Abstract
Background
Hard water is associated with atopic dermatitis (eczema). We wanted to determine if a baby cleanser and its individual components altered free ionized calcium (Ca2+) in a simulated hard water baby bath. For these studies, an in vitro determination of free Ca2+ in a simulated hard water baby bath, and an in vivo exploratory study of free Ca2+ absorption into skin from hard water were performed.
Methods
Free Ca2+ was measured with an ion-sensitive electrode in vitro in hard water (100–500 ppm, Ca2+) before and after addition of the cleanser and/or its components. In an exploratory study, absorption of Ca2+ into skin from hard water was determined in three female participants (aged 21–29 years).
Results
At an in-use dilution of 1%, the test cleanser reduced free Ca2+ from ~500 ppm to <200 ppm; a 10% in-use dilution bound virtually all free Ca2+. The anionic surfactant component contributed the most to this effect. In the exploratory in vivo study, we measured a reduction of ~15% in free Ca2+ from simulated hard water over 10 minutes.
Conclusion
Baby cleansers can bind free Ca2+ and reduce the effective water hardness of bath water. Reducing the amount of free Ca2+ in the water will reduce the availability of the ion for binding to the skin. Altering or reducing free Ca2+ concentrations in bath water may be an important parameter in creating the ideal baby bath.
Keywords: bath, cleanser, hard water, infant, neonate, surfactant
Introduction
Cleansing approaches, routines, and products must be carefully considered for infants; infant skin is different from the skin of older children and adults, and continues to gradually mature in structure, composition, and function for several years after birth.1,2 The stratum corneum (SC) corneocyte cells are smaller and the SC is much thinner.1 Although infant skin is better hydrated than adult skin, it has lower concentrations of natural moisturizing factor.3 Transepidermal water loss is also higher in infants, and infant skin can both absorb and lose water at a faster rate than adult skin.3 Skin pH is more neutral at birth, but quickly becomes more acidic, with the skin’s “acid mantle” providing a more protective barrier.4 The fact that infant skin is not fully mature may place it at greater risk for the disruption of skin barrier integrity. These differences between adult and infant skin underlie the research and guidelines on factors that constitute an ideal bath for newborns and infants.5
Water alone is limited in its ability to gently and effectively cleanse, particularly for the removal of oily or fatty substances like feces and associated enzymes.5–7 Cleansers can emulsify and dislodge oily materials, soils, and microorganisms more effectively than water, so that these materials can be more easily removed.6,8 Appropriately formulated mild cleansers can prevent drying of the baby’s skin and help support the development of the skin’s natural pH.5,9 Guidelines and expert opinion indicate that infant skin should be cleansed with mild liquid cleansers that are neutral in pH or mildly acidic (pH 5.5–7.0),5,6,9–11 or with those that have minimal impact on the baby’s skin surface pH8,9,12 and have a record of safety.6,9,13 A warm (~105°F) immersion bath (as opposed to a sponge bath), ideally 2 hours after birth, when the infant is stable (thermal, cardiorespiratory), with a mild cleanser that does not disrupt the skin barrier has been found to be a good first bath for newborns.14–16
Hard water has been defined by the US Geological Survey as water containing divalent cations, primarily ionized calcium (Ca2+) and magnesium (Mg2+) at concentrations >120 ppm.17 Water hardness varies by geography and mineral content of the water supply.17,18 Several observational studies suggest that hard water is associated with the development of atopic dermatitis (AD).19–24 Although the relationship between water hardness and AD is not well characterized, reducing water hardness may help in reducing the potential for developing AD. In an arm washing study with different solid bars (sodium soap, triethanolamine soap, and synthetic detergent bar), harder water was found to be more irritating.19
Some common surfactants (soaps, sodium dodecyl sulfate, and polydisperse nonylphenol polyethoxylate [Igepal CO-660, Solvay, Brussels, Belgium]) are known to interact with Ca2+ and Mg2+ ions present in hard water, resulting in precipitation of the surfactant, alteration of micelle behavior, and potentially altering the composition of the solution.25–30 The aim of this study was to investigate whether cleansers formulated for use in a baby bath have the potential to alter the free Ca2+ in the bath and reduce the effective water hardness, thereby improving bath conditions.
Methods
Materials
In order to simulate baby bath water, a solution of deionized water and calcium chloride (CaCl2) was created at various concentrations to reach water hardness equivalents between 100 and 500 ppm. Calcium chloride salt was obtained from Sigma-Aldrich (St Louis, MO, USA). Molar concentrations of calcium chloride solutions were obtained from Ricca Chemical Company (Arlington, TX, USA).
Test solutions
Three test cleansers (commercial baby wash products) and four individual ingredients, components of the test cleaners, were tested for their Ca+2 binding. The three test cleansers were obtained from www.drugstore.com (USA): Johnson’s® Head-To-Toe® Baby Wash (HTT; Johnson & Johnson Consumer Inc., Skillman, NJ, USA), Burt’s Bees® (BB; Burt’s Bees Baby Bee Shampoo & Wash, Durham, NC, USA), and California Baby® (CB; California Baby Super Sensitive™ Shampoo & Bodywash, Los Angeles, CA, USA). As stated on the label, HTT contained water, cocamidopropyl betaine (CAPB), polyethylene glycol (PEG)-80 sorbitan laurate, sodium laureth sulfate (SLES), PEG-150 distearate, glycerin, polyquaternium-10, tetrasodium ethylenediaminetetraacetic acid (EDTA), citric acid, sodium hydroxide, sodium benzoate, ethylhexylglycerin, phenoxyethanol, and fragrance. Four individual components as aqueous solutions, made using deionized water, were also tested: SLES, PEG-80 sorbitan laurate (PEG80SL), EDTA, and decyl glucoside (obtained from Sigma-Aldrich). Additionally, a four component aqueous solution comprising SLES, CAPB, and PEG80SL (SLES/CAPB/PEG80SL) in a 1:1:1 weight ratio was created and tested.
Calcium measurements
All measurements were performed using a Mettler-Toledo DC420 calcium-selective electrode and Mettler-Toledo S47-K SevenMulti™ with ion-selective expansion unit (Mettler-Toledo, LLC, Columbus, OH, USA). The probe was calibrated with commercially prepared calcium carbonate molar solutions (Sigma-Aldrich) from 10 to 1,000 ppm Ca2+ ions. Stirring and measurement were done at room temperature. For measurements, the calcium probe was lowered into the glass beaker (50 mL) containing the test solution, and the concentration was recorded continuously. A reading was obtained when the measurement had stabilized, usually after 30 seconds.
In vitro
The effect of the test cleansers on apparent Ca2+ ion concentrations in solution were made after adding the test cleansers at typical in-use cleanser dilutions of 1% and 10% concentration in deionized water containing 100, 200, 300, and 500 ppm Ca2+. EDTA and test cleanser components were also tested at typical in-use dilutions of 1% and 10% relative to their concentration in commercially supplied test cleansers using 200 and 500 ppm Ca2+ ion solutions. EDTA is typically used at a concentration of 0.5% in products. In this study, EDTA was tested at a dilution of 1% and 10% of the 0.5% stock solution (0.05 wt% and 0.005 wt% of EDTA). Surfactants in typical baby cleansers are about 10 wt% active. Individual surfactant or combinations of surfactants were tested at 1% and 10% dilutions of 10% solution (0.1 wt% and 1.0 wt% of surfactant). Each solution was independently created and measured twice.
In vivo
After providing verbal consent to participate, an exploratory pilot study was performed in three female participants (coauthors KC, MCM, and SA, aged 21–29 years) to determine the absorption of Ca2+ ions into the skin from a simulated baby bath. The authors did not obtain IRB approval for the in-vivo aspect of the study. It was very exploratory in nature and was carried out by the three authors who conceived of the specific experiments, and who are acknowledged as the participants/subjects. Simulated bath water containing 200 and 500 ppm free Ca2+ ions was placed on the skin of the volar forearm over a diameter of 2.5 cm (an area of 4.9 cm2) in a volume of 25 or 50 mL in a glass vial (Fisher Scientific, Waltham, MA, USA). Separate chambers were used for each sampling time of 1, 5, or 10 minutes. The bath solution was added to the chamber and the skin of the arm was pressed against the open top of the chamber (held in place by the participant’s other hand). The participant’s arm was then rotated over so that the solution was against the skin. After the indicated time (1, 5, or 10 minutes), the arm and chamber were rotated back over and the entire sample, in the chamber, was removed from the skin, and then the free Ca2+ ion in solution was determined by placing the ion-selective probe into the chamber. An additional larger 50 mL chamber was used for a 10 minute exposure to confirm the findings from the 25 mL chamber. This volume provided a larger reservoir of Ca2+ to control for possible depletion of the supply of Ca2+ available for absorption by the skin.
Concentrations were monitored for up to 10 minutes as the recommended length of a newborn baby bath is 5–10 minutes.9 Absolute free Ca2+ concentrations as well as change from baseline concentrations were determined. Each exposure was repeated three times for each participant, and the mean concentration for each exposure was recorded.
Reduction in Ca2+ from donor solution was assumed to be due to absorption into the skin. The differences between starting concentration and ending concentration at the different time points were reported as a positive absorption per area of skin exposed (mg/mm2).
Results
In vitro effect of test cleansers and their components on free Ca2+ concentrations in a simulated baby bath
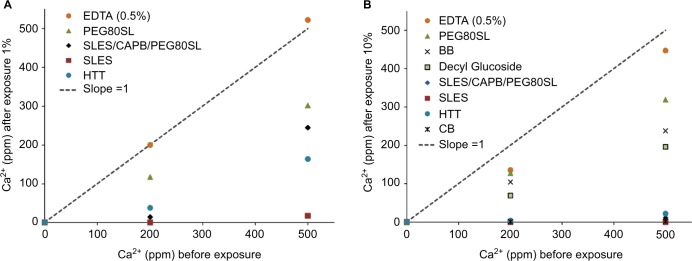
At typical in-use cleanser dilutions of 1% and 10%, the HTT test cleanser reduced measured free Ca2+ in solution at all tested calcium concentrations (Figure 1). The effect of HTT test cleanser components on free Ca2+ ion in solution are shown after an in-use dilution in simulated bath water of 1% (Figure 2A) and 10% (Figure 2B). EDTA had only a slight effect on free Ca2+ ion in solution. The surfactant component SLES appeared to have the greatest effect on free Ca2+. At the 1% in-use dilution, the SLES/CAPB/PEG80SL solution had an effective concentration of 0.33 wt% SLES, 0.33 wt% CAPB, and 0.33 wt% PEG80SL. The 1:1:1 blend resulted in a lower SLES concentration in the final solution, and correspondingly less of an effect on free Ca2+ (Figure 2A). At a 10% in-use dilution, the SLES/CAPB/PEG80SL solution had an effective concentration of 3.3 wt% SLES, 3.3 wt% CAPB, and 3.3 wt% PEG80SL, and reduced free Ca2+ to 17 and 289 ppm from the 200 and 500 ppm CaCl2 water solutions, respectively (Figure 2B).
Figure 1.
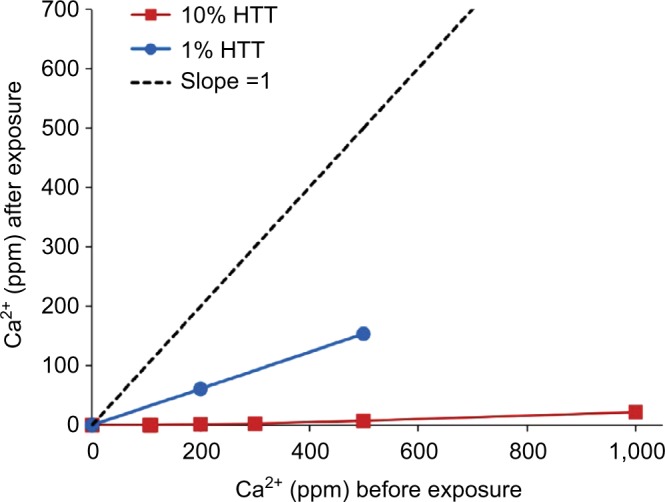
Effect of HTT at 1% and 10% dilution on calcium concentration in simulated baby bath.
Notes: The dotted line with slope =1 illustrates the starting conditions. HTT = Johnson’s® Head-To-Toe® Baby wash (Johnson & Johnson Consumer Inc., Skillman, NJ, USA).
Abbreviation: Ca2+, ionized calcium.
Figure 2.
The effect of HTT cleanser components on free Ca2+ ion in solution.
Notes: (A) In-use dilution 1% and (B) 10%. The line with slope =1 illustrates the starting conditions. BB= Burt’s Bees® (Burt’s Bees Baby Bee Shampoo & Wash, Durham, NC, USA). HTT= Johnson’s® Head-To-Toe® Baby Wash (Johnson & Johnson Consumer Inc., Skillman, NJ, USA. CB= California Baby® Super Sensitive™ Shampoo & Bodywash (Los Angeles, CA, USA).
Abbreviations: Ca2+, ionized calcium; EDTA, ethylenediaminetetraacetic acid; PEG80SL, PEG-80 sorbitan laurate; SLES, sodium laureth sulfate; SLES/CAPB/PEG80SL, sodium laureth sulfate, cocamidopropyl betaine, and PEG-80 sorbitan laurate in a 1:1:1 weight ratio.
HTT, CB, and BB were evaluated at a 10% dilution in a simulated baby bath containing 200 and 500 ppm free Ca2+ (Figure 2B). HTT reduced free Ca2+ to 29 and 4.9 ppm in 200 and 500 ppm CaCl2 test solutions, respectively. CB was as effective as HTT in reducing free Ca2+ to 0.8 and 2.0 ppm. BB and decyl glucoside alone (a component of CB and BB) had similar efficacy and reduced free Ca2+ to 103 ppm in the 200 ppm CaCl2 test solution and 136 ppm in the 500 ppm CaCl2 test solution.
In vivo absorption of calcium into skin
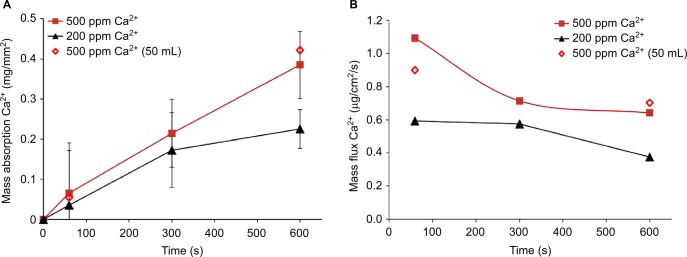
Exposure of Ca2+-containing simulated baby bath to skin for up to 10 minutes (600 seconds) in adult volunteers resulted in a reduction of free Ca2+ in solution, presumably through absorption into the skin (Figure 3A). The absorption of Ca2+ was similar from both 25 and 50 mL exposure chambers, demonstrating that the free Ca2+ in solution was not being depleted in the smaller chamber. For 200 and 500 ppm Ca2+ solutions, the amount of Ca2+ that absorbed into the skin increased with time across the 10 minute test period. Ca2+ absorption appeared to occur faster from the 500 ppm solution compared with the 200 ppm solution.
Figure 3.
Absorption of Ca2+ from donor solutions into volar forearm skin of human volunteers.
Notes: (A) Mass flux (± standard deviation) into the skin per mm2 exposed area. (B) Mass flux per second. Closed symbols are from 25 mL chambers. Open symbols are from 50 mL chambers.
Abbreviation: Ca2+, ionized calcium.
The driving force for absorption of Ca2+ into the skin decreased over the time course of the experiment (Figure 3B). The flux of Ca2+ into the skin decreased slightly over time for the 200 and 500 ppm Ca2+ solutions. As Ca2+ absorbed into the skin, the concentration in the source solution decreased over the time course of the experiment. At 10 minutes (600 seconds), the Ca2+ concentration was ~15% lower than the initial Ca2+ concentration.
Discussion
This study demonstrated that specially formulated baby cleansers can reduce free Ca2+ and thus reduce water hardness of bath water. HTT and CB at in-use dilutions of 1% and 10% were the most effective of the tested cleansers in reducing free Ca2+ in defined hard water solutions. The surfactant components of HTT, SLES, and PEG80SL appeared to be responsible for the majority of the effect of HTT on Ca2+. CB provided reductions in free Ca2+ similar to those observed with HTT. BB reduced free Ca2+, but was not as effective as HTT or CB. Unexpectedly, EDTA alone, a well-known chelator of calcium, did not have much impact on free Ca2+ at the concentrations used in this study.
In vivo, it was observed that higher concentrations of free Ca2+ in water were associated with higher rates of Ca2+ absorption into the skin surface in adults. Thus, bathing in hard water in the absence of a cleanser might result in excess calcium absorption into the skin. The implications of this process for skin health is unclear, however, as the properties of adult and pediatric skin are different. In use (during cleansing and/or in the bath), Ca2+ is likely held within a complex of surfactant molecules. Synthetic detergents, such as alkyl sulfates, form stable soluble complexes with Ca2+,31,32 (over certain concentration ranges of Ca2+)33 and, unlike bar soaps, do not form insoluble complexes that come out of solution and produce soap scum. Furthermore, the addition of the ethylene oxide to alkyl sulfate (eg, SLES) further increases the solubility in hard water.34 As this Ca2+ complex remains stable in solution, the Ca2+ is likely to be washed away during rinsing. Thereby, the Ca2+ likely does not end up on or in the skin after the cleansing, as can happen during washing with soap. Reducing the amount of free Ca2+ in bath water could thereby reduce exposure of the skin to the uncomplexed Ca2+ ion.
Increased exposure to Ca2+ may interfere with normal epidermal calcium distribution/calcium gradient. Normal calcium gradient has been shown to be necessary for terminal differentiation of corneocytes and SC barrier formation.35–37 Interference with skin-barrier formation may be related to development of susceptibility for skin irritation in the presence of hard water.19–21,24
In the US, geological survey data indicate significant variation in water hardness, with hard water (CaCl2 121–180 mg/L) generally localized to the Midwestern states and very hard water (CaCl2 181–250 mg/L) generally localized to the Upper Plains and Rocky Mountain areas.17 Similarly, hard water is found in many areas throughout the world.18
Several studies have demonstrated an association between hard water and the incidence of AD. McNally et al studied atopic eczema prevalence in primary school-aged children in the UK and found a positive association between prevalence of atopic eczema and water hardness.23 No significant association was seen in secondary school-aged children, leading the authors to speculate that the risk was greater in younger children. Similar increased risk of this disease in areas of hard water exposure was noted in Japanese and Spanish children.21,22 The increased susceptibility of younger children to develop AD in the presence of hard water suggested that there might be a critical window of opportunity to protect skin integrity/skin health over the long term, and that hard water softening may offer the most benefit for younger children.
There are only a few studies that attempted to directly evaluate the effect of hard water exposure on AD. In one study in which ion exchange water softeners were installed in participants’ homes, no consistent evidence was presented that hard water softening could reduce the incidence of eczema in areas with naturally hard water.38–40 In another study, ion exchange water softening systems (replacing Ca2+ and Mg2+ with sodium ions) were installed in participants’ homes for 12 weeks, again with little effect on their eczema symptoms or amount of drug usage (eg, steroids, calcineurin inhibitors).38,39 However, a more recent, 6-week, blinded crossover study in participants with less severe AD did show significant symptom improvement40 after installation of an ion exchange water softening system compared with a placebo system, suggesting a possible benefit in participants with moderate severity of disease. The latter study suggests, but does not prove, that reducing water hardness may improve AD. Additional studies must be performed to confirm a possible effect of hard water on AD.
Conclusion
Altering or reducing free Ca2+ concentrations in bath water is an additional parameter in creating the ideal baby bath. Although the relationship between water hardness and development of AD is not well characterized, water softening properties of cleansers may help reduce water hardness that the skin experiences. Additional studies are needed to identify the contribution of specific ingredients, combinations of ingredients, and formulation parameters to achieve water softening in typical baby bath conditions. Also, a larger clinical study is needed to confirm a possible benefit to the skin of cleanser-induced water softening in bath water.
Acknowledgments
Medical writing and editorial assistance were provided by Alex Loeb, PhD, CMPP, Evidence Scientific Solutions, Philadelphia, PA, USA, and was funded by Johnson & Johnson Consumer Inc.
Footnotes
Disclosure
These studies were fully supported by Johnson & Johnson Consumer Inc., Skillman, NJ, USA. The authors report no other conflicts of interest in this work.
References
- 1.Stamatas GN, Nikolovski J, Luedtke MA, Kollias N, Wiegand BC. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol. 2010;27(2):125–131. doi: 10.1111/j.1525-1470.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 2.Lund C, Kuller J, Lane A, Lott JW, Raines DA. Neonatal skin care: the scientific basis for practice. J Obstet Gynecol Neonatal Nurs. 1999;28(3):241–254. doi: 10.1111/j.1552-6909.1999.tb01989.x. [DOI] [PubMed] [Google Scholar]
- 3.Nikolovski J, Stamatas GN, Kollias N, Wiegand BC. Barrier function and water-holding and transport properties of infant stratum corneum are different from adult and continue to develop through the first year of life. J Invest Dermatol. 2008;128(7):1728–1736. doi: 10.1038/sj.jid.5701239. [DOI] [PubMed] [Google Scholar]
- 4.Kanti V, Bonzel A, Stroux A, et al. Postnatal maturation of skin barrier function in premature infants. Skin Pharmacol Physiol. 2014;27(5):234–241. doi: 10.1159/000354923. [DOI] [PubMed] [Google Scholar]
- 5.Blume-Peytavi U, Lavender T, Jenerowicz D, et al. Recommendations from a European roundtable meeting on best practice healthy infant skin care. Pediatr Dermatol. 2016;33(3):311–321. doi: 10.1111/pde.12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blume-Peytavi U, Hauser M, Stamatas GN, Pathirana D, Garcia Bartels N. Skin care practices for newborns and infants: review of the clinical evidence for best practices. Pediatr Dermatol. 2012;29(1):1–14. doi: 10.1111/j.1525-1470.2011.01594.x. [DOI] [PubMed] [Google Scholar]
- 7.Walters R, Fevola M, LiBrizzi J, Martin K. Designing cleansers for the unique needs of baby skin. Cosm Toil. 2008;123(12):53–60. [Google Scholar]
- 8.Kuehl BL, Fyfe KS, Shear NH. Cutaneous cleansers. Skin Therapy Lett. 2003;8(3):1–4. [PubMed] [Google Scholar]
- 9.Blume-Peytavi U, Cork MJ, Faergemann J, Szczapa J, Vanaclocha F, Gelmetti C. Bathing and cleansing in newborns from day 1 to first year of life: recommendations from a European round table meeting. J Eur Acad Dermatol Venereol. 2009;23(7):751–759. doi: 10.1111/j.1468-3083.2009.03140.x. [DOI] [PubMed] [Google Scholar]
- 10.Lund CH, Kuller J, Lane AT, Lott JW, Raines DA, Thomas KK. Neonatal skin care: evaluation of the AWHONN/NANN research-based practice project on knowledge and skin care practices. Association of Women’s Health, Obstetric and Neonatal Nurses/National Association of Neonatal Nurses. J Obstet Gynecol Neonatal Nurs. 2001;30(1):30–40. [PubMed] [Google Scholar]
- 11.Association of Women’s Health Obstetric and Neonatal Nurses . Neonatal Skin Care: Evidence-based Clinical Practice Guideline. 3rd ed. Washington, DC: Association of Women’s Health, Obstetric and Neonatal Nurses; 2013. [Google Scholar]
- 12.Gfatter R, Hackl P, Braun F. Effects of soap and detergents on skin surface pH, stratum corneum hydration and fat content in infants. Dermatology. 1997;195(3):258–262. doi: 10.1159/000245955. [DOI] [PubMed] [Google Scholar]
- 13.Dizon MV, Galzote C, Estanislao R, Mathew N, Sarkar R. Tolerance of baby cleansers in infants: a randomized controlled trial. Indian Pediatr. 2010;47(11):959–963. doi: 10.1007/s13312-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 14.Varda KE, Behnke RS. The effect of timing of initial bath on newborn’s temperature. J Obstet Gynecol Neonatal Nurs. 2000;29(1):27–32. doi: 10.1111/j.1552-6909.2000.tb02753.x. [DOI] [PubMed] [Google Scholar]
- 15.Loring C, Gregory K, Gargan B, et al. Tub bathing improves thermoregulation of the late preterm infant. J Obstet Gynecol Neonatal Nurs. 2012;41(2):171–179. doi: 10.1111/j.1552-6909.2011.01332.x. [DOI] [PubMed] [Google Scholar]
- 16.Bryanton J, Walsh D, Barrett M, Gaudet D. Tub bathing versus traditional sponge bathing for the newborn. J Obstet Gynecol Neonatal Nurs. 2004;33(6):704–712. doi: 10.1177/0884217504270651. [DOI] [PubMed] [Google Scholar]
- 17.United States Geological Survey Office of Water Safety Water Hardness and Alkalinity. 2012. [Accessed April 3, 2013]. Available from: http://water.usgs.gov/owq/hardness-alkalinity.html.
- 18.United Nations Environment Programme Global Environment Monitoring System/Water Programme Water Quality for Ecosystem and Human Health. 2008. [Accessed February 19, 2015]. Available from: http://www.unwater.org/wwd10/downloads/water_quality_human_health.pdf.
- 19.Warren R, Ertel KD, Bartolo RG, Levine MJ, Bryant PB, Wong LF. The influence of hard water (calcium) and surfactants on irritant contact dermatitis. Contact Dermatitis. 1996;35(6):337–343. doi: 10.1111/j.1600-0536.1996.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsai TF, Maibach HI. How irritant is water? An overview. Contact Dermatitis. 1999;41(6):311–314. doi: 10.1111/j.1600-0536.1999.tb06990.x. [DOI] [PubMed] [Google Scholar]
- 21.Arnedo-Pena A, Bellido-Blasco J, Puig-Barbera J, et al. Domestic water hardness and prevalence of atopic eczema in Castellon (Spain) school children. Salud Publica Mex. 2007;49(4):295–301. doi: 10.1590/s0036-36342007000400009. Spanish. [DOI] [PubMed] [Google Scholar]
- 22.Miyake Y, Yokoyama T, Yura A, Iki M, Shimizu T. Ecological association of water hardness with prevalence of childhood atopic dermatitis in a Japanese urban area. Environ Res. 2004;94(1):33–37. doi: 10.1016/s0013-9351(03)00068-9. [DOI] [PubMed] [Google Scholar]
- 23.McNally NJ, Williams HC, Phillips DR, et al. Atopic eczema and domestic water hardness. Lancet. 1998;352(9127):527–531. doi: 10.1016/s0140-6736(98)01402-0. [DOI] [PubMed] [Google Scholar]
- 24.Osborne DW. Hard water and skin irritation. J Am Acad Dermatol. 1987;16(6):1263–1264. doi: 10.1016/s0190-9622(87)80029-4. [DOI] [PubMed] [Google Scholar]
- 25.Hu P, Tuvell M. Effect of water hardness ions on the solution properties of an anionic surfactant. J Am Oil Chem Soc. 1988;65(8):1340–1345. [Google Scholar]
- 26.Parkhurst HJ. Toilet soaps, soap substitutes and hard water: A study of various combinations by patch tests. Arch Dermatol Syphilol. 1941;43(2):299–310. [Google Scholar]
- 27.Rodriguez CH, Chintanasathien C, Scamehorn JF, Saiwan C, Chavadej S. Precipitation in solutions containing mixtures of synthetic anionic surfactant and soap. I. Effect of sodium octanoate on hardness tolerance of sodium dodecyl sulfate. J Surfactants Deterg. 1998;1(3):321–328. [Google Scholar]
- 28.Sammalkorpi M, Karttunen M, Haataja M. Ionic surfactant aggregates in saline solutions: sodium dodecyl sulfate (SDS) in the presence of excess sodium chloride (NaCl) or calcium chloride (CaCl2) J Phys Chem B. 2009;113(17):5863–5870. doi: 10.1021/jp901228v. [DOI] [PubMed] [Google Scholar]
- 29.Stellner KL, Scamehorn JF. Hardness tolerance of anionic surfactant solutions. 1. Anionic surfactant with added monovalent electrolyte. Langmuir. 1989;5(1):70–77. [Google Scholar]
- 30.Stellner KL, Scamehorn JF. Hardness tolerance of anionic surfactant solutions. 2. Effect of added nonionic surfactant. Langmuir. 1989;5(1):77–84. [Google Scholar]
- 31.Draelos Z. Cosmetics and Dermatologic Problems and Solutions. 3rd ed. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- 32.Rieger M, Rhein L. Surfactants in Cosmetics. 2nd ed. New York: Marcel Decker; 1997. [Google Scholar]
- 33.Homendra N, Devi CI. Turbidity studies on mixed surfactant systems in hard water: A new method for estimation of water hardness. Indian J Chem Technol. 2004;11:783–786. [Google Scholar]
- 34.Zoller U. Handbook of Detergents, Part E: Applications. Boca Raton, FL: CRC Press; 2008. [Google Scholar]
- 35.Celli A, Sanchez S, Behne M, Hazlett T, Gratton E, Mauro T. The epidermal Ca2+ gradient: Measurement using the phasor representation of fluorescent lifetime imaging. Biophys J. 2010;98(5):911–921. doi: 10.1016/j.bpj.2009.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicanová J, Boelsma E, Mommaas AM, et al. Normalization of epidermal calcium distribution profile in reconstructed human epidermis is related to improvement of terminal differentiation and stratum corneum barrier formation. J Invest Dermatol. 1998;111(1):97–106. doi: 10.1046/j.1523-1747.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- 37.Elias PM, Nau P, Hanley K, et al. Formation of the epidermal calcium gradient coincides with key milestones of barrier ontogenesis in the rodent. J Invest Dermatol. 1998;110(4):399–404. doi: 10.1046/j.1523-1747.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- 38.Thomas KS, Dean T, O’Leary C, et al. A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLoS Med. 2011;8(2):e1000395. doi: 10.1371/journal.pmed.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas KS, Koller K, Dean T, et al. A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: The Softened Water Eczema Trial (SWET) Health Technol Assess. 2011;15(8):v–vi. 1–156. doi: 10.3310/hta15080. [DOI] [PubMed] [Google Scholar]
- 40.Togawa Y, Kambe N, Shimojo N, et al. Ultra-pure soft water improves skin barrier function in children with atopic dermatitis: A randomized, double-blind, placebo-controlled, crossover pilot study. J Dermatol Sci. 2014;76(3):269–271. doi: 10.1016/j.jdermsci.2014.10.009. [DOI] [PubMed] [Google Scholar]