Abstract
Background
The regulation of mucociliary clearance is a key part of the defense mechanisms developed by the airway epithelium. If a high aggregate quality of evidence shows the clinical effectiveness of nasal irrigation, there is a lack of studies showing the intrinsic role of the different irrigation solutions allowing such results. This study investigated the impact of solutions with different pH and ionic compositions, eg, normal saline, non‐diluted seawater and diluted seawater, on nasal mucosa functional parameters.
Methods
For this randomized, controlled, blinded, in vitro study, we used airway epithelial cells obtained from 13 nasal polyps explants to measure ciliary beat frequency (CBF) and epithelial wound repair speed (WRS) in response to 3 isotonic nasal irrigation solutions: (1) normal saline 0.9%; (2) non‐diluted seawater (Physiomer®); and (3) 30% diluted seawater (Stérimar). The results were compared to control (cell culture medium).
Results
Non‐diluted seawater enhanced the CBF and the WRS when compared to diluted seawater and to normal saline. When compared to the control, it significantly enhanced CBF and slightly, though nonsignificantly, improved the WRS. Interestingly, normal saline markedly reduced the number of epithelial cells and ciliated cells when compared to the control condition.
Conclusion
Our results suggest that the physicochemical features of the nasal wash solution is important because it determines the optimal conditions to enhance CBF and epithelial WRS thus preserving the respiratory mucosa in pathological conditions. Non‐diluted seawater obtains the best results on CBF and WRS vs normal saline showing a deleterious effect on epithelial cell function.
Keywords: seawater, saline, ciliary beat frequency, wound repair, nasal mucosa
Nasal mucosa plays a particularly important protective role. The mucociliary clearance mechanism acts as a highly effective, nonspecific waste disposal system that is sometimes insufficient to prevent allergic response or microbial infection to airborne allergens, pollutants or pathogens.
In vitro and in vivo studies have revealed the air pollutants attenuating properties on ciliary beat frequency (CBF).1, 2, 3 Other studies have shown an impaired CBF in patients with allergic rhinitis or asthma.4, 5, 6, 7 Certain topical antibiotics have been shown to reduce the CBF and thus cannot be recommended in treatment of local infections.8 Pathogens targeting the airway like Pseudomonas aeruginosa, Streptococcus pneumoniae, or Haemophilus influenzae produce various cilio‐inhibitory factors.9, 10, 11, 12 This is clearly illustrated in cystic fibrosis or primary ciliary dyskinesia in which the impaired ciliary beat and/or thicker mucus result in a defective mucociliary clearance that may participate in repeated lung infections and pathophysiology.13, 14, 15, 16 It is thus important to restore an efficient ciliary beat to ensure the protective role of the respiratory mucosa.
Furthermore, contact prolongation of airborne allergens, pollutants, and pathogens with the nasal mucosa worsen the inflammatory reaction leading to epithelial lesions that threaten mucosal integrity. Mucosal surface wounds become entry points for bacteria and viruses that target airway epithelial cells and may result in respiratory infections. In order to restore its functionality, the airway epithelium enters in turn in a process of repair and regeneration.17 In cases of inflammatory respiratory diseases such as asthma,17 it is becoming apparent that normal airway epithelium repair is compromised. Ensuring the proper conditions of wound repair process represents therefore a great therapeutic interest for any respiratory conditions associated with mucosal inflammation.
Nasal irrigation with saline solutions is commonly used as adjunctive treatment in upper respiratory conditions as well as in postsurgical follow‐up.18 A 2012 systematic review and meta‐analysis by Hermelingmeier et al.19 showed the benefit of nasal irrigation in symptom relief and drug reduction in cases of allergic rhinitis. It has also been recommended as add‐on therapy in pediatric allergic rhinitis by European experts.20 In addition, nasal irrigation was helpful in improving the nasal peak expiratory flow rates and quality of life scores in children with atopic21 and nonatopic22 acute sinusitis. A Cochrane review by Kassel et al.23 of data in adults with acute upper respiratory tract infections (URTIs) revealed benefit in quicker symptom resolution and return to work, though the results were not statistically significant. Recently, several international expert groups recommended nasal saline irrigation in chronic rhinosinusitis with or without nasal polyps,24, 25, 26 and after endonasal surgery.24
If the efficacy of nasal irrigation is no longer questioned in vivo, the literature on nasal irrigation composition specifically, remains limited and does not provide any evidence on its potential impact. The aim of this study is to investigate and compare the functional impact of 3 commonly used isotonic nasal irrigation solutions, reflecting the diversity of products available on the market: (1) normal saline (0.9%); (2) isotonic non‐diluted seawater solution; and (3) isotonic 30% diluted seawater solution. We hypothesize that the non‐diluted seawater solution is more effective on the CBF and wound repair speed (WRS) vs other solutions.
Patients and methods
Study design
Prospective, randomized, controlled, and blinded in vitro study using biological materials from nasal polyp explants.
Material
The bioethical law N° 2004–800 of the French Public Health Code authorizes the use of human tissues. Local Institutional Review Board approved this study and informed consent was obtained from all patients. Three centers provided nasal polyps explants: University Hospital Hautepierre (Strasbourg, France); University Hospital Robert Debré (Paris, France); and private clinic Courlancy (Reims, France). Nasal polyps were removed from patients diagnosed with sinonasal polyposis without any other comorbidities (asthma or nonsteroidal anti‐inflammatory drugs allergies). Age, sex, and clinical characteristics are summarized in Table 1. Some of the patients received corticoids and/or antibiotics. Treatments are described in Table 1.
Table 1.
Demographic and clinical data of patients
| Patient number | Age (years) | Sex | Allergy history | Intolerance history | Treatment |
|---|---|---|---|---|---|
| 01 | 75 | M | None | None | None |
| 02 | 34 | M | None | None | None |
| 03 | 69 | F | None | None | None |
| 04 | 44 | F | None | None | Beclomethasone |
| 05 | 53 | M | Latex | None | Beclomethasone |
| 06 | 52 | M | None | None | Pristinamycin |
| 07 | 77 | M | None | None | None |
| 08 | 63 | M | None | None | Prednisolone; pristinamycin |
| 09 | 36 | M | None | None | Beclomethasone |
| 10 | 67 | F | None | None | None |
| 11 | 56 | M | None | None | Beclomethasone |
| 12 | 50 | M | None | None | None |
| 13 | 39 | M | None | None | None |
| 14 | 51 | M | None | None | Mometasone furoate |
Randomization
Biological materials were randomly assigned (using random letters A, B, C) into either the normal saline group, the non‐diluted seawater group, and the 30% diluted seawater group.
Blinding
Blinding of tested solutions except control medium was strictly maintained for researchers including laboratory staff. The composition of the tested solutions was revealed by the sponsor after study completion. Study solutions were provided and prepackaged by the sponsor's head pharmacist (located at a distant site) into anonymous kits and lettered accordingly.
Tested solutions
Non‐diluted seawater (Physiomer®, Laboratoire de la Mer, Saint‐Malo, France) is a sterile, isotonic 100% non‐diluted seawater solution. Isotonicity is achieved by selective electrodialysis that removes NaCl ions while preserving seawater full content in other minerals. Physiomer® is sterilized aseptically through microfiltration at 0.2 μm.
Diluted seawater (Stérimar®, Laboratoires Fumouze, Levallois‐Perret, France) is a sterile, isotonic, seawater solution diluted at 30%. It is rendered isotonic through dilution with purified water. Its minerals content is reduced by two‐thirds vs seawater. Stérimar® is sterilized by gamma irradiation.
Normal saline 0.9% (CDM Lavoisier, Paris, France) is a sterile, ready‐for‐injection solution. It contains 3500 mg/L of sodium ions and 5500 mg/L of chloride ions. Normal saline conforms to pharmaceutical quality standards.
Table 2 summarizes the different test solutions and their physicochemical characteristics.
Table 2.
pH and osmolarity of solutions
| Tested solution | Batch number | Measured pH |
Measured osmolarity (mOsm/kg) |
|---|---|---|---|
| Physiomer® | P1201024A | 7.9 | 308 |
| Stérimar® | FE2234 | 7.28 | 302 |
| Lavoisier normal saline (0.9%) | 2F258; 2F220 | 5.21 | 308 |
Cell cultures
For the measurement of CBF, nasal epithelial tissue from nasal polyps from 10 patients (1 to 2 mm2 in size) were seeded on 12‐well culture plates (BD Falcon, Franklin Lakes, NJ, USA) coated with type IV collagen (Sigma Aldrich, St. Louis, MO, USA) and incubated in Bronchial Epithelial cell Growth MediumTM (BEGM; Lonza, Verviers, Belgium) at 37°C for 4 to 6 days until explants were surrounded by a cell outgrowth that contained well‐visible ciliated cells.
BEGMTM was used for cell cultures as control. Saline solution is usually considered as a reference product for nasal irrigation; however, it is not used and dedicated for cell culture. BEGMTM contains equal proportions of Bronchial Epithelial Basal Medium (Lonza) and Dulbecco's Modified Eagle Medium (Lonza). The medium is supplemented with 0.1 mM of retinoic acid, 0.5 mg/L of human epidermal growth factor, 5 mg/L of epinephrine, 0.13 g/L of bovine pituitary extract, 0.5 mg/L of hydrocortisone, 5 mg/L of insulin, 6.5 mg/L of triiodothyronine, 0.5 mg/L of transferrin (all from Lonza, Verviers, Belgium), 200 U/mL of penicillin, 200 mg/L of streptomycin, and 1.5 mg/L of bovine serum albumin (all from Sigma‐Aldrich, Lyon, France).
For the WRS assay, nasal epithelial cells were isolated from nasal polyps from 13 patients by incubation with 0.1% type XIV collagenase (Sigma‐Aldrich) in Roswell Park Memorial Institute (RPMI) 1640 culture medium medium (Life Technologies, Saint Aubin, France) supplemented with 200 U/mL penicillin and 200 μg/mL streptomycin (Life Technologies) overnight at 4°C.
Isolated epithelial cells were washed, suspended in BEGM medium, counted, and then seeded on 12‐well culture plates coated with type IV collagen at a density of 6 × 104 cells/cm2 and cultured in BEGM medium at 37°C until confluence was reached.
CBF measurement
After a culture period of 4 to 6 days, once explants were surrounded by a cell outgrowth and contained well‐visible ciliated cells, wells were rinsed with either BEGM medium, non‐diluted seawater, diluted seawater, or normal saline. Plates were then incubated in the corresponding solutions for 30 minutes, under an inverted microscope (Axiovert 200; Zeiss, Germany) equipped with an environmental chamber maintained at 37°C and 5% CO2 and a CoolSNAP CCD camera (Roper Scientific, Inc., Tucson, AZ, USA) allowing live‐cell imaging. For each condition, 20 areas with ciliated cells were recorded over 10 seconds with an acquisition frequency of 50 images/second (×32 magnification). CBF was quantified using an in‐house–developed plug‐in on the ImageJ software (NIH, Bethesda, MD; http://imagej.nih.gov/ij/index.html).27 The control condition was achieved by incubating the cells with BEGM medium. Values were expressed as mean frequency in hertz (Hz) for each condition.
WRS measurement
At confluence, cultures were rinsed and incubated with BEGM medium, non‐diluted seawater, diluted seawater, or normal saline. The control condition was achieved by incubating cells with BEGM medium. After a 4‐hour incubation period, an epithelial wound was realized by scratching each epithelial cell monolayer in a linear pattern with a pipette tip (100 μL).
Wells were then rinsed with BEGM medium to eliminate debris and cells were incubated with fresh culture medium. Three areas per condition and culture were controlled by live‐cell imaging (×10 magnification) until wound closure. Phase contrast images were captured every 10 minutes and then analyzed with an in‐house–developed plug‐in on ImageJ software. WRS was expressed as mean speed for each condition and culture in μm2/hour.
Statistical analysis
Statistical analysis was carried out independently by the research unit INSERM UMRS‐S 903 using the nonparametric Wilcoxon test in Prism 5©. CBF and WRS were expressed as median ± standard errors of the mean. Statistical significance was defined as p < 0.05.
Results
CBF in response to non‐diluted seawater (Physiomer®), diluted seawater (Stérimar®), and normal saline
After a 4‐day to 6‐day culture period, explant outgrowths show visible and functional ciliated cells. Supporting Video 1 presents CBF in the 3 different nasal irrigation solutions compared to control. Mean values and standard deviations for each culture and condition are presented in Table 3. The CBF mean was 12.80 ± 1.18 Hz for the control condition, 13.83 ± 1.46 Hz for cells exposed to non‐diluted seawater, 12.61 ± 1.13 Hz for cells exposed to diluted seawater, and 11.16 ± 1.80 Hz for cells exposed to normal saline. Figure 1 shows the means and the distribution of the measured CBF values for each condition. Incubation of ciliated cells with non‐diluted seawater significantly increased the CBF compared to the control condition (p = 0.0039). Diluted seawater, on the other hand, did not modify the CBF compared to the control (p > 0.05). Surprisingly, incubation with normal saline induced greater ciliated cell death compared with control, non‐diluted seawater, and diluted seawater (p = 0.0039). Moreover, after 30 minutes, 5 of the 10 nasal explant cultures incubated with normal saline exhibited no ciliated cells and CBF could not be determined (Table 3). Finally, ciliated cells incubated with non‐diluted seawater exhibited a significantly better CBF compared to cells incubated with diluted seawater (p = 0.0059).
Table 3.
Ciliary beat frequency for each explants and solutions
| Explant number |
Control (Hz) |
Physiomer® (Hz) |
Stérimar® (Hz) |
0.9% NaCl (Hz) |
|---|---|---|---|---|
| 1 | 13.69 | 15.31 | 14.16 | 13.14 |
| 2 | 12.98 | 13.21 | 12.90 | 11.32 |
| 3 | 12.49 | 15.44 | 13.37 | nd |
| 4 | 14.09 | 15.04 | 12.61 | nd |
| 5 | 13.07 | 13.58 | 13.54 | nd |
| 6 | 10.74 | 12.11 | 11.36 | nd |
| 7 | 11.79 | 13.12 | 11.75 | nd |
| 8 | 14.78 | 15.92 | 13.51 | 9.59 |
| 9 | 12.28 | 12.12 | 12.37 | 12.67 |
| 10 | 12.10 | 12.47 | 10.50 | 9.08 |
| Mean | 12.80 | 13.83** | 12.61 | 11.16** |
| SD | 1.18 | 1.46 | 1.13 | 1.80 |
** p < 0.01, comparison of Physiomer® and 0.9% NaCl vs CTRL.
CTRL = control; nd = not determined (death cell); SD = standard deviation.
Figure 1.
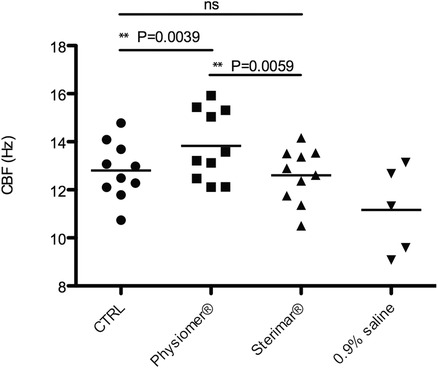
CBF: mean and distribution of the values of CBF for the control (•), Physiomer® (■), Stérimar® (▲), and normal saline 0.9% (▼) conditions. CBF is expressed in hertz (Hz). **p = 0.0039, **p = 0.0059, CBF = ciliary beat frequency; CTRL = control; ns = nonsignificant.
WRS in response to non‐diluted seawater (Physiomer®), diluted seawater (Stérimar®), and normal saline
Our WRS model exposed to different conditions is shown in Supporting Video 2. Mean values and standard deviations for each culture and conditions are presented in Table 4. The mean WRS was 69825.18 ± 26634.36 μm2/hour for the control condition, 74793.38 ± 24298.26 μm2/hour for cells exposed to non‐diluted seawater, 49237.74 ± 28228.66 μm2/hour for cells exposed to diluted seawater, and 5036.18 ± 10374.19 μm2/hour for cells exposed to normal saline. Figure 2 shows the WRS means and the distribution of the measured values for each condition.
Table 4.
Wound repair speed for each explant and solution
| Explant number |
Control (μm2/hour) |
Physiomer® (μm2/hour) |
Stérimar® (μm2/hour) |
0.9% NaCl (μm2/hour) |
|---|---|---|---|---|
| 1 | 42068.83 | 76953.81 | 37498.06 | −3707.19 |
| 2 | 53085.60 | 72525.33 | 52519.79 | 125.43 |
| 3 | 108927.93 | 90072.95 | 100699.66 | 8232.33 |
| 4 | 59098.62 | 62144.68 | 48757.18 | 2013.14 |
| 5 | 35406.18 | 20563.92 | 18061.37 | 3243.46 |
| 6 | 61025.22 | 70991.49 | 30805.66 | 1283.73 |
| 7 | 84179.58 | 63026.18 | 18131.67 | 9497.80 |
| 8 | 40599.71 | 62023.00 | 23785.74 | 15311.40 |
| 9 | 106692.41 | 114806.43 | 39197.86 | −6140.88 |
| 10 | 59580.00 | 75823.75 | 45010.63 | 1961.25 |
| 11 | 105500.62 | 116436.27 | 97488.66 | −3296.17 |
| 12 | 94326.33 | 79993.78 | 85877.69 | 3243.46 |
| 13 | 57236.36 | 66952.39 | 42256.76 | 33702.66 |
| Mean | 69825.18 | 74793.38 | 49237.74*** | 5036.18*** |
| SD | 26634.36 | 24298.26 | 28228.66 | 10374.19 |
*** p < 0.001, comparison of Stérimar® and 0.9% NaCl vs CTRL.
CTRL = control; SD = standard deviation.
Figure 2.
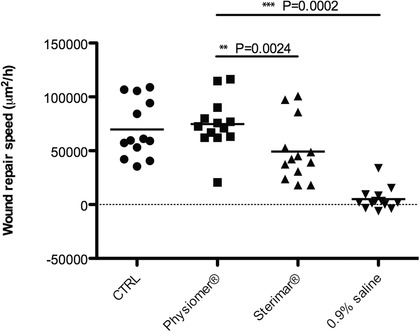
WRS: mean and distribution of the values of WRS for the control (•), Physiomer® (■), Stérimar® (▲), and normal saline 0.9% (▼) conditions. WRS is expressed in μm2 per hour. **p = 0.0024, ***p = 0.0002. CTRL = control; WRS = wound repair speed.
Incubation of epithelial cells with non‐diluted seawater slightly raised the WRS compared to the control condition, but the results did not reach significance. Diluted seawater on the other hand, reduced the WRS compared to the control (p = 0.0002). Nasal epithelial cells exposed to non‐diluted seawater exhibited a significantly better WRS compared to cells exposed to diluted seawater (p = 0.0024) and normal saline (p = 0.0002). Interestingly, incubation with normal saline greatly reduced the WRS, showing a deleterious effect on epithelial cell function (p = 0.0002). In fact, the reduced WRS was exacerbated by a high cell death rate in normal saline (data not shown).
Discussion
Our in vitro results showed that non‐diluted seawater markedly enhanced the CBF and slightly but nonsignificantly raised the WRS when compared to the control condition. Both enhancements were significant when compared to normal saline and diluted seawater. Moreover, our results confirmed previous findings28, 29 demonstrating that normal saline is potentially deleterious for nasal mucosa as it induced nasal epithelial cell death in vitro. A recent review of the literature showed the effects of selected ions on epithelial cells such as magnesium on the control of local inflammation resulting from allergy, calcium in the regulation of the ciliary beat frequency, and the implication of potassium on healing.30
Many models have been described for the in vitro evaluation of the CBF and other features of the nasal mucosa. In addition to cell lines, primary cells have been extensively used. They are isolated from different regions of the nasal mucosa, grown as explant outgrowth cultures or dissociated tissue cultures, with coated or uncoated supports, in a perfusion system or CO2 incubator. The various methods and time of CBF recording result in a stable, coordinated CBF ranging from 7 to 13 Hz according to the literature,31, 32, 33, 34 12 to 13 Hz being the CBF recorded in vivo.35, 36 In the present study, the mean baseline CBF (12.80 Hz) from the nasal polyps explants outgrowth is in accordance with previous published reports using similar methods in vitro and in vivo.34, 37, 38 As for the wound repair capacity of the nasal mucosa, in vitro studies using a similar model are lacking.
The difficulty of this kind of investigation lies in the high interpatient variability, which may explain why the difference between non‐diluted seawater and the control medium did not reach statistical significance. Our results confirm that non‐diluted seawater unlike normal saline and other diluted seawater solutions, does not inhibit the physiological process of wound repair.
Previous studies28, 29 demonstrated in vitro that the viability of bronchial epithelial cells (BECs) incubated in normal saline decreases by 40% and 20% after 2‐hour and 4‐hour incubation periods, respectively. In contrast, incubation in non‐diluted seawater (Physiomer®) maintains a healthy BEC culture with adherent cells and intercellular junctions. Protein contents are also higher in BEC cultures with non‐diluted seawater (Physiomer®) compared to normal saline, or compared to diluted seawater (Stérimar®). Our results show that, in vitro, non‐diluted seawater gives better results than normal saline and diluted seawater solutions on CBF and WRS. This suggest that in vivo superiority of mineral‐rich solutions could rely on such an in vitro effect on CBF and WRS.
Nasal irrigation with normal saline has been recommended and performed for many years. Although there has not been reported any deleterious effect on the nasal mucosa like in our model, it shows the impact of nasal solutions specifically in terms of composition. This corroborates recent findings showing that nasal irrigation was proven more effective when performed with seawater‐derived or mineral‐rich solutions compared to a normal saline 0.9% solution in allergic rhinitis,39 chronic rhinosinusitis,40, 41 and post–endonasal surgery.42, 43, 44 Recently, Low et al.44 showed in vivo moderate, though statistically better and faster symptom resolution with nasal irrigation with lactated Ringer's solution after endoscopic sinus surgery when compared with normal saline. Lactated Ringer's solution is an isotonic solution, composed of a sodium lactate as buffer (2500 mg/L), sodium (3000 mg/L), chloride (3900 mg/L), calcium (120 mg/L), and potassium (150 mg/L).
The effect of non‐diluted seawater can be explained by the preservation of seawater mineral composition. Indeed, calcium ions are well known for their implication in ciliary beat regulation.45, 46, 47 Potassium, magnesium, and zinc ions have been shown to assist in epithelial wound repair.48, 49, 50 Diluted seawater solutions contain far lower mineral content due to dilution. Moreover non‐diluted seawater alkaline pH (7.9) is more favorable to the ciliary beat, as previously shown in vitro,51 whereas normal saline acidic pH (5.21) is deleterious for the in vitro ciliary beat.51
Conclusion
Our results suggest that the physicochemical features of the nasal wash solution is important because it determines the optimal conditions to enhance CBF and epithelial WRS, thus preserving the respiratory mucosa in pathological conditions. Further in vivo studies will be needed to confirm the superiority of non‐diluted seawater vs normal saline and diluted seawater in pathological conditions such as chronic rhinosinusitis and/or allergic rhinitis.
Supporting information
Supporting Video 1. Effect of culture medium, 0.9% saline, undiluted (Physiomer®) and diluted (Sterimar®) seawater solutions on the CBF of explant outgrowths with functional ciliated cells.
Supporting Video 2. Effect of culture medium, 0.9% saline, undiluted (Physiomer®) and diluted (Sterimar®) seawater solutions on the WRS of nasal epithelial cell cultures after an epithelial wound.
Acknowledgments
We thank Pr Christian Debry, Dr Jean‐Claude Mérol and Dr. Talal Nasser for the delivery of polyps, as well as Pr. Philippe Birembaut for his general support in the implementation of the study as former head of INSERM UMRS‐S 903.
How to Cite this Article: Bonnomet A, Luczka E, Coraux C, de Gabory L. Non‐diluted seawater enhances nasal ciliary beat frequency and wound repair speed compared to diluted seawater and normal saline. Int Forum Allergy Rhinol. 2016;6:1062‐1068.
Funding sources for the study: Laboratoire de la Mer to INSERM UMRS‐S 903.
Potential conflict of interest: None provided.
References
- 1. Bayram H, Devalia JL, Sapsford RJ, et al. The effect of diesel exhaust particles on cell function and release of inflammatory mediators from human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol. 1998;18:441–448. [DOI] [PubMed] [Google Scholar]
- 2. Calderon‐Garciduenas L, Valencia‐Salazar G, Rodriguez‐Alcaraz A, et al. Ultrastructural nasal pathology in children chronically and sequentially exposed to air pollutants. Am J Respir Cell Mol Biol. 2001;24:132–138. [DOI] [PubMed] [Google Scholar]
- 3. Sakai N, Tamaoki J, Chiyotani A, et al. [Inhibitory effect on sulfur dioxide on ciliary motility in rabbit tracheal epithelium and its prevention by intracellular cyclic AMP]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993;31:733–737. Japanese [PubMed] [Google Scholar]
- 4. Holmström M, Lund V, Scadding G. Nasal ciliary beat frequency after nasal allergen challenge. Am J Rhinol. 1992;6:101–105. [Google Scholar]
- 5. Maurizi M, Paludetti G, Todisco T, et al. Ciliary ultrastructure and nasal mucociliary clearance in chronic and allergic rhinitis. Rhinology. 1984;22:233–240. [PubMed] [Google Scholar]
- 6. Mezey RJ, Cohn MA, Fernandez RJ, et al. Mucociliary transport in allergic patients with antigen‐induced bronchospasm. Am Rev Respir Dis. 1978;118:677–684. [DOI] [PubMed] [Google Scholar]
- 7. Ohashi Y, Nakai Y, Kihara S, et al. Ciliary activity in patients with nasal allergies. Arch Otorhinolaryngol. 1985;242:141–147. [DOI] [PubMed] [Google Scholar]
- 8. Gosepath J, Grebneva N, Mossikhin S, Mann WJ. Topical antibiotic, antifungal, and antiseptic solutions decrease ciliary activity in nasal respiratory cells. Am J Rhinol. 2002;16:25–31. [PubMed] [Google Scholar]
- 9. Janson H, Carlén B, Cervin A, et al. Effects on the ciliated epithelium of protein D‐producing and ‐nonproducing nontypeable Haemophilus influenzae in nasopharyngeal tissue cultures. J Infect Dis. 1999;180:737–746. [DOI] [PubMed] [Google Scholar]
- 10. Read RC, Roberts P, Munro N, et al. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol. 1992;72:2271‐2277. [DOI] [PubMed] [Google Scholar]
- 11. Read RC, Rutman AA, Jeffery PK, et al. Interaction of capsulate Haemophilus influenzae with human airway mucosa in vitro. Infect Immun. 1992;60:3244–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinfort C, Wilson R, Mitchell T, et al. Effect of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect Immun. 1989;57:2006–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sagel SD, Davis SD, Campisi P, Dell SD. Update of respiratory tract disease in children with primary ciliary dyskinesia. Proc Am Thorac Soc. 2011;8:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Worlitzsch D, Tarran R, Ulrich M, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol. 2007;35:116–129. [DOI] [PubMed] [Google Scholar]
- 17. Grainge CL, Davies DE. Epithelial injury and repair in airways diseases. Chest. 2013;144:1906–1912. [DOI] [PubMed] [Google Scholar]
- 18. Tomooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope. 2000;110:1189–1193. [DOI] [PubMed] [Google Scholar]
- 19. Hermelingmeier KE, Weber RK, Hellmich M, et al. Nasal irrigation as an adjunctive treatment in allergic rhinitis: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2012;26:e119–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68:1102–1116. [DOI] [PubMed] [Google Scholar]
- 21. Wang YH, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in atopic children. J Microbiol Immunol Infect. 2014;47:63–69. [DOI] [PubMed] [Google Scholar]
- 22. Wang YH, Yang CP, Ku MS, et al. Efficacy of nasal irrigation in the treatment of acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 2009;73:1696–1701. [DOI] [PubMed] [Google Scholar]
- 23. Kassel JC, King D, Spurling GK. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev. 2010;(3):CD006821. [DOI] [PubMed] [Google Scholar]
- 24. Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):3 p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 25. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 Suppl):S1–S31. [DOI] [PubMed] [Google Scholar]
- 26. Wei CC, Adappa ND, Cohen NA. Use of topical nasal therapies in the management of chronic rhinosinusitis. Laryngoscope. 2013;123:2347–2359. [DOI] [PubMed] [Google Scholar]
- 27. Zahm JM, Dionisius JP, Pierrot D, et al. [Measuring the frequency of ciliary beating on video screen.] Mesure de la fréquence de battement ciliaire sur écran vidéo. Innov Techn Biol Med. 1990;2:118–129. [Google Scholar]
- 28. Tabary O, Muselet C, Miesch MC, et al. Reduction of chemokine IL‐8 and RANTES expression in human bronchial epithelial cells by a sea‐water derived saline through inhibited nuclear factor‐kappaB activation. Biochem Biophys Res Commun. 2003;309:310–316. [DOI] [PubMed] [Google Scholar]
- 29. Traissac L, Bordenave L, Bareille R, et al. In vitro study of the effect of sea water by‐products on the respiratory mucosa. Rev Soc Fr ORL. 1995;32:43–49. [Google Scholar]
- 30. Bastier PL, Lechot A, Bordenave L, et al. Nasal irrigation: From empiricism to evidence‐based medicine. A review. Eur Ann Otorhinolaryngol Head Neck Dis. 2015;132:281–285. [DOI] [PubMed] [Google Scholar]
- 31. Birk R, Aderhold C, Stern‐Strater J, et al. Polyhexanide‐containing solution reduces ciliary beat frequency of human nasal epithelial cells in vitro. Eur Arch Otorhinolaryngol. 2015;272:377–383. [DOI] [PubMed] [Google Scholar]
- 32. Carson JL, Lu TS, Brighton L, et al. Phenotypic and physiologic variability in nasal epithelium cultured from smokers and non‐smokers exposed to secondhand tobacco smoke. In Vitro Cell Dev Biol Anim. 2010;46:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiao J, Meng N, Wang H, Zhang L. Comparison of human nasal epithelial cells grown as explant outgrowth cultures or dissociated tissue cultures in vitro. Front Med. 2013;7:486–491. [DOI] [PubMed] [Google Scholar]
- 34. Shaari J, Palmer JN, Chiu AG, et al. Regional analysis of sinonasal ciliary beat frequency. Am J Rhinol. 2006;20:150–154. [PubMed] [Google Scholar]
- 35. Rutland J, Cole PJ. Nasal mucociliary clearance and ciliary beat frequency in cystic fibrosis compared with sinusitis and bronchiectasis. Thorax. 1981;36:654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stanley PJ, Wilson R, Greenstone MA, et al. Effect of cigarette smoking on nasal mucociliary clearance and ciliary beat frequency. Thorax. 1986;41:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chevillard M, Hinnrasky J, Zahm JM, et al. Proliferation, differentiation and ciliary beating of human respiratory ciliated cells in primary culture. Cell Tissue Res. 1991;264:49–55. [DOI] [PubMed] [Google Scholar]
- 38. Zahm JM, Pierrot D, Hinnrasky J, et al. Functional activity of ciliated outgrowths from cultured human nasal and tracheal epithelia. Biorheology. 1990;27:559–565. [DOI] [PubMed] [Google Scholar]
- 39. Cordray S, Harjo JB, Miner L. Comparison of intranasal hypertonic dead sea saline spray and intranasal aqueous triamcinolone spray in seasonal allergic rhinitis. Ear Nose Throat J. 2005;84:426–430. [PubMed] [Google Scholar]
- 40. Friedman M, Hamilton C, Samuelson CG, et al. Dead Sea salt irrigations vs saline irrigations with nasal steroids for symptomatic treatment of chronic rhinosinusitis: a randomized, prospective double‐blind study. Int Forum Allergy Rhinol. 2012;2:252–257. [DOI] [PubMed] [Google Scholar]
- 41. Friedman M, Vidyasagar R, Joseph N. A randomized, prospective, double‐blind study on the efficacy of dead sea salt nasal irrigations. Laryngoscope. 2006;116:878–882. [DOI] [PubMed] [Google Scholar]
- 42. Keerl R, Weber R, Muller C, Schick B. [Effectiveness and tolerance of nasal irrigation following paranasal sinus surgery]. Laryngorhinootologie. 1997;76:137–141. [DOI] [PubMed] [Google Scholar]
- 43. Michel O, Charon J. [Postoperative inhalation treatment after paranasal sinus interventions. A placebo‐controlled, double‐blind and randomized study]. HNO. 1991;39:433–438. [PubMed] [Google Scholar]
- 44. Low TH, Woods CM, Ullah S, Carney AS. A double‐blind randomized controlled trial of normal saline, lactated Ringer's, and hypertonic saline nasal irrigation solution after endoscopic sinus surgery. Am J Rhinol Allergy. 2014;28:225–231. [DOI] [PubMed] [Google Scholar]
- 45. Di Benedetto G, Magnus CJ, Gray PT, Mehta A. Calcium regulation of ciliary beat frequency in human respiratory epithelium in vitro. J Physiol. 1991;439:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmid A, Salathe M. Ciliary beat co‐ordination by calcium. Biol Cell. 2011;103:159–169. [DOI] [PubMed] [Google Scholar]
- 47. Zsembery A, Boyce AT, Liang L, et al. Sustained calcium entry through P2X nucleotide receptor channels in human airway epithelial cells. J Biol Chem. 2003;278:13398–13408. [DOI] [PubMed] [Google Scholar]
- 48. Bardou O, Trinh NT, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L145–L155. [DOI] [PubMed] [Google Scholar]
- 49. Tesfaigzi Y. Roles of apoptosis in airway epithelia. Am J Respir Cell Mol Bio. 2006;34:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zsembery A, Fortenberry JA, Liang L, et al. Extracellular zinc and ATP restore chloride secretion across cystic fibrosis airway epithelia by triggering calcium entry. J Biol Chem. 2004;279:10720–10729. [DOI] [PubMed] [Google Scholar]
- 51. Luk CK, Dulfano MJ. Effect of pH, viscosity and ionic‐strength changes on ciliary beating frequency of human bronchial explants. Clin Sci (Lond). 1983;64:449–451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Video 1. Effect of culture medium, 0.9% saline, undiluted (Physiomer®) and diluted (Sterimar®) seawater solutions on the CBF of explant outgrowths with functional ciliated cells.
Supporting Video 2. Effect of culture medium, 0.9% saline, undiluted (Physiomer®) and diluted (Sterimar®) seawater solutions on the WRS of nasal epithelial cell cultures after an epithelial wound.


