Abstract
The Notch pathway represents a highly conserved signaling network with essential roles in regulation of key cellular processes and functions, many of which are critical for development. Accumulating evidence indicates that it is also essential for fibrosis and thus the pathogenesis of chronic fibroproliferative diseases in diverse organs and tissues. Different effects of Notch activation are observed depending on cellular and tissue context as well as in both physiologic and pathologic states. Close interactions of Notch signaling pathway with other signaling pathways have been identified. In this review, current knowledge on the role of the Notch signaling with special focus on fibrosis and its potential as a therapeutic target is summarized.
Keywords: Notch, fibrosis, myofibroblast
Graphical abstract
Introduction
Fibrosis is characterized by excessive deposition of connective tissue often in conjunction with a reparative or reactive process [1]. Fibroblast proliferation, emergence of myofibroblasts, ECM deposition and tissue remodeling are additional key features [1]. Chronic progressive fibrosis can occur in virtually all organs including the lung[2], kidney[3], liver[4], skin[5] and heart[6]. It is commonly a result of excessive, prolonged or repeated injury with associated chronic inflammation [1-6]. Extensive research have uncovered complex mechanisms underlying fibrosis, which involve multiple cell types, factors, signaling pathways and genes[1-6]. In particular and germane to this review is recent mounting evidence from both animal model and human studies implicating the Notch signaling pathway in the pathogenesis of fibrosis [7-19].
Notch signaling
The Notch signaling network is an evolutionarily conserved intercellular signaling pathway that regulates interactions between physically adjacent cells [20]. Five ligands, namely Delta-like 1, Delta-like 3, Delta-like 4, Jagged-1 and Jagged-2 [21], were identified for the four notch receptor members Notch 1, Notch2, Notch3 and Notch4 in mammals[20-23]. The Notch receptors are single transmembrane polypeptides synthesized in the endoplasmic reticulum and transported to the cell surface through the trans-Golgi network [23]. They share structural elements containing an extracellular domain with multiple epidermal growth factor-like (EGF) repeats, transmembrane domain, and an intracellular domain with multiple subdomains[20-23]. The Notch proteins are cleaved in the trans-Golgi network, and presented on the cell surface as a heterodimer[20-23].
Binding of ligands from the surface of neighboring cells to the receptor on the adjacent cell induces the conformational change of Notch, leading to the exposure of S2 site and triggers sequentially proteolytic cleavage by A Disintegrin and Metalloprotease (ADAM) and the γ secretase complex[20-24]. Cleavage by ADAM produces a substrate for the second cleavage by the presenilin-containing γ-secretase complex, releasing the Notch intracellular domain (NICD) [23, 24]. The cleaved NICD is then translocated to the nucleus where it binds with the transcription factor CBF1/Suppressor of hairless/Lag1 (CSL) and modulates gene expression [23, 24]. Without NICD, CBF1 (also known as RBPJ) protein binds to the consensus DNA sequence in association with SMART/HDACs complex, acting as a transcriptional repressor [25, 26]. Interaction between NICD and CBF1 displaces the SMART/HDACs corepressor complex, which is replaced with a co-activator complex (MAML1-3, EP300 and SNW1). This results in the transcriptional activation of the target genes primarily involving two families of helix-loop-helix transcription factors Hes (Hairy enhance of split) and Hey (Hairy/enhancer of spit related with YRPW motif) [25]. In addition to this canonical signaling pathway, non-canonical Notch signaling independent of either CBF1 or γ-secretase cleavage, or both have been identified [20-22, 25]. Post-translational modifications including O-fucosylation and O-glycosylation via fringe proteins (lunatic, radical, and manic) regulate the specificity of Notch receptor-ligand binding, and are also critical for its function [27].
Termination of Notch signaling in the cell can occur naturally at or downstream of the Notch receptor[28-31]. The Notch receptor can undergo lysosomal degradation involving the ubiquitin ligase Itch/AIP4 or Nedd4, which act together with Numb [30] and Itch/AIP4 [28-30]. GSK3 controls NICD1 ubiquitination and proteasome-mediated degradation by phosphorylation of the NICD and regulates the NICD interaction with the E3 ubiquitin ligase CDC4/FBW7 [32, 33].
Notch and myofibroblast differentiation
Myofibroblasts are cells with phenotype between fibroblasts and smooth muscle cells [34, 35]. They express α-smooth muscle actin (ACTA2) and other general mesenchymal markers such as vimentin, and arise de novo in response to tissue injury [34, 35]. Myofibroblasts are the major extracellular matrix producing cell[34, 35]. They are enriched in injured tissue undergoing repair/remodeling and are thought to promote repair by contracting the edges of the wound[34, 35]. Additionally, myofibroblasts produce matrix to facilitate the the repair process [1, 34-36]. If they do not undergo apoptosis upon successful repair[37], excessive matrix production by persistent myofibroblasts can result in exuberant scar formation and fibrosis [1, 34, 35]. Thus chronic fibrotic lesions in diverse tissues are characterized by persistence of these myofibroblasts [1-6, 37]. Thus targeting this de novo genesis of the myofibroblast and/or its survival have been considered in therapeutic approaches for controlling chronic progressive fibrotic diseases.
Myofibroblasts have multiple origins based on their organ or tissue locations, and include resident fibroblasts, activated stellate cells, stromal tissue/mesenchymal progenitor/stem cells, epithelial/endothelial cells undergo epithelial/endothelial to mesenchymal transition (EMT/EndMT), as well as circulating mesenchymal precursors, fibrocytes, etc [34, 35, 37]. TGFβ and other fibrogenic cytokines/factors are known inducers of myofibroblast differentiation from these diverse precursors [38-40]. Recent evidence further indicates that the Notch signaling pathway is also involved in the regulation of myofibroblast differentiation in chronic fibrosis including in the lung [7, 15, 18, 41], kidney [42-46], liver [47-51], heart [12, 14] and skin [16, 52].
Notch and epithelial-mesenchymal transition
Epithelial-mesenchymal transition (EMT), as well as a similar transition occurring in vascular endothelial cells referred to as endothelial-mesenchymal transition (EndMT), are associated with the induction of transcription factors causing alterations in expression of genes that are involved in regulation of cell-cell adhesion, cytoskeletal dynamics[53]. These changes reflect the transition from epithelial/endothelial morphology and physiology to the mesenchymal phenotype [53]. In the case of epithelial cells there is gradual loss of E-cadherin expression and apical-basal polarity accompanied by reorganization of their cytoskeleton to acquire a motile phenotype and eventual acquisition of the myofibroblast phenotype characterized by expression of ACTA2 [53]. While EMT has been well studied in embryonic development, it is suspected also to play some role in the genesis of new fibroblasts during the development of organ fibrosis in adult tissues[53, 54]. Indeed, in mature tissues, epithelium can undergo EMT following epithelial stress such as inflammation or wounding, leading to fibroblast proliferation and fibrogenesis [53, 54]. Transforming growth factor-β (TGF-β), one of the major profibrotic cytokines, induces EMT in vitro and has been associated with EMT in vivo[54]. However it remains unclear as to the level of contribution of EMT or EndMT to the overall fibroblast/myofibroblast population in tissues undergoing fibrosis relative to that from other cellular sources.
The role of Notch signaling in the regulation of EMT is suggested by indirect and direct studies. Thus Notch signaling molecules are reported to activate TGFβ in rat mesangial cells under hyperglycemic conditions [55]. Given the role of TGFβ in promotion of EMT, the potential significance of Notch signaling in this process is suggested [53, 55]. Since EMT is associated with chronic fibrosis in the kidney [54], lung [56-59], liver [49, 60] and heart[61-63] evidence for Notch signaling in EMT focused on epithelial cells derived these tissues. For example, a lung-related study used the rat alveolar epithelial cell line, RLE-6TN, to document Notch involvement[15]. In that study activation of Notch, either by ectopic expression of the NICD or by co-culture with Jagged1 expression cells, induces the expression of mesenchymal marker genes including ACTA2, collagen I and vimentin with concomitant reduction in the expression of epithelial marker genes such as E-cadherin, occludin, and zonula occludens-1[15]. In addition to these direct effects mediated by its intracellular domain, Notch can indirectly regulate EMT through other signaling pathways, including TGFβ [15],NF-κB [64] and β-catenin [65], and through the action of various regulatory miRNAs [66-70]. These mechanisms implicate Notch signaling in potential regulation of fibrosis by their impact on genesis of the fibroblast/myofibroblast. Thus therapeutic targeting of this pathway may represent a feasible approach for control of fibrosis in diverse tissues and chronic progressive fibroproliferative diseases.
Notch and Pulmonary Fibrosis
Notch signaling is required for lung development, and Notch receptors and ligands are present in both epithelial and mesenchymal compartments of the developing lung[71]. It is essential for cell differention and mobilization during lung alveogenesis [72], and in the mesenchyme, RBPJ is critical for recruitment and specification of arterial vascular smooth muscle cells, mesothelial epithelial-mesenchymal transition, and selection of Clara versus ciliated cell fate [73]. NOTCH1 is also essential for regeneration of Clara cells during repair of airway injury [74].
In the adult lung, activation of the Notch pathway is reported in patients with chronic obstructive pulmonary disease (COPD)[75], idiopathic pulmonary arterial hypertension [76] and idiopathic pulmonary fibrosis (IPF) [15]. Animal model studies confirmed activation of Notch signaling in pulmonary fibrosis, consistent with similar reports in lung specimens from patients with idiopathic interstitial pneumonias or IPF [7, 15, 18]. The majority of known Notch-related genes are expressed in human small airway epithelium [75] with evidence of elevated Notch expression in lung myofibroblasts in the bleomycininduced model of pulmonary fibrosis and in lung specimens from patients with idiopathic interstitial pneumonias [15]. Mucus cells from patients with chronic obstructive pulmonary disease, idiopathic pulmonary artery hypertension or IPF express the Notch downstream transcription factor, HES1 [77]. Aberrantly sustained Notch activity in injured lungs results in an alveolar cyst architecture that is indicative of a fibrotic phenotype [78]. Moreover differential expression of miRNAs targeting the Notch signaling pathway is observed in rapidly or slowly progressive IPF patients compared to healthy controls, further implicating a role for Notch signaling in IPF pathogenesis [79]. Support is provided by evidence that Notch1 can upregulate type I collagen promoter activity through a Hes1-dependent mechanism [80]. Additionally in the bleomycin model, Jagged1/Notch-signaling is upregulated and found to be essential for myofibroblast differentiation induced by FIZZ-1, a profibrotic factor expressed mainly in IL-4/IL-13 activated alveolar epithelial cells [18]. Impairment of Notch signaling due to impaired fucosylation in FXKO mice inhibits myofibroblast differentiation and bleomycin induced fibrosis [18]. Additionally mesenchymal-specific conditional Notch1 deficient mice exhibited significant reduction of pulmonary fibrosis in the same bleomycin model [81].
In addition to the regulation of myofibroblast differentiation from fibroblasts in the mesenchymal compartment, Notch also regulates EMT in the lung [15]. The pharmacologic inhibition of Notch signaling significantly inhibits TGF-β-induced expression of ACTA2. Furthermore Notch induces the expression of TGF-β family members as well as the phosphorylation of Smad3, an important mediator for TGFβ signal [15]. A771726, an active metabolite of leflunomide known to induce ILD stimulates the expression of Jagged-1, 2, Dll-1 and Notch-1, 3, 4 mRNAs in a dose-dependent manner accompanied by increased NICD in the nuclear extract. This effect was diminished by N-[N-(3,5-difluorophenacetyl-L-alanyl)]-Sphenylglycine t-butyl ester (DAPT), an inhibitor of γ-secretase [82].
In addition to direct regulation of myofibroblast differentiation and EMT, Notch can also interact with other signal pathways to regulate pulmonary fibrosis. In animal model studies lung injury is accompanied with suppression of CXCR7 expression and recruitment of vascular endothelial growth factor receptor 1 (VEGFR1) expressing perivascular macrophages [83]. This recruitment stimulates Wnt/β-catenin-dependent increase of Jagged1 expression in pulmonary capillary endothelial cells, which in turn stimulates Notch signaling in fibroblasts and enhances fibrosis [83]. Treatment with a CXCR7 agonist or pulmonary capillary endothelial cell targeted Jag1 shRNA after lung injury promotes alveolar repair and reduces fibrosis [83]. Notch signaling can also stimulate IGF1R expression to induce AKT phosphorylation and cooperate with HIF1α to promote pulmonary fibrosis [84].
Chronic Kidney Disease
Renal fibrosis is characterized by the increased deposition of collagen and extracellular matrix, de novo genesis of myofibroblasts, migration of leukocytes, dysfunctions of epithelial cells and loss of capillaries [85]. Both Notch1 and Notch2 are expressed during kidney development [86, 87]. But unlike in the lung, Notch activity is not detectable in mature human and rodent kidneys [88]. It appears that in various renal disorders in humans and in animal models Notch signaling is reactivated. In patients with diverse fibrotic kidney diseases, cleaved Notch1, Notch2, and Jagged1 are expressed on podocytes and their level of expression correlates with the amount of proteinuria across all disease groups [89]. Notch3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury [90]. The degree of glomerulosclerosis correlated with podocyte expression of cleaved Notch1, while the severity of tubulointerstitial fibrosis and the estimated glomerular filtration rate correlated with expression of cleaved Notch1 in the tubulointerstitium [89]. Renal tubular Notch signaling triggers a prosenescent state after acute kidney injury [91]. Mutation of NOTCH3 is related to the kidney fibrosis found in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), a disease mainly characterized by nonspecific neointimal fibrosis and hyalinosis of the arterial wall [92]. Increased Jag1/Notch1/HeyL expression in a folic acid–induced kidney injury model and in kidneys of tubulointerstitial fibrosis/chronic kidney disease patients have been reported [45]. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans [45].
In vivo experiments with genetically modified mice and inhibitors of Notch signaling confirm the role of Notch signal in kidney fibrosis. Genetic deletion of the Notch pathway in tubular epithelial cells reduced renal fibrosis [45]. Notch3 deficient mice exhibit less proteinuria, uremia, and inflammatory infiltration [93]. Tubular epithelial cell-specific expression of active Notch1 caused rapid development of tubulo-interstitial fibrosis [45]. Transgenic mice expressing the active Notch1 in developing podocytes showed severe proteinuria and progressive glomerulosclerosis at 2 weeks after birth [94]. Over-expression of NICD in proximal tubules results in aggravated tubular damage and a fibrotic phenotype [94]. In contrast, pharmacologic inhibition of γ-secretase using DAPT reduces tubulointerstitial fibrosis and peritoneal dialysis fluid-induced peritoneal fibrosis in rats [95].
In vitro, activation of Notch signaling with Delta-like 4 caused prosenescent changes in tubular cells while inhibition with DAPT attenuated these changes. Activation of the Notch3 receptor in the glomeruli induces phenotypic changes in podocytes promoting renal inflammation and fibrosis and leading to disease progression [93]. Cross talk between TGFβ and Notch signaling has also been reported in the kidney. Thus inhibition of γ-secretase ameliorates kidney fibrosis via inhibition of TGFβ/Smad2/3 signaling pathway activation [96]. In addition, TGFβ induces renal epithelial Jagged-1 expression in fibrosis [46], while Notch mediated EMT is associated with increased expression of the Snail transcription factor [17]. Depletion of Notch4 in shear-stimulated proximal tubule epithelial cells attenuates collagen accumulation via effects on TGFβ signaling [97]. The vitamin D analogue, paricalcitol attenuates TGF-β1 induced Smad2 phosphorylation and upregulation of the Notch ligand Jagged-1, ACTA2 and thrombospondin-1 and prevented the TGF-β1-mediated loss of E-Cadherin [98]. Additionally, miRNA regulation of Notch signaling affects EMT in tubular epithelial cells [99].
Notch and Skin fibrosis
Constitutive expression of the NICD is reported in normal keratinocytes of the epidermis, hair follicles, sebaceous gland endothelial cells, and immune cells [100-103]. Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis [101-103]. In fibroproliferative skin diseases, the NICD is highly expressed in fibroblasts of keloids and moderately to highly expressed in hypertrophic scars and dermatofibromas, whereas low or no expression is detected in the fibroblasts of normal skin specimens and morpheas [101]. In hypertrophic scar, more Notch1 and Jagged1 positive keratinocytes were found than in normal skin [100]. The Notch pathway was hyperactivated in skin biopsy samples from patients with scleroderma and animal models[104]. Increased expression of ADAM-17, a proteinase involved in Notch activation, is also overexpressed in these skin samples [104]. Accumulation of the NICD and increased HES-1 transcription are noted in animal models of scleroderma [52, 104].
Inhibition of Notch signaling using the γ-secretase inhibitor DAPT or with Notch-1 antisense strategy reduces fibrosis [16, 52]. In addition to prevention of fibrosis, delayed targeting of Notch signaling is also effective in causing almost complete regression of established experimental fibrosis [52]. In systemic sclerosis, activation of Notch signaling is detected in lesional skin as well as in cultured fibroblasts isolated from the skin samples [16]. This systemic sclerosis dermal fibroblast phenotype resembles that of healthy dermal fibroblasts with Jagged-1 induced Notch activation [16].
Therapeutic targeting of Notch signaling, alone or in combination with other interacting signaling pathways has shown some success in animal model translational studies. In one study targeting multiple morphogen (Notch/Hedgehog/Wnt) pathways is shown to have additive antifibrotic effects with improved tolerability [105]. Their results indicate that inhibition of Hedgehog, Wnt and Notch signaling dose-dependently ameliorated animal models of fibrosis. Combination therapies with low doses of Hedgehog/Wnt inhibitors or Hedgehog/Notch inhibitors demonstrate additive anti-fibrotic effects in preventive as well as in therapeutic regimens. In contrast to high dose monotherapies, combination therapies are well tolerated and may help to overcome dose-limiting toxicity of Hedgehog, Wnt and Notch signalling.
Notch and Liver fibrosis
The first evidence for a role of the Notch signaling pathway in hepatic disease was the finding that mutation of the Notch ligand, Jagged1, result in Alagille syndrome (AGS) which correlated with a variety of pediatric disorders including biliary atresia, congenital hepatic fibrosis, sclerosing cholangitis, cystic fibrosis, fulminant hepatic failure, tyrosinemia, and chronic rejection [106]. Jagged1 deficiency results in absence of reactive ductular cells and accumulation of hepatobiliary cells lacking the biliary-specific transcription factor, hepatic nuclear factor 1β, which prevents the switch to a biliary phenotype [107]. In contrast to undetectable expression of Jagged-2, Delta like-1, and Delta like-3, Jagged-1 and Delta like-4 are expressed in normal and diseased liver tissue with primary biliary cirrhosis, primary sclerosing cholangitis, or alcoholic liver disease [108]. Jagged-1 expression is significantly up-regulated in diseased liver tissue [108]. In primary liver cell isolates, Jagged-1 is expressed in all cell types, while Delta like-4 expression is localized to biliary epithelial and liver endothelial cells, but not in hepatocytes [108]. Genomic analysis of differentially expressed genes in liver and biliary epithelial cells reveals overexpression of Notch transcripts in patients with primary biliary cirrhosis liver [109] or hepatocellular carcinoma[110, 111]. Notch3 and Notch4 are not expressed in normal liver [50, 112] but Notch4 is expressed by hepatocytes at the edge of regenerative nodules and in cell planes adjacent to fibrous septa [112], While Notch3 is significantly up-regulated in fibrotic liver tissues from patients with chronic active hepatitis [50]. Expression of Notch1 and Hes1 is reduced in activated hepatic stellate cells (HSCs) [9] but hepatitis B virus (HBV) infection drives increased Notch1 expression in intrahepatic T cells in cirrhosis [113]. Notch 2, Notch 3, Hey2 and HeyL expression increases significantly during activation of quiescent HSCs to myofibroblastic HSCs [49]. High activation of Notch signaling is also observed in hepatic progenitor cells isolated from tissue with primary biliary cirrhosis [114]. Moreover the numbers of Notch1, Notch3, and Notch4 positive cells are significantly increased in fibrotic areas in the CCl4 injured rats [115]. Notch signaling is also highly activated in a rat model of liver fibrosis induced by CCl4 [116] while TGFβ treated hepatic stellate cell line (HSC-T6) [116] exhibited elevated expression of Jagged1, Notch3, and Hes1 [116]. In the ethanol-induced zebrafish fibrosis model, a subset of Notch-responsive HPCs retains its capacity to regenerate as hepatocytes is identified [117]. More direct evidence documenting a role for Notch signaling in liver fibrosis is revealed by studies in a variety of mouse models. The AlbCre+/− transgenic mice were crossed with HNF-6 (HNF-6flox/flox, HNF-6 KO)[118], RBPJ (RBPflox/flox, RBP KO)[119], or both HNF-6 and RBPJ (DKO) mice to knocked out HNF-6 or RBPJ alone or simultaneously in the bipotential hepatoblast progenitor cell [120]. While fibrosis is not observed when HNF-6 or RBPJ is knocked out individually in the bipotential hepatoblast progenitor cell, simultaneous knockout of both these genes results in cholestasis, hepatic necrosis, and fibrosis [120]. In contrast, myeloid-specific disruption of RBPJ attenuates fibrosis in CCl4 induced hepatic fibrosis along with reduction in inflammation, HSC activation and expression of inflammatory and profibrotic factors including platelet-derived growth factor (PDGF)-B and TGFβ1 [121].
In vitro studies with primary isolated cells or immortalized cell lines further confirm the role of Notch in myofibroblast differentiation. In HSC-T6 cells, an immortalized rat liver stellate cell line, Notch1 inhibits but Hes1 stimulates the promoter activities of α-SMA, COL1α1 and COL1α2 [9]. Co-culture of bone marrow-derived mesenchymal stem cells with HSCs inhibits HSC proliferation that requires cell-cell contact and is partially mediated by Notch pathway activation [51]. Notch signaling stimulates Sox9b expression, which is required to maintain Notch signaling in intrahepatic biliary cells [122].
Based on the ample evidence implicating Notch signaling in liver fibrosis, a number of approaches targeting this pathway have been attempted to control the fibrotic response and/or enhance regeneration. A number of Wnt and Notch antagonists have been identified to facilitate hepatocyte regeneration in the fibrotic liver by chemical screens [117]. The Wnt agonists attenuate Notch signaling by inducing Numb, a membrane-associated protein that inhibits Notch signaling [117]. On the anti-fibrotic side, blocking Notch signaling activation by a γ-secretase inhibitor, DAPT, significantly attenuates rat liver fibrosis induced by injection of CCl4, accompanied by suppression of EMT [116]. Additionally DAPT treatment does not affect hepatocyte proliferation in vivo but affords some protection from hepatocyte apoptosis [116]. Interestingly inhibition of Notch signaling in activated HSCs or myofibroblasts induces a mesenchymal-to-epithelial-like transition[116]. In a rat model of cholestatic liver fibrosis, inhibition of Notch signaling with DAPT is also effective in reducing fibrosis [48]. Treatment with another Notch inhibitor, RO4929097 reduces liver fibrosis partly through its effects on hepatic cell differentiation [123]. Another selective γ-secretase inhibitor, Avagacestat, is found to inhibit TGFβ-induced HSC activation and contractility [124]. Therapeutic use of this inhibitor in vivo significantly attenuates fibrogenesis in the CCl4-induced mouse liver fibrosis model [124]. Transfection of Notch3 shRNA in a rat CCl4 induced liver fibrosis model reverses EMT in fibrotic livers by reducing TGFβ1 expression [125]. Consistent with the Notch inhibitor study[48, 116, 123, 124], the inhibition of Notch3 has no effect on hepatocyte proliferation but reduces hepatocyte apoptosis [125]. Inhibiting RBPJ function with a synthetic decoy oligodeoxynucleotide (ODN) in the 3,5-Diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet mouse fibrosis model is also effective in reducing fibrosis [47]. Finally, resveratrol (3,5,4’-trihydroxy-trans-stilbene), a phytoalexin produced naturally by several plants in response to injury or when the plant is under attack by pathogens[126], protects from liver fibrosis by decreasing lipid peroxidation and suppression of Notch1 and Notch3 gene expression [115]. Further studies are necessary to evaluate the therapeutic potential of these agents used in the animal model and in vitro studies.
Conclusion remarks
There is ample evidence for the role of the Notch signaling pathway in regulation of key cellular processes and functions essential for the pathogenesis of fibrosis. However, different effects of Notch activation are observed depending on cellular and tissue context as well as in both physiologic and pathologic states. In addition, Notch interacts closely with other signaling pathways, indicating that combination therapies targeting the other signaling (for example Hedgehog) pathways may be required for effective treatment of diseases with chronic progressive fibrosis. Therefore, investigation of the molecular mechanisms regulating Notch activation in a specific cell context and the complex interplay with additional signaling partners involved is important for a successful therapeutic strategy. Although clinical trials targeting the Notch signaling pathway have not been conducted for treating chronic fibrotic diseases, many have been conducted for treating cancers. The compounds and strategies developed in these cancer studies may be useful and applicable for future studies in developing novel therapies for chronic fibrotic diseases that currently have no effective therapy.
Table 1.
Current strategies used in control of the Notch pathway in fibrosis. (1) blocking the activation of Notch receptors by γ-secretase inhibitors, (2) targeting the ligand or (3) targeting NICD activity, and (4) inhibiting signal transduction.
| Reagent | Organ/Tissue | Species | Reference |
|---|---|---|---|
| (1)Targeting Notch receptor activation* | |||
| DAPT | Liver, Kidney, Skin, Peritoneum | Rat, Mouse and Human |
[52, 91, 95, 116, 127, 128] |
| (2)Targeting ligand* | |||
| Jagged1 shRNA | Lung | Mouse | [83] |
| (3)Targeting NICD | |||
| Notch1 anti-sense construct | Skin | Mouse | [52] |
| Notch3 anti-sense oligodeoxynucleotides |
Kidney | Mouse | [93] |
| Notch3 shRNA | Liver | Rat | [125] |
| mir-148a | Liver | Mouse | [123] |
| mir-34a | Kidney | Human | [99] |
| astragaloside | Liver | Rat | [129] |
| astragalus | Lung | Rat | [130] |
| (4)Targeting signal transduction | |||
| RBPj decoy oligodeoxynucleotide | Liver | Mouse | [47] |
| resveratrol | Liver | Rat | [115] |
| scutellarin | Heart | Rat | [131] |
| Dibenzazepine | Kidney | Mouse | [96] |
| olmesartan | Heart | Mouse | [128] |
Targeting the ligand and receptor using monoclonal antibodies has also been used in other studies but not in fibrosis.
Acknowledgements
We thank and acknowledge the excellent assistance of Robin Kunkel in the drawing of the cartoon depicted in Figure 1.
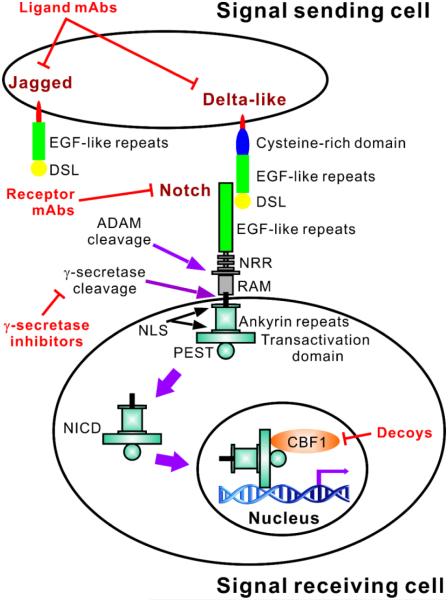
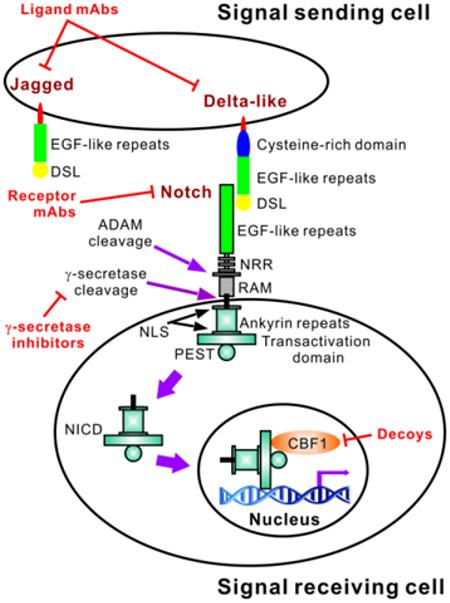
Figure 1.
Brief overview of the Notch pathway with indication of sites for targeted control of the Notch signaling pathway. Notch receptors have an extracellular domain comprised of epidermal growth factor (EGF)-like repeats and three Lin-12 Notch repeats (LNR). Ligand binding triggers sequential receptor cleavages involving ADAM family metalloproteases and the γ-secretase complex, ultimately leading to the cytoplasmic release of the intracellular domain (NICD). This comprises a RAM domain, six ankyrin repeats between two nuclear localization sequences (NLS) and a transactivation domain. A PEST sequence is also present at the C-terminus of all four Notch receptors, with a transactivation domain present in Notch1 and 2. After cytoplasmic release, the NICD translocates (solid purple arrows) into the nucleus where it exerts its transcriptional activity to regulate target genes, such as ACTA2. Both Delta-like and Jagged ligands contain Delta-serrate-Lag2 (DSL) domains followed by a variable number of EGF-like repeats (8 for Delta-like1 and 4, and 6 for Delta-like3). An additional cysteine-rich domain is present in Jagged ligands, which also have 18 EGF-like repeats. The sites currently targeted for control of the Notch signal pathway in fibrosis are indicated in red.
This work was supported by grants HL052285 and HL112880 from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wilson MD. Fibrogenesis: Mechanisms, Dynamics and Clinical Implications. Iran J Pathol. 2015;10(2):83–88. [PMC free article] [PubMed] [Google Scholar]
- [2].Gharaee-Kermani M, Hu B, Thannickal VJ, Phan SH, Gyetko MR. Current and emerging drugs for idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs. 2007;12(4):627–646. doi: 10.1517/14728214.12.4.627. [DOI] [PubMed] [Google Scholar]
- [3].Kuma A, Tamura M, Otsuji Y. Mechanism of and Therapy for Kidney Fibrosis. J UOEH. 2016;38(1):25–34. doi: 10.7888/juoeh.38.25. [DOI] [PubMed] [Google Scholar]
- [4].Toosi AE. Liver Fibrosis: Causes and Methods of Assessment, A Review. Rom J Intern Med. 2015;53(4):304–314. doi: 10.1515/rjim-2015-0039. [DOI] [PubMed] [Google Scholar]
- [5].Desbois AC, Cacoub P. Systemic sclerosis: An update in 2016. Autoimmun Rev. 2016 doi: 10.1016/j.autrev.2016.01.007. [DOI] [PubMed] [Google Scholar]
- [6].Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fibroblast Awakens. Circulation research. 2016;118(6):1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu XD, Zhang LY, Zhu TC, Zhang RF, Wang SL, Bao Y. Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. Int J Clin Exp Pathol. 2015;8(5):4525–4534. [PMC free article] [PubMed] [Google Scholar]
- [8].Le Guen L, Marchal S, Faure S, de Santa Barbara P. Mesenchymal-epithelial interactions during digestive tract development and epithelial stem cell regeneration. Cell Mol Life Sci. 2015;72(20):3883–3896. doi: 10.1007/s00018-015-1975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang K, Zhang YQ, Ai WB, Hu QT, Zhang QJ, Wan LY, Wang XL, Liu CB, Wu JF. Hes1, an important gene for activation of hepatic stellate cells, is regulated by Notch1 and TGF-beta/BMP signaling. World J Gastroenterol. 2015;21(3):878–887. doi: 10.3748/wjg.v21.i3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhou XL, Liu JC. Role of Notch signaling in the mammalian heart. Braz J Med Biol Res. 2014;47(1):1–10. doi: 10.1590/1414-431X20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xie G, Karaca G, Swiderska-Syn M, Michelotti GA, Kruger L, Chen Y, Premont RT, Choi SS, Diehl AM. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology. 2013;58(5):1801–1813. doi: 10.1002/hep.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nemir M, Metrich M, Plaisance I, Lepore M, Cruchet S, Berthonneche C, Sarre A, Radtke F, Pedrazzini T. The Notch pathway controls fibrotic and regenerative repair in the adult heart. Eur Heart J. 2014;35(32):2174–2185. doi: 10.1093/eurheartj/ehs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hu B, Phan SH. Myofibroblasts. Curr Opin Rheumatol. 2013;25(1):71–77. doi: 10.1097/BOR.0b013e32835b1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fan YH, Dong H, Pan Q, Cao YJ, Li H, Wang HC. Notch signaling may negatively regulate neonatal rat cardiac fibroblast-myofibroblast transformation. Physiol Res. 2011;60(5):739–748. doi: 10.33549/physiolres.932149. [DOI] [PubMed] [Google Scholar]
- [15].Aoyagi-Ikeda K, Maeno T, Matsui H, Ueno M, Hara K, Aoki Y, Aoki F, Shimizu T, Doi H, Kawai-Kowase K, Iso T, Suga T, Arai M, Kurabayashi M. Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor-{beta}-Smad3 pathway. American journal of respiratory cell and molecular biology. 2011;45(1):136–144. doi: 10.1165/rcmb.2010-0140oc. [DOI] [PubMed] [Google Scholar]
- [16].Dees C, Tomcik M, Zerr P, Akhmetshina A, Horn A, Palumbo K, Beyer C, Zwerina J, Distler O, Schett G, Distler JH. Notch signalling regulates fibroblast activation and collagen release in systemic sclerosis. Ann Rheum Dis. 2011;70(7):1304–1310. doi: 10.1136/ard.2010.134742. [DOI] [PubMed] [Google Scholar]
- [17].Saad S, Stanners SR, Yong R, Tang O, Pollock CA. Notch mediated epithelial to mesenchymal transformation is associated with increased expression of the Snail transcription factor. Int J Biochem Cell Biol. 2010;42(7):1115–1122. doi: 10.1016/j.biocel.2010.03.016. [DOI] [PubMed] [Google Scholar]
- [18].Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, Lowe JB, Phan SH. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 2009;174(5):1745–1755. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ono Y, Sensui H, Okutsu S, Nagatomi R. Notch2 negatively regulates myofibroblastic differentiation of myoblasts. J Cell Physiol. 2007;210(2):358–369. doi: 10.1002/jcp.20838. [DOI] [PubMed] [Google Scholar]
- [20].Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- [21].D'Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Artavanis-Tsakonas S, Muskavitch MA. Notch: the past, the present, and the future. Curr Top Dev Biol. 2010;92:1–29. doi: 10.1016/S0070-2153(10)92001-2. [DOI] [PubMed] [Google Scholar]
- [23].Kovall RA, Blacklow SC. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol. 2010;92:31–71. doi: 10.1016/S0070-2153(10)92002-4. [DOI] [PubMed] [Google Scholar]
- [24].Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- [25].Yuan X, Wu H, Han N, Xu H, Chu Q, Yu S, Chen Y, Wu K. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J Hematol Oncol. 2014;7:87. doi: 10.1186/s13045-014-0087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Oswald F, Kostezka U, Astrahantseff K, Bourteele S, Dillinger K, Zechner U, Ludwig L, Wilda M, Hameister H, Knochel W, Liptay S, Schmid RM. SHARP is a novel component of the Notch/RBP-Jkappa signalling pathway. The EMBO journal. 2002;21(20):5417–5426. doi: 10.1093/emboj/cdf549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Blair SS. Notch signaling: Fringe really is a glycosyltransferase. Curr Biol. 2000;10(16):R608–612. doi: 10.1016/s0960-9822(00)00633-3. [DOI] [PubMed] [Google Scholar]
- [28].Chastagner P, Israel A, Brou C. AIP4/Itch regulates Notch receptor degradation in the absence of ligand. PLoS One. 2008;3(7):e2735. doi: 10.1371/journal.pone.0002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fortini ME, Bilder D. Endocytic regulation of Notch signaling. Curr Opin Genet Dev. 2009;19(4):323–328. doi: 10.1016/j.gde.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McGill MA, McGlade CJ. Mammalian numb proteins promote Notch1 receptor ubiquitination and degradation of the Notch1 intracellular domain. J Biol Chem. 2003;278(25):23196–23203. doi: 10.1074/jbc.M302827200. [DOI] [PubMed] [Google Scholar]
- [31].Wilkin MB, Carbery AM, Fostier M, Aslam H, Mazaleyrat SL, Higgs J, Myat A, Evans DA, Cornell M, Baron M. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr Biol. 2004;14(24):2237–2244. doi: 10.1016/j.cub.2004.11.030. [DOI] [PubMed] [Google Scholar]
- [32].Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8(2):83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- [33].Pancewicz J, Nicot C. Current views on the role of Notch signaling and the pathogenesis of human leukemia. BMC Cancer. 2011;11:502. doi: 10.1186/1471-2407-11-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57(4):376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170(6):1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Krafts KP. Tissue repair: The hidden drama. Organogenesis. 2010;6(4):225–233. doi: 10.4161/org.6.4.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Micallef L, Vedrenne N, Billet F, Coulomb B, Darby IA, Desmouliere A. The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S5. doi: 10.1186/1755-1536-5-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hu B, Liu J, Wu Z, Liu T, Ullenbruch MR, Ding L, Henke CA, Bitterman PB, Phan SH. Reemergence of hedgehog mediates epithelial-mesenchymal crosstalk in pulmonary fibrosis. American journal of respiratory cell and molecular biology. 2015;52(4):418–428. doi: 10.1165/rcmb.2014-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hu B, Wu Z, Liu T, Ullenbruch MR, Jin H, Phan SH. Gut-enriched Kruppel-like factor interaction with Smad3 inhibits myofibroblast differentiation. American journal of respiratory cell and molecular biology. 2007;36(1):78–84. doi: 10.1165/rcmb.2006-0043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. American journal of respiratory cell and molecular biology. 2003;29(3):397–404. doi: 10.1165/rcmb.2003-0063OC. Pt 1. [DOI] [PubMed] [Google Scholar]
- [41].Xu K, Moghal N, Egan SE. Notch signaling in lung development and disease. Adv Exp Med Biol. 2012;727:89–98. doi: 10.1007/978-1-4614-0899-4_7. [DOI] [PubMed] [Google Scholar]
- [42].Sweetwyne MT, Tao J, Susztak K. Kick it up a notch: Notch signaling and kidney fibrosis. Kidney Int Suppl. 2011;2014;4(1):91–96. doi: 10.1038/kisup.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sirin Y, Susztak K. Notch in the kidney: development and disease. J Pathol. 2012;226(2):394–403. doi: 10.1002/path.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Leask A. Targeting the jagged/notch pathway: a new treatment for fibrosis? J Cell Commun Signal. 2010;4(4):197–198. doi: 10.1007/s12079-010-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bielesz B, Sirin Y, Si H, Niranjan T, Gruenwald A, Ahn S, Kato H, Pullman J, Gessler M, Haase VH, Susztak K. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J Clin Invest. 2010;120(11):4040–4054. doi: 10.1172/JCI43025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Morrissey J, Guo G, Moridaira K, Fitzgerald M, McCracken R, Tolley T, Klahr S. Transforming growth factor-beta induces renal epithelial jagged-1 expression in fibrotic disease. J Am Soc Nephrol. 2002;13(6):1499–1508. doi: 10.1097/01.asn.0000017905.77985.4a. [DOI] [PubMed] [Google Scholar]
- [47].Lee SJ, Kim KH, Pak SC, Kang YN, Yoon GS, Park KK. Notch signaling affects biliary fibrosis via transcriptional regulation of RBP-jkappa in an animal model of chronic liver disease. Int J Clin Exp Pathol. 2015;8(10):12688–12697. [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang X, Du G, Xu Y, Li X, Fan W, Chen J, Liu C, Chen G, Zern MA, Mu Y, Liu P. Inhibition of notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes. Lab Invest. 2016;96(3):350–360. doi: 10.1038/labinvest.2015.149. [DOI] [PubMed] [Google Scholar]
- [49].Zhang QD, Xu MY, Cai XB, Qu Y, Li ZH, Lu LG. Myofibroblastic transformation of rat hepatic stellate cells: the role of Notch signaling and epithelial-mesenchymal transition regulation. Eur Rev Med Pharmacol Sci. 2015;19(21):4130–4138. [PubMed] [Google Scholar]
- [50].Chen YX, Weng ZH, Zhang SL. Notch3 regulates the activation of hepatic stellate cells. World J Gastroenterol. 2012;18(12):1397–1403. doi: 10.3748/wjg.v18.i12.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chen S, Xu L, Lin N, Pan W, Hu K, Xu R. Activation of Notch1 signaling by marrow-derived mesenchymal stem cells through cell-cell contact inhibits proliferation of hepatic stellate cells. Life Sci. 2011;89(25-26):975–981. doi: 10.1016/j.lfs.2011.10.012. [DOI] [PubMed] [Google Scholar]
- [52].Dees C, Zerr P, Tomcik M, Beyer C, Horn A, Akhmetshina A, Palumbo K, Reich N, Zwerina J, Sticherling M, Mattson MP, Distler O, Schett G, Distler JH. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 2011;63(5):1396–1404. doi: 10.1002/art.30254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Stahl PJ, Felsen D. Transforming growth factor-beta, basement membrane, and epithelial-mesenchymal transdifferentiation: implications for fibrosis in kidney disease. Am J Pathol. 2001;159(4):1187–1192. doi: 10.1016/s0002-9440(10)62503-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liu L, Gao C, Chen G, Li X, Li J, Wan Q, Xu Y. Notch Signaling Molecules Activate TGF-beta in Rat Mesangial Cells under High Glucose Conditions. J Diabetes Res. 2013;2013:979702. doi: 10.1155/2013/979702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xi Y, Tan K, Brumwell AN, Chen SC, Kim YH, Kim TJ, Wei Y, Chapman HA. Inhibition of epithelial-to-mesenchymal transition and pulmonary fibrosis by methacycline. American journal of respiratory cell and molecular biology. 2014;50(1):51–60. doi: 10.1165/rcmb.2013-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hosper NA, van den Berg PP, de Rond S, Popa ER, Wilmer MJ, Masereeuw R, Bank RA. Epithelial-to-mesenchymal transition in fibrosis: collagen type I expression is highly upregulated after EMT, but does not contribute to collagen deposition. Exp Cell Res. 2013;319(19):3000–3009. doi: 10.1016/j.yexcr.2013.07.014. [DOI] [PubMed] [Google Scholar]
- [58].Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- [59].Kolosova I, Nethery D, Kern JA. Role of Smad2/3 and p38 MAP kinase in TGF-beta1-induced epithelial-mesenchymal transition of pulmonary epithelial cells. J Cell Physiol. 2011;226(5):1248–54. doi: 10.1002/jcp.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ikegami T, Zhang Y, Matsuzaki Y. Liver fibrosis: possible involvement of EMT. Cells Tissues Organs. 2007;185(1-3):213–221. doi: 10.1159/000101322. [DOI] [PubMed] [Google Scholar]
- [61].Bronnum H, Andersen DC, Schneider M, Sandberg MB, Eskildsen T, Nielsen SB, Kalluri R, Sheikh SP. miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving Programmed Cell Death 4 and Sprouty-1. PLoS One. 2013;8(2):e56280. doi: 10.1371/journal.pone.0056280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Liu Y, Du J, Zhang J, Weng M, Li X, Pu D, Gao L, Deng S, Xia S, She Q. Snail1 is involved in de novo cardiac fibrosis after myocardial infarction in mice. Acta Biochim Biophys Sin (Shanghai) 2012;44(11):902–910. doi: 10.1093/abbs/gms085. [DOI] [PubMed] [Google Scholar]
- [63].von Gise A, Pu WT. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circulation research. 2012;110(12):1628–1645. doi: 10.1161/CIRCRESAHA.111.259960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gopalakrishnan N, Sivasithamparam ND, Devaraj H. Synergistic association of Notch and NFkappaB signaling and role of Notch signaling in modulating epithelial to mesenchymal transition in colorectal adenocarcinoma. Biochimie. 2014;107:310–318. doi: 10.1016/j.biochi.2014.09.020. Pt B. [DOI] [PubMed] [Google Scholar]
- [65].Leong KG, Niessen K, Kulic I, Raouf A, Eaves C, Pollet I, Karsan A. Jagged1-mediated Notch activation induces epithelial-to-mesenchymal transition through Slug-induced repression of E-cadherin. J Exp Med. 2007;204(12):2935–2948. doi: 10.1084/jem.20071082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kang J, Kim E, Kim W, Seong KM, Youn H, Kim JW, Kim J, Youn B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. J Biol Chem. 2013;288(38):27343–27357. doi: 10.1074/jbc.M113.490482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zaravinos A. The Regulatory Role of MicroRNAs in EMT and Cancer. J Oncol. 2015;2015:865816. doi: 10.1155/2015/865816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Engelsvold DH, Utheim TP, Olstad OK, Gonzalez P, Eidet JR, Lyberg T, Troseid AM, Dartt DA, Raeder S. miRNA and mRNA expression profiling identifies members of the miR-200 family as potential regulators of epithelial-mesenchymal transition in pterygium. Exp Eye Res. 2013;115:189–198. doi: 10.1016/j.exer.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yang Y, Ahn YH, Gibbons DL, Zang Y, Lin W, Thilaganathan N, Alvarez CA, Moreira DC, Creighton CJ, Gregory PA, Goodall GJ, Kurie JM. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200-dependent pathway in mice. J Clin Invest. 2011;121(4):1373–1385. doi: 10.1172/JCI42579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, Brabletz T. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. The EMBO journal. 2011;30(4):770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kong Y, Glickman J, Subramaniam M, Shahsafaei A, Allamneni KP, Aster JC, Sklar J, Sunday ME. Functional diversity of notch family genes in fetal lung development, American journal of physiology. Lung cellular and molecular physiology. 2004;286(5):L1075–L1083. doi: 10.1152/ajplung.00438.2002. [DOI] [PubMed] [Google Scholar]
- [72].Xu K, Nieuwenhuis E, Cohen BL, Wang W, Canty AJ, Danska JS, Coultas L, Rossant J, Wu MY, Piscione TD, Nagy A, Gossler A, Hicks GG, Hui CC, Henkelman RM, Yu LX, Sled JG, Gridley T, Egan SE. Lunatic Fringe-mediated Notch signaling is required for lung alveogenesis, American journal of physiology. Lung cellular and molecular physiology. 2010;298(1):L45–L56. doi: 10.1152/ajplung.90550.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 2010;123:213–224. doi: 10.1242/jcs.058669. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Xing Y, Li A, Borok Z, Li C, Minoo P. NOTCH1 is required for regeneration of Clara cells during repair of airway injury. Stem cells. 2012;30(5):946–955. doi: 10.1002/stem.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tilley AE, Harvey BG, Heguy A, Hackett NR, Wang R, O'Connor TP, Crystal RG. Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2009;179(6):457–466. doi: 10.1164/rccm.200705-795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nature medicine. 2009;15(11):1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Plantier L, Crestani B, Wert SE, Dehoux M, Zweytick B, Guenther A, Whitsett JA. Ectopic respiratory epithelial cell differentiation in bronchiolised distal airspaces in idiopathic pulmonary fibrosis. Thorax. 2011;66(8):651–657. doi: 10.1136/thx.2010.151555. [DOI] [PubMed] [Google Scholar]
- [78].Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, Tan K, Tan V, Liu FC, Looney MR, Matthay MA, Rock JR, Chapman HA. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yang G, Yang L, Wang W, Wang J, Wang J, Xu Z. Discovery and validation of extracellular/circulating microRNAs during idiopathic pulmonary fibrosis disease progression. Gene. 2015;562(1):138–144. doi: 10.1016/j.gene.2015.02.065. [DOI] [PubMed] [Google Scholar]
- [80].Hu M, Ou-Yang HF, Wu CG, Qu SY, Xu XT, Wang P. Notch signaling regulates col1alpha1 and col1alpha2 expression in airway fibroblasts. Exp Biol Med (Maywood) 2014;239(12):1589–1596. doi: 10.1177/1535370214538919. [DOI] [PubMed] [Google Scholar]
- [81].Hu B, Wu Z, Bai D, Liu T, Ullenbruch MR, Phan SH. Mesenchymal deficiency of Notch1 attenuates bleomycin-induced pulmonary fibrosis. Am J Pathol. 2015;185(11):3066–3075. doi: 10.1016/j.ajpath.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Namba T, Tanaka KI, Ito Y, Hoshino T, Matoyama M, Yamakawa N, Isohama Y, Azuma A, Mizushima T. Induction of EMT-like phenotypes by an active metabolite of leflunomide and its contribution to pulmonary fibrosis. Cell Death Differ. 2010;17(12):1882–1895. doi: 10.1038/cdd.2010.64. [DOI] [PubMed] [Google Scholar]
- [83].Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, Fong GH, Sakmar TP, Rafii S, Ding BS. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nature medicine. 2016;22(2):154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Eliasz S, Liang S, Chen Y, De Marco MA, Machek O, Skucha S, Miele L, Bocchetta M. Notch-1 stimulates survival of lung adenocarcinoma cells during hypoxia by activating the IGF-1R pathway. Oncogene. 2010;29(17):2488–2498. doi: 10.1038/onc.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124(6):2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288(5):F939–F952. doi: 10.1152/ajprenal.00369.2004. [DOI] [PubMed] [Google Scholar]
- [87].Piscione TD, Wu MY, Quaggin SE. Expression of Hairy/Enhancer of Split genes. Hes1 and Hes5, during murine nephron morphogenesis, Gene Expr Patterns. 2004;4(6):707–711. doi: 10.1016/j.modgep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- [88].Niranjan T, Bielesz B, Gruenwald A, Ponda MP, Kopp JB, Thomas DB, Susztak K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nature medicine. 2008;14(3):290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- [89].Murea M, Park JK, Sharma S, Kato H, Gruenwald A, Niranjan T, Si H, Thomas DB, Pullman JM, Melamed ML, Susztak K. Expression of Notch pathway proteins correlates with albuminuria, glomerulosclerosis, and renal function. Kidney international. 2010;78(5):514–522. doi: 10.1038/ki.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Djudjaj S, Chatziantoniou C, Raffetseder U, Guerrot D, Dussaule JC, Boor P, Kerroch M, Hanssen L, Brandt S, Dittrich A, Ostendorf T, Floege J, Zhu C, Lindenmeyer M, Cohen CD, Mertens PR. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J Pathol. 2012;228(3):286–299. doi: 10.1002/path.4076. [DOI] [PubMed] [Google Scholar]
- [91].Sorensen-Zender I, Rong S, Susnik N, Zender S, Pennekamp P, Melk A, Haller H, Schmitt R. Renal tubular Notch signaling triggers a prosenescent state after acute kidney injury. Am J Physiol Renal Physiol. 2014;306(8):F907–F915. doi: 10.1152/ajprenal.00030.2014. [DOI] [PubMed] [Google Scholar]
- [92].Guerrot D, Francois A, Boffa JJ, Boulos N, Hanoy M, Legallicier B, Triquenot-Bagan A, Guyant-Marechal L, Laquerriere A, Freguin-Bouilland C, Ronco P, Godin M. Nephroangiosclerosis in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: is NOTCH3 mutation the common culprit? Am J Kidney Dis. 2008;52(2):340–345. doi: 10.1053/j.ajkd.2008.04.017. [DOI] [PubMed] [Google Scholar]
- [93].El Machhour F, Keuylian Z, Kavvadas P, Dussaule JC, Chatziantoniou C. Activation of Notch3 in Glomeruli Promotes the Development of Rapidly Progressive Renal Disease. J Am Soc Nephrol. 2015;26(7):1561–1575. doi: 10.1681/ASN.2013090968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Waters AM, Wu MY, Onay T, Scutaru J, Liu J, Lobe CG, Quaggin SE, Piscione TD. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19(6):1139–1157. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhu F, Li T, Qiu F, Fan J, Zhou Q, Ding X, Nie J, Yu X. Preventive effect of Notch signaling inhibition by a gamma-secretase inhibitor on peritoneal dialysis fluid-induced peritoneal fibrosis in rats. Am J Pathol. 2010;176(2):650–659. doi: 10.2353/ajpath.2010.090447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Xiao Z, Zhang J, Peng X, Dong Y, Jia L, Li H, Du J. The Notch gamma-secretase inhibitor ameliorates kidney fibrosis via inhibition of TGF-beta/Smad2/3 signaling pathway activation. Int J Biochem Cell Biol. 2014;55:65–71. doi: 10.1016/j.biocel.2014.08.009. [DOI] [PubMed] [Google Scholar]
- [97].Grabias BM, Konstantopoulos K. Notch4-dependent antagonism of canonical TGF-beta1 signaling defines unique temporal fluctuations of SMAD3 activity in sheared proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2013;305(1):F123–F133. doi: 10.1152/ajprenal.00594.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Nolan KA, Brennan EP, Scholz CC, Cullen C, Ryan A, Taylor CT, Godson C. Paricalcitol protects against TGF-beta1-induced fibrotic responses in hypoxia and stabilises HIF-alpha in renal epithelia. Exp Cell Res. 2015;330(2):371–381. doi: 10.1016/j.yexcr.2014.07.034. [DOI] [PubMed] [Google Scholar]
- [99].Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L, Wang H, Huang C, Sun S. Hypoxia-induced down-regulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells. PLoS One. 2012;7(2):e30771. doi: 10.1371/journal.pone.0030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Xia W, Pan BH, Liu B, Zhang X, Ma FC, Wang YM, Yang XT, Liu D, Guo SZ. Expression of Notch receptors, ligands and downstream target genes in epidermis of hypertrophic scar. Zhonghua zheng xing wai ke za zhi = Zhonghua zhengxing waike zazhi = Chinese journal of plastic surgery. 2009;25(1):41–45. [PubMed] [Google Scholar]
- [101].Kim JE, Lee JH, Jeong KH, Kim GM, Kang H. Notch intracellular domain expression in various skin fibroproliferative diseases. Annals of dermatology. 2014;26(3):332–337. doi: 10.5021/ad.2014.26.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Lin HY, Kao CH, Lin KM, Kaartinen V, Yang LT. Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis. PLoS One. 2011;6(1):e15842. doi: 10.1371/journal.pone.0015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Okuyama R, Tagami H, Aiba S. Notch signaling: its role in epidermal homeostasis and in the pathogenesis of skin diseases. Journal of dermatological science. 2008;49(3):187–194. doi: 10.1016/j.jdermsci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- [104].Kavian N, Servettaz A, Mongaret C, Wang A, Nicco C, Chereau C, Grange P, Vuiblet V, Birembaut P, Diebold MD, Weill B, Dupin N, Batteux F. Targeting ADAM-17/notch signaling abrogates the development of systemic sclerosis in a murine model. Arthritis Rheum. 2010;62(11):3477–3487. doi: 10.1002/art.27626. [DOI] [PubMed] [Google Scholar]
- [105].Distler A, Lang V, Del Vecchio T, Huang J, Zhang Y, Beyer C, Lin NY, Palumbo-Zerr K, Distler O, Schett G, Distler JH. Combined inhibition of morphogen pathways demonstrates additive antifibrotic effects and improved tolerability. Ann Rheum Dis. 2014;73(6):1264–1268. doi: 10.1136/annrheumdis-2013-204221. [DOI] [PubMed] [Google Scholar]
- [106].Louis AA, Van Eyken P, Haber BA, Hicks C, Weinmaster G, Taub R, Rand EB. Hepatic jagged1 expression studies. Hepatology. 1999;30(5):1269–1275. doi: 10.1002/hep.510300512. [DOI] [PubMed] [Google Scholar]
- [107].Fabris L, Cadamuro M, Guido M, Spirli C, Fiorotto R, Colledan M, Torre G, Alberti D, Sonzogni A, Okolicsanyi L, Strazzabosco M. Analysis of liver repair mechanisms in Alagille syndrome and biliary atresia reveals a role for notch signaling. Am J Pathol. 2007;171(2):641–653. doi: 10.2353/ajpath.2007.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Nijjar SS, Wallace L, Crosby HA, Hubscher SG, Strain AJ. Altered Notch ligand expression in human liver disease: further evidence for a role of the Notch signaling pathway in hepatic neovascularization and biliary ductular defects. Am J Pathol. 2002;160(5):1695–1703. doi: 10.1016/S0002-9440(10)61116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tanaka A, Leung PS, Kenny TP, Au-Young J, Prindiville T, Coppel RL, Ansari AA, Gershwin ME. Genomic analysis of differentially expressed genes in liver and biliary epithelial cells of patients with primary biliary cirrhosis. Journal of autoimmunity. 2001;17(1):89–98. doi: 10.1006/jaut.2001.0522. [DOI] [PubMed] [Google Scholar]
- [110].Zekri AR, Hafez MM, Bahnassy AA, Hassan ZK, Mansour T, Kamal MM, Khaled HM. Genetic profile of Egyptian hepatocellular-carcinoma associated with hepatitis C virus Genotype 4 by 15 K cDNA microarray: preliminary study. BMC research notes. 2008;1:106. doi: 10.1186/1756-0500-1-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, Bottinger E, Friedman S, Waxman S, Llovet JM. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45(4):938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- [112].Gramantieri L, Giovannini C, Lanzi A, Chieco P, Ravaioli M, Venturi A, Grazi GL, Bolondi L. Aberrant Notch3 and Notch4 expression in human hepatocellular carcinoma. Liver Int. 2007;27(7):997–1007. doi: 10.1111/j.1478-3231.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- [113].Trehanpati N, Shrivastav S, Shivakumar B, Khosla R, Bhardwaj S, Chaturvedi J, Sukriti, Kumar B, Bose S, Mani Tripathi D, Das T, Sakhuja P, Rastogi A, Bhihari C, Singh S, Gupta S, Kottilil S, Sarin SK. Analysis of Notch and TGF-beta Signaling Expression in Different Stages of Disease Progression During Hepatitis B Virus Infection. Clin Transl Gastroenterol. 2012;3:e23. doi: 10.1038/ctg.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Spee B, Carpino G, Schotanus BA, Katoonizadeh A, Vander Borght S, Gaudio E, Roskams T. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut. 2010;59(2):247–257. doi: 10.1136/gut.2009.188367. [DOI] [PubMed] [Google Scholar]
- [115].Tanriverdi G, Kaya-Dagistanli F, Ayla S, Demirci S, Eser M, Unal ZS, Cengiz M, Oktar H. Resveratrol can prevent CCl(4)-induced liver injury by inhibiting Notch signaling pathway. Histol Histopathol. 2016:11720. doi: 10.14670/HH-11-720. [DOI] [PubMed] [Google Scholar]
- [116].Chen Y, Zheng S, Qi D, Zheng S, Guo J, Zhang S, Weng Z. Inhibition of Notch signaling by a gamma-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS One. 2012;7(10):e46512. doi: 10.1371/journal.pone.0046512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Huang M, Chang A, Choi M, Zhou D, Anania FA, Shin CH. Antagonistic interaction between Wnt and Notch activity modulates the regenerative capacity of a zebrafish fibrotic liver model. Hepatology. 2014;60(5):1753–1766. doi: 10.1002/hep.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhang H, Ables ET, Pope CF, Washington MK, Hipkens S, Means AL, Path G, Seufert J, Costa RH, Leiter AB, Magnuson MA, Gannon M. Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech Dev. 2009;126(11-12):958–973. doi: 10.1016/j.mod.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Han H, Tanigaki K, Yamamoto N, Kuroda K, Yoshimoto M, Nakahata T, Ikuta K, Honjo T. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol. 2002;14(6):637–645. doi: 10.1093/intimm/dxf030. [DOI] [PubMed] [Google Scholar]
- [120].Vanderpool C, Sparks EE, Huppert KA, Gannon M, Means AL, Huppert SS. Genetic interactions between hepatocyte nuclear factor-6 and Notch signaling regulate mouse intrahepatic bile duct development in vivo. Hepatology. 2012;55(1):233–243. doi: 10.1002/hep.24631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].He F, Guo FC, Li Z, Yu HC, Ma PF, Zhao JL, Feng L, Li WN, Liu XW, Qin HY, Dou KF, Han H. Myeloid-specific disruption of recombination signal binding protein Jkappa ameliorates hepatic fibrosis by attenuating inflammation through cylindromatosis in mice. Hepatology. 2015;61(1):303–314. doi: 10.1002/hep.27394. [DOI] [PubMed] [Google Scholar]
- [122].Delous M, Yin C, Shin D, Ninov N, Debrito Carten J, Pan L, Ma TP, Farber SA, Moens CB, Stainier DY. Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS genetics. 2012;8(6):e1002754. doi: 10.1371/journal.pgen.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jung KH, Zhang J, Zhou C, Shen H, Gagea M, Rodriguez-Aguayo C, Lopez-Berestein G, Sood AK, Beretta L. Differentiation therapy for hepatocellular carcinoma: Multifaceted effects of miR-148a on tumor growth and phenotype and liver fibrosis. Hepatology. 2016;63(3):864–879. doi: 10.1002/hep.28367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bansal R, van Baarlen J, Storm G, Prakash J. The interplay of the Notch signaling in hepatic stellate cells and macrophages determines the fate of liver fibrogenesis. Sci Rep. 2015;5:18272. doi: 10.1038/srep18272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zheng SP, Chen YX, Guo JL, Qi D, Zheng SJ, Zhang SL, Weng ZH. Recombinant adeno-associated virus-mediated transfer of shRNA against Notch3 ameliorates hepatic fibrosis in rats. Exp Biol Med (Maywood) 2013;238(6):600–609. doi: 10.1177/1535370213480698. [DOI] [PubMed] [Google Scholar]
- [126].Fremont L. Biological effects of resveratrol. Life Sci. 2000;66(8):663–73. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- [127].Hellerbrand C, Massoumi R. Cylindromatosis-A Protective Molecule against Liver Diseases. Medicinal research reviews. 2016;36(2):342–359. doi: 10.1002/med.21381. [DOI] [PubMed] [Google Scholar]
- [128].You J, Wu J, Jiang G, Guo J, Wang S, Li L, Ge J, Zou Y. Olmesartan attenuates cardiac remodeling through DLL4/Notch1 pathway activation in pressure overload mice. J Cardiovasc Pharmacol. 2013;61(2):142–151. doi: 10.1097/FJC.0b013e31827a0278. [DOI] [PubMed] [Google Scholar]
- [129].Yongping M, Zhang X, Xuewei L, Fan W, Chen J, Zhang H, Chen G, Liu C, Liu P. Astragaloside prevents BDL-induced liver fibrosis through inhibition of notch signaling activation. Journal of ethnopharmacology. 2015;169:200–209. doi: 10.1016/j.jep.2015.04.015. [DOI] [PubMed] [Google Scholar]
- [130].Zhou Y, Liao S, Zhang Z, Wang B, Wan L. Astragalus injection attenuates bleomycin-induced pulmonary fibrosis via down-regulating Jagged1/Notch1 in lungs. The Journal of pharmacy and pharmacology. 2016 doi: 10.1111/jphp.12518. [DOI] [PubMed] [Google Scholar]
- [131].Zhou H, Chen X, Chen L, Zhou X, Zheng G, Zhang H, Huang W, Cai J. Anti-fibrosis effect of scutellarin via inhibition of endothelial-mesenchymal transition on isoprenaline-induced myocardial fibrosis in rats. Molecules. 2014;19(10):15611–15623. doi: 10.3390/molecules191015611. [DOI] [PMC free article] [PubMed] [Google Scholar]