl-b and c-Cbl in insulin-stimulated glucose transport. J. Biol. Chem. 278:36754-36762. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J., A. Kimura, C. A. Baumann, and A. R. Saltiel. 2002. APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol. Cell. Biol. 22:3599-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minami, A., M. Iseki, K. Kishi, M. Wang, M. Ogura, N. Furukawa, S. Hayashi, M. Yamada, T. Obata, Y. Takeshita, Y. Nakaya, Y. Bando, K. Izumi, S. A. Moodie, F. Kajiura, M. Matsumoto, K. Takatsu, S. Takaki, and Y. Ebina. 2003. Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes 52:2657-2665. [DOI] [PubMed] [Google Scholar]
- 23.Moodie, S. A., J. Alleman-Sposeto, and T. A. Gustafson. 1999. Identification of the APS protein as a novel insulin receptor substrate. J. Biol. Chem. 274:11186-11193. [DOI] [PubMed] [Google Scholar]
- 24.Myers, M. G., Jr., Y. Zhang, G. A. Aldaz, T. Grammer, E. M. Glasheen, L. Yenush, L. M. Wang, X. J. Sun, J. Blenis, J. H. Pierce, and M. F. White. 1996. YMXM motifs and signaling by an insulin receptor substrate 1 molecule without tyrosine phosphorylation sites. Mol. Cell. Biol. 16:4147-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelms, K., T. J. O'Neill, S. Li, S. R. Hubbard, T. A. Gustafson, and W. E. Paul. 1999. Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm. Genome 10:1160-1167. [DOI] [PubMed] [Google Scholar]
- 26.Ohtsuka, S., S. Takaki, M. Iseki, K. Miyoshi, N. Nakagata, Y. Kataoka, N. Yoshida, K. Takatsu, and A. Yoshimura. 2002. SH2-B is required for both male and female reproduction. Mol. Cell. Biol. 22:3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pronk, G. J., J. McGlade, G. Pelicci, T. Pawson, and J. L. Bos. 1993. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J. Biol. Chem. 268:5748-5753. [PubMed] [Google Scholar]
- 28.Qian, X., and D. D. Ginty. 2001. SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol. Cell. Biol. 21:1613-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian, X., A. Riccio, Y. Zhang, and D. D. Ginty. 1998. Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21:1017-1029. [DOI] [PubMed] [Google Scholar]
- 30.Riedel, H., J. Wang, H. Hansen, and N. Yousaf. 1997. PSM, an insulin-dependent, pro-rich, PH, SH2 domain containing partner of the insulin receptor. J. Biochem. (Tokyo) 122:1105-1113. [DOI] [PubMed] [Google Scholar]
- 31.Rui, L., V. Aguirre, J. K. Kim, G. I. Shulman, A. Lee, A. Corbould, A. Dunaif, and M. F. White. 2001. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J. Clin. Investig. 107:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rui, L., and C. Carter-Su. 1999. Identification of SH2-bbeta as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc. Natl. Acad. Sci. USA 96:7172-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rui, L., and C. Carter-Su. 1998. Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bbeta with PDGF receptor and phosphorylation of SH2-Bbeta. J. Biol. Chem. 273:21239-21245. [DOI] [PubMed] [Google Scholar]
- 34.Rui, L., T. L. Fisher, J. Thomas, and M. F. White. 2001. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J. Biol. Chem. 276:40362-40367. [DOI] [PubMed] [Google Scholar]
- 35.Rui, L., J. Herrington, and C. Carter-Su. 1999. SH2-B is required for nerve growth factor-induced neuronal differentiation. J. Biol. Chem. 274:10590-10594. [DOI] [PubMed] [Google Scholar]
- 36.Rui, L., L. S. Mathews, K. Hotta, T. A. Gustafson, and C. Carter-Su. 1997. Identification of SH2-Bbeta as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 17:6633-6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rui, L., M. Yuan, D. Frantz, S. Shoelson, and M. F. White. 2002. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 277:42394-42398. [DOI] [PubMed] [Google Scholar]
- 38.Sakaue, H., W. Ogawa, M. Takata, S. Kuroda, K. Kotani, M. Matsumoto, M. Sakaue, S. Nishio, H. Ueno, and M. Kasuga. 1997. Phosphoinositide 3-kinase is required for insulin-induced but not for growth hormone- or hyperosmolarity-induced glucose uptake in 3T3-L1 adipocytes. Mol. Endocrinol. 11:1552-1562. [DOI] [PubMed] [Google Scholar]
- 39.Sharma, P. M., K. Egawa, Y. Huang, J. L. Martin, I. Huvar, G. R. Boss, and J. M. Olefsky. 1998. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J. Biol. Chem. 273:18528-18537. [DOI] [PubMed] [Google Scholar]
- 40.Sun, X. J., P. Rothenberg, C. R. Kahn, J. M. Backer, E. Araki, P. A. Wilden, D. A. Cahill, B. J. Goldstein, and M. F. White. 1991. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352:73-77. [DOI] [PubMed] [Google Scholar]
- 41.Sun, X. J., L. M. Wang, Y. Zhang, L. Yenush, M. G. Myers, Jr., E. Glasheen, W. S. Lane, J. H. Pierce, and M. F. White. 1995. Role of IRS-2 in insulin and cytokine signalling. Nature 377:173-177. [DOI] [PubMed] [Google Scholar]
- 42.Tamemoto, H., T. Kadowaki, K. Tobe, T. Yagi, H. Sakura, T. Hayakawa, Y. Terauchi, K. Ueki, Y. Kaburagi, S. Satoh, et al. 1994. Insulin resistance and growth retardation in mice lacking insulin receptor substrate-1. Nature 372:182-186. [DOI] [PubMed] [Google Scholar]
- 43.Wang, J., and H. Riedel. 1998. Insulin-like growth factor-I receptor and insulin receptor association with a Src homology-2 domain-containing putative adapter. J. Biol. Chem. 273:3136-3139. [DOI] [PubMed] [Google Scholar]
- 44.White, M. F. 1997. The insulin signalling system and the IRS proteins. Diabetologia 40(Suppl. 2):S2-S17. [DOI] [PubMed] [Google Scholar]
- 45.White, M. F. 1998. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol. Cell. Biochem. 182:3-11. [PubMed] [Google Scholar]
- 46.Withers, D. J., J. S. Gutierrez, H. Towery, D. J. Burks, J. M. Ren, S. Previs, Y. Zhang, D. Bernal, S. Pons, G. I. Shulman, S. Bonner-Weir, and M. F. White. 1998. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900-904. [DOI] [PubMed] [Google Scholar]
- 47.Wojtaszewski, J. F., B. F. Hansen, B. Urso, and E. A. Richter. 1996. Wortmannin inhibits both insulin- and contraction-stimulated glucose uptake and transport in rat skeletal muscle. J. Appl. Physiol. 81:1501-1509. [DOI] [PubMed] [Google Scholar]
- 48.Yousaf, N., Y. Deng, Y. Kang, and H. Riedel. 2001. Four PSM/SH2-B alternative splice variants and their differential roles in mitogenesis. J. Biol. Chem. 276:40940-40948. [DOI] [PubMed] [Google Scholar]
- 49.Yu, C., Y. Chen, G. W. Cline, D. Zhang, H. Zong, Y. Wang, R. Bergeron, J. K. Kim, S. W. Cushman, G. J. Cooney, B. Atcheson, M. F. White, E. W. Kraegen, and G. I. Shulman. 2002. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277:50230-50236. [DOI] [PubMed] [Google Scholar]