Abstract
We report herein the use of an exonuclease III and G-quadruplex probe to construct a G-quadruplex-based luminescence detection platform for Hg2+. Unlike common DNA-based Hg2+ detection methods, when using the dsDNA probe to monitor the hairpin formation, the intercalation of the dsDNA probe may be influenced by the distortion of dsDNA. This ‘mix-and-detect’ methodology utilized the G-quadruplex probe as the signal transducer and is simple, rapid, convenient to use and can detect down to 20 nM of Hg2+.
Keywords: iridium(III), exonuclease III, G-quadruplex, mercury(II) ion
1. Introduction
Mercury ions (Hg2+) are a hazardous pollutant produced by refineries, factories, power station or runoff from landfills. Long-term absorption of mercury may result in kidney damage, memory impairment and other severe health problems [1, 2]. Living organisms will absorb and then accumulate mercury in fatty tissue via eating and drinking. Therefore, exposure of the human body to mercury should be minimized due to potential metal accumulation. According to the United States Environmental Protection Agency, the maximum safe concentration of mercury in drinking water is 2 ppb, the quantitative detection of which would require a very sensitive instrument [3–5]. Unfortunately, existing analytical methods for the sensitive detection of Hg2+ are not satisfactory for in-field use due to expensive instrumentation and labor-intensive sample preparation protocols. These instrumental methods include atomic absorption spectroscopy (AAS), inductively-coupled plasma mass spectrometry (ICP-MS) [6] and ion-selective electrodes [7, 8].
In 2004, Ono and co-workers discovered that Hg2+ is able to coordinate with thymine nucleobases [9–12]. This discovery has sparked a wave of novel Hg2+ detection methods, such as oligonucleotide-based luminescence [13–28], colorimetric [29–40], electrochemical [41, 42] and surface-enhanced Raman scattering methods [43–46]. Later on, they reported the crystal structure of a DNA duplex containing the T−HgII−T mismatch, which showed that the T−HgII−T bond largely distorts DNA structure and also stabilizes B-form DNA [12]. Moreover, C−AgI−A [47] and C−AgI−T mismatches have also been reported [11].
The G-quadruplex is a DNA secondary structure which is formed from a guanine-rich DNA sequence with four or more G-tracts. It is stabilized by monovalent cations such as K+, Na+ or NH4+ to form square-planar arrangements of guanine residues [48, 49]. Because of its rich structural polymorphism, the G-quadruplex has been widely used to construct analytical detection platforms [43, 44, 50–64] or various types of logic gates [65–67].
Exonuclease III (ExoIII) is a nuclease that catalyzes the stepwise removal of mononucleotides from the 3′-terminus of duplex DNA. Exo III has been used as a component of oligonucleotide-based detection platforms for different targets or as a tool for signal amplification [68, 69]. Interestingly, ExoIII has been reported to cleave DNA duplexes containing T−Hg2+−T mismatches and release Hg2+ into solution [42].
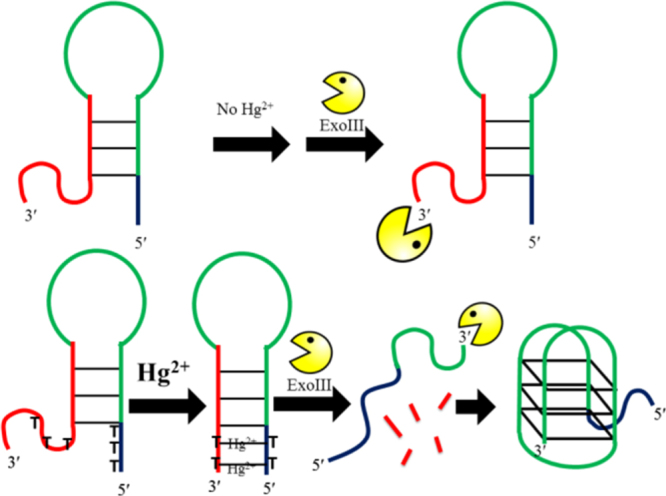
In our previous study, we constructed AND, OR and INHIBIT logic gates utilizing HgII and AgI ions as signal inputs. The ‘Klenow fragment’ polymerase was utilized as one of the signal transducing elements that catalyzes the extension of designed DNA in the 5′ to 3′ direction to form a duplex product and displace the split G-quadruplex sequence in the presence of Ag+ and Hg2+ [70]. Moreover, previous investigations demonstrated that the photophysical properties of iridium(III) complexes could be fine-tuned by changing the CˆN or NˆN donor ligand. We report herein a novel detection platform for Hg2+ using a novel iridium(III) complex. Unlike common detection methods using dsDNA probes to monitor the hairpin formation, which could be influenced by the distortion of dsDNA by mismatches, our approach utilized a G-quadruplex probe and is based on the newly discovered phenomenon that ExoIII cleaves DNA duplexes containing T−Hg2+−T mismatches. The detection mechanism of this platform is outlined in scheme 7. The DNA probe contains a G-quadruplex-forming sequence (green line), and its complementary sequence (red line), as well as 3′ and 5′ polyT (poly thymine) overhangs. The overall DNA structure assumes a hairpin formation with unhybridized 3′ and 5′-termini. Since ExoIII is unable to recognize single-stranded DNA (ssDNA) as substrate, it will not cleave the unhybridized 3′ overhang and the hairpin DNA will not be digested in the absence of Hg2+. Upon exposure to Hg2+ however, the 3′ and 5′ polyT overhangs form T−Hg2+−T bridges, creating a duplex DNA structure that is vulnerable to ExoIII digestion from the 3′-terminus (red line). ExoIII cleavage releases the G-quadruplex-forming sequence. The G-quadruplex structure binds with the luminescent G-quadruplex-selective iridium(III) complex 1, with a switch-on emission response. Furthermore, the Hg2+ may be recycled as they are re-released into solution following the ExoIII cleavage step.
Scheme 1. Schematic representation of the ExoIII-assisted label-free G-quadruplex-based assay for Hg2+.
2. Experimental details
2.1. Materials
Reagents, unless specified, were purchased from Sigma Aldrich (St. Louis, MO, USA) and used as received. Iridium chloride hydrate (IrCl3 · xH2O) was purchased from Precious Metals Online (Australia). All oligonucleotides were synthesized by Techdragon Inc. (Hong Kong, China).
2.2. Synthesis
Complex 1 was prepared according to (modified) literature methods and was characterized by proton nuclear magnetic resonance (1H-NMR), 13C-NMR and high resolution mass spectrometry (HRMS). Complex 1. 1H NMR (400 MHz, acetone-d6) δ 8.80 (d, J = 8.8 Hz, 2H), 8.53 (d, J = 8.8 Hz, 2H), 8.14-8.09 (m, 4H), 7.89 (d, J = 8.8 Hz, 2H), 7.83-7.81 (m, 2H), 7.67-7.63 (m, 2H), 7.42-7.38 (m, 2H), 7.31-7.27 (m, 2H), 7.21-7.17 (m, 2H), 7.15-7.06 (m, 6H), 6.58 (d, J = 7.6 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ 181.0, 161.3, 148.9, 148.8, 147.8, 142.1, 140.0, 132.5, 132.1, 131.7, 131.3, 129.9, 129.7, 129.6, 128.5, 127.5, 127.3, 126.9, 125.0, 123.9, 122.1, 118.1; HRMS: calculated for C44H28IrN4S2 [M–PF6]+ 869.1385; found 869.1332; analyzed (C44H28IrN4S2PF6). C, H, N: calculated 52.12, 2.78, 5.53; found 52.34, 2.77, 5.58.
3. Results and discussion
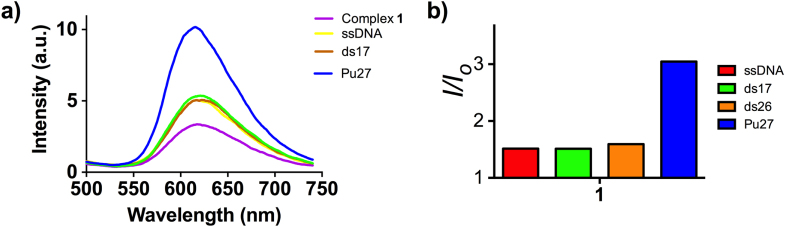
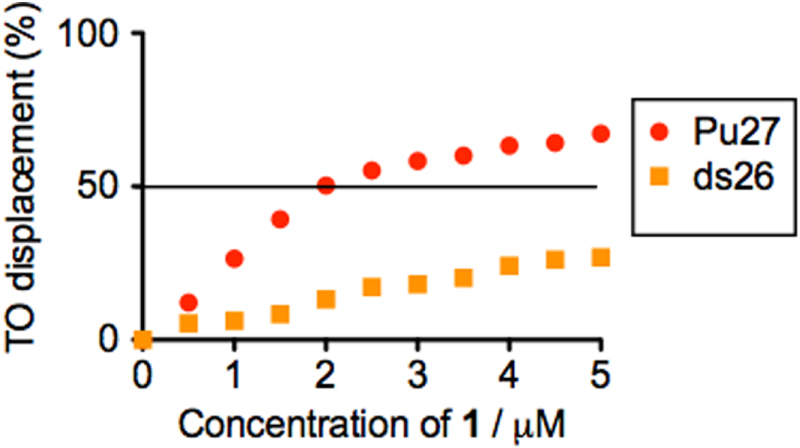
Complex 1 is a cyclometalated iridium(III) complex: [Ir(pbt)2(biq)]PF6 (1, where pbt = 2-phenylbenzo[d]thiazole, biq = 2,2′-biquinoline, figure 1). The synthesis, characterization and photophysical properties of 1 are given in the supplementary information (table S1, figure S1). No significant change in the UV–visible absorption spectrum of 1 was observed in aqueous buffered solution over 48 h, indicating that the complex is stable in aqueous solution and may be suitable for long term use. By itself, complex 1 was weakly emissive in aqueous buffered solution (20 mM Tris, pH 7.0). Encouragingly, we found that a significant increase in luminescence response of 1 can be triggered by the presence of G-quadruplex DNA, as shown by emission titration experiments. We then explored the effect of the addition of various types of DNA (figure 2). ssDNA or double-stranded DNA (ds17, ds26) had only a minor effect on the luminescence of 1. However, in the presence of the Pu27 G-quadruplex, the luminescence of 1 was significantly increased (about 3-fold). We anticipate that the G-quadruplex shields the metal center of 1 from solvent quenching by non-radiative decay of the excited state, thus recovering 3MLCT phosphorescence. To validate this hypothesis, the G-quadruplex fluorescent intercalator displacement (G4-FID) assay was utilized to investigate the binding affinity of 1 for G-quadruplex and dsDNA. The result showed that only 2 μM of 1 could displace 50% of thiazole orange (TO) from G-quadruplex-TO assemble. On the other hand, only about 30% of TO could be displaced from dsDNA-TO assemble in the presence of 5 μM of complex 1 (figure 3). It outlines the G-quadruplex selective property of complex 1.
Figure 1. Chemical structure of cyclometalated iridium(III) complex 1.
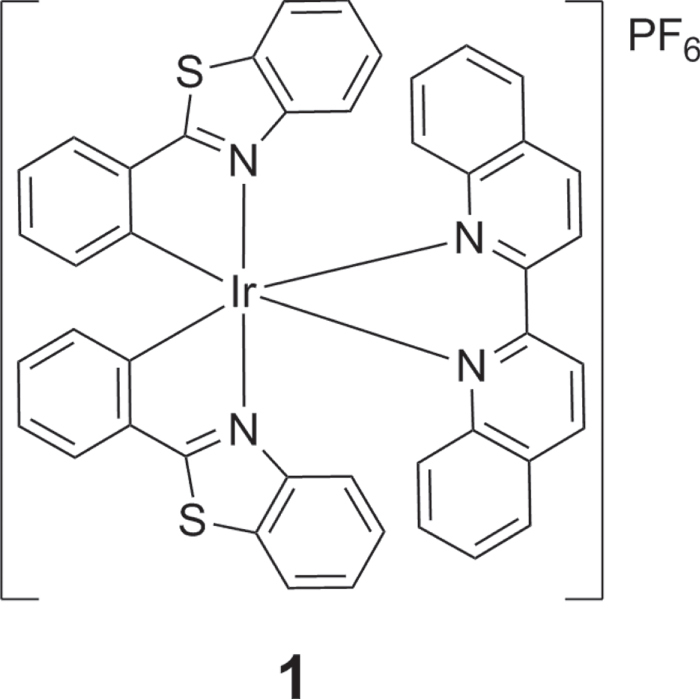
Figure 2. (a) Emission spectrum and (b) luminescence enhancement of complex 1 (1 μM) in the presence of 5 μM of ssDNA, ds17, ds26 or Pu27 G-quadruplex.
Figure 3. Percentage of TO displaced from DNA duplex ds26 or Pu27 G-quadruplex upon addition of complex 1.
As complex 1 displays the property of a G-quadruplex-selective probe, we sought to apply it in our Hg2+ sensing platform. Encouragingly, we observed that the presence of Hg2+ enhanced the luminescence intensity of the complex 1–hairpin DNA system (figure 4). To validate that the luminescence enhancement is due to the switching of the DNA structure, we also sought to exclude the possibility of direct interaction between complex 1 and Hg2+ as a contributor to the response. We found that no luminescence increase was observed for the system lacking hairpin DNA (figure S2). We propose that the observed luminescence enhancement of the system is due to the formation of the T−Hg2+−T mismatched duplex in the 3′ and 5′ overhangs, which allows ExoIII digestion of the hairpin DNA, resulting in the release of the G-quadruplex-forming sequence and the formation of a G-quadruplex structure that is recognized by the G-quadruplex-selective complex 1 with a switch-on emission response.
Figure 4. Emission enhancement of the system ([1] = 1 μM, [DNA] = 1.5 μM, [K+] = 100 mM) with increasing concentration of Hg2+.
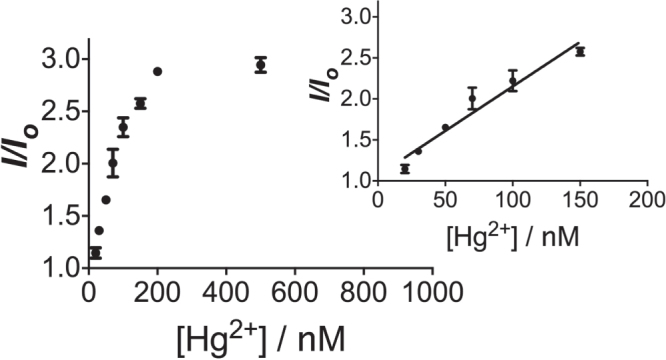
The linear detection range of the system was found to be from 20 to 200 nM of Hg2+ (figure 4). Maximal luminescence was reached at 200 nM. The detection limit of the present assay was determined to be 20 nM of Hg2+ by the 3σ method. The selectivity of this detection platform for Hg2+ over other metal ions was also evaluated. The results showed that the luminescence response of the system for Hg2+ was significantly stronger than that for 5-fold excess concentrations of the other metal ions (figure 5).
Figure 5. Relative luminescence intensity of the system ([1] = 1 μM, [DNA] = 2 μM) in the presence of 10 nM Hg2+ or 5-fold excess of other metal ions.
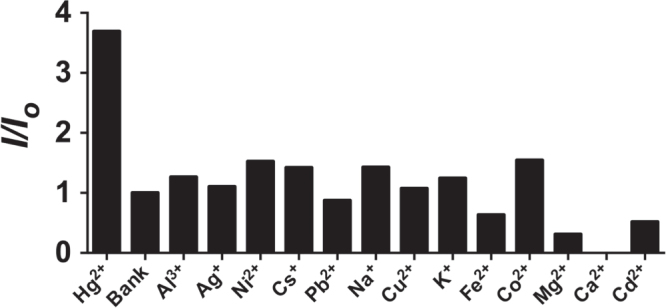
Figure 6. Luminescence response of the system to increasing concentrations (20, 50, 100, 200 and 500 nM) of Hg2+ in a 50-fold diluted river water sample.
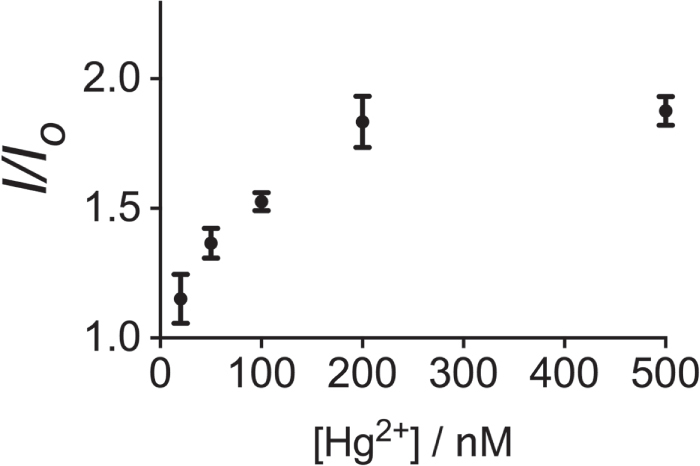
In order to investigate the effectiveness of this Hg2+ assay in a practical application, we applied it to the detection of Hg2+ in spiked natural water samples. No Hg2+ was detected in the natural water samples by ICP-MS. Therefore, the natural water samples (Nam Sang Wai River in Hong Kong) were diluted 50-fold with Tris buffer and spiked with various concentrations (20, 50, 100, 200 and 500 nM) of Hg2+. The samples showed a gradual increase in luminescence intensity with Hg2+ (figure 6). This result demonstrates that our detection platform could potentially be further developed as a sensitive probe for natural water sample analysis of Hg2+.
4. Conclusions
In conclusion, a novel luminescent iridium(III) complex which shows the G-quadruplex probe property, was investigated and utilized for the construction of an exonuclease-assisted, label-free G-quadruplex-based luminescence detection platform for Hg2+. Compared with the modified DNA, the cost label-free approach using unmodified DNA is relatively low. Unlike common detection methods, when using the dsDNA probe to monitor the hairpin formation, the intercalation of the dsDNA probe may be influenced by the distortion of dsDNA. This ‘mix-and-detect’ methodology utilized the G-quadruplex probe as the signal transducer and it is simple, rapid, convenient to use and can detect down to 20 nM of Hg2+, which is comparable to recently reported label-free DNA-based Hg2+ ion detection methods. For comparison, we have also summarized those reported methods in table S2. Furthermore, the potential application in a real water sample was also demonstrated, which shows the robustness of the system.
Acknowledgments
This work is supported by Hong Kong Baptist University (FRG2/14-15/004), the Health and Medical Research Fund (HMRF/13121482 and HMRF/14130522), the Research Grants Council (HKBU/201811, HKBU/204612 and HKBU/201913), the French National Research Agency/Research Grants Council Joint Research Scheme (A-HKBU201/12), State Key Laboratory of Environmental and Biological Analysis Research Grant (SKLP-14-15-P001), National Natural Science Foundation of China (21575121), Guangdong Province Natural Science Foundation (2015A030313816), Hong Kong Baptist University Century Club Sponsorship Scheme 2015, Interdisciplinary Research Matching Scheme (RC-IRMS/14-15/06), the State Key Laboratory of Synthetic Chemistry, the Science and Technology Development Fund, Macao SAR (098/2014/A2 and 007/2014/AMJ), the University of Macau (MYRG091(Y3-L2)-ICMS12-LCH, MYRG2015-00137-ICMS-QRCM and MRG023/LCH/2013/ICMS).
References
- 1.Sekowski J W, Malkas L H, Wei Y. and Hickey R J. Mercuric ion inhibits the activity and fidelity of the human cell DNA synthesome. Toxicol. Appl. Pharm. 1997;145:268–76. doi: 10.1006/taap.1997.8185. [DOI] [PubMed] [Google Scholar]
- 2.Tchounwou P B, Ayensu W K, Ninashvili N. and Sutton D. Review: environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003;18:149–75. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- 3.Nolan E M. and Lippard S J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008;108:3443–80. doi: 10.1021/cr068000q. [DOI] [PubMed] [Google Scholar]
- 4.Ma D-L, Chan D S-H, Man B Y-W. and Leung C-H. Oligonucleotide-based luminescent detection of metal ions. Chem. Asian. J. 2011;6:986–1003. doi: 10.1002/asia.201000870. [DOI] [PubMed] [Google Scholar]
- 5.Ma D-L, He H-Z, Leung K-H, Zhong H-J, Chan D S-H. and Leung C-H. Label-free luminescent oligonucleotide-based probes. Chem. Soc. Rev. 2013;42:3427–40. doi: 10.1039/c2cs35472a. [DOI] [PubMed] [Google Scholar]
- 6.Hatch W R. and Ott W L. Determination of submicrogram quantities of mercury by atomic absorption spectrophotometry. Anal. Chem. 1968;40:2085–7. doi: 10.1021/ac50158a025. [DOI] [Google Scholar]
- 7.Salaün P. and van den Berg C M G. Voltammetric detection of mercury and copper in seawater using a gold microwire electrode. Anal. Chem. 2006;78:5052–60. doi: 10.1021/ac060231+. [DOI] [PubMed] [Google Scholar]
- 8.Passariello B, Barbaro M, Quaresima S, Casciello A. and Marabini A. Determination of mercury by inductively coupled plasma—mass spectrometry. Microchem. J. 1996;54:348–54. doi: 10.1006/mchj.1996.0110. [DOI] [PubMed] [Google Scholar]
- 9.Ono A. and Togashi H. Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions. Angew. Chem. Int. Edn. 2004;43:4300–2. doi: 10.1002/anie.200454172. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka Y, Oda S, Yamaguchi H, Kondo Y, Kojima C. and Ono A. 15N−15N J-coupling across HgII: direct observation of HgII-mediated T−T base pairs in a DNA duplex. J. Am. Chem. Soc. 2006;129:244–5. doi: 10.1021/ja065552h. [DOI] [PubMed] [Google Scholar]
- 11.Kondo J, Yamada T, Hirose C, Okamoto I, Tanaka Y. and Ono A. Crystal structure of metallo DNA duplex containing consecutive Watson–Crick-like T–HgII–T base pairs. Angew. Chem. Int. Edn. 2014;53:2385–8. doi: 10.1002/anie.201309066. [DOI] [PubMed] [Google Scholar]
- 12.Funai T, Nakamura J, Miyazaki Y, Kiriu R, Nakagawa O, Wada S-i, Ono A. and Urata H. Regulated incorporation of two different metal ions into programmed sites in a duplex by DNA polymerase catalyzed primer extension. Angew. Chem. Int. Edn. 2014;53:6624–7. doi: 10.1002/anie.201311235. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Wang Q, Shi X, Hornak L A. and Wu N. Detection of mercury(II) by quantum dot/DNA/gold nanoparticle ensemble based nanosensor via nanometal surface energy transfer. Anal. Chem. 2011;83:7061–5. doi: 10.1021/ac2019014. [DOI] [PubMed] [Google Scholar]
- 14.Wang J. and Liu B. Highly sensitive and selective detection of Hg2+ in aqueous solution with mercury-specific DNA and Sybr green I. Chem. Commun. 2008:4759–61. doi: 10.1039/b806885b. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Heon Lee J. and Lu Y. Highly sensitive ‘turn-on’ fluorescent sensor for Hg2+ in aqueous solution based on structure-switching DNA. Chem. Commun. 2008:6005–7. doi: 10.1039/b812755g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Li T, Li B, Li J. and Wang E. Carbon nanotube-DNA hybrid fluorescent sensor for sensitive and selective detection of mercury(II) ion. Chem. Commun. 2010;46:1476–8. doi: 10.1039/b921191h. [DOI] [PubMed] [Google Scholar]
- 17.Chan D S-H, Lee H-M, Che C-M, Leung C-H. and Ma D-L. A selective oligonucleotide-based luminescent switch-on probe for the detection of nanomolar mercury(II) ion in aqueous solution. Chem. Commun. 2009:7479–81. doi: 10.1039/b913995h. [DOI] [PubMed] [Google Scholar]
- 18.Liu J. and Lu Y. Rational design of ‘turn-on’ allosteric DNAzyme catalytic beacons for aqueous mercury ions with ultrahigh sensitivity and selectivity. Angew. Chem. Int. Edn. 2007;46:7587–90. doi: 10.1002/anie.200702006. [DOI] [PubMed] [Google Scholar]
- 19.Chiang C-K, Huang C-C, Liu C-W. and Chang H-T. Oligonucleotide-based fluorescence probe for sensitive and selective detection of mercury(II) in aqueous solution. Anal. Chem. 2008;80:3716–21. doi: 10.1021/ac800142k. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Wang Z, Zong S, Chen H, Zhu D, Wu L, Hu G. and Cui Y. SERS Detection and removal of mercury(II)/silver(I) using oligonucleotide-functionalized core/shell magnetic silica sphere@Au nanoparticles. ACS Appl. Mater. Interfaces. 2014;6:7371–9. doi: 10.1021/am5006282. [DOI] [PubMed] [Google Scholar]
- 21.Dave N, Chan M Y, Huang P-J J, Smith B D. and Liu J. Regenerable DNA-functionalized hydrogels for ultrasensitive, instrument-free mercury(II) detection and removal in water. J. Am. Chem. Soc. 2010;132:12668–73. doi: 10.1021/ja106098j. [DOI] [PubMed] [Google Scholar]
- 22.Deng L, Ouyang X, Jin J, Ma C, Jiang Y, Zheng J, Li J, Li Y, Tan W. and Yang R. Exploiting the higher specificity of silver amalgamation: selective detection of mercury(II) by forming Ag/Hg amalgam. Anal. Chem. 2013;85:8594–600. doi: 10.1021/ac401408m. [DOI] [PubMed] [Google Scholar]
- 23.Zhu G, Li Y. and Zhang C-Y. Simultaneous detection of mercury(II) and silver(I) ions with picomolar sensitivity. Chem. Commun. 2014;50:572–4. doi: 10.1039/C3CC46884D. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Zhu X, Ye H, Yu L, Liu X. and Chen G. A simple ‘molecular beacon’-based fluorescent sensing strategy for sensitive and selective detection of mercury(II) Chem. Commun. 2011;47:12158–60. doi: 10.1039/c1cc14265h. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Zhan S, Xu L, Shi W, Xi T, Zhan X. and Zhou P. A simple and label-free sensor for mercury(II) detection in aqueous solution by malachite green based on a resonance scattering spectral assay. Chem. Commun. 2011;47:6027–9. doi: 10.1039/c1cc10563a. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Zhai J. and Sun X. Nano-C60 as a novel, effective fluorescent sensing platform for mercury(II) ion detection at critical sensitivity and selectivity. Nanoscale. 2011;3:2155–7. doi: 10.1039/c1nr10052a. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Z, Xu L, Zhou X, Qin J. and Yang C. Designing label-free DNA sequences to achieve controllable turn-off/on fluorescence response for Hg2+ detection. Chem. Commun. 2011;47:8010–2. doi: 10.1039/c1cc12384j. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Chen L, Lin Z, Qiu B. and Chen G. A highly sensitive and selective ‘signal-on’ electrochemiluminescent biosensor for mercury. Chem. Commun. 2010;46:3149–51. doi: 10.1039/b926319e. [DOI] [PubMed] [Google Scholar]
- 29.Li T, Dong S. and Wang E. Label-free colorimetric detection of aqueous mercury ion (Hg2+) using Hg2+−modulated G-quadruplex-based DNAzymes. Anal. Chem. 2009;81:2144–9. doi: 10.1021/ac900188y. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Wang J, Jiao K. and Yang X. Colorimetric detection of mercury ion (Hg2+) based on DNA oligonucleotides and unmodified gold nanoparticles sensing system with a tunable detection range. Biosens. Bioelectron. 2009;24:3153–8. doi: 10.1016/j.bios.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Liu C-W, Hsieh Y-T, Huang C-C, Lin Z-H. and Chang H-T. Detection of mercury(II) based on Hg2+−DNA complexes inducing the aggregation of gold nanoparticles. Chem. Commun. 2008:2242–4. doi: 10.1039/b719856f. [DOI] [PubMed] [Google Scholar]
- 32.Li T, Li B, Wang E. and Dong S. G-quadruplex-based DNAzyme for sensitive mercury detection with the naked eye. Chem. Commun. 2009:3551–3. doi: 10.1039/b903993g. [DOI] [PubMed] [Google Scholar]
- 33.Xue X, Wang F. and Liu X. One-step, room temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J. Am. Chem. Soc. 2008;130:3244–5. doi: 10.1021/ja076716c. [DOI] [PubMed] [Google Scholar]
- 34.Darbha G K, Singh A K, Rai U S, Yu E, Yu H. and Chandra Ray P. Selective detection of mercury (II) ion using nonlinear optical properties of gold nanoparticles. J. Am. Chem. Soc. 2008;130:8038–43. doi: 10.1021/ja801412b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C-W, Huang C-C. and Chang H-T. Control over surface DNA density on Gold nanoparticles allows selective and sensitive detection of mercury(II) Langmuir. 2008;24:8346–50. doi: 10.1021/la800589m. [DOI] [PubMed] [Google Scholar]
- 36.Chen G-H, Chen W-Y, Yen Y-C, Wang C-W, Chang H-T. and Chen C-F. Detection of mercury(II) ions using colorimetric gold nanoparticles on paper-based analytical devices. Anal. Chem. 2014;86:6843–9. doi: 10.1021/ac5008688. [DOI] [PubMed] [Google Scholar]
- 37.Zuo X, Wu H, Toh J. and Li S F Y. Mechanism of mercury detection based on interaction of single-strand DNA and hybridized DNA with gold nanoparticles. Talanta. 2010;82:1642–6. doi: 10.1016/j.talanta.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 38.Wei Q, Nagi R, Sadeghi K, Feng S, Yan E, Ki S J, Caire R, Tseng D. and Ozcan A. Detection and spatial mapping of mercury contamination in water samples using a smart-phone. ACS Nano. 2014;8:1121–9. doi: 10.1021/nn406571t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You J, Hu H, Zhou J, Zhang L, Zhang Y. and Kondo T. Novel cellulose polyampholyte–gold nanoparticle-based colorimetric competition assay for the detection of cysteine and mercury(II) Langmuir. 2013;29:5085–92. doi: 10.1021/la3050913. [DOI] [PubMed] [Google Scholar]
- 40.Kanayama N, Takarada T. and Maeda M. Rapid naked-eye detection of mercury ions based on non-crosslinking aggregation of double-stranded DNA-carrying gold nanoparticles. Chem. Commun. 2011;47:2077–9. doi: 10.1039/c0cc05171c. [DOI] [PubMed] [Google Scholar]
- 41.Wu D, Zhang Q, Chu X, Wang H, Shen G. and Yu R. Ultrasensitive electrochemical sensor for mercury(II) based on target-induced structure-switching DNA. Biosens. Bioelectron. 2010;25:1025–31. doi: 10.1016/j.bios.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Xuan F, Luo X. and Hsing I M. Conformation-dependent exonuclease III activity mediated by metal ions reshuffling on thymine-rich DNA duplexes for an ultrasensitive electrochemical method for Hg2+ detection. Anal. Chem. 2013;85:4586–93. doi: 10.1021/ac400228q. [DOI] [PubMed] [Google Scholar]
- 43.Du Y, Li B. and Wang E. ‘Fitting’ makes ‘sensing’ simple: label-free detection strategies based on nucleic acid aptamers. Acc. Chem. Res. 2012;46:203–13. doi: 10.1021/ar300011g. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Lu C-H. and Willner I. From cascaded catalytic nucleic acids to enzyme–DNA nanostructures: controlling reactivity, sensing, logic operations, and assembly of complex structures. Chem. Rev. 2014;114:2881–941. doi: 10.1021/cr400354z. [DOI] [PubMed] [Google Scholar]
- 45.Lu C-H, Willner B. and Willner I. DNA nanotechnology: from sensing and DNA machines to drug-delivery systems. ACS Nano. 2013;7:8320–32. doi: 10.1021/nn404613v. [DOI] [PubMed] [Google Scholar]
- 46.Willner I, Shlyahovsky B, Zayats M. and Willner B. DNAzymes for sensing, nanobiotechnology and logic gate applications. Chem. Soc. Rev. 2008;37:1153–65. doi: 10.1039/b718428j. [DOI] [PubMed] [Google Scholar]
- 47.Funai T, Miyazaki Y, Aotani M, Yamaguchi E, Nakagawa O, Wada S-i, Torigoe H, Ono A. and Urata H. Ag(I) ion mediated formation of a C–A mispair by DNA polymerases. Angew. Chem. Int. Edn. 2012;51:6464–6. doi: 10.1002/anie.201109191. [DOI] [PubMed] [Google Scholar]
- 48.Davis J T. G-quartets 40 years later: from 5′-GMP to molecular biology and supramolecular chemistry. Angew. Chem. Int. Edn. 2004;43:668–98. doi: 10.1002/anie.200300589. [DOI] [PubMed] [Google Scholar]
- 49.Zhou J, Bourdoncle A, Rosu F, Gabelica V. and Mergny J-L. Tri-G-quadruplex: controlled assembly of a G-quadruplex structure from three G-rich strands. Angew. Chem. Int. Edn. 2012;51:11002–5. doi: 10.1002/anie.201205390. [DOI] [PubMed] [Google Scholar]
- 50.Hu D, Pu F, Huang Z, Ren J. and Qu X. A Quadruplex-based, label-free, and real-time fluorescence assay for RNase H activity and inhibition. Chem. Eur. J. 2010;16:2605–10. doi: 10.1002/chem.200902166. [DOI] [PubMed] [Google Scholar]
- 51.Zhao C, Wu L, Ren J. and Qu X. A label-free fluorescent turn-on enzymatic amplification assay for DNA detection using ligand-responsive G-quadruplex formation. Chem. Commun. 2011;47:5461–3. doi: 10.1039/c1cc11396h. [DOI] [PubMed] [Google Scholar]
- 52.Qu K, Zhao C, Ren J. and Qu X. Human telomeric G-quadruplex formation and highly selective fluorescence detection of toxic strontium ions. Mol. Biosyst. 2012;8:779–82. doi: 10.1039/C2MB05446A. [DOI] [PubMed] [Google Scholar]
- 53.He H-Z, Chan D S-H, Leung C-H. and Ma D-L. G-quadruplexes for luminescent sensing and logic gates. Nucleic Acids Res. 2013;41:4345–59. doi: 10.1093/nar/gkt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z, Lin Y, Zhao C, Ren J. and Qu X. Silver metallization engineered conformational switch of G-quadruplex for fluorescence turn-on detection of biothiols. Chem. Commun. 2012;48:11428–30. doi: 10.1039/c2cc36690h. [DOI] [PubMed] [Google Scholar]
- 55.Peng Y, Wang X, Xiao Y, Feng L, Zhao C, Ren J. and Qu X. i-Motif quadruplex DNA-based biosensor for distinguishing single- and multiwalled carbon nanotubes. J. Am. Chem. Soc. 2009;131:13813–8. doi: 10.1021/ja9051763. [DOI] [PubMed] [Google Scholar]
- 56.Hu D, Huang Z, Pu F, Ren J. and Qu X. A label-free, quadruplex-based functional molecular beacon (LFG4-MB) for fluorescence turn-on detection of DNA and nuclease. Chem. Eur. J. 2011;17:1635–41. doi: 10.1002/chem.201001331. [DOI] [PubMed] [Google Scholar]
- 57.Xu H, Gao S, Yang Q, Pan D, Wang L. and Fan C. Amplified fluorescent recognition of G-quadruplex folding with a cationic conjugated polymer and DNA intercalator. ACS Appl. Mater. Interfaces. 2010;2:3211–6. doi: 10.1021/am1006854. [DOI] [PubMed] [Google Scholar]
- 58.Zhu C, Wen Y, Li D, Wang L, Song S, Fan C. and Willner I. Inhibition of the in vitro replication of DNA by an aptamer–protein complex in an autonomous DNA machine. Chem. Eur. J. 2009;15:11898–903. doi: 10.1002/chem.200901275. [DOI] [PubMed] [Google Scholar]
- 59.Li D, Song S. and Fan C. Target-responsive structural switching for nucleic acid-based sensors. Acc. Chem. Res. 2010;43:631–41. doi: 10.1021/ar900245u. [DOI] [PubMed] [Google Scholar]
- 60.Zhou J, Amrane S, Korkut D N, Bourdoncle A, He H-Z, Ma D-L. and Mergny J-L. Combination of i-Motif and G-quadruplex structures within the same strand: formation and application. Angew. Chem. Int. Edn. 2013;125:7896–900. doi: 10.1002/ange.201301278. [DOI] [PubMed] [Google Scholar]
- 61.Ma D-L, Chan D S-H. and Leung C-H. Group 9 organometallic compounds for therapeutic and bioanalytical applications. Acc. Chem. Res. 2014;47:3614–31. doi: 10.1021/ar500310z. [DOI] [PubMed] [Google Scholar]
- 62.Leung C-H, Chan D S-H, He H-Z, Cheng Z, Yang H. and Ma D-L. Luminescent detection of DNA-binding proteins. Nucleic Acids Res. 2012;40:941–55. doi: 10.1093/nar/gkr763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin S, Gao W, Tian Z, Yang C, Lu L, Mergny J-L, Leung C-H. and Ma D-L. Luminescence switch-on detection of protein tyrosine kinase-7 using a G-quadruplex-selective probe. Chem. Sci. 2015;6:4284–90. doi: 10.1039/c5sc01320h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leung K-H, He H-Z, He B, Zhong H-J, Lin S, Wang Y-T, Ma D-L. and Leung C-H. Label-free luminescence switch-on detection of hepatitis C virus NS3 helicase activity using a G-quadruplex-selective probe. Chem. Sci. 2015;6:2166–71. doi: 10.1039/c4sc03319a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji L, Guo Y, Hong S, Wang Z, Wang K, Chen X, Zhang J, Hu J. and Pei R. Label-free detection of Pb2+ based on aggregation-induced emission enhancement of Au-nanoclusters. RSC Adv. 2015;5:36582–6. doi: 10.1039/C5RA03449C. [DOI] [Google Scholar]
- 66.Guo Y, Zhou L, Xu L, Zhou X, Hu J. and Pei R. Multiple types of logic gates based on a single G-quadruplex DNA strand. Sci. Rep. 2014;4:7315. doi: 10.1038/srep07315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L, Hong S, Sun N, Wang K, Zhou L, Ji L. and Pei R. Berberine as a novel light-up i-Motif fluorescence ligand and its application to design molecular logic systems. Chem. Commun. 2015 doi: 10.1039/C5CC08242K. at press. [DOI] [PubMed] [Google Scholar]
- 68.Su X, Zhu X, Zhang C, Xiao X. and Zhao M. In situ, real-time monitoring of the 3′ to 5′ exonucleases secreted by living cells. Anal. Chem. 2012;84:5059–65. doi: 10.1021/ac300745f. [DOI] [PubMed] [Google Scholar]
- 69.Song C. and Zhao M. Real-time monitoring of the activity and kinetics of T4 polynucleotide kinase by a singly labeled DNA-hairpin smart probe coupled with λ exonuclease cleavage. Anal. Chem. 2009;81:1383–8. doi: 10.1021/ac802107w. [DOI] [PubMed] [Google Scholar]
- 70.Ma D-L, Lin S, Lu L, Wang M, Hu C, Liu L-J, Ren K. and Leung C-H. G-quadruplex-based logic gates for HgII and AgI ions employing a luminescent iridium(III) complex and extension of metal-mediated base pairs by polymerase. J. Mater. Chem. B. 2015;3:4780–5. doi: 10.1039/C5TB00718F. [DOI] [PubMed] [Google Scholar]