Abstract
Physical exercise reduces anxiety-like behavior in adult mice. The specific mechanisms that mediate this anxiolytic effect are unclear, but adult neurogenesis in the dentate gyrus has been implicated because it is robustly increased by running and has been linked to anxiodepressive-like behavior. We therefore tested the effects of long-term wheel running on anxiety-like behavior in GFAP-TK (TK) mice, a transgenic mouse with completely ablated neurogenesis. Five weeks of running reduced anxiety-like behavior equally in both TK mice and wild type (WT) control mice on two tests of anxiety-like behavior, elevated plus-maze and novelty-suppressed feeding. WT and TK mice also had similar patterns of c-fos expression in the hippocampus following anxiety testing. Following testing on the elevated plus-maze, running reduced c-fos expression in the dorsal dentate gyrus and CA3 in both WT and TK mice. Following testing on novelty-suppressed feeding, running reduced c-fos expression throughout the dentate gyrus and CA3 in both WT and TK mice. Interestingly, following testing on a less anxiogenic version of novelty-suppressed feeding, running also reduced c-fos expression in the dorsal dentate gyrus in both WT and TK mice, supporting earlier suggestions that the dorsal hippocampus is less important for emotional behavior than the ventral region. These results suggest that although running increases adult neurogenesis, new neurons are not involved in the decreased anxiety-like behavior or hippocampal activation produced by running.
Keywords: adult neurogenesis, anxiety, exercise, immediate early genes
Introduction
Physical exercise is anxiolytic in adult mice (Duman et al., 2008; Schoenfeld et al., 2014), however the specific mechanisms underlying its effects on anxiety are unclear. The hippocampus, and the ventral hippocampus in particular, is important for regulating mood (Bannerman et al., 2003). One possibility is that running, which robustly increases neurogenesis in the dentate gyrus of the hippocampus in adult mice (van Praag et al., 1999) may exert its anxiolytic effects through new neurons. Increases in neurogenesis are associated with positive effects of running on mood (Bjørnebekk et al., 2006). In addition, knockout of growth factors and neurotrophins that regulate adult neurogenesis prevent running-induced increases in adult neurogenesis and decreases in anxiodepressive-like behavior (Li et al., 2008; Trejo et al., 2008; Yau et al., 2014), suggesting that increases in adult neurogenesis may be functionally important for the anxiolytic actions of physical exercise. However, simply increasing adult neurogenesis does not reduce anxiety-like behavior in mice (Sahay et al., 2011). New neurons have been linked with improvements in learning following running (Clark et al., 2008; Yau et al., 2011), but the relationship between adult neurogenesis and the decreased anxiety-like behavior seen with running has not been directly tested.
To test the functional role of new neurons in the anxiolytic effects of exercise, we used GFAP-TK (TK) mice, a pharmacogenetic model for specifically inhibiting neurogenesis in adulthood (Snyder et al., 2011). TK mice and wild type (WT) littermate controls on a CD-1 background were group housed and given ad libitum access to food and water. Starting at eight-weeks of age, all mice were given valganciclovir (VGCV; p.o., 0.3% in powdered chow) to eliminate cell proliferation and immature neurons. After eight weeks of treatment, half of the mice of each genotype were given access to running wheels for five weeks, before testing on either the elevated plus maze (EPM; n = 7 for all groups) or novelty-suppressed feeding (NSF bright light; n = 10 for control groups, n = 9 for running groups; NSF dim light: n = 6–9 for all groups) tests (Walf and Frye, 2007; Samuels and Hen, 2011).
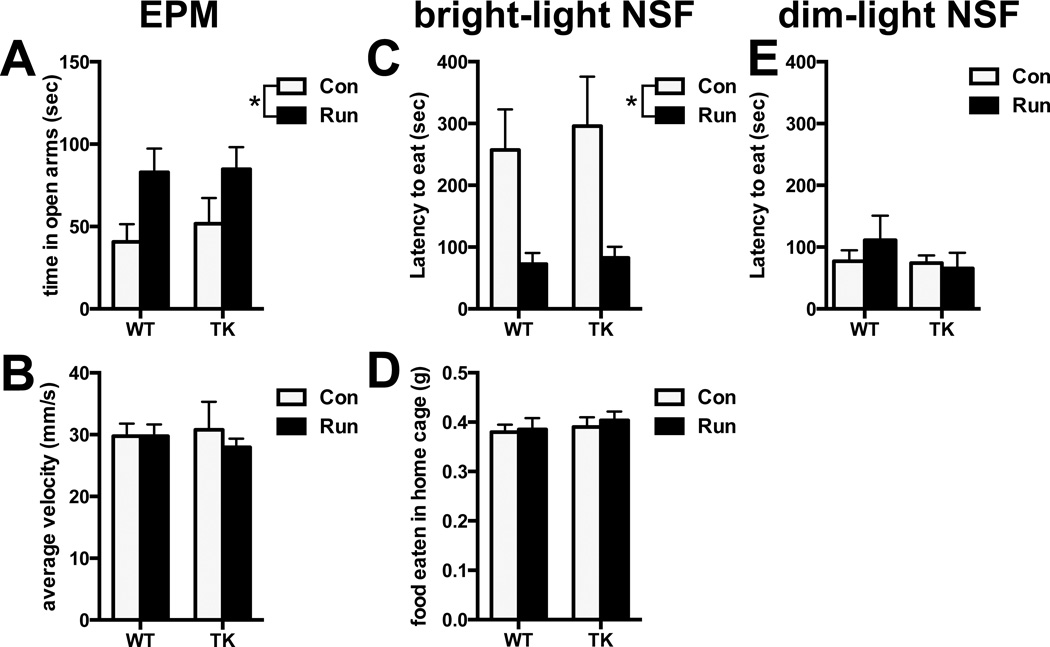
In the EPM under dim white light conditions (Fig 1A), running increased time in the open arms, suggesting reduced anxiety-like behavior, in both WT and TK mice (time in open arms: running effect: F1,24 = 7.62, P = 0.01; genotype effect: F1,24 = 0.22, P = 0.64). Neither running nor genotype affected the average velocity or total number of arm entries in the maze (average velocity: running effect: F1,24 = 0.31, P = 0.59; genotype effect: F1,24 = 0.02, P = 0.88, Fig 1B; total arm entries: mean ± SEM – WT control (con): 18.43 ± 1.17, TK con: 18.29 ± 2.02, WT running (run): 22.57 ± 1.23, TK run: 18.00 ± 1.95 running effect: F1,24 = 2.06, P = 0.16; genotype effect: F1,24 = 1.38, P = 0.25; running×genotype interaction: F1,24 = 1.82, P = 0.19). In the NSF under bright light conditions (700 lux), we saw similar results (Fig 1C,D). Independent of neurogenesis, running decreased the latency to eat in a novel environment, indicative of decreased anxiety without affecting home cage consumption (latency to eat: running effect: F1,34 = 12.66, P = 0.001; genotype effect: F1,34 = 0.19, P = 0.67; home cage consumption: running effect: F1,34 = 0.24, P = 0.63; genotype effect: F1,34 = 0.56, P = 0.46). To test motivated exploration under less anxiety-inducing conditions, we tested control and running WT and TKs on NSF with dim white light (100 lux). Under these conditions, mice displayed very little neophagia (Fig 1E), which did not differ across groups (latency to eat: running effect: F1,26 = 0.68, P = 0.42; genotype effect: F1,26 = 0.19, P = 0.67).
Figure 1.
Effect of running on anxiety-like behavior in WT and TK mice. A) Running increases time spent in the open arms of the elevated plus-maze in both WT and TK mice, without affecting overall locomotion, measured by average velocity (B). C) Running decreases the latency to eat in the novelty-suppressed feeding test under bright light in both WT and TK mice, without affecting general hunger, measured by a 10-minute home cage consumption test after testing (D). E) Under dim light, all mice display non-anxious phenotypes and eat quickly. *p < .05, main effect of running.
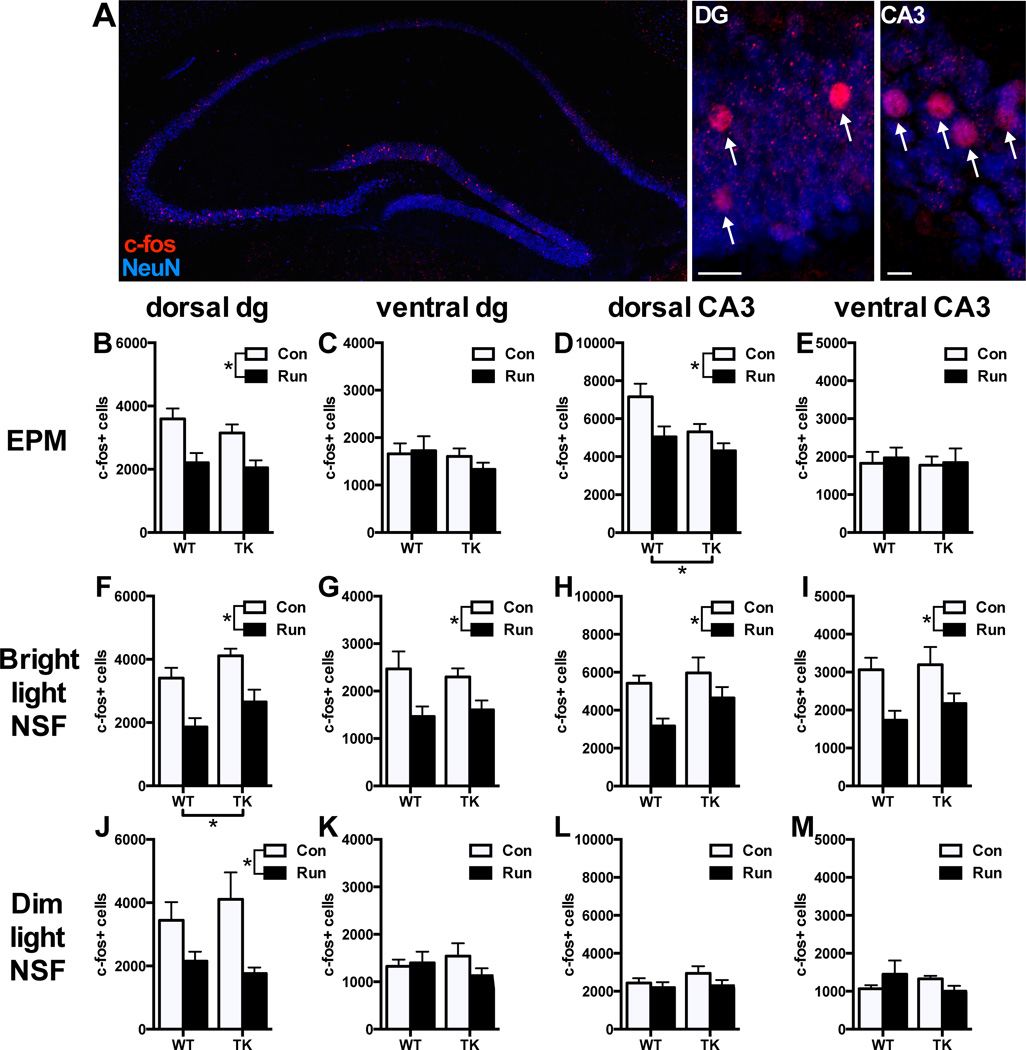
In addition to its behavioral effects, exercise also decreases the activation of excitatory neurons in the ventral hippocampus normally occurring in response to stress (Snyder et al., 2009; Schoenfeld et al., 2013). However, it is unknown how the loss of new neurons affects stress-induced activation of the hippocampus or whether such activation occurs during more mildly stressful anxiety tests. Therefore, we also asked whether physical exercise alters neuronal activation in the hippocampus following anxiety testing by examining the expression of c-fos, an immediate early gene induced by synaptic activation that has been used for mapping neuronal activation (Snyder et al., 2009; Schoenfeld et al., 2013). Wheels were removed from cages 24-hours before behavioral testing to prevent running from inducing c-fos expression at the time point examined. Mice were perfused 60 minutes after anxiety testing, near the peak of c-fos protein expression in the hippocampus (Peng and Houser, 2005) and processed and stained for double-labeling of c-fos and NeuN as previously described (Snyder et al., 2009). All c-fos+ cells throughout the entire dorsal and ventral dentate gyrus and CA3 were counted on an epifluorescent microscope (Olympus BX-51). Statistical analysis of c-fos+ cell counts were performed using 2x2 between-subjects ANOVA, and a 2x2x2 between-subjects ANOVA comparing bright and dim light NSF conditions, with interactions analyzed by Tukey post-hoc tests.
After elevated plus-maze testing (Fig 2B–E), running WTs and TKs both had lower c-fos expression than sedentary controls in the dorsal dentate gyrus and CA3. In the dorsal CA3, TKs also had less c-fos expression than WTs (running effect: dorsal dg: F1,23 = 18.18, P = 0.0003; dorsal CA3: F1,23 = 8.29, P = 0.009; genotype effect: dorsal CA3: F1,23 = 5.67, P = 0.03). There were no running by genotype interactions, and no effects of any kind in the ventral subregions after EPM (P > 0.05).
Figure 2.
Effect of running on neuronal activation following anxiety testing in WT and TK mice. A) Representative example of c-fos labeling throughout the hippocampus (left), with higher magnification photos depicting c-fos+ cells (arrows) in the dentate gyrus (middle) and CA3 (right). B-E) Following EPM testing, running reduced c-fos expression in the dorsal dentate gyrus (B) and CA3 (D), but had no effect in the ventral dentate gyrus (C) and CA3 (E). F–I) Following bright-light NSF testing, running reduced c-fos expression in the dorsal dentate gyrus (F), ventral dentate gyrus (G), dorsal CA3 (H), and ventral CA3 (I). J–M) Following dim-light NSF testing, running reduced c-fos expression in the dorsal dentate gyrus (J), but there was little c-fos expression in all mice in the ventral dentate gyrus (K), dorsal CA3 (L), and ventral CA3 (M). *p < .05, main effect of either running (legends) or genotype (x-axis). Scale bars are 20µm.
After the novelty-suppressed feeding test under anxiogenic, bright-light conditions (Fig 2F–I), running WTs and TKs both had lower c-fos expression than sedentary controls in dorsal and ventral dentate gyrus and CA3. In the dorsal dentate gyrus, TK mice also had greater c-fos expression than WTs overall (running effect: dorsal dg: F1,33 = 23.96, P < 0.0001; ventral dg: F1,33 = 11.91, P = 0.002; dorsal CA3: F1,33 = 9.43, P = 0.004; ventral CA3: F1,33 = 11.44, P = 0.002; genotype effect: dorsal dg: F1,33 = 5.91, P = 0.02 There were no other effects of genotype or interactions of running and genotype on c-fos expressing after bright-light NSF (P > 0.05).
After being tested in the novelty-suppressed feeding test under non-anxiogenic, dim-light conditions (Fig 2J–M), running mice had lower c-fos expression in dorsal dentate gyrus (running effect: dorsal dg: F1,26 = 14.21, P = 0.0009), however there were no effects of running in other hippocampal regions nor any genotype or interaction effects (P > 0.05). Three-way ANOVAs were used to compare bright- and dim-light conditions of NSF. A main effect of running reduced c-fos expression in the dorsal dentate gyrus in both light conditions (running effect: dorsal dg: F1,59 = 37.64, P < 0.000001). In the ventral dentate gyrus, a running by light interaction approached significance suggesting that running only affected c-fos expression under bright conditions (running×light interaction: F1,59 = 3.70, P = 0.059). In the ventral CA3, a significant running by light interaction indicated that running reduced c-fos expression only under bright conditions (running×light interaction: F1,59 = 6.43, P = 0.01). In the dorsal CA3, the running by light interaction was not significant (F1,59 = 2.62, P = 0.11), but the pattern appeared similar to that seen in the other regions. Because mice were removed from the NSF arena as soon as they began to eat, neuronal activation could potentially reflect differences in the amount of time spent exploring the arena rather than differences in emotionality. However, there were no consistent correlations between time spent in the arena (latency to eat) and c-fos expression among any of the conditions (Table 1).
Table 1.
Correlations between time in arena and c-fos expression
| Experimental Condition |
Hippocampal Region |
|||
|---|---|---|---|---|
| dorsal dg | ventral dg | dorsal CA3 | ventral CA3 | |
|
Bright light sedentary |
0.28 (.23) |
0.39 (.09) |
−0.35 (.13) |
0.57 (.008) |
|
Bright light running |
−0.02 (.93) |
0.26 (.30) |
−0.08 (.76) |
0.18 (.46) |
|
Dim light sedentary |
0.21 (.48) |
0.00 (.99) |
0.11 (.71) |
0.41 (.16) |
|
Dim light running |
−0.37 (.15) |
0.26 (.31) |
−0.11 (.66) |
0.33 (.20) |
values are Pearson’s correlation coefficient with p-values in parentheses
Our results indicate that new neurons are not involved in the reduction of anxiety-like behavior by exercise, as seen in both the elevated plus-maze and novelty-suppressed feeding tests. Although adult neurogenesis has been suggested as a mechanism for the anxiolytic effects of exercise, based on correlations and factors influencing both in parallel (Bjørnebekk et al., 2006; Trejo et al., 2008; Yau et al., 2014), we find that mice with complete ablation of new neurons in the hippocampus show the same reduction in anxiety-like behavior following running seen in mice with intact neurogenesis. Running produces many changes in the hippocampus and throughout the brain that could be responsible for the shift in emotional behavior. One consequence of running is that it increases GABAergic signaling in the hippocampus in response to stress. Diminished GABAergic inhibition in the hippocampus has been implicated in mediating anxiety-like behavior in mice (Earnheart et al., 2007), and the anxiolytic effects of exercise can be blocked using GABA antagonists in the ventral hippocampus (Schoenfeld et al., 2013), suggesting that changes in GABAergic signaling may mediate reductions in anxiety-like behavior. Another consequence of running is the proliferation and growth of astrocytes in the hippocampus of exercised mice (Li et al., 2005; Brockett et al., 2015), which have lower hippocampal glutamate levels (Biedermann et al., 2012). Anxiety-like behavior is inversely correlated with astrocytic glutamate uptake in the hippocampus (Zimmer et al., 2015), so changes in astrocytic mechanisms involving glutamate in the hippocampus may also mediate decreases in anxiety-like behavior following exercise. Exercise also promotes dendritic growth in the hippocampus (Stranahan et al., 2007), facilitates LTP (van Praag et al., 1999), and affects a wide range of neurotrophic factors, neuropeptides, and neurotransmitters (Bjørnebekk et al., 2006), all of which could potentially affect hippocampal function and alter anxiety-like behavior. Although it is difficult to point to a specific change that is most likely to mediate the anxiolytic effects of running, there are clearly many plausible targets.
Although running is anxiolytic in many studies it, somewhat paradoxically, increases anxiety in others, in a neurogenesis-dependent manner (Fuss et al., 2010; Onksen et al., 2012). Therefore, new neurons are functionally important for running effects on anxiety-like behavior under some conditions. One key difference between these studies and those showing anxiolytic effects of running (Schoenfeld et al., 2013; 2014)) is that the mice used in studies in which running was anxiogenic were all single-housed during running episodes and anxiety testing. Social isolation in control adult mice increases corticosteroid levels and anxiety-like behavior (Kamal et al., 2014; Ieraci et al., 2016). In running mice, isolation increases circulating corticosteroid levels while running (Grégoire et al., 2014), and while not all socially isolated mice display an anxiogenic phenotype following exercise (Duman et al., 2008), isolation potentially increases the stressful aspects of running relative to positive effects. One reason many running studies have been conducted in isolated animals is that it allows for monitoring of individual activity tracking, though this should soon be possible in socially-housed animals with new tracking technologies (Freund et al., 2013).
Activation of dentate gyrus and CA3 neurons by anxiety testing was decreased in runners, independent of neurogenesis, paralleling the effects on anxiety-like behavior. Activation of hippocampal principal neurons by stress is also inhibited by running (Fuss et al., 2010; Schoenfeld et al., 2013), consistent with the anxiogenic testing situations being mildly stressful. Significant activation of the ventral dentate gyrus and CA3 occurred in the anxiogenic bright light version of the NSF test, but very little activation occurred in the non-anxiogenic version of the test, suggesting that activity in these brain regions may encode emotional information. In contrast, the granule neurons in the dorsal dentate gyrus were equally activated in the anxiogenic tasks (EPM and bright light NSF test) and in the non-anxiogenic low light version of the NSF test, providing novel support for functional segmentation of the dentate gyrus into an emotional ventral portion and a cognitive, or spatial, dorsal portion (Bannerman et al., 1999; 2004; Fanselow and Dong, 2010).
The required role for adult neurogenesis in some antidepressant effects, and the strong effects of physical exercise on both emotional behavior and neurogenesis, suggest that new neurons could play a critical role in the anxiolytic effects of exercise. However, we find that running has the same anxiety-reducing effect in mice that completely lack new neurons that it has in normal mice. Activation of hippocampal neurons during anxiety-like behavior is similarly unaffected by the loss of adult neurogenesis. These findings indicate a dissociation between neurogenic effects and behavioral effects of running. Future research will be needed to identify the specific mediators of exercise-induced reductions in anxiety-like behavior.
Acknowledgments
Grant sponsor: This work was supported by the Intramural Program of the NIH, National Institute of Mental Health, ZIAMH002784 (H.A.C).
References
- Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, Rawlins JNP. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang W-N, Pothuizen HHJ, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–1188. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- Biedermann S, Fuss J, Zheng L, Sartorius A, Falfán-Melgoza C, Demirakca T, Gass P, Ende G, Weber-Fahr W. In vivo voxel based morphometry: Detection of increased hippocampal volume and decreased glutamate levels in exercising mice. Neuroimage. 2012;61:1206–1212. doi: 10.1016/j.neuroimage.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Bjørnebekk A, Mathé AA, Brené S. Running has differential effects on NPY, opiates, and cell proliferation in an animal model of depression and controls. Neuropsychopharmacology. 2006;31:256–264. doi: 10.1038/sj.npp.1300820. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, Lüscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J, Brandmaier AM, Lewejohann L, Kirste I, Kritzler M, Krüger A, Sachser N, Lindenberger U, Kempermann G. Emergence of individuality in genetically identical mice. Science. 2013;340:756–759. doi: 10.1126/science.1235294. [DOI] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NMB, Hensley FW, Weber K-J, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon C-H, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onksen JL, Briand LA, Galante RJ, Pack AI, Blendy JA. Running-induced anxiety is dependent on increases in hippocampal neurogenesis. Genes Brain Behav. 2012;11:529–538. doi: 10.1111/j.1601-183X.2012.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Houser CR. Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci. 2005;25:7210–7220. doi: 10.1523/JNEUROSCI.0838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BA, Hen R. Novelty-Suppressed Feeding in the Mouse. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice Characterization Using Behavioral Tests, Volume II. Vol. 63. Neuromethods. Totowa, NJ: Humana Press; 2011. pp. 107–121. [Google Scholar]
- Schoenfeld TJ, Kloth AD, Hsueh B, Runkle MB, Kane GA, Wang SS-H, Gould E. Gap junctions in the ventral hippocampal-medial prefrontal pathway are involved in anxiety regulation. J Neurosci. 2014;34:15679–15688. doi: 10.1523/JNEUROSCI.3234-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Rada P, Pieruzzini PR, Hsueh B, Gould E. Physical exercise prevents stress-induced activation of granule neurons and enhances local inhibitory mechanisms in the dentate gyrus. J Neurosci. 2013;33:7770–7777. doi: 10.1523/JNEUROSCI.5352-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2009;19:360–370. doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martín MV, Torres-Aleman I. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci. 2008;37:402–411. doi: 10.1016/j.mcn.2007.10.016. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau S-Y, Lau BW-M, Tong J-B, Wong R, Ching Y-P, Qiu G, Tang S-W, Lee TMC, So K-F. Hippocampal neurogenesis and dendritic plasticity support running-improved spatial learning and depression-like behaviour in stressed rats. PLoS ONE. 2011;6:e24263. doi: 10.1371/journal.pone.0024263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau S-Y, Li A, Hoo RLC, Ching Y-P, Christie BR, Lee TMC, Xu A, So K-F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci USA. 2014;111:15810–15815. doi: 10.1073/pnas.1415219111. [DOI] [PMC free article] [PubMed] [Google Scholar]