Abstract
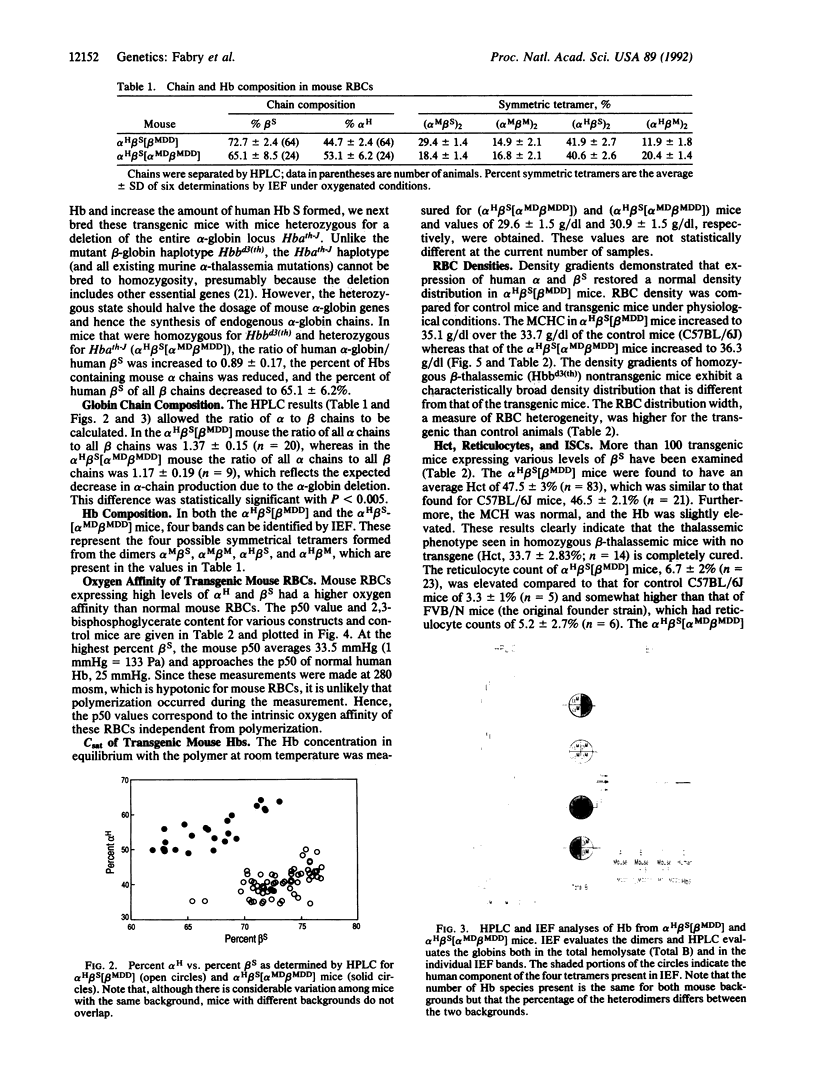
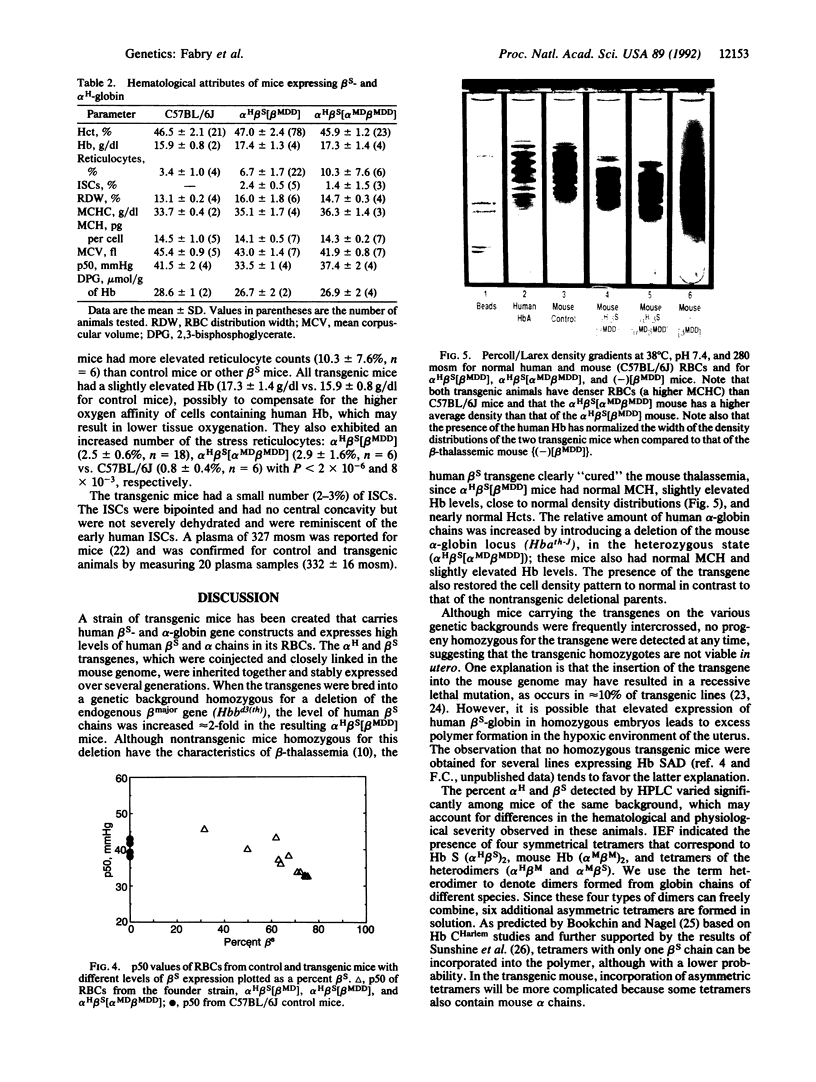
A line of transgenic mice (alpha H beta S-11; where alpha H is human alpha-globin) was created in which the human beta S and human alpha 2 globin genes, each linked to the beta-globin locus control region, were cointegrated into the mouse genome. On a normal genetic background, the transgenic mice produced 36% human beta S-globin chains with an alpha H/beta S ratio of 1.3. Higher levels of beta S were achieved by breeding the transgenic mice with mutant mice carrying a mouse beta major-globin gene deletion. Mice heterozygous for the beta major deletion (alpha H beta S[beta MD]; MD, mouse deletion) had 54% beta S with an alpha H/beta S ratio of 1.0; mice homozygous for the beta major deletion (alpha H beta S[beta MDD]) had 72.5% beta S and an alpha H/beta S ratio of 0.73. Because mouse alpha chains inhibit hemoglobin (Hb) S polymerization, we bred the mice to heterozygosity for a mouse alpha-globin deletion. These mice (alpha H beta S[alpha MD beta MDD]) had an increased alpha H/beta S ratio of 0.89 but expressed 65% beta S. Expression of the human genes cured the thalassemic phenotype associated with the murine beta major deletion. Transgenic alpha H beta S[beta MDD] mice had normal hematocrit and Hb and somewhat elevated reticulocytes (6% vs. 3% for control), whereas the mice carrying the alpha-globin deletion (alpha H beta S[alpha MD beta MDD]) had a normal hematocrit and Hb and more elevated reticulocytes (10.3 +/- 7.6% vs. 3.4 +/- 1.0%). Expression of the transgene restored a normal distribution of erythrocyte densities when compared to thalassemic mice; however, the average mean corpuscular Hb concentration of alpha H beta S[beta MDD] mice increased to 35.7 g/dl (vs. control 33.7 g/dl) whereas that of alpha H beta S[alpha MD beta MDD] mice was further elevated to 36.3 g/dl. The intrinsic oxygen affinity was increased in transgenic mouse erythrocytes at 280 milliosmolal, and the PO2 at midsaturation of alpha H beta S[alpha MD beta MDD] erythrocytes was higher than that of alpha H beta S[beta MDD] cells (37.4 +/- 2 vs. 33.5 +/- 1 mmHg). The higher values of the mean corpuscular Hb concentration and intrinsic PO2 at midsaturation, which favor in vivo sickling, may explain the slightly more severe hematological picture in alpha H beta S[alpha MD beta MDD] mice. We conclude that the transgenic mouse with high Hb S expression does not exhibit adult anemia but does have abnormal hematological features: increased erythrocyte density, high oxygen affinity, and reticulocytosis with increased stress reticulocytes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barabino G. A., McIntire L. V., Eskin S. G., Sears D. A., Udden M. Rheological studies of erythrocyte-endothelial cell interactions in sickle cell disease. Prog Clin Biol Res. 1987;240:113–127. [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Townes T. M. Synthesis of functional human hemoglobin in transgenic mice. Science. 1989 Sep 1;245(4921):971–973. doi: 10.1126/science.2772649. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L. Ligand-induced conformational dependence of hemoglobin in sickling interactios. J Mol Biol. 1971 Sep 14;60(2):263–270. doi: 10.1016/0022-2836(71)90292-0. [DOI] [PubMed] [Google Scholar]
- Fabry M. E., Costantini F., Pachnis A., Suzuka S. M., Bank N., Aynedjian H. S., Factor S. M., Nagel R. L. High expression of human beta S- and alpha-globins in transgenic mice: erythrocyte abnormalities, organ damage, and the effect of hypoxia. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12155–12159. doi: 10.1073/pnas.89.24.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry M. E., Romero J. R., Buchanan I. D., Suzuka S. M., Stamatoyannopoulos G., Nagel R. L., Canessa M. Rapid increase in red blood cell density driven by K:Cl cotransport in a subset of sickle cell anemia reticulocytes and discocytes. Blood. 1991 Jul 1;78(1):217–225. [PubMed] [Google Scholar]
- Forrester W. C., Novak U., Gelinas R., Groudine M. Molecular analysis of the human beta-globin locus activation region. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5439–5443. doi: 10.1073/pnas.86.14.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. Evidence for a locus activation region: the formation of developmentally stable hypersensitive sites in globin-expressing hybrids. Nucleic Acids Res. 1987 Dec 23;15(24):10159–10177. doi: 10.1093/nar/15.24.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester W. C., Thompson C., Elder J. T., Groudine M. A developmentally stable chromatin structure in the human beta-globin gene cluster. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1359–1363. doi: 10.1073/pnas.83.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D. R., Fraser P., Vidal M. A., Hedges M. J., Ropers D., Luzzatto L., Grosveld F. A transgenic mouse model of sickle cell disorder. Nature. 1990 Jan 11;343(6254):183–185. doi: 10.1038/343183a0. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hanscombe O., Vidal M., Kaeda J., Luzzatto L., Greaves D. R., Grosveld F. High-level, erythroid-specific expression of the human alpha-globin gene in transgenic mice and the production of human hemoglobin in murine erythrocytes. Genes Dev. 1989 Oct;3(10):1572–1581. doi: 10.1101/gad.3.10.1572. [DOI] [PubMed] [Google Scholar]
- Kaul D. K., Fabry M. E., Nagel R. L. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc Natl Acad Sci U S A. 1989 May;86(9):3356–3360. doi: 10.1073/pnas.86.9.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Lawrence C., Fabry M. E., Nagel R. L. The unique red cell heterogeneity of SC disease: crystal formation, dense reticulocytes, and unusual morphology. Blood. 1991 Oct 15;78(8):2104–2112. [PubMed] [Google Scholar]
- Martinell J., Whitney J. B., 3rd, Popp R. A., Russell L. B., Anderson W. F. Three mouse models of human thalassemia. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5056–5060. doi: 10.1073/pnas.78.8.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin E. M., Witkowska H. E., Spangler E., Curtin P., Lubin B. H., Mohandas N., Clift S. M. Hypoxia-induced in vivo sickling of transgenic mouse red cells. J Clin Invest. 1991 Feb;87(2):639–647. doi: 10.1172/JCI115041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Townes T. M., Palmiter R. D., Brinster R. L. High-level erythroid expression of human alpha-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):37–41. doi: 10.1073/pnas.86.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Townes T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Behringer R. R. Human sickle hemoglobin in transgenic mice. Science. 1990 Feb 2;247(4942):566–568. doi: 10.1126/science.2154033. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. B., Shelton J. R., Huynh V., Teplow D. B. High performance liquid chromatographic separation of the globin chains of non-human hemoglobins. Hemoglobin. 1985;9(5):461–482. doi: 10.3109/03630268508997024. [DOI] [PubMed] [Google Scholar]
- Scott A. F., Bunn H. F., Brush A. H. The phylogenetic distribution of red cell 2,3 diphosphoglycerate and its interaction with mammalian hemoglobins. J Exp Zool. 1977 Aug;201(2):269–288. doi: 10.1002/jez.1402010211. [DOI] [PubMed] [Google Scholar]
- Silverstein E., Sokoloff L., Mickelsen O., Jay G. E. Primary Polydipsia and Hydronephrosis in an Inbred Strain of Mice. Am J Pathol. 1961 Feb;38(2):143–159. [PMC free article] [PubMed] [Google Scholar]
- Skow L. C., Burkhart B. A., Johnson F. M., Popp R. A., Popp D. M., Goldberg S. Z., Anderson W. F., Barnett L. B., Lewis S. E. A mouse model for beta-thalassemia. Cell. 1983 Oct;34(3):1043–1052. doi: 10.1016/0092-8674(83)90562-7. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Eaton W. A. Gelation of sickle cell hemoglobin in mixtures with normal adult and fetal hemoglobins. J Mol Biol. 1979 Oct 9;133(4):435–467. doi: 10.1016/0022-2836(79)90402-9. [DOI] [PubMed] [Google Scholar]
- Trudel M., Saadane N., Garel M. C., Bardakdjian-Michau J., Blouquit Y., Guerquin-Kern J. L., Rouyer-Fessard P., Vidaud D., Pachnis A., Roméo P. H. Towards a transgenic mouse model of sickle cell disease: hemoglobin SAD. EMBO J. 1991 Nov;10(11):3157–3165. doi: 10.1002/j.1460-2075.1991.tb04877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Solomon W., Li Q., London I. M. The "beta-like-globin" gene domain in human erythroid cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6384–6388. doi: 10.1073/pnas.82.19.6384. [DOI] [PMC free article] [PubMed] [Google Scholar]