This study aimed to evaluate the therapeutic effect of mesenchymal stromal cell (MSC)-based cell transplantation as an alternative treatment for liver fibrosis. When injected into the spleen of thioacetamide-injured mice, human placental amnion membrane-derived MSCs can engraft into the injury site, ameliorate liver fibrosis, and restore liver function,
Keywords: Human amnion membrane-derived CD34+ MSC, Transplantation, Antifibrotic, Thioacetamide-induced liver fibrosis
Abstract
Liver fibrosis represents the end stage of chronic liver inflammatory diseases and is defined by the abnormal accumulation of extracellular matrix in the liver. Advanced liver fibrosis results in cirrhosis, liver failure, and portal hypertension. Liver transplantation has been the most effective treatment for these diseases, but the procedure is limited by the shortage of suitable donors. Mesenchymal stromal cells (MSCs) have shown great potential in the treatment of chronic inflammatory diseases associated with fibrosis. This study aimed to evaluate the therapeutic effect of MSC-based cell transplantation as an alternative treatment for liver fibrosis. A CD34-positive subpopulation of human placental amnion membrane-derived stem/progenitor cells (CD34+ AMSPCs) was isolated through the depletion of CD34-negative stromal fibroblasts (CD34− AMSFCs) facilitated by CD34 fluorescence-activated cell sorting, enriched and expanded ex vivo. These cells express pluripotency markers and demonstrate multidirectional differentiation potentials. Comparative analysis was made between CD34+ AMSPCs and CD34− AMSFCs in terms of the expressions of stemness surface markers, embryonic surface antigens, and multilineage differentiation potentials. A mouse model of liver fibrosis was established by thioacetamide (TAA) administration. When injected into the spleen of TAA-injured mice, human placental amnion membrane-derived MSCs (hAM-MSCs) can engraft into the injury site, ameliorate liver fibrosis, and restore liver function, as shown by pathological and blood biochemical analysis and downregulated gene expressions associated with liver damage. CD34+ AMSPCs represent a more primitive subset of hAM-MSCs and could be a suitable candidate with a potentially better safety profile for cell-based therapy in treatment of liver diseases associated with fibrosis.
Significance
In this study, a CD34+ subpopulation of stem/progenitor cells derived from neonatal placental amnion membrane, denoted as CD34+ AMSPCs, were identified, enriched, and characterized. These cells are highly proliferative, express mesenchymal stromal cells and pluripotent stem cell markers, and demonstrate multidirectional differentiation potentials, indicating their promising application in clinical regenerative therapies. CD34+ AMSPC transplantation ameliorated liver fibrosis in mice with drug-induced liver injury. These cells represent a potential therapeutic agent for treating liver diseases associated with fibrosis.
Introduction
Liver fibrosis is a histological change caused by liver inflammation and is characterized by the disturbed balance between extracellular matrix (ECM) synthesis and degradation resulting in excessive accumulation of ECM components in the extracellular space in the liver [1]. Once considered unidirectional, liver fibrosis is now viewed as reversible when the underlining causes are removed or treated with therapeutic agents to attenuate the inflammation and promote ECM degradation and endogenous healing. The use of stem/progenitor cells as therapeutic agents is deemed promising, owing not only to their highly proliferative capacity and multilineage differentiation potential but also to their functionality in secretion of trophic molecules to promote cell survival and growth.
Despite the isolation of human embryonic stem cells and the more recently discovered induced pluripotent stem cells [2, 3], many critical issues still preclude prevalent clinical use of these pluripotent stem cells, including the ethical concerns of human embryonic stem cells and tumorigenic potential of pluripotent stem cells.
To efficiently repair or regenerate defective organs, one would expect the stem/progenitor cells to possess desirable therapeutic properties, for example, minimum side effects, ability to integrate into host tissue, differentiation into desired cell lineages, paracrine effect, immunomodulation, regulation of tissue remodeling, and activation of endogenous repair/regeneration mechanisms.
Several studies have reported that bone marrow-derived mesenchymal stem cells (BM-MSCs) can differentiate into hepatocyte-like cells, restoring liver function after injury or chronic fibrogenesis [4–11]. Although BM-MSCs provide an attractive therapeutic candidate for treating liver diseases, their use is limited by several factors, such as low cell yields from donor BM, lack of donors, dependence on donor age, and invasive procedure for patients [4, 12, 13]. Searching for alternative cell sources to overcome the drawbacks of BM-MSCs is therefore warranted.
The multipotent differentiation capacity of human amniotic membrane-derived mesenchymal stem cells (hAM-MSCs) has been reported recently, and studies support their candidacy as a suitable source for cell-based therapy [14–18]. Analogous to bone marrow-derived cells, hAM-MSCs exhibit self-renewal ability, strong immunosuppressive function, freedom from ethical concerns, and easy accessibility and can be induced to various cell types, including the hepatic lineage [19]. Furthermore, superior to bone marrow-derived cells, they can be obtained noninvasively from the amnion membrane of the placenta and cultured. Another good source of cell-based therapy is adipose tissue-derived stem cells. The adipose tissue represents an accessible, abundant pool of adult stem cells for potential applications in regenerative medicine. Adipose tissue-derived mesenchymal stem cells (ADMSCs) are very similar to bone marrow-derived mesenchymal stem cells (BM-MSCs), and they share many common traits [20–23].
BM-MSCs have been explored in a number of clinical trials for several indications [24]. More recent and ongoing human studies are using marrow MSCs, bone marrow mononuclear cells, skeletal myoblasts, endothelial progenitor cells (EPCs), and ADMSCs. Some of these studies have yielded promising results [25–31], but applications in clinical trials have been controversial, with potential risk of tumorigenicity [32]. The mixed results indicate that the heterogeneity of MSCs may play a role in the outcome of MSC-based therapy.
MSCs in tissues are represented by a mixture of the stem/progenitor cells (MSPCs) and the mature mesenchymal stromal fibroblast populations (MSFs). The mature MSFs have been shown to cause tumor activation and cancer metastasis [32]. In order to increase the safety and efficacy of MSC-based therapy, it is conceptually rational to deplete the MSFs from MSCs.
CD34 hematopoietic stem/progenitor marker has been found expressed in most of blood vessel-rich mesenchymal connective tissues, as shown by the existence of a CD34+ common mesodermal stem/progenitor cell population in the human blood vessel tissues [33, 34]. Previously, we identified and characterized a CD34-positive subpopulation of stem/progenitor cells from human neonatal placental amnion membrane (CD34+ AMSPCs) with multipotent differentiation potentials [35]. But maintaining CD34 expression during in vitro expansion has been a challenge, as manifested by the quick loss of its expression during early passages. Recently, we have further demonstrated that CD34-positive mesenchymal stem/progenitor cells (CD34+ MSPCs) isolated from various human neonatal and adult somatic stromal tissues can be successfully sustained during extensive in vitro culture [36].
In this study, CD34+ AMSPCs were separated from the CD34-negagive mesenchymal stromal fibroblasts (CD34− AMSFCs) in an effort to increase safety and efficacy. We present evidence that CD34+ AMSPCs represent the more primitive subpopulation in hAM-MSCs with multidirectional differentiation potentials, including adipogenic, osteogenic, chondrogenic, neurogenic, myogenic, and hepatogenic differentiations. The highly proliferative property and plasticity of CD34+ AMSPCs indicate their promising application in clinical regenerative therapies. To further explore their therapeutic potential, we investigated the antifibrotic activities of CD34+ AMSPC transplantation in thioacetamide (TAA)-treated NOD.CB17-Prkdcscid/NcrCrl (NOD-SCID) mice. Attempts were made to compare the preclinical therapeutic potentials among transplantations with CD34+ AMSPCs, CD34− AMSFCs, and ADMSCs in liver regeneration in the injured mice.
Materials and Methods
Isolation of Amnion Mesenchymal Cells and Adipose Tissue MSCs
Human tissues for this study were obtained by a protocol approved by the institutional review boards of Cathay General Hospital and Taipei Medical University. Primary amnion mesenchymal cells were essentially isolated from the membranes of donors by using a modified method [37–39]. Amnion membrane (approximately 300 cm2; n = 9) was stripped from chorion and washed in 3× 150 ml of 1× Hanks’ buffer to remove blood. To deplete the amnion epithelial cells (Am-EPCs), we cut washed amnion membrane into 2- to 3-cm2 fragments, dispensed in 100 ml 1× Hanks’ balanced salt solution with 0.1% Trypsin-EDTA (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com; catalog no. 14185-052, Thermo Fisher Scientific, Grand Island, NY, https://www.thermofisher.com) and incubated in a water bath at 37°C for 15 minutes. The process was repeated four times.
For the isolation of the amnion mesenchymal cells (AM-MSCs), the Am-EPC depleted amnion membrane was subjected to washing with Hanks’ buffer 1 time and digested with collagenase 1A (1 mg/ml in Hank’s balanced salt solution) (catalog no. C9891; Sigma-Aldrich) at 37°C for 45–60 minutes. An appropriate amount of Hanks’ buffer and a 40-μm nylon cell strainer (catalog no. 352235, Becton, Dickinson and Company, Detroit MI, http://www.bd.com) were used to collect the AM-MSCs. Adipose tissue-derived MSCs were isolated, expanded, and characterized, as has been previously reported [40].
Enrichment and Expansion of CD34+ AMSPCs in Culture
After centrifugation at 170g, AM-MSCs were plated in CELL-BIND T75 flasks (catalog no. 3290, Corning, Corning, NY, http://www.corning.com) at a density of 5 × 104 cells per cm2 and incubated at 37°C in Medium199 (catalog no. 12-118F, Lonza, Basel, Switzerland, http://www.lonza.com), supplemented with fetal bovine serum (FBS) (10%) (catalog no. SH 30071.03, GE Life Sciences/Hyclone, Logan, UT), epidermal growth factor (EGF) (20 ng/ml) (catalog no. 236-EG, R&D Systems, Minneapolis, MN, https://www.rndsystems.com), and hydrocortisone (0.4 μg/ml) (catalog no. 07904; StemCell Technologies, St. Katharinen, Germany, https://www.stemcell.com), with 5% CO2. Primary AM-MSCs were put into fresh medium on day 5 after isolation, and cells were expanded to 80% confluence in 7 days. They were then harvested with 0.1% Trypsin-EDTA (a 4-minute digestion) and split into the new passage culture at the seeding density of 100,000 cells per T75 flask after quantification under the microscope. Alternatively, the collected AM-MSCs could be cultured in phenol red-free RPMI-1640 (catalog no. 11835-030; Thermo Fisher), supplemented with FBS (10%) (catalog no. SH30071.03; GE Life Sciences/Hyclone), sodium pyruvate (0.1 mM) (catalog no. S8636; Sigma-Aldrich), fibroblast growth factor basic (bFGF) (10 ng/ml), and EGF (10 ng/ml) (catalog nos; 233 FB and 236 EG, R&D Systems). Cells were split when they reached 70%∼80% confluence, the culture medium was changed every 3∼4 days.
CD34+ AMSPC Flow Cytometry Sorting
To separate the CD34+ AMSPC subpopulation, we labeled the expanded primary AM-MSCs cultured at passages 2–3 with CD34 antibody. Up to 3 × 106 cells were sorted by the FACSAria flow cytometer (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com), following the manufacturer’s instructions. CD34-positive (CD34+) and CD34-negative (CD34−) cells were then analyzed, sorted, and collected. In brief, 3–4 × 106 harvested third-passage AM-MSCs were trypsinized and labeled with PE-conjugated CD34 in 100 μl phosphate buffer at room temperature for 15 minutes, as suggested by the manufacturer. Cells were then filtered through a 40-μm nylon cell strainer (catalog no. 352235, Becton, Dickinson and Company), and CD34+ AMSPC and CD34− AMSFC subpopulations were separated by BD Biosciences FACSAria. After sorting, the CD34+ AMSPC and CD34− AMSFC subpopulations were reanalyzed for the positive fraction and expanded in M199 condition or RPMI condition, as described above. The CD34 expression of CD34+ AMSPCs was checked every five passages in the following days.
Flow Cytometry Analysis
For FACS analysis, freshly harvested AM-MSCs were trypsinized and incubated with aliquot fluorescein (or R-phycoerythrin) conjugated monoclonal antibodies for 30 minutes at 4°C in 100 μl phosphate buffer, following the manufacturer’s suggestion. Cells were analyzed using the FACS autoflow cytometry system (BD Bioscience). The flow-cytometric data were processed with FCS Express V3 software (De Novo Software, Glendale, CA, http://www.denovosoftware.com).
Induction of Adipogenic, Osteogenic, Chondrogenic, Neurogenic, Cardiomyogenic, and Hepatic Differentiations
Sorted CD34+ AMSPCs from passages 5–6 were used for multilineage differentiation inductions. The adipogenic, osteogenic, and chondrogenic differentiation protocols were adopted from the work of Zuk et al. [41], as briefly described below. The CD34+ AMSPCs and CD34− AMSFCs preconditioned in Dulbecco’s modified Eagle’s medium (DMEM/LG, Thermo Fisher), supplemented with 10% FBS (GE Life Sciences/Hyclone), were used for the following lineage differentiations: (a) Adipogenesis: DMEM/LG medium supplemented with 10% FBS, 0.5 mM isobutyl-methylxanthine, 1 μM dexamethasone, 10 μM insulin, and 200 μM indomethacin; (b) Osteogenesis: DMEM/LG medium supplemented with 10% FBS, 0.1 μM dexamethasone, 50 μM ascorbate-2-phosphate, and 10 mM β-glycerolphosphate; (c) Chondrogenesis: DMEM/LG medium supplemented with 1% FBS, 6.25 μg/ml insulin, 10 ng/ml transforming growth factor (TGF)-β1 (R&D Systems), and 50 nM ascorbate-2-phosphate; (d) Neurogenesis: cells were cultured in preinduction medium containing serum-free DMEM-HG/F12 (1:1) supplement with 1× B27, 20 ng/ml EGF, 20 ng/ml bFGF, and 2 μg/ml heparin for 7 days at 37°C 5% CO2. The formed sphere-like cell clusters were dissociated into single cells by HyQtase. The cells were then cultured in a poly-d-lysine (PDL)-coated chamber slide with DMEM-HG/F12 (1:1) supplement with 10 ng/ml platelet-derived growth factor (PDGF)-BB, 50 ng/ml brain-derived neurotrophic factor (BDNF), and 50 ng/ml glial cell line-derived neurotrophic factor (GDNF) for 7 to 10 days at 5% CO2, 37°C. The medium was changed every 3–4 days. Expression of neuron-specific marker TuJ1 was identified by immunofluorescent staining. (All reagents used for the differentiation were from Sigma-Aldrich.) For adipogenic, osteogenic, and neurogenic differentiations, the cell density was 3 × 104 cells per square centimeter. For chondrogenic differentiation, a higher cell density of 1–2 × 105 per 10 μl was used for chondrosphere formation. Medium was changed every 3 to 4 days for all differentiation assays, and cells were fixed for histochemical staining after 14 days for adipogenic, osteogenic, and chondrogenic differentiations, and 28 days for neurogenic differentiation, respectively; (e) Cardiomyogenesis: Sorted CD34+ AMSPC cells at passages 4–6 were harvested for induction of cardiomyogenic differentiation, as described by Smits et al. [42]. The CD34+ AMSPCs were incubated overnight in the growth medium (EGM-2:M199 [vol/vol 1:3], supplemented with 10% FBS, and MEM nonessential amino acids [1×]; Thermo Fisher). The following morning, cells were transferred into the cardiomyogenic differentiation medium (Iscove's modified Dulbecco's medium [IMDM] [Thermo Fisher], Ham’s F12 nutrient mixture with GlutaMAX-1 [Thermo Fisher] [vol/vol 1:1], supplemented with 2% horse serum [Thermo Fisher], 1× MEM nonessential amino acids and 1× insulin-transferring-selenium [Thermo Fisher]) at a cell density of 104 per square centimeter. After 6–8 hours, a cardiomyogenic differentiation agent, 5-azacytidine (Sigma-Aldrich) was added into the medium at the final concentration of 5 μM/ml. Four microliters of 5-azacytidine (0.25 mM) stock solution was added into the differentiation medium daily and was changed back to the differentiation medium without 5-azacytidine on day 4. On day 6 of the differentiation assay, ascorbic acid (10−4 M) (Sigma-Aldrich) and TGF-β1 (1 ng/ml) (PeproTech, Rocky Hill, NJ, http://www.peprotech.com) were added to the medium. From this point forward, ascorbic acid and TGF-β1 were supplemented every other day and twice weekly, respectively. The medium was changed every 2–3 days, depending on the medium pH changes. When changing the medium, cell debris was removed by phosphate-buffered saline (PBS) washes. The cardiomyogenic AM-MSCs were fixed for histochemical staining after 28 days in the differentiation culture; (f) Hepatic differentiation: AM-MSCs were seeded at a density of 0.75 × 106 cells per well in DMEM Std + 10% FBS + 10 ng/ml EGF and kept for 24 hours. The cultures were then each overlaid with 0.44 mg/ml of the respective matrix and kept for another 24 hours. At day 2, the medium was switched to IMDM Std + 10% FBS + 10 ng/ml EGF + 10 ng/ml FGF-2 for 48 hours and then supplemented with 20 ng/ml hepatocyte growth factor (HGF), 1 μM Dex, 1× insulin/ transferrin/selenium (Thermo Fisher) for the following 5 days. The treatment was maintained for an additional week with the exception of FGF-2, which was replaced by 20 ng/ml Oncostatin-M.
Generation of Mouse Liver Fibrosis Model
All animal experimental protocols were approved by the National Taiwan University College of Medicine and the College of Public Health’s Institutional Animal Care and Use Committee. Four- to 6-week-old NOD-SCID mice were injected intraperitoneally with TAA (200 mg/kg, twice a week) for 8 weeks. An equal volume of 1 × PBS was administered to animals in the normal control group. After final TAA injection at week 8, animals were randomly divided into four groups (six mice in each group): control group (nontransplantation [TP]), CD34+ group (TP with CD34+ AMSPCs), CD34− group (TP with CD34− AMSFCs), and adipose group (TP with ADMSCs). One day after the final injection of TAA, 1 × 106 human MSCs or equal volume of saline for the control were injected into the spleen under anesthesia with isoflurane.
Liver Histology
Liver tissue was fixed in 10% formaldehyde, embedded in paraffin, and sectioned to thickness of 5 μm. The tissue sections were stained with Masson trichrome for collagen deposition analysis. Liver fibrosis in TAA-injured mice was scored according to the histopathological criteria of Nanji et al. and Goodman [43, 44] with modifications: 0, no fibrosis; 1, fibrosis confined to enlarged portal zones; 2, periportal or portal-portal septa with intact architecture; 3, distorted architecture (septal fibrosis, bridging) without obvious cirrhosis; and 4, probable or definite cirrhosis.
Quantification of Liver Fibrosis
In order to quantify the fibrosis level, we used BioVision Hydroxyproline Elisa Assay Kit (Biovision, Milpitas, CA, http://www.biovision.com) to measure the absorbance of hydroxyproline at 560 nm by SpetraMax multidetection Elisa Reader (Molecular Devices, Sunnyvale, CA, http://www.moleculardevices.com), following the manufacturer’s instructions.
Blood Biochemistry Analysis
The levels of glutamate-oxaloacetate transaminase/aspartate transaminase (GOT/AST), glutamate-pyruvate transaminase/alanine transaminase (GPT/ALT), and total bilirubin (TBIL) in the blood collected from the animals at 3 weeks posttransplantation were measured using a SPOTCHEM Auto Dry Chemistry Analyzer SP-4410. In addition, albumin level was examined by SpetraMax multidetection Elisa Reader using the AlPCO Mouse Albumin Elisa Kit (ALPCO, Salem, NH, http://www.alpco.com).
Detection of Transplanted PKH26 Red-Labeled Mesenchymal Stem Cells (PKH26-Labeled MSCs)
In order to track the transplanted MSCs in TAA-injured mice, we labeled MSCs by using a PKH26 Red Fluorescent Cell Linker kit (Sigma-Aldrich) according to the manufacturer’s instructions. The PKH26-labeled MSCs (1 × 106) were directly transplanted into the spleen of each TAA-injured mouse by injection at a depth of 5 mm. Liver tissues were collected at 3 weeks posttransplantation and were embedded in Tissue-Tek O.C.T. compound. The presence of grafted MSCs in the liver sections of TAA-injured mice was observed and pictured by AXIO Imager A1, AX10 microscope (Zeiss, Oberkochen, Germany, http://www.zeiss.com).
RNA Isolation and Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was isolated from sorted or differentiated cells using the RNAqueous-Micro RNA isolation kit (Thermo Fisher/Ambion), as per kit protocol. Quantitative reverse transcription-polymerase chain reaction (RT-qPCR) was done to analyze the relative expression of the following genes: transcription factors: Oct-4, Nanog, Rex-1, and Sox-2; lineage-specific markers: adipogenic (PPAR-γ and GLUT4), osteogenic (c-module binding factor-1 and alkaline phosphatase), and chondrogenic (collagen II and aggrecan). Primer sequences are shown in supplemental online Figure 3.
Mouse liver tissue was homogenized and lysed. Total RNA was prepared using TRIzol reagent (Thermo Fisher). Total RNA samples (1 mg) were reverse transcribed using RevertAid Reverse Transcriptase (Thermo Fisher). The resulting cDNA was amplified in an Applied Biosystem machine with RT-qPCR using purchased Taqman primers for the following genes: α-smooth muscle actin (α-SMA), collagen I, TGF-β, HGF, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The settings were 2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Relative gene expression levels were determined using the comparative threshold cycle CT (ΔΔCT) method with GAPDH used as an endogenous control and normal control as calibrator. The results were collected and analyzed by StepOne Software version 2.2.2 (Thermo Fisher/Applied Biosystems).
Statistical Analysis
Data are presented as the mean ± SD. Unpaired Student’s t test was used when comparisons were made between only two groups, whereas a one-way analysis of variance (ANOVA) followed by post hoc Tukey’s test were applied when comparing more than two groups. Difference was considered statistically significant at p < .05.
Results
The Multipotent Stem/Progenitor Characteristics of CD34+ AMSPCs
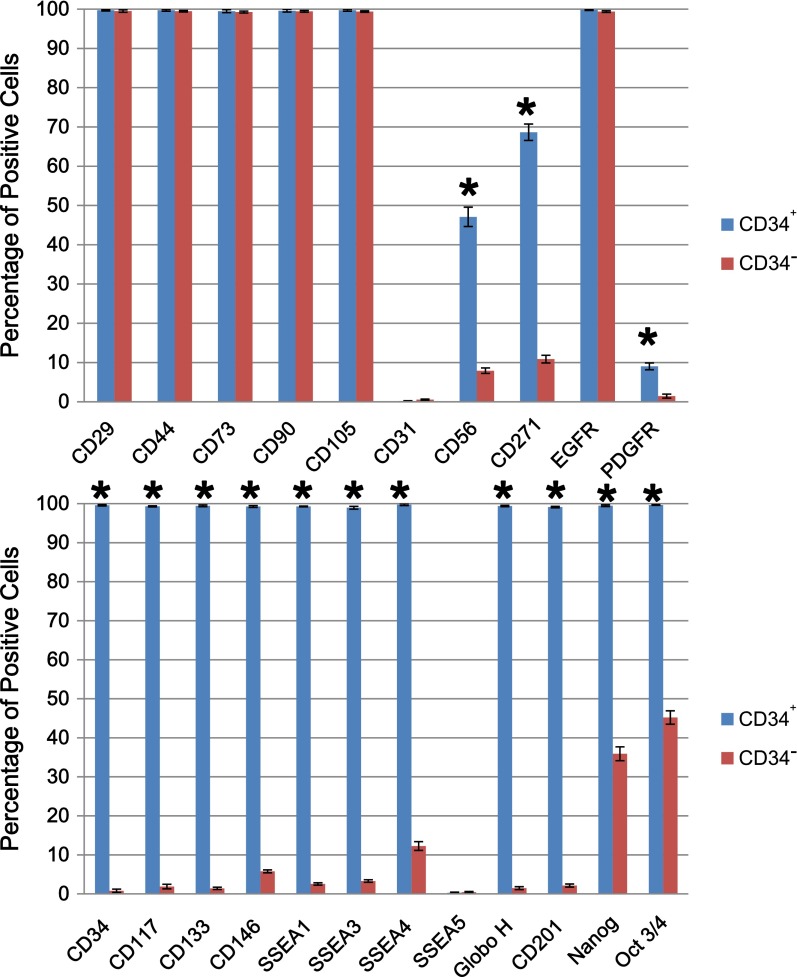
Cell phenotype of CD34+ AMSPCs and CD34− AMSFCs: Both CD34+ AMSPCs and CD34− AMSFCs express common MSC surface markers (CD29, CD44, CD73, CD90, and CD105) (Fig. 1). In comparison with CD34− AMSFCs, the isolated CD34+ AMSPCs express higher levels of (a) stem cell transcription factors Oct-3/4, Nanog, Sox-2, and Rex-1 (FLOW data in Fig. 1 and RT-qPCR data in supplemental online Fig. 2); (b) embryonic antigens (SSEA-1, SSEA-3, and SSEA-4, but not SSEA-5); (c) stem cell biomarkers (CD34, CD133, CD117, CD146, CD201, and CD271); and (d) other cell surface markers and receptors (CD56, EGFR, and PDGF receptor) (Fig. 1; supplemental online Fig. 1).
Figure 1.
Comparison of cell phenotype between CD34+ amnion membrane-derived stem/progenitor cells and CD34− amnion membrane-derived stromal fibroblast cells. Flow cytometry analysis of common mesenchymal stromal cell makers (CD29, CD44, CD73, CD90, and CD105), stem cell transcription factors (Nanog and Oct-3/4), embryonic antigens (SSEA-1, SSEA-3, SSEA-4, SSEA-5, and GloboH), stem cell biomarkers (CD34, CD133, CD117, CD146, and CD201), and other cell surface markers and receptors (CD31, CD56, CD271, EGFR, and PDGFR). n = 3; ∗, p < .05. Abbreviations: CD34+, CD34+ amnion membrane-derived stem/progenitor cells; CD34−, CD34− amnion membrane-derived stromal fibroblast cells; EGFR, epidermal growth factor receptor; PDGFR, platelet-derived growth factor receptor.
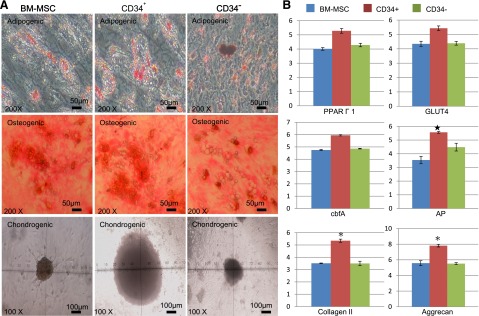
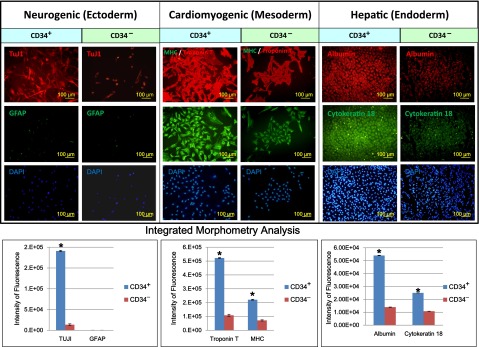
Mesenchymal and multidirectional differentiation capacities of CD34+ AMSPCs and CD34− AMSFCs: (a) Both CD34+ AMSPCs and CD34− AMSFCs have demonstrated similar characteristic MSC mesenchymal (adipogenic, osteogenic, and chondrogenic) differentiation capacities as shown by the histochemical staining and RT-qPCR. Relative expressions of some lineage-specific genes (alkaline phosphatase for osteogenesis; collagen II and aggrecan for chondrogenesis) are slightly higher in CD34+ AMSPCs (Fig. 2); (b) CD34+ AMSPCs exhibited better multidirectional differentiation capacities than did CD34− AMSFCs, as shown by immunohistostaining studies, including ectodermal neuron differentiation, mesodermal cardiomyogenic differentiation, and endodermal hepatic differentiations (Fig. 3). These data suggest that CD34+ AMSPCs represent a more primitive population than do CD34− AMSFCs with higher transdifferentiation potentials.
Figure 2.
Mesenchymal differentiation capacities of CD34+ amnion membrane-derived stem/progenitor cells and CD34− amnion membrane-derived stromal fibroblast cells. After 14 days’ induction in respective differentiation medium, histochemical staining and quantitative reverse transcription-polymerase chain reaction (RT-qPCR) were performed to confirm the differentiation and quantify the relative gene expressions representative to each lineage. (A): Adipogenic: intracelluar oil droplets were seen under Oil Red O staining; osteogenic: calcified extracellular matrix was observed by Von Kossa staining; chondrogenic: chondrosphere was formed and photographed. (B): Relative expression of adipogenic, osteogenic, and chondrogenic lineage-specific genes by RT-qPCR. n = 3; ∗, p < .05. Abbreviations: AP, alkaline phosphatase; BM-MSC, bone marrow mesenchymal stromal cells; cbfA, core binding factor-α; CD34+, CD34+ amnion membrane-derived stem/progenitor cells; CD34−, CD34− amnion membrane-derived stromal fibroblast cells.
Figure 3.
Comparison of multidirectional differentiation capacities between CD34+ AMSPCs and CD34− amnion membrane-derived stromal fibroblast cells. Immunohistostaining analysis was done to detect markers for cell types from three germ layers. TuJ1: marker for neuron differentiation (ectoderm); troponin T and major histocompatibility complex: markers for cardiomyogenic differentiation (mesoderm); secretory albumin and cytokeratin 18: markers for hepatic differentiation (endoderm). Integrated morphometry analysis was done to quantify the fluorescence intensity. Scale bar = 100 μm. ∗, p < .05. Abbreviations: AMSPCs, amnion membrane-derived stem/progenitor cells; CD34+, CD34+ amnion membrane-derived stem/progenitor cells; CD34−, CD34− amnion membrane-derived stromal fibroblast cells; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; MHC, major histocompatibility complex; TUJ1, neuron-specific class III β tubulin.
Generation of the TAA-Induced Liver Fibrosis Model
To evaluate the regenerative function of CD34+ AMSPCs, we introduced an in vivo model of chronic liver damage, the induced liver injury in mice through repeated administrations of a high concentration of TAA (approximately 200 mg/kg, twice a week) over the course of 8 weeks (supplemental online Fig. 4A). By comparison with the livers of normal control animals, the livers of TAA-injured mice displayed a range of severe damages, including bridging fibrosis, connecting neighboring portal veins to central veins, various lipid changes, and increased inflammation (supplemental online Fig. 4B).
hMSC Transplantation Alleviated TAA-Induced Liver Fibrosis
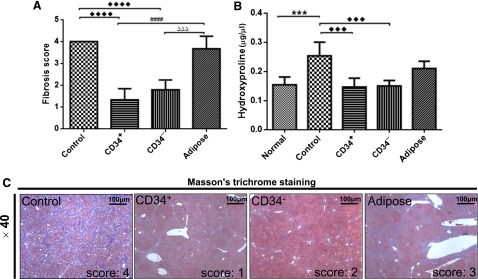
Liver damage scores assigned according to the four-tiered cirrhosis criteria were significantly lower in CD34+ AMSPC- and CD34− AMSFC-transplanted (TP) mice but not in ADMSC TP mice (Fig. 4A). In comparison with the control, the collagen accumulation levels were significantly lower in CD34+ AMSPC- and CD34− AMSFC-transplanted mice but not in ADMSC TP mice, as quantified by hydroxyproline assay (Fig. 4B), with a 50% reduction from CD34+ AMSPC and CD34− AMSFC transplantations in comparison with the control. The fibrosis scores obtained through Masson’s trichrome staining are shown in Figure 4C for all experimental groups. The results showed that hAM-MSC transplantations have significantly reversed the progression of liver fibrosis.
Figure 4.
Human mesenchymal stromal cell transplantation alleviated thioacetamide (TAA)-induced liver fibrosis. (A): The degree of liver injury from TAA-treated mice scored as 0–4 by histopathological criteria (Masson trichome staining), modified from Nanji et al. [43] as follows: 0 representing no fibrosis, 1 representing fibrosis confined to enlarged portal zones, 2 representing periportal or portal septa with intact architecture, 3 representing architectural distortion (septal fibrosis, bridging) without obvious cirrhosis, and 4 representing probable or definite cirrhosis. (B): The collagen accumulation in the mice quantified by hydroxyproline assay. (C): Histopathological analysis of the livers from CD34+, CD34−, and adipose tissue-derived mesenchymal stem cell-transplanted mice by Masson's trichrome staining. One-way analysis of variance, followed by Tukey’s multiple-comparisons test, was used to analyze the data; statistical significances were identified by adjusted p values; p < .05 was considered statistically different. Scale bar = 100 μm. ♦♦♦♦ and ####, p < .0001; ♦♦♦, ∗∗∗, and △△△, p < .001. Abbreviations: adipose, adipose tissue-derived mesenchymal stem cells transplantation; CD34+, CD34+ amnion membrane-derived stem/progenitor cells; CD34−, CD34− amnion membrane-derived stromal fibroblast cells; Control: nontransplantation.
Recovery and Restoration of the Liver Function in TAA-Injured Mice by CD34+ hAMSPC Transplantation
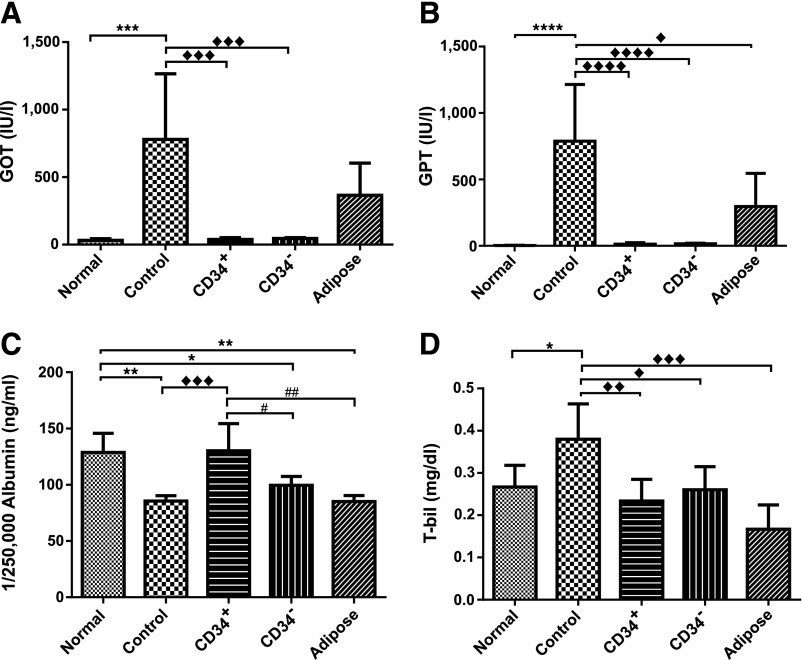
Mice treated with TAA alone had more than a 10-fold increase of serum GOT and more than a 20-fold increase of serum GPT in comparison with the normal group (Fig. 5A, 5B). In comparison with the control group, both hAM-MSC and ADMSC transplantations lowered serum GOT and GTP, but ADMSCs’ effect on GOT did not reach statistically significant levels (Fig. 5A, 5B). Only transplantation of CD34+ AMSPCs could effectively elevate albumin level to normal in comparison with TPs of CD34− AMSFCs and ADMSCs (Fig. 5C). All TP mice were able to restore serum TBIL level to normal (Fig. 5D). These results suggested that human MSC transplantation ameliorated the TAA-induced deterioration of liver function. Under our current experimental settings, hAM-MSCs have better therapeutic effect than does ADMSC, and CD34+ AMSPCs have a slight edge over CD34− AMSFCs in terms of restoring serum albumin level. In summary, we confirmed the suitability of MSC treatment on the TAA-induced liver failure as a model for studying fibrosis by pathological analyses and functional blood biochemistry.
Figure 5.
Human mesenchymal stromal cell transplantation improved the hepatic function of thioacetamide-injured mice. Liver function was evaluated by biochemical parameters. (A): Glutamate-oxaloacetate transaminase; (B): glutamate-pyruvate transaminase; (C): albumin; (D): total bilirubin. One-way analysis of variance, followed by Tukey’s multiple-comparisons test, was used to analyze the data; statistical significances were identified by adjusted p value; p < .05 was considered as statistically different. ♦♦♦♦ and ∗∗∗∗, p < .0001; ♦♦♦ and ∗∗∗, p < .001; ♦♦, ∗∗, and ##, p < .01; ♦, ∗, and #, p < .05. Abbreviations: Adipose, transplantation with adipose tissue-derived mesenchymal stem cells; CD34+, transplantation with CD34+ amnion membrane-derived stem/progenitor cells; CD34−, transplantation with CD34− amnion membrane-derived stromal fibroblast cells; Control, nontransplantation; GOT, glutamate oxaloacetate transaminase; T-bil, total bilirubin.
hMSCs Engraftment in TAA-Injured Liver and Effects of Transplanted hMSCs on Gene Expression Associated With Liver Damage and Suppression of Hepatic Stellate Cell Activation
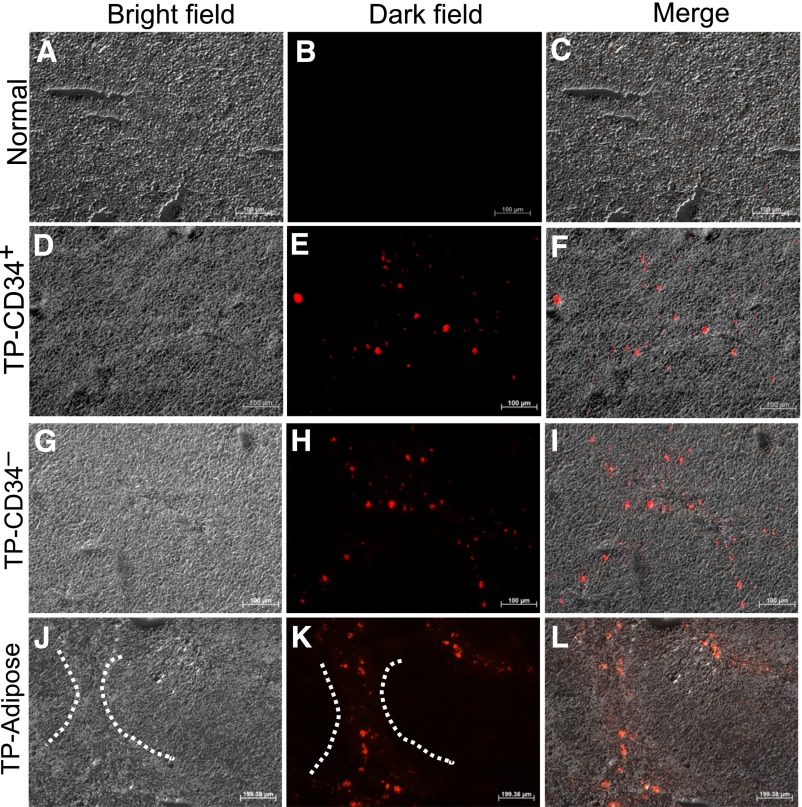
Three weeks after hMSC transplantation, PKH26-labeled stem cells were found around the portal tracts, in the fibrotic areas and around inflammatory sites of the liver (Fig. 6). These observations suggest that the transplanted cells had migrated through the blood circulation from the spleen into the sinusoid and liver parenchymal tissue.
Figure 6.
Engraftment of human mesenchymal stromal cells in the thioacetamide (TAA)-injured mice liver. The frozen sections of normal liver (A–C), CD34+ amnion membrane-derived stem/progenitor cells (AMSPC) liver (D–F), CD34− amnion membrane-derived stromal fibroblast cell (AMSFC) liver (G–I), and adipose tissue-derived mesenchymal stem cells (ADMSC) liver (J–L) were shown under a bright field (A, D, G, J), a fluorescent field (B, E, H, K), and a merged field (C, F, I, L). PKH26-labeled CD34+ AMSPCs, CD34− AMSFCs, and ADMSCs engrafted into TAA-injured mice tissues were shown in (E), (H), and (K). The dashed lines in (J) and (K) showed the exemplified bridging fibrosis area of the stem/progenitor cells homing. Scale bar = 100 μm. Abbreviation: TP, transplantation.
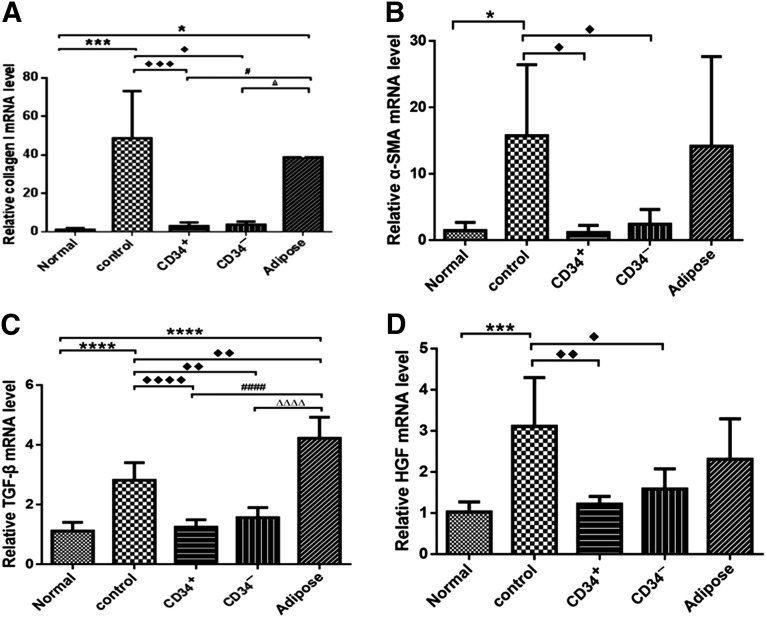
To further determine whether hMSCs can induce regeneration of damaged liver tissues, we analyzed the expressions of Col I, α-SMA, TGF-β, and HGF, which are markers of liver damage, in the livers of TP and non-TP mice (Fig. 7). The expressions of both Col I and α-SMA were dramatically increased in TAA-injured livers in comparison with those in the normal group. Col I and α-SMA mRNA expressions were significantly lower in CD34+ AMSPC and CD34− AMSFC mice than in non-TP animals at 3 weeks posttransplantation (p < .05; Fig. 7A, 7B). α-SMA is a marker of activated hepatic stellate cell (HSC). Thus, human amnion membrane- derived MSC transplantation has inhibited the activation of HSC. TGF-β mRNA expression was also significantly lower in hAM-MSCs TP mice than in non-TP mice at 3 weeks posttransplantation (p < .05; Fig. 7C). TGF-β has been shown to activate hepatic stellate cells to stimulate Col I synthesis by inducing the transcription factor Snail1 and activating Smad2/3 signaling [45]. On the basis of these data, we concluded that activation of hepatic stellate cells was suppressed in mice with hAM-MSC transplantation. When liver damage occurs, HGF is activated to stimulate hepatocyte to replicate and inhibit apoptosis. HGF expression was downregulated in hAM-MSC TP groups in comparison with the control, as shown in Figure 7D, indicating that the liver damage had been alleviated through hAM-MSC transplantation. The effect of ADMSC transplantation did not reach statistically significant levels in three of the four genes that we investigated.
Figure 7.
The effects of human mesenchymal stromal cell transplantation on the mRNA expressions of Col I (A), α-smooth muscle actin (B), transforming growth factor-β (C), and hepatocyte growth factor (D) in the livers of thioacetamide-injured mice. The relative mRNA levels were measured by quantitative reverse-transcription polymerase chain reaction using glyceraldehyde-3-phosphate dehydrogenase as endogenous control. One-way analysis of variance, followed by Tukey’s multiple-comparisons test, was used to analyze the data; statistical significance was identified by adjusted p value; p < .05 was considered statistically different. ♦♦♦♦, ∗∗∗∗, ####, and △△△△, p < .0001; ♦♦♦ and ∗∗∗, p < .001; ♦♦, p < .01; ♦, ∗, #, and △, p < .05. Abbreviations: α-SMA, α-smooth muscle actin; Adipose, transplantation with adipose tissue-derived mesenchymal stem cells; CD34+, transplantation with CD34+ amnion membrane-derived stem/progenitor cells; CD34−, transplantation with CD34− amnion membrane-derived stromal fibroblast cell; Control, nontransplantation; HGF, hepatocyte growth factor; TGF-β, transforming growth factor-β.
Discussion
HSC activation is the earliest step in hepatic fibrogenesis, collagen deposition, and progressive fibrosis leading to cirrhosis. When activated, HSCs transform into myofibroblast-like cells, the major producers of extracellular matrix collagen in the liver. The expression of α-SMA is a reliable and widely used marker for the activation of HSCs [46, 47]. In this study, we investigated the effect of three sets of MSC transplantation on HSC activation in TAA-injured mice. Our results have shown that both CD34+ AMSPCs and CD34− AMSFCs dramatically inhibited HSC activation after transplantation, as indicated by the downregulated expressions of α-SMA and Col I.
BM-MSCs had been shown to engraft into damaged tissues of bone marrow, lung, liver, heart, and brain and to facilitate the recovery of tissue function by reducing inflammation and remodeling the tissue [48, 49]. Although a significant reversion of liver fibrosis was observed in CCl4-injured rats treated with BM-MSCs [4], the underlying mechanism remains poorly understood. Furthermore, some reports claimed that BM-MSCs had no therapeutic effect on liver injury in a rat model [50]. They had the potential to transform into fibrogenic liver cells [51] and may be involved in the development of cancer [52]. However, MSCs derived from the umbilical cord had shown therapeutic potential in a rat model of liver fibrosis [53].
In this TAA-injured mice study, we investigated the effect of transplantation of CD34+ AMSPCs, which express stem cell markers and embryonic antigens, display multidifferentiation potentials, and show ability to diminish the fibrosis in the injured liver. The major findings are as follows [1]: The administration of neonatal amnion stem cells into TAA-induced fibrosis mouse by the spleen delayed disease progression and restored liver function [2]; human amnion stem cell transplantation inhibited the HSC activation [3]; transplanted hMSCs migrated into injured liver and restored hepatic albumin expression [4]. The therapeutic effect of CD34+ AMSPCs in the fibrotic liver was slightly better than that of CD34− AMSFCs. The antifibrotic effect of the CD34+ AMSPC transplantation was proven to alleviate the injury in the mice with severely damaged livers. It is worth noting that ADMSCs have been reported to exert beneficial effects in other animal models of acute liver failure when infused through the tail vein [54]. Administration through the spleen may not be the ideal route for ADMSCs. This could possibly explain the limited therapeutic benefits we observed in our study for ADMSC transplantation.
Of note, the CD34-sorted MSPCs coexpress multiepitopes GloboH_CD133_CD117_CD144_CD201 surface markers, characteristic of the stem/progenitor cells, indicating their highly proliferative and multipotent differentiation potential. These findings suggest that CD34+ AMSPCs may mediate the healing process of damaged hepatocytes and improve the function of the injured liver through antifibrotic effects. Taken together, CD34+ AMSPCs would be a superior cell source for transplantation in treating liver disease associated with fibrosis.
Two major mechanisms are proposed to be underlying the therapeutic effect of CD34+ AMSPC transplantation on the hepatic fibrosis: replacement of damaged cells and local delivery of bioactive molecules [55]. Replacement of cells is the goal of cell therapy for regenerative medicine, and the release of biological signals, as mediators or receptors, is the basis for many cellular processes, including immunomodulation induced by mesenchymal stem cells. Study reported that the hepatic progenitors can be raised by exposing the MSPCs to the hepatic progenitor factors such as Hnf4a and Faxa [56]. Our study has also shown that CD34+ AMSPCs can differentiate into hepatocyte-like cells in vitro, indicating their potential contribution to replace the damaged liver cells. MSC-derived growth factors and cytokines have been extensively investigated [57]. These MSC-derived bioactive molecules exert a wide range of functions, including anti-inflammatory (IL-1R antagonist), liver regeneration (HGF), reducing hepatocyte apoptosis (vascular endothelial growth factor and insulin-like growth factor-binding protein), suppressing HSC activation (tumor necrosis factor-α [TNF-α] and nerve growth factor), and degrading ECM (MMP9). It is very likely that both mechanisms attributed to the reported antifibrotic effects after infusion of allogeneic MSCs.
Liver fibrosis is caused by various injuries. After injury, blood mononuclear cells migrate to the injured site. The activated monocytes may differentiate into M1 macrophages and dendritic cells. Along with recruited neutrophils, they phagocytose the dead cells and debris. In coordination with pericyte activation, they transform into myofibroblasts by secreting TGF-β, platelet-derived growth factor subunit B, TNF-α, and Galactin-3 to initiate tissue repairing. After the innate and adaptive immune system insults and M1-to-M2 conversion, the MSCs are recruited to repair and regenerate the damaged tissue. Blood monocytes are converted into EPCs. Together with MSCs, they facilitate the neurogenic and vascular regeneration under the EGF stimulation, mediated by the plasma cytokines [58]. Thus, the antifibrotic regenerative effect involves the integration of both circulating hematopoietic and stromal stem/progenitor cells.
Conclusion
In conclusion, our current study has demonstrated that the multipotent neonatal CD34+ AMSPCs are a superior cell candidate for application in regenerative medicine. Their transplantation can effectively ameliorate hepatic fibrosis and restore liver function to normal by deactivating hepatic stellate cells and reducing the net collagen deposition. These findings will contribute to the development of safe and effective therapeutic treatment for liver injury associated with fibrosis and potentially other inflammatory diseases.
Supplementary Material
Acknowledgments
This research was funded in part by grants from NSC 103-2314-B-002-125-MY2 (principal investigator, P.-H.L.) and NSC 101-2314-B-038-039-MY2 and SL&T Ltd. (principal investigator, D.T.-b.S.). We thank Zhiqin Zou for editing the manuscript.
Author Contributions
P.-H.L.: conception and design, financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript; C.-T.T. and C.-C.H.: collection and assembly of data, data analysis and interpretation, manuscript writing; M.-S.T. and C.-M.H.: provision of study materials, final approval of manuscript; N.-C.C.: provision of study materials (Ad-MSC), final approval of manuscript; T.-M.H.: conception and design, grant application, final approval of manuscript; D.T-b.S.: conception and design, financial support, administrative support, provision of study materials, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Crawford JM. Pathologic basis of disease: The liver and biliary tract. Philadelphia, PA: Saunders; 1999. pp. 845–891. [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304–1311. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 5.Chernykh ER, Starostina NM, Paltsev AI, et al. Autologous bone marrow cells in the treatment of cirrhosis of the liver. Bull Exp Biol Med. 2007;144:640–645. doi: 10.1007/s10517-007-0393-3. [DOI] [PubMed] [Google Scholar]
- 6.Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431–3440. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 8.Oyagi S, Hirose M, Kojima M, et al. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Mohamadnejad M, Namiri M, Bagheri M, et al. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13:3359–3363. doi: 10.3748/wjg.v13.i24.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. 2008;135:438–450. doi: 10.1053/j.gastro.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Salama H, Zekri AR, Zern M, et al. Autologous hematopoietic stem cell transplantation in 48 patients with end-stage chronic liver diseases. Cell Transplant. 2010;19:1475–1486. doi: 10.3727/096368910X514314. [DOI] [PubMed] [Google Scholar]
- 12.Hüttmann A, Li CL, Dührsen U. Bone marrow-derived stem cells and “plasticity”. Ann Hematol. 2003;82:599–604. doi: 10.1007/s00277-003-0713-2. [DOI] [PubMed] [Google Scholar]
- 13.Stolzing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Bailo M, Soncini M, Vertua E, et al. Engraftment potential of human amnion and chorion cells derived from term placenta. Transplantation. 2004;78:1439–1448. doi: 10.1097/01.tp.0000144606.84234.49. [DOI] [PubMed] [Google Scholar]
- 15.Zhao P, Ise H, Hongo M, et al. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79:528–535. doi: 10.1097/01.tp.0000149503.92433.39. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji H, Miyoshi S, Ikegami Y, et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res. 2010;106:1613–1623. doi: 10.1161/CIRCRESAHA.109.205260. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One. 2011;6:e16789. doi: 10.1371/journal.pone.0016789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikane S, Hosoda H, Yamahara K, et al. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation. 2013;96:697–706. doi: 10.1097/TP.0b013e31829f753d. [DOI] [PubMed] [Google Scholar]
- 19.Marcus AJ, Coyne TM, Rauch J, et al. Isolation, characterization, and differentiation of stem cells derived from the rat amniotic membrane. Differentiation. 2008;76:130–144. doi: 10.1111/j.1432-0436.2007.00194.x. [DOI] [PubMed] [Google Scholar]
- 20.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 21.Katz AJ, Tholpady A, Tholpady SS, et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 22.Prunet-Marcassus B, Cousin B, Caton D, et al. From heterogeneity to plasticity in adipose tissues: Site-specific differences. Exp Cell Res. 2006;312:727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Banas A, Teratani T, Yamamoto Y, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 24.Patel AN, Genovese J. Potential clinical applications of adult human mesenchymal stem cell (Prochymal®) therapy. Stem Cells Cloning. 2011;4:61–72. doi: 10.2147/SCCAA.S11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mias C, Lairez O, Trouche E, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27:2734–2743. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 26.Wu HJ, Yiu WH, Li RX, et al. Mesenchymal stem cells modulate albumin-induced renal tubular inflammation and fibrosis. PLoS One. 2014;9:e90883. doi: 10.1371/journal.pone.0090883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton JA, Hudak KE, Chung EJ, et al. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells. 2013;31:2231–2241. doi: 10.1002/stem.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadi Gorji S, Karimpor Malekshah AA, Hashemi-Soteh MB, et al. Effect of mesenchymal stem cells on Doxorubicin-induced fibrosis. Cell J. 2012;14:142–151. [PMC free article] [PubMed] [Google Scholar]
- 29.Asanuma H, Vanderbrink BA, Campbell MT, et al. Arterially delivered mesenchymal stem cells prevent obstruction-induced renal fibrosis. J Surg Res. 2011;168:e51–e59. doi: 10.1016/j.jss.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kisseleva T, Gigante E, Brenner DA. Recent advances in liver stem cell therapy. Curr Opin Gastroenterol. 2010;26:395–402. doi: 10.1097/MOG.0b013e32833a6bec. [DOI] [PubMed] [Google Scholar]
- 31.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 32.Pan Q, Fouraschen SM, de Ruiter PE, et al. Detection of spontaneous tumorigenic transformation during culture expansion of human mesenchymal stromal cells. Exp Biol Med (Maywood) 2014;239:105–115. doi: 10.1177/1535370213506802. [DOI] [PubMed] [Google Scholar]
- 33.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Sidney LE, Branch MJ, Dunphy SE, et al. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem Cells. 2014;32:1380–1389. doi: 10.1002/stem.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DTB, Hsiao CC, Tsai MS. Identification and enrichment of ESC like clonogenic mesenchymal stem cells in human placenta (AM-cMSC) for regenerative medicine. Poster presented at 10th International Society of Stem Cell Research (ISSCR) Annual Meeting; June 18, 2012; Yokohama, Japan. [Google Scholar]
- Shih DTB, Hsiao CC, Hwang SM et al. Chemical enrichment of somatic tissue stromal CD 34 +MSCs for regenerative medicine. Poster presented at 12th International Society of Stem Cell Research (ISSCR) Annual Meeting; June 18, 2014; Vancouver, British Columbia, Canada. [Google Scholar]
- 37.Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: A property of the mesenchymal cells. Biol Reprod. 1996;55:1253–1260. doi: 10.1095/biolreprod55.6.1253. [DOI] [PubMed] [Google Scholar]
- 38.Whittle WL, Gibb W, Challis JR. The characterization of human amnion epithelial and mesenchymal cells: The cellular expression, activity and glucocorticoid regulation of prostaglandin output. Placenta. 2000;21:394–401. doi: 10.1053/plac.1999.0482. [DOI] [PubMed] [Google Scholar]
- 39.Moore RM, Silver RJ, Moore JJ. Physiological apoptotic agents have different effects upon human amnion epithelial and mesenchymal cells. Placenta. 2003;24:173–180. doi: 10.1053/plac.2002.0886. [DOI] [PubMed] [Google Scholar]
- 40.Cheng N-C, Wang S, Young T-H. The influence of spheroid formation of human adipose-derived stem cells on chitosan films on stemness and differentiation capabilities. Biomaterials. 2012;33:1748–1758. doi: 10.1016/j.biomaterials.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 41.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smits AM, van Laake LW, den Ouden K, et al. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res. 2009;83:527–535. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
- 43.Nanji AA, Mendenhall CL, French SW. Beef fat prevents alcoholic liver disease in the rat. Alcohol Clin Exp Res. 1989;13:15–19. doi: 10.1111/j.1530-0277.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 44.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47:598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Kaimori A, Potter J, Kaimori JY, et al. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem. 2007;282:22089–22101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 46.Paik YH, Kim JK, Lee JI, et al. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut. 2009;58:1517–1527. doi: 10.1136/gut.2008.157420. [DOI] [PubMed] [Google Scholar]
- 47.Roderfeld M, Rath T, Voswinckel R, et al. Bone marrow transplantation demonstrates medullar origin of CD34+ fibrocytes and ameliorates hepatic fibrosis in Abcb4-/- mice. Hepatology. 2010;51:267–276. doi: 10.1002/hep.23274. [DOI] [PubMed] [Google Scholar]
- 48.Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 49.Schmelzer E, Zhang L, Bruce A, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho AB, Quintanilha LF, Dias JV, et al. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells. 2008;26:1307–1314. doi: 10.1634/stemcells.2007-0941. [DOI] [PubMed] [Google Scholar]
- 51.Higashiyama R, Inagaki Y, Hong YY, et al. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2006;45:213–222. doi: 10.1002/hep.21477. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz Y, Lazova R, Qumsiyeh M, et al. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant. 2005;35:1021–1024. doi: 10.1038/sj.bmt.1704939. [DOI] [PubMed] [Google Scholar]
- 53.Tsai PC, Fu TW, Chen YM, et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484–495. doi: 10.1002/lt.21715. [DOI] [PubMed] [Google Scholar]
- 54.Deng L, Liu G, Wu X, et al. Adipose derived mesenchymal stem cells efficiently rescue carbon tetrachloride-induced acute liver failure in mouse. Sci World J . 2014;10:1155–1163. doi: 10.1155/2014/103643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genovese JA, Spadaccio C, Rivello HG, et al. Electrostimulated bone marrow human mesenchymal stem cells produce follistatin. Cytotherapy. 2009;11:448–456. doi: 10.1080/14653240902960445. [DOI] [PubMed] [Google Scholar]
- 56.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 57.Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med. 2015;30:580–589. doi: 10.3904/kjim.2015.30.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.