Abstract
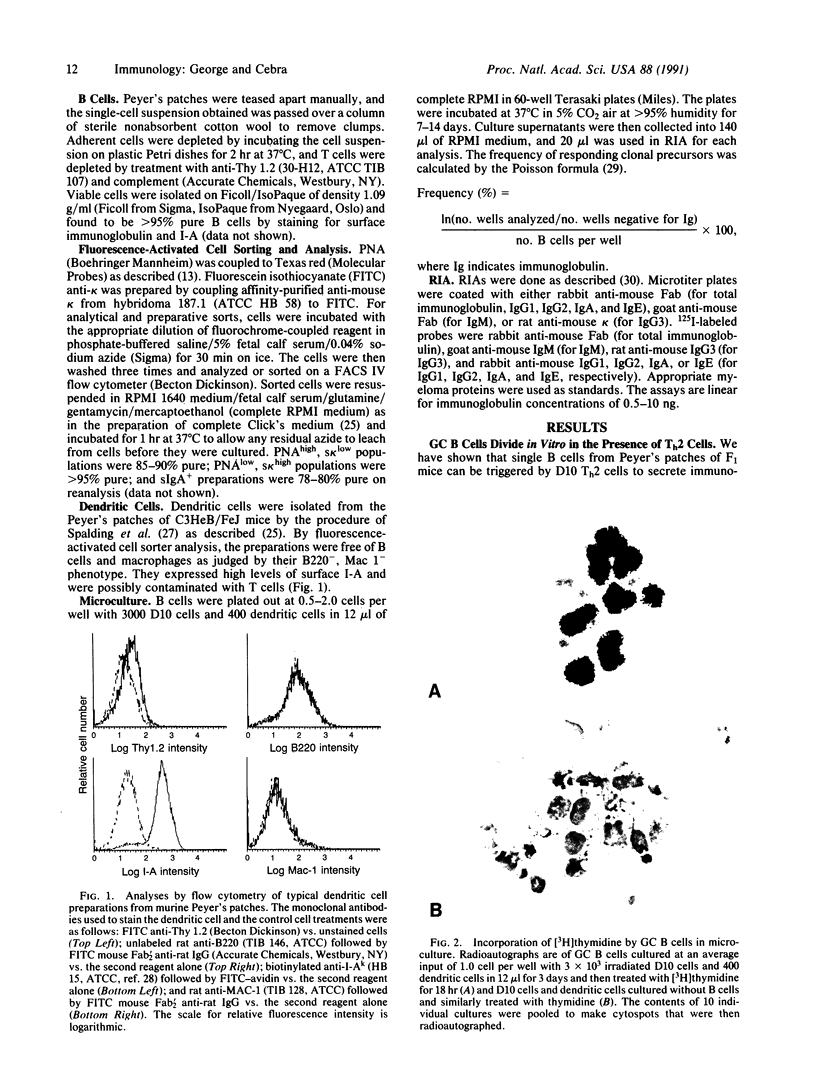
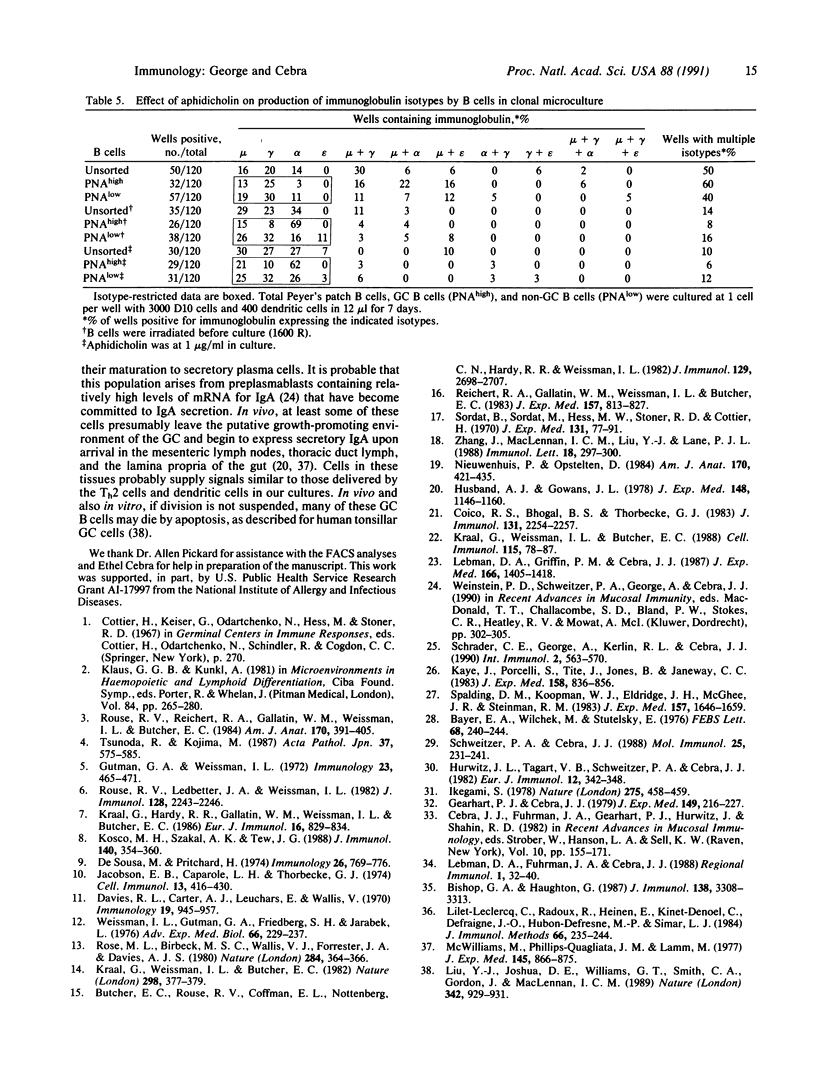
B cells purified from the germinal centers (GCs) of murine Peyer's patches can be stimulated in a clonal microculture containing helper T cells and dendritic cells to divide and secrete immunoglobulin. Intraclonal isotype switching occurs, and a variety of immunoglobulin isotypes, including IgA, is secreted. Memory cells, which generate clones secreting IgA exclusively, are only rarely identified in the GC B-cell subset. Such memory cells can, however, be readily identified among unfractionated Peyer's patch B cells, and in non-GC subsets of B cells. The results suggest that the GC does not contain IgA memory cells that can be restimulated in vitro to secrete only IgA. When division of GC B cells is prevented by irradiation or aphidicholin treatment, a large subset that secretes IgA as the sole immunoglobulin isotype is seen, and the output of presumably single B cells is large enough to be scored by RIA. Both helper T cells and dendritic cells are required for the phenomenon. The data indicate that commitment to IgA secretion occurs in Peyer's patch GCs and suggest that the prolific cell division known to be supported in GCs may forestall terminal differentiation of preplasmablasts to immunoglobulin secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Wilchek M., Skutelsky E. Affinity cytochemistry: the localization of lectin and antibody receptors on erythrocytes via the avidin-biotin complex. FEBS Lett. 1976 Oct 1;68(2):240–244. doi: 10.1016/0014-5793(76)80445-0. [DOI] [PubMed] [Google Scholar]
- Bishop G. A., Haughton G. Role of the interleukin 2 receptor in differentiation of a clone of Ly-1+ B cells. J Immunol. 1987 May 15;138(10):3308–3313. [PubMed] [Google Scholar]
- Butcher E. C., Rouse R. V., Coffman R. L., Nottenburg C. N., Hardy R. R., Weissman I. L. Surface phenotype of Peyer's patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982 Dec;129(6):2698–2707. [PubMed] [Google Scholar]
- Coico R. F., Bhogal B. S., Thorbecke G. J. Relationship of germinal centers in lymphoid tissue to immunologic memory. VI. Transfer of B cell memory with lymph node cells fractionated according to their receptors for peanut agglutinin. J Immunol. 1983 Nov;131(5):2254–2257. [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V., Dietrich F. M. The morphology of immune reactions in normal, thymectomized and reconstituted mice. 3. Response to bacterial antigens: salmonellar flagellar antigen and pneumococcal plysaccharide. Immunology. 1970 Dec;19(6):945–957. [PMC free article] [PubMed] [Google Scholar]
- De Sousa M., Pritchard H. The cellular basis of immunological recovery in nude mice after thymus grafting. Immunology. 1974 Apr;26(4):769–776. [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Cebra J. J. Differentiated B lymphocytes. Potential to express particular antibody variable and constant regions depends on site of lymphoid tissue and antigen load. J Exp Med. 1979 Jan 1;149(1):216–227. doi: 10.1084/jem.149.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman G. A., Weissman I. L. Lymphoid tissue architecture. Experimental analysis of the origin and distribution of T-cells and B-cells. Immunology. 1972 Oct;23(4):465–479. [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J. L., Tagart V. B., Schweitzer P. A., Cebra J. J. Patterns of isotype expression by B cell clones responding to thymus-dependent and thymus-independent antigens in vitro. Eur J Immunol. 1982 Apr;12(4):342–348. doi: 10.1002/eji.1830120416. [DOI] [PubMed] [Google Scholar]
- Husband A. J., Gowans J. L. The origin and antigen-dependent distribution of IgA-containing cells in the intestine. J Exp Med. 1978 Nov 1;148(5):1146–1160. doi: 10.1084/jem.148.5.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Jacobson E. B., Caporale L. H., Thorbecke G. J. Effect of thymus cell injections on germinal center formation in lymphoid tissues of nude (thymusless) mice. Cell Immunol. 1974 Sep;13(3):416–430. doi: 10.1016/0008-8749(74)90261-5. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosco M. H., Szakal A. K., Tew J. G. In vivo obtained antigen presented by germinal center B cells to T cells in vitro. J Immunol. 1988 Jan 15;140(2):354–360. [PubMed] [Google Scholar]
- Kraal G., Hardy R. R., Gallatin W. M., Weissman I. L., Butcher E. C. Antigen-induced changes in B cell subsets in lymph nodes: analysis by dual fluorescence flow cytofluorometry. Eur J Immunol. 1986 Jul;16(7):829–834. doi: 10.1002/eji.1830160718. [DOI] [PubMed] [Google Scholar]
- Kraal G., Weissman I. L., Butcher E. C. Germinal centre B cells: antigen specificity and changes in heavy chain class expression. Nature. 1982 Jul 22;298(5872):377–379. doi: 10.1038/298377a0. [DOI] [PubMed] [Google Scholar]
- Kraal G., Weissman I. L., Butcher E. C. Memory B cells express a phenotype consistent with migratory competence after secondary but not short-term primary immunization. Cell Immunol. 1988 Aug;115(1):78–87. doi: 10.1016/0008-8749(88)90163-3. [DOI] [PubMed] [Google Scholar]
- Lebman D. A., Fuhrman J. A., Cebra J. J. Intraduodenal application of cholera holotoxin increases the potential of clones from Peyer's patch B cells of relevant and unrelated specificities to secrete IgG and IgA. Reg Immunol. 1988 Jul-Aug;1(1):32–40. [PubMed] [Google Scholar]
- Lebman D. A., Griffin P. M., Cebra J. J. Relationship between expression of IgA by Peyer's patch cells and functional IgA memory cells. J Exp Med. 1987 Nov 1;166(5):1405–1418. doi: 10.1084/jem.166.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilet-Leclercq C., Radoux D., Heinen E., Kinet-Denoël C., Defraigne J. O., Houben-Defresne M. P., Simar L. J. Isolation of follicular dendritic cells from human tonsils and adenoids. I. Procedure and morphological characterization. J Immunol Methods. 1984 Feb 10;66(2):235–244. doi: 10.1016/0022-1759(84)90334-x. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Joshua D. E., Williams G. T., Smith C. A., Gordon J., MacLennan I. C. Mechanism of antigen-driven selection in germinal centres. Nature. 1989 Dec 21;342(6252):929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- McWilliams M., Phillips-Quagliata J. M., Lamm M. E. Mesenteric lymph node B lymphoblasts which home to the small intestine are precommitted to IgA synthesis. J Exp Med. 1977 Apr 1;145(4):866–875. doi: 10.1084/jem.145.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis P., Opstelten D. Functional anatomy of germinal centers. Am J Anat. 1984 Jul;170(3):421–435. doi: 10.1002/aja.1001700315. [DOI] [PubMed] [Google Scholar]
- Reichert R. A., Gallatin W. M., Weissman I. L., Butcher E. C. Germinal center B cells lack homing receptors necessary for normal lymphocyte recirculation. J Exp Med. 1983 Mar 1;157(3):813–827. doi: 10.1084/jem.157.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. L., Birbeck M. S., Wallis V. J., Forrester J. A., Davies A. J. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980 Mar 27;284(5754):364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- Rouse R. V., Ledbetter J. A., Weissman I. L. Mouse lymph node germinal centers contain a selected subset of T cells--the helper phenotype. J Immunol. 1982 May;128(5):2243–2246. [PubMed] [Google Scholar]
- Rouse R. V., Reichert R. A., Gallatin W. M., Weissman I. L., Butcher E. C. Localization of lymphocyte subpopulations in peripheral lymphoid organs: directed lymphocyte migration and segregation into specific microenvironments. Am J Anat. 1984 Jul;170(3):391–405. doi: 10.1002/aja.1001700313. [DOI] [PubMed] [Google Scholar]
- Schrader C. E., George A., Kerlin R. L., Cebra J. J. Dendritic cells support production of IgA and other non-IgM isotypes in clonal microculture. Int Immunol. 1990;2(6):563–570. doi: 10.1093/intimm/2.6.563. [DOI] [PubMed] [Google Scholar]
- Schweitzer P. A., Cebra J. J. Quality of antibodies secreted by clones in microcultures from B cells enriched on haptenated gelatin: isotypes and avidities. Mol Immunol. 1988 Mar;25(3):231–241. doi: 10.1016/0161-5890(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Sordat B., Sordat M., Hess M. W., Stoner R. D., Cottier H. Specific antibody within lymphoid germinal center cells of mice after primary immunization with horseradish peroxidase: a light and electron microscopic study. J Exp Med. 1970 Jan 1;131(1):77–91. doi: 10.1084/jem.131.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding D. M., Koopman W. J., Eldridge J. H., McGhee J. R., Steinman R. M. Accessory cells in murine Peyer's patch. I. Identification and enrichment of a functional dendritic cell. J Exp Med. 1983 May 1;157(5):1646–1659. doi: 10.1084/jem.157.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda R., Kojima M. A light microscopical study of isolated follicular dendritic cell-clusters in human tonsils. Acta Pathol Jpn. 1987 Apr;37(4):575–585. doi: 10.1111/j.1440-1827.1987.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Weissman I. L., Gutman G. A., Friedberg S. H., Jerabek L. Lymphoid tissue architecture. III. Germinal centers, T cells, and thymus-dependent vs thymus-independent antigens. Adv Exp Med Biol. 1976;66:229–237. doi: 10.1007/978-1-4613-4355-4_35. [DOI] [PubMed] [Google Scholar]
- Zhang J., MacLennan I. C., Liu Y. J., Lane P. J. Is rapid proliferation in B centroblasts linked to somatic mutation in memory B cell clones? Immunol Lett. 1988 Aug;18(4):297–299. doi: 10.1016/0165-2478(88)90178-2. [DOI] [PubMed] [Google Scholar]




