Abstract
Study Objectives:
Prospective evidence on the association of sleep duration and midday napping with metabolic syndrome (MetS) is limited. We aimed to examine the associations of sleep duration and midday napping with risk of incidence and reversion of MetS and its components among a middle-aged and older Chinese population.
Methods:
We included 14,399 subjects from the Dongfeng-Tongji (DFTJ) Cohort Study (2008–2013) who were free of coronary heart disease, stroke, and cancer at baseline. Baseline data were obtained by questionnaires and health examinations. Odds ratios (ORs) and 95% confidence interval (CI) were derived from multivariate logistic regression models.
Results:
After controlling for potential covariates, longer sleep duration (≥ 9 h) was associated with a higher risk of MetS incidence (OR, 1.29; 95% CI, 1.08–1.55) and lower reversion of MetS (OR, 0.80; 95% CI, 0.66–0.96) compared with sleep duration of 7 to < 8 h; whereas shorter sleep duration (< 6 h) was not related to incidence or reversion of MetS. For midday napping, subjects with longer napping (≥ 90 min) was also associated with a higher risk of MetS incidence and a lower risk of MetS reversion compared with those with napping of 1 to < 30 min (OR, 1.48; 95% CI, 1.05–2.10 and OR, 0.70; 95% CI, 0.52–0.94, respectively). Significance for incidence or reversion of certain MetS components remained in shorter and longer sleepers but disappeared across napping categories.
Conclusions:
Both longer sleep duration and longer midday napping were potential risk factors for MetS incidence, and concurrently exert adverse effects on MetS reversion.
Citation:
Yang L, Xu Z, He M, Yang H, Li X, Min X, Zhang C, Xu C, Angileri F, Légaré S, Yuan J, Miao X, Guo H, Yao P, Wu T, Zhang X. Sleep duration and midday napping with 5-year incidence and reversion of metabolic syndrome in middle-aged and older Chinese. SLEEP 2016;39(11):1911–1918.
Keywords: incidence, metabolic syndrome, midday napping, reversion, sleep duration
Significance.
The current study identifies significant associations of longer sleep duration and midday napping with risk of MetS incidence and reversion among middle-aged and older Chinese. Independent of other risk factors, longer sleepers (≥ 9 h) or longer nap takers (≥ 90 min) exhibited 29% or 48% increased risk of MetS incidence as well as 20% or 30% lower MetS reversion, compared with individuals who reported sleeping 7 to < 8 h or napping 1 to < 30 min. No significant associations were observed between shorter sleep duration (< 6 h) and incidence or reversion of MetS. Our findings underscore the importance of appropriate sleep and nap in the prevention and reversal of MetS.
INTRODUCTION
Metabolic syndrome (MetS) is characterized by a set of cardiomet abolic risk factors, including central obesity, dyslipidemia, elevated blood pressure, and impaired fasting glucose.1 This condition confers an increased risk of cardiovascular diseases, type 2 diabetes, and all-cause mortality.2–4 In China, the prevalence of MetS is increasing and MetS becomes a major public health concern. The International Collaborative Study of Cardiovascular Disease in Asia reported that the prevalence of MetS was approximately 13.7% among Chinese adults aged 35–74 y.5 It is therefore important to identify the potential risk factors and provide management strategies.
Both sleep and midday napping are important health-related factors and closely linked to hormonal and metabolic systems.6,7 A growing body of epidemiological evidence showed that shorter (< 6 h) or longer sleep duration (≥ 9 h) and midday napping (more than 1 h) are independently related to increased risks of MetS and/or its components.8–10 However, most of the previous studies are cross-sectional and several prospective studies on the relation of sleep duration with MetS incidence have produced heterogeneous findings. Three studies conducted in Korea and Japan indicated a higher risk of MetS incidence in shorter sleepers, but not in longer sleepers,11–13 while a recent study reported a U-shaped relationship between sleep hours and the risk of MetS incidence in the general Chinese population.14 However, the relationship of midday napping with incident MetS has not been confirmed in longitudinal studies. Furthermore, no study has examined the relationship of sleep duration or midday napping on the reversion of MetS and its components.
Therefore, we aimed to explore whether sleep duration or midday napping was independently associated with incidence and reversion of MetS and its components over a 5-y period in a middle-aged and older Chinese population.
METHODS
Study Population
Our research was derived from the Dongfeng-Tongji (DFTJ) Cohort Study launched in 2008 among retired workers of Dongfeng Motor Corporation (DMC) in Shiyan city, Hubei province. The details of the DFTJ cohort have been described previously.15 Briefly, the primary aim of the DFTJ cohort was to investigate a wide range of lifestyle, dietary, psychosocial, occupational, environmental, and biochemical factors in relation to the development of chronic diseases. The Retirement Office and the Social Insurance Center at DMC provided a list of the retired workers and invited them to participate in this study. All retired employees are covered by DMC's health-care service system and each participant has a unique medical insurance card number and ID. A total of 27,009 retirees were included in the cohort and agreed to answer questionnaires and performed health examinations between September 2008 and June 2010. Five years later, the participants were invited to the follow-up investigation via telephone. At the follow-up survey, participants repeated questionnaire interview, physical examinations, and blood collection as those in the baseline survey. Among 25.978 individuals (96.2% of those at baseline) who completed the follow-up until October, 2013, we excluded individuals with self-reported coronary heart disease, stroke, or cancer at baseline (n = 5,865) as those with these chronic conditions may spend more time sleeping or napping and thus overestimate the sleep/nap/MetS associations.8 In addition, we also excluded those with missing information on sleep duration at baseline (n = 101), or components of MetS at baseline and during the follow-up (n = 5,613). Because the amount of missing data varied for each component of MetS, to preserve statistical power as much as possible, we used all available data for individual component analyses. Thus, the sample size differed for each component of MetS incidence: n = 8,904 for central obesity, 8,500 for hypertriglyceridemia, 8,249 for reduced high-density lipoprotein (HDL) cholesterol, 6,696 for elevated blood pressure, 7,124 for impaired fasting glucose; and the sample size also differed for each component of MetS reversion: n = 4,111 for central obesity, 3,919 for hypertriglyceridemia, 3,578 for reduced HDL cholesterol, 4,429 for elevated blood pressure, and 4,138 for impaired fasting glucose. The final analysis sample consisted of 14,399 subjects (9,275 without MetS and 5,124 with MetS at baseline). Compared to the analytical sample, the excluded subjects were older, tended to be males, had longer sleep duration and midday napping, poorer sleep quality, and higher levels of waist circumference, triglycerides, systolic blood pressure, diastolic blood pressure, and fasting blood glucose (Table S1 in the supplemental material). The study was approved by the Ethics and Human Subject committee of Tongji Medical College, and Dongfeng General Hospital, DMC. All study participants provided informed consent.
Assessment of MetS
The MetS was defined using the consensus statement as the presence of three or more of the following16: (1) central obesity: waist circumference ≥ 90 cm in men or ≥ 80cm in women; (2) hypertriglyceridemia: triglycerides ≥ 1.7 mmol/L (drug treatment for elevated triglycerides is an alternate indicator); (3) reduced HDL cholesterol levels: HDL cholesterol < 1.0 mmol/L in men, or < 1.3 mmol/L in women (drug treatment for reduced HDL cholesterol is an alternate indicator); (4) elevated blood pressure: blood pressure ≥ 130/85 mmHg or current use of antihypertensive medications; or (5) impaired fasting glucose: fasting glucose ≥ 5.6 mmol/L or the use of diabetes medication.
Incidence of MetS was defined as not having MetS at baseline but having MetS after 5 y of follow-up, whereas reversion was defined as having MetS at baseline but not at the 5-y assessment.
Assessment of Sleep Duration and Midday Napping
Sleep duration was assessed by asking: “What time did you usually go to sleep at night and wake up in the morning over the previous 6 mo?” We categorized sleep duration into five groups: < 6 h, 6 to < 7 h, 7 to < 8 h, 8 to < 9 h, ≥ 9 h. Habit nap takers were defined as those who had taken a planned or regular nap as a habit more than three times per week after lunch over the past 6 mo. Midday napping was assessed by asking “Did you have a habit of taking midday nap over the previous 6 mo?” Those who responded ‘yes’ were further asked about the duration of their naps. Midday napping was thereby categorized as no napping (0 min), 1 to < 30 min, 30 to < 60 min, 60 to < 90 min, and ≥ 90 min. Additionally, we computed total sleep time by summing up nighttime sleep duration and midday napping, and we also categorized it into 5 groups: < 7.5 hours, 7.5 to < 8.5 hours, 8.5 to < 9.5 hours, 9.5 to < 10.5 hours, ≥ 10.5 h.
Assessment of Covariates
Semistructured questionnaires were used to collect information on demographics (age, sex, education, and marital status), diets, lifestyle habits such as smoking status (current, former, never), drinking status (current, former, never) and physical activity, occupational history, environmental exposures, and family and medical histories. Participants who were smoking at least one cigarette per day for more than half a year were considered as current smokers; those who were drinking at least one time per week for more than half a year were defined as current drinkers. Physical activity was defined as regular exercise more than 20 min per day over the past 6 mo.17 Participants were also asked to report the average times spent per week and the average hours spent per time on physical activity. The duration of physical activity (hours per week) was calculated according to the following formula: frequency (times per week) × duration (hours per time). Dietary data were categorized as six major food groups: eggs, meat, fish, fruits, vegetables, and beans. Participants were asked about the frequency of habitual consumption during the previous 12 mo and selected among four categories of frequency (times per day, times per week, times per month, and times per year). The frequency was transformed into times per week in the subsequent analysis. Sleep quality was assessed by asking whether the individuals experienced any of the following three types of sleep problems: experiencing difficulties in falling asleep lasting for 30 min or longer, waking up frequently during the night, or waking up too early in the morning and being unable to get back to sleep.18 Participants were asked to choose from three responses: never, one to two times per week, and three or more times per week, which corresponded to good, fair, and poor sleep quality, respectively. Standing height, body weight, and waist circumference were measured with subjects in light indoor clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Fasting blood in the morning was drawn before physical examinations using standardized procedures. Blood lipids [total cholesterol, triglycerides, HDL-cholesterol, and low-density lipoprotein (LDL) cholesterol], fasting glucose, hepatic function, renal function, a complete blood count, and tumor-associated antigens were measured specifically for the DFTJ cohort study at the hospital's laboratory.
Statistical Analysis
The distributions of the numerical data were assessed by the one-sample Kolmogorov-Smirnov test. The differences of baseline characteristics between groups according to MetS incidence and reversion status were evaluated by t-test for continuous variables and chi-square test for categorical variables. Multivariate logistic regression models were used to examine the associations of sleep duration and midday napping with incidence and reversion of MetS and of its components calculated with odds ratios (ORs) and 95% confidence interval (95% CI). We performed two separate analyses: the first on the incidence of MetS in participants who did not have the condition at baseline, and the second on reversion of MetS in participants who had the condition at baseline. We used 7 to < 8 h of nighttime sleep duration and 1 to < 30 min of midday napping as the reference groups based on published literature showing their beneficial effects for adults.19,20 Potential confounders were adjusted including age, sex, BMI, education (primary school or below, middle school, high school or beyond), smoking status (never/former/current), drinking status (never/former/current), physical activity (hours per week), sleep quality (good/fair/ poor), use of hypnotics (yes/no), and diet frequency (times per week, for eggs, meat, fish, fruits, vegetables, and beans). We further adjusted for midday napping for the sleep-MetS incidence or reversion relationships and nighttime sleep duration for the nap-MetS incidence or reversion relationships. All statistical analysis was performed using SAS version 9.3 (SAS institute Inc., Cary, NC) and the significance tests were two-tailed with P values below 0.05.
RESULTS
Table 1 shows the baseline characteristics of participants according to changes in the MetS status. The mean age of the study population at baseline was 62.5 ± 7.4 y, and the majority are females (56.5%). Among the 9,275 participants having no MetS at baseline, 2,447 (26.4%) occurred at follow-up, and among the 5,124 participants with MetS at baseline, 1,462 (28.5%) recovered by follow-up. Participants with MetS incidence were more likely to be female and overweight than those without MetS. In contrast, participants with MetS reversion tended to be male and of normal weight than those with MetS at baseline. As expected, the changes of all components contributing to MetS varied significantly by different MetS incidence and reversion status.
Table 1.
Baseline characteristics and 5-y changes in metabolic syndrome components among the study population.
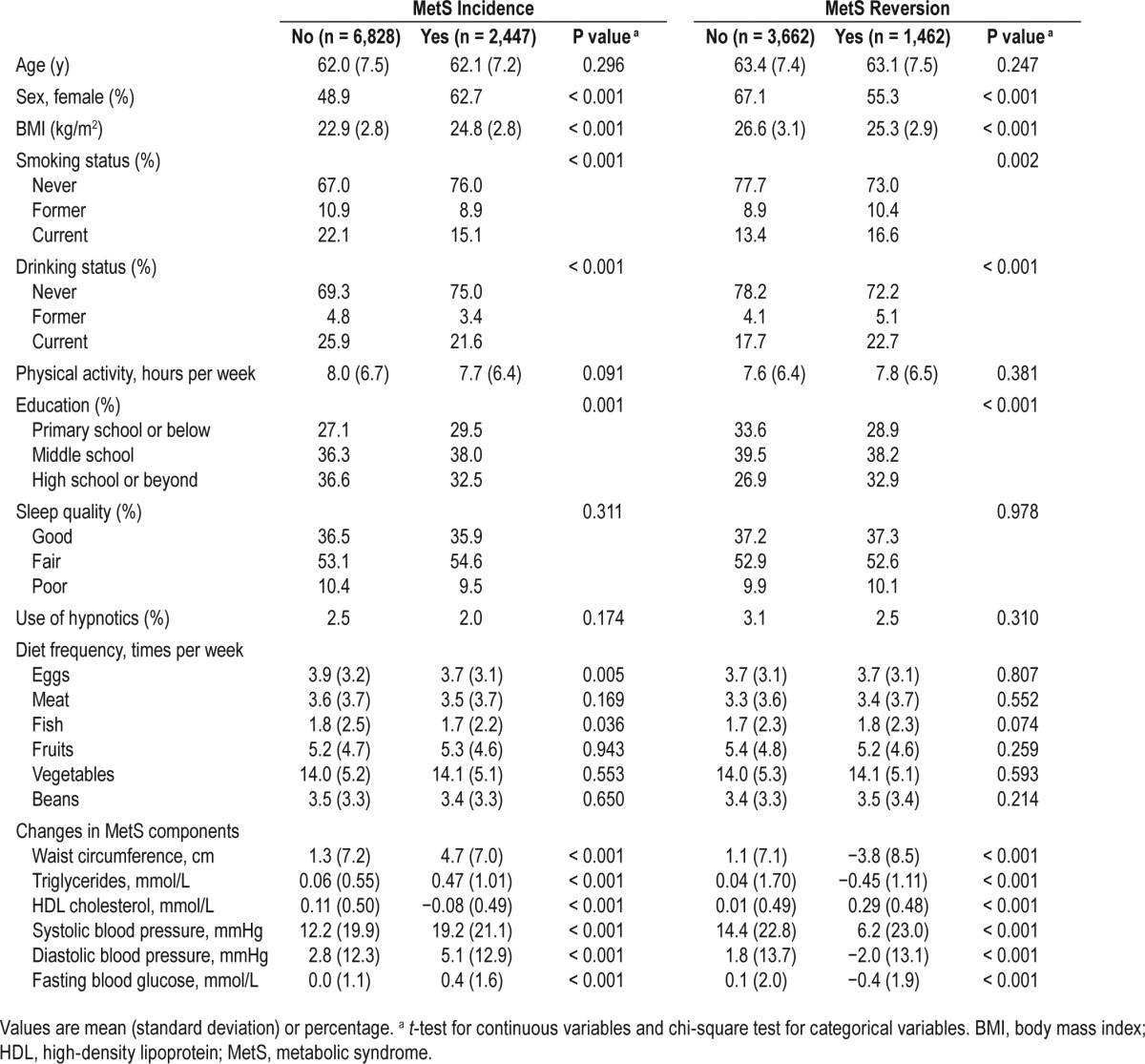
Table 2 lists the adjusted ORs for the incident risk of MetS and its individual components by categories of nighttime sleep duration. After adjustment for potential confounders, we found that longer nighttime sleep duration was significantly associated with an increased risk of incident MetS. Compared with the reference group of 7 to < 8 h per night, the adjusted OR (95% CI) for MetS incidence was 1.29 (1.08–1.55) for sleeping ≥ 9 h per night, while other groups of sleep duration were not significantly related to MetS incidence. Analyses of the individual MetS components revealed that both shorter (< 6 h) and longer (≥ 9 h) nighttime sleep duration were associated with a greater risk of developing central obesity compared with sleep duration of 7 to < 8 h per night, and the ORs were 2.36 (95% CI, 1.32–4.23) and 1.28 (95% CI, 1.03–1.60), respectively; associations between other components and nighttime sleep duration categories were not significant.
Table 2.
Odds ratio for the 5-y incidence of metabolic syndrome and its individual components by nighttime sleep duration.
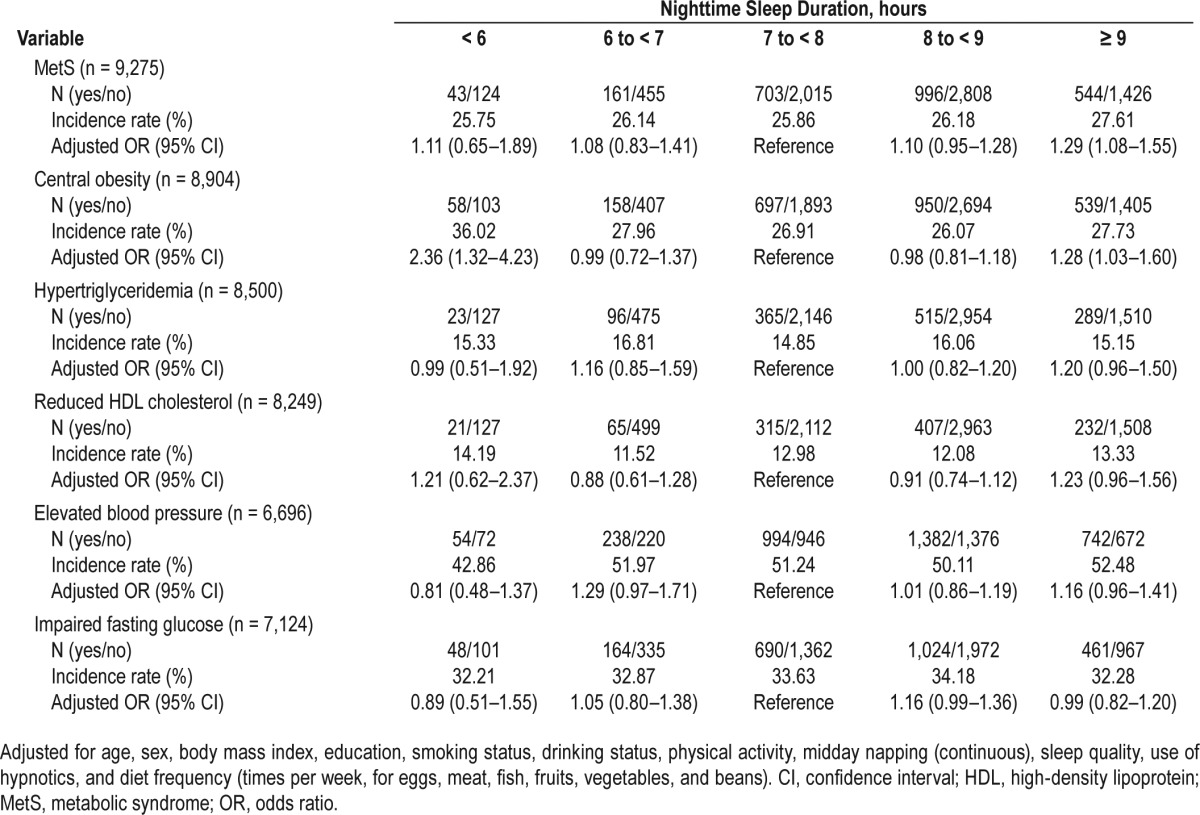
As shown in Table 3, longer nighttime sleep duration ≥ 9 h was also associated with lower reversion of MetS. The OR (95% CI) for the 5-y MetS reversion in individuals with nighttime sleep duration ≥ 9 h compared with those of 7 to < 8 h was 0.80 (0.66–0.96). With respect to the components of MetS, longer sleepers (≥ 9 h) showed lower reversion of central obesity (OR, 0.73; 95% CI, 0.56–0.94) and hypertriglyceridemia (OR, 0.80; 95% CI, 0.66–0.96); while shorter sleepers (< 6 h) were less likely to revert reduced HDL cholesterol (OR, 0.54; 95% CI, 0.31–0.93).
Table 3.
Odds ratios for the 5-y reversion of metabolic syndrome and its individual components by nighttime sleep duration.
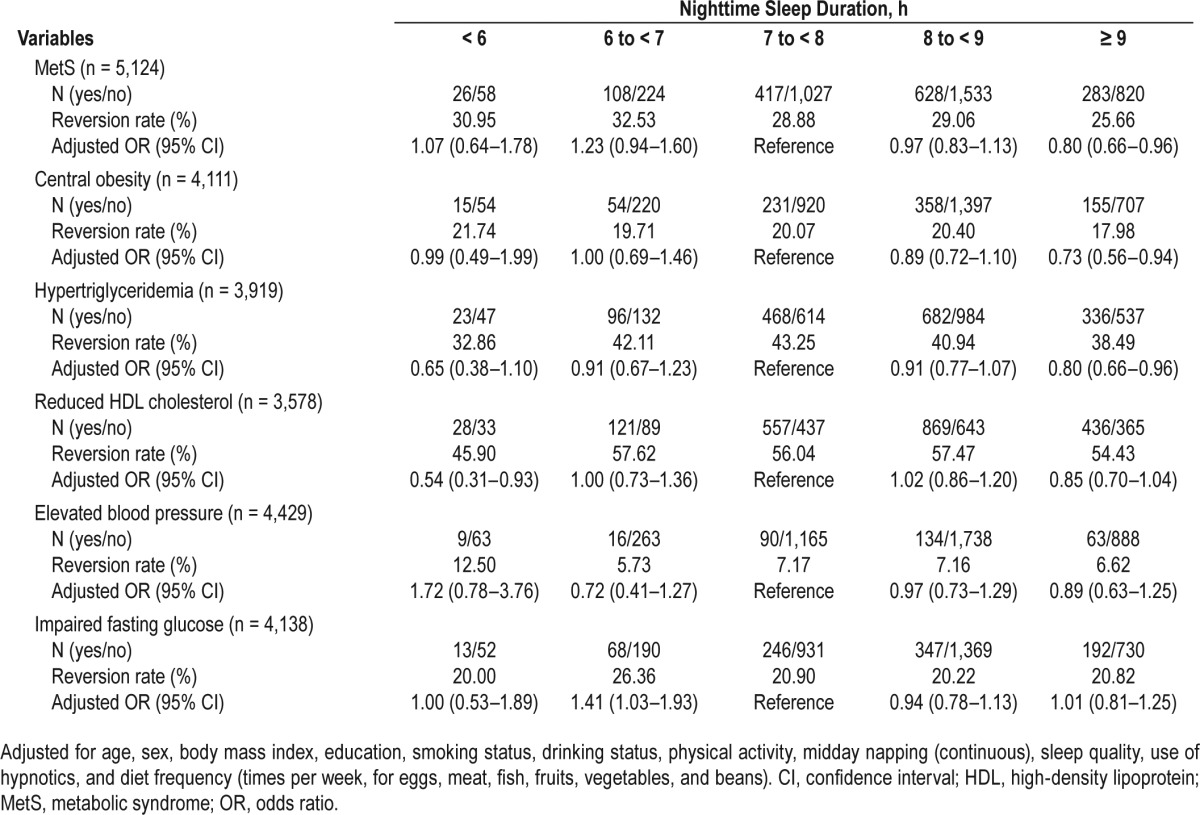
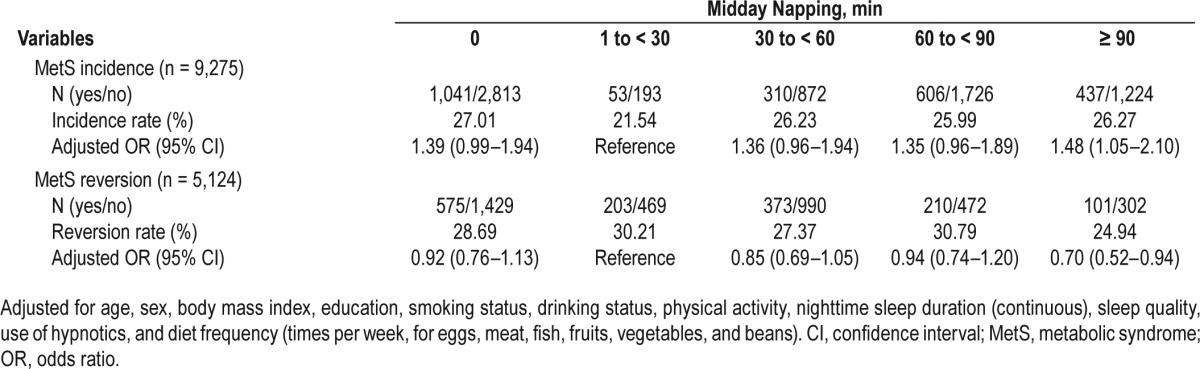
Compared to subjects who napped for 1 to < 30 min, subjects with no napping and napping ≥ 90 min exhibited a 39% and 48% increased risk of MetS incidence, and the ORs were 1.39 (95% CI, 0.99–1.94) and 1.48 (95% CI, 1.05–2.10), respectively, whereas longer nap takers who napped ≥ 90 min had a 30% decreased risk of MetS reversion (OR, 0.70; 95% CI, 0.52–0.94), respectively (Table 4). No statistically significant associations were detected between midday napping and the 5-y incidence and reversion of all MetS components (Table S2 and Table S3 in the supplemental material).
Table 4.
Odds ratios for the 5-y incidence and reversion of metabolic syndrome by midday napping.
Finally, we also examined the associations of total sleep time with incidence and reversion of MetS and its components in additional analyses. Compared with subjects reporting 7.5 to < 8.5 h of sleep per day, those with longer total sleep time (9.5 to < 10.5 h and ≥ 10.5 h) showed a higher incidence and a lower reversion of MetS, whereas those with longer total sleep time (≥ 10.5 h) exhibited a higher incidence and a lower reversion of central obesity (Table S4 and Table S5 in the supplemental material).
DISCUSSION
To the best of our knowledge, this is the first study to simultaneously study the relationships of sleep duration and midday nap with MetS incidence and reversion in middle-aged and older adults who have a high prevalence of MetS. Our findings indicated that the incidence of MetS was higher and the reversion of MetS was lower by longer sleep duration and midday napping.
Recently, Li et al. showed that both shorter (< 6 h) and longer (8–9 and ≥ 9 h) sleep duration were associated with a greater incidence of MetS in the general Chinese adult population aged 30–65 y.14 Our results supported the longitudinal relationship of longer sleep duration and MetS incidence with the larger sample size. Moreover, we excluded chronic conditions (such as self-reported coronary heart disease, stroke, and cancer at baseline) from analyses to rule out residual confounding due to chronic conditions, and to examine whether there was a genuine link between sleep duration or midday napping and MetS incidence. In addition, our findings are novel and strengthen evidence of the adverse effect of longer sleep duration on MetS reversion.
Regarding the components of MetS, the increased OR of central obesity associated with longer sleep duration is consistent with previously reported positive relations of longer sleep duration and adiposity.9,21 Central obesity is the most prevalent manifestation of MetS, and a growing number of evidence suggests a close correlation of increased abdominal fat with MetS and other related metabolic abnormalities.22,23 In long sleepers, the physical inactivity due to increased nighttime sleep may reduce energy expenditure, which may contribute to obesity and development of MetS.24 In agreement with a previous study reporting an OR of 1.13 (95% CI, 1.02–1.24) for elevated triglycerides among individuals with prolonged sleep duration,9 we also found that longer nighttime sleep duration (≥ 9 h) was significantly associated with lower reversion of hypertriglyceridemia. We then speculate that the effect of longer sleep duration on the incidence or reversion of MetS may be predominantly mediated through changes in abnormal fat distribution and dyslipidemia.
Shorter sleep duration (< 6 h) was not related to the incidence or reversion of MetS in the current study, which was in contrast with previous cohort reports.11–13 Choi et al.11 observed an association between shorter sleep duration (< 6 h) and MetS incidence among Korean females aged 40–55 y (n = 1,107). In another study of worksite males aged 35–63 y in Japan (n = 948), sleep deprivation was also related to an elevated risk of MetS incidence.12 Moreover, shorter sleep duration (< 6 h) was also an independent risk factor for incident MetS among 2,579 adults aged about 55 y in Korea.13 Our study consisted of retired employees who were generally older than those in the three former cohorts. Because of reduced participation in social or physical activities due to retirement, the elderly have more opportunities to go to bed earlier and sleep longer than younger adults. However, long sleep duration may not actually be long sleep as it could be long time in bed but with poor sleep quality. Self-reported sleep duration assessed by questionnaire did not allow us to differentiate time asleep from time in bed. The relatively small number of shorter sleepers (n = 251) may be one explanation for the absence of associations between shorter sleep duration and incidence or reversion of MetS. However, we observed that subjects who slept < 6 h per night exhibited higher incidence of central obesity and lower reversion of reduced HDL-cholesterol. Taheri et al.25 previously showed that short sleep duration was significantly associated with reduced leptin and elevated ghrelin levels, which would increase appetite and lead to the development of obesity. The preliminary findings related to short sleep duration and incidence or reversion of MetS and its components should be confirmed in future studies.
Our results further revealed that prolonged midday napping was also associated with an increased risk of MetS incidence and reduced reversion of MetS. Midday napping is a common practice in China, especially among older adults, and a short nap is traditionally considered as a healthy habit.26 However, conflicting results regarding the rationality of midday napping have been generated in epidemiologic studies. Some studies have reported that napping is associated with a higher risk for diabetes and all-causes mortality among older adults.27,28 In line with previous cross-sectional studies showing a positive relationship of longer napping with a higher risk for MetS,10,29 we found that excessive napping (≥ 90 min) was also an independent predictor of incident MetS and might exert an adverse effect on the MetS reversion. Nonetheless, the findings should be interpreted cautiously because we did not see any signifi-cant difference between napping categories and incidence or reversion of MetS components.
The potential mechanisms underlying these findings remain to be investigated. Longer sleep duration or napping could be related to disrupted circadian rhythms and/or hormonal alterations, which may predispose to insulin resistance, a key characteristic in MetS pathophysiology.9,30,31 In addition, chronic inflammation, generation of reactive oxygen species, and sympathetic activation may also play a central role in the relationship between excessive sleep or napping and MetS.32,33 Furthermore, it could be argued that longer sleep duration or midday napping may be a consequence of an underlying illness, and therefore the observed sleep/nap/MetS relationship may be caused by preexisting diseases.
Limitations
Some limitations of the current study should be addressed. First, self-reported sleep duration and midday napping were used in our analyses and they could have been prone to misclassification. Second, the measurements of snoring, obstructive sleep apnea, or neck circumference were not available in the questionnaire and physical examination, so we were unable to further adjust for these factors. Third, information on physical activity was obtained from questionnaires. Self-reported physical activity is also a major confounder that may be affected by bias (social desirability and recall bias, particularly in older individuals). Finally, the study was performed in middle-aged and older Chinese adults, and thus the findings cannot be extrapolated to other age groups or ethnics.
In conclusion, our data suggest that longer sleep duration (≥ 9 h) and midday napping (≥ 90 min) are independently associated with a higher risk of MetS incidence and lower reversion of MetS. The significant risks for incidence or reversion of certain components of MetS persist in shorter and longer sleep duration but diminish according to napping categories. Future prospective studies and randomized clinical trials using objective measures of sleep depth, continuity, and sleep disordered breathing are needed to corroborate these findings and to identify the biologic pathways by which sleep or nap affects the development or reversal of MetS.
DISCLOSURE STATEMENT
This was not an industry-supported study. This work was supported by the Natural National Scientific Foundation of China (NNSFC 81373093,81230069, and 81390542), 111 Project (No. B12004); and the Program for Changjiang Scholars; and Innovative Research Team in University of Ministry of Education of China (No. IRT1246); China Medical Board (No.12-113), and Outstanding Leaders Training Program of Pudong Health and Family Planning Commission of Shanghai (Grant No.PWRL2014-01). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: L. Yang, Z. Xu., X. Zhang analyzed the data, and wrote the manuscript. L. Yang, Z. Xu, H. Yang, X. Li, X. Min, C. Zhang, C. Xu contributed to the data collection. L. Yang, Z. Xu, M. He, F. Angileri, S. Légaré, J. Yuan, X. Miao, H. Guo, P. Yao, T. Wu, X. Zhang designed the study, contributed to the discussion, and reviewed and edited the manuscript. T. Wu, X. Zhang were the guarantors of this work, and as such, had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–90. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–14. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Gu D, Reynolds K, Wu X, et al. Prevalence of the metabolic syndrome and overweight among adults in China. Lancet. 2005;365:1398–405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 6.Van Cauter E, Holmback U, Knutson K, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- 7.Woods DL, Kim H, Yefimova M. To nap or not to nap: excessive daytime napping is associated with elevated evening cortisol in nursing home residents with dementia. Biol Res Nurs. 2013;15:185–90. doi: 10.1177/1099800411420861. [DOI] [PubMed] [Google Scholar]
- 8.Wu MC, Yang YC, Wu JS, Wang RH, Lu FH, Chang CJ. Short sleep duration associated with a higher prevalence of metabolic syndrome in an apparently healthy population. Prev Med. 2012;55:305–9. doi: 10.1016/j.ypmed.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Arora T, Jiang CQ, Thomas GN, et al. Self-reported long total sleep duration is associated with metabolic syndrome: the Guangzhou Biobank Cohort Study. Diabetes Care. 2011;34:2317–9. doi: 10.2337/dc11-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin D, Sun K, Li F, et al. Association between habitual daytime napping and metabolic syndrome: a population-based study. Metabolism. 2014;63:1520–7. doi: 10.1016/j.metabol.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Choi JK, Kim MY, Kim JK, et al. Association between short sleep duration and high incidence of metabolic syndrome in midlife women. Tohoku J Exp Med. 2011;225:187–93. doi: 10.1620/tjem.225.187. [DOI] [PubMed] [Google Scholar]
- 12.Otsuka T, Kawada T, Yanai M, Kitagawa Y, Kan H. [Incidence of metabolic syndrome and associated lifestyle factors in a worksite male population] Sangyo Eiseigaku Zasshi. 2011;53:78–86. doi: 10.1539/sangyoeisei.b10013. [DOI] [PubMed] [Google Scholar]
- 13.Kim JY, Yadav D, Ahn SV, et al. A prospective study of total sleep duration and incident metabolic syndrome: the ARIRANG study. Sleep Med. 2015;16:1511–5. doi: 10.1016/j.sleep.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Lin L, Lv L, et al. U-shaped relationships between sleep duration and metabolic syndrome and metabolic syndrome components in males: a prospective cohort study. Sleep Med. 2015;16:949–54. doi: 10.1016/j.sleep.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Zhu J, Yao P, et al. Cohort Profile: the Dongfeng-Tongji cohort study of retired workers. Int J Epidemiol. 2013;42:731–40. doi: 10.1093/ije/dys053. [DOI] [PubMed] [Google Scholar]
- 16.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto N, Nakatani T, Morita N, Saeki K, Kurumatani N. Home-based walking improves cardiopulmonary function and health-related QOL in community-dwelling adults. Int J Sports Med. 2007;28:1040–5. doi: 10.1055/s-2007-965073. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki E, Yorifuji T, Ueshima K, et al. Sleep duration, sleep quality and cardiovascular disease mortality among the elderly: a population-based cohort study. Prev Med. 2009;49:135–41. doi: 10.1016/j.ypmed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 20.Tamaki M, Shirota A, Hayashi M, Hori T. Restorative effects of a short afternoon nap (< 30 min) in the elderly on subjective mood, performance and eeg activity. Sleep Res Online. 2000;3:131–9. [PubMed] [Google Scholar]
- 21.Lopez-Garcia E, Faubel R, Leon-Munoz L, Zuluaga MC, Banegas JR, Rodriguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87:310–6. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 22.Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–49. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 23.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 24.Magee L, Hale L. Longitudinal associations between sleep duration and subsequent weight gain: a systematic review. Sleep Med Rev. 2012;16:231–41. doi: 10.1016/j.smrv.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan TY, Lan TH, Wen CP, Lin YH, Chuang YL. Nighttime sleep, Chinese afternoon nap, and mortality in the elderly. Sleep. 2007;30:1105–10. doi: 10.1093/sleep/30.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33:78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leng Y, Wainwright NW, Cappuccio FP, et al. Daytime napping and the risk of all-cause and cause-specific mortality: a 13-year follow-up of a British population. Am J Epidemiol. 2014;179:1115–24. doi: 10.1093/aje/kwu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Xu G, Shen L, et al. Daily sleep duration and risk of metabolic syndrome among middle-aged and older Chinese adults: cross-sectional evidence from the Dongfeng-Tongji cohort study. BMC Public Health. 2015;15:178. doi: 10.1186/s12889-015-1521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23:372–81. doi: 10.1016/j.cub.2013.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santos RV, Tufik S, De Mello MT. Exercise, sleep and cytokines: is there a relation? Sleep Med Rev. 2007;11:231–9. doi: 10.1016/j.smrv.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Mancia G, Bousquet P, Elghozi JL, et al. The sympathetic nervous system and the metabolic syndrome. J Hypertens. 2007;25:909–20. doi: 10.1097/HJH.0b013e328048d004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


