Abstract
BACKGROUND
The Australian National Cervical Screening Program, introduced more than 20 years ago, does not record the Indigenous status of screening participants. This article reports the first population‐based estimates of participation in cervical screening for Indigenous and non‐Indigenous Australian women.
METHODS
This was a retrospective, population‐based study of 1,334,795 female Queensland residents, aged 20 to 69 years, who participated in cervical screening from 2000 to 2011; 26,829 were identified as Indigenous through linkage to hospitalization records. Participation rates were calculated as the number of women screened divided by the average estimated resident population, with adjustments made for hysterectomies, for each 2‐, 3‐, and 5‐year screening period. Multivariate logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs), which were adjusted for age group, place of residence, and socioeconomic disadvantage.
RESULTS
In 2010‐2011, the 2‐year participation rate was 55.7% (95% CI, 55.6%‐55.9%) for non‐Indigenous women and 33.5% (95% CI, 32.9%‐34.1%) for Indigenous women; this represented a decrease from 2000‐2001 (57.7% [95% CI, 57.6%‐57.9%] and 35.3% [95% CI, 34.5%‐36.1%], respectively). The difference between Indigenous and non‐Indigenous women was greatest for those aged 45 to 49 years. The 3‐ and 5‐year participation rates were higher within both groups, and the absolute differences between the 2 groups were larger. Significant interactions between the Indigenous status and the place of residence and socioeconomic disadvantage highlight that the Indigenous/non‐Indigenous differential was evident in all places of residence except for very remote areas (OR, 0.99; 95% CI, 0.95‐1.02) and was greatest in the most affluent areas (OR, 0.26; 95% CI, 0.24‐0.27).
CONCLUSIONS
Indigenous Australian women participate less than non‐Indigenous women, and this gap has not closed. These results provide important benchmarks for the new Australian cervical screening program commencing in 2017, which will provide opportunities to reduce inequities for Indigenous women and address longstanding data deficiencies in the collection of the Indigenous status. Cancer 2016;122:1560–9. © 2016 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: cancer, cervical screening, early detection, health care disparities, Indigenous Australian, Papanicolaou (Pap) test
Short abstract
Despite over 20 years of organized cervical screening in Australia, participation is much lower among Indigenous Australian women than non‐Indigenous women in Queensland, and this gap has not closed. This report of population‐based screening participation provides benchmark figures for Indigenous Australian women before major changes to the Australian national cervical screening program which will be implemented in 2017.
INTRODUCTION
The Australian National Cervical Screening Program (NCSP) includes the Pap Smear Registers (PSRs), which record all Papanicolaou (Pap) tests performed in Australia and send reminders for overdue Pap tests to women and their primary care providers.1 Since the introduction of the NCSP in 1991, cervical cancer incidence and mortality have been halved,1 and they are among the lowest in the world.2 The NCSP currently recommends 2‐year Pap tests for women aged 20 to 69 years, regardless of their human papillomavirus (HPV) vaccination status, sexual orientation, ethnicity, or religion. The PSRs provide data for detailed national reporting of screening participation; in 2012‐2013, 58% of eligible Australian women underwent a Pap test.1
Cervical cancer has a greater impact on Aboriginal and Torres Strait Islander women (hereafter respectfully called Indigenous women) than non‐Indigenous women. The cervical cancer incidence is approximately 2 times higher (20 vs 9 per 100,000) and the mortality rates are 4 times higher (8 vs 2 deaths per 100,000) for Indigenous women versus non‐Indigenous women.1 Indigenous women are more likely to have advanced disease when they are diagnosed3 and lower survival rates than non‐Indigenous women (5‐year survival rate, 57.6% vs 78.3%).4
Several localized studies, mostly in remote communities or regions, have reported much lower screening participation for Indigenous women than non‐Indigenous women.5, 6, 7, 8, 9 Participation varied considerably and ranged from 20% to 64% in Queensland and from 17% to 75% in the Northern Territory (NT) for Indigenous women.5, 6 Since its inception, the NCSP has been unable to report on screening participation or other outcomes for Indigenous women because the Indigenous status is not routinely collected on pathology request and report forms (PSRs' primary data source). Consequently, no information is available to assess the effectiveness of different access and service delivery models or the effectiveness of interventions to increase participation for Indigenous women.1
We identified Indigenous women in the NCSP by linking PSR records to hospital inpatient records, for which the quality of the Indigenous status is known to be reasonably high.10 For the first time, we can report on screening participation for Indigenous women versus non‐Indigenous women for an entire state, and this includes time trends, variation by age group, place of residence, and area‐level socioeconomic status.
MATERIALS AND METHODS
Data Sources and Linkage
As previously described,11 records were extracted from the Queensland PSR for all Pap tests and cervical biopsies performed between February 8, 1999 (the date on which the Queensland PSR commenced) and December 31, 2011, with the following inclusion criteria: a woman aged 20 to 69 years at the time of the Pap test who was a Queensland resident and had not opted out of the PSR. The PSR extract included the date of birth (month/year), the place of residence (suburb/postcode), test date, test type (cytology/histology), and test result. Women were excluded if insufficient address details meant that a Queensland statistical local area (SLA) could not be assigned to their place of residence.
Records were extracted from the Queensland Health Admitted Patient Data Collection (QHAPDC) for all women who were identified as Indigenous for an inpatient episode in a Queensland public hospital during 1995‐2011. In the most recent audit of Indigenous status data in 2011‐2012 in Queensland, 87% (95% confidence interval [CI], 84%‐91%) of people who self‐reported being Indigenous were recorded as Indigenous in hospital records.10 The QHAPDC extract was probabilistically matched to the PSR extract on the basis of weighted key variables (eg, name, birth date, and place of residence) and clerical review. Women in the PSR who were linked to the QHAPDC extract of Indigenous women were classified as Indigenous if they were identified as Indigenous in the majority (>50%) of their inpatient episodes.11, 12 Remaining women, including those whose PSR record or records did not link to the QHAPDC extract, were classified as non‐Indigenous.
Participation
Participation rates were calculated with the NCSP method1 and were measured as the percentage of women in the population aged 20 to 69 years who had been screened at least once within a specific time period. To calculate this, the number of women who were recorded in the PSR with at least 1 Pap test within a specific time period (numerator) was divided by the average estimated resident population (ERP) for the same time period (denominator); this was adjusted to include only those who required cervical screening (females aged 20‐69 years with an intact cervix) because women who have undergone a hysterectomy with their cervix removed do not require cervical screening. Although the exact number of women with an intact cervix is not known, national hysterectomy fractions (all women combined) have been calculated to estimate these values.1 Because these are not available separately for Indigenous and non‐Indigenous women, the same fractions were used for both Indigenous and non‐Indigenous ERPs. When a woman had more than 1 Pap test in the specified time period, only her first Pap test was counted.
The 2‐year participation rate was calculated for each 5‐year age group (20‐24, 25‐29, …, 65‐69 y) separately for Indigenous and non‐Indigenous women for each 2‐year period between 2000‐2001 and 2010‐2011. This analysis was also stratified by the remoteness of residence and area‐level disadvantage. The same method was used for calculating participation for 3‐year intervals (ie, 2000‐2002, 2003‐2005, 2006‐2008, and 2009‐2011) and 5‐year intervals (ie, 2000‐2004 and 2005‐2009).
Five‐year participation rates were calculated with the NCSP method, and to demonstrate how the numerator affected the estimates, 2 alternative methods for deriving the numerator were used: 1) counting the last Pap test (rather than their first) of a woman in the reporting period and 2) identifying a cohort of women who were 20 to 69 years old at the start of the 5‐year period and counting their first Pap test. The denominator in each case was the average ERP, adjusted by hysterectomy.
Geographical Variables
Residential suburb and postcode information at the time of each Pap test was mapped to the 2011 SLAs. If the address information was insufficient to assign an SLA, information from the woman's next available record was used. At the time of each Pap test, SLAs were used to classify the place of residence as 1 of the 5 categories of the Accessibility/Remoteness Index of Australia13 and to determine an area‐level socioeconomic disadvantage measure with the Index of Relative Socio‐Economic Advantage and Disadvantage (IRSAD).14
Statistical Analyses
Distributions of characteristics are presented as proportions for categorical variables, with chi‐square tests used to compare groups. To account for differences in age structures between Indigenous and non‐Indigenous women, we directly age‐standardized the participation rates according to the age distribution for women in the 2001 Australian ERP.15
To assess the screening history, we looked at the experience of all women aged 30 to 69 years who had undergone a Pap test in 2010‐2011 (and likely should have undergone screening in the previous 10 years). Using a 10‐year look‐back period (2000‐2009), we classified their screening history as any screening versus no previous screening.
Logistic regression was used to examine the association (expressed as an odds ratio [OR]) between Indigenous status and participation in screening. Because the PSR is population‐based, counts of nonscreened women in the population are assumed to be the difference between the ERP and the number of women screened. The proportion of women who opt out of the PSR (and, therefore, are counted as nonscreeners) has been reported to be less than 1% in other states, but it has not been reported for Queensland.16, 17 The models for each outcome included 5 a priori, categorical, independent variables of interest: Indigenous status, age at Pap test, 5‐year age group (reference category, 20‐24 y), place of residence (reference category, major city), and IRSAD (reference category, most disadvantaged). Each independent variable was also assessed for the presence of an interaction with Indigenous status; only Indigenous status × place of residence and Indigenous status × IRSAD were statistically significant (P < .01) and were included. To report the impact of the interactions, using the margins and lincom commands in Stata, we calculated adjusted ORs for Indigenous women versus non‐Indigenous women for each category of IRSAD and place of residence. Simple linear regression was used to graphically represent the association of participation rates and time periods stratified by Indigenous status.
All analyses were conducted with Stata 14.0 (StataCorp, College Station, Texas). Approvals from the ethics committees of Queensland Health (HREC/15/QCH/19‐957), the NT Department of Health and Menzies School of Health Research (HOMER‐2012‐1737), and Charles Darwin University (H12093) were obtained, with approvals for data access and linkage from data custodians, the Queensland Research Linkage Group, and the Director General of Queensland Health.
RESULTS
After the exclusion of 1545 women with conflicting dates of birth (2.0% Indigenous) and the exclusion of 3164 women (3.9% Indigenous) with insufficient address details (11,072 Pap tests), the final data set contained 1,334,795 women residing in Queensland with 4,565,250 Pap tests performed during 2000‐2011. Of these women, 26,829 (2%) were identified as Indigenous (87,372 Pap tests). Indigenous and non‐Indigenous women in the cohort were younger than women in the respective ERPs (likely because of women captured at their first Pap test), with minor differences in the place of residence for Indigenous women (Table 1).
Table 1.
Demographic Characteristics of Women at Their First Recorded Papanicolaou Test in the Queensland Pap Smear Register and Corresponding Proportions of Women in the Estimated Resident Population, 2000–2011
| Variable | Indigenous, % | Non‐Indigenous, % | ||
|---|---|---|---|---|
| Study Cohort (n = 26,829 or 2.0%)a | ERP (n = 36,306 or 2.8%)b | Study Cohort (n = 1,307,966 or 98.0%)a | ERP (n = 1,256,567 or 97.2%)b | |
| Age group | ||||
| 20–24 y | 31.6 | 16.8 | 21.7 | 10.8 |
| 25–29 y | 17.2 | 14.9 | 14.7 | 10.8 |
| 30–34 y | 14.1 | 14.4 | 13.6 | 11.1 |
| 35–39 y | 11.3 | 13.6 | 12.4 | 11.6 |
| 40–44 y | 8.6 | 11.6 | 10.8 | 11.6 |
| 45–49 y | 6.7 | 9.5 | 8.9 | 11.2 |
| 50–54 y | 4.4 | 7.5 | 7.1 | 10.4 |
| 55–59 y | 3.0 | 5.6 | 5.0 | 9.2 |
| 60–64 y | 2.1 | 3.8 | 3.5 | 7.6 |
| 65–69 y | 1.0 | 2.5 | 2.3 | 5.9 |
| IRSAD | ||||
| Most disadvantaged | 27.4 | 38.5 | 11.4 | 13.3 |
| Quintile 2 | 34.6 | 26.9 | 21.8 | 21.8 |
| Quintile 3 | 18.2 | 19.8 | 23.1 | 24.5 |
| Quintile 4 | 15.2 | 10.6 | 25.0 | 23.5 |
| Most affluent | 4.6 | 4.2 | 18.8 | 17.0 |
| Place of residence | ||||
| Major city | 25.0 | 29.4 | 62.2 | 63.5 |
| Inner regional | 15.9 | 17.3 | 19.3 | 19.9 |
| Outer regional | 39.5 | 31.0 | 16.7 | 14.9 |
| Remote | 7.9 | 7.9 | 1.1 | 0.9 |
| Very remote | 11.7 | 14.4 | 0.7 | 0.8 |
Abbreviations: ERP, estimated resident population; IRSAD, Index of Relative Socio‐Economic Advantage and Disadvantage.
Number of women by their characteristics at their first Papanicolaou test as a proportion of the total number of women by Indigenous status.
Average number of women as a proportion divided by the average ERP from 2000 to 2011.
Two‐Year Participation
Age‐standardized 2‐year participation rates decreased between 2000‐2001 and 2010‐2011 from 35.3% (95% CI, 34.5%‐36.1%) to 33.5% (95% CI, 32.9%‐34.1%) for Indigenous women and from 57.7% (95% CI, 57.6%‐57.9%) to 55.7% (95% CI, 55.6%‐55.9%) for non‐Indigenous women (Table 2). Two‐year participation was more than 20 percentage points lower (range, 21.1‐23.6) for Indigenous women versus non‐Indigenous women throughout the study (Fig. 1). For 2010‐2011 screening rates to be equal for both groups, 8606 extra Indigenous women would have needed to be screened.
Table 2.
Age‐Standardized Participation Rates of Women Aged 20 to 69 Years Over Time by Indigenous Status, 2000–2001 to 2010–2011, in Queensland
| Year | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) |
|---|---|---|
| 2‐y participation | ||
| 2000–2001 | 35.3 (34.5–36.1) | 57.7 (57.6–57.9) |
| 2002–2003 | 36.3 (35.5–37.1) | 57.4 (57.3–57.6) |
| 2004–2005 | 35.5 (34.8–36.2) | 56.5 (56.4–56.7) |
| 2006–2007 | 35.1 (34.4–35.8) | 57.9 (57.8–58.0) |
| 2008–2009 | 34.3 (33.7–35.0) | 58.0 (57.8–58.1) |
| 2010–2011 | 33.5 (32.9–34.1) | 55.7 (55.6–55.9) |
| 3‐y participation | ||
| 2000–2002 | 44.0 (43.1–44.8) | 69.2 (69.0–69.4) |
| 2003–2005 | 44.7 (43.8–45.5) | 69.9 (69.8–70.1) |
| 2006–2008 | 43.2 (42.4–43.9) | 69.8 (69.7–70.0) |
| 2009–2011 | 41.8 (41.1–42.5) | 68.3 (68.2–68.5) |
| 5‐y participation | ||
| 2000–2004 | 51.6 (50.7–52.6) | 80.6 (80.4–80.8) |
| 2005–2009a | 50.6 (49.8–51.4) | 79.8 (79.6–79.9) |
| 2007–2011a | 50.1 (49.3–50.8) | 79.7 (79.5–79.8) |
Abbreviation: CI, confidence interval.
Rates were age‐standardized with the estimated resident population for Australia for 2001.
Both ranges include 2007 and 2008.
Figure 1.
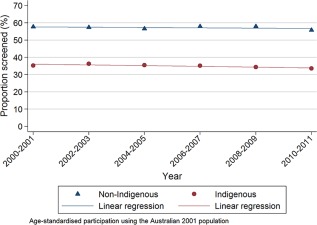
Two‐year participation rates of women aged 20 to 69 years for cervical screening by Indigenous status, 2000‐2001 to 2010‐2011, in Queensland, Australia.
Participation was lower for Indigenous women than non‐Indigenous women in every age group in every time period (Fig. 2); the differential was largest for women aged 45 – 49 years. Participation was highest for non‐Indigenous women in the 45‐ to 49‐year age group; in 2010‐2011, 60.8% (95% CI, 60.4%‐61.3%) of non‐Indigenous women and 33.4% (95% CI, 31.5%‐35.4%) of Indigenous women participated (a 27 percentage point difference). In contrast, Indigenous women's participation rate was highest among those aged 30 to 34 years; in 2010‐2011, 37.1% (95% CI, 35.5%‐38.3%) of Indigenous women and 56.5% (95% CI, 56.1%‐56.9%) of non‐Indigenous women participated (a 19.4 percentage point difference).
Figure 2.
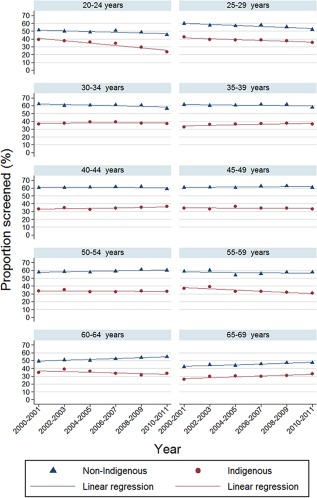
Age‐specific 2‐year participation rates by Indigenous status, 2000‐2001 to 2010‐2011, in Queensland, Australia.
Over time, participation increased among Indigenous women aged 65 to 69 years from 21.3% (vs 42.2% for non‐Indigenous women) in 2000‐2001 to 32.9% (vs 47.5% for non‐Indigenous women) in 2010‐2011; this makes the 14 percentage point differential in 2010‐2011 the smallest observed across all age groups and time periods. For both groups, participation in the 20‐ to 24‐year age group and, to a lesser extent, the 25‐ to 29‐year age group decreased between 2000‐2001 and 2010‐2011 (Fig. 2), most notably among Indigenous women aged 20 to 24 years.
Indigenous women participated less than non‐Indigenous women in every remoteness category and screening period (Table 3). Outer regional areas had the highest participation for Indigenous and non‐Indigenous women in each screening period. In 2010‐2011, participation in outer regional areas was 44.0% (95% CI, 42.8%‐45.3%) for Indigenous women and 64.4% (95% CI, 64.1%‐64.8%) for non‐Indigenous women (Fig. 3). In the same time period, participation in major cities was 53.3% (95% CI, 53.1%‐53.4%) among non‐Indigenous women and 26.7% (95% CI, 25.7%‐27.8%) among Indigenous women. Participation increased with increasing affluence for both Indigenous and non‐Indigenous women (Table 4). In 2010‐2011, participation among women living in the most disadvantaged areas was 25.2% (95% CI, 24.3%‐26.0%) for Indigenous women and 49.1% (95% CI, 48.8%‐49.5%) for non‐Indigenous women.
Table 3.
Two‐Year Participation in Cervical Screening by Place of Residence and Indigenous Status, 2000–2001 to 2010–2011
| Year | Major Cities | Inner Regional | Outer Regional | Remote | Very Remote | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | |
| 2000–2001 | 31.4 (30.0–32.9) | 55.9 (55.7–56.1) | 30.7 (29.0–32.6) | 58.8 (58.5–59.2) | 44.5 (43.0–46.1) | 63.9 (63.5–64.3) | 29.4 (27.0–32.0) | 61.1 (59.5–62.8) | 31.7 (29.9–33.7) | 47.9 (46.4–49.4) |
| 2002–2003 | 30.2 (28.8–31.6) | 55.2 (55.0–55.3) | 32.1 (30.4–34.0) | 59.4 (59.1–59.8) | 46.8 (45.2‐ 48.4) | 64.5 (64.5–65.3) | 30.1 (27.8–32.6) | 57.4 (55.9–59.0) | 34.2 (32.3–36.2) | 48.6 (47.1–50.1) |
| 2004–2005 | 30.9 (29.6–32.2) | 54.4 (54.2–54.6) | 31.0 (29.4–32.7) | 58.8 (58.5–59.1) | 45.6 (44.2–47.1) | 63.1 (62.7–63.5) | 31.4 (29.1–33.9) | 57.6 (56.1–59.1) | 30.8 (29.1–32.6) | 47.5 (46.0–48.9) |
| 2006–2007 | 31.0 (29.8–32.3) | 56.0 (55.8–56.2) | 30.8 (29.3–32.3) | 59.3 (59.0–59.6) | 45.1 (43.8–46.5) | 64.8 (64.4–65.2) | 33.2 (30.9–35.7) | 59.0 (57.5–60.5) | 28.6 (27.0–30.3) | 47.3 (45.9–48.8) |
| 2008–2009 | 28.7 (27.6–29.9) | 55.6 (55.4–55.7) | 32.0 (30.6–33.6) | 60.2 (59.9–60.5) | 44.6 (43.3–46.0) | 66.1 (65.7–66.5) | 31.9 (29.7–34.3) | 60.8 (59.3–62.3) | 28.0 (26.4–29.6) | 47.5 (46.0–49.0) |
| 2010–2011 | 26.7 (25.7–27.8) | 53.3 (53.1–53.4) | 29.3 (27.9–30.7) | 57.9 (57.6–58.2) | 44.0 (42.8–45.3) | 64.4 (64.1–64.8) | 32.1 (29.9–34.5) | 58.5 (57.1–60.0) | 30.9 (29.3–32.5) | 47.0 (45.6–48.5) |
Abbreviation: CI, confidence interval.
Rates were age‐standardized with the estimated resident population for Australia for 2001.
Figure 3.
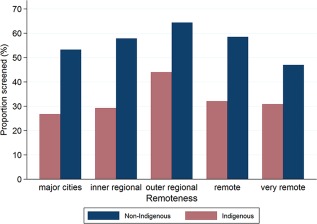
Age‐standardized proportions of screened women aged 20 to 69 years by remoteness category and Indigenous status, 2010‐2011, in Queensland, Australia.
Table 4.
Two‐Year Participation in Cervical Screening by Area‐Level Disadvantage and Indigenous Status, 2000–2001 to 2010–2011
| Year | Most Disadvantaged | Quintile 2 | Quintile 3 | Quintile 4 | Most Advantaged | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | |
| 2000–2001 | 25.2 (24.1–26.2) | 48.4 (48.0–48.8) | 47.1 (45.3–48.9) | 59.3 (59.0–59.6) | 32.9 (31.2–34.7) | 54.6 (54.3–54.9) | 48.1 (45.1–51.3) | 60.3 (60.0–60.7) | 40.0 (35.7–44.7) | 64.4 (64.0–64.8) |
| 2002–2003 | 27.1 (26.0–28.2) | 49.3 (48.9–49.6) | 47.5 (45.8–49.3) | 59.9 (59.5–60.2) | 32.2 (30.5–33.8) | 54.1 (53.9–54.4) | 50.3 (47.2–53.4) | 58.6 (58.3–59.0) | 40.1 (35.9–44.7) | 63.8 (63.4–64.2) |
| 2004–2005 | 27.0 (26.0–28.0) | 49.1 (48.7–49.5) | 46.3 (44.7–47.9) | 58.8 (58.5–59.1) | 30.5 (29.1–32.0) | 53.1 (52.8–53.4) | 47.8 (45.1–50.6) | 57.8 (57.5–58. 1) | 40.7 (36.7–44.9) | 62.8 (62.4–63.2) |
| 2006–2007 | 25.7 (24.7–26.6) | 50.6 (50.2–50.9) | 46.2 (44.7–47.7) | 60.0 (59.7–60.3) | 31.3 (29.9–32.8) | 54.6 (54.3–54.9) | 46.9 (44.4–49.5) | 58.6 (58.3–58.9) | 41.0 (37.2–45.1) | 64.8 (64.5–65.2) |
| 2008–2009 | 24.6 (23.7–25.5) | 50.7 (50.4–51.1) | 45.8 (44.4–47.3) | 60.1 (59.8–60.4) | 29.7 (28.4–31.1) | 55.6 (55.3–55.9) | 47.1 (44.7–49.6) | 58.3 (58.0–58.6) | 39.3 (35.6–43.3) | 63.9 (63.6–64.3) |
| 2010–2011 | 25.2 (24.3–26.0) | 49.1 (48.8–49.5) | 43.8 (42.4–45.1) | 57.8 (57.5–58.1) | 28.6 (27.3–29.9) | 53.0 (52.8–53.3) | 43.6 (41.3–45.9) | 56.1 (55.8–56.4) | 44.6 (40.0–49.5) | 61.7 (61.3–62.0) |
Abbreviation: CI, confidence interval.
Rates were age‐standardized with the estimated resident population for Australia for 2001.
Longer Screening Intervals
Three‐year participation was lower for Indigenous women than non‐Indigenous women by an average of 26 percentage points (range, 24.6‐27.4), and it decreased between 2000‐2002 and 2009‐2011 from 44.0% (95% CI, 43.1%‐44.8%) to 41.8% (95% CI, 41.1%‐42.5%) for Indigenous women and from 69.2% (95% CI, 69.0%‐69.4%) to 68.3% (95% CI, 68.2%‐68.5%) for non‐Indigenous women (Table 2). Five‐year participation was lower for Indigenous women by an average of 29 percentage points (range, 28.2‐29.4). Approximately 50% of Indigenous women and 80% of non‐Indigenous women were screened during 2007‐2011. The estimated 5‐year rate varied with the method used to estimate the numerator, although the differences were similar for Indigenous and non‐Indigenous women (Table 5).
Table 5.
Comparison of Methods for Calculating 5‐Year Participation in Cervical Screening by Indigenous Status, 2007–2011
| Method | Indigenous, % (95% CI) | Non‐Indigenous, % (95% CI) | Indigenous/Non‐Indigenous Percentage Point Difference |
|---|---|---|---|
| First Papanicolaou test | 50.1 (49.3–50.8) | 79.7 (79.5–79.8) | 29.6 |
| Last Papanicolaou test | 52.3 (51.5–53.1) | 81.7 (81.5–81.9) | 29.4 |
| Age of 20–69 y at beginning of 2007, first Papanicolaou test | 48.5 (47.7–49.2) | 76.8 (76.6–77.0) | 28.3 |
Abbreviation: CI, confidence interval.
Rates were age‐standardized with the estimated resident population for Australia for 2001.
Multivariate Analysis
Associations between screening participation and the 2‐year calendar period, age group, and Indigenous status both by place of residence and by IRSAD are shown in Table 6. Participation was lower for women younger than 30 years and those older than 50 years, and this trend was similar for Indigenous and non‐Indigenous women. There was evidence of a decrease in participation from 2000 to 2011: 1% (OR, 0.99; P < .001) and 2% (OR, 0.98; P < .001) per 2‐year period among non‐Indigenous and Indigenous women, respectively. However, according to the interaction between year and Indigenous status, these trends were not significantly different. The significant interaction between Indigenous status and both place of residence and IRSAD highlighted how the Indigenous differential varied by location. The Indigenous/non‐Indigenous differential was greatest for Indigenous women living in the most affluent areas (OR, 0.26, 95% CI, 0.24–0.27) and was evident for all place‐of‐residence categories except for very remote areas, where the likelihood of being screened was similar for both groups after adjustments.
Table 6.
Multivariate Analysis of 2‐Year Participation, 2000–2001 to 2010–2011, in Queensland
| Variable | Odds Ratio (95% CI)a |
|---|---|
| 2‐y periodb, c | 0.99 (0.99–0.99) |
| Age groupc, d | |
| 20–24 y | 0.64 (0.63–0.64) |
| 25–29 y | 0.86 (0.86–0.87) |
| 30–39 y | 1.0 |
| 40–49 y | 1.03 (1.02–1.03) |
| 50–59 y | 0.92 (0.92–0.93) |
| 60–69 y | 0.65 (0.65–0.66) |
| IRSAD: Indigenous/non‐Indigenous | |
| Most disadvantaged | 0.53 (0.53–0.54) |
| Quintile 2 | 0.54 (0.53–0.55) |
| Quintile 3 | 0.47 (0.46–0.48) |
| Quintile 4 | 0.62 (0.60–0.64) |
| Most affluent | 0.26 (0.24–0.27) |
| Place of residence: Indigenous/non‐Indigenous | |
| Major cities | 0.49 (0.48–0.49) |
| Inner regional | 0.34 (0.33–0.34) |
| Outer regional | 0.41 (0.40–0.42) |
| Remote | 0.48 (0.46–0.50) |
| Very remote | 0.99 (0.95–1.02) |
Abbreviation: CI, confidence interval; IRSAD, Index of Relative Socio‐Economic Advantage and Disadvantage.
The logistic regression compares women who participated in cervical screening with the population; adjustments have been made for all other variables in the table, and interaction terms for the Indigenous status and IRSAD and for the Indigenous status and remoteness are included.
The odds ratio compares one 2‐year period with the next.
There was no significant difference between Indigenous and non‐Indigenous women.
The odds ratio compares the age group with the referent category (30–39 years).
Screening History
In 2010‐2011, 548,549 women aged 30 to 69 years had a Pap test (1.6% Indigenous). Of these women (both groups), 90.7% had a history of a previous Pap test in 2000‐2009. The proportions of women who had a Pap test in 2010‐2011 without a history of a Pap test in 2000‐2009 were similar for Indigenous (8.5%) and non‐Indigenous women (9.3%).
DISCUSSION
Overall participation in cervical screening has remained 20 percentage points lower for Indigenous women versus non‐Indigenous women in Queensland for more than a decade. Furthermore, participation appears to be declining over time. This is the first time that population‐based data on cervical screening participation have been available for Indigenous women in Australia. Previous studies used indirect estimates to report on Indigenous women's participation by investigating screening in Indigenous communities (Indigenous population > 70%).5, 6, 18 In the NT, Indigenous women's participation was increased from approximately 30% in 1997‐1998 to 44% in 1999‐2000.5 Although the cervical cancer incidence was declining before the NT PSR commenced in 1996, it is plausible that an increase in screening contributed to this decline.18 National evidence suggests that cervical cancer incidence and mortality are decreasing for Indigenous women;19 along with trends in the NT, this suggests that cervical cancer prevention efforts have had some positive effects for Indigenous women. With methods similar to those used in the NT,5 Queensland Indigenous women's participation was estimated to be 17 percentage points lower than that of non‐Indigenous women during the early years of the Queensland PSR.6 Since then, there has been no further information about Indigenous women's participation in the program. There is also no evidence of cervical cancer incidence trends among Indigenous women in Queensland.
The decline in participation rates among younger women in our study is consistent with national reports1 and reports showing that participation is lower among HPV‐vaccinated women.20 The effect of vaccination status on Indigenous women's cervical screening participation is unknown.
The linkage of data sets was deemed successful,11 although it is inevitable that we did not identify all Indigenous women in the PSR because some true matches may have been missed, the Indigenous status on some hospital records may have been misclassified as non‐Indigenous (particularly in urban areas10), and some Indigenous women in the Queensland PSR would not have had a public hospital record during the study. As such, it is likely that we underestimated the participation rates for Indigenous women. We were unable to quantify the extent of the underestimation for Indigenous women who had not been to a hospital but were recorded in the PSR or for Indigenous women who had a record in the PSR but failed to link to their true QHAPDC record or records.14, 21 However, to account for misclassification in the QHAPDC, the Australian Institute of Health and Welfare (AIHW) recommends using a correction factor of 8% for Queensland.10 Therefore, 2‐year participation for Indigenous women in Queensland could be up to a maximum of 38% rather than 35%. The AIHW recommends different correction factors by remoteness within Queensland (1.17 in major cities and 0.97 in very remote areas) because the accuracy of Indigenous identification improves with increasing remoteness.10 If we applied the correction factor of 1.17, the participation rate for Indigenous women in major cities in 2010‐2011 could be 31.2% rather than 26.7%.10 The accuracy in recording Indigenous status has improved over time in the QHAPDC, so greater underestimation may have occurred in the early periods of the study. However, using a majority‐based algorithm to determine Indigenous status may have helped us to overcome this. We are reasonably confident that most Indigenous women eligible for our study had a record in the QHAPDC. The number of Indigenous women aged 20 to 69 years at any time between 1995 and 2011 in the extract from the QHAPDC used to link to the PSR in our study exceeded the Queensland resident 2011 ERP of Indigenous women aged 20 to 69 years at any time between June 1995 and December 2011.11, 22, 23 Two percent of women in the PSR were linked to an Indigenous identified record in the QHAPDC. This is lower than the proportion of Indigenous women in the Queensland female population (2.95% of Queensland females in the 20‐ to 69‐year age group24) but plausible because participation rates are not 100%.1 We found declining participation rates for younger Indigenous women in the later years of the study, but careful consideration is needed when one is interpreting these results. Because our study was able to identify only Indigenous women hospitalized in a public hospital during 1995‐2011, younger Indigenous women who entered our study later may have been less likely to have been to a hospital and, therefore, more likely to be incorrectly classified as non‐Indigenous. Linking the PSR to another source of data (eg, immunization records) could possibly improve the accuracy of the Indigenous status for younger women. Indigenous women identified in our study cohort are possibly somewhat different from Indigenous women who have never been hospitalized, and these differences may be related to their screening behavior.25 The incidence of hysterectomies may be lower among Indigenous women than non‐Indigenous women.26 However, we were unable to use different hysterectomy fractions for Indigenous women because adequate data were not available. A sensitivity analysis calculating participation rates for women aged 25 to 40 years (<5% of the population have had a hysterectomy,1 and any differential effects should be minimal) showed little difference in the estimates compared with those for women aged 20 to 69 years (data not shown). The Queensland PSR does not record the hysterectomy status of women in the register. Because women who have undergone a partial hysterectomy or hysterectomy not for a benign reason may still require screening, we may have counted women in the numerator who did not have an intact cervix but excluded them from the denominator. We are unable to quantify this and acknowledge this as a potential limitation.
Recently, Australia announced that a renewed cervical screening program (known as the Renewal) will be implemented in 2017, and this will change the current 2‐year screening by Pap test to 5‐year primary HPV screening for women aged 25 to 69 years. Further reductions of 15% to 22% in cervical cancer incidence and mortality rates in the population are expected because of the higher sensitivity and better negative predictive value of HPV testing.21 The Renewal will offer self‐collection of HPV samples for underscreened and never screened women and move from the current reminder‐based screening system to a call and recall system. The call and recall system will actively send invitations before the rescreening due date and invite women to start screening on their 25th birthday.
Our study findings provide an important benchmark for screening behavior before the implementation of the Renewal. Our results indicate that Indigenous women who are screened do so regularly (although not as frequently as non‐Indigenous women), and this may indicate that Indigenous women who do not undergo screening never undergo screening. Age‐specific participation varied among Indigenous and non‐Indigenous women; this included much lower screening participation among Indigenous women in the age group in which cervical cancer is likely to develop (mean age of diagnosis, 48.7 years27). Declining participation among all younger women highlights the importance of HPV vaccination in this age group to increase protection against the most common oncogenic HPV types and the need to emphasize to young women the importance of participating in cervical screening regardless of their HPV vaccination status.
Our findings indicate that participation is highest in outer regional areas for both Indigenous and non‐Indigenous women. Participation in very remote areas is relatively low for all women, and this signifies that access to services is likely difficult and needs improvement. Within major cities, participation is higher for non‐Indigenous women than Indigenous women; this indicates that locally available services are not meeting the needs of Indigenous women. Rates in urban areas are likely to be underestimated because the identification of Indigenous women is less accurate in urban areas than remote areas.
Importantly, our estimated 5‐year participation indicated that at least 50% of Indigenous women in 2007‐2011 were screened. Although this is promising for the Renewal (50% coverage is possible), it is concerning that the Indigenous/non‐Indigenous differential increased. With the Renewal's call and recall system, which includes the opportunity to make personalized and culturally relevant invitation letters and options for self‐collection, it is possible that screening coverage among Indigenous women will be considerably improved.
We used alternative methods to calculate 5‐year participation because those previously used by the AIHW are limited by their reliance on aggregated data.1 For example, for the period of 2007‐2011, a woman who had a Pap test at the age of 20 years in 2010 would be included in the numerator and the denominator, even though she would have been in the at‐risk population for only part of the period. Our cohort‐based estimates are based on individual‐level data, and including only women who were 20 years old in 2007 ensured greater consistency between the numerator and the denominator. Although the method affected the actual estimates, it had a negligible impact on the Indigenous/non‐Indigenous differential. A consistent and accurate method needs to be considered before the Renewal.
After reports of indirectly estimated low participation in Central Australia, successful efforts to increase screening were already evident by the next reporting period, and this gives hope that improvement is possible within Queensland.18 However, without ongoing surveillance, improvements in the participation and evaluation of initiatives are difficult. It is imperative that the national data collection and register proposed by the Renewal establish mechanisms to collect the Indigenous status and ultimately report on Indigenous women. Furthermore, it is important that Indigenous women and their health care providers understand why Indigenous identification at the point of screening is relevant and important for improving the health of Indigenous people. Even once high‐quality Indigenous status data are achieved, participation rate estimates produced from the national register may be biased if there are inconsistencies between the register's Indigenous status and the comparable population information from the census. As has been done in New Zealand,28 validation by linkage of the Australian register and the census would ensure consistent identification of Indigenous women in both data sets.
In conclusion, in the state of Queensland, Indigenous women participate less often than non‐Indigenous women, and there has been no improvement in closing this gap. Ultimately, our findings reflect the current cervical screening program but also provide critical benchmarks before major program changes in 2017. Our findings reinforce the critical need for the new national register to adequately collect the Indigenous status to monitor and evaluate screening participation and ultimately reduce inequities for Indigenous women.
FUNDING SUPPORT
The National Indigenous Cervical Screening Project is funded by a National Health and Medical Research Council (NHMRC) Project Grant (#104559). This project is part of a NHMRC Centre of Research Excellence in Discovering Indigenous Strategies to improve Cancer Outcomes via Engagement, Research Translation and Training (DISCOVER‐TT CRE) (#1041111) and Cancer Council NSW (#SRP13‐01) Strategic Research Partnership to Improve Cancer Control for Indigenous Australians (STREP Ca‐CIndA). The authors also acknowledge the ongoing support of the Lowitja Institute, Australia's National Institute for Aboriginal and Torres Strait Islander Health Research. Lisa J. Whop was supported by a Sidney Myer Health scholarship, a Menzies Enhanced Living scholarship, and a Lowitja Institute scholarship. Abbey Diaz was supported by a National Health and Medical Research Council postgraduate scholarship (1055587) and a DISCOVER‐TTCRE–funded Menzies Enhanced Living scholarship. The National Health and Medical Research Council supported Joan Cunningham with a research fellowship (1058244), Patricia C. Valery with a career development fellowship (1083090), and Karen Canfell with a career development award (1082989). The views expressed in this publication are those of the authors and do not necessarily reflect the views of the funding agencies. The funding bodies did not have any role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
CONFLICT OF INTEREST DISCLOSURES
Karen Canfell, Dorota Gertig, and Julia M. L. Brotherton are investigators in a trial of cytology and primary human papillomavirus screening in Australia (Compass), which is conducted and funded by the Victorian Cytology Service, a government‐funded health promotion charity, and by Karen Canfell's research group (formerly at the University of New South Wales and now at the Cancer Council New South Wales). The Victorian Cytology Service received equipment and a funding contribution for the Compass pilot study and for the main trial from Roche Molecular Systems and Ventana, Inc.
Lisa J. Whop, John R. Condon, Peter Baade, Gail Garvey, Joan Cunningham, Patricia C. Valery, Dianne L. O'Connell, Julia M. L. Brotherton, Karen Canfell, Kamalini Lokuge, David Roder, and Dorota Gertig conceptualized the study and contributed to the development of the study design and methodology. Lisa J. Whop, Gail Garvey, and John R. Condon conducted the approval process and initial data acquisition along with Suzanne P. Moore and Abbey Diaz for additional data requests. Lisa J. Whop analyzed the data and was supported by John R. Condon, Peter Baade, Joan Cunningham, and Abbey Diaz. All authors contributed to the interpretation of findings. Lisa J. Whop drafted the initial manuscript; all authors contributed revisions and read and approved the final draft.
We acknowledge the staff and registrars at the Queensland Health Pap Smear Register, Queensland Cancer Registry, and Queensland Health Admitted Patient Data Collection for assistance in data provision, Catherine Taylor (Queensland Record Linkage Group) for the record linkage, and Tegan Harris for database design and management contributions.
REFERENCES
- 1. Australian Institute of Health and Welfare . Cervical Screening in Australia 2012–2013. Canberra, Australia: Australian Institute of Health and Welfare; 2015. [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 3. Diaz A, Moore SP, Martin JH, Green AC, Garvey G, Valery PC. Factors associated with cancer‐specific and overall survival among Indigenous and non‐Indigenous gynecologic cancer patients in Queensland, Australia: a matched cohort study. Int J Gynecol Cancer. 2015;25:542–547. [DOI] [PubMed] [Google Scholar]
- 4. Condon JR, Zhang X, Baade P, et al. Cancer survival for Aboriginal and Torres Strait Islander Australians: a national study of survival rates and excess mortality. Popul Health Metr. 2014;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Binns PL, Condon JR. Participation in cervical screening by Indigenous women in the Northern Territory: a longitudinal study. Med J Aust. 2006;185:490–494. [DOI] [PubMed] [Google Scholar]
- 6. Coory MD, Fagan PS, Muller JM, Dunn NA. Participation in cervical cancer screening by women in rural and remote Aboriginal and Torres Strait Islander communities in Queensland. Med J Aust. 2002;177:544–547. [DOI] [PubMed] [Google Scholar]
- 7. Panaretto KS, Dallachy D, Manessis V, et al. Cervical smear participation and prevalence of sexually transmitted infections in women attending a community‐controlled Indigenous health service in north Queensland. Aust N Z J Public Health. 2006;30:171–176. [DOI] [PubMed] [Google Scholar]
- 8. Gilles MT, Crewe S, Granites IN, Coppola A. A community‐based cervical screening program in a remote Aboriginal community in the Northern Territory. Aust J Public Health. 1995;19:477–481. [DOI] [PubMed] [Google Scholar]
- 9. Hunt JM, Gless GL, Straton JA. Pap smear screening at an urban aboriginal health service: report of a practice audit and an evaluation of recruitment strategies. Aust N Z J Public Health. 1998;22:720–725. [DOI] [PubMed] [Google Scholar]
- 10. Australian Institute of Health and Welfare . Indigenous Identification in Hospital Separations Data—Quality Report. Canberra, Australia: Australian Institute of Health and Welfare; 2013. [Google Scholar]
- 11. Whop LJ, Diaz A, Baade P, et al. Using probabilistic record linkage methods to identify Australian Indigenous women on the Queensland Pap Smear Register: the National Indigenous Cervical Screening Project. BMJ Open. 2016;6(2):e009540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Australian Institute of Health and Welfare and Australian Bureau of Statistics . National Best Practice Guidelines for Data Linkage Activities Relating to Aboriginal and Torres Strait Islander People. Canberra, Australia: Australian Institute of Health and Welfare; 2012. [Google Scholar]
- 13. Australian Institute of Health and Welfare . Rural, Regional and Remote Health: A Guide to Remoteness Classifications. Canberra, Australia: Australian Institute of Health and Welfare; 2004. [Google Scholar]
- 14. Australian Bureau of Statistics . Technical Paper Socio‐Economic Indexes for Areas (SEIFA) 2011. Canberra, Australia: Australian Bureau of Statistics; 2011. [Google Scholar]
- 15. Australian Institute of Health and Welfare . Principles on the Use of Direct Age‐Standardisation in Administrative Data Collections: for Measuring the Gap Between Indigenous and Non‐Indigenous Australians. Canberra, Australia: Australian Institute of Health and Welfare; 2011.
- 16. Cancer Institute New South Wales . Cervical Cancer Screening in New South Wales: Annual Statistical Report 2009–2010. Sydney, Australia: Cancer Institute New South Wales; 2013. [Google Scholar]
- 17. Victorian Cytology Service . Annual Report 2011. Melbourne, Australia: Victorian Cytology Service Inc; 2011. [Google Scholar]
- 18. Zhang X, Condon JR, Douglas F, et al. Women's Cancers and Cancer Screening in the Northern Territory. Darwin, Australia: Northern Territory Department of Health; 2012. [Google Scholar]
- 19. Zhang X, Condon JR, Rumbold AR, Cunningham J, Roder DM. Estimating cancer incidence in Indigenous Australians. Aust N Z J Public Health. 2011;35:477–485. [DOI] [PubMed] [Google Scholar]
- 20. Budd AC, Brotherton JM, Gertig DM, Chau T, Drennan KT, Saville M. Cervical screening rates for women vaccinated against human papillomavirus. Med J Aust. 2014;201:279–282. [DOI] [PubMed] [Google Scholar]
- 21. Medical Services Advisory Committee . MSAC Application No. 1276: National Cervical Screening Program Renewal. Canberra, Australia: Medical Services Advisory Committee; 2014. [Google Scholar]
- 22. Australian Bureau of Statistics . 3238.0—Estimates and Projections, Aboriginal and Torres Strait Islander Australians, 2001 to 2026. Canberra, Australia: Australian Bureau of Statistics; 2014.
- 23. Queensland Treasury and Trade (Government Statistician) . Population Estimates by Indigenous Status, Statistical Local Area and Hospital and Health Services, Age, Sex, as at June 2000 to 2011. Brisbane, Australia: Queensland Treasury and Trade; 2012.
- 24. Office of Economic and Statistical Research . Census 2011: Women in Queensland. Brisbane, Australia: Queensland Treasury and Trade; 2012. [Google Scholar]
- 25. Aminisani N, Armstrong BK, Canfell K. Cervical cancer screening in Middle Eastern and Asian migrants to Australia: a record linkage study. Cancer Epidemiol. 2012;36:e394–e400. [DOI] [PubMed] [Google Scholar]
- 26. Spilsbury K, Semmens JB, Hammond I, Bolck A. Persistent high rates of hysterectomy in Western Australia: a population‐based study of 83 000 procedures over 23 years. BJOG. 2006;113:804–809. [DOI] [PubMed] [Google Scholar]
- 27. Australian Institute of Health and Welfare . Cancer in Australia: an overview 2014. Cancer series No 90. Cat. no. CAN 88. Canberra: AIHW;2014. [Google Scholar]
- 28. Department of Public Health . New Zealand census mortality study. http://www.otago.ac.nz/wellington/departments/publichealth/research/hirp/otago020541.html#aboutus, Accessed November 16, 2015.


