Abstract
Acute or chronic injury of the adult mammalian brain is often associated with persistent functional deficits as its potential for regeneration and capacity to rebuild lost neural structures is limited. However, the discovery that neural stem cells (NSCs) persist throughout life in discrete regions of the brain, novel approaches to induce the formation of neuronal and glial cells, and recently developed strategies to generate tissue for exogenous cell replacement strategies opened novel perspectives how to regenerate the adult brain. Here, we will review recently developed approaches for brain repair and discuss future perspectives that may eventually allow for developing novel treatment strategies in acute and chronic brain injury.
Key Words: Stem cells, Brain, Brain injury, Regeneration
Introduction
The brain is at constant risk to be hurt and impaired in its function by traumatic injuries or chronic diseases, associated with inflammation and neurodegeneration. In contrast to many other organs and tissues such as the skin or liver in mammals that retain the capacity to regenerate and regain at least partially lost functions, the brain cannot simply regrow injured areas that become functionally connected to the unharmed regions of the brain [1]. The inability of the mammalian brain to regenerate has been associated with its complex functions and the required need for stability, which is in contrast to lower species such as certain invertebrates that retain the capacity to regrow substantial parts of the brain upon injury [2,3]. However, even the human brain holds substantial potential for at least partial compensation and thus functional repair by a process called functional neuroplasticity: for example, motor or sensory skills that are lost in the course of an ischemic stroke can be - with time and training - taken over by other brain regions that had not been involved in those motor or sensory functions before the injury [4].
But even though injury-induced functional regeneration and compensation can have a substantial impact on life quality, it often fails to allow for an acceptable level of functionality leaving patients suffering acute or chronic injury with severe disabilities. Thus, strategies need to be developed that aim to support the brain's attempts to regenerate. In principle, three different approaches have been pursued over the last decades. In the following, we will review the main approaches that are designed to improve functional regeneration of the injured brain and that are based on i) activation and recruitment of endogenous neural stem cells (NSCs), ii) reprogramming of neural cells for tailored cell replacement therapies, and iii) exogenous transplantation-based approaches (fig. 1). Finally, we discuss potential next steps to move forward innovative regenerative approaches for brain repair because, despite substantial advances in the last years, the vast majority of potential therapies are currently far from being used in the clinical routine.
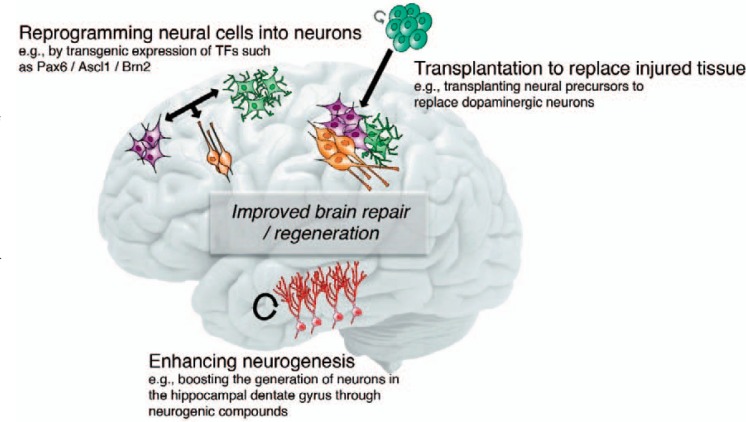
Fig. 1.
Enhancing brain repair and regeneration. Shown are three main aspects how regeneration of adult brain may be achieved in the future. This may become possible by enhancing the activity of endogenous NSCs to generate neurons, for example by using compounds that enhance the formation of new neurons. Further, the ectopic generation of neurons (or glial cells such as myelinating oligodendrocytes) outside the neurogenic niches may be achieved by expression of transcription factors inducing fate changes such as SOX2 and Ascl1. Furthermore, the development of novel cell sources may improve the benefits of studies based on the transplantation of neural cells to replace lost tissue where the aim is to either transplant stem cells or their differentiated progeny to enhance brain repair. For details please refer to the main text.
Harnessing Endogenous Neural Stem Cells for Brain Repair
It has been a longstanding dogma in the neurosciences that all neurons in the mammalian brain are generated during embryonic and early postnatal neurogenesis. However, first evidence generate in the mid-1960s suggested that even in the adult brain new neurons are generated throughout life in distinct regions [5]. However, it took almost another 30 years and the advancement of technical approaches such as the thymidine analogue BrdU and confocal microscopy to unambiguously show that i) a cell is newborn in the adult brain and ii) differentiates into a neuron [6]. It is now fully accepted that new neurons are generated throughout life in mammals [7].
However, there seem to be substantial species differences: whereas in rodents (and also non-human primates) a substantial number of NSCs persist in the subventricular zone (SVZ) that generate new cells migrating via the rostral migratory stream (RMS) towards the olfactory bulb (OB) where they differentiate into different types of olfactory neurons, this neurogenic system seems to be absent/inactive in the adult human brain [8,9,10,11]; but see also [12]. In contrast, new neurons are born throughout life in the hippocampal dentate gyrus (DG) both in rodents and primates including humans [13,14,15]. The hippocampus is required for certain forms of learning and in simple terms serves as a filter station that is required for many types of memory and determines which of these memories are transferred into long-term storage [16]. Furthermore, the hippocampus is involved in the regulation of mood [17].
In the DG, NSCs reside in the subgranular zone where they divide and generate cells that differentiate into excitatory, glutamatergic granule cells and that integrate into the DG over the course of several weeks [18,19]. This process, called adult hippocampal neurogenesis, is important for proper hippocampal function and critically involved in dentate computation of incoming information [20,21,22]. Thus, the main purpose of neurogenesis in the DG is certainly not to serve as a backup system for repair in the case of injury. Neurogenesis is required for normal brain function.
However, failing or altered neurogenesis has been associated with several neuropsychiatric diseases. For example, it has been shown that stress, one of the key components in the etiology of affective disorders, substantially decreases the number of newborn neurons [23]. Furthermore, it has been shown that certain antidepressants such as selective serotonin-reuptake inhibitor (SSRI; e.g., fluoxetine) enhance neurogenesis and require proper neurogenesis for their antidepressant action, at least in rodents [24,25,26]. Thus, altered neurogenesis may participate in the disease process of affective disorders such as major depression. Given this, novel compounds that specifically target the activity of NSCs or later steps of neuronal differentiation/integration may have beneficial effects in the treatment of affective disorders.
However, neurogenesis cannot only be reduced, but there are also circumstances where the integration of newborn granule cells is affected: epileptic seizures induce the ectopic generation of granule cells that do not show proper integration into the DG circuit [27,28]. They extend aberrant processes and show aberrant migration, for example into the hilus of the DG. Thus, it has been speculated that seizure-induced, aberrant neurogenesis may interfere with proper circuit function in epilepsy patients and may also contribute to the process of epileptogenesis [29]. Targeting NSCs and the neurogenic process in depression or epilepsy may hold the potential to ameliorate disease symptoms or to attenuate disease progression.
Normalizing or enhancing neurogenesis in the context of diseases affecting this process is only one aspect how the neurogenic permissiveness of adult NSCs may be useful to ameliorate brain function. Clearly, the finding that NSCs persist in the adult brain has also opened new possibilities for targeted induction of neurogenesis in the adult brain. When can this be helpful? Many degenerative diseases of the nervous system are associated with substantial neuronal cell death in the chronic phase of the disease although neural death and degeneration also affects early and subclinical phases of many brain diseases. Even though novel treatment options appear on the horizon that for example attenuate disease progression in Alzheimer's disease (AD) by reducing the amyloid load in affected patients [30,31], it is also clear that neurons that are lost at the stage of diagnosis/treatment will be chronically lost. Thus, enhancing neurogenesis in diseases such as AD that dramatically affect hippocampal function may be useful to improve brain function by providing new neurons for the injured hippocampus. If enhanced neurogenesis is efficient to enhance cognitive function in humans remains unclear but is certainly an exciting area where the combination of approaches to attenuate disease progression (e.g., lowering amyloid load) together with regenerative strategies (i.e., enhancing neurogenesis) may turn out to be effective and clinically relevant in the future [32].
As outlined above, neurogenesis in the SVZ/OB seems to extremely low or completely absent in the human brain [11]. However, there is evidence that NSCs may be also retained in the human SVZ but that they are just not generating cells that migrate to the OB [10,12,33]. Interestingly, it has been recently shown that neuroblasts are present also in the adult human SVZ and neighboring striatum and that they are able to generate new striatal interneurons throughout life in the human brain [34]. Strikingly, this type of neurogenesis appears to be substantially lower in patients with Huntington's disease (HD), suggesting that approaches to enhance striatal neurogenesis may represent a novel therapeutic approach in HD [34]. Thus, enhancing neurogenesis in the SVZ may also hold the potential for repair in the human brain. Basic research in the SVZ aiming to understand how NSCs become activated may turn out to be helpful to induce neurogenesis also in the human SVZ [35].
NSCs may not represent the only source for neurons generated in the human brain. Whereas in the rodent SVZ NSCs are capable to respond to injuries such as ischemic strokes that lesion the neighboring striatum resulting in the enhanced generation and subsequent striatal migration of new neurons generated in the SVZ, strokes in human seem not to directly affect neurogenesis in the SVZ [36,37,38]. However, recent data in mice showed that local astroglial cells carry a latent neurogenic program (e.g., being capable to generate neuronal cells) that becomes activated upon ischemic stroke [39]. Mechanistically, the latent neurogenic program depends on Notch signaling, providing an entry point how this novel route for neurogenesis may also be targeted in human diseases such as stroke [39].
Taken together, the finding that NSCs remain active in the adult mammalian brain and that new neurons are generated throughout life has opened novel approaches to either ameliorate disease symptoms or to truly induce neurogenesis in areas where neurons are lost in the context of acute or chronic degenerative disease.
Generating Neuronal and Glial Cells at the Site of Injury
Apart from strategies to utilize the neurogenic potential of endogenous NSCs to locally generate new neuronal cells, an alternative approach has been to ectopically induce the generation of neuronal (and glial) cells to support brain repair [40,41]. This is based on a vast amount of data from basic research aiming to characterize the transcriptional programs that guide neurogenesis during embryonic development and in the adult neurogenic niches. The idea was to use this knowledge to redirect the fate of newborn cells towards a neuronal fate (or any desired cell fate). Thus, key neurogenic transcription factors used alone or in combinations with other fate determinants have been successfully used in the rodent brain to induce functional neurogenesis, for example after experimental lesions of the cortex [42]. Starting cells that were targeted - mostly by retroviral vectors expressing key transcription factors - include astroglial (i.e., astrocytes) and oligodendroglial (i.e., oligodendrocyte precursors) cells that were directed to generate neurons or other required cell types such as oligodendrocytes in the context of demyelinating disease [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57]. So far the generation of several distinct neuronal subtypes has been achieved. This is obviously important to replace the exact neuronal subtype that is lost in the respective diseases. One example, where neuronal cell replacement may turn out to be feasible is Parkinson's disease (PD) where mostly dopaminergic neurons are lost. Current attempts aim to generate dopaminergic neurons by targeted overexpression of single transcription factors or cocktails of previously identified key regulators within the striatum (the target area of most dopaminergic neurons extending their axons) or directly in the substantia nigra (where dopaminergic neurons are lost in PD) [56,58].
Notably, the tailored generation of cells for brain repair is not restricted to neuronal cells. Clearly, a variety of brain diseases are associated with a loss of function of glial cells. Of particular interest are oligodendrocytes that form myelin sheets around axons, which are required for proper neuronal function. Impaired myelin function and subsequent demyelination is a common feature of a variety of diseases ranging from multiple sclerosis (MS) to epilepsy [59,60]. Death of myelinating oligodendrocytes leads ultimately to neuronal loss and functional impairments. In the context of MS, immune cells attack myelinating oligodendrocytes and subsequently kill them. Whereas the therapeutic possibilities and treatment options of the initial inflammatory phase of the disease have substantially improved over the last decades, there is still no regenerative approach available that may help to induce remyelination and thus prevent functional loss that is associated with secondary neuronal cell death [59,61]. Thus, inducing the formation of myelinating oligodendrocytes either by activating oligodendrocytes precursor cells or by reprogramming other neural cells into myelinating oligodendrocytes may represent a promising approach [62,63]. In this context, it has also been shown that NSCs in the DG that do not generate oligodendrocytes under normal conditions retain the potential for oligodendrocyte differentiation and can be redirected to adopt an oligodendroglial fate [64,65]. Those newborn oligodendrocytes have at least in animal models of demyelinating disease the potential for remyelination [66].
Reprogramming cell fate has the exciting potential to tailor cell generation depending on the missing cell type. At the same time, all those approaches are currently rather invasive (e.g., virus-based overexpression of transcription factors) and thus relatively far away from clinical applications. But the experimental approaches currently tested in animal models of disease may identify novel routes how to replace lost neural cells in the human brain.
Transplantation of Neural Stem Cells and Their Progeny to the Injured Brain
Boosting endogenous NSC activity or targeting neural cells to redirect them towards a neuronal fate represent approaches to target endogenous cell sources for neural cell replacement and repair. In addition, the strategy to transplant either neuronal cells or their precursors has been investigated over the last decades and has been also clinically used [67,68]. Transplantation-based approaches are probably most suitable for diseases in which selected neuronal subtypes are affected, such as dopaminergic neurons in PD or striatal interneurons in HD, and to a lesser extent when more widespread degeneration occurs, as for example in AD. In fact, clinical trials have been performed for PD in which dopaminergic precursor cells were isolated from the fetal mesencephalon followed by transplantation into the diseased brain. However, results were mixed [69,70,71]. As it turned out, one of the key factors in predicting the success of transplants was the quality and quantity of the transplant. Given that the cell source was fetal mesencephalic precursors (derived from aborted fetuses), restricting the availability and also containing progenitors for cells that may have adverse effects upon transplantation (e.g., GABAergic neurons), it became clear that a source of transplantable cells that is i) available at large quantities for standardization and ii) contains exactly the cell type required (in this case dopaminergic neurons/precursors) will be desirable [72].
Substantial efforts have been undertaken in the last years to generate experimental protocols to yield pure dopaminergic neuronal populations derived from embryonic NSCs or pluripotent stem cells such as embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs). In fact, there are several promising approaches identified that at least in non-human primates showed exciting results in the context of PD models [58,73,74]. At this time new clinical trials are prepared that will use novel cell sources to test again the feasibility and efficacy of cell transplants to treat neurological disease [72]. This is not limited to PD but may also turn out to be applicable to other chronic degenerative diseases such as HD.
Perspectives and Conclusions
Regenerating the injured brain to allow for functional recovery remains a currently unmet challenge. Even though progress has been made in the last decades to ameliorate disease symptoms and to slow down disease progression for some diseases, the large majority of acute or chronic neurodegenerative diseases are still associated with substantial functional impairments in the everyday life of affected patients. Thus, novel approaches to regenerate the injured adult brain are needed [1]. The finding that NSCs persist in the adult brain and that neurogenesis occurs throughout life has spurred new hopes that the permissiveness of the adult brain to support life-long neurogenesis can be harnessed for repair. In addition, substantial progress has been made to understand the molecular and cellular mechanisms regulating neuronal differentiation and subsequent integration [75].
Thus, it is probably realistic to hope that we will be able in the future to either recruit endogenous neurogenic cells such as NSCs or to reprogram neural cells into neurons. However, a key challenge will remain that neurons are not only generated but that they are capable to find their proper way into the preexisting circuit allowing for meaningful and correct integration. It is plausible to speculate that a wrongly integrated neuron or the wrong neuronal subtype may do more harm than improving brain function [76,77]. Even though regional specifics are likely to exist, the adult brain gave us with the DG that harbors neurogenic NSCs under physiological conditions a system where we can study neuronal differentiation and integration of newborn neurons that apparently find their way into the circuit. In the future, we will have to understand more details how new neurons achieve this. These experiments will aim to discover more molecular details of adult neurogenesis, but we also need improved methods to study the process on a cellular level, e.g., using advanced imaging approaches to study the neurogenic process directly within the adult mammalian brain. The knowledge derived from these experiments will not only be important to develop strategies to recruit/activate endogenous neural cells for cell replacement. They will be also important to optimize transplantation-based approaches with the aim to achieve the best function of transplants in the adult brain. However, strategies aiming to transplant exogenous cells for brain repair will require further improved and optimized approaches to deliver cells safely and efficiently.
One apparent problem in the field is the dependence on animal models of acute or chronic diseases of the brain. This will not change, and in vivo experimental studies will remain a key pillar of brain regeneration research. However, the hope is that these experiments will be complemented in the future with approaches using human tissues grown in the culture dish that are derived from human NSCs or ESCs/iPSCs. Great progress has been made over the last years, and it became possible to grow organoids that resemble structurally at least early steps of brain development and that will allow for testing novel approaches directly in human cells/tissues [78]. Such organoid-based approaches will need to be standardized and improved, but they may represent a novel direction allowing for faster translation of basic research into human treatment strategies. Potential applications are the use of human organoids to screen for compounds enhancing NSC activity or redirecting the fate of newborn cells. In combination with conventional experimental research using animal models of disease, the novel tools will hopefully bring us one step closer to achieve significant and functional brain regeneration in the future.
Disclosure Statement
The author declares no competing financial interests.
Acknowledgments
The work in our laboratory is supported by the Swiss National Science Foundation, the European Research Council, the Novartis Foundation, and the EMBO Young Investigator program.
References
- 1.Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron. 2013;80:588–601. doi: 10.1016/j.neuron.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 2.Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2012;72:429–461. doi: 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- 3.Barbosa JS, Sanchez-Gonzalez R, Di Giaimo R, Baumgart EV, Theis FJ, Gotz M, Ninkovic J. Neurodevelopment. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science. 2015;348:789–793. doi: 10.1126/science.aaa2729. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen PM, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Aphasia in acute stroke: incidence, determinants, and recovery. Ann Neurol. 1995;38:659–666. doi: 10.1002/ana.410380416. [DOI] [PubMed] [Google Scholar]
- 5.Altman J, Das GD. Autoradiographic and histologic evidence of postnatal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 9.Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MS, Steier P, Kutschera W, Johnson L, Landen M, Druid H, Spalding KL, Frisen J. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 13.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 14.Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, Possnert G, Mash DC, Druid H, Frisen J. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 16.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. Erratum in Psychol Rev. 1992;99:582. [DOI] [PubMed] [Google Scholar]
- 17.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun SM, Jessberger S. Adult neurogenesis: mechanisms and functional significance. Development. 2014;141:1983–1986. doi: 10.1242/dev.104596. [DOI] [PubMed] [Google Scholar]
- 20.Clelland CD, Choi M, Romberg C, Clemenson GDJr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 24.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 25.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 26.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/ca3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: Functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jessberger S, Parent JM. Epilepsy and adult neurogenesis. Cold Spring Harb Perspect Biol. 2015;7:a020677. doi: 10.1101/cshperspect.a020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, Davey G, Moritz E, Nitsch RM. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nat Med. 2002;8:1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- 31.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 32.Jessberger S, Gage FH. Adult neurogenesis: bridging the gap between mice and humans. Trends Cell Biol. 2014;24:558–563. doi: 10.1016/j.tcb.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 34.Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 35.Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Huttner HB, Bergmann O, Salehpour M, Racz A, Tatarishvili J, Lindgren E, Csonka T, Csiba L, Hortobagyi T, Mehes G, Englund E, Solnestam BW, Zdunek S, Scharenberg C, Strom L, Stahl P, Sigurgeirsson B, Dahl A, Schwab S, Possnert G, Bernard S, Kokaia Z, Lindvall O, Lundeberg J, Frisen J. The age and genomic integrity of neurons after cortical stroke in humans. Nat Neurosci. 2014;17:801–803. doi: 10.1038/nn.3706. [DOI] [PubMed] [Google Scholar]
- 37.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nature Med. 2002;5:5. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 38.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 39.Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisen J. A latent neurogenic program in astrocytes regulated by notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- 40.Berninger B, Jessberger S. Engineering of adult neurogenesis and gliogenesis. Cold Spring Harb Perspect Biol. 2016;8:a018861. doi: 10.1101/cshperspect.a018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinrich C, Spagnoli FM, Berninger B. In vivo reprogramming for tissue repair. Nat Cell Biol. 2015;17:204–211. doi: 10.1038/ncb3108. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Wernig M, Berninger B, Nakafuku M, Parmar M, Zhang CL. In vivo reprogramming for brain and spinal cord repair. eNeuro. 2015;2 doi: 10.1523/ENEURO.0106-15.2015. ENEURO-0106-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Miao Q, Yuan J, Han S, Zhang P, Li S, Rao Z, Zhao W, Ye Q, Geng J, Zhang X, Cheng L. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J Neurosci. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Islam MM, Smith DK, Niu W, Fang S, Iqbal N, Sun G, Shi Y, Zhang CL. Enhancer analysis unveils genetic interactions between TLX and SOX2 in neural stem cells and in vivo reprogramming. Stem Cell Reports. 2015;5:805–815. doi: 10.1016/j.stemcr.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu W, Zang T, Smith DK, Vue TY, Zou Y, Bachoo R, Johnson JE, Zhang CL. SOX2 reprograms resident astrocytes into neural progenitors in the adult brain. Stem Cell Reports. 2015;4:780–794. doi: 10.1016/j.stemcr.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinrich C, Bergami M, Gascon S, Lepier A, Vigano F, Dimou L, Sutor B, Berninger B, Gotz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014;3:1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Gotz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Gotz M. Glial cells generate neurons: the role of the transcription factor pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 51.Heinrich C, Blum R, Gascon S, Masserdotti G, Tripathi P, Sanchez R, Tiedt S, Schroeder T, Gotz M, Berninger B. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8:e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang CL. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nature Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor olig2 in response to brain injuries: Implications for neuronal repair. Proc Natl Acad Sci U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grande A, Sumiyoshi K, Lopez-Juarez A, Howard J, Sakthivel B, Aronow B, Campbell K, Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karow M, Sanchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascon S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Gotz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torper O, Ottosson DR, Pereira M, Lau S, Cardoso T, Grealish S, Parmar M. In vivo reprogramming of striatal ng2 glia into functional neurons that integrate into local host circuitry. Cell Reports. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franklin RJ, French-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 60.Kremer D, Gottle P, Hartung HP, Kury P. Pushing forward: remyelination as the new frontier in CNS diseases. Trends Neurosci. 2016;39:246–263. doi: 10.1016/j.tins.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Naegele M, Martin R. The good and the bad of neuroinflammation in multiple sclerosis. Handb Clin Neurol. 2014;122:59–87. doi: 10.1016/B978-0-444-52001-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 62.Huang JK, Fancy SP, Zhao C, Rowitch DH, French-Constant C, Franklin RJ. Myelin regeneration in multiple sclerosis: targeting endogenous stem cells. Neurotherapeutics. 2011;8:650–658. doi: 10.1007/s13311-011-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rafalski VA, Ho PP, Brett JO, Ucar D, Dugas JC, Pollina EA, Chow LM, Ibrahim A, Baker SJ, Barres BA, Steinman L, Brunet A. Expansion of oligodendrocyte progenitor cells following sirt1 inactivation in the adult brain. Nature Cell Biol. 2013;15:614–624. doi: 10.1038/ncb2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jessberger S, Gage FH. Fate plasticity of adult hippocampal progenitors: Biological relevance and therapeutic use. Trends i Pharmacol Sci. 2009;30:61–65. doi: 10.1016/j.tips.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun GJ, Zhou Y, Ito S, Bonaguidi MA, Stein-O'Brien G, Kawasaki NK, Modak N, Zhu Y, Ming GL, Song H. Latent tri-lineage potential of adult hippocampal neural stem cells revealed by nf1 inactivation. Nat Neurosci. 2015;18:1722–1724. doi: 10.1038/nn.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Braun SM, Pilz GA, Machado RA, Moss J, Becher B, Toni N, Jessberger S. Programming hippocampal neural stem/progenitor cells into oligodendrocytes enhances remyelination in the adult brain after injury. Cell Reports. 2015;11:1679–1685. doi: 10.1016/j.celrep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 67.Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–544. doi: 10.1038/75705. [DOI] [PubMed] [Google Scholar]
- 68.Lindvall O. Cerebral implantation in movement disorders: State of the art. Mov Disord. 1999;14:201–205. doi: 10.1002/1531-8257(199903)14:2<201::aid-mds1001>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 69.Lindvall O, Sawle G, Widner H, Rothwell JC, Bjorklund A, Brooks D, Brundin P, Frackowiak R, Marsden CD, Odin P, et al. Evidence for long-term survival and function of dopaminergic grafts in progressive Parkinson's disease. Ann Neurol. 1994;35:172–180. doi: 10.1002/ana.410350208. [DOI] [PubMed] [Google Scholar]
- 70.Wenning GK, Odin P, Morrish P, Rehncrona S, Widner H, Brundin P, Rothwell JC, Brown R, Gustavii B, Hagell P, Jahanshahi M, Sawle G, Bjorklund A, Brooks DJ, Marsden CD, Quinn NP, Lindvall O. Short- and long-term survival and function of unilateral intrastriatal dopaminergic grafts in Parkinson's disease. Ann Neurol. 1997;42:95–107. doi: 10.1002/ana.410420115. [DOI] [PubMed] [Google Scholar]
- 71.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 72.Barker RA, Barrett J, Mason SL, Bjorklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 2013;12:84–91. doi: 10.1016/S1474-4422(12)70295-8. [DOI] [PubMed] [Google Scholar]
- 73.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acady Sci U S A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 75.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scharfman HE, Hen R. Neuroscience. Is more neurogenesis always better? Science. 2007;315:336–338. doi: 10.1126/science.1138711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hagell P, Piccini P, Bjorklund A, Brundin P, Rehncrona S, Widner H, Crabb L, Pavese N, Oertel WH, Quinn N, Brooks DJ, Lindvall O. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 78.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]