Abstract
The tropomyosin receptor kinase (Trk) receptor family comprises 3 transmembrane proteins referred to as Trk A, B and C (TrkA, TrkB and TrkC) receptors that are encoded by the NTRK1, NTRK2 and NTRK3 genes, respectively. These receptor tyrosine kinases are expressed in human neuronal tissue and play an essential role in the physiology of development and function of the nervous system through activation by neurotrophins. Gene fusions involving NTRK genes lead to transcription of chimeric Trk proteins with constitutively activated or overexpressed kinase function conferring oncogenic potential. These genetic abnormalities have recently emerged as targets for cancer therapy, because novel compounds have been developed that are selective inhibitors of the constitutively active rearranged proteins. Developments in this field are being aided by next generation sequencing methods as tools for unbiased gene fusions discovery. In this article, we review the role of NTRK gene fusions across several tumour histologies, and the promises and challenges of targeting such genetic alterations for cancer therapy.
Keywords: NTRK, gene fusions, Molecular oncology, Target therapy
Find the Fact Sheet on NTRK/TrkB here: http://oncologypro.esmo.org/Publications/Glossary-of-Molecular-Biology/Glossary-in-Molecular-Biology
Introduction
The treatment of solid tumours is dramatically changing in recent years thanks to the enhancement of molecular diagnostic technologies leading to identification of an increasing number of specific actionable oncogenic abnormalities such as gene activating point mutations, in-frame insertions/deletions and amplification or rearrangements. The concept of precision medicine consists in the accomplishment of therapy individualised to each tumour by exploiting these alterations as predictive biomarkers as well as targets of therapy. Neurotrophic tropomyosin receptor kinase (NTRK) gene rearrangements have recently emerged as targets for cancer therapy, because novel compounds have been developed that are selective inhibitors of the constitutively active fusion proteins that arise from these molecular alterations. Developments in this field are being aided by next generation sequencing methods as tools for unbiased gene fusion discovery. In this article, we review the role of NTRK gene fusions across several tumour histologies, and the promises and challenges of targeting such genetic alterations for cancer therapy.
Tropomyosin receptor kinase (Trk) family of receptors
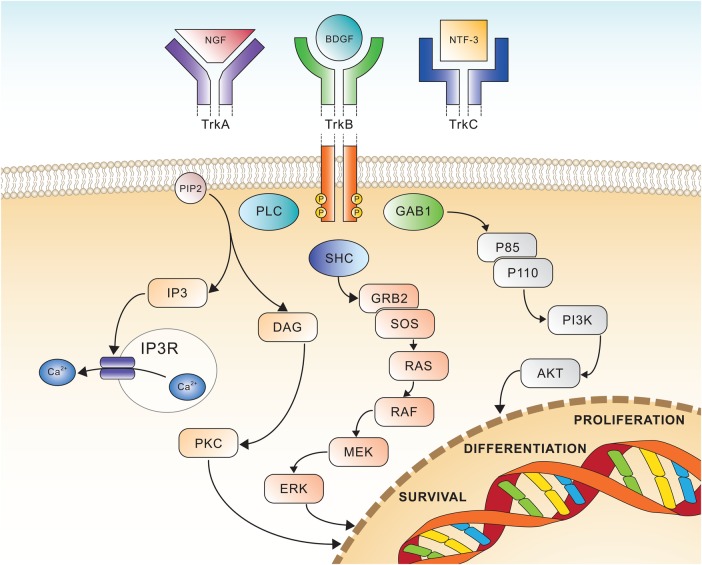
The Trk receptor family comprises three transmembrane proteins referred to as Trk A, B and C (TrkA, TrkB and TrkC) receptors, and are encoded by the NTRK1, NTRK2 and NTRK3 genes, respectively. These receptor tyrosine kinases (TK) are expressed in human neuronal tissue, and play an essential role in both the physiology of development and function of the nervous system through activation by neurotrophins (NTs).1 The latter are specific ligands known as nerve growth factor (NGF) for TrkA, brain-derived growth factor (BDGF), and NT-4/5 for TrkB and NT3 for TrkC, respectively.2 All three Trk receptors are structured with an extracellular domain for ligand binding, a transmembrane region and an intracellular domain with a kinase domain. The binding of the ligand to the receptor triggers the oligomerisation of the receptors and phosphorylation of specific tyrosine residues in the intracytoplasmic kinase domain. This event results into the activation of signal transduction pathways leading to proliferation, differentiation and survival in normal and neoplastic neuronal cells1 (figure 1). The binding of TrkA receptor by NGF causes the activation of the Ras/Mitogen activated protein kinase (MAPK) pathway, which leads to increased proliferation and cellular growth through extracellular signal-regulated kinase (ERK) signalling. Other pathways such as phospholipase C-γ (PLCγ) and PI3K are also activated. TrkC coupling with NT3 causes preferential activation of the PI3/AKT pathway preventing apoptosis and increasing cell survival, whereas TrkB transduces the BDNF signal via Ras-ERK, PI3K and PLCγ pathway, resulting in neuronal differentiation and survival.1 The Trk receptor kinases play a key role in central and peripheral nervous system development as well as in cell survival. The proper regulation of Trk receptor levels and their activation is critically important in cell functioning, and the upregulation of Trk receptors has been reported in several central nervous system-related disorders (eg, TrkB in epilepsy, neuropathic pain, or depression).3
Figure 1.
Schematic view of Trk receptors signalling, showing the three major pathways involved in cell differentiation and survival. AKT, v-akt murine thymoma viral oncogene homologue; BDGF, brain-derived growth factor; DAG, diacyl-glycerol; ERK, extracellular signal-regulated kinase; GAB1, GRB2-associated-binding protein 1; GRB2, growth factor receptor-bound protein 2; IP3, inositol trisphosphate; MEK, mitogen-activated protein kinase; NGF, nerve growth factor; NTF-3, neurotrophin 3; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C; RAF, rapidly accelerated fibrosarcoma kinase; RAS, rat sarcoma kinase; SHC, Src homology 2 domain containing.
The NTRK1 gene is located on chromosome 1q21-q22,4 and its mutations disrupting the function of the TrkA protein are found in patients affected by congenital insensitivity to pain with anhidrosis (CIPA) syndrome.5 In 1999 Indo et al cloned the full-length NTRK1 human gene encoding a 790-residue or 796-residue protein (TrkA receptor) with an intracellular domain containing a juxtamembrane region, a TK domain and a short C terminal tail.6
The NTRK2 gene is mapped on chromosome 9q22.17 and contains 24 exons,8 coding for a protein of 822 amino acid residues (TrkB receptor). The full-length TrkB receptor contains an N-terminal signal sequence, followed by a cysteine-rich domain, a leucine-rich domain, a second cysteine-rich domain, 2 immunoglobulin (Ig)-like domains that make up the BDNF-binding region, a transmembrane domain, a Src homology 2 domain containing (SHC)-binding motif, a TK domain near the C terminus and a C-terminal PLCγ-docking site.
The NTRK3 gene is located on chromosome 15q25,9 and its transcription product known as TrkC was isolated and characterised by Lamballe et al10 in 1991. TrkC receptor is a glycoprotein of 145 kD preferentially expressed in the human hippocampus, cerebral cortex and in the granular cell layer of the cerebellum.
Molecular alterations of NTRK genes in various malignancies across histologies
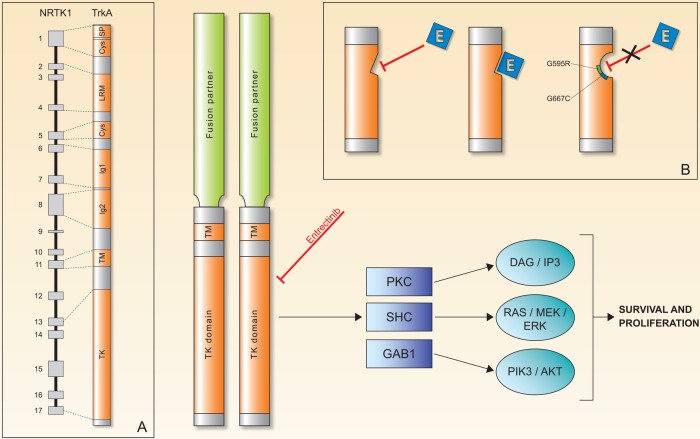
Gene fusions of NTRK genes represent the main molecular alterations with known oncogenic and transforming potential.11 Less common oncogenic mechanisms that have been described are in-frame deletion of NTRK1 in acute myeloid leukaemia12 and a TrkA alternative splicing in neuroblastoma.13 In all reported Trk oncogenic gene fusions, the 3’ region of the NTRK gene is joined with a 5’ sequence of a fusion partner gene by an intrachromosomal or interchromosomal rearrangement, and the oncogenic chimaera is typically a constitutively activated or overexpressed kinase (figure 2). Table 1 shows the NTRK fusions reported to date and their associated cancer types.
Figure 2.
The chimeric Trk protein, composed by the TK domain with ATPase activity and a TM loop along with a fusion partner. Oligomerisation of the chimeric protein is the main proposed mechanisms for increased tumour cell survival and proliferation via the known pathways of Trk receptors. (A) NTRK1 gene with 17 exon sequences and respective TrkA protein regions.5 (B) Mechanism of entrectinib (E) action and the known mechanisms of acquired resistance (point mutation G595R and G567C) to E.50 Cys, cysteine clusters; Ig1 and Ig2, first and second immunoglobulin-like motifs, respectively; LRM, leucine-rich motifs; SP, signal peptide; TK, tyrosine kinase; TM, transmembrane.
Table 1.
Reported gene fusions involving NTRK genes along with the corresponding tumour
| Gene fusion | Cancer type | Authors (year) |
|---|---|---|
| NTRK1 | ||
| LMNA-NTRK1 | Colorectal | Sartore-Bianchi et al (2016) |
| Soft tissue sarcoma | Doebele et al (2015) | |
| Spitzoid melanomas | Wiesner et al (2014) | |
| AYA sarcoma | Morosini et al (2015) | |
| Congenital infantile fibrosarcoma | Wong et al (2015) | |
| TPM3-NTRK1 | Colorectal | Lee et al (2015), Créancier et al (2015), Ardini et al (2014) |
| Papillary thyroid carcinomas | Bongarzone et al (1989), Butti et al (1995) | |
| Glioblastoma | Wu et al (2014) | |
| SQSTM1-NTRK1 | NSCLC | Farago et al (2015) |
| NTRK1-SQSTM1 | NSCLC | Siena et al (2015) |
| NFASC-NTRK1 | Glioblastoma multiforme | Frattini et al (2013), Kim et al (2014) |
| BCAN-NTRK1 | Glioblastoma multiforme | Kim et al (2014), Frattini et al (2013) |
| PPL-NTRK1 | Thyroid carcinoma | Farago et al (2015) |
| RFWD2-NTRK1 | Large cell neuroendocrine tumour (lung) | Fernandez-Cuesta et al (2014) |
| CD74-NTRK1 | Lung adenocarcinomas | Vaishnavi et al (2013) |
| MPRIP-NTRK1 | Lung adenocarcinomas | Vaishnavi et al (2013) |
| RABGAP1L-NTRK1 | ICC | Ross et al (2014) |
| TFG-NTRK1 | Thyroid carcinomas | Greco et al (1995) |
| TP53-NTRK1 | Spitzoid melanomas | Wiesner et al (2014) |
| NTRK2 | ||
| Unknown-NTRK1 | Appendiceal adenocarcinoma | Braghiroli et al (2016) |
| AFAP1-NTRK2 | Low-grade glioma | Stransky et al (2014) |
| AGBL4-NTRK2 | Glioblastoma | Wu et al (2014) |
| NACC2-NTRK2 | Pilocytic astrocytomas | Jones et al (2013) |
| PAN3-NTRK2 | Head and neck squamous cell carcinoma | Wu et al (2014) |
| QKI-NTRK2 | Pilocytic astrocytomas | Jones et al (2013) |
| TRIM24-NTRK2 | Lung adenocarcinoma | Wu et al (2014) |
| VCL-NTRK2 | Glioblastoma | Wu et al (2014) |
| NTRK3 | ||
| ETV6-NTRK3 | Glioblastoma | Zhang et al (2013) |
| Glioblastoma | Wu et al (2014) | |
| MASC | Tognon et al (2002), Ito et al (2015), Del Castillo et al (2015) | |
| Ductal carcinoma | Makretsov et al (2004), Arce et al (2005), Lagree et al (2011), Pinto et al (2014) | |
| Fibrosarcoma | Morerio et al (2004), Punnett et al (2000) | |
| Congenital mesoblastic nephroma | Watanabe et al (2002) | |
| Radiation-associated thyroid cancer | Leeman-Neill et al (2014) | |
| AML | Kralik et al (2011), Eguchi et al (1999), Knezevich et al (1998) | |
| GIST | Brenca et al (2015) | |
| MASC of salivary gland | Urano et al (2015), Skàlovà et al (2015) | |
| Papillary thyroid cancer | Leeman-Neill et al (2014), Seungbok Lee et al (2014) | |
| Colorectal | Hechtman et al (2015) | |
| BTBD1-NTRK3 | Glioblastoma | Wu et al (2014) |
AFAP1, actin filament-associated protein 1; AGBL4, ATP/GTP-binding protein-like 4; AML, acute myeloid leukaemia; AYA, adolescents and young adults; BCAN, brevican; BTBD1, BTB (POZ) domain containing 1; CD74, CD74 molecule; ETV6, ets variant 6; GIST, gastrointestinal stromal tumor; ICC, intrahepatic cholangiocarcinoma; LMNA, lamin A/C; MASC, mammary secretory breast carcinoma; MPRIP, myosin phosphatase Rho interacting protein; NACC2, NACC family member 2, BEN and BTB (POZ) domain containing; NFASC, neurofascin; NSCLC, non-small cell lung cancer; PAN3, PAN3 poly(A) specific ribonuclease subunit; PPL, periplakin; QKI, KH domain containing RNA binding; RABGAP1L, RAB GTPase activating protein 1-like; RFWD2, ring finger and WD repeat domain 2, E3 ubiquitin protein ligase; SQSTM1, sequestosome 1; TFG, TRK-fused gene; TP53, tumour protein p53; TPM3, tropomyosin 3; TRIM24, tripartite motif containing 24; VCL, vinculin.
Colorectal adenocarcinoma: It has been believed for more than three decades since the seminal work of Fearon and Vogelstein14 on molecular carcinogenesis of colorectal carcinoma (CRC), that rearrangement of oncogenes are of such low prevalence, as compared with DNA sequencing alteration or amplifications, that their role is almost negligible in the genesis of this tumour type. The first published report of a NTRK rearrangement in CRC dates back to 1986,15 when a TPM3-NTRK1 translocation was detected in a tumour biopsy, and thereafter very little has been reported about these gene defects in CRC. However, the NTRK gene fusions and their oncogenic potential in this tumour as oncogenes may have been underestimated, mostly because of the absence, until recently, of targeted therapies exploiting these gene abnormalities. These circumstances resemble what previously occurred with ALK (Anaplastic Lymphoma Kinase) gene fusions in non-small cell lung cancer (NSCLC), in which despite evidence of ALK fusions since 2007,16 the scientific interest was raised only after synthesis of compounds with ALK-specific inhibitory activity.17 In particular, in 2014, Ardini et al reported the characterisation of the TPM3-NTRK1 gene rearrangement as a recurring, although rare, event in CRC, and also discovered entrectinib (NMS-P626; RXDX-101) as a novel, highly potent and selective pan-Trk inhibitor. Entrectinib suppressed TPM3-TRKA phosphorylation and downstream signalling in KM12 cells and showed remarkable antitumour activity in mice bearing KM12 tumours. Also, in 2015, Créancier et al18 reported the 0.5% prevalence of NTRK fusions in 408 CRC clinical samples, including a TPM3-NTRK1 (TRK-T2 fusion). Recently, in the molecular screening within the phase I first-in-human study of entrectinib (EudraCT number: 2012-000148-88), an abnormal expression of the TrkA protein was identified in tumour and liver metastases of a patient with CRC refractory to standard therapy, and molecular characterisation unveiled a novel LMNA-NTRK1 rearrangement within chromosome 1, with oncogenic potential. The patient was treated with entrectinib, achieving objective partial response with decrease of metastatic lesions in the liver and adrenal gland.19 Further, as a part of the molecular screening of the Memorial Sloan Kettering IMPACT (MSK-IMPACT) programme,20 Braghiroli et al21 reported a 4% (2 of the 49 cases) of incidence of NTRK unidentified fusions in appendiceal adenocarcinoma.
Lung adenocarcinoma: Gene rearrangements have already emerged as therapeutic targets in NSCLC,22 since Food and Drug Administration (FDA) and European Medicines Evaluation Agency (EMEA) approval of crizotinib for patients with NSCLC harbouring EML4-ALK translocations. In 2013, Vaishnavi et al23 described two different gene fusions involving the NTRK1 gene that lead to constitutive TrkA TK domain activation. The first was characterised by a rearrangement of the 5’ portion of the myosin phosphatase Rho-interacting protein (MPRIP) gene fused to the 3’ portion of NTRK1; the resultant protein (RIP-TrkA) encoded by this fusion showed in cultured cells autophosphorylation of the fusion protein at critical tyrosine residues in cultured cells, implying its constitutive activation. The second gene fusion was characterised by a rearrangement between the CD74 and NTRK1 gene. In the same study, the authors also reported a TPM53-NTRK1 fusion (similar to that already described in CRC). A total of 3.3% patients in this study (3/91) harboured NTRK rearrangements potentially susceptible to TrkA inhibitors. In 2014, Stransky et al24 identified a novel TRIM24-NTRK2 gene fusion in lung adenocarcinoma through an unbiased computational pipeline designed for the identification of gene fusions in the data set from The Cancer Genome Atlas (TCGA).
Papillary thyroid carcinoma (PTC): A few years after the first published paper of a NTRK rearrangement in colorectal cancer, Bongarzone et al25 in 1989 described an oncogenic version of NTRK1 in PTC. Further works showed that oncogenic NTRK1 rearrangements in PTC are the consequence of the fusion of the TK domain of NTRK1 oncogene with 5-terminal sequences of at least three different genes. TRK-T1 and TRK-T2 are two different hybrid forms derived from chromosome inversion and different portions of TPR (Translocated Promoter Region) gene on chromosome 1q25 activate them. Another NTRK1 oncogene obtained by fusion with the TFG gene (TRK fused gene) on chromosome 3 is TRK–T3. All these oncogenic forms of NTRK1 gene encode cytoplasmic hybrid proteins that are constitutively phosphorylated at tyrosine residues.26 Somatic rearrangements of the NTRK1 gene in PTC usually do not exceed 12%, but range quite widely across different populations (from 15% to 50% in the Italian population27 to <10% in French,28 Japanese29 and Chinese30). A rare chromosomal rearrangement in sporadic thyroid cancer, but more frequent in radiation-related tumours, is ETV6-NTRK3, which results from an interchromosomal translocation t(12;15)(p13;q25) that juxtaposes exons 1–4 of ETV6 to exons 12–18 of NTRK3. The breakpoint differs from those reported in congenital fibrosarcomas and secretory breast cancers.31 Recently, via unbiased genomic approaches, novel gene rearrangements have been described in PTC, such as the PPL-NTRK1 (from periplakin, PPL)32 and the RBPMS-NTRK3 (from the RNA-binding protein with multiple splicing, RBPMS) gene fusion.24
Human secretory breast carcinoma: This is a rare but distinct subtype of infiltrating ductal carcinoma that was originally described in children and adolescents, but is now known to occur with equal incidence in adults. In 2002, Tognon et al33 reported the ETV6-NTRK3 gene fusion t(12;15)(p12;q26.1) as a pathognomonic genetic feature of this rare carcinoma.
Glioblastoma: Gene fusions occur in approximately 30–50% of patient with glioblastoma (GBM) samples and the Trk family could play a very important role. In 2013, Frattini et al34 analysed 185 GBM samples and found two in-frame fusions involving NTRK1 (BCAN-NTRK1 and NFASC-NTRK1). In the same year, Shah et al35 confirmed such genomic events as recurrent in a cohort of 24 GBM samples. In their work based on wide genomic screening of formalin-fixed paraffin-embedded (FFPE) specimens, Zheng et al32 reported one in-frame fusion involving exon 21 of ARHGEF2 (encoding Rho/Rac guanine nucleotide exchange factor 2) and exon 10 of NTRK1, and two in-frame fusions involving exon 5 of CHTOP (encoding the chromatin target of PRMT1) and exon 10 of NTRK1, of the 115 brain tumour FFPE analyses. In 2014, Wu et al36 applied a whole genome, whole exome and/or transcriptome sequencing to 127 samples of paediatric high-grade glioma (HGG), identifying recurrent fusions involving the neurotrophin receptor genes NTRK1, 2, or 3 in 40% of non-brainstem HGG.
Miscellaneous tumours: The recent efforts for identifying targetable genomic alterations through the use of next generation sequencing (NGS) are leading to the identification of novel and recurrent gene fusions in other various types of cancer. Ross et al37 reported a NGS screening of 28 FFPE samples of intrahepatic cholangiocarcinoma, in which a novel gene fusion RABGAP1L-NTRK1 was identified from a liver biopsy of a 62-year-old woman. Further, a recurrent gene fusion involving the ETV6 and the NTRK3 gene (ETV6-NTRK3) has been described in congenital fibrosarcoma.38
Trk inhibitors
The growing number of identified gene fusions involving the NTRK gene enhanced the interest of the scientific community in the development of drugs with inhibitory capacity for the TK domain of Trk. Bertrand et al39 published the high-resolution crystal structures of TrkA and TrkB in their apo-forms, as well as in complex with three nanomolar inhibitors. At least 40 residues of the kinase domain in its Asp-Phe-Gly (DFG)-in conformation, which potentially interact with a ligand in the ATP-binding site, are highly conserved between Trk proteins. Only 2 of the 40 residues are different between TrkA and TrkB, whereas the TrkB and TrkC ATP-binding sites are identical,39 suggesting an easier design of pan-, rather than selective, inhibitors of the three distinct isoforms of Trk receptors. Even though this may be an issue in developing selective Trk agonists for the treatment of neurological diseases,3 40 it may be indeed advantageous for cancer treatment where inhibition of all three Trk members’ gene fusions translates into wider antitumour activity.41
There is a limited number of reported Trk receptor tyrosine-kinase inhibitors in the literature and only a few of these have been tested in clinical trials (table 1).
Entrectinib, formerly RXDX-101 and NMS-E628, is an orally bioavailable inhibitor of the TK TrkA, TrkB and TrkC, as well as of C-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK). Entrectinib can cross the blood–brain barrier, and could thus potentially be effective in the treatment of brain metastases and GBM by activating gene fusions of NTRK, ROS1 or ALK. Currently, two phase I trials are ongoing with this drug;42–44 the RP2D of this drug is known and initial reports of substantial antitumour activity in cancers harbouring NTRK1 gene fusions have been published.19 45 During screening for accrual in the European phase I trial of entrectinib (EudraCT Number: 2014-001326-15), the investigators identified a novel LMNA–NTRK1 gene fusion in a patient with metastatic CRC who was enrolled in the trial. The patient achieved a meaningful response after only 1 month of treatment, suggesting for the first time that the NTRK gene may play a role as actionable oncogenic driver in this tumour type as well.19 In February 2015, entrectinib was granted FDA Orphan Drug Designation for the treatment of TrkA, TrkB and TrkC positive non-small cell lung and colorectal tumours.41
LOXO-101 is a pan-Trk inhibitor with highly selective activity against the Trk kinase family. To date, in the phase I study, the maximum tolerated dose (MTD) has not yet been reached and pharmacokinetics show good systemic exposure of LOXO-101 after oral dosing, reaching approximately 98% inhibition of TrkA/B/C at peak concentrations with once daily dosing of 50 or 100 mg.46 The clinical activity of this inhibitor was recently reported47 in a case of a 41-year-old woman enrolled in the phase I trial who showed remarkable tumour response after treatment with LOXO-101.47 The report by Doebele et al47 also identified a LMNA–NTRK1 gene fusion, previously unreported in sarcoma.
Altiratinib (DCC-2701)48 and sitravatinib (MGCD516)49 are multikinase inhibitors with reported in vitro activity against TrkA and TrkB, and were both recently tested in phase I clinical trials. Other multikinase inhibitors with claimed anti-Trk activity include TSR-011, PLX7486, DS-6051b, F17752 and cabozantinib (XL184), all in development in phase I/II trials (table 2).
Table 2.
Ongoing phase I/II trials involving drugs with known inhibitory activity of NTRK-related kinases
| NCT/EudraCT number | Title | Drug | Targets | Phases | Patients | Start date |
|---|---|---|---|---|---|---|
| NCT02219711 | Phase 1/1b study of MGCD516 in patients with advanced cancer | MGCD516 | MET, AXL, c-kit, MER, DDR2, VEGFR, PDGFR, RET, Trk, Eph |
1 | 120 | August 2014 |
| NCT02568267 | Basket study of entrectinib (RXDX-101) for the treatment of patients with solid tumors harboring NTRK1/2/3, ROS1, or ALK gene rearrangements (fusions) | Entrectinib (RXDX-101) |
TrkA, TrkB, TrkC, ROS1, ALK |
2 | 300 | October 2015 |
| NCT02097810 | Study of oral RXDX-101 in adult patients with locally advanced or metastatic cancer targeting NTRK1, NTRK2, NTRK3, ROS1, or ALK molecular alterations | 1/2 | 175 | June 2014 | ||
| NCT02650401 | Study of RXDX-101 in children with recurrent or refractory solid tumors and primary CNS tumors | 1 | 80 | December 2015 | ||
|
NCT02048488/ 2013–000686–37 |
A phase I/IIa open-label, dose escalation and cohort expansion trial of oral TSR-011 in patients with advanced solid tumors and lymphomas | TSR-011 | TrkA, ALK | 1/2 | 150 | October 2012 |
| NCT02637687 | Oral TRK inhibitor LOXO-101 for treatment of advanced pediatric solid or primary central nervous system tumors | LOXO-101 | TrkA, TrkB, TrkC | 1 | 36 | December 2015 |
| NCT02122913 | Oral TRK inhibitor LOXO-101 for treatment of advanced adult solid tumors | 1 | 108 | April 2014 | ||
| NCT02576431 | Study of LOXO-101 in subjects with NTRK fusion positive solid tumors | 2 | 151 | October 2015 | ||
| NCT01639508 | Cabozantinib in patients with RET fusion-positive advanced non-small cell lung cancer and those with other genotypes: ROS1 or NTRK fusions or increased MET or AXL activity | Cabozantinib (XL184) |
TrkA, RET, ROS1, MET, AXL | 2 | 68 | July 2012 |
| NCT01804530 | Phase 1 study of PLX7486 as single agent and with gemcitabine plus nab-paclitaxel in patients with advanced solid tumors | PLX7486 | TrkA, TrkB, TrkC, FMS | 1 | 160 | August 2013 |
| NCT02279433 | A first-in-human study to evaluate the safety, tolerability and pharmacokinetics of DS-6051b | DS-6051b | TrkA,TrkB,TrkC, ROS1 | 1 | 70 | September 2014 |
| 2013–003009–24 | Phase I-II study of F17752 in patients with advanced solid tumours | F17752 | ALK, ROS1, Trk | 1/2 | 112 | September 2015 |
| NCT02228811 | A study of DCC-2701 in participants with advanced solid tumors | Altiratinib (DCC-2701) |
TrkA, TrkB, TrkC, MET, TIE2, VEGFR |
1 | 48 | June 2014 |
ALK, anaplastic lymphoma receptor tyrosine kinase; AXL, AXL receptor tyrosine kinase; c-kit, mast/stem cell growth factor receptor; CNS, central nervous system; DDR2, discoidin domain receptor 2; Eph, ephrin receptor tyrosine kinases; FMS, McDonough Feline Sarcoma Viral; MER, MER receptor tyrosine kinase; MET, hepatocyte growth factor receptor; PDGFR, platelet-derived growth factor receptor; RET, rearranged during transfection; ROS1, ROS proto-oncogene 1; TIE2, TEK receptor tyrosine kinase; TRK, tropomyosin-related kinases (also known as TrkA,B,C for kinase A, B and C); VEGFR, vascular endothelial growth factor receptor.
Acquired resistance to Trk inhibitors
As with other targeted therapies, the onset of acquired (secondary) resistance may limit the efficacy of Trk inhibitors. Given their recent introduction into the therapeutic armamentarium of cancer treatment, very limited data are available regarding mechanisms underlying resistance. Recently, a study by Russo et al unveiled gene alterations associated with entrectinib-acquired resistance. In particular, it has been reported that entrectinib induced a remarkable clinical response in mCRC carrying a LMNA-NTRK1 translocation, and tumour resistance developed after 4 months of response to discontinuous treatment (the cycle on which the patient was treated consisted of 4 days on, 3 days off, for 3 weeks, followed by a 1 week break in dosing), when the patient experienced progression of disease.19 To characterise the molecular basis of acquired resistance to entrectinib, circulating tumour DNA (ctDNA) was collected longitudinally (liquid biopsies) during treatment in this individual case, and a tumour biopsy was obtained before entrectinib treatment and transplanted into immunodeficient mice in order to obtain a patient-derived xenograft (PDX), which was challenged with the same entrectinib regimen until resistance.50 The study of ctDNA extracted from plasma samples collected before treatment initiation and at clinical relapse revealed two novel NTRK1 genetic alterations, p.G595R and p.G667C, in the kinase domain of the protein, which were not detected in the ctDNA pretreatment but emerged in the circulation as early as 4 weeks from beginning of treatment. This finding was strengthened by the observation of the emergence of one of the same molecular alterations, the NTRK1 p.G695R, in the engrafted tumour biopsy expanded to multiple cohorts of mice treated with dosage and schedule of entrectinib matching those of the patient. The establishment of independent preclinical models with acquired resistance to entrectinib finally reinforced the conclusion that p.G595R or p.G667C NTRK1 mutations are mechanisms of resistance to entrectinib. The authors also concluded that there is probably a dose-dependent effect affecting the emergence of each of the two mutations: NTRK1 p.G667C emerged with exposure of low concentration of the inhibitor (absent with higher dose), and it is weaker than p.G595R in conferring resistance.50 In the reported case,19 indeed, the Trk pan-inhibitor entrectinib was administered with an intermittent dosing regimen that may have promoted or anticipated the development of resistance. Nevertheless, it is still unknown whether continuous or intermittent dosing will affect the emergence and/or the type of acquired mutations.
Conclusions and future perspectives
Gene fusions have been recognised as drivers in tumours for more than three decades, providing useful insights into carcinogenic processes. New deep-sequencing technologies are now illuminating a landscape of previously undetected gene fusions in a multitude of cancers.51 In such an expanding scenario, NTRK gene fusions are emerging as novel targets across multiple tumour types, due to the growing availability of new drugs with anti-Trk activity.
Clearly, a major challenge in the development of these inhibitors is the low incidence in each single tumour histology that is counterbalanced by a wide distribution of Trk alterations across multiple histologies, thus increasing the number of patients who can be reached and treated with matched therapeutics in basket trials at the front edge of present clinical cancer research.20 52
Acknowledgments
The authors thank Pratik Multani, MD, and colleagues at Ignyta Inc, and Antonella Isacchi, PhD, and colleagues at Nerviano Medical Sciences, for a fascinating discussion.
Footnotes
Funding: Authors at Niguarda Cancer Center are supported by the following grants: Terapia Molecolare dei Tumori from Fondazione Oncologia Niguarda Onlus; and Associazione Italiana per la Ricerca sul Cancro (AIRC) 2010 Special Program Molecular Clinical Oncology 5×1000, project 9970.
Competing interests: None declared.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1.Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett 2001;169:107–14. 10.1016/S0304-3835(01)00530-4 [DOI] [PubMed] [Google Scholar]
- 2.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 2003;72:609–42. 10.1146/annurev.biochem.72.121801.161629 [DOI] [PubMed] [Google Scholar]
- 3.Boulle F, Kenis G, Cazorla M et al. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog Neurobiol 2012;98:197–206. 10.1016/j.pneurobio.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 4.Weier HU, Rhein AP, Shadravan F et al. Rapid physical mapping of the human trk protooncogene (NTRK1) to human chromosome 1q21-q22 by P1 clone selection, fluorescence in situ hybridization (FISH), and computer-assisted microscopy. Genomics 1995;26:390–3. 10.1016/0888-7543(95)80226-C [DOI] [PubMed] [Google Scholar]
- 5.Indo Y, Mardy S, Miura Y et al. Congenital insensitivity to pain with anhidrosis (CIPA): novel mutations of the TRKA (NTRK1) gene, a putative uniparental disomy, and a linkage of the mutant TRKA and PKLR genes in a family with CIPA and pyruvate kinase deficiency. Hum Mutat 2001;18:308–18. 10.1002/humu.1192 [DOI] [PubMed] [Google Scholar]
- 6.Mardy S, Miura Y, Endo F et al. Congenital insensitivity to pain with anhidrosis: novel mutations in the TRKA (NTRK1) gene encoding a high-affinity receptor for nerve growth factor. Am J Hum Genet 1999;64:1570–9. 10.1086/302422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawara A, Liu XG, Ikegaki N et al. Cloning and chromosomal localization of the human TRK-B tyrosine kinase receptor gene (NTRK2). Genomics 1995;25:538–46. 10.1016/0888-7543(95)80055-Q [DOI] [PubMed] [Google Scholar]
- 8.Yeo GS, Connie Hung CC, Rochford J et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci 2004;7:1187–9. 10.1038/nn1336 [DOI] [PubMed] [Google Scholar]
- 9.Valent A, Danglot G, Bernheim A. Mapping of the tyrosine kinase receptors trkA (NTRK1), trkB (NTRK2) and trkC (NTRK3) to human chromosomes 1q22, 9q22 and 15q25 by fluorescence in situ hybridization. Eur J Hum Genet 1997;5:102–4. [PubMed] [Google Scholar]
- 10.Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell 1991;66:967–79. 10.1016/0092-8674(91)90442-2 [DOI] [PubMed] [Google Scholar]
- 11.Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov 2015;5:25–34. 10.1158/2159-8290.CD-14-0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomasson MH, Xiang Z, Walgren R et al. Somatic mutations and germline sequence variants in the expressed tyrosine kinase genes of patients with de novo acute myeloid leukemia. Blood 2008;111:4797–808. 10.1182/blood-2007-09-113027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacconelli A, Farina AR, Cappabianca L et al. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell 2004;6:347–60. 10.1016/j.ccr.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 14.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. 10.1016/0092-8674(90)90186-I [DOI] [PubMed] [Google Scholar]
- 15.Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature 1986;319:743–8. 10.1038/319743a0 [DOI] [PubMed] [Google Scholar]
- 16.Soda M, Choi YL, Enomoto M et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–6. 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- 17.Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 18.Créancier L, Vandenberghe I, Gomes B et al. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma. Cancer Lett 2015;365:107–11. 10.1016/j.canlet.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 19.Sartore-Bianchi A, Ardini E, Bosotti R et al. Sensitivity to entrectinib associated with a novel LMNA-NTRK1 gene fusion in metastatic colorectal cancer. J Natl Cancer Inst 2016;108:pii: djv306 10.1093/jnci/djv306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng DT, Mitchell TN, Zehir A et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015;17:251–64. 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braghiroli MI. Genomic profiling and efficacy of anti-EGFR therapy in appendiceal adenocarcinoma. J Clin Oncol 2016;34(Suppl 4S):abstr 574. [Google Scholar]
- 22.Solomon BJ, Mok T, Kim DW et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167–77. 10.1056/NEJMoa1408440 [DOI] [PubMed] [Google Scholar]
- 23.Vaishnavi A, Capelletti M, Le AT et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med 2013;19:1469–72. 10.1038/nm.3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stransky N, Cerami E, Schalm S et al. The landscape of kinase fusions in cancer. Nat Commun 2014;5:4846 10.1038/ncomms5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bongarzone I, Pierotti MA, Monzini N et al. High frequency of activation of tyrosine kinase oncogenes in human papillary thyroid carcinoma. Oncogene 1989;4:1457–62. [PubMed] [Google Scholar]
- 26.Greco A, Miranda C, Pierotti MA. Rearrangements of NTRK1 gene in papillary thyroid carcinoma. Mol Cell Endocrinol 2010;321:44–9. 10.1016/j.mce.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 27.Bongarzone I, Vigneri P, Mariani L et al. RET/NTRK1 rearrangements in thyroid gland tumors of the papillary carcinoma family: correlation with clinicopathological features. Clin Cancer Res 1998;4:223–8. [PubMed] [Google Scholar]
- 28.Delvincourt C, Patey M, Flament JB et al. Ret and trk proto-oncogene activation in thyroid papillary carcinomas in French patients from the Champagne-Ardenne region. Clin Biochem 1996;29:267–71. 10.1016/0009-9120(96)00006-9 [DOI] [PubMed] [Google Scholar]
- 29.Wajjwalku W, Nakamura S, Hasegawa Y et al. Low frequency of rearrangements of the ret and trk proto-oncogenes in Japanese thyroid papillary carcinomas. Jpn J Cancer Res 1992;83: 671–5. 10.1111/j.1349-7006.1992.tb01963.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu RT, Chou FF, Wang CH et al. Low prevalence of RET rearrangements (RET/PTC1,RET/PTC2,RET/PTC3, and ELKS-RET) in sporadic papillary thyroid carcinomas in Taiwan Chinese. Thyroid 2005;15:326–35. 10.1089/thy.2005.15.326 [DOI] [PubMed] [Google Scholar]
- 31.Ricarte-Filho JC, Li S, Garcia-Rendueles ME et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest 2013;123:4935–44. 10.1172/JCI69766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Z, Liebers M, Zhelyazkova B et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med 2014;20:1479–84. 10.1038/nm.3729 [DOI] [PubMed] [Google Scholar]
- 33.Tognon C, Knezevich SR, Huntsman D et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002;2:367–76. 10.1016/S1535-6108(02)00180-0 [DOI] [PubMed] [Google Scholar]
- 34.Frattini V, Trifonov V, Chan JM et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet 2013;45:1141–9. 10.1038/ng.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah N, Lankerovich M, Lee H et al. Exploration of the gene fusion landscape of glioblastoma using transcriptome sequencing and copy number data. BMC Genomics 2013;14:818 10.1186/1471-2164-14-818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu G, Diaz AK, Paugh BS et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 2014;46:444–50. 10.1038/ng.2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross JS, Wang K, Gay L et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014;19:235–42. 10.1634/theoncologist.2013-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knezevich SR, McFadden DE, Tao W et al. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet 1998;18:184–7. 10.1038/ng0298-184 [DOI] [PubMed] [Google Scholar]
- 39.Bertrand T, Kothe M, Liu J et al. The crystal structures of TrkA and TrkB suggest key regions for achieving selective inhibition. J Mol Biol 2012;423:439–53. 10.1016/j.jmb.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 40.Raeppel SL, Gaudette F, Nguyen H et al. Identification of a novel series of potent TrkA receptor tyrosine kinase inhibitors. Int J Med Chem 2012;2012:412614 10.1155/2012/412614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolfo C, Ruiz R, Giovannetti E et al. Entrectinib: a potent new TRK, ROS1, and ALK inhibitor. Expert Opin Investig Drugs 2015;24:1493–500. 10.1517/13543784.2015.1096344 [DOI] [PubMed] [Google Scholar]
- 42.Ardini E, Meninchicheri M, Banfi P et al. The ALK inhibitor NMS-E628 also potently inhibits ROS1 and induces tumor regression in ROS-driven models. Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013;73(8 Supp):Abstract # 2092. [Google Scholar]
- 43.Siena S, Drillon A, Ou SH et al. Entrectinib (RXDX-101), an oral pan-Trk, ROS1, and ALK inhibitor in patients with advanced solid tumors harboring gene rearrangements. Eur J Cancer 2015;51:S724–S725. 10.1016/j.ejca.2015.03.026 [DOI] [Google Scholar]
- 44.De Braud F, Pilla L, Niger M et al. RXDX-101, an oral Pan-Trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations. Ann Oncol 2014;25(Suppl 4):iv146–64. [Google Scholar]
- 45.Farago AF, Le LP, Zheng Z et al. Durable clinical response to entrectinib in NTRK1-rearranged non-small cell lung cancer. J Thorac Oncol 2015;10:1670–4. 10.1097/01.JTO.0000473485.38553.f0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burris HA, Shaw AT, Bauer TM et al. Pharmacokinetics (PK) of LOXO-101 during the first-in-human phase I study in patients with advanced solid tumors: interim update. Cancer Res 2015;75 (15 Suppl):Abstract 4529.
- 47.Doebele RC, Davis LE, Vaishnavi A et al. An Oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov 2015;5:1049–57. 10.1158/2159-8290.CD-15-0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith BD, Kaufman MD, Leary CB et al. Altiratinib inhibits tumor growth, invasion, angiogenesis, and microenvironment-mediated drug resistance via balanced inhibition of MET, TIE2, and VEGFR2. Mol Cancer Ther 2015;14:2023–34. 10.1158/1535-7163.MCT-14-1105 [DOI] [PubMed] [Google Scholar]
- 49.Patwardhan PP, Ivy KS, Musi E et al. Significant blockade of multiple receptor tyrosine kinases by MGCD516 (Sitravatinib), a novel small molecule inhibitor, shows potent anti-tumor activity in preclinical models of sarcoma. Oncotarget 2015;7:4093–109. 10.18632/oncotarget.6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russo M, Misale S, Wei G et al. Acquired resistance to the TRK inhibitor entrectinib in colorectal Cancer. Cancer Discov 2016;6:36–44. 10.1158/2159-8290.CD-15-0940 [DOI] [PubMed] [Google Scholar]
- 51.Mertens F, Johansson B, Fioretos T et al. The emerging complexity of gene fusions in cancer. Nat Rev Cancer 2015;15: 371–81. 10.1038/nrc3947 [DOI] [PubMed] [Google Scholar]
- 52.Abrams J, Conley B, Mooney M et al. National cancer institute's precision medicine initiatives for the new national clinical trials network. Am Soc Clin Oncol Educ Book 2014:71–6. 10.14694/EdBook_AM.2014.34.71 [DOI] [PubMed] [Google Scholar]