Abstract
Background
Maternal obesity is emerging as a public health problem, recently highlighted together with maternal under-nutrition as a ‘double burden’, especially in African countries undergoing social and economic transition. This systematic review was conducted to investigate the current evidence on maternal obesity in Africa.
Methods
MEDLINE, EMBASE, Scopus, CINAHL and PsycINFO were searched (up to August 2014) and identified 29 studies. Prevalence, associations with socio-demographic factors, labour, child and maternal consequences of maternal obesity were assessed. Pooled risk ratios comparing obese and non-obese groups were calculated.
Results
Prevalence of maternal obesity across Africa ranged from 6.5 to 50.7%, with older and multiparous mothers more likely to be obese. Obese mothers had increased risks of adverse labour, child and maternal outcomes. However, non-obese mothers were more likely to have low-birthweight babies. The differences in measurement and timing of assessment of maternal obesity were found across studies. No studies were identified either on the knowledge or attitudes of pregnant women towards maternal obesity; or on interventions for obese pregnant women.
Conclusions
These results show that Africa's levels of maternal obesity are already having significant adverse effects. Culturally adaptable/sensitive interventions should be developed while monitoring to avoid undesired side effects.
Keywords: obesity, population-based and preventative services, pregnancy and childbirth disorders
Introduction
Obesity is a worldwide epidemic.1 Prevalence is higher in wealthy countries,2,3 but increasing in developing countries,3,4 with severe consequences.5–8
Pregnancy is a recognized obesity trigger.9 Maternal obesity incidence is increasing worldwide10–17 and associated with short- and long-term complications for mothers14,18–22 and children23–26 during pregnancy, delivery and post-delivery.
Many developing countries now experience a double burden of malnutrition4,27–29 with increased maternal overweight and obesity.12,30 In Africa, the obesity increase in women has been steeper than in Asia with more than 40% reproductive aged women being overweight or obese.12 In addition, sub-Saharan Africa has the highest global neonatal mortality rate31 but the slowest progress in reducing maternal mortality.32 Approximately, one in four maternal deaths results from pre-existing medical conditions including obesity and diabetes.32,33
Maternal obesity is assessed differently worldwide, but pre-pregnancy or first trimester body mass index (BMI) is widely recommended.34–36 Other measures include weight and mid-arm circumference.37–39 There is also a lack of consensus on recommendations for managing obese pregnant women but in any case guidelines that do exist may not be applicable to countries with inadequate healthcare services.40,41
While maternal under-nutrition effects are well known, there is a paucity of data on maternal obesity in African countries.23,42,43 A scoping exercise identified one study utilizing pooled data from demographic health surveys in sub-Saharan Africa focussing on maternal obesity effects on neonatal death.44 This indicates a need for a comprehensive literature review to assess the prevalence and burden of maternal obesity in Africa in order to develop policies and interventions to improve maternal health.3,33
The aim of this systematic review was to investigate maternal obesity in Africa assessing prevalence, socio-demographic associations, adverse pregnancy outcomes in mother and child, existing interventions along with knowledge and attitudes of pregnant women, and healthcare providers towards maternal obesity.
Methodology
A comprehensive search of MEDLINE, EMBASE, Scopus, CINAHL and PsycINFO was conducted in August 2014 with no restriction on language or publication year (Supplementary data, Table S1). Google Scholar and references of relevant articles were also searched. Mesh terms and keywords for maternal obesity and geographical location were combined using Boolean operators. Studies, irrespective of design, conducted in Africa recording maternal obesity either by maternal BMI or by other weight measures at any time during pregnancy or immediately after delivery were included. All maternal and child outcomes with at least one obese group and a comparison group were assessed. Intervention studies to increase maternal weight, studies conducted in non-pregnant women and studies that targeted women with specific disease conditions such as HIV were excluded (Supplementary data, Table S2).
A data extraction form was developed and piloted, and quality assessment carried out using the Effective Public Health Practice Project quality assessment tool.45 Study quality was graded strong, moderate or weak based on selection bias, study design, confounders, data collection method and dropouts.45
All studies reporting maternal obesity prevalence in some way were included in prevalence comparisons using their criteria. Although BMI was preferred some studies reported more than one obesity measure. Maternal obesity was measured at different time points in different studies, while two studies adjusted BMI for gestational age. Where obesity was measured at different points in pregnancy in the same study, the earliest measurement was used. ‘Obese’ groups were mainly defined as BMI ≥30 kg/m2 with the comparator ‘non-obese’ group being a combination of overweight and normal weight participants. This was possible for all studies reporting BMI except one,46 where overweight and obese data were presented separately for prevalence but not for other outcomes, so this study was excluded from meta-analysis. One study47 measured obesity as BMI 27.6–41.8 kg/m2, another study48 as ≥28 kg/m2 and four other studies used weight ≥80 kg49,50 and ≥90 kg51,52 as obesity cut-off points. The results from these studies were included in meta-analysis for the respective outcome(s) measured.
Pooling of evidence using meta-analyses
Raw data within obese and non-obese groups were extracted for each outcome. ‘Obese’ group sizes were sufficient making relative risks (RRs) appropriate for dichotomous outcomes and the mean difference (MD) for continuous measures with their 95% confidence interval. Pooled estimates for each outcome were calculated using meta-analysis where appropriate (two or more studies, similarly measured). Otherwise, results were described along with their individual estimated effects, subject to sufficient information and put in context.
A Health Technology Assessment report highlighted a lack of consensus on the important outcomes of maternal obesity,14 so this review assessed all outcomes reported within the included studies. Separate meta-analyses were considered for each using Review Manager Software (version 5.2) with heterogeneity assessed by the chi-squared and the I2 statistic. Fixed effect models were used unless otherwise indicated but for outcomes with moderate heterogeneity (I2 >50%, P ≥ 0.10), random effects models (REM) were used. In cases of substantial heterogeneity (I2 >75%), possible causes were explored and sensitivity analyses performed resulting in some studies being excluded from meta-analyses due to heterogeneity.53 Given the number of outcomes, only forest plots of outcomes meeting at least two of three criteria are presented in the online document (Supplementary data, Figs S1–9): those assessed by a large number of studies (≥5), with highly significant results and being a significant cause of maternal or child mortality in Africa.31,33
Results of the literature search
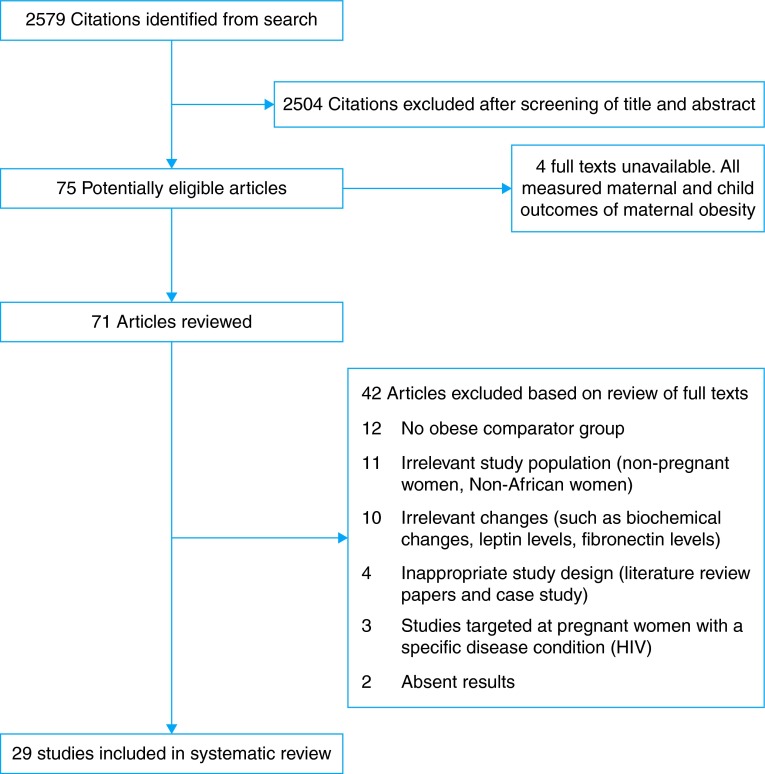
A total of 2579 titles and abstracts were identified. Initially, 300 were screened by two independent reviewers (O.J.O. and A.S.P.) refereed by a third reviewer (D.M.). Consistency established, the remainder were scanned by one reviewer (O.J.O.). Full texts for 75 potentially eligible papers were retrieved except for four studies (Fig. 1). After reading the full texts, 29 studies were included in this review, basic characteristics of which are presented in Table 1.
Fig. 1.
Flow diagram of the selection process for the review.
Table 1.
Basic characteristics of included studies
| Author, year, country, study quality | Study type | Sample size | Outcomes | Enrolment dates | Obesity measure | Gestational age |
|---|---|---|---|---|---|---|
| Efiong, 197549
Nigeria Strong |
Cohort | 200 | Labour outcomes: Prolonged labour, precipitate labour, caesarean section, cephalopelvic disproportion Child outcomes: Macrosomia (>4 kg), stillbirth, low birthweight, death Maternal outcomes: Pre-eclampsia, urinary tract infection, malpresentation, antepartum haemorrhage, postpartum haemorrhage, retained placenta, death |
February 1972 to January 1975 | Weight >80 kg | 14th week of pregnancy |
| Lawoyin, 199354
Nigeria Moderate |
Cohort | 492 | Other outcomes: Pre-pregnancy weight | Not specified | BMIa | Delivery |
| Khan, 199655
Egypt Moderate |
Cohort | 80 | Prevalence Socio-demographic outcomes: Age, parity |
1983–1985 | BMI | Pre-pregnancy |
| Mahomed, 199847
Zimbabwe Strong |
Case control | 338 | Maternal outcome: Pre-eclampsia | June 1995 to April 1996 | BMI | Immediately post-delivery |
| Olayemi, 200250
Nigeria Moderate |
Cross-sectional | 3104 | Prevalence Socio-demographic outcomes: Parity, marital status, ethnicity Labour outcomes: Cephalopelvic disproportion, prolonged labour, shoulder dystocia, retained placenta, genital laceration, caesarean section Child outcomes: Macrosomia (>4 kg), low birthweight, asphyxia (1 min) Maternal outcomes: Pre-eclampsia, gestational diabetes mellitus, urinary tract infection, anaemia, antepartum haemorrhage, malpresentation, thromboembolic disease, preterm gestation, post-term gestation, wound infection, postpartum haemorrhage, eclampsia, death |
1 January 1995 to 31 December 1999 | Weight ≥90 kg | Last antenatal visit before delivery |
| Adesina, 200351
Nigeria Weak |
Case control | 190 | Child outcome: Macrosomia (≥4 kg) | 1 January 1998 to 31 December 2000 | Weight >90 kg | Term |
| Anorlu, 200552
Nigeria Moderate |
Case control | 368 | Maternal outcome: Pre-eclampsia | February 2001 to August 2002 | Weight ≥80 kg | Pre-pregnancy |
| Van Bogaert, 200556
South Africa Weak |
Cross-sectional | 2042 | Socio-demographic outcome: Parity | Not specified | Ponderal index | End pregnancy |
| Edomwonyi, 200657
Nigeria Weak |
Cross-sectional | 300 | Prevalence Socio-demographic outcome: Age Labour outcomes: Caesarean section, type of caesarean section, intra-operative complications |
June 2004 to June 2005 | BMI | Not stated |
| Villamor, 200658
Tanzania Strong |
Interrupted time series | 73 689 | Prevalence Socio-demographic outcomes: Parity, education, cohabitation, employment |
1995–2004 | BMI | ≤14 weeks and <28 weeks |
| Adebami, 200759
Nigeria Weak |
Cross-sectional | 473 | Prevalence Child outcome: Foetal malnutrition |
January to August 2001 | BMI | Likely third trimester |
| Mamabolo, 200760
South Africa Moderate |
Cross-sectional | 262 | Prevalence Socio-demographic outcomes: Age, parity |
May–August 1999 and February–April 2000 | BMI | Third trimester (28–36 weeks) |
| Ward, 200761
South Africa Moderate |
Cohort | 98 | Socio-demographic outcomes: Age Maternal outcomes: Haemoglobin levels |
Not specified | BMI | Pre-pregnancy |
| Abdul, 200962
Nigeria Weak |
Case control | 425 | Child outcomes: Macrosomia (≥4 kg) | January 2001 to December 2005 | BMI | Booking |
| Kamanu, 200948
Nigeria Weak |
Cross-sectional | 9040 | Child outcomes: Macrosomia (>4.5 kg) | 1 January 1999 to 31 December 2003 | BMI Weight |
Third trimester before delivery |
| Ngoga, 200963
South Africa Weak |
Case control | 309 | Socio-demographic outcomes: Parity Labour outcomes: Induction of labour, epidural during labour, instrumental delivery, episiotomy, perineal tear, caesarean section, wound sepsis Child outcomes: Macrosomia (>4.5 kg), asphyxia (<7 at 5 min), neonatal death Maternal outcomes: Urinary tract infections, anaemia |
Not specified | BMI | Booking |
| Addo, 201046
Ghana Weak |
Retrospective cohort | 1755 | Prevalence Socio-demographic outcome: Age |
1 January to 31 December 1992 | BMI | First trimester |
| Basu, 201064
South Africa Moderate |
Cross-sectional | 767 | Prevalence Labour outcomes: Preterm labour, preterm rupture of membranes, induction of labour, caesarean section, failed induction of labour, longitudinal skin incision Maternal outcomes: Pregnancy-induced hypertension, gestational diabetes mellitus, urinary tract infection |
February and September 2006 | BMI | Booking (median 28 weeks) |
| Ugwuja, 201065
Nigeria Weak |
Cohort | 349 | Socio-economic outcomes: Age, parity, education, living accommodation Labour outcomes: Instrumental delivery, caesarean section, preterm delivery Child outcomes: Low birthweight, stillbirth Maternal outcomes: Anaemia, post-term delivery |
Not specified | BMI | At recruitment (≤25 weeks) |
| Adesina, 201166
Nigeria Moderate |
Matched case control | 236 | Socio-demographic outcomes: Age, education, marital status, ethnicity, parity, social status Child outcomes: Low birthweight, macrosomia (≥4.2 kg), perinatal asphyxia (5 min APGAR <7), birth trauma, neonatal admission. Maternal outcomes: Hypertension in pregnancy, gestational diabetes, infection, pre-eclampsia, gestational age at delivery, assisted vaginal delivery, caesarean section, cephalopelvic disproportion, perineal laceration, postpartum haemorrhage, prolonged/obstructed labour |
Not specified | BMI | ≤32 weeks |
| Chigbu, 201167
Nigeria Moderate |
Cross-sectional | 3167 | Prevalence Socio-demographic outcomes: Age, parity, employment status, educational level, rural/urban residence Other outcomes: Pre-pregnancy weight |
April 2009 to January 2010 | BMI | First trimester (Mean 11.0 ±2.2 weeks) |
| Ezeanochie, 201168
Nigeria Moderate |
Cross-sectional Then Case control |
2086 402-case control |
Prevalence Socio-demographic outcomes: Age, parity, social class, marital status Maternal outcomes: Gestational diabetes, pregnancy-induced hypertension, antenatal admission, birth before 37 weeks, birth before 34 weeks Labour outcomes: Augmentation of labour, mode of delivery (spontaneous vaginal delivery, instrumental delivery, caesarean section), episiotomy, perineal tear, postpartum haemorrhage, maternal mortality. Child outcomes: sex, low birthweight, macrosomia (not specified), stillbirth, admission into neonatal special care unit, severe birth asphyxia (5 min APGAR <3) |
January 2006 to December 2008 | BMI | Booking |
| Jeremiah, 201169
Nigeria Weak |
Cohort | 300 | Prevalence Socio-demographic outcomes: Age, parity, educational status Maternal outcomes: antepartum haemorrhage, anaemia, malaria, urinary tract infection, sickle cell disease, malpresentation, twin gestation, preterm delivery, prolonged pregnancy, maternal death, gestational DM Labour outcomes: Caesarean section, genital lacerations or episiotomies, postpartum haemorrhage, wound infection Child outcomes: Macrosomia (≥4 kg), intrauterine foetal death, birth asphyxia (1 min APGAR), birth trauma, congenital abnormality, admission to special baby care unit, perinatal mortality |
May 2006 and April 2007 | BMI | Booking |
| Davies, 201270
South Africa Strong |
Data from previous cluster-RCT | 1145 | Prevalence Socio-demographic outcomes: Age, parity, education, marital status, employment, income, housing type |
2009–2010 | BMI | Adjusted BMI for gestational age. |
| El-Makhangy, 201271
Egypt Moderate |
Prospective cohort | 250 | Maternal outcome: Pre-eclampsia | Not specified | BMI | At 20 weeks and 28 weeks of gestation |
| Okafor, 201272
Nigeria Weak |
Cross-sectional | 250 | Prevalence | May 2008 to December 2010 | BMI | Last weight before delivery |
| Koyanagi, 201373
7 countries Moderate |
Cross-sectional | 78 545 | Prevalence Child outcome: Macrosomia (≥4 kg) |
2004–2005 | BMI | Booking |
| Iyoke, 201374
Nigeria Strong |
Retrospective cohort | 1806 648 (cohort) |
Prevalence Socio-demographic outcomes: Occupation, educational status, marital status, residence Maternal outcomes: Premature rupture of membranes, pre-eclampsia/eclampsia, antepartum haemorrhage, gestational diabetes, caesarean section, postpartum haemorrhage Child outcomes: Macrosomia (not specified), severe birth asphyxia, newborn intensive care admission |
1 January 2010 to 31 December 2011 | BMI | First trimester |
| Davies, 201375
South Africa Strong |
Data from previous cluster-RCT | 1058 | Prevalence Maternal outcomes: Maternal death, caesarean section, maternal hospital stay, preterm labour, post-term labour, gestational diabetes, pregnancy-induced hypertension Child outcomes: Stillbirth, neonatal death, low birthweight, macrosomia (≥4.5 kg) |
2009–2010 | BMI | Adjusted BMI for gestational age |
BMI, body mass index; RCT, randomized controlled trial.
aCalculated as weight in kilograms divided by height in metre squared.
Included studies were from Nigeria (n = 16), South Africa (n = 7), Egypt (n = 2), Ghana (n = 1), Tanzania (n = 1), Zimbabwe (n = 1) and a final study conducted in seven African countries. With respect to quality, 11 studies were weak,46,48,51,56,57,59,62,63,65,69,72 12 moderate50,52,54,55,60,61,64,66,67,68,71,73 and 6 studies were classified as strong.47,49,58,70,74,75 Most studies gave weak descriptions about controlling for confounders and/or about data collection methods. All outcomes were extracted and categorized into four major groups—prevalence, socio-demographic characteristics, labour, and child and maternal outcomes (Supplementary data, Table S3).
Results of the review
Prevalence of maternal obesity
Prevalence of maternal obesity was assessed by 16 studies, ranging between 6.5 and 50.7% (Table 2). Fifteen studies used BMI, while one study used a weight cut-off point at ≥90 kg.50
Table 2.
Prevalence of maternal obesity
| Study, year | Sample size | Country | Timing of measurement | Measure | Prevalence of Obesity |
|---|---|---|---|---|---|
| Khan, 199655 | 80 | Egypt | Pre-pregnancy | BMIa percentiles ≥95th percentile | 10% (8/80) |
| Villamor, 200658 | 4068 | Tanzania (2004 data chosen) | First trimester (gestational week ≤14 weeks) | BMI ≥30 | 7.3% (298/4068) |
| Addo, 201046 | 1755 | Ghana | First trimester | BMI ≥30.1 | 17.9% (314/1755) |
| Chigbu, 201167 | 3167 | Nigeria | First trimester | BMI ≥30 | 10.7% (339/3167) |
| Iyoke, 201374 | 1806 | Nigeria | First trimester | BMI ≥30 | 17.9% (340/1806) |
| Basu, 201064 | 767 | South Africa | Antenatal booking (median 28 weeks) | BMI ≥30 | 44% (337/767) |
| Ezeanochei, 201168 | 2086 | Nigeria | Antenatal booking | BMI >30 | 9.6% (201/2086) |
| Jeremiah, 201169 | 4832 | Nigeria | Antenatal booking | BMI >30 | 7.4% (357/4832) |
| Koyanagi, 201373 | 78 545 | 7 African countries | Antenatal booking | BMI ≥30 | Algeria: 24.9% (3672/14 761) Angola: 9.6% (328/3424) DRC: 6.5% (544/8375) Kenya 20.3% (544/2680) Niger: 11.1% (888/8018) Nigeria: 31.7% (2415/7621) Uganda 13.0% (1367/10558) |
| Olayemi, 200250 | 3104 | Nigeria | Third trimester | Weight ≥90 kg | 7.4% (230/3104) |
| Edomwonyi, 200657 | 300 | Nigeria | Third trimester | BMI >30 | 50.7% (152/300) |
| Mamabolo, 200760 | 262 | South Africa | Third trimester | BMI ≥30 | 24.05% (63/262) |
| Okafor, 201272 | 250 | Nigeria | Third trimester | BMI ≥35 | 14.8% (37/250) |
| Davies, 201270 | 1145 | South Africa | Adjusted BMI for gestational age (GBMI) | BMI >29 to <50 | 33.53% (384/1145) |
| Davies, 201375 | 1058 | South Africa | Adjusted BMI for gestational age (GBMI) | BMI ≥29 | 33.1% (350/1058) |
| Adebami, 200759 | 465 | Nigeria | Not stated | BMI >30 | 17.8% (83/465) |
BMI, body mass index; DRC, Democratic Republic of Congo.
aCalculated as weight in kilograms divided by height in metre squared.
Studies using pre-pregnancy or first trimester measurements suggest obesity prevalence between 9.0 and 17.9%.46,55,58,67,74 Those using ‘booking’ dates (antenatal registration) imply 6.5–44.0%,64,68,69,73 while third trimester reports indicate prevalence between 14.0 and 50.7%.50,57,60,72 The study by Adebami et al.59 while not specifying gestational age, reported a prevalence of 17.8%. Two other studies using ‘BMI adjusted for gestational age’ reported obesity prevalence at 33.1 and 33.5%, respectively.70,75 The study with the highest prevalence was measured among pregnant women scheduled for caesarean section.57
Worth highlighting is the study73 assessing maternal obesity at booking in seven countries: obesity prevalence was estimated as 6.5% in the Democratic Republic of Congo up to 31.7% in Nigeria. Also, of note, maternal obesity (using BMI measured in first trimester) increased from 2.4 to 7.3% over a 9-year period in Tanzania.58
Socio-demographic associations of maternal obesity
Eight socio-demographic outcomes were considered for separate meta-analyses. Obese mothers were significantly older than non-obese mothers (number of studies n = 555,57,60,65,68; MD 2.67 years, 2.12–3.22). Five studies were excluded from meta-analysis on age due to varied data types,58,63 unavailable data64,67 and heterogeneity.71 Maternal obesity was significantly associated with increasing age in three of these studies58,63,64 but not in two.67,71 One study excluded for heterogeneity71 may have had different results, because the women were younger (20–30 years) than the other studies.
The RR of obesity was increased in multiparous women (n = 4,50,65,68,69 REM, RR 1.49, 1.19–1.87) but not significantly in mothers older than 35 years (n = 2,68,69 REM, RR 1.32, 0.96–1.82), married mothers (n = 4,50,66,68,74 RR 1.17, 1.00–1.38), HIV-infected mothers (n = 2,69,70 RR 0.93, 0.77–1.13), mothers with tertiary education (n = 4,65,66,69,70 REM, RR 1.21, 0.92–1.58) or employed mothers (n = 3,65,70,74 RR 0.95, 0.84–1.08).
Meta-analysis was not performed on seven studies investigating parity due to varied data types,55,60 different study group characteristics,56,58 unavailable data64,67 and heterogeneity.70 In these, obesity was positively associated with parity in three studies,55,58,60 but had no association in the other four.56,64,67,70 For one study excluded from meta-analysis on marital status due to different study group characteristics,58 obesity was not associated with marital status. Three studies excluded from the tertiary education meta-analysis because of different study group characteristics,58 unavailable data67 and heterogeneity74 showed one positive relationship between maternal education and obesity58 another negative relationship,74 while the third study showed no relationship between maternal obesity and education.67 Out of two studies not included in the unemployment meta-analysis due to different study group characteristics58 and unavailable data,67 one found that employed women were more likely to be obese,58 while the other reported no relationship between maternal obesity and employment.67
Although two studies67,74 investigated obesity and urban dwelling, one did not provide data.67 However, both reported that urban mothers67,74 were more likely to be obese than rural women. Similarly, although varied data types prevented meta-analysis for social class one study reported mothers in higher social classes were more likely to be obese66 and another showed no significant relationship.68 Although meta-analysis was also not conducted for ethnicity,50,66 because of multiple ethnic groups, individually neither showed significance between ethnicity and obesity.
Nine outcomes were measured by single studies. These indicated no significant association between maternal obesity and wealth,70 type of living accommodation,65 smoking (RR 0.81, 0.42–1.59),70 possession of identity document (RR 1.02, 0.98–1.06),70 booked antenatal clinic (RR 1.00, 0.93–1.07),70 income (RR 0.90, 0.78–1.03),70 formal housing (RR 0.90, 0.74–1.08),70 electricity (RR 1.00, 0.95–1.04)70 or positive tuberculosis infection (RR 1.83, 0.26–12.96).70
Effects of maternal obesity on labour outcomes
Meta-analysis was conducted on six labour outcomes (Supplementary data, Table S4). Obese mothers had increased risks of caesarean section (n = 8,49,50,65,66,68,69,74,75 RR 1.87, 1.64–2.12) (Supplementary data, Fig. S1) and instrumental delivery (n = 8,49,50,63–66,68,69 REM, RR 2.72, 1.29–5.72) (Supplementary data, Fig. S2). Two studies were excluded from the caesarean section meta-analysis due to heterogeneity.63,64 In one, there was no relationship between obesity and caesarean section,64 while in the other, morbidly obese mothers (BMI ≥40 kg/m2) had significantly increased caesarean section rates compared with non-obese mothers (20–25 kg/m2).63 In the only study57 categorizing caesarean section into elective and emergency, obese mothers had marginally increased risks of elective caesarean section (RR 1.22, 1.01–1.47) and reduced risks of emergency caesarean section (RR 0.74, 0.56–0.99).
Overall, there was no significant relationship between maternal obesity and induction of labour, prolonged labour, episiotomy/perineal tear or cephalopelvic disproportion. However, one study (excluded from meta-analysis based on heterogeneity) reported significantly increased risks of induction of labour and perineal tear among morbidly obese mothers compared with non-obese mothers.63
Ten other labour outcomes reported in single studies indicate obese mothers more likely to have intra-operative complications (RR 2.92, 1.77–4.82)57 and less likely to have general anaesthesia (RR 0.32, 0.21–0.51) compared with non-obese mothers.57 Morbidly obese mothers were more likely to require epidural pain relief (RR 60.30, 3.63–1000.71).63 There was no significant relationship between maternal obesity and any indication for caesarean section,57 longitudinal skin incision (RR 1.32, 0.83–2.11),64 failed induction of labour (RR 2.25, 0.44–11.63),64 shoulder dystocia (RR 9.04, 0.49–166.91),50 precipitate labour (RR 0.50, 0.09–2.67),49 difficult laparotomy64 or difficult delivery of neonate during caesarean section.64
Effects of maternal obesity on child outcomes
Several studies with sufficient homogeneity reported on seven child outcomes (Supplementary data, Table S5). Obese mothers had increased risks of macrosomia (n = 9,49,51,62,63,68,69,73–75 REM, RR 1.83, 1.51–2.21) (Supplementary data, Fig. S3) admission of neonate into special care baby or intensive care unit (n = 4,66,68,69,74 RR 1.56, 1.19–2.06), and reduced risks of low-birthweight babies (n = 6,49,50,65,66,68,75 RR 0.73, 0.53–0.99) (Supplementary data, Fig. S4) compared with non-obese mothers. Three studies excluded from the macrosomia meta-analysis based on heterogeneity, also showed positive relationships between maternal obesity and macrosomia.48,50,66 There was no significant association between maternal obesity and stillbirth, perinatal mortality, birth asphyxia using 5 and 1 min APGAR scores (Supplementary data, Fig. S5) or birth injuries.
Eight other child outcomes were assessed by single studies. In these maternal obesity was not associated with foetal malnutrition using the Clinical Assessment of Foetal Nutritional Status score (RR 0.88, 0.44–1.69)59 or congenital abnormality (RR 5.00, 0.24–103.28).69 However, obese mothers had increased risks of ‘baby stay for over 24 h in the hospital’ (RR 1.63, 1.25–2.13),75 increased number of days stayed in the hospital,75 and higher birthweight z-scores, birth length z-scores and head circumference z-scores.75 The risk of infant death (RR 0.28, 0.08–0.93)75 was significantly reduced among obese mothers.
Effects of maternal obesity on maternal outcomes
Meta-analyses were possible on 17 maternal outcomes (Supplementary data, Table S6). Compared with normal weight women, obese mothers were found to have increased risks of wound infection (n = 3,50,63,69 RR 3.21, 1.28–8.06), gestational diabetes mellitus (n = 6,64,66,68,69,74,75 RR 2.42, 1.47–3.98) (Supplementary data, Fig. S6), pregnancy-induced hypertension (n = 3,64,68,75 REM, RR 1.59, 1.02–2.50), pre-eclampsia (n = 9,47,49,50,52,63,66,69,71,74 REM, RR 2.19, 1.58–3.03) (Supplementary data, Fig. S7), antepartum haemorrhage (n = 4,49,50,69,74 RR 3.67, 1.77–7.62) (Supplementary data, Fig. S8), postpartum haemorrhage (n = 6,49,50,66,68,69,74 RR 1.86, 1.18–2.92) (Supplementary data, Fig. S9), maternal hospital admission (n = 3,63,68,75 RR 1.38, 1.21–1.57), urinary tract infection (n = 5,50,63,64,66,69 RR 1.74, 1.05–2.88), postdate pregnancy (n = 6,50,64–66,69,75 RR 1.22, 1.01–1.47), malpresentation (n = 3,49,50,69 RR 3.01, 1.43–6.32), preterm rupture of membranes (n = 2,64,74 RR 2.88, 1.78–4.67) and pre-existing diabetes mellitus (n = 3,50,63,65 RR 2.98, 1.21–7.34). The risks of maternal anaemia were lower among obese mothers, although not significantly (n = 3,50,65,69 RR 0.90, 0.75–1.07). There were no significant associations between maternal obesity and maternal mortality, preterm labour, retained placenta and chronic or essential hypertension. Two studies excluded from meta-analyses based on heterogeneity reported significantly increased risks of pregnancy-induced hypertension in obese66 and morbidly obese mothers,63 and significantly lower risks of maternal anaemia among morbidly obese mothers63 compared with non-obese mothers.
Eleven maternal outcomes assessed by single studies indicate that obese mothers had significantly higher haemoglobin levels (MD 0.95 g/dl, 0.01–1.89),61 and morbidly obese mothers had longer gestation duration (MD 1.20 weeks, 0.76–1.64)63 than non-obese mothers. However, there was no association between maternal obesity and sickle cell anaemia (RR 0.33, 0.01–8.12),69 thromboembolic disease (RR 3.01, 0.12–73.57),50 eclampsia (RR 3.01, 0.12–73.57),50 glycosuria (RR 6.00, 0.74–48.94),49 twin gestation (RR 0.67, 0.11–3.93),69 hyperemesis gravidarum (RR 3.00, 0.12–72.77),49 malaria (RR 0.67, 0.11–3.93),69 miscarriages (RR 0.87, 0.38–2.00)75 or termination of a pregnancy (RR 1.23, 0.21–7.35).75
With respect to weight gain during pregnancy, one study showed that obese mothers were more likely to gain less weight than non-obese mothers (RR 0.60, 0.44–0.83).49 In another study,74 obese mothers had lower risks of gaining either excessive (RR 0.43, 0.30–0.60) or inadequate weight (RR 0.11, 0.06–0.20) based on the Institute of Medicine standards, compared with non-obese mothers. In yet another study, it was reported that weight gain in pregnancy was not significantly different between obese, overweight and normal weight women.55
Discussion
Main finding of this study
This review confirms maternal obesity as an emerging major public health issue in Africa, which is increasing in many African countries but varies between countries. When measured in the third trimester, obesity levels are high (up to 50.7%); however, even when measured in the first trimester, levels in Africa are comparable (up to 17.9%) with some developed countries, where approximately one in five pregnant women are obese.76
Obesity was measured at different time points by individual studies, using different measures and cut-offs points. Two studies used BMI adjusted for gestational age. From this review and other African studies,43,77 pregnant women generally register late for antenatal care, usually in the second trimester. In addition, only urban women with tertiary education knew their pre-pregnancy weights.54,67 Therefore, it may not ever be feasible to use pre-pregnancy or first trimester BMI,14 for diagnosing maternal obesity in these settings. Antenatal booking measurements were used by some studies, but due to varying times of booking across studies, these measurements may be heterogeneous. Several studies have investigated adjusting BMI for gestational age,75,78 BMI centile charts79 and weight gain charts,43 for assessing maternal obesity. It is also worth mentioning that apart from BMI, other anthropometric measurements such as maternal weight and mid-upper arm circumference are associated with adverse pregnancy outcomes.38,43 This warrants further exploration in order to reach a consensus on appropriate standardized measures for maternal obesity. Ideally, women should be encouraged to register early for antenatal care.
This review and others have identified several detrimental effects of obesity on both mother and child.19,20,80,81 The outcomes of maternal obesity could be viewed differently in terms of importance,14 both from a cultural and health system point of view. For example, many African women are averse to caesarean section82 and have poor access to safe caesarean section.83 Some adverse outcomes identified in this study such as haemorrhage and pre-eclampsia are important causes of maternal mortality in sub-Saharan Africa84 and hence might be even more critical in an African setting.
Among the socio-demographic associations, maternal obesity was found to be higher in older, multiparous women and among urban settlements in Africa but not associated with wealth. Obesity was previously seen as a disease of the affluent in developing settings, but recent evidence shows increasing obesity among both poor and rich.85
While addressing the issue of obesity in pregnant women, clinicians and researchers still need to be mindful of maternal under-nutrition. Preterm labour, low birthweight and anaemia were more common in non-obese pregnant women, although only low birthweight was significant in this review. Other studies have shown that preterm labour and anaemia are associated with underweight in pregnancy,86,87 while others88 found that maternal obesity was not independently associated with increased preterm deliveries. While high gestational weight gain might be helpful in preventing low birthweight, this must be balanced against other maternal and child health risks.41
Despite the broad and robust search strategy, this review did not identify any studies in Africa investigating knowledge and attitudes of healthcare professionals relating to maternal obesity let alone for mothers. It is crucial that health professionals and mothers are educated on issues of maternal obesity, as inadequate knowledge and poor attitudes of either group can create barriers to effective obesity interventions.89–91
What is already known on this topic
Several observational studies have been conducted in Africa assessing the prevalence and/or associations between maternal obesity and various health outcomes.
What this study adds
To our knowledge, this is the first comprehensive systematic review on maternal obesity in Africa. It highlights the high prevalence, problems with measurement and adverse effects of obesity for pregnant women across Africa. It also highlights the urgent need for exploratory study(s) with both mothers and healthcare professionals to assess their knowledge and perceptions towards maternal obesity. This will help to develop measurement tools, and tailor interventions incorporating all stakeholders' views for management of maternal obesity in African countries.
Limitations of this study
For the various meta-analyses, the crude study estimates could have introduced bias due to confounding factor effects in the different studies. Owing to different maternal obesity definitions, there is potential for misclassification of obesity with possible overestimation or underestimation of effect sizes. Both pre-pregnancy obesity and excessive gestational weight gain are independently associated with poor pregnancy outcomes.13,20,25,92 This review did not differentiate between pre-pregnancy obesity and excessive gestational weight; therefore, the results should be interpreted in context. Additionally, these studies represent only a few African countries and may not generalize all African women. Finally, four full text articles not retrieved should none-the-less be considered while interpreting results. All four studies (Supplementary data, Table S2) found that maternal obesity in Africa was associated with adverse labour, child or maternal outcomes.
Conclusion
Maternal obesity in Africa is of significant prevalence, with important adverse effects. Culturally adaptable/sensitive interventions should be developed while monitoring to avoid undesired side effects such as low birthweight.
Supplementary data
Funding
This work was supported by the Commonwealth Scholarship Commission in the United Kingdom.
Supplementary Material
References
- 1. Obesity and overweight-fact sheet [WWWdocument] http://www.who.int/mediacentre/factsheets/fs311/en/ (10 December 2012, date last accessed).
- 2.Racusin D, Stevens B, Campbell G et al. Obesity and the risk and detection of fetal malformations. Semin Perinatol 2012;36 (3):213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M, Fleming T, Robinson M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384 (9945):766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popkin BM. Part II. What is unique about the experience in lower- and middle-income less-industrialised countries compared with the very-high-income industrialised countries? The shift in stages of the nutrition transition in the developing world differs from past experiences. Public Health Nutr 2002;5 (1A):205–14. [DOI] [PubMed] [Google Scholar]
- 5.Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity 2009;17 (5):941–64. [DOI] [PubMed] [Google Scholar]
- 6.Field AE, Coakley EH, Must A et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001;161 (13):1581–6. [DOI] [PubMed] [Google Scholar]
- 7.Knight JA. Diseases and disorders associated with excess body weight. Ann Clin Lab Sci 2011;41 (2):107–21. [PubMed] [Google Scholar]
- 8.Kulie T, Slattengren A, Redmer J et al. Obesity and women's health: an evidence-based review. J Am Board Fam Med 2011;24 (1):75–85. [DOI] [PubMed] [Google Scholar]
- 9.Davis EM, Zyzanski SJ, Olson CM et al. Racial, ethnic, and socioeconomic differences in the incidence of obesity related to childbirth. Am J Public Health 2009;99 (2):294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopelman PG. Obesity as a medical problem. Nature 2000;404 (6778):635–43. [DOI] [PubMed] [Google Scholar]
- 11.Heslehurst N, Rankin J, Wilkinson JR et al. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int J Obes 2010;34 (3):420–8. [DOI] [PubMed] [Google Scholar]
- 12.Black RE, Victora CG, Walker SP et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382 (9890):427–51. [DOI] [PubMed] [Google Scholar]
- 13.Guelinckx I, Devlieger R, Beckers K et al. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obesity Reviews 2008;9 (2):140–50. [DOI] [PubMed] [Google Scholar]
- 14.Thangaratinam S, Rogozinska E, Jolly K et al. Interventions to reduce or prevent obesity in pregnant women: a systematic review. Health Technol Assess 2012;16 (31):1–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SY, Dietz PM, England L et al. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity 2007;15 (4):986–93. [DOI] [PubMed] [Google Scholar]
- 16.Callaway LK, Prins JB, Chang AM et al. The prevalence and impact of overweight and obesity in an Australian obstetrics population. Med J Aust 2006;184 (2):56–9. [DOI] [PubMed] [Google Scholar]
- 17.Huda SS, Brodie LE, Sattar N. Obesity in pregnancy: prevalence and metabolic consequences. Semin Fetal Neonatal Med 2010;15 (2):70–6. [DOI] [PubMed] [Google Scholar]
- 18.Brockelsby J, Dresner M. Obesity and pregnancy. Curr Anaesth Crit Care 2006;17 (3–4):125–9. [Google Scholar]
- 19.Bhattacharya S, Campbell DM, Liston WA et al. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health 2007;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heslehurst N, Simpson H, Ells LJ et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev 2008;9 (6):635–83. [DOI] [PubMed] [Google Scholar]
- 21.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol 2002;100 (2):245–52. [DOI] [PubMed] [Google Scholar]
- 22.Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynecol Obstet 2006;93 (3):269–74. [DOI] [PubMed] [Google Scholar]
- 23.Poston L. Gestational weight gain: influences on the long-term health of the child. Curr Opin Clin Nutr Metab Care 2012;15 (3):252–7. [DOI] [PubMed] [Google Scholar]
- 24.Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal fetal health. Rev Obstet Gynaecol 2008;1 (4):170–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Aune D, Saugstad OD, Henriksen T et al. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta-analysis. J Am Med Assoc 2014;311 (15):1536–46. [DOI] [PubMed] [Google Scholar]
- 26.Meehan S, Beck CR, Mair-Jenkins J et al. Maternal obesity and infant mortality: a meta-analysis. Pediatrics 2014;133 (5):863–71. [DOI] [PubMed] [Google Scholar]
- 27.Black RE, Allen LH, Bhutta ZA et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371 (9608):243–60. [DOI] [PubMed] [Google Scholar]
- 28.Haddad L, Cameron L, Barnett I. The double burden of malnutrition in SE Asia and the Pacific: priorities, policies and politics. Health Policy Plan; advance access publication 15 October 2014, doi:10.1093/heapol/czu110. [DOI] [PubMed] [Google Scholar]
- 29.Steyn NP, Mchiza ZJ. Obesity and the nutrition transition in Sub-Saharan Africa. Ann NY Acad Sci 2014;1311 (1):88–101. [DOI] [PubMed] [Google Scholar]
- 30.Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. Am J Clin Nutr 2005;81 (3):714–21. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Oza S, Hogan D et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385 (9966):430–40. [DOI] [PubMed] [Google Scholar]
- 32.Requejo JH, Bryce J, Barros AJ et al. Countdown to 2015 and beyond: fulfilling the health agenda for women and children. Lancet 2015;385 (9966):466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Say L, Chou D, Gemmill A et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2 (6):e323–33. [DOI] [PubMed] [Google Scholar]
- 34.Davies GA, Maxwell C, McLeod L et al. SOGC Clinical Practice Guidelines: obesity in pregnancy. No. 239, February 2010. Int J Gynaecol Obstet 2010;110 (2):167–73. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynaecologists should know. Curr Opin Obstet Gynecol 2009;21 (6):521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleinman KP, Oken E, Radesky JS et al. How should gestational weight gain be assessed? A comparison of existing methods and a novel method, area under the weight gain curve. Int J Epidemiol 2007;36 (6):1275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heslehurst N. Symposium I: consequences of obesity and overweight during pregnancy: identifying at risk women and the impact of maternal obesity on National Health Service maternity services. Proc Nutr Soc 2011;70 (4):439–49. [DOI] [PubMed] [Google Scholar]
- 38.Robinson HE, O'Connell CM, Joseph KS et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol 2005;106 (6):1357–64. [DOI] [PubMed] [Google Scholar]
- 39.Okereke C, Anyaehie U, Dim C et al. Evaluation of some anthropometric indices for the diagnosis of obesity in pregnancy in Nigeria: a cross-sectional study. Afr Health Sci 2014;13 (4):1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott C, Andersen CT, Valdez N et al. No global consensus: a cross-sectional survey of maternal weight policies. BMC Pregnancy Childbirth 2014;14:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alavi N, Haley S, Chow K et al. Comparison of national gestational weight gain guidelines and energy intake recommendations. Obes Rev 2013;14 (1):68–85. [DOI] [PubMed] [Google Scholar]
- 42.Kandala N-B, Stranges S. Geographic variation of overweight and obesity among women in Nigeria: a case for nutritional transition in Sub-Saharan Africa. PLoS ONE 2014;9 (6):e101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruger HS. Maternal anthropometry and pregnancy outcomes: a proposal for the monitoring of pregnancy weight gain in outpatient clinics in South Africa. Curationis 2005;28 (4):40–9. [DOI] [PubMed] [Google Scholar]
- 44.Cresswell JA, Campbell OM, De Silva MJ et al. Effect of maternal obesity on neonatal death in Sub-Saharan Africa: multivariable analysis of 27 national datasets. Lancet 2012;380 (9850):1325–30. [DOI] [PubMed] [Google Scholar]
- 45.Jackson N, Waters E, Guidelines for Systematic Reviews in Health Promotion and Public Health Taskforce. Criteria for the systematic review of health promotion and public health interventions. Health Promot Int 2005;20 (4):367–74. [DOI] [PubMed] [Google Scholar]
- 46.Addo V. Body mass index, weight gain during pregnancy and obstetric outcomes. Ghana Med J 2010;44 (2):64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahomed K, Williams MA, Woelk GB et al. Risk factors for pre-eclampsia among Zimbabwean women: maternal arm circumference and other anthropometric measures of obesity. Paediatr Perinat Epidemiol 1998;12 (3):253–62. [DOI] [PubMed] [Google Scholar]
- 48.Kamanu CI, Onwere S, Chigbu B et al. Fetal macrosomia in African women: a study of 249 cases. Arch Gynecol Obstet 2009;279 (6):857–61. [DOI] [PubMed] [Google Scholar]
- 49.Efiong EI. Pregnancy in the overweight Nigerian. Brit J Obstet Gynaec 1975;82 (11):903–6. [DOI] [PubMed] [Google Scholar]
- 50.Olayemi OO, Umuerri CO, Aimakhu CO. Obstetric performance of Nigerian obese parturients. Trop J Obstet Gynaecol 2002;19 (1):17–20. [Google Scholar]
- 51.Adesina OA, Olayemi O. Fetal macrosomia at the University College Hospital, Ibadan: a 3-year review. J Obstet Gynaecol 2003;23 (1):30–3. [DOI] [PubMed] [Google Scholar]
- 52.Anorlu RI, Iwuala NC, Odum CU. Risk factors for pre-eclampsia in Lagos, Nigeria. Aust NZ J Obstet Gynaecol 2005;45 (4):278–82. [DOI] [PubMed] [Google Scholar]
- 53.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1. 0. The Cochrane Collaboration, updated 2011 www.cochrane-handbook.org (19 June 2015, date last accessed).
- 54.Lawoyin TO. A prospective study on some factors which influence the delivery of large babies. J Trop Med Hyg 1993;96 (6):352–6. [PubMed] [Google Scholar]
- 55.Khan LK, Harrison GG, Galal OM et al. Prevalence and functional correlates of obesity in an Egyptian village. Ecol Food Nutr 1996;34 (4):311–25. [Google Scholar]
- 56.Van Bogaert L. Maternal end-pregnancy ponderal index and birth weight. J Obstet Gynaecol 2005;25 (4):353–4. [DOI] [PubMed] [Google Scholar]
- 57.Edomwonyi NP, Osaigbovo PE. Incidence of obesity in parturients scheduled for caesarean section, intra-operative complications, management and outcome. East Afr Med J 2006;83 (4):112–9. [DOI] [PubMed] [Google Scholar]
- 58.Villamor E, Msamanga G, Urassa W et al. Trends in obesity, underweight, and wasting among women attending prenatal clinics in urban Tanzania, 1995–2004. Am J Clin Nutr 2006;83 (6):1387–94. [DOI] [PubMed] [Google Scholar]
- 59.Adebami O, Oyedeji G, Aderinsola J. The influence of maternal socio-economic and nutritional status on foetal malnutrition in Nigeria. Internet J Third World Med 2007;4 (1) DOI:10.5580/fa1 [Google Scholar]
- 60.Mamabolo RL, Alberts M, Levitt NS et al. Prevalence of gestational diabetes mellitus and the effect of weight on measures of insulin secretion and insulin resistance in third-trimester pregnant rural women residing in the central region of Limpopo province, South Africa. Diabetic Med 2007;24 (3):233–9. [DOI] [PubMed] [Google Scholar]
- 61.Ward E, Kruger HS, van Graan A. The influence of pre-pregnancy BMI and weight gain during pregnancy on pregnancy outcomes. South Afr J Clin Nutr 2007;20 (3):112–7. [Google Scholar]
- 62.Abdul M, Nasir S, Shittu S et al. Maternal risks factors and delivery outcome of fetal macrosomia in Zaria, northern Nigeria. Niger Med Pract 2009;55 (4):64–8. [Google Scholar]
- 63.Ngoga E, Hall D, Mattheyse F et al. Outcome of pregnancy in the morbidly obese woman. South Afr Family Practice 2009;51 (1):39–41. [Google Scholar]
- 64.Basu JK, Jeketera CM, Basu D. Obesity and its outcomes among pregnant South African women. Int J Gynecol Obstet 2010;110 (2):101–4. [DOI] [PubMed] [Google Scholar]
- 65.Ugwuja EI, Akubugwo EI, Obidoa O et al. Maternal BMI during pregnancy: effect on trace elements status and pregnancy outcomes. In J Health Res 2010;3 (2):71–8. [Google Scholar]
- 66.Adesina K, Aderibigbe S, Fawole A et al. Pregnancy outcome of the obese in Ilorin. Obstet Med 2011;4 (4):160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chigbu CO, Aja LO. Obesity in pregnancy in southeast Nigeria. Ann Med Health Serv Sci Res 2011;1 (2):135–40. [PMC free article] [PubMed] [Google Scholar]
- 68.Ezeanochie MC, Ande AB, Olagbuji BN. Maternal obesity in early pregnancy and subsequent pregnancy outcome in a Nigerian population. Afr J Reprod Health 2011;15 (4):55–9. [PubMed] [Google Scholar]
- 69.Jeremiah I, Nyeche S, Akani C et al. Pregnancy outcome among obese parturients at the university of Port Harcourt teaching hospital, Nigeria. J Med Med Sci 2011;2 (10):1152–6. [Google Scholar]
- 70.Davies HR, Visser J, Tomlinson M et al. An investigation into the influence of socioeconomic variables on gestational body mass index in pregnant women living in a peri-urban settlement, South Africa. Matern Child Health J 2012;16 (8):1732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.El-Makhangy IM, Moeity F, Anwer MY. Relationship between maternal obesity and increased risk of preeclampsia. Bull Alex Fac Med 2010;46 (2):207–19. [Google Scholar]
- 72.Okafor UV, Efetie ER, Nwoke O et al. Anaesthetic and obstetric challenges of morbid obesity in caesarean deliveries—a study in south-eastern Nigeria. Afr Health Sci 2012;12 (1):54–7. [PMC free article] [PubMed] [Google Scholar]
- 73.Koyanagi A, Zhang J, Dagvadorj A et al. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet 2013;381 (9865):476–83. [DOI] [PubMed] [Google Scholar]
- 74.Iyoke CA, Ugwu GO, Ezugwu FO et al. Retrospective cohort study of the effects of obesity in early pregnancy on maternal weight gain and obstetric outcomes in an obstetric population in Africa. Int J Women's Health 2013;5:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies HR, Visser J, Tomlinson M et al. An investigation into utilising gestational body mass index as a screening tool for adverse birth outcomes and maternal morbidities in a group of pregnant women in Khayelitsha. South Afr J Clin Nutr 2013;26 (3):116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf) 2013;78 (1):9–16. [DOI] [PubMed] [Google Scholar]
- 77.Ndidi E, Oseremen I. Reasons given by pregnant women for late initiation of antenatal care in the Niger Delta, Nigeria. Ghana Med J 2010;44 (2):47–51. [PMC free article] [PubMed] [Google Scholar]
- 78.Cruz MLS, Harris DR, Read JS et al. Association of body mass index of HIV-1-infected pregnant women and infant birth weight, body mass index, length, and head circumference: the National Institute of Child Health and Human Development International Site Development Initiative Perinatal Study. Nutr Res 2007;27 (11):685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ochsenbein-Kölble N, Roos M, Gasser T et al. Cross-sectional study of weight gain and increase in BMI throughout pregnancy. Eur J Obstet Gynecol Reprod Biol 2007;130 (2):180–6. [DOI] [PubMed] [Google Scholar]
- 80.Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of macrosomia. Obstet Gynecol 2004;191 (3):964–8. [DOI] [PubMed] [Google Scholar]
- 81.Kalk P, Guthmann F, Krause K et al. Impact of maternal body mass index on neonatal outcome. Eur J Med Res 2009;14 (5):216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sunday-Adeoye I, Kalu C. Pregnant Nigerian women's view of cesarean section. Niger J Clin Pract 2011;14 (3):276–9. [DOI] [PubMed] [Google Scholar]
- 83.Chu K, Cortier H, Maldonado F et al. Cesarean section rates and indications in Sub-Saharan Africa: a multi-country study from médecins sans frontières. PLoS ONE 2012;7 (9):e44484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harrison KA. The struggle to reduce high maternal mortality in Nigeria. Afr J Reprod Health 2009;13 (3):9–20. [PubMed] [Google Scholar]
- 85.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70 (1):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Han Z, Mulla S, Beyene J et al. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol 2011;40 (1):65–101. [DOI] [PubMed] [Google Scholar]
- 87.Al-Mehaisen L, Khader Y, Al-Kuran O et al. Maternal anemia in rural Jordan: room for improvement. Anemia 2011;2011:381812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nohr EA. Maternal obesity and neonatal mortality in an African setting. Lancet 2012;380 (9850):1292–3. [DOI] [PubMed] [Google Scholar]
- 89.Smith DM, Cooke A, Lavender T. Maternal obesity is the new challenge: a qualitative study of health professionals’ views towards suitable care for pregnant women with a body mass index (BMI) ≥30 kg/m2 . BMC Pregnancy Childbirth 2012;12 (1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furness P, McSeveny K, Arden M et al. Maternal obesity support services: a qualitative study of the perspectives of women and midwives. BMC Pregnancy Childbirth 2011;11 (1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shub A, Huning EY, Campbell KJ et al. Pregnant women's knowledge of weight, weight gain, complications of obesity and weight management strategies in pregnancy. BMC Res Notes 2013;6 (1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramachenderan J, Bradford J, Mclean M. Maternal obesity and pregnancy complications: a review. Aust NZ J Obstet Gynaecol 2008;48 (3):228–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.