OsERF71 alters root structure to enhance drought resistance.
Abstract
Plant responses to drought stress require the regulation of transcriptional networks via drought-responsive transcription factors, which mediate a range of morphological and physiological changes. AP2/ERF transcription factors are known to act as key regulators of drought resistance transcriptional networks; however, little is known about the associated molecular mechanisms that give rise to specific morphological and physiological adaptations. In this study, we functionally characterized the rice (Oryza sativa) drought-responsive AP2/ERF transcription factor OsERF71, which is expressed predominantly in the root meristem, pericycle, and endodermis. Overexpression of OsERF71, either throughout the entire plant or specifically in roots, resulted in a drought resistance phenotype at the vegetative growth stage, indicating that overexpression in roots was sufficient to confer drought resistance. The root-specific overexpression was more effective in conferring drought resistance at the reproductive stage, such that grain yield was increased by 23% to 42% over wild-type plants or whole-body overexpressing transgenic lines under drought conditions. OsERF71 overexpression in roots elevated the expression levels of genes related to cell wall loosening and lignin biosynthetic genes, which correlated with changes in root structure, the formation of enlarged aerenchyma, and high lignification levels. Furthermore, OsERF71 was found to directly bind to the promoter of OsCINNAMOYL-COENZYME A REDUCTASE1, a key gene in lignin biosynthesis. These results indicate that the OsERF71-mediated drought resistance pathway recruits factors involved in cell wall modification to enable root morphological adaptations, thereby providing a mechanism for enhancing drought resistance.
Since plants first colonized land 465 million years ago, they have evolved a variety of strategies to avoid or resist the stresses associated with terrestrial habitats (Wellman et al., 2003). The abiotic stress resulting from drought conditions has been studied extensively, as water deficits severely affect plant development and productivity (Atkinson and Urwin, 2012). Moreover, water-deficient areas increasingly include agriculturally important land, as a consequence of global climate change and an explosive increase in the human population (Mittler, 2006). This has motivated efforts to improve crops through the manipulation of drought resistance mechanisms, which include escape, avoidance, and tolerance to drought stress (Moore et al., 2008; Yoshimura et al., 2008). Drought escape and avoidance involve aspects of growth and development to evade drought stress, while drought tolerance typically utilizes inherent physiological or physical capacity to overcome drought stress. However, because plant drought resistance occasionally results from a combination of developmental and physiological changes, the categorization of the drought resistance mechanisms is not always easy.
Recent studies have uncovered several drought resistance mechanisms, including the regulation of transcriptional networks by drought-responsive transcription factors (Zhu, 2002; Shinozaki et al., 2003; Chinnusamy et al., 2004). Members of transcription factor families, such as AP2/ERF, bZIP, MYB, and NAC, have been proposed as key regulators of plant drought resistance mechanisms (Kang et al., 2002; Abe et al., 2003; Fujita et al., 2004; Tran et al., 2004; Oh et al., 2009; Jeong et al., 2010; Seo et al., 2011), since drought stress induces their expression, followed by the activation of downstream drought-responsive genes that are required for developmental and physiological responses. Several studies have shown that overexpression of drought-responsive transcription factors can lead to enhanced drought resistance. For example, plants overexpressing Arabidopsis (Arabidopsis thaliana) AtMYB96 or HARDY (an AP2/ERF transcription factor) or transgenic rice (Oryza sativa) lines overexpressing OsAP37, OsNAC5, OsNAC9, OsNAC10, OsbZIP12, OsbZIP12, or OsbZIP23 all showed strong resistance to drought stress (Karaba et al., 2007; Oh et al., 2009; Jeong et al., 2010, 2013; Seo et al., 2011; Redillas et al., 2012; Joo et al., 2014; Park et al., 2015). Drought-responsive genes are often categorized into abscisic acid (ABA)-dependent or ABA-independent groups. The first group contains ABA-responsive elements in their promoters and requires interaction with ABA-responsive element-binding bZIP transcription factors, whereas ABA-independent genes contain dehydration-responsive elements/C-repeats (DRE/CRTs) in their promoters and are regulated by DRE/CRT-binding AP2/ERF transcription factors (DREB; Uno et al., 2000; Kang et al., 2002; Sakuma et al., 2002; Fujita et al., 2005; Yoshida et al., 2010).
Transcription factors that contain a highly conserved AP2/ERF DNA-binding domain are widespread in the plant kingdom (Rashid et al., 2012). Since AP2 was first identified and studied in the context of Arabidopsis flower development (Jofuku et al., 1994), 122 and 170 AP2/ERF genes have been predicted in the Arabidopsis (Nakano et al., 2006) and rice (Rashid et al., 2012) genomes, respectively. This family of transcription factors has been implicated in regulating plant growth and development (Elliott et al., 1996; Chuck et al., 1998; Boutilier et al., 2002), but members also are known to be components of mechanisms that provide protection from abiotic stresses, including drought (Stockinger et al., 1997; Jaglo-Ottosen et al., 1998; Liu et al., 1998). For example, DREB genes, which encode DRE/CRT-binding AP2/ERF transcription factors, have been shown to be required in Arabidopsis for the transcriptional activation of drought-responsive genes, and overexpression of DREBs enhances tolerance to drought stress (Liu et al., 1998; Haake et al., 2002). In rice, 42 out of 170 AP2/ERF genes have been identified as abiotic stress-responsive genes (Oh et al., 2009).
The plant organs to detect drought stress conditions are roots, and upon drought perception, they release signals to induce resistance and/or adapt their architecture for optimal growth under these conditions (Sieburth and Lee, 2010; Atkinson and Urwin, 2012). Split-root experiments in drought conditions and drought-inducible maize (Zea mays) root signals indicate that roots generate uncharacterized drought-inducible signals that move to the aerial parts of the plant and confer drought resistance to the shoots (Went, 1943; Passioura, 1988; Saab and Sharp, 1989; Gowing et al., 1990; Chazen and Neumann, 1994). Previous studies also have elucidated structural adaptations of root architecture to overcome drought stress. For example, rice inbred lines (IR20 × MGL-2) with long, thick roots showed enhanced drought tolerance (Ekanayake et al., 1985), and root-specific overexpression of OsNAC10 promoted radial root growth, which in turn enhanced drought tolerance (Jeong et al., 2010). In addition, overexpression of DEEPER ROOTING1 conferred drought avoidance capacity by altering rice root architecture (Uga et al., 2013), while drought stress was reported to cause an accumulation of lignin in roots, thereby modifying cell wall architecture to enhance growth under drought conditions (Yoshimura et al., 2008). These studies indicate that root architecture is closely related to plant drought resistance; however, the molecular mechanisms that confer root-mediated drought resistance are not well understood.
In this study, we investigated the role of the rice AP2/ERF transcription factor OsERF71 in drought resistance. Overexpressing OsERF71 under the control of two different promoters, driving expression in either the whole plant body or the root, showed that the latter was sufficient to enhance drought resistance. We also characterized the spatiotemporal expression patterns of OsERF71 and identified downstream genes that constitute the OsERF71-mediated drought resistance pathway. OsERF71 was found to control lignin biosynthesis in roots by directly regulating the expression of OsCCR1, a key gene in lignin biosynthesis. These data provide insights into plant drought resistance mechanisms via root structural adaptations.
RESULTS
OsERF71 Expression Is Induced by Abiotic Stresses in an ABA-Independent Manner
Of the 170 rice AP2/ERF family genes, 42 were identified previously as stress inducible, including OsERF71 (Os06g0194000; Oh et al., 2009). The OsERF71 gene belongs to the group VII AP2/ERF genes, which have a unique and uncharacterized N-terminal MCGGAI(I/L) motif (Nakano et al., 2006). Some of the group VII members are known to be involved in developmental and stress-related processes in rice (OsERF70), barley (Hordeum vulgare; BERF1), wheat (Triticum aestivum; TaERF1), and cotton (Gossypium hirsutum; EREB1; Zhu et al., 2003; Xu et al., 2007; Meng et al., 2010; Osnato et al., 2010; Rashid et al., 2012); however, the associated molecular mechanisms are not well understood. In this study, we focused on investigating the molecular mechanism of OsERF71 in response to abiotic stresses.
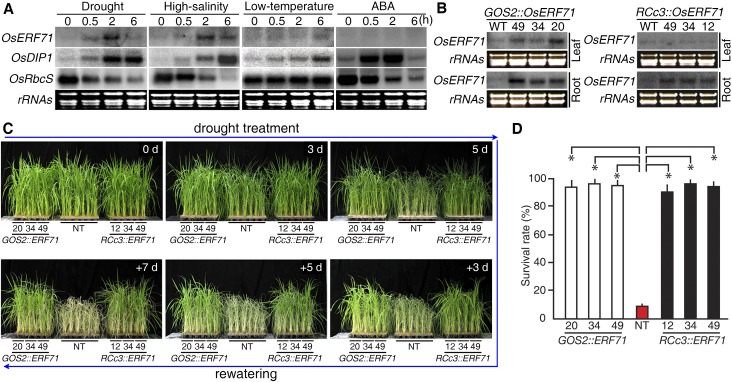
An RNA-gel blot analysis was performed using total RNAs from leaves collected from 2-week-old rice plants (Nipponbare) exposed to drought, high salinity, low temperature, or ABA in order to determine the expression patterns of OsERF71 in response to different stress conditions. Two molecular markers were used to monitor the status of the stress-treated rice plants: DEHYDRATION-INDUCIBLE PROTEIN1 (OsDIP1) and SMALL SUBUNIT OF RUBISCO (OsRbcS), which show opposite patterns of expression in response to abiotic stresses and ABA (Jang et al., 2003). The validity of these markers was confirmed, as OsDIP1 expression was detected after 0.5 h of each of the treatments and was highly induced after 2 h (Fig. 1A) as well as being induced by ABA. In contrast, OsRbcS expression was substantially reduced after 0.5 h of drought, salt, and ABA treatment but was unresponsive to cold treatment. We observed that OsERF71 expression was elevated after 0.5 h of drought treatment and showed a further major increase after 2 h (Fig. 1A). Likewise, OsERF71 expression was highly induced after 2 h of exposure to high-salinity conditions but showed only a slightly increase after 6 h of low-temperature conditions, and ABA treatment did not affect its expression (Fig. 1A). These results suggested that OsERF71 expression is induced by abiotic stresses in an ABA-independent manner and is highly responsive to drought stress.
Figure 1.
OsERF71 overexpression in rice enhances drought resistance. A, RNA gel-blot analyses showing transcriptional expression of OsERF71 in response to various stress conditions. An OsDIP1 gene probe was used as a positive control for abiotic stresses and the OsRbcS gene as a negative control. Ribosomal RNAs (rRNAs) were used as an internal control to determine equal loading of total RNA. B, RNA gel-blot analyses showing the expression levels and patterns of OsERF71 transcript accumulation in three independent homozygous lines of GOS2::OsERF71 (lines 20, 34, and 49) and RCc3:OsERF71 (lines 12, 34, and 49) plants. Ribosomal RNAs were used as an internal control to determine equal loading of total RNA. C, Drought resistance of GOS2::OsERF71 and RCc3:OsERF71 transgenic rice plants at the vegetative stage. Three independent homozygous GOS2::OsERF71 and RCc3:OsERF71 transgenic lines and NT control plants were grown in soil for 1 month and exposed to drought for 5 d, followed by rewatering. Numbers on the images indicate the duration over which drought was imposed and rewatering was applied. D, Survival rate at 15 d after rewatering. Values represent means + sd of three repeated tests. Each test was used with 38 to 40 plants for each transgenic line and 115 to 128 plants for NT. Asterisks indicate significant differences compared with NT (P < 0.05 by Student’s t test).
OsERF71 Overexpression in Rice Roots Enhances Drought Resistance at the Vegetative Stage
To understand the mechanism by which drought stress rapidly induces OsERF71 expression, two different types of overexpressing plants, GOS2::OsERF71 and RCc3::OsERF71, were generated for functional analyses. The GOS2 (rice eukaryotic translation initiation factor1-like gene) promoter has been shown to drive expression throughout the whole plant body (de Pater et al., 1992), while the RCc3 (rice lipid transfer protein-like gene) promoter does so in a root-specific manner (Xu et al., 1995). Fifty independent lines for each construct were generated, and to eliminate somaclonal variation, successive field selection of T1 to T4 plants (4 years) was performed to identify elite lines that grew normally, without stunting. Three independent homozygous lines for GOS2::OsERF71 (lines 20, 34, and 49) and RCc3::OsERF71 (lines 12, 34, and 49) were randomly selected from each population for evaluation of the levels and patterns of OsERF71 expression using RNA-gel blot analysis. Total RNA was isolated separately from leaves and roots of 2-week-old seedlings of each line grown under normal conditions, and in agreement with the nature of the promoters, elevated levels of OsERF71 transcript were seen in the roots of RCc3::OsERF71 plants but in both leaves and roots of GOS2::OsERF71 plants (Fig. 1B).
We next compared the performance of 1-month-old GOS2::OsERF71 and RCc3::OsERF71 plants with that of nontransgenic (NT) plants following exposure to drought conditions by monitoring drought-induced visual symptoms (Fig. 1C). Water content in soils during the drought treatment was uniform at each time point and gradually decreased over time between 1 and 5 d after drought treatment (Supplemental Fig. S1). After 3 d of drought treatment, the NT plants started to show evidence of leaf rolling, wilting, and loss of greening. However, after 5 d of drought exposure, the GOS2::OsERF71 and RCc3::OsERF71 plants appeared more viable than the NT controls, and after rewatering for up to 7 d, the transgenic plants recovered better from the drought-induced damage than did the NT plants, which wilted and finally died (Fig. 1C). After 15 d of rewatering, the transgenic plants showed a 94% to 96% survival rate, whereas the NT plants showed an approximately 10% survival rate (Fig. 1D). These data suggest that OsERF71 overexpression enhanced drought resistance in rice at the vegetative growth stage and that OsERF71 overexpression in roots was sufficient to promote drought resistance.
Root-Specific Overexpression of OsERF71 Increases Grain Yield under Drought Conditions
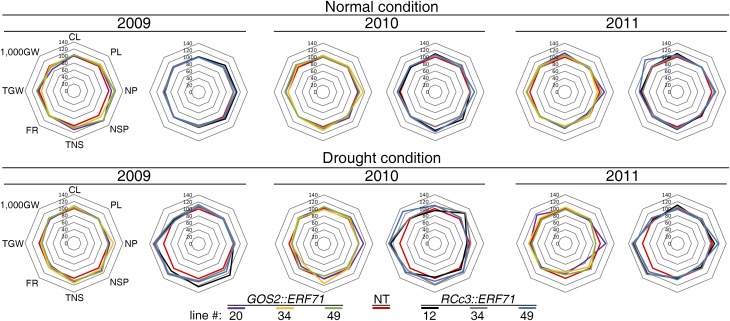
Yield components under drought conditions are thought to be an important criterion to evaluate plant drought resistance at the reproductive stage of growth, because grain productivity is seriously affected by drought stress (Atkinson and Urwin, 2012). Accordingly, yield parameters of the GOS2::OsERF71 and RCc3::OsERF71 plants were measured under both normal and drought field conditions in three cultivating seasons. Three independent lines of T5 (2009), T6 (2010), and T7 (2011) homozygous GOS2::OsERF71 and RCc3::OsERF71 transgenic lines, together with NT controls, were transplanted in a paddy field and grown to maturity. Rice plants at the transition stage from vegetative to reproductive development were exposed to drought stress conditions twice by removing water and discontinuing watering, followed by rewatering for further growth. Yield parameters were scored in 30 plants per independent line with three replicates. All yield parameters, including the grain-filling rate and total grain weight, were similar in the transgenic and NT control plants under normal field conditions (Fig. 2; Supplemental Table S1), indicating that overexpression of OsERF71 does not affect crop grain productivity under normal growth conditions. In contrast, exposure of the plants to the drought stress conditions resulted in an increased total grain weight and grain-filling rate in the RCc3::OsERF71 plants commonly in 3-year field tests but inconsistent variation in other yield parameters of three independent lines (Fig. 2; Supplemental Table S1). Specifically, total grain weight of the RCc3::OsERF71 plants was 23% to 32% higher than that of the NT control plants, whereas total grain weight of GOS2::OsERF71 plants was similar to that of the NT controls. Similarly, the grain-filling rate of the RCc3::OsERF71 plants was 18% greater than that of the NT control plants, but the grain-filling rate was similar in the GOS2::OsERF71 and NT control plants (Fig. 2; Supplemental Table S1). The higher grain-filling rate and total grain weight in the drought-treated RCc3::OsERF71 plants suggested that root-specific overexpression of OsERF71 enhanced grain yield under drought conditions.
Figure 2.
Agronomic traits of GOS2::OsERF71 and RCc3::OsERF71 transgenic rice plants grown in the field under both normal and drought conditions. Spider plots represent the agronomic traits from three independent homozygous T4 to T6 lines (2009–2011, respectively). Each data point represents the percentage of the mean values (n = 30 for each line) listed in Supplemental Table S1. Mean measurements from the NT controls were assigned a 100% reference value. CL, Culm length; FR, filling rate; NP, number of panicles per hill; NSP, number of spikelets per panicle; PL, panicle length; TGW, total grain weight; TNS, total number of spikelets; 1,000GW, 1,000 grain weight.
OsERF71 Is Expressed Predominantly in the Rice Root Endodermis and Pericycle
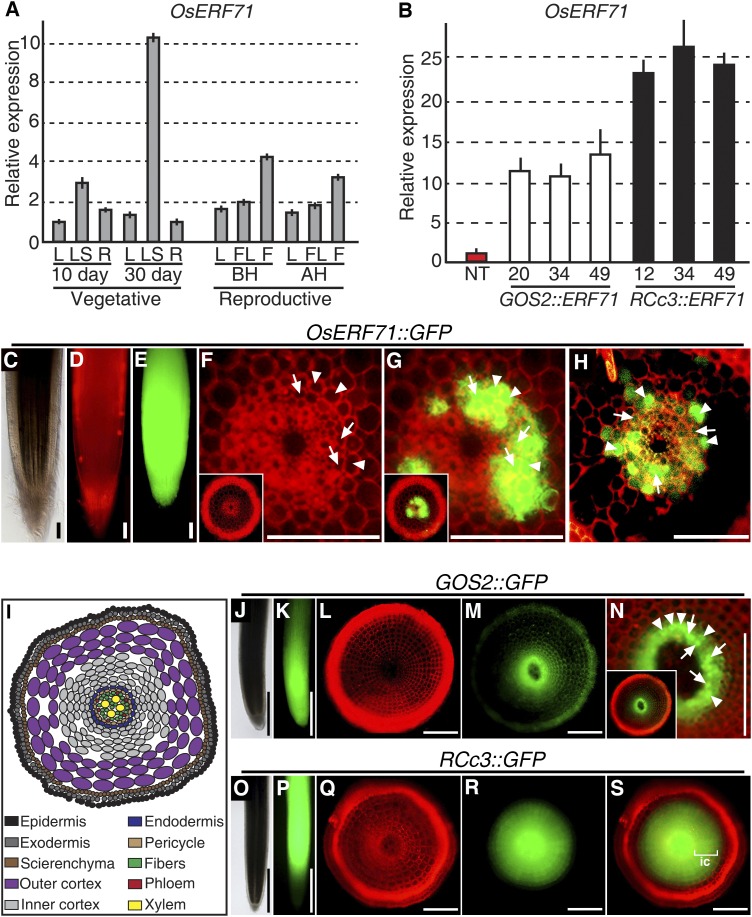
OsERF71 is expressed at low levels in 2-week-old leaves and roots under normal growth conditions (Fig. 1, A and B). To better understand the temporal expression patterns of the gene, its expression at various developmental stages of leaf blade, leaf sheath, root, flag leaf, and flower was investigated by quantitative real-time (qRT)-PCR (Fig. 3A). OsERF71 transcripts were detected at all developmental stages and sampled tissues, with the highest levels detected in 30-d-old leaf sheaths. The spatial expression in the roots was then determined by generating transgenic rice plants expressing the transcriptional fusion, OsERF71::GFP. GFP fluorescence was found to be high in the root apical meristem (Fig. 3, C–E) but was lower in the root elongation zones (Fig. 3, F and G). In this area (3 mm above the root apical tip), the signal was detected predominantly in the pericycle (arrows in Fig. 3) and endodermal cell layers (arrowheads in Fig. 3), while very low or no expression was detected in the cortex or epidermis (Fig. 3, F and G). In the root maturation zone (1 cm above the root apical tip), GFP fluorescence also was visible only in the pericycle and endodermal cell layers (Fig. 3H), suggesting that OsERF71 functions in the root meristem, pericycle, and endodermis.
Figure 3.
OsERF71 spatiotemporal expression and subcellular expression patterns of GOS2::GFP and RCc3::GFP. A, qRT-PCR analysis of OsERF71 expression in rice tissues of 10- and 30-d-old NT rice plants at the vegetative developmental stage and before heading (BH) and after heading (AH) in NT rice plants at the reproductive stage. F, Flower; FL, flag leaf; L, leaf blade; LS, leaf sheath; R, root. UBIQUITIN1 expression was used as an internal control. Data are shown as means ± sd of three biological and two technical replicates. B, qRT-PCR analysis of OsERF71 expression in 2-week-old GOS2::OsERF71 (lines 20, 34, and 49), RCc3:OsERF71 (lines 12, 34, and 49), and NT roots. UBIQUITIN1 expression was used as an internal control. Data are shown as means + sd of three biological and two technical replicates. C to H, Confocal microscopy images of 2-week-old OsERF71::GFP roots. C to E, Root apex in bright-field image (C), propidium iodide (PI)-stained image (D), and GFP expression (E). F and G, Cross sections of a root in the elongation zone, 3 mm above the root tip, in PI-stained image (F) and GFP merged image (G). Insets in F and G are whole images. H, Cross section of a root in the maturation zone, 1 cm above the apical tips. Bars in C to H = 100 μm. I, Schematic diagram representing the organization of an NT root in the elongation zone in transverse section. J to N, Confocal microscopy images of 2-week-old GOS2::GFP roots. J and K, Root apex in bright-field image (J) and GFP expression (K). L to N, Cross sections of a root in the elongation zone, 3 mm above the root tip, in PI-stained image (L), GFP expression (M), and merged image (N). Inset in N is the whole image. Bars = 1 mm in J and K and 100 μm in L to N. O to S, Confocal microscopy images of 2-week-old RCc3::GFP roots. O and P, Root apex in bright-field image (O) and GFP expression (P). Q to S, Cross sections of a root in the elongation zone, 3 mm above the root tip, in PI-stained image (Q), GFP expression (R), and merged image (S). Bars = 1 mm in O and P and 100 μm in Q to S. Arrowheads, Endodermis; arrows, pericycle; ic, inner cortex.
OsERF71 Expression Levels and Patterns in GOS2::OsERF71 and RCc3::OsERF71 Roots
Both GOS2::OsERF71 and RCc3::OsERF71 plants showed drought resistance at the vegetative stage of growth. Interestingly, however, the grain yield of the RCc3::OsERF71 plants was significantly enhanced under drought conditions as compared with that of the GOS2::OsERF71 plants. These observations and the fact that OsERF71 transcript levels were elevated in the roots of both overexpressors led us to speculate that the levels and/or patterns of this expression might be different. We examined the expression levels in 2-week-old GOS2::OsERF71 and RCc3::OsERF71 roots (Fig. 3B) and found that the expression in RCc3::OsERF71 roots was about 2-fold higher than in GOS2::OsERF71 roots. Using 2-week-old GOS2::GFP and RCc3::GFP plants (Fig. 3, J–S), the spatial distribution of GFP fluorescence in GOS2::GFP was found to be in the root apical meristem and decreasing gradually along the root elongation and maturation zones (Fig. 3, J and K), whereas no RCc3::GFP was detected in the root apical meristem (around 1 mm above the apical tip) but rather in the root elongation and maturation zones (Fig. 3, O and P). In the elongation zone (3 mm above the apical root tips), the GOS2::GFP plants showed a high GFP signal in the pericycle and endodermis but a low signal in the epidermis, cortex, and inner cells of the vasculature (compare Fig. 3, L–N, with Fig. 3I). In contrast, the RCc3::GFP plants showed high GFP signal in the pericycle, endodermis, vasculature, and inner cortex layers of the elongation zone and low GFP signal in the outer cortex cell layer and the epidermis of the elongation zone (compare Fig. 3, Q–S, with Fig. 3I). Thus, different OsERF71 expression patterns and/or levels in the RCc3::OsERF71 and GOS2::OsERF71 roots might be responsible for the different grain yield under drought stress conditions at the reproductive stage of growth.
Root Overexpression of OsERF71 Alters Radial Root Growth
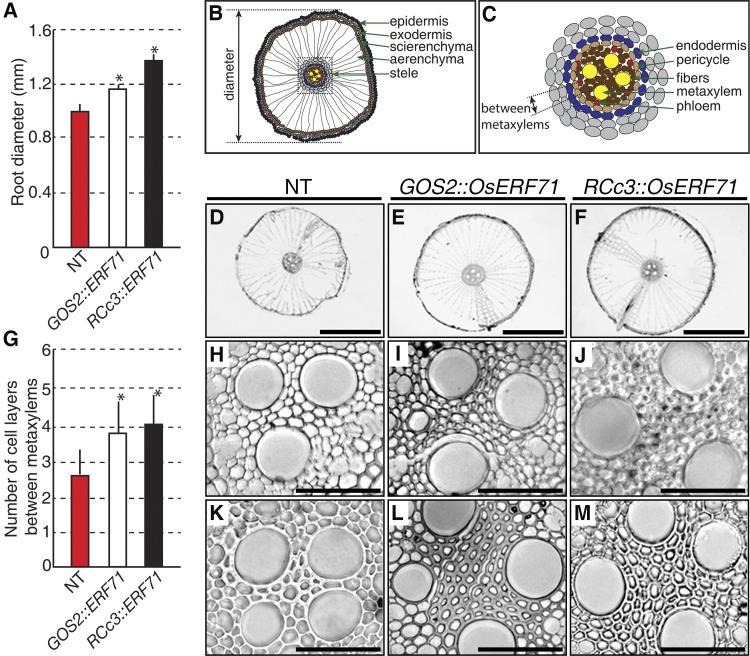
To observe possible morphological changes in the roots caused by OsERF71 overexpression, fully developed roots of 3-month-old GOS2::OsERF71, RCc3::OsERF71, and NT control plants were examined. Distinct differences in root thickness were observed (Fig. 4A), with GOS2::OsERF71 and RCc3::OsERF71 roots being 20% and 32% thicker than NT roots, respectively. In transverse sections, GOS2::OsERF71 and RCc3::OsERF71 roots had larger aerenchyma (Fig. 4, B and D–F), which are air spaces between epidermal and endodermal cell layers that provide an internal pathway for oxygen movement (Shiono et al., 2011). They also developed approximately four cell layers between the mature metaxylem cells (Fig. 4G) compared with the two to three cell layers in the NT control plants (Fig. 4, C and H–M). In summary, OsERF71 overexpression gave rise to larger aerenchyma in the roots, more cell layers in the root vasculature, and, accordingly, thicker radial root growth.
Figure 4.
Root phenotypes of GOS2::OsERF71 and RCc3::OsERF71 transgenic plants. A, Quantification of root diameter (n = 50 for each genotype). B, Schematic diagram representing the organization of a maturate NT root in transverse section. C, Schematic diagram showing an enlarged representation of the root stele in B. D to F, Transverse section images showing the internal anatomy of 3-month-old maturate roots of an NT control plant (D), GOS2::OsERF71 (E), and RCc3::OsERF71 (F). G, Quantification of the number of cell layers between mature metaxylem cells (n = 38 for NT, 39 for GOS2::OsERF71, and 42 for RCc3::OsERF71 plants). H to M, Transverse section images showing cell layers between mature metaxylem cells in roots containing three and four metaxylem cells from 3-month-old NT control (H and K), GOS2::OsERF71 (I and L), and RCc3::OsERF71 (J and M) plants. Error bars indicate sd, and asterisks indicate significant differences compared with NT (P < 0.05 by Student’s t test). Bars = 50 μm in H to M and 500 μm in D to F.
Identification of Genes Regulated by OsERF71
GOS2::OsERF71 and RCc3::OsERF71 plants exhibited drought resistance phenotypes, suggesting that an OsERF71-mediated drought resistance pathway was constitutively active in the overexpressing lines, even when no drought stress was imposed. To identify genes in this pathway, the Rice 3′-Tiling microarray was used to isolate gene expression profiles in 2-week-old GOS2::OsERF71 and RCc3::OsERF71 roots grown under normal growth conditions compared with NT roots. A cutoff change of at least 3-fold was used to identify up- and down-regulated genes, and Student’s t test (P < 0.05) was used to discard data for genes that were not statistically relevant. This analysis revealed that GOS2::OsERF71 contained 266 up-regulated and 159 down-regulated genes compared with NT, while RCc3::OsERF71 had 258 up-regulated and 374 down-regulated genes compared with NT (Supplemental Fig. S2A; Supplemental Table S2). As expected, the two profiles shared many genes, such that 73% of the differentially expressed genes from GOS2::OsERF71 were identical to 49% of the differentially expressed genes from RCc3::OsERF71. Specifically, there was an overlap of 192 up-regulated and 116 down-regulated genes between gene expression profiles from the GOS2::OsERF71 and RCc3::OsERF71 roots (Supplemental Fig. S2B; Supplemental Table S3). The 308 common genes were considered to be candidate genes in an OsERF71-mediated drought resistance pathway and were categorized using Gene Ontology analysis (PANTHER Classification System; http://pantherdb.org). Many of these genes were assigned to a metabolic process category (30%) in a biological process class or to hydrolase activity (15%) and catalytic activity (28%) in a molecular process class (Supplemental Fig. S2C).
OsERF71 Promotes Lignification by Up-Regulating the Expression of Lignin Biosynthetic Genes
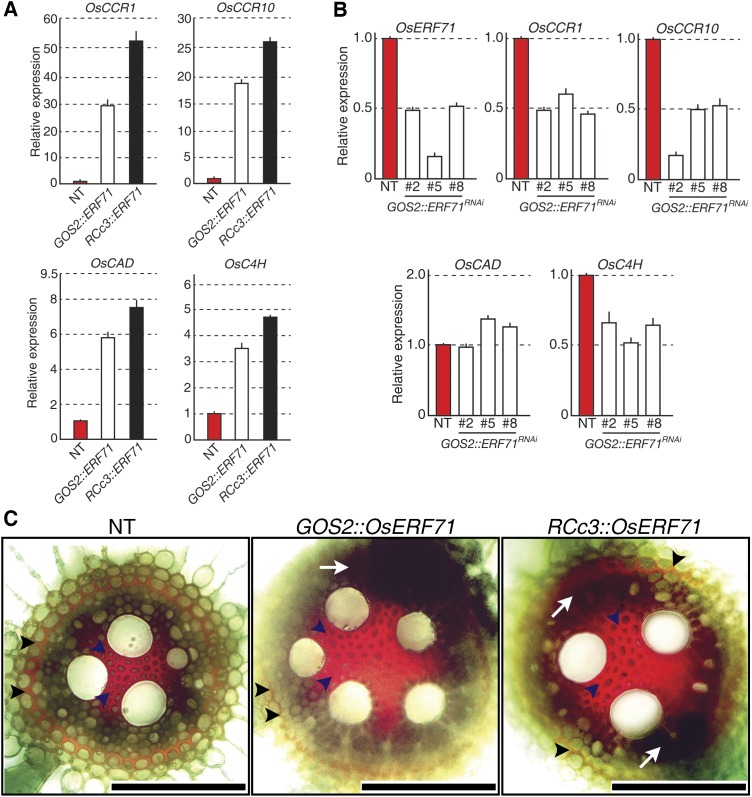
The 192 up-regulated genes were associated mainly with general stress responses, cell wall organization, or lignin biosynthesis (Table I; Supplemental Table S4). Cell wall-associated proteins such as EXPANSIN (EXP), CHITINASE, PECTINESTERASE, and XYLOGLUCAN ENDOTRANSGLUCOSYLASE (XTH) are thought to be important for plant adaptation to drought stress by modifying plant growth (Moore et al., 2008; Ghosh and Xu, 2014). In addition, plant lignification is known to be an abiotic stress symptom, including drought stress, since lignin biosynthesis can be triggered in response to abiotic stresses (Fan et al., 2006; Yoshimura et al., 2008). The up-regulated genes putatively involved in lignin biosynthesis included CINNAMOYL-COENZYME A REDUCTASE (OsCCR1 and OsCCR10) and CINNAMYL ALCOHOL DEHYDROGENASE (OsCAD), which showed a greater than 10-fold increase in expression compared with NT, and CINNAMATE-4-HYDROXYLASE (OsC4H), PHENYLALANINE AMMONIA LYASE (OsPAL), and PEROXIDASE (OsPRX), whose expression increased more than 3-fold compared with NT (Table I). PAL, C4H, CCR, CAD, and PRX are key enzymes required for lignin biosynthesis (Meyer et al., 1998; Rohde et al., 2004; Sibout et al., 2005; Kawasaki et al., 2006; Bonawitz and Chapple, 2010; Liu et al., 2010; Lee et al., 2013). To verify the up-regulation of lignin biosynthesis genes identified by the microarray analysis, their transcript levels in 2-week-old GOS2::OsERF71 and RCc3::OsERF71 roots were analyzed by qRT-PCR. This analysis confirmed that OsERF71 overexpression caused the transcript levels of OsCCR1, OsCCR10, OsCAD, and OsC4H to increase (Fig. 5A), such that they were 53-, 26-, 7.5-, and 4.8-fold higher, respectively, in RCc3::OsERF71 roots and 29-, 18-, 5.8-, and 3.5-fold higher, respectively, in GOS2::OsERF71 roots than in NT roots. To show whether OsERF71 is necessary to regulate the expression of lignin biosynthesis genes, we generated a knockdown rice, GOS2::OsERF71RNAi. Based on reduced expression of OsERF71, three independent homozygous GOS2::OsERF71RNAi lines (2, 5, and 8) were selected for further analysis (Fig. 5B). The morphology of GOS2::OsERF71RNAi remained similar to that of NT. The transcript levels of lignin biosynthesis genes, OsCCR1, OsCCR10, and OsC4H, were significantly down-regulated in 2-week-old GOS2::OsERF71RNAi roots compared with NT roots, whereas transcript levels of OsCAD remained similar in GOS2::OsERF71RNAi and NT roots (Fig. 5B). Overall, these data indicate that OsERF71 is required to regulate the expression of lignin biosynthesis genes in roots.
Table I. List of lignin biosynthesis genes up-regulated (greater than 3-fold) in both GOS2::OsERF71 and RCc3::OsERF71 roots compared with NT roots based on microarray data.
| Gene Name | Identifier |
GOS2::OsERF71 |
RCc3::OsERF71 |
||
|---|---|---|---|---|---|
| Fold Change | Pa | Fold Change | Pa | ||
| PAL | Os04g0518400 | 4.34 | 1.96E-05 | 3.37 | 1.95E-05 |
| C4H | Os02g0467600 | 4.04 | 1.93E-05 | 4.09 | 9.70E-06 |
| C4H | Os02g0467000 | 3.35 | 4.68E-05 | 3.16 | 3.59E-05 |
| CCR10 | Os02g0811800 | 14.44 | 7.25E-06 | 14.21 | 2.98E-06 |
| CCR1 | Os02g0808800 | 13.77 | 7.25E-06 | 19.15 | 2.07E-06 |
| CAD | Os04g0612700 | 14.04 | 2.07E-05 | 15.22 | 1.00E-05 |
| PRX | Os06g0521500 | 5.42 | 2.12E-05 | 6.28 | 9.19E-06 |
| PRX | Os01g0963000 | 4.25 | 1.00E-04 | 5.76 | 5.49E-05 |
| PRX | Os01g0327100 | 3.07 | 4.73E-05 | 3.07 | 3.27E-05 |
P value based on one-way ANOVA.
Figure 5.
Regulation of lignin biosynthesis genes in OsERF71-overexpressing and RNAi knockdown transgenic rice plants. A, qRT-PCR analysis showing the transcript levels of OsCCR1, OsCCR10, OsCAD, and OsC4H in 2-week-old GOS2::OsERF71 and RCc3::OsERF71 roots. UBIQUITIN1 expression was used as an internal control. Data are shown as means + sd of three biological and two technical replicates. B, qRT-PCR analysis showing the transcript levels of OsERF71, OsCCR1, OsCCR10, OsCAD, and OsC4H in 2-week-old GOS2::OsERF71RNAi roots (lines 2, 5, and 8). UBIQUITIN1 expression was used as an internal control. Data are shown as means + sd of three biological and two technical replicates. C, Images of phloroglucinol-stained 3-month-old maturate roots of NT (left), GOS2::OsERF71 (middle), and RCc3::OsERF71 (right) plants. Black arrowheads indicate the Casparian strip; blue arrowheads indicate the cells between mature metaxylem cells; and white arrows indicate additional phloroglucinol-stained regions. Bars = 100 μm.
The up-regulation of the lignin biosynthesis genes by OsERF71 overexpression prompted us to examine lignin accumulation in roots using phloroglucinol staining, which specifically stains lignin red (Fig. 5C). Cells between the mature metaxylem and the Casparian strip of transgenic and NT roots all showed staining (Fig. 5C, arrowheads); however, RCc3::OsERF71 and GOS2::OsERF71 contained additional areas that stained red (Fig. 5C, arrows). Taken together, these results indicate that OsERF71 overexpression promoted lignification by up-regulating the expression of lignin biosynthetic genes.
OsERF71 Directly Regulates OsCCR1, a Key Gene in Lignin Biosynthesis
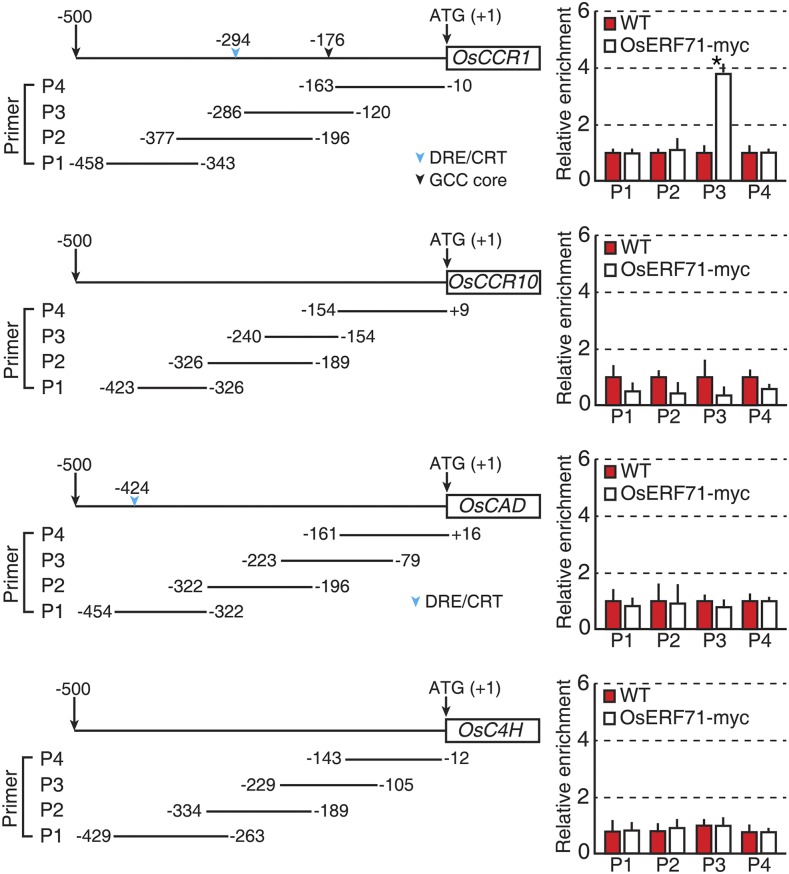
Since lignin biosynthesis is triggered by drought stress and OsERF71 overexpression up-regulated the expression of genes involved in lignin biosynthesis under normal growth conditions, we hypothesized that OsERF71 directly regulates the expression of these genes in response to drought. To confirm this, transgenic plants expressing a myc-tagged version of OsERF71, RCc3::OsERF71-myc, were generated and OsERF71-myc expression in roots was confirmed. Two-week-old RCc3::OsERF71-myc plants showed strong transcriptional expression of OsERF71-myc as well as of lignin biosynthetic genes in their roots when grown under normal conditions, consistent with the expression patterns obtained from the RCc3:OsERF71 plants (Supplemental Fig. S3A). Expression of the OsERF71-myc protein was detected in 2-week-old RCc3::OsERF71-myc roots using an anti-myc antibody (Supplemental Fig. S3B). To identify possible OsERF71 targets among the lignin biosynthetic genes, a chromatin immunoprecipitation (ChIP) assay using the anti-myc antibody was performed, followed by quantitative PCR (qPCR) analysis. Among the up-regulated lignin biosynthetic genes, only an OsCCR1 promoter showed interaction with the OsERF71-myc protein (Fig. 6). This promoter has a GCC core cis-element, which is known as an AP2/ERF transcription factor-binding site (Mizoi et al., 2012), suggesting that OsERF71 binds directly to the OsCCR1 promoter and regulates OsCCR1 expression to activate lignin biosynthesis in rice roots.
Figure 6.
ChIP-qPCR for OsERF71 interacting with the promoters of the selected lignin biosynthesis genes. Two-week-old RCc3::OsERF71-myc and NT roots were used in ChIP-qPCR experiments with an anti-myc antibody. Left, ChIP-qPCR analysis showing the promoters of four lignin biosynthesis genes with four PCR-amplified regions (P1–P4). Right, ChIP-qPCR data indicate each PCR amplification region of each gene. The fold enrichment was normalized to ChIP-qPCR data of wild-type (WT) roots. Data are shown as means + sd of three technical replicates. The asterisk indicates a significant difference based on a 95% confidence interval. DRE, G/ACCGAC; CRT, GTCGAC; and GCC core, GCCGCC.
DISCUSSION
OsERF71 was identified previously as a stress-responsive AP2/ERF gene (Oh et al., 2009). Here, it was found to be more sensitive to drought stress than other abiotic stresses (Fig. 1A). Both whole-plant (GOS2::OsERF71) and root-specific (RCc3::OsERF71) overexpression of OsERF71 in transgenic rice plants gave rise to drought resistance phenotypes at the vegetative stages of growth (Fig. 1C), indicating that root-specific overexpression alone was sufficient to confer the phenotype. Unexpectedly, RCc3::OsERF71 plants showed enhanced grain yield under drought conditions, whereas GOS2::OsERF71 plants did not (Fig. 2). This difference may reflect different OsERF71 expression patterns and levels in the RCc3::OsERF71 and GOS2::OsERF71 roots. Indeed, the expression levels of OsERF71 were higher in RCc3::OsERF71 roots than in GOS2::OsERF71 roots (Fig. 3B). GFP signal from the GOS2::GFP and RCc3::GFP roots was detected in the pericycle and endodermis of both genotypes, while only RCc3::GFP-expressing roots showed a signal in the vasculature and inner cortex layers (Fig. 3, N and S). The similar expression patterns in the pericycle and endodermis observed in the overexpressing lines were consistent with the expression patterns of the endogenous OsERF71 gene (Fig. 3, G and H), suggesting that overexpression of OsERF71 in the pericycle and endodermis is sufficient to confer drought resistance at the vegetative stage of growth. OsERF71 overexpression in the root meristem, as evidenced by GFP fluorescence in the GOS2::GFP roots, did not appear to contribute to the enhanced drought resistance, since the RCc3 promoter has no activity in the root meristem (Fig. 3P). Consequently, OsERF71 overexpression in the vasculature and inner cortex layers, and/or higher expression levels, may lead to the increased grain yield under drought stress conditions at the reproductive stage of growth. This conclusion was supported in part by the microarray data, since the number of downstream genes affected by RCc3::OsERF71 overexpression in roots was larger than that in GOS2::OsERF71 roots (Supplemental Fig. S2). Similar observations were made when OsNAC10 was overexpressed under the control of the GOS2 and RCc3 promoters in rice (Jeong et al., 2010).
Roots play key roles in water and mineral uptake and in the perception of drought conditions, since they are in direct contact with the drying soil. Thus, root development adapts quickly to drought stress to optimize growth and development. GOS2::OsERF71- and RCc3::OsERF71-overexpressing roots had thicker radial growth due to larger aerenchyma and more cell layers in the vasculature (Fig. 4). Similar phenotypes have been reported in previous studies. Specifically, vigorous root radial growth was shown to be closely connected to high plant water potential under drought conditions in rice (Ekanayake et al., 1985; Price et al., 1997), and overexpression of OsNAC5, OsNAC9, and OsNAC10 promoted thicker radial root growth and enhanced drought resistance in rice (Jeong et al., 2010, 2013; Redillas et al., 2012). Interestingly, root aerenchyma were recently reported to improve drought tolerance by reducing root metabolic costs and optimizing root growth and water uptake from drying soil (Zhu et al., 2010; Yang et al., 2012). Thus, the larger aerenchyma induced by OsERF71 overexpression may be considered to be a structural adaptation of the roots to optimize growth under drought conditions. In addition to radial root growth, other structurally modified roots such as long roots, more lateral roots, and angle change of root growth contribute to drought tolerance for plants. Overexpression of TaNAC2 and HARDY in Arabidopsis promotes primary root growth and lateral root development that induced tolerance to drought (Karaba et al., 2007; Mao et al., 2012). Recently, DEEPER ROOTING1 conferred rice tolerance to drought by changing the root architecture through increased root growth angle (Uga et al., 2013). Taken together, modulation of root structure enhances tolerance to water deficit conditions, and radial root growth with large aerenchyma is one of structural adaptations to survive under drought conditions.
Microarray experiments identified 192 up-regulated downstream genes common in both RCc3::OsERF71 and GOS2::OsERF71 roots compared with NT roots (Supplemental Fig. S2). Many of these could be divided into three groups: general stress-inducible genes, cell wall-associated genes, and lignin biosynthesis genes. The stress-inducible genes included 9-CIS-EPOXYCAROTENOID DIOXYGENASE, a key enzyme in ABA biosynthesis that can improve plant drought tolerance when overexpressed (Iuchi et al., 2001; Qin and Zeevaart, 2002). LATE EMBRYOGENESIS ABUBDANT PROTEIN and UNIVERSAL STRESS PROTEIN also were found among the up-regulated genes, both of which have been shown to improve drought tolerance when overexpressed (Xu et al., 1996; Sivamani et al., 2000; Loukehaich et al., 2012). Among cell wall-associated genes, cell wall-loosening genes such as EXP and XTH are known to be involved in cell enlargement. GmEXP1 overexpression in transgenic tobacco (Nicotiana tabacum) plants accelerated root growth and cell enlargement (Lee et al., 2003), and loss of AtXTH18 function in Arabidopsis by RNA interference (RNAi)-based suppression caused a reduction in root length and cell size (Osato et al., 2006). Thus, it is possible that increased expression of cell wall-loosening enzymes promotes the formation and enlargement of aerenchyma and that this structural change in root architecture reverberates enhanced drought resistance.
Interestingly, we observed that OsERF71 overexpression elevated root lignification levels. Lignin is a hydrophobic biopolymer composed of three kinds of monolignols: p-coumaryl, coniferyl, and sinapyl alcohols (Boerjan et al., 2003). Each monolignol is produced from the aromatic amino acids Phe and Tyr by the activity of enzymes encoded by genes such as PAL, C4H, 4CL, CCR, and CAD (Bonawitz and Chapple, 2010). Microarray and qRT-PCR analyses of RCc3::OsERF71 and GOS2::OsERF71 roots revealed that genes such as OsCCR1, OsC4H, OsCCR10, and OsCAD (Fig. 5A; Table I) were up-regulated in the overexpression transgenic lines, whereas OsCCR1, OsCCR10, and OsC4H were down-regulated in RNAi knockdown lines (Fig. 5B). Unexpectedly, OsCAD remained similar in RNAi knockdown lines and NT roots. It is possible that low OsERF71 expression in GOS2::OsERF71RNAi may be enough to regulate OsCAD expression, or OsERF71 may not primarily regulate OsCAD expression. OsERF71 overexpression transgenic roots also were highly lignified, as evidenced by staining with phloroglucinol (Fig. 5C). Lignin biosynthesis in roots is known to be triggered by drought stress (Fan et al., 2006; Yoshimura et al., 2008), and the expression levels of lignin biosynthetic genes were greater in the roots of both transgenic genotypes, even in the absence of imposed drought stress. We hypothesized that those genes are direct downstream targets of OsERF71, and indeed, we found that OsERF71 binds directly to the promoter of OsCCR1 (Fig. 6). Another line of evidence that OsERF71 regulates root lignification was that the expression levels of lignin biosynthetic genes correlated with those of OsERF71 in the transgenic roots. The OsERF71 expression levels in RCc3::OsERF71 roots were about 2-fold higher than in GOS2::OsERF71 roots (Fig. 3B), and in agreement with this, the expression levels of lignin biosynthetic genes were higher in RCc3::OsERF71 roots than in GOS2::OsERF71 roots (Fig. 5A). In addition, 2OG-Fe(II) OXYGENASE also was found to be up-regulated by OsERF71 overexpression. A mutation in RL14, which encodes a 2OG-Fe(II) OXYGENASE protein, has been shown to result in reduced lignin content in rice (Fang et al., 2012); and, taken together with our data, this suggests that the increased expression of 2OG-Fe(II) OXYGENASE contributed to the increased lignification in the OsERF71 transgenic roots. Lignin is a key component of secondary cell walls, where it provides mechanical strength (Boerjan et al., 2003). In addition, the hydrophobic property of lignin has been suggested to have an inhibitory influence on water transpiration from plant tissues under drought conditions (Barros et al., 2015). Hu et al. (2009) reported that drought-tolerant maize inbred lines displayed greater lignification under drought stress conditions than drought-sensitive inbred lines, further indicating a role for lignin in drought resistance. How the OsERF71-mediated lignification affects radial root growth and enlarged aerenchyma remains unknown, although combinatory regulation by its downstream genes was sufficient to induce radial root growth when overexpressed. Taken together, the OsERF71-mediated structural adaptation of root architecture inhibits water transpiration under drought conditions, and this root adaptation and growth optimization enhance drought resistance.
In conclusion, the data presented here provide new insights into the molecular mechanisms of drought resistance in plants. OsERF71 modulates downstream genes, including general stress inducible genes, cell wall-associated genes, and lignin biosynthesis genes, which together contribute to improved drought resistance, and gain of function of OsERF71 in rice roots is sufficient for drought resistance phenotypes.
MATERIALS AND METHODS
Plasmid Construction and Rice Transformation
The coding region of rice (Oryza sativa) OsERF71 (AK069833; Os06g0194000) was amplified using the PrimeSTAR HS DNA Polymerase (Takara) and Reverse Transcription System (Promega), according to the manufacturer’s instructions, from total RNAs extracted from whole plants of 2-week-old japonica rice ‘Nipponbare’ grown in a greenhouse (16-h-light/8-h-dark cycle) at 28°C. The primers used for cloning into the entry vector, pENTR/SD (Invitrogen), were the forward primer 5′-CACCATGTGCGGCGGCGCCATCCT-3′ and the reverse primer 5′-TCAGTAGAACTCGGCCGACA-3′. To generate OsERF71-overexpressing transgenic rice, the OsERF71 complementary DNA (cDNA) was subcloned into the rice transformation vector, p700-GOS2 (Jeong et al., 2010), carrying the 2.2-kb GOS2 (Os07g0529800) promoter, upstream region from ATG start codon using the Gateway system (Invitrogen; Supplemental Fig. S4). This construct was named GOS2::OsERF71 and was used for constitutive overexpression (Supplemental Fig. S4). For root-specific expression, the cDNA was cloned into the rice transformation vector, p700-RCc3 (Jeong et al., 2010), carrying 1.3 kb of the RCc3 (Os02g0662000) promoter, upstream region from ATG start codon and was named RCc3::OsERF71. For an RNAi construct named GOS2::OsERF71RNAi, 378 bp from the OsERF71 coding region (between 571 and 899 from ATG) was cloned into two sites of the rice transformation vector, p700-GOS2-RNAi, which is separated by a GUS sequence (Supplemental Fig. S4). For promoter-driven GFP expression, the OsERF71::GFP, GOS2::GFP, and RCc3::GFP constructs were made, containing the promoters of OsERF71 (2.1 kb), GOS2 (2.2 kb), and RCc3 (1.3 kb) that had been amplified by PCR using Nipponbare genomic DNAs. The primers used for cloning into the pENTR/SD vector were the forward primer 5′-CACCCAGACCAGCACCAAGTGGTG-3′ and the reverse primer 5′-GGTGGGCTAGGCTATGGTGCT-3′ for the OsERF71 promoter, the forward primer 5′-CACCTCTAGAATCCGAAAAGTTTCTG-3′ and the reverse primer 5′-TATGGAACTTTGCTGGTGAAAGTGGC-3′ for the GOS2 promoter, and the forward primer 5′-CACCTCGACGCTACTCAAGTGGTGGG-3′ and the reverse primer 5′-GATCACAAGCGCAGCTAATC-3′ for the RCc3 promoter. Finally, the promoters were subcloned into the rice transformation vector, pMJ401 (Park et al., 2010), carrying the GFP using the Gateway system (Supplemental Fig. S4). Transgenic plants (Nipponbare) were obtained by Agrobacterium tumefaciens (LBA4404)-mediated cocultivation, as described previously (Jang et al., 1999).
RNA Gel-Blot Analysis
‘Nipponbare’ rice seeds were germinated in soil and grown in a greenhouse (16-h-light/8-h-dark cycle) at 28°C. For drought treatment, 2-week-old seedlings were air dried by removing the plants from their pots for the indicated times under continuous light of approximately 1,000 μmol m−2 s−1. For high-salinity and ABA treatments, 2-week-old seedlings were transferred to a nutrient solution including 400 mm NaCl and 100 μm ABA, respectively. For low-temperature treatments, 2-week-old seedlings were placed in a 4°C cold chamber under continuous light of 150 μmol m−2 s−1. Total RNA samples were extracted using Plant RNA Purification Reagent (Invitrogen). Ten micrograms of each total RNA sample was loaded on a 1.2% denatured agarose gel and blotted onto Hybond N+ nylon membranes (Amersham Bioscience). These were incubated with an OsERF71 cDNA probe corresponding to a 360-bp fragment of the C-terminal region of OsERF71, which was generated by PCR amplification (forward primer 5′-GAACGCGATTGAGGATACTTTCG-3′ and reverse primer 5′-TCAGTAGAACTCGGCCGACAC-3′). OsDIP1 and OsRbcS probes were used as markers for stress treatments as described previously (Jang et al., 2003). To determine the expression pattern and OsERF71 transcript levels in OsERF71-overexpressing transgenic plants, total RNA was extracted from the roots and leaves of three independent T5 homozygous lines of 2-week-old RCc3::OsERF71 and GOS2::OsERF71 plants. Ten micrograms of each total RNA sample was used for RNA gel-blot analysis.
Drought Stress Treatment at the Vegetative Stage
Transgenic and NT (‘Nipponbare’) rice plants were germinated on Murashige and Skoog medium at 28°C for 4 d, and 18 seedlings of each transgenic and NT line were transplanted in soil pots (4 × 4 × 6 cm; four plants per pot) and grown for 4 weeks in a greenhouse at 28°C to 30°C. Each pot had the same size of holes in the bottom, and they were located in one big tray to synchronize watering. Drought stress was imposed simultaneously on all rice plants by sequentially removing water from the soil pots for 5 d and rewatering. Drought-induced symptoms were visualized by imaging RCc3::OsERF71, GOS2::OsERF71, and NT plants at the indicated time points using an NEX-5N camera (Sony).
Grain Yield Evaluation of Rice Plants Grown in the Field
To evaluate the yield components of transgenic rice plants under normal field conditions, three independent lines of T5 (2009), T6 (2010), and T7 (2011) homozygous RCc3::OsERF71 and GOS2::OsERF71 transgenic rice plants were planted in a rice paddy field at the Rural Development Administration, Suwon (127:01E/37:16N), Korea (2009), and subsequently at the Kyungpook National University, Gunwi (128:34E/36:15N), Korea (2010 and 2011). A randomized design was employed for three replicates using three different 10-m2 plots. Approximately 100 plants for each genotype were sown per year, and 60 seedlings per line were transplanted into the plots 25 d after sowing (30 for normal field conditions and 30 for drought field conditions). Fertilizer was applied at 70:40:70 (nitrogen:phosphorus:potassium) kg ha−1 after the last paddling. Yield parameters were scored with the 30 plants per line collected from three different plots for normal field conditions. To evaluate the yield components of the transgenic plants under drought field conditions, we built rain-off shelters to cover rice plants and made a semifield condition before drought treatment (Supplemental Fig. S5A). Intermittent drought stress was applied during panicle development twice by draining the water from the bottom of the container. To monitor drought treatment, we checked water content in soils (randomly selected 100 spots for each time point) with the SM150 Soil Moisture Sensor (AT Delta-T Devices; Supplemental Fig. S5B). When full leaf rolling was observed in the NT plants after the first drought treatment, they were irrigated overnight and subjected again to a second round of drought stress until another complete leaf rolling occurred. After two drought stress treatments, the plants were irrigated until harvesting. Yield parameters were scored with the 30 plants for each line collected from three different plots for drought field conditions. The results from three independent lines were compared with those of NT controls, using analysis by one-way ANOVA.
qRT-PCR Analysis
For qRT-PCR experiments, a SuperScript III Platinum One-Step qRT-PCR system (Invitrogen) was used to generate first-strand cDNAs. qRT-PCR was carried out using a Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) and an Mx3000p Real-Time PCR machine (Stratagene). For OsERF71 temporal expression, total RNA was extracted from several tissues of 10-d-old and 30-d-old NT rice plants at different vegetative stages and several tissues before heading and after heading at the reproductive stage. To measure OsERF71 expression levels in OsERF71-overexpressing and knockdown RNAi transgenic rice plants and to validate the microarray data, total RNA samples were extracted from the roots of 2-week-old transgenic and NT rice seedlings grown under normal growth conditions. Rice UBIQUITIN1 (Os06g0681400) transcript abundance was used as the normalizing internal control, and three biological and two technical replicates were analyzed for all quantitative experiments. Gene-specific primers used for qRT-PCR are listed in Supplemental Table S5.
Confocal Microscopy
The GFP localization of OsERF71::GFP, GOS2::GFP, and RCc3::GFP transgenic rice plants was analyzed in 10 µm propidium iodide-stained roots of 2-week-old transgenic rice plants using an SP8 STED laser scanning confocal microscope (Leica). For root elongation and maturation zones, hand-cut transverse sections were used. GFP and propidium iodide were excited at 488 nm, and then emitted light was detected between 512 and 560 nm and between 610 and 650 nm, respectively.
Root Phenotypic Analysis and Phloroglucinol Staining
RCc3::OsERF71, GOS2::OsERF71, and NT rice plants were transplanted in five polyvinyl chloride tubes (1.2 μm in length and 0.2 μm in diameter) that were filled with natural paddy soil and placed into containers filled with water. Roots at the panicle development stage (approximately 3 months after germination) were used for quantification of root diameter and anatomical analysis. Fifty roots were randomly collected from five individual RCc3::OsERF71 (lines 12, 34, and 9), GOS2::OsERF71 (lines 20, 34, and 49), and NT plants, and root diameter was measured using an Olympus SZX12 stereomicroscope. To visualize the internal anatomy of the transgenic rice roots, cross sections were made using the Technovit 7100 system, as described previously (Jang et al., 2011) with slight modification. Technovit saturation was carried out for 3 d. Sections (3 μm) were taken from an ultramicrotome (MTX; RMC), and images were captured with an Olympus DP70 camera mounted on an Olympus BX 500 light microscope. Phloroglucinol staining of 3-month-old RCc3::OsERF71, GOS2::OsERF71, and NT roots (25–27 roots for each genotype) was as described previously (Jensen, 1962).
Rice 3′-Tiling Microarray Analysis
Gene expression profiling of RCc3::OsERF71 and GOS2::OsERF71 was performed using the Rice 3′-Tiling microarray manufactured by NimbleGen (http://www.nimblegen.com). This microarray chip contains 27,448 genes, the sequences of which are deposited at the International Rice Genome Sequencing Project Rice Annotation Project 1 database (http://rapdb.lab.nig.ac.jp). Further information about this microarray, including statistical analysis, can be found at http://www.ggbio.com (GreenGene Biotech). Total mRNA samples were extracted from the roots for each genotype that were randomly collected from 2-week-old RCc3::OsERF71 (lines 12, 34, and 49), GOS2::OsERF71 (lines 20, 34, and 49), and NT plants grown under normal growth conditions and then purified with a Qiagen Oligotex column system according to the manufacturer’s instructions. Data were obtained from two biological replicates for each genotype. To generate downstream gene profiles for RCc3::OsERF71 and GOS2::OsERF71, we calculated the fold change for each transcript compared with NT, using a cutoff change of at least 3-fold to identify up- and down-regulated genes. Student’s t test (P < 0.05) and one-way ANOVA (P < 0.01) were used to eliminate genes that showed no significant differences in expression between the expression profiles of a transgenic line and NT. These microarray data can be found at http://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE81325).
ChIP-qPCR Assay
RCc3::OsERF71-myc transgenic rice plants were generated for ChIP-qPCR. For RCc3::OsERF71-myc, the coding sequence of OsERF71 was amplified using PrimeSTAR DNA polymerase (TaKaRa) with the OsERF71-B forward primer (5′-GGATCCATGTGCGGCGGCGCCATCCT-3′) and the OsERF71-N reverse primer (5′-GCGGCCGCGTAGAATCGGCCGACACGG-3′). After enzyme digestion of the construct with BamHI and NotI, the coding sequence was ligated into the multiple cloning site of the pE3c vector, which is flanked with a 6×Myc tag coding sequence (Dubin et al., 2008). Finally, the OsERF71-6×Myc sequence from pE3c-OsERF71 was subcloned into the p700-RCc3 vector carrying a 1.3-kb RCc3 promoter sequence using the Gateway system (Supplemental Fig. S4). Two-week-old NT and RCc3::OsERF71-myc roots were harvested and then immediately immersed in cross-linking buffer (0.4 m Suc, 10 mm Tris-HCl [pH 8], 5 mm β-mercaptoethanol, and 1% formaldehyde) under vacuum for 15 min. After cross-linking, ChIP-qPCR assays were performed as described by Chung et al. (2009), except that an anti-myc antibody (sc-788; Santa Cruz Biotechnology; http://www.scbt.com) was used. Three technical replicates were performed. Primers used for qPCR are listed in Supplemental Table S5.
Accession Numbers
Genes from this article can be found in the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) under the following accession numbers: OsERF71 (Os06g0194000), OsDIP1 (Os02g0669100), OsRbcS (Os12g0274700), OsUBI1 (Os06g0681400), OsRCc3 (Os02g0662000), OsGOS2 (Os07g0529800), OsPAL (Os04g0518400), OsC4H (Os02g0467600), OsC4H (Os02g0467000), OsCCR10 (Os02g0811800), OsCCR1 (Os02g0808800), OsCAD (Os04g0612700), OsPRX (Os06g0521500), OsPRX (Os01g0963000), and OsPRX (Os01g0327100).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Soil moisture during drought treatment at the vegetative growth stage.
Supplemental Figure S2. OsERF71-regulated genes in GOS2::OsERF71 and RCc3::OsERF71 roots.
Supplemental Figure S3. Expression analysis of RCc3::OsERF71-myc plants.
Supplemental Figure S4. Vector maps for GOS2::OsERF71, RCc3::OsERF71, GOS2::GFP, RCc3::GFP, OsERF71::GFP, RCc3::OsERF71-myc, and GOS2::ERF71RNAi.
Supplemental Figure S5. Photograph of the field experiments and water content in soils during drought treatment in the field.
Supplemental Table S1. Agronomic traits of RCc3::OsERF71 and GOS2::OsERF71 transgenic rice plants grown in the field.
Supplemental Table S2. List of up- and down-regulated genes from GOS2::OsERF71 and RCc3::OsERF71 microarray compared with NT (greater than 3-fold, less than −3-fold, P < 0.05).
Supplemental Table S3. List of up- and down-regulated genes common between RCc3::OsERF71 and GOS2::OsERF71 (greater than 3-fold, less than −3-fold, P < 0.05) compared with NT.
Supplemental Table S4. List of stress-inducible and cell wall-associated genes up-regulated (greater than 3-fold) by both GOS2::OsERF71 and RCc3::OsERF71 based on microarray data.
Supplemental Table S5. List of gene-specific primers for qRT-PCR and ChIP-qPCR.
Supplementary Material
Acknowledgments
We thank the Rural Development Administration and Kyungpook National University for providing rice paddy fields, M. Redillas and S.-H. Park for critical reading of the article, and S.W. Bang, P.J. Chung, and J.H. Kang for discussion.
Glossary
- ABA
abscisic acid
- NT
nontransgenic
- qRT
quantitative real-time
- ChIP
chromatin immunoprecipitation
- qPCR
quantitative PCR
- RNAi
RNA interference
- cDNA
complementary DNA
Footnotes
This work was supported by the Rural Development Administration under the Next-Generation BioGreen 21 Program (grant no. PJ011135 to J.-K.K.) and by the Basic Science Research Program through the National Research Foundation of Korea, Ministry of Education (grant no. NRF–2014R1A6A3A04053795 to D.-K.L.).
References
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63: 3523–3543 [DOI] [PubMed] [Google Scholar]
- Barros J, Serk H, Granlund I, Pesquet E (2015) The cell biology of lignification in higher plants. Ann Bot (Lond) 115: 1053–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Bonawitz ND, Chapple C (2010) The genetics of lignin biosynthesis: connecting genotype to phenotype. Annu Rev Genet 44: 337–363 [DOI] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AA, Miki BL, et al. (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14: 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazen O, Neumann PM (1994) Hydraulic signals from the roots and rapid cell-wall hardening in growing maize (Zea mays L.) leaves are primary responses to polyethylene glycol-induced water deficits. Plant Physiol 104: 1385–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55: 225–236 [DOI] [PubMed] [Google Scholar]
- Chuck G, Meeley RB, Hake S (1998) The control of maize spikelet meristem fate by the APETALA2-like gene indeterminate spikelet1. Genes Dev 12: 1145–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung PJ, Kim YS, Jeong JS, Park SH, Nahm BH, Kim JK (2009) The histone deacetylase OsHDAC1 epigenetically regulates the OsNAC6 gene that controls seedling root growth in rice. Plant J 59: 764–776 [DOI] [PubMed] [Google Scholar]
- de Pater BS, van der Mark F, Rueb S, Katagiri F, Chua NH, Schilperoort RA, Hensgens LA (1992) The promoter of the rice gene GOS2 is active in various different monocot tissues and binds rice nuclear factor ASF-1. Plant J 2: 837–844 [DOI] [PubMed] [Google Scholar]
- Dubin MJ, Bowler C, Benvenuto G (2008) A modified Gateway cloning strategy for overexpressing tagged proteins in plants. Plant Methods 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayake IJ, O’Toole JC, Garrity DP, Masajo TM (1985) Inheritance of root characters and their relations to drought resistance in rice. Crop Sci 25: 927–933 [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ, Gerentes D, Perez P, Smyth DR (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8: 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Linker R, Gepstein S, Tanimoto E, Yamamoto R, Neumann PM (2006) Progressive inhibition by water deficit of cell wall extensibility and growth along the elongation zone of maize roots is related to increased lignin metabolism and progressive stelar accumulation of wall phenolics. Plant Physiol 140: 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Zhao F, Cong Y, Sang X, Du Q, Wang D, Li Y, Ling Y, Yang Z, He G (2012) Rolling-leaf14 is a 2OG-Fe (II) oxygenase family protein that modulates rice leaf rolling by affecting secondary cell wall formation in leaves. Plant Biotechnol J 10: 524–532 [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Maruyama K, Seki M, Hiratsu K, Ohme-Takagi M, Tran LS, Yamaguchi-Shinozaki K, Shinozaki K (2004) A dehydration-induced NAC protein, RD26, is involved in a novel ABA-dependent stress-signaling pathway. Plant J 39: 863–876 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17: 3470–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Xu J (2014) Abiotic stress responses in plant roots: a proteomics perspective. Front Plant Sci 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowing DJG, Davies WJ, Jones HG (1990) A positive root-sourced signal as an indicator of soil drying in apple, Malus × domestica Borkh. J Exp Bot 41: 1535–1540 [Google Scholar]
- Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF, Zhang JZ (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130: 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Li WC, Xu YQ, Li GJ, Liao Y, Fu FL (2009) Differential expression of candidate genes for lignin biosynthesis under drought stress in maize leaves. J Appl Genet 50: 213–223 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF (1998) Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 280: 104–106 [DOI] [PubMed] [Google Scholar]
- Jang G, Yi K, Pires ND, Menand B, Dolan L (2011) RSL genes are sufficient for rhizoid system development in early diverging land plants. Development 138: 2273–2281 [DOI] [PubMed] [Google Scholar]
- Jang IC, Nahm BH, Kim JK (1999) Subcellular targeting of green fluorescent protein to plastids in transgenic rice plants provides a high-level expression system. Mol Breed 5: 453–461 [Google Scholar]
- Jang IC, Oh SJ, Seo JS, Choi WB, Song SI, Kim CH, Kim YS, Seo HS, Choi YD, Nahm BH, et al. (2003) Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol 131: 516–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen WA. (1962) Botanical Histochemistry: Principles and Practices. WH Freeman, San Francisco, pp 198–202 [Google Scholar]
- Jeong JS, Kim YS, Baek KH, Jung H, Ha SH, Do Choi Y, Kim M, Reuzeau C, Kim JK (2010) Root-specific expression of OsNAC10 improves drought tolerance and grain yield in rice under field drought conditions. Plant Physiol 153: 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JS, Kim YS, Redillas MCFR, Jang G, Jung H, Bang SW, Choi YD, Ha SH, Reuzeau C, Kim JK (2013) OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol J 11: 101–114 [DOI] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6: 1211–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo J, Lee YH, Song SI (2014) Overexpression of the rice basic leucine zipper transcription factor OsbZIP12 confers drought tolerance to rice and makes seedlings hypersensitive to ABA. Plant Biotechnol Rep 8: 431–441 [Google Scholar]
- Kang JY, Choi HI, Im MY, Kim SY (2002) Arabidopsis basic leucine zipper proteins that mediate stress-responsive abscisic acid signaling. Plant Cell 14: 343–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch-Martinez N, Krishnan A, Nataraja KN, Udayakumar M, Pereira A (2007) Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci USA 104: 15270–15275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Koita H, Nakatsubo T, Hasegawa K, Wakabayashi K, Takahashi H, Umemura K, Umezawa T, Shimamoto K (2006) Cinnamoyl-CoA reductase, a key enzyme in lignin biosynthesis, is an effector of small GTPase Rac in defense signaling in rice. Proc Natl Acad Sci USA 103: 230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Ahn JH, Song SK, Choi YD, Lee JS (2003) Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol 131: 985–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Rubio MC, Alassimone J, Geldner N (2013) A mechanism for localized lignin deposition in the endodermis. Cell 153: 402–412 [DOI] [PubMed] [Google Scholar]
- Liu F, Xu W, Wei Q, Zhang Z, Xing Z, Tan L, Di C, Yao D, Wang C, Tan Y, et al. (2010) Gene expression profiles deciphering rice phenotypic variation between Nipponbare (japonica) and 93-11 (indica) during oxidative stress. PLoS ONE 5: e8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukehaich R, Wang T, Ouyang B, Ziaf K, Li H, Zhang J, Lu Y, Ye Z (2012) SpUSP, an annexin-interacting universal stress protein, enhances drought tolerance in tomato. J Exp Bot 63: 5593–5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Zhang H, Qian X, Li A, Zhao G, Jing R (2012) TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J Exp Bot 63: 2933–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Li F, Liu C, Zhang C, Wu Z, Chen Y (2010) Isolation and characterization of an ERF transcription factor gene from cotton (Gossypium barbadense L.). Plant Mol Biol Rep 28: 176–183 [Google Scholar]
- Meyer K, Shirley AM, Cusumano JC, Bell-Lelong DA, Chapple C (1998) Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis. Proc Natl Acad Sci USA 95: 6619–6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11: 15–19 [DOI] [PubMed] [Google Scholar]
- Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta 1819: 86–96 [DOI] [PubMed] [Google Scholar]
- Moore JP, Vicré-Gibouin M, Farrant JM, Driouich A (2008) Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol Plant 134: 237–245 [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SJ, Kim YS, Kwon CW, Park HK, Jeong JS, Kim JK (2009) Overexpression of the transcription factor AP37 in rice improves grain yield under drought conditions. Plant Physiol 150: 1368–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osato Y, Yokoyama R, Nishitani K (2006) A principal role for AtXTH18 in Arabidopsis thaliana root growth: a functional analysis using RNAi plants. J Plant Res 119: 153–162 [DOI] [PubMed] [Google Scholar]
- Osnato M, Stile MR, Wang Y, Meynard D, Curiale S, Guiderdoni E, Liu Y, Horner DS, Ouwerkerk PBF, Pozzi C, et al. (2010) Cross talk between the KNOX and ethylene pathways is mediated by intron-binding transcription factors in barley. Plant Physiol 154: 1616–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Jeong JS, Lee KH, Kim YS, Choi YD, Kim JK (2015) OsbZIP23 and OsbZIP45, members of the rice basic leucine zipper transcription factor family, are involved in drought tolerance. Plant Biotechnol Rep 9: 89–96 [Google Scholar]
- Park SH, Yi N, Kim YS, Jeong MH, Bang SW, Choi YD, Kim JK (2010) Analysis of five novel putative constitutive gene promoters in transgenic rice plants. J Exp Bot 61: 2459–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura JB. (1988) Root signals control leaf expansion in wheat seedling growing in drying soil. Aust J Plant Physiol 15: 687–693 [Google Scholar]
- Price AH, Tomos AD, Virk DS (1997) Genetic dissection of root growth in rice (Oryza sativa L.). I. A hydrophonic screen. Theor Appl Genet 95: 132–142 [Google Scholar]
- Qin X, Zeevaart JA (2002) Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol 128: 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid M, Guangyuan H, Guangxiao Y, Hussain J, Xu Y (2012) AP2/ERF transcription factor in rice: genome-wide canvas and syntenic relationships between monocots and eudicots. Evol Bioinform Online 8: 321–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redillas MCFR, Jeong JS, Kim YS, Jung H, Bang SW, Choi YD, Ha SH, Reuzeau C, Kim JK (2012) The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol J 10: 792–805 [DOI] [PubMed] [Google Scholar]
- Rohde A, Morreel K, Ralph J, Goeminne G, Hostyn V, De Rycke R, Kushnir S, Van Doorsselaere J, Joseleau JP, Vuylsteke M, et al. (2004) Molecular phenotyping of the pal1 and pal2 mutants of Arabidopsis thaliana reveals far-reaching consequences on phenylpropanoid, amino acid, and carbohydrate metabolism. Plant Cell 16: 2749–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab IN, Sharp RE (1989) Non-hydraulic signals from maize roots in drying soil: inhibition of leaf elongation but not stomatal conductance. Planta 179: 466–474 [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Biophys Res Commun 290: 998–1009 [DOI] [PubMed] [Google Scholar]
- Seo PJ, Lee SB, Suh MC, Park MJ, Go YS, Park CM (2011) The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 23: 1138–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K, Seki M (2003) Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol 6: 410–417 [DOI] [PubMed] [Google Scholar]
- Shiono K, Ogawa S, Yamazaki S, Isoda H, Fujimura T, Nakazono M, Colmer TD (2011) Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Ann Bot (Lond) 107: 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibout R, Eudes A, Mouille G, Pollet B, Lapierre C, Jouanin L, Séguin A (2005) CINNAMYL ALCOHOL DEHYDROGENASE-C and -D are the primary genes involved in lignin biosynthesis in the floral stem of Arabidopsis. Plant Cell 17: 2059–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE, Lee DK (2010) BYPASS1: how a tiny mutant tells a big story about root-to-shoot signaling. J Integr Plant Biol 52: 77–85 [DOI] [PubMed] [Google Scholar]
- Sivamani E, Bahieldin A, Wraith JM, Al-Niemi T, Dyer WE, Ho TD, Qu R (2000) Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci 155: 1–9 [DOI] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LS, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16: 2481–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CH, Osterloff PL, Mohiuddin U (2003) Fragments of the earliest land plants. Nature 425: 282–285 [DOI] [PubMed] [Google Scholar]
- Went FW. (1943) Effect of the root system on tomato stem growth. Plant Physiol 18: 51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Duan X, Wang B, Hong B, Ho T, Wu R (1996) Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110: 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Buchholz WG, DeRose RT, Hall TC (1995) Characterization of a rice gene family encoding root-specific proteins. Plant Mol Biol 27: 237–248 [DOI] [PubMed] [Google Scholar]
- Xu ZS, Xia LQ, Chen M, Cheng XG, Zhang RY, Li LC, Zhao YX, Lu Y, Ni ZY, Liu L, et al. (2007) Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol Biol 65: 719–732 [DOI] [PubMed] [Google Scholar]
- Yang X, Li Y, Ren B, Ding L, Gao C, Shen Q, Guo S (2012) Drought-induced root aerenchyma formation restricts water uptake in rice seedlings supplied with nitrate. Plant Cell Physiol 53: 495–504 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J 61: 672–685 [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K (2008) Programmed proteome response for drought avoidance/tolerance in the root of a C(3) xerophyte (wild watermelon) under water deficits. Plant Cell Physiol 49: 226–241 [DOI] [PubMed] [Google Scholar]
- Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33: 740–749 [DOI] [PubMed] [Google Scholar]
- Zhu JK. (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Cai XL, Wang ZY, Hong MM (2003) An interaction between a MYC protein and an EREBP protein is involved in transcriptional regulation of the rice Wx gene. J Biol Chem 278: 47803–47811 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.