Abstract
Background
Adolescence is a critical period for emotional maturation and is a time when clinically significant symptoms of anxiety and depression increase, particularly in females. However, few studies relate developmental differences in symptoms of anxiety and depression to brain development. Cerebral blood flow (CBF) is one brain phenotype that is known to have marked developmental sex differences.
Methods
We investigated whether developmental sex differences in CBF mediated sex differences in anxiety and depression symptoms by capitalizing upon a large sample of 875 youths who completed cross-sectional imaging as part of the Philadelphia Neurodevelopmental Cohort. Perfusion was quantified on a voxelwise basis using arterial spin labeled MRI at 3T. Perfusion images were related to trait and state anxiety using a general additive model with penalized splines, while controlling for gray matter density on a voxelwise basis. Clusters found to be related to anxiety were evaluated for interactions with age, sex, and puberty.
Results
Trait anxiety was associated with elevated perfusion in a network of regions including the amygdala, anterior insula, and fusiform cortex, even after accounting for pre-scanner state anxiety. Notably, these relationships strengthened with age and the transition through puberty. Moreover, higher trait anxiety in post-pubertal females was mediated by elevated perfusion of the left amygdala.
Conclusions
Taken together, these results demonstrate that differences in the evolution of cerebral perfusion during the adolescent period may be a critical element of the affective neurobiology underlying sex differences in anxiety and mood symptoms.
Keywords: cerebral blood flow, perfusion, amygdala, insula, adolescence, anxiety, depression
INTRODUCTION
Adolescence is a critical developmental period where sex differences in behavior and appearance intensify. Additionally, increasing evidence suggests that adolescence also signals the emergence of sex differences in brain structure (1-3) and function (4-14). Furthermore, the disproportionate prevalence of anxiety and mood disorders in females also manifests in adolescence (15). Although anxiety is apparent in children, the incidence rates and diagnostic severity of anxiety and depression increase in adolescence and early adulthood (15-17). However, few studies use large samples to link sex differences in brain function to sex differences in psychiatric symptoms. The current study uses a measure of cerebral blood flow (CBF) to examine such relationships.
Prior studies on the functional neuroanatomy of anxiety disorders implicate a network of brain regions associated with emotion processing, affect regulation, and salience detection (18-20). Previous functional magnetic resonance imaging (fMRI) work links both anxiety and depressive disorders to greater activation in the amygdala, insula, and anterior cingulate cortex (ACC) compared to healthy participants (21,22). The concordance between findings in anxiety and depressive disorders is supported by factor analyses of clinical psychopathology data, which indicate the presence of an “anxious-misery” dimension of psychopathology encompassing highly comorbid mood and anxiety symptoms (23). The presence of anxious-misery symptoms is associated with ACC and medial prefrontal cortex dysfunction (24). Additionally, studies link brain activation measured with task-based fMRI paradigms to both transient, state anxiety (25,26) and long-standing, trait anxiety (27,28). Trait anxiety in particular is associated with both anxiety and mood symptoms and can be conceptualized as a measure of anxious-misery (29-31). Thus, convergent evidence across multiple studies suggests that anxiety and mood symptoms are associated with abnormalities in affective regions such as the amygdala and insula.
Despite such research, evidence regarding sex differences in affective circuitry relevant to anxiety and mood disorders is relatively limited. One relevant prior study suggested that higher anxiety is associated with greater amygdala responses to unattended fearful faces in females, but not males (32). Similarly, another report documented greater activation in the amygdala and anterior cingulate in women compared with men during fear conditioning (33). However, it remains unknown whether sex differences in brain development might in part explain differential vulnerability to anxiety and mood symptoms in females. Furthermore, most studies have considered relatively small samples, which limit joint analyses of anxiety, brain development, and sex differences during adolescence.
Prior studies have principally examined affective circuitry using task-based or resting-state fMRI, which measures changes in evoked or correlated brain function. Research suggests that CBF, which is tightly coupled to regional brain metabolism (34,35), may be a potentially important brain phenotype for understanding developmental sex differences in anxiety and mood symptoms. Previous work in adults has established that females have greater CBF than males (36-38). We recently demonstrated that this sex difference in CBF unfolds with puberty (13). Furthermore, prior research in adults also links regional CBF in affective regions such as the amygdala to symptoms of anxiety and depression (39-42). When considered together, these related findings suggest that age and sex-related differences in the risk of anxiety and mood disorders may be correlated with corresponding changes in CBF.
Accordingly, in this study we evaluated the hypothesis that emerging sex differences in the perfusion of affective circuits during adolescence mediates sex differences in anxiety. We leveraged a large sample of children, adolescents, and young adults imaged using arterial spin labeled (ASL) MRI as part of the Philadelphia Neurodevelopmental Cohort (PNC; 43) to test three specific predictions. First, we predicted that greater levels of anxiety would be associated with greater CBF in affective regions such as the amygdala. Second, following our prior work, we predicted that females would have higher perfusion in these affective regions as adolescence progresses. Third, we examined whether higher CBF in affective regions mediates higher levels of anxiety in females.
METHODS AND MATERIALS
Participants
A total of 1,601 youths were imaged as part of the PNC (43-45). Of these participants, 170 were excluded for the following reasons: medical disorders that could impact brain functioning (n=73), non-psychiatric medication use that could affect CNS functioning (n=78), or substantial structural brain abnormalities resulting in frankly abnormal brain anatomy (n=20) (46); one subject was excluded on the basis of two criteria. Of the remaining 1,431 subjects, children under the age of 12 years were not considered in the present analyses (n=373) due to the recommendation of a 5th grade reading level for the State-Trait Anxiety Inventory (STAI; 47), which was our primary measure of anxiety and mood symptoms. Of these 1,058 participants, 183 participants were excluded for missing clinical data, missing STAI data, failure to complete perfusion imaging, or failing to meet image quality assurance protocols; many subjects were excluded for multiple reasons. This yielded a final sample of 875 participants (mean age = 16.5 years; range = 12-23 years; SD = 2.7 years; 398 males), with similar numbers of males and females excluded. Among this final sample, 105 participants (12%) were taking psychotropic medications at the time of imaging. The impact of medication use was evaluated in sensitivity analyses, as described below.
Clinical assessment
Lifetime psychopathology was determined using Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision criteria (48). Demographic data by screening category are summarized in Table 1. As for prior work in this dataset (13), puberty was coded as a categorical variable with three classes: early pubertal (Tanner stages 1-3), mid-pubertal (Tanner stage 4), and post-pubertal (Tanner stage 5); for the n in each pubertal stage, see supplementary Table S1. For additional details regarding the clinical assessment, see the Supplementary Methods section.
Table 1.
Summary of demographic data by screening category.
| n | Mean (S.D.) Age |
Female (%) |
Caucasian (%) |
Mean (S.D.) Years Maternal Education |
|
|---|---|---|---|---|---|
|
Typically
Developing |
236 | 16.85 (2.88) | 47 | 56.8 | 14.76 (2.62) |
| ADHD | 140 | 15.55 (2.36) | 42.1 | 38.6 | 14.07 (2.45) |
| Agoraphobia | 62 | 16.39 (2.26) | 75.8 | 29 | 13.63 (2.15) |
| Anorexia | 10 | 16.47 (2.27) | 70 | 40 | 13.1 (1.85) |
| Bulimia | 4 | 18.31 (1.04) | 100 | 100 | 15.5 (2.52) |
| Conduct Disorder | 85 | 16.47 (2.31) | 49.4 | 14.1 | 12.83 (2.02) |
| Major Depression | 146 | 17.66 (2.17) | 67.8 | 43.2 | 13.79 (2.34) |
| GAD | 16 | 16.86 (2.47) | 62.5 | 56.2 | 14.19 (2.83) |
| Mania | 8 | 17.17 (3.06) | 75 | 25 | 14.14 (1.86) |
| OCD | 29 | 17.47 (2.42) | 72.4 | 34.5 | 13.34 (2.33) |
| ODD | 320 | 16.04 (2.37) | 51.6 | 32.5 | 13.69 (2.39) |
| Panic | 9 | 15.5 (3.02) | 55.6 | 33.3 | 13.11 (1.45) |
| Specific Phobias | 273 | 16.37 (2.52) | 69.6 | 39.9 | 13.95 (2.32) |
| Psychosis-spectrum | 302 | 16.24 (2.78) | 54 | 29.1 | 13.67 (2.14) |
| PTSD | 125 | 17.03 (2.55) | 70.4 | 32 | 13.52 (2.26) |
| Separation Anxiety | 41 | 16.79 (2.22) | 68.3 | 56.1 | 14.1 (2.44) |
| Social Phobia | 242 | 16.28 (2.52) | 60.3 | 34.3 | 13.79 (2.37) |
Note. Due to comorbidity, individual participants may be present in more than one category.
Dimensional characterization of mood and anxiety symptoms
The STAI measures transient, situational levels of anxiety (state anxiety) as well as general and long-standing levels of anxiety (trait anxiety). Past work examining the STAI has shown that trait anxiety is comprised of a mix of both anxiety and mood symptoms (29-31), consistent with the dimension of anxious-misery (23). To obtain a measure of state and trait anxiety free from response bias shown in previous research (30,49), we conducted separate factor analyses of each scale using a confirmatory bifactor model implemented in MPlus (50-53; see Supplementary Methods and supplementary Tables S2 and S3 for factor loadings). Relationships between the summary scores and demographic data such as age, sex, and pubertal phase were explored using linear models; all puberty analyses included age as a covariate. Additionally, we examined how summary scores of state and trait anxiety related to psychopathology screening categories.
Image acquisition, preprocessing, and perfusion quantification
Image acquisition and processing are reported in detail elsewhere (43,54); see Supplementary Methods for details. Briefly, a custom spin-echo pseudocontinuous arterial spin labeling (pCASL) sequence was used to image brain perfusion. Following distortion correction, data were processed with FSL (55) including skull removal, motion-correction, spatial smoothing (6mm FWHM), and intensity normalization. CBF was quantified from control-label pairs using ASL Toolbox (56). As prior (13), the T1 relaxation parameter was modeled on an age- and sex-specific basis (57). This model accounts for the fact that T1 relaxation time differs according to age and sex, and has been shown to enhance the accuracy and reliability of results in developmental samples (58). Participant-level CBF images were co-registered to T1 images using boundary based registration (59), normalized to the Montreal Neurologic Institute 152 1mm template using the top-performing SyN deformable registration included in ANTs (60-62), and downsampled to 2mm voxels prior to analysis. All transformations were concatenated so that only one interpolation was performed.
Group-level analyses
As a first step, in order to identify regions where perfusion was related to summary scores of state or trait anxiety, we conducted a whole-brain voxelwise analysis. Both linear and nonlinear age effects were flexibly modeled using penalized splines within a generalized additive model (GAM; 63,64). Furthermore, in order to control for confounding effects of brain structure on estimates of CBF (65), gray matter density calculated using Atropos (60) was modeled on a voxelwise basis.
where vox = voxels. Type I error for voxelwise whole-brain analyses was controlled using AFNI AlphaSim (66) with a minimum voxel significance of z > 2.58 and a corrected cluster significance of p < 0.0001 (minimum cluster size k = 146).
Supplementary analyses tested alternative models and covariates such as: excluding participants taking psychoactive medication, including race and maternal education as covariates, examining the effect of the motion covariate, modeling state and trait in separate models, and using raw STAI scores that were not factor analyzed to remove response bias. For details regarding group level analyses, see the Supplementary Methods section. Additionally, we examined whether regions associated with dimensional symptom severity were specifically linked to certain diagnostic groups with at least 100 participants (see Supplementary Methods for details); results are reported as covariate-adjusted effect sizes (Cohen’s d).
Analyses of interactions with age and puberty
Based on our previous work in this dataset showing that CBF is higher in females than males during puberty (13), we next evaluated whether interactive effects with age and sex were present within regions identified by the voxelwise analysis as anxiety-relevant. Analyses included modeling interactions between anxiety and age, between sex and age, as well as between anxiety and pubertal stage (while controlling for age). For additional details regarding each of these analyses, see the Supplementary Methods section. To control for multiple comparisons across clusters, we used the False Discovery Rate (FDR; Q < 0.05).
Mediation analysis
As described below, the above analyses revealed that post-pubertal females have higher trait anxiety and also that higher CBF in affective regions was associated with greater trait anxiety. Accordingly, as a final step, we investigated whether higher levels of anxiety in post-pubertal females were mediated by higher CBF. The indirect effect of sex on trait anxiety through the proposed mediator (perfusion) was tested using both the Sobel test and bootstrapping procedures (67; see Supplementary Methods for details).
Amygdala resting state functional connectivity
As a final step, in order to further evaluate the functional significance of increased left amygdala perfusion, we conducted a seed-based functional connectivity analysis in a sample of 592 participants who also received resting-state imaging as part of the PNC, using previously-detailed methods (54,68-70; see Supplementary Methods for details). Group level analyses used the same procedures, models, and type I error correction as for ASL data.
RESULTS
Post-pubertal females have higher trait anxiety
Sex differences in trait anxiety levels were found in post-pubertal adolescents, with females endorsing higher levels of trait anxiety than males (t(449) = 2.50, p = .013). No significant sex differences were found for trait anxiety in the early- or mid-pubertal groups (p ≥ .194). Females also endorsed higher levels of state anxiety in both the post-pubertal (t(449) = 2.09, p = .038) and mid-pubertal groups (t(274) = 2.18, p = .030) but not in the early-pubertal group (p = .54). As displayed in Figure S1, the levels of state and trait anxiety were high across all screening diagnostic categories (which due to comorbidity are not mutually exclusive categories) compared to typically developing youth.
Trait but not state anxiety is associated with elevated perfusion of affective regions
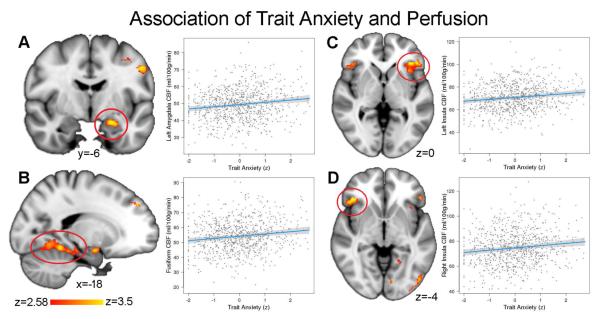
We used voxelwise mass-univariate analyses to test the prediction that greater levels of anxiety would be associated with greater CBF in affective circuitry. Even while controlling for state anxiety, higher levels of trait anxiety were associated with increased perfusion in a network of regions involved in emotion processing including the left amygdala, bilateral insula, and bilateral fusiform gyrus (Figure 1; see Table 2 for a complete list of regions). The dorsal anterior cingulate was significant at a slightly more liberal cluster threshold (Table S4).
Figure 1.
Elevated perfusion in affective regions is associated with trait anxiety. Higher perfusion was associated with higher trait anxiety in the a) left amygdala, b) fusiform gyrus, c) left anterior insula, and d) right anterior insula (see Table 2 for a complete list). Images thresholded at z > 2.58, cluster corrected p < 0.0001.
Table 2.
Regions where trait anxiety was associated with increased perfusion.
| Region | k | Maximum z | Peak MNI coordinates |
||
|---|---|---|---|---|---|
| Fusiform | 1036 | 4.39 | −36 | −58 | −14 |
| Left insula | 560 | 4.00 | −44 | 30 | 2 |
| Left precentral gyrus | 275 | 3.70 | −48 | −10 | 38 |
| Left frontal pole | 263 | 3.82 | −26 | 44 | 36 |
| Right insula | 218 | 3.99 | 46 | 30 | −8 |
| Left inferior lateral occipital cortex | 193 | 3.62 | −38 | −88 | −8 |
| Left amygdala | 168 | 3.63 | −20 | −4 | −16 |
| Right frontal pole | 148 | 3.75 | 30 | 40 | −12 |
Note. Clusters considered significant if z > 2.58, k > 146 in a sample of 875 subjects.
State anxiety was associated with decreased perfusion in lateral occipital cortex (k = 206, maximum z = 3.6, peak MNI coordinates: −40 −86 −10), but this result was not stable in supplementary analyses. In contrast, trait anxiety results were consistent over a range of models, sub-samples, and covariates. Specifically, trait anxiety results was similar when state and trait anxiety were analyzed in separate models (Table S5), when participants taking psychotropic medications were excluded and participant race and maternal education were also included in the model (n = 766; Table S6), and when raw total scores from STAI were used instead of factor scores (Table S7). Notably, these results could not be attributed to motion: CBF in left amygdala was related to motion (see Figure S2) but motion was not related to trait anxiety (r = −.01, p = .86).
We additionally examined whether elevated amygdala perfusion was associated with specific screening diagnostic categories. Notably, differences in amygdala perfusion between screening diagnostic categories and typically developing youth showed small to medium effect sizes for all categories (d = .15-.30). See supplementary Table S8 for covariate-adjusted Cohen’s d for each screening category.
Brain regions associated with trait anxiety show developmental sex differences
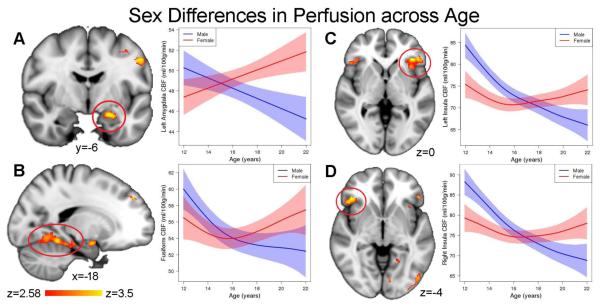
In our prior work in this dataset, we have demonstrated developmental sex differences in cerebral perfusion, whereby CBF becomes higher in females than males as adolescence progresses (13). Accordingly, we next tested the prediction that there would be prominent sex differences in the regions identified by their significant association between CBF and trait anxiety. As expected, perfusion in these regions showed a divergent pattern between males and females in adolescence (Figure 2 and Table 3): while perfusion declined throughout adolescence in males, perfusion displayed a quadratic or increasing pattern in females. As a result, perfusion was greater in females than males in the post-pubertal period but not in other pubertal stages. These results demonstrate that regions where trait anxiety is associated with CBF show marked sex differences in their developmental patterns.
Figure 2.
Regions where CBF is linked to trait anxiety also show marked developmental sex differences. CBF declined over time in males (blue), while perfusion increased in females (red) in the a) left amygdala, b) fusiform gyrus, c) left anterior insula, and d) right anterior insula (also see Table 3).
Table 3.
Developmental sex differences (age by sex interaction) in regions where a significant association between trait anxiety and perfusion was present.
| Region | F | p | pfdr |
|---|---|---|---|
| Fusiform | 9.05 | 0.003 | 0.003 |
| Left insula | 29.82 | <0.001 | <0.001 |
| Left precentral gyrus | 20.57 | <0.001 | <0.001 |
| Left frontal pole | 2.86 | 0.055 | 0.055 |
| Right insula | 25.45 | <0.001 | <0.001 |
| Left inferior lateral occipital cortex | 12.70 | <0.001 | 0.001 |
| Left amygdala | 14.81 | <0.001 | <0.001 |
| Right frontal pole | 4.02 | 0.006 | 0.007 |
Note. Bold = significant at α < .05; pfdr = FDR corrected p-values
The association between perfusion and trait anxiety increases with development
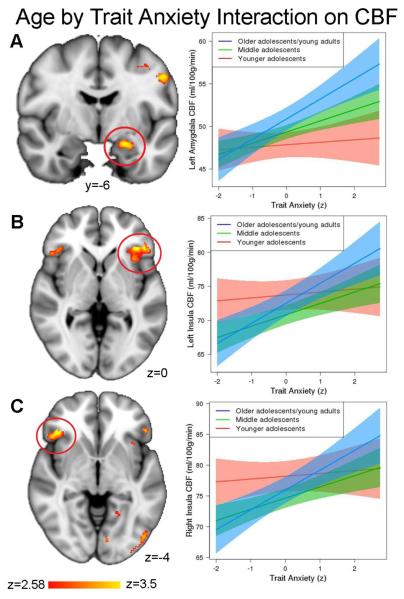
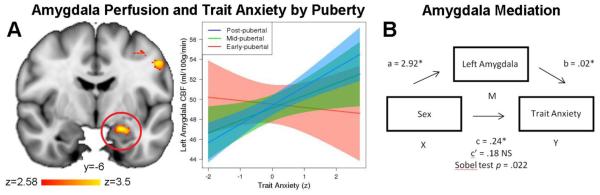
Next, we investigated how the association between trait anxiety and CBF differs with age and puberty within those regions. Multiple comparison-corrected results revealed significant trait anxiety-by-age interactions in the left amygdala (t(864) = 2.66, pfdr = .017), left insula (t(864) = 2.50, pfdr = .017), and right insula (t(864) = 2.40, pfdr = .017; Figure 3). No other regions had a significant trait anxiety-by-age interaction. These results suggest that the association between trait anxiety and perfusion of affective regions becomes stronger with age. Next, in clusters where a significant anxiety-by-age effect was present, we examined whether this effect could be specifically attributable to puberty, while controlling for age. Notably, there was a significant trait anxiety-by-puberty interaction in the left amygdala following correction for multiple comparisons (t(854) = 2.48, pfdr = .039). As displayed in Figure 4a, this interaction suggests that the relationship between amygdala perfusion and anxiety is higher in later pubertal stages in this sample, above and beyond the effects of age. No significant interactions between trait anxiety and puberty were found in the left or right insula (pfdr ≥ .073). All sensitivity analyses revealed the same significant pattern of results.
Figure 3.
The association between trait anxiety and regional perfusion becomes stronger with increasing age. An age-by-trait anxiety interaction was significant in the a) left amygdala, b) left anterior insula, and c) right anterior insula.
Figure 4.
Amygdala perfusion mediates trait anxiety in post-pubertal females. A) The relationship between amygdala perfusion and anxiety increases with pubertal stage (early, mid, or post-pubertal), above and beyond effects of age. B) The relationship between higher trait anxiety levels in post-pubertal females was mediated by higher perfusion in the left amygdala. Mediation results show the unstandardized regression coefficients for the post-pubertal group in the left amygdala; when controlling for mean perfusion in the left amygdala, the relationship between sex and trait anxiety becomes non-significant (c’).
Elevated levels of anxiety in post-pubertal females is mediated by amygdala perfusion
Having established that post-pubertal females have higher levels of both anxiety and amygdala perfusion, as a final step we tested the prediction that amygdala perfusion mediates post-pubertal sex differences in trait anxiety. Mediation analysis revealed a significant indirect effect (95% CI [.02, .12]; z = 2.29, SE = .03, p = .022), suggesting that higher trait anxiety levels in post-pubertal females may be mediated in part by higher perfusion in the left amygdala (Figure 4b). The significance of this mediation effect is emphasized by the fact that, when controlling for mean perfusion in the left amygdala, the relationship between sex and trait anxiety became non-significant.
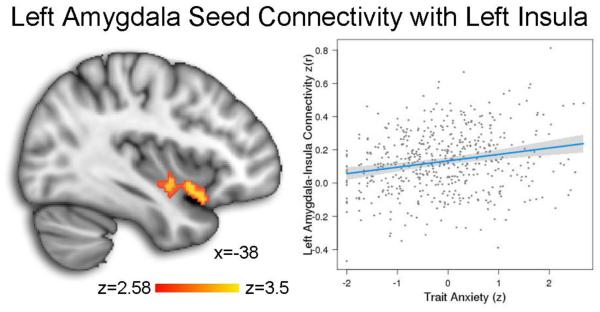
Trait anxiety is associated with left amygdala-insula connectivity
A seed analysis using the left amygdala cluster where CBF was associated with anxiety revealed that higher levels of trait anxiety were associated with increased connectivity with the left insula (k = 153, maximum z = 3.49, peak MNI coordinates: −40 6 −10; see Figure 5). No other clusters achieved significance. Additionally, there were no age by sex, anxiety by age, or anxiety by puberty interactions within this cluster.
Figure 5.
Higher levels of trait anxiety were associated with increased connectivity between the left amygdala seed and the left insula. Analyses were conducted in a sample of n=592 PNC participants who also received a resting-state functional connectivity scan.
DISCUSSION
The current study related sex differences in perfusion in the amygdala, insula, and other regions to sex differences in trait anxiety that emerge in adolescence. Leveraging a large sample of youth imaged as part of the PNC, we delineate five inter-related results describing how the development of brain perfusion may relate to anxiety. First, as expected, sex differences in anxiety were apparent, with post-pubertal females showing higher levels of trait anxiety than post-pubertal males. Second, trait anxiety was associated with greater perfusion in a network of regions including the amygdala, anterior insula, and fusiform cortex. This effect was present even while accounting for in-scanner state anxiety. Third, these regions showed substantial developmental sex differences, with females demonstrating higher perfusion than males in the post-pubertal period. Fourth, across both males and females, the relationship between trait anxiety and perfusion in affective regions increased in strength with age and puberty. Finally, higher trait anxiety levels observed in post-pubertal females were mediated by elevated perfusion of the left amygdala. Taken together, these results suggest a new mechanism for understanding sex differences in anxiety and mood symptoms.
The results of this study are convergent with and extend results from prior studies that link elevated perfusion to clinically-diagnosed anxiety disorders (39,40) or to task-induced anxiety/stress (41,42). Trait anxiety encompasses symptoms of both anxiety and depression (30,49,71), and thus may be conceptualized as a measure of “anxious misery,” which is consistently identified by factor analyses of large-scale studies of psychopathology (23). Remarkably, the regions we found associated with trait anxiety are localized within the social cognition network and are critical for processes including face perception, emotion identification, detection of social threat, and emotion regulation (72-75). Furthermore, many of the regions implicated including the anterior insula belong to the cingulo-opercular network (76-78), which has frequently been implicated in anxiety disorders in the past (79).
Prior research has demonstrated that CBF is tightly linked to regional metabolism, and thus local neural activity (80,81). Current results suggest that heightened activity in affective circuitry associated with anxiety may result in higher regional metabolism and CBF. Along with prior reports from clinical samples (39,40), these results suggest that perfusion in affective regions may reflect the presence of long-standing symptoms of anxiety, and thus may be a candidate biomarker for longitudinal studies of development, drug discovery, and clinical trials of novel interventions for ameliorating anxiety. Furthermore, connectivity analyses showed that the left amygdala cluster where CBF was associated with anxiety was related to increased connectivity with the left insula, suggesting that amygdala-insula coupling is an important circuit associated with trait anxiety.
As suggested by previous work in adults (82), sex differences in were marked. As in our prior work (13), CBF diverged with pubertal stage, such that females showed higher perfusion than males by the end of adolescence. While the PNC did not include hormonal measures such as circulating androgen or estrogen levels, based on animal models (83,84) and studies of ovarian hyperstimulation in the context of fertility treatment (85,86), we speculate that such developmental sex differences may be associated with rising estrogen levels in females. The amygdala has both estrogen and androgen receptors (87-89) and the influx of estrogen enhances the acquisition of fear conditioning in female mice (83,84). Therefore, the amygdala may be particularly susceptible to the hormonal changes that occur during pubertal development. Likewise, developmental sex differences may also be influenced by the increase in testosterone in males, as testosterone treatment reduces anxious behaviors in rodents (90,91).
In addition to the presence of developmental sex differences, the relationship between perfusion in affective regions and trait anxiety was stronger in the older adolescents/young adults. Thus, perfusion of affective regions may be associated with differential risk in specific developmental epochs. Of note, greater trait anxiety was associated with higher perfusion only in the left amygdala and not the right amygdala in the current study. This lateralization of amygdala perfusion is consistent with previous work showing greater activity in the left but not the right amygdala in response to negative emotional information (82,92). Furthermore, prior studies of major depression has also shown a greater left amygdala activity (93) which may suggest a potential common marker for anxious-misery symptoms across disorders.
While increased amygdala perfusion was associated with higher anxiety in the post-pubertal period in both males and females, post-pubertal females have both higher anxiety and higher perfusion. We tested the link between these effects using a mediation analysis, and found that higher trait anxiety levels in post-pubertal females were mediated by higher perfusion in the left amygdala. This suggests that greater perfusion in affective regions in post-pubertal females may represent an important developmental vulnerability to trait anxiety. Intriguingly, during adolescence females also display enhanced development of social cognition, demonstrating clear superiority over males (8). Future studies could evaluate whether elevated perfusion in affective regions during adolescence may allow for improvements in social cognition while also conferring vulnerability to anxiety and mood disorders.
Examination of specific screening diagnostic categories demonstrated that elevated CBF in the amygdala was relatively similar across diagnostic screen categories, with small to medium effect sizes. PTSD showed the largest effect size, consistent with previous research demonstrating that PTSD shows a closer relationship with the anxious-misery dimension than the fear dimension (94). However, all diagnostic categories (including ODD and ADHD) showed similar effect sizes, suggesting that these findings may potentially be indicative of general psychopathology rather than specific to anxiety or depression. It should be noted that the observed effect sizes may in part result from the use of a community-based sample with lower levels of psychopathology than treatment-seeking clinical populations. However, this approach provides enhanced power to investigate important dimensional effects that are present across traditional diagnostic categories.
Several limitations of the present work should be noted. First and foremost, direct causal effects regarding sex differences cannot be inferred from the results of this study. Sex differences in brain functioning are the result of a complex interaction between both genetics and environmental factors (95,96). The associations described here are not “sexually dimorphic,” consistent with research showing that brains show a mosaic of male and female features rather than being sexually dimorphic (97). Perfusion of affective regions is a continuous variable, with highly overlapping distributions among males and females. Indeed, the association between anxiety and perfusion was present for both sexes in this cross-sectional sample. Nonetheless, the presence of significant sex differences in perfusion does suggest particular relevance for females, and may represent a potentially important mechanism for sex differences in anxiety and mood disorders.
Second, while the PNC benefitted from a large sample size that allowed examination of interactive effects of age, sex, and anxiety, cross-sectional data limits the ability to infer developmental patterns. Future research would benefit from longitudinal designs that can better assess changes in the relationship between trait anxiety and perfusion over time and account for the temporal precedence between variables. Third, the pubertal assessment included in the PNC does not allow us to fully disentangle chronologic age and puberty. Future studies should gather measures of circulating hormones and also assess menstrual phase, which may impact functional brain phenotypes (98). Lastly, although generalizability of these results is enhanced by the use of a representative community sample, it would also be useful to investigate the association between anxiety and perfusion in clinically ascertained samples or in samples of children at risk for psychopathology because of environmental stressors or family history.
These limitations notwithstanding, our data suggest a novel mechanism for sex differences in anxiety and mood symptoms, which are associated with high morbidity and mortality in females worldwide. To the degree that elevated perfusion of affective brain regions may represent a risk phenotype for subsequent clinical disorders, it may aid in early identification of youth at risk for disabling symptoms. Through incorporation within clinical trials, elevated perfusion may become a useful biomarker for treatment of anxiety and mood disorders.
Supplementary Material
ACKNOWLEDGMENTS
Thanks to the acquisition and recruitment team: Karthik Prabhakaran, Jeff Valdez, Raphael Gerraty, Marisa Riley, Jack Keefe, Elliott Yodh, and Rosetta Chiavacci. Thanks to Chad Jackson and Larry Macy for data management and systems support. Thanks to Kathleen Merikangas and Marcy Burstein at the NIMH. Supported by RC2 grants from the National Institute of Mental Health MH089983 and MH089924 and P50MH096891. Additional support was provided by R01MH107703 and K23MH098130 to TDS, R01MH101111 to DHW, K01MH102609 to DRR, K08MH079364 to MEC, R01NS085211 to RTS, T32MH065218 to SNV, and the Dowshen Program for Neuroscience. Support for developing statistical analyses (RTS & TDS) was provided by a seed grant by the Center for Biomedical Computing and Image Analysis (CBICA) at Penn. Data deposition: The data reported in this paper have been deposited in database of Genotypes and Phenotypes (dbGaP), www.ncbi.nlm.nih.gov/gap (accession no. phs000607.v1.p1).
Footnotes
FINANCIAL DISCLOSURES: Dr. Raquel E. Gur participated in an advisory board for Otsuka Pharmaceuticals. Dr. Ruben C. Gur receives royalties from the Brain Resource Centre and serves without compensation on an advisory board for Lumosity. Dr. Edna B. Foa receives royalties from the sale of the books, “Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide,” and “Reclaiming your Life from a Traumatic Experience Workbook” by Oxford University Press. Dr. Foa also receives payment for training workshops she conducts on prolonged exposure therapy. All other authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. The Journal of Neuroscience. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Human brain mapping. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, et al. Sex differences in frontal lobe white matter microstructure: a DTI study. Neuroreport. 2003;14:2469–2473. doi: 10.1097/00001756-200312190-00035. [DOI] [PubMed] [Google Scholar]
- 4.Blakemore S-J. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- 5.Blanton RE, Cooney RE, Joormann J, Eugène F, Glover GH, Gotlib IH. Pubertal stage and brain anatomy in girls. Neuroscience. 2012;217:105–112. doi: 10.1016/j.neuroscience.2012.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4--18 years. Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. Journal of neuroscience methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26:251. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpern DF. Sex Differences in Cognitive Abilities. 3rd Edition. Psychology Press; 2000. 3, revised. [Google Scholar]
- 10.Peper JS, Brouwer RM, Schnack HG, van Baal GC, van Leeuwen M, van den Berg SM, et al. Sex steroids and brain structure in pubertal boys and girls. Psychoneuroendocrinology. 2009;34:332–342. doi: 10.1016/j.psyneuen.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Peper JS, Schnack HG, Brouwer RM, Van Baal GCM, Pjetri E, Szekely E, et al. Heritability of regional and global brain structure at the onset of puberty: a magnetic resonance imaging study in 9-year-old twin pairs. Human brain mapping. 2009;30:2184–2196. doi: 10.1002/hbm.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satterthwaite TD, Vandekar S, Wolf DH, Ruparel K, Roalf DR, Jackson C, et al. Sex differences in the effect of puberty on hippocampal morphology. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:341–350. doi: 10.1016/j.jaac.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliott MA, Vandekar SN, et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proceedings of the National Academy of Sciences. 2014;111:8643–8648. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. The Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- 15.Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen H-U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International journal of methods in psychiatric research. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roza SJ, Hofstra MB, van der Ende J, Verhulst FC. Stable prediction of mood and anxiety disorders based on behavioral and emotional problems in childhood: a 14-year follow-up during childhood, adolescence, and young adulthood. American Journal of Psychiatry. 2014;160:2116–2121. doi: 10.1176/appi.ajp.160.12.2116. [DOI] [PubMed] [Google Scholar]
- 17.Zahn-Waxler C, Shirtcliff EA, Marceau K. Disorders of childhood and adolescence: Gender and psychopathology. Annu. Rev. Clin. Psychol. 2008;4:275–303. doi: 10.1146/annurev.clinpsy.3.022806.091358. [DOI] [PubMed] [Google Scholar]
- 18.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical--subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 20.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 21.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. American Journal of Psychiatry. 2014;169:693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 23.Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 24.Etkin A. Neurobiology of anxiety: from neural circuits to novel solutions? Depression and anxiety. 2012;29:355–358. doi: 10.1002/da.21957. [DOI] [PubMed] [Google Scholar]
- 25.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. The Journal of neuroscience. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cerebral cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- 27.Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American journal of psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 29.Bieling PJ, Antony MM, Swinson RP. The State--Trait Anxiety Inventory, Trait version: structure and content re-examined. Behaviour research and therapy. 1998;36:777–788. doi: 10.1016/s0005-7967(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 30.Bados A, Gómez-Benito J, Balaguer G. The state-trait anxiety inventory, trait version: does it really measure anxiety? Journal of personality assessment. 2010;92:560–567. doi: 10.1080/00223891.2010.513295. [DOI] [PubMed] [Google Scholar]
- 31.Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognitive Therapy and Research. 2001;25:1–22. [Google Scholar]
- 32.Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: interaction between sex and trait anxiety. Psychiatry Research: Neuroimaging. 2008;162:51–57. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bücking A, Zeidan MA, et al. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biol. Mood Anxiety Disord. 2012;2:1–10. doi: 10.1186/2045-5380-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Current opinion in neurology. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- 35.Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magnetic Resonance in Medicine. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- 36.Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, et al. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217:659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Zhu X, Feinberg D, Guenther M, Gregori J, Weiner MW, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magnetic Resonance in Medicine. 2012;68:912–922. doi: 10.1002/mrm.23286. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez G, Warkentin S, Risberg J, Rosadini G. Sex differences in regional cerebral blood flow. J Cereb Blood Flow Metab. 1988;8:783–789. doi: 10.1038/jcbfm.1988.133. [DOI] [PubMed] [Google Scholar]
- 39.Andreescu C, Gross JJ, Lenze E, Edelman KD, Snyder S, Tanase C, et al. Altered cerebral blood flow patterns associated with pathologic worry in the elderly. Depression and anxiety. 2011;28:202–209. doi: 10.1002/da.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. Neuroimage. 2011;54:S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, et al. Gender difference in neural response to psychological stress. Social cognitive and affective neuroscience. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, et al. The philadelphia neurodevelopmental cohort: a publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. 2016;124:1115–1119. doi: 10.1016/j.neuroimage.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. Journal of Child Psychology and Psychiatry. 2015;56:1356–1369. doi: 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gur RE, Kaltman D, Melhem ER, Ruparel K, Prabhakaran K, Riley M, et al. Incidental findings in youths volunteering for brain MRI research. American Journal of Neuroradiology. 2013;34:2021–2025. doi: 10.3174/ajnr.A3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spielberger CD. State-Trait Anxiety Inventory for Adults: Manual and Sample : Manual, Instrument and Scoring Guide. Consulting Psychologists Press; 1983. [Google Scholar]
- 48.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. American Psychiatric Association; 2000. [Google Scholar]
- 49.Balsamo M, Romanelli R, Innamorati M, Ciccarese G, Carlucci L, Saggino A. The state-trait anxiety inventory: shadows and lights on its construct validity. Journal of Psychopathology and Behavioral Assessment. 2013;35:475–486. [Google Scholar]
- 50.Reise SP. The rediscovery of bifactor measurement models. Multivariate Behavioral Research. 2012;47:667–696. doi: 10.1080/00273171.2012.715555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reise SP, Moore TM, Haviland MG. Bifactor models and rotations: Exploring the extent to which multidimensional data yield univocal scale scores. Journal of personality assessment. 2010;92:544–559. doi: 10.1080/00223891.2010.496477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holzinger KJ, Swineford F. The bi-factor method. Psychometrika. 1937;2:41–54. [Google Scholar]
- 53.Muthén LK, Muthén BO. Mplus. The comprehensive modelling program for applied researchers: Users guide. 2012. p. 5.
- 54.Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, et al. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magnetic resonance imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu W-C, Jain V, Li C, Giannetta M, Hurt H, Wehrli FW, et al. In vivo venous blood T1 measurement using inversion recovery true-FISP in children and adults. Magnetic Resonance in Medicine. 2010;64:1140–1147. doi: 10.1002/mrm.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain V, Duda J, Avants B, Giannetta M, Xie SX, Roberts T, et al. Longitudinal reproducibility and accuracy of pseudo-continuous arterial spin--labeled perfusion MR imaging in typically developing children. Radiology. 2012;263:527–536. doi: 10.1148/radiol.12111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klein A, Ghosh SS, Avants B, Yeo BTT, Fischl B, Ardekani B, et al. Evaluation of volume-based and surface-based brain image registration methods. Neuroimage. 2010;51:214–220. doi: 10.1016/j.neuroimage.2010.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association. 2004;99:673–686. [Google Scholar]
- 64.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2011;73:3–36. [Google Scholar]
- 65.Taki Y, Hashizume H, Sassa Y, Takeuchi H, Wu K, Asano M, et al. Correlation between gray matter density-adjusted brain perfusion and age using brain MR images of 202 healthy children. Human brain mapping. 2011;32:1973–1985. doi: 10.1002/hbm.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 67.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 68.Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, et al. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, et al. Linked sex differences in cognition and functional connectivity in youth. Cerebral cortex. 2014 doi: 10.1093/cercor/bhu036. bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kennedy BL, Schwab JJ, Morris RL, Beldia G. Assessment of state and trait anxiety in subjects with anxiety and depressive disorders. Psychiatric Quarterly. 2001;72:263–276. doi: 10.1023/a:1010305200087. [DOI] [PubMed] [Google Scholar]
- 72.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Adolphs R. The neurobiology of social cognition. Current opinion in neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 74.Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia research. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 76.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cerebral cortex. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sylvester CM, Corbetta M, Raichle ME, Rodebaugh TL, Schlaggar BL, Sheline YI, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends in neurosciences. 2012;35:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Detre John A. Physiology of Functional Activation. In: Wilson DF, Evans SM, Biaglow J, Pastuszko A, editors. Oxygen Transport To Tissue XXIII: Oxygen Measurements in the 21st Century: Basic Techniques and Clinical RelevanceVolume 510 of Advances in Experimental Medicine and Biology. Springer Science & Business Media; 2012. pp. 365–368. illustrated. [Google Scholar]
- 81.He X, Raichle ME, Yablonskiy DA. Transmembrane dynamics of water exchange in human brain. Magnetic resonance in medicine. 2012;67:562–571. doi: 10.1002/mrm.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, et al. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiology of learning and memory. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- 83.Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Hormones and behavior. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Hormones and Behavior. 2001;40:472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- 85.Nevo O, Soustiel JF, Thaler I. Cerebral blood flow is increased during controlled ovarian stimulation. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293:H3265–H3269. doi: 10.1152/ajpheart.00633.2007. [DOI] [PubMed] [Google Scholar]
- 86.Shamma FN, Fayad P, Brass L, Sarrel P. Middle cerebral artery blood velocity during controlled ovarian hyperstimulation. Fertility and sterility. 1992;57:1022–1025. [PubMed] [Google Scholar]
- 87.Clark AS, MacLusky NJ, Goldman-Rakic PS. Androgen Binding and Metabolism in the Cerebral Cortex of the Developing Rhesus Monkey*. Endocrinology. 1988;123:932–940. doi: 10.1210/endo-123-2-932. [DOI] [PubMed] [Google Scholar]
- 88.Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biological psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 89.Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. Journal of Comparative Neurology. 2001;439:208–223. doi: 10.1002/cne.1343. [DOI] [PubMed] [Google Scholar]
- 90.Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABA A receptors in the rat. Hormones and behavior. 1993;27:568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- 91.Aikey JL, Nyby JG, Anmuth DM, James PJ. Testosterone rapidly reduces anxiety in male house mice (Mus musculus) Hormones and Behavior. 2002;42:448–460. doi: 10.1006/hbeh.2002.1838. [DOI] [PubMed] [Google Scholar]
- 92.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 93.Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 94.Cox BJ, Clara IP, Enns MW. Posttraumatic stress disorder and the structure of common mental disorders. Depression and Anxiety. 2002;15:168–171. doi: 10.1002/da.10052. [DOI] [PubMed] [Google Scholar]
- 95.Joel D, Yankelevitch-Yahav R. Reconceptualizing sex, brain and psychopathology: interaction, interaction, interaction. British journal of pharmacology. 2014;171:4620–4635. doi: 10.1111/bph.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rippon G, Jordan-Young R, Kaiser A, Fine C. Recommendations for sex/gender neuroimaging research: key principles and implications for research design, analysis, and interpretation. Frontiers in human neuroscience. 2014;8:1–13. doi: 10.3389/fnhum.2014.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joel D, Berman Z, Tavor I, Wexler N, Gaber O, Stein Y, et al. Sex beyond the genitalia: The human brain mosaic. Proceedings of the National Academy of Sciences. 2015;112:15468–15473. doi: 10.1073/pnas.1509654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. The Journal of Neuroscience. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.