ABSTRACT
Undecaprenyl phosphate (Und-P) is a member of the family of essential polyprenyl phosphate lipid carriers and in the Gram-negative bacterium Escherichia coli is required for synthesizing the peptidoglycan (PG) cell wall, enterobacterial common antigen (ECA), O antigen, and colanic acid. Previously, we found that interruption of ECA biosynthesis indirectly alters PG synthesis by sequestering Und-P via dead-end intermediates, causing morphological defects. To determine if competition for Und-P was a more general phenomenon, we determined if O-antigen intermediates caused similar effects. Indeed, disrupting the synthesis of O antigen or the lipopolysaccharide core oligosaccharide induced cell shape deformities, which were suppressed by preventing the initiation of O-antigen biosynthesis or by manipulating Und-P metabolism. We conclude that accumulation of O-antigen intermediates alters PG synthesis by sequestering Und-P. Importantly, many previous experiments addressed the physiological functions of various oligosaccharides and glycoconjugates, but these studies employed mutants that accumulate deleterious intermediates. Thus, conclusions based on these experiments must be reevaluated to account for possible indirect effects of Und-P sequestration.
IMPORTANCE Bacteria use long-chain isoprenoids like undecaprenyl phosphate (Und-P) as lipid carriers to assemble numerous glycan polymers that comprise the cell envelope. In any one bacterium, multiple oligosaccharide biosynthetic pathways compete for a common pool of Und-P, which means that disruptions in one pathway may produce secondary consequences that affect the others. Using the Gram-negative bacterium Escherichia coli as a model, we demonstrate that interruption of the biogenesis of O antigen, a major outer membrane component, indirectly impairs peptidoglycan synthesis by sequestering Und-P into dead-end intermediates. These results strongly argue that the functions of many Und-P-utilizing pathways must be reevaluated, because much of our current understanding is based on experiments that did not control for these unintended secondary effects.
INTRODUCTION
Long-chain isoprenoids are widely conserved lipid carriers that translocate glycan intermediates across biological membranes, where they are assembled onto other polymers or released (reviewed in reference 1). In bacteria, the primary lipid carrier is undecaprenyl phosphate (Und-P), a 55-carbon isoprenoid (C55-P) also referred to as bactoprenol, which is the dephosphorylated product of undecaprenyl pyrophosphate (Und-PP), as synthesized by UppS (2, 3). Homologues with shorter chains serve the same function in species of mycobacteria and corynebacteria (C50-P) (4, 5) and Paracoccus denitrificans (C45-P) (6). Und-P is required for synthesizing numerous bacterial glycans, including cell wall peptidoglycan (PG) (7, 8), wall teichoic acids (WTA) (9), enterobacterial common antigen (ECA) (10), O antigen (11), capsular polysaccharides (12), several commercially important exopolysaccharides (13), and a variety of other carbohydrates (14–17). These glycan polymers are synthesized on the cytoplasmic side of the plasma membrane, where individual sugar residues are transferred sequentially from sugar-nucleotide precursors onto Und-P (18–22). The resulting Und-P-linked oligosaccharides are translocated (“flipped”) across the plasma membrane and transferred from the lipid carrier onto individual glycan chains (23). Either before or after being dephosphorylated, the leftover Und-PP carrier is then recycled into the cytoplasm, where it rejoins the pool of free Und-P (24).
Mutations that prevent Und-P recycling lead to the accumulation of Und-PP-linked intermediates, resulting in a variety of deleterious or toxic effects (24–28). Because any one organism normally uses Und-P to synthesize multiple products, either these intermediates could be toxic in and of themselves or they might exert their effects indirectly by sequestering Und-P so that it becomes unavailable for use in other pathways. Although there is a long history of ascribing these effects to Und-P sequestration, only a few experiments have addressed this question directly. For example, some Corynebacterium glutamicum mutants accumulate lethal amounts of arabinogalactan and lipoarabinomannan intermediates, but the cells survive if the pool of decaprenyl phosphate is increased (5). Similarly, several Staphylococcus aureus mutants produce lethal WTA intermediates, but an increase in the pool of Und-P restores cell growth (29). Lastly, an increase in the amount of Und-P reverses the morphological and membrane defects caused by dead-end Und-PP-linked ECA intermediates in Escherichia coli (30). In all these cases the intermediates were not overtly toxic. That is, they did not directly inhibit a particular reaction. Instead, the results strongly argue that these dead-end intermediates sequester so much lipid carrier that they impede other, essential biosynthetic pathways (e.g., PG synthesis). Thus, cell death and many other physiological deficiencies were simply secondary effects caused by the inability to synthesize multiple products.
Apart from these examples, little is known about how different biosynthetic pathways compete for Und-P or how mutations in each pathway affect the others, including those which disrupt O-antigen biosynthesis. Thus, we sought to determine if the PG and O-antigen biosynthetic pathways from E. coli compete for Und-P and, if so, to describe the physiological effects of this competition. By using a genetic approach, we demonstrate that disruption of O-antigen biogenesis indirectly alters cell wall synthesis by interfering with Und-P metabolism, and we conclude that the O-antigen and PG biosynthetic pathways compete for a common pool of Und-P. More broadly, we extend this conclusion to interactions among all Und-P-utilizing pathways. An important implication of these observations is that much work regarding the physiological functions of various glycans should be reevaluated, because many of these experiments employed mutants that accumulate similar dead-end Und-PP-linked intermediates. This means that these previous experiments may not reflect the true biological roles of individual glycan products. Instead, the phenotypes observed in such mutants may be contaminated by unintended secondary effects caused by Und-P sequestration.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains, plasmids, and primers used in this study are listed in Tables S1 to S3 in the supplemental material, respectively. Bacteria were grown in LB broth (5 g/liter yeast extract, 10 g/liter tryptone, 10 g/liter NaCl). When appropriate, kanamycin was used at 50 μg/ml.
Strain construction.
The parent strain for this study was MG1655 wbbL+ (31). Restoration of O-antigen production in MG1655 wbbL+ obscures the lipopolysaccharide (LPS) core oligosaccharide, preventing P1-mediated generalized transduction. Consequently, all gene deletions were generated by using bacteriophage lambda Red recombination (32). Kanamycin resistance markers were evicted via the FLP recombinase produced from pCP20 (33). All gene deletions were verified by colony PCR.
Plasmid construction.
Plasmids for rescue of ΔwaaL and ΔwaaC cells were constructed in pDSW361, which is a kanamycin-resistant derivative of pDSW204 (34). pMAJ41 (P204::waaL) was constructed by amplifying waaL from MG1655 DNA with primers P229 and P251. The 1,275-bp product was cut with EcoRI and PstI and ligated to the same sites of pDSW361. Plasmids pMAJ42 (P204::waaC), pMAJ44 (P204::wecG), and pMAJ45 (P204::uppS) were similarly constructed using the following primer pairs: P231/P252 (pMAJ42), P233/P253 (pMAJ44), and P21/P254 (pMAJ45). pMAJ43 was constructed by digesting pMAJ19 (30) with EcoRI and PstI and ligating the murA insert to the same sites of pDSW361. All reference sequences were obtained from the EcoGene (version 3.0) database (35). All cloning was verified by sequencing at the UAMS DNA Sequencing Core Facility.
Suppression of shape defects in ΔwaaL and ΔwaaC mutants.
Overnight cultures were diluted 1:2,000 into LB medium containing kanamycin and 100 μM IPTG (isopropyl-β-d-thiogalactopyranoside) (unless otherwise noted) and grown at 37°C to an optical density at 600 nm (OD600) of 0.5 to 0.6 (approximately 10 doublings). Cells were fixed with 4% paraformaldehyde, spotted onto 1% agarose pads, and visualized by phase-contrast microscopy. Our microscope and camera have been described previously (36).
Labeling of O16 antigen by concanavalin A-AF488.
Overnight cultures were diluted 1:2,000 into LB medium and grown at 37°C to an OD600 of 0.5 to 0.6. Cells were pelleted by centrifugation, washed twice in phosphate-buffered saline (PBS; 137 mM NaCl, 3 mM KCl, 9 mM NaH2PO4, 2 mM KH2PO4, pH 7.4), and incubated with 2 μM concanavalin A conjugated to Alexa Fluor 488 (AF488) (Thermo Fisher Scientific) to label the O16 antigen. After 30 min, the cells were pelleted, washed twice in PBS, and visualized by phase-contrast and fluorescence microscopy.
Flow cytometry analysis.
Live cells were prepared for flow cytometry as described previously (30). Cell size was assayed by using the forward scatter detector mode of a BD LSRFortessa flow cytometer, housed in the UAMS Flow Cytometry Core Facility. All flow data were analyzed using FlowJo (version 10.1) software.
RESULTS
Mutations that interrupt O-antigen biosynthesis induce morphological defects.
The accumulation of unprocessed Und-PP-linked ECA intermediates disrupts cell shape by restricting the availability of Und-P for PG synthesis (30). To determine if the interruption of other Und-P-utilizing pathways would provoke similar effects, we constructed a series of mutations in the O-antigen biosynthesis pathway of E. coli MG1655 and assayed their effects on cell shape. One complicating factor was that commonly used derivatives of E. coli K-12 (e.g., MG1655) normally do not produce O antigen because the rhamnosyl transferase gene (wbbL) is disrupted by an insertion element (wbbL::IS5) (37). Rendueles et al. recently constructed a derivative of E. coli MG1655 in which the wild-type wbbL allele was reintroduced (MG1655 wbbL+) (31). We obtained this strain and confirmed that it produced the O16 antigen (Fig. 1). We then made stepwise deletions in the O-antigen biosynthesis pathway (with the exception of wbbL) and evaluated the mutants for changes in cell shape by microscopy and flow cytometry.
FIG 1.
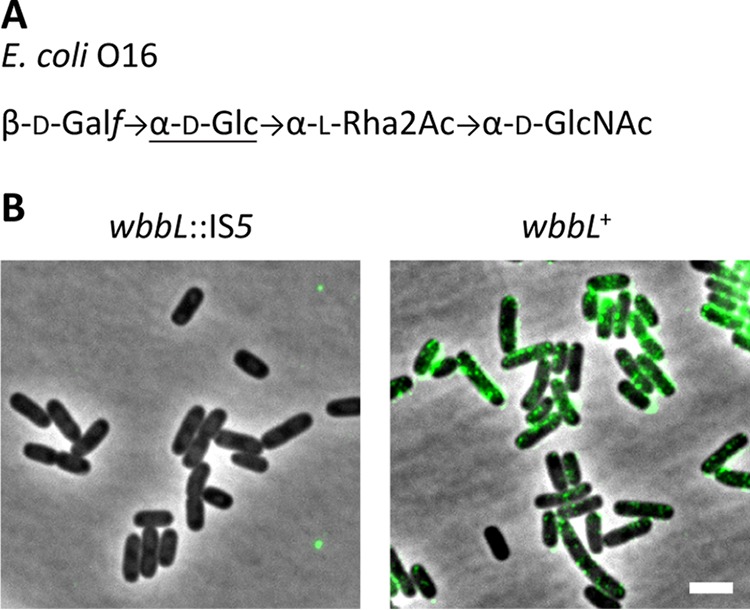
Restoring O-antigen biosynthesis to E. coli K-12. (A) Structure of the O16 antigen from E. coli K-12 (reviewed in reference 43). Abbreviations: Galf, galactofuranose; Glc, glucose; Rha, rhamnose; GlcNAc, N-acetylglucosamine. (B) Detection of O antigen with concanavalin A-AF488 in cells with the indicated genotypes. Cells were labeled with concanavalin A-AF488, washed, and photographed by phase-contrast and fluorescence microscopy (green signal). Bar, 3 μm. Concanavalin A binds α-glucose (underlined in panel A) and α-mannose residues. The strains tested were MAJ1 (wbbL::IS5) and MAJ330 (wbbL+).
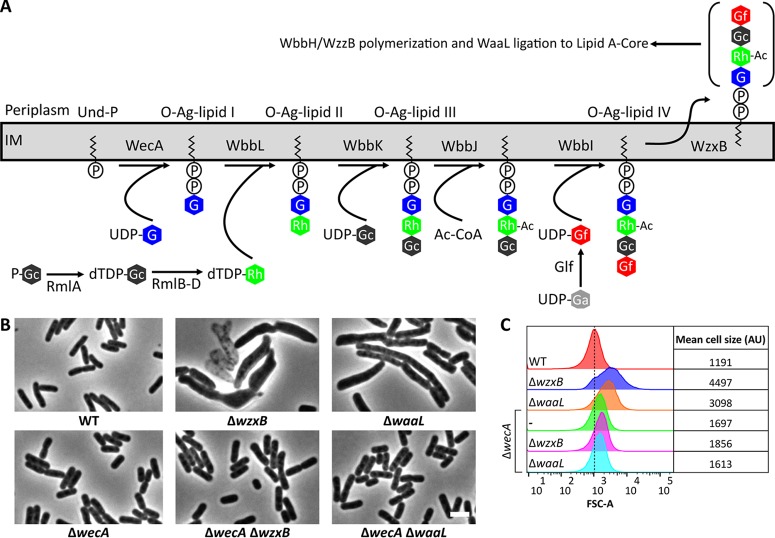
Of the mutants that were predicted to accumulate Und-PP-linked O-antigen intermediates, those lacking either the O-antigen flippase, WzxB [also known as Wzx(O16)], or the WaaL ligase produced the most severe morphological alterations (Fig. 2B; see also Fig. S1A in the supplemental material). Cells lacking wzxB were swollen, filamentous, or chained (Fig. 2B; see also Fig. S1A). When examined by flow cytometry a population of ΔwzxB cells exhibited a 3.8-fold greater distribution in forward scatter area (FSC-A) (Fig. 2C), confirming that the cells were enlarged. In addition, ΔwzxB cells lysed frequently (e.g., see the empty cells in Fig. 2B). The morphological defects associated with ΔwzxB cells were unstable, and the mutant readily developed suppressor mutations that reversed these shape abnormalities (see Fig. S2). Among these suppressors were insertion sequence (IS) insertions into wbbL and rmlC (see Fig. S2C), which prevent the formation of O-antigen intermediates (Fig. 2A). These results parallel the genetic instability associated with mutations in the Pseudomonas aeruginosa wzx gene, a homologue of E. coli wzxB (38). The P. aeruginosa wzx mutant also acquires second-site mutations in wbpL, which eliminates the initial step of O-antigen synthesis and prevents the accumulation of Und-PP-linked O-antigen intermediates. Finally, E. coli cells lacking the WaaL ligase also induced morphological alterations in E. coli, causing cells to grow, on average, 40% wider and 62% longer than wild-type cells (n = 254) (Fig. 2B and C and data not shown). ΔwaaL cells also lysed, but they did so less frequently than the ΔwzxB mutant cells (not shown).
FIG 2.
Disrupting O-antigen biosynthesis induces morphological defects in E. coli. (A) O-antigen biosynthesis pathway. Abbreviations: IM, inner membrane; O-Ag, O antigen; P-Gc, glucose 1-phosphate; Und-P, undecaprenyl phosphate; G, N-acetylglucosamine; Ac, acetyl; Ac-CoA, acetyl coenzyme A; Rh, rhamnose; Gf, galactofuranose. Note that WzxB and WbbH are also known as Wzx(O16) and Wzy(O16), respectively (62). (B) Micrographs of cells with the indicated genotypes. Cells were grown at 37°C in LB for approximately 10 doublings until the culture reached an OD600 of 0.5 to 0.6. The cells were then fixed and photographed by phase-contrast microscopy. Additional O-antigen mutants are shown in Fig. S1 in the supplemental material. Micrographs of ΔwzxB cells were from overnight cultures because the strain readily develops suppressor mutations that correct the shape defect, as shown in Fig. S2. WT, wild type. Bar, 3 μm. (C) Flow cytometry data from live cells in panel B. Histograms of the forward scatter area (FSC-A) from 100,000 events (cells) are shown. The mean cell size of the wild type is represented by the dashed line and is expressed in arbitrary units (AU). Data are representative of those from two independent experiments. The strains tested were MAJ330 (wild type), MAJ339 (ΔwzxB), MAJ345 (ΔwaaL), MAJ343 (ΔwecA), MAJ369 (ΔwecA ΔwzxB), and MAJ370 (ΔwecA ΔwaaL).
Interestingly, aside from the morphological changes observed for the ΔwzxB and ΔwaaL mutants (Fig. 2B), the other O-antigen biosynthesis mutants exhibited little to no change in cell shape (see Fig. S1A and B in the supplemental material). This was surprising because the accumulation of Und-PP-linked ECA intermediates, particularly later-stage intermediates, induces cell shape defects (30). However, in the E. coli MG1655 strain in which the O-antigen pathway was reconstituted, the O-antigen and ECA biosynthesis pathways compete for a common starting substrate, Und-PP–N-acetylglucosamine (Und-PP–GlcNAc). It was therefore possible that Und-PP–GlcNAc was being redirected into the ECA pathway and that this side reaction was reducing the accumulation of Und-PP-linked O-antigen intermediates. To test this possibility, we deleted O-antigen pathway genes (wbbK and wbbJ) in an ECA-negative ΔwecB mutant. WecB helps convert UDP-GlcNAc to UDP–N-acetylmannosaminuronic acid (Fig. 3A) (39, 40), so that in the absence of WecB Und-PP–GlcNAc cannot be utilized in ECA biosynthesis (Fig. 3A). Deletion of wecB, wbbK, or wbbJ individually had little effect on cell shape (Fig. 3B and C). However, cells of double mutants lacking wecB and wbbK or wecB and wbbJ were greatly enlarged and occasionally branched (Fig. 3B), and the mean cell sizes increased substantially (Fig. 3C). Like the ΔwzxB mutant, ΔwecB ΔwbbJ cells were also genetically unstable, readily accumulating suppressor mutations (data not shown). In short, the presence of a functional ECA pathway suppressed the negative effects caused by disruption of the early stages of O-antigen biosynthesis.
FIG 3.
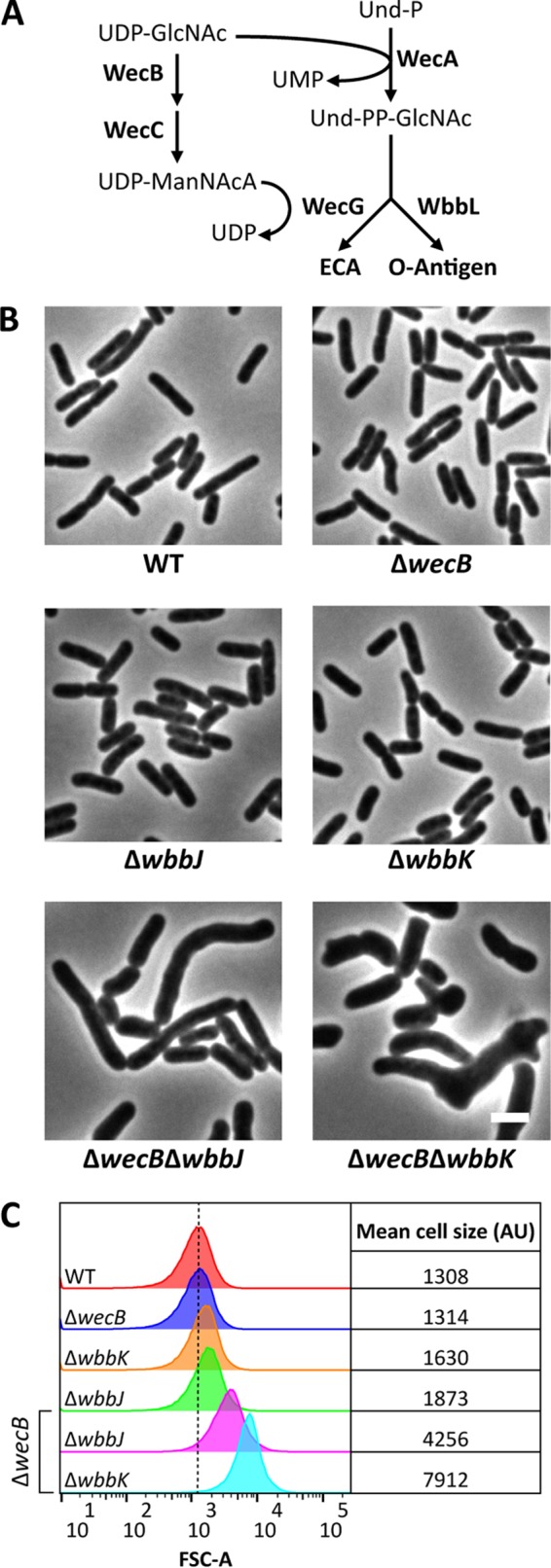
Synthetically misshapen mutant combinations in the O-antigen and ECA pathways. (A) Biosynthetic pathway illustrating how the ECA and O-antigen synthesis pathways compete for Und-PP–GlcNAc. Abbreviations: GlcNAc, N-acetylglucosamine; ManNAcA, N-acetylmannosaminuronic acid; Und-P, undecaprenyl phosphate; Und-PP, undecaprenyl pyrophosphate; ECA, enterobacterial common antigen. (B) Micrographs of cells with the indicated genotypes. Cells were grown and imaged as described in the legend to Fig. 2. Bar, 3 μm. (C) Flow cytometry data from live cells in panel B. Histograms of the forward scatter area from 100,000 events (cells) are shown. The mean cell size for wild-type cells (red graph) is represented by the dashed line and is expressed in arbitrary units (AU). Data are representative of those from two independent experiments. The strains tested were MAJ330 (wild type), MAJ356 (ΔwecB), MAJ346 (ΔwbbJ), MAJ344 (ΔwbbK), MAJ398 (ΔwecB ΔwbbJ), and MAJ397 (ΔwecB ΔwbbK).
Taken together, these results strongly indicate that interruption of the O-antigen biosynthesis pathway induces morphological defects by producing dead-end intermediates.
Disruption of O-antigen assembly limits Und-P availability.
Most studies characterizing cell shape in E. coli have been conducted in strains lacking O antigen. However, restoration of O-antigen synthesis had no impact on cell shape (see Fig. S1 in the supplemental material; compare wild-type cells to cells of the O-antigen-negative wbbL::IS5 mutant). Because cells lacking WbbL do not make O antigen (Fig. 2A), it was clear that the O antigen itself was not required to maintain a normal morphology. Thus, the morphological defects provoked by the deletion of other genes in the pathway (Fig. 2B and 3B; see also Fig. S1A) cannot be explained by the absence of O antigen but are instead best explained by the accumulation of Und-PP-linked intermediates. If so, then deletion of wecA, which initiates O-antigen biosynthesis by transferring GlcNAc-1-phosphate onto the Und-P carrier (Fig. 2A) (41), should reverse the shape defects by eliminating these intermediates. Indeed, the removal of wecA reversed the shape defects observed in ΔwzxB and ΔwaaL cells (Fig. 2B and C), confirming that the morphological defects were not caused by the absence of O antigen but were caused by the presence of deleterious Und-PP-linked intermediates. (We note, though, that cells lacking WecA were slightly larger than wild-type cells [Fig. 2B and C] [30], being, on average, 7% longer and 9% wider [n = 219]. What causes this small difference is unknown.)
At this point, we considered it likely that the morphological defects caused by accumulating dead-end O-antigen intermediates were due to impaired PG synthesis caused by Und-P sequestration. If so, then artificially increasing the pool of Und-P should suppress shape defects in O-antigen mutants. To test this possibility, we overproduced the undecaprenyl pyrophosphate synthase UppS (also known as IspU) to increase the pool of Und-P in a ΔwaaL mutant, which cannot ligate O antigen to the lipid A core and therefore accumulates several pathway intermediates. As predicted, the presence of additional UppS returned ΔwaaL cells to the shape and size of wild-type cells (Fig. 4; compare the puppS-containing strain to cells containing pwaaL and those containing vector only). Thus, cell shape defects that accompany interruptions of the O-antigen biosynthetic pathway are most likely caused by diminished levels of Und-P.
FIG 4.
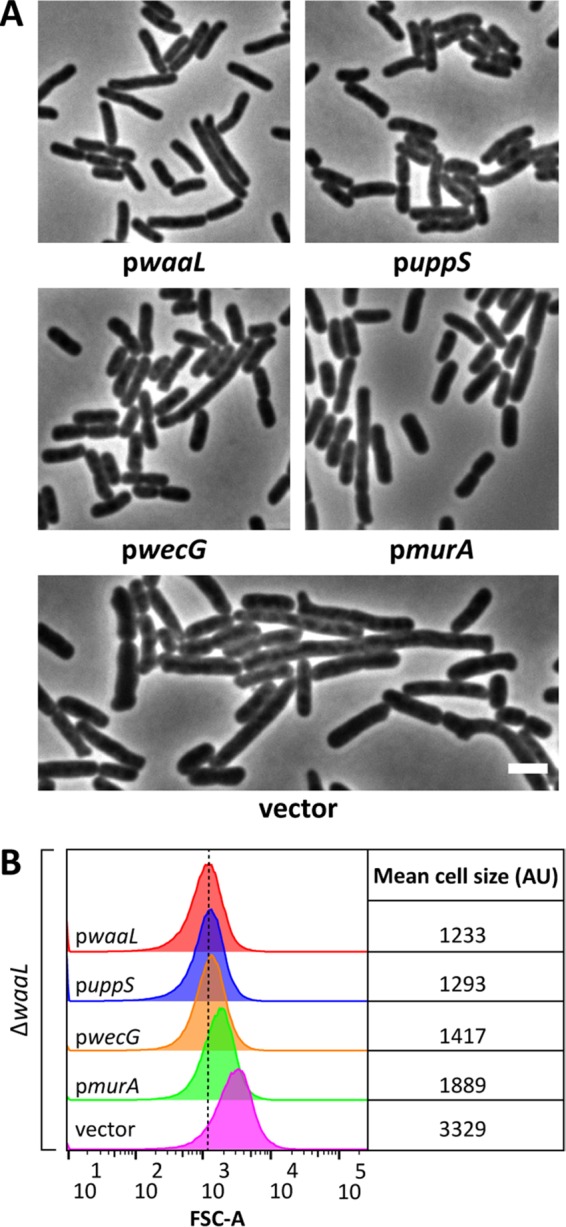
Suppression of ΔwaaL shape defects. (A) Micrographs of ΔwaaL cells containing derivatives of pDSW361 that express the indicated genes. Cells were grown at 37°C in LB containing 100 μM IPTG (0 μM IPTG for pwecG) until the culture reached an OD600 of 0.5 to 0.6. The cells were then fixed and photographed by phase-contrast microscopy. Bar, 3 μm. Further characterization of wecG overexpression is found in Fig. S3 in the supplemental material. (B) Flow cytometry data from live cells in panel A. Histograms of the forward scatter area from 100,000 events (cells) are shown. The mean cell size for ΔwaaL cells expressing waaL in trans (red graph) is represented by the dashed line and is expressed in arbitrary units (AU). Data are representative of those from two independent experiments. The strains tested were MAJ434 (pwaaL), MAJ437 (puppS), MAJ436 (pwecG), MAJ435 (pmurA), and MAJ433 (vector). The effect of expression of the aforementioned derivatives of pDSW361 on wild-type cells is shown in Fig. S4.
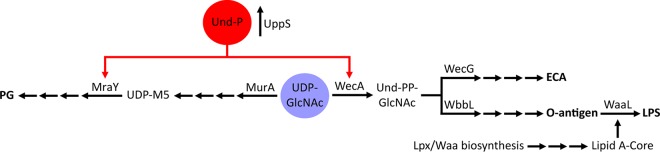
To further confirm that a lack of Und-P altered PG synthesis and caused shape defects, we skewed the balance of shared metabolites in favor of the PG synthesis pathway as another way of alleviating Und-P sequestration. For many E. coli strains, including MG1655 (42), GlcNAc is incorporated at the reducing end of the O antigen (Fig. 1A) (reviewed in reference 43). This is important because both the PG and O-antigen biosynthetic pathways compete for the nucleotide precursor of this sugar, UDP-GlcNAc. Since the first committed step for PG synthesis is the transfer of enolpyruvate to UDP-GlcNAc by MurA (44), we reasoned that overexpression of murA would increase the flux of UDP-GlcNAc into the PG synthesis pathway at the expense of O-antigen production, thereby alleviating the shape defect of ΔwaaL cells. Indeed, overexpression of murA reversed the morphological defects of ΔwaaL cells (Fig. 4), though the reversal was not quite as effective as that after uppS overexpression (discussed above). MurA activity is feedback inhibited by UDP–N-acetylmuramic acid, the product of the second step in PG synthesis (45). This regulatory circuit probably limited the amount of UDP-GlcNAc that could be diverted into the PG pathway and, thus, the extent of shape recovery. (Feedback inhibition of MurA presumably ensures that sufficient levels of UDP-GlcNAc are available for synthesizing other products, like O antigen.)
Finally, we employed a third strategy for increasing the amount of Und-P available for PG synthesis in ΔwaaL cells. The glycosyltransferases WecG and WbbL compete for Und-PP–GlcNAc to elongate ECA and O antigen, respectively (18, 42) (Fig. 3A). We reasoned that overexpression of wecG would bolster the available pool of Und-P in the ΔwaaL mutant by diverting more Und-P into the ECA pathway, thereby increasing Und-P turnover and reducing the accumulation of dead-end O-antigen intermediates. As predicted, additional WecG reversed the shape defects of ΔwaaL cells (Fig. 4), consistent with the idea that Und-P was recycled and made available for PG synthesis. Interestingly, leaky expression of wecG from the plasmid was sufficient to suppress the shape defects in ΔwaaL cells (Fig. 4), but further increases in wecG expression caused these cells to grow as longer and longer filaments (e.g., see Fig. S3A and B in the supplemental material; compare the results obtained with 0 μM IPTG to those obtained with 100 μM IPTG). This profound filamentation strongly suggests that the diversion of excessive amounts of Und-PP–GlcNAc into the ECA pathway also sequestered the Und-P pool, thereby inhibiting PG synthesis and cell division (although we cannot completely rule out other indirect effects). We note that overexpression of wecG caused only moderate elongation of wild-type cells (see Fig. S4; compare the results for pwecG to those for the vector only), an effect not nearly as profound as that seen in the ΔwaaL mutant (see Fig. S3A and B). Also, with the exception of wecG, overexpression of other genes had no effect on the morphology of wild-type cells (see Fig. S4).
In sum, the preceding results reinforce the hypothesis that the general accumulation of Und-PP-linked intermediates indirectly impairs PG synthesis, most likely by limiting the availability of Und-P.
Disruptions in LPS core biosynthesis sequester Und-P.
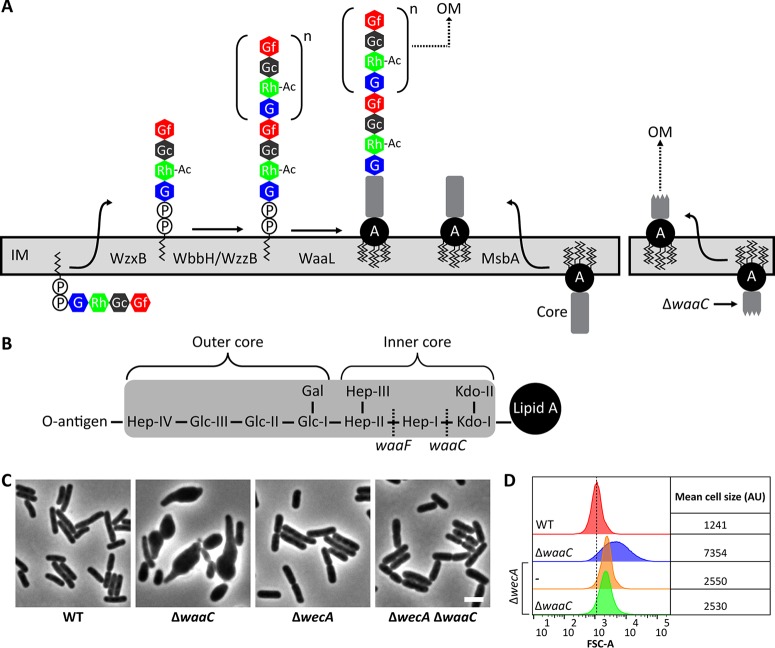
During LPS assembly, the WaaL ligase connects O-antigen subunits to the core oligosaccharide (Fig. 5A) (reviewed in reference 46). Thus, in cells producing O antigen, any mutation that produces an incomplete core oligosaccharide should also accumulate dead-end Und-PP-linked O-antigen intermediates (Fig. 5A). Because the PG and O-antigen biosynthetic pathways compete for Und-P, the sequestration of Und-P in this way should impair PG synthesis and produce cell shape defects in mutants that lack a complete core oligosaccharide. To test this idea, we deleted waaC and waaF from MG1655 wbbL+. WaaC and WaaF add the first and second heptose residues, respectively, to the Kdo-2 moiety of the LPS inner core (Fig. 5B) (47, 48). The loss of WaaC or WaaF caused cells to swell, usually beginning at one end, so that the cells adopted a shape resembling a bowling pin or tadpole (Fig. 5C; see also Fig. S5 in the supplemental material). These defects were distinct from those observed in ΔwzxB or ΔwaaL cells (Fig. 2B; see also Fig. S1A). Three lines of evidence indicated that these morphological changes were probably due to Und-P sequestration. First and foremost, preventing the synthesis of O antigen by deleting wecA suppressed the shape defects in ΔwaaC cells (Fig. 5C and D) and ΔwaaF cells (see Fig. S5). Second, increasing the pool of Und-P by overexpressing uppS reversed the shape defects of ΔwaaC cells (Fig. 6). Third, restricting the biosynthesis of O antigen either by increasing the flux of UDP-GlcNAc into the PG synthesis pathway (by overexpressing murA) or by redirecting Und-PP–GlcNAc to the ECA pathway (by overexpressing wecG) returned ΔwaaC cells to a nearly normal wild-type morphology (Fig. 6). These three results were identical to those obtained by the approach in which the morphological effects exhibited by mutants in the O-antigen biosynthetic pathway were suppressed, as discussed above. Similarly, the suppression of shape defects in the ΔwaaC mutant paralleled the suppression of defects observed in the ΔwaaL mutant. In particular, overexpression of murA suppressed but did not fully restore the wild-type morphology to ΔwaaC cells (Fig. 6), and expression of wecG, too, highly induced cell filamentation (see Fig. S3C and D). We note, though, that we do not understand why mutants lacking Wzx, WaaL, or WaaC differ from one another in morphology. Presumably, these mutations have comparable effects on the pool of Und-P, and therefore, the mutants should have similar phenotypes. The fact that their phenotypes differ suggests that additional factors are at play. Collectively, the data demonstrate that shape defects associated with disruptions of the LPS core are mostly likely caused not by the loss of LPS per se but are instead indirect effects caused by the sequestration of Und-P and interference with PG synthesis.
FIG 5.
Disrupting LPS core biosynthesis induces morphological defects in E. coli. (A) Maturation of Und-PP-linked O-antigen intermediates and their ligation to the lipid A core. Mutations that truncate the core oligosaccharide (e.g., ΔwaaC) prevent attachment of the O antigen (right). Abbreviations: OM, outer membrane; A, lipid A. The definitions of the other abbreviations are listed in the legend to Fig. 2. (B) General structure of the E. coli K-12 core oligosaccharide (shaded in gray) (reviewed in reference 77). WaaC and WaaF transfer Hep-I and Hep-II, respectively, onto the growing O-antigen chain. (C) Micrographs of cells with the indicated genotypes. Cells were grown and imaged as described in the legend to Fig. 2. The cells were fixed and photographed by phase-contrast microscopy. Bar, 3 μm. Similar results were obtained for mutants of waaF, as shown in Fig. S5 in the supplemental material. (D) Flow cytometry data from live cells in panel C. Histograms of the FSC-A from 100,000 events (cells) are shown. The mean cell size of the wild-type strain (red graph) is represented by the dashed line and is expressed in arbitrary units (AU). Data are representative of those from two independent experiments. The strains tested were MAJ330 (wild type), MAJ374 (ΔwaaC), MAJ343 (ΔwecA), and MAJ384 (ΔwecA ΔwaaC).
FIG 6.
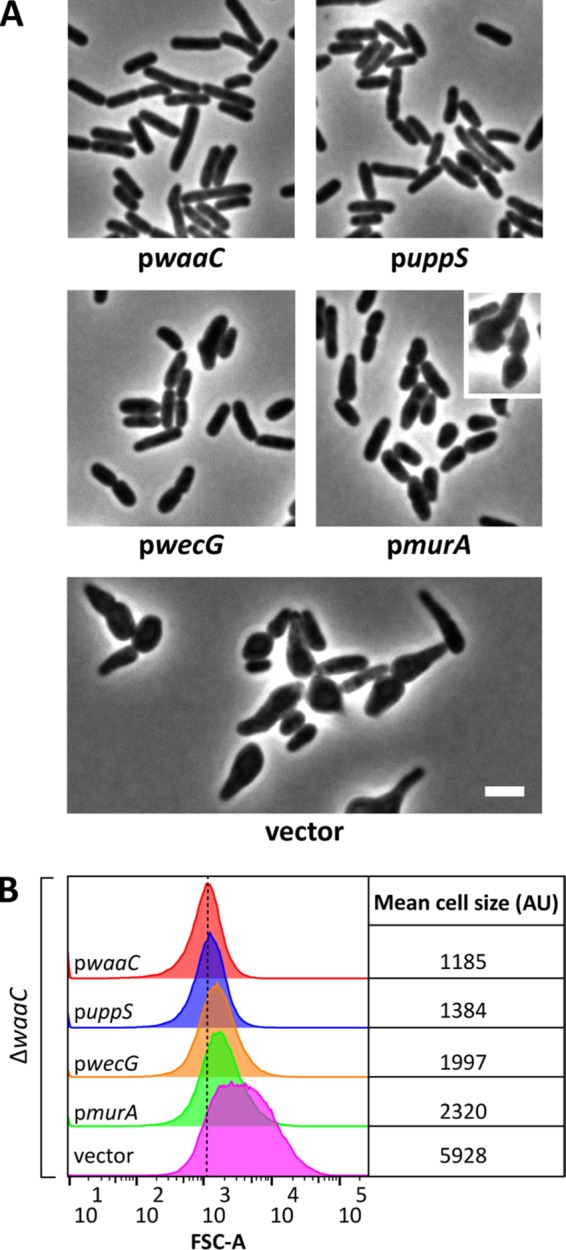
Suppression of ΔwaaC shape defects. (A) Micrographs of ΔwaaC cells containing derivatives of pDSW361 that express the indicated genes. Cells were grown and imaged as described in the legend to Fig. 4, except that IPTG was added to 25 μM for the pwecG strain. Overexpression of murA does not fully suppress the shape defects of ΔwaaC cells (e.g., pmurA cells, inset). Bar, 3 μm. (B) Flow cytometry data from live cells in panel A. Histograms of the forward scatter area from 100,000 events (cells) are shown. The mean cell size area for ΔwaaC cells expressing waaC in trans (red graph) is represented by the dashed line and is expressed in arbitrary units (AU). Data are representative of those from two independent experiments. The strains tested were MAJ439 (pwaaC), MAJ442 (puppS), MAJ441 (pwecG), MAJ440 (pmurA), and MAJ438 (vector).
DISCUSSION
Und-P and its variants are universal lipid carriers employed by all bacteria for synthesizing numerous glycan polymers (reviewed in reference 1). Because it frequently serves as an intermediate in multiple synthetic pathways, Und-P unites what are otherwise biochemically and functionally diverse oligosaccharides. Consequently, disruptions of one Und-P-dependent pathway may have indirect effects on the others. Here, we demonstrate that disruption of the biogenesis of the E. coli O antigen compromises PG synthesis by sequestering Und-P. We conclude that the O-antigen and PG synthetic pathways compete for a common pool of Und-P (Fig. 7). We further posit that because of this competition, the phenotypes exhibited by many previous O-antigen and LPS mutants may not accurately reflect the physiological functions of these cellular components. Instead, because Und-P will be sequestered, the behaviors of these mutants may represent an unknown combination of contributing factors, including those caused by defects in cell wall synthesis. Of course, these considerations would not apply to most mutations in E. coli K-12, which does not synthesize O antigen.
FIG 7.
Model of competition for Und-P in E. coli. The PG, ECA, and O-antigen biosynthesis pathways compete for pools of Und-P (red) and UDP-GlcNAc (blue). Interruption of the biosynthesis of ECA, O antigen, or the lipid A core sequesters part of the pool of Und-P and therefore restricts its availability for PG synthesis. Note that the colanic acid biosynthesis pathway, which also utilizes Und-P, is omitted from this illustration because this compound is not appreciably produced under normal growth conditions, though it is highly expressed during stress. Abbreviations: PG, peptidoglycan; UDP-M5, UDP–N-acetylmuramic acid–l-alanine–d-glutamate–meso-diaminopimelic acid–d-alanine–d-alanine; Und-P, undecaprenyl phosphate; GlcNAc, N-acetylglucosamine; Und-PP, undecaprenyl pyrophosphate; ECA, enterobacterial common antigen; LPS, lipopolysaccharide.
Mutations that disrupt LPS biosynthesis sequester Und-P.
To our knowledge, the present study describes the first controlled experiments to examine the secondary effects of Und-P sequestration as it relates to LPS biosynthesis. However, several lines of independent evidence corroborate the results reported here. One of the best examples centers on the study of the O-antigen flippase, WzxB. The loss of WzxB causes the accumulation of Und-PP-linked O-antigen intermediates (49), which in turn limits the availability of Und-P for PG synthesis. Because of this, wzxB mutants are notoriously difficult to isolate (38, 49, 50) and wzxB mutations are deleterious in the presence of a fully functional O-antigen-producing machinery (51, 52). We recreated both of these circumstances in the present study, and in both instances the accumulation of Und-PP-linked O-antigen intermediates induced morphological defects that were reversed by eliminating the production of O antigen. Thus, it is not surprising that wzxB flippase mutants acquire suppressing mutations that prevent the formation of O-antigen intermediates (38). Indeed, investigators who study WzxB function in Salmonella enterica do so in a genetic background where expression of O antigen is controlled by a galE mutation, which removes the first committed step in the biosynthetic pathway so that no Und-PP-linked O-antigen intermediates can accumulate until galactose is supplied (49, 51, 52). The deleterious effects of Und-P sequestration have also been noted for other transport mutants (53, 54), as well as mutants that are unable to ligate O-antigen intermediates to the lipid A core (24) or that truncate the core oligosaccharide (55). In toto, then, our LPS and O-antigen findings are consistent with the conditional phenotypes that Und-P sequestration produces in strains with mutations that disrupt the synthesis of wall teichoic acids (56, 57), capsular polysaccharides (58–60), exopolysaccharide (61), and ECA (30, 62).
The present results, especially when combined with previous observations (29, 30), strongly suggest that the accumulation of any Und-PP-linked intermediate interferes with PG synthesis. This possibility, which we consider highly probable, has significant implications for past and future experiments that explore the physiological relevance of any glycan polymer whose synthesis requires a Und-P intermediate. Here, we point out that this consideration is of special concern with regard to understanding the physiological roles played by LPS and O antigen. Many experiments have investigated the biological functions of these compounds by studying mutants which we now predict also accumulate dead-end O-antigen intermediates. It is very likely that the phenotypes of such mutants represent a combination of behaviors: those caused by the loss of LPS or O antigen plus those caused by secondary defects due to Und-P sequestration. A further complication is that one of these secondary effects, impaired cell wall synthesis, itself induces widespread cell envelope stress responses (63–65). Thus, the phenotypes exhibited by such mutants are also contaminated by stress behaviors. In short, mutants that accumulate Und-PP-linked intermediates probably exhibit phenotypes with a complicated mixture of causes, many of which are unrelated to the direct functions of LPS or O antigen. More broadly, we wish to highlight the danger of drawing conclusions about the physiological function of any compound or cellular component on the basis of the behavior of mutants that accumulate similar Und-PP-linked intermediates (e.g., see references 30 and 66).
Finally, to be clear, we note that Und-P sequestration does not explain all previous phenotypes associated with disruptions in the biosynthesis of LPS or O antigen. For example, the absence of LpxL (formerly HtrB), a lauroyl acyltransferase required for the maturation of Kdo-2–lipid A (67), induces cell shape defects in E. coli, even though that mutant does not produce O antigen (68). Similarly, depletion of LPS transport proteins induces cell shape defects in the absence of O antigen (69). Und-PP-linked O-antigen intermediates are not present in either of these cases, so no Und-P should be sequestered. These results, along with many others, clearly indicate that LPS plays a distinct physiological role. What we are urging here is that the mutants to be studied must be carefully selected to avoid unnecessary confusion in interpreting the results of similar experiments.
Evidence for relaxed substrate specificity or reversible reactions.
As mentioned earlier, loss of the O-antigen flippase, WzxB, is extremely deleterious, with the results including gross morphological changes and lysis (Fig. 2B; see also Fig. S1A in the supplemental material). These effects are much greater than those in strains with mutations that disrupt earlier steps in the O-antigen biosynthetic pathway (Fig. 3B; see also Fig. S1A). Interestingly, WzxB can translocate different repeat units (70, 71), and under certain conditions, mutants lacking WzxB express LPS linked to O units (51, 62), suggesting that other Wzx translocases exhibit substrate cross-reactivity. This seemingly relaxed substrate specificity may mean that more than one Und-PP-linked intermediate accumulates in a wzxB mutant. If so, then PG synthesis may be compromised to a greater degree than if WzxB flipped O-antigen intermediates exclusively, which may explain why a wzxB mutation is so deleterious. Alternatively, the reaction catalyzed by WbbI may be the first irreversible reaction during elongation of O-antigen intermediates. Thus, any mutation before or at the WbbI step (Fig. 2) would accumulate fewer Und-PP-linked intermediates than it would if the reactions were not reversible. That O-antigen glycosyltransferase reactions might be reversible would not be too surprising, given that initiation of O-antigen synthesis by WbaP (a WecA homologue) is reversible in Salmonella enterica serovar Typhimurium (72). In summary, the absence of reaction reversibility could explain why disruptions in WzxB and later steps are so deleterious.
Is the level of Und-P defined?
Competition for Und-P among a variety of biochemical pathways raises the interesting question of whether the steady-state levels of Und-P can be changed. In fact, increasing the amount of cellular Und-P would seem to be the simplest way to bypass the deleterious consequences of any dead-end intermediates, as we show in principle by producing more UppS, which reverses the effects of Und-P sequestration. The answer was partially uncovered by Barreteau et al., who determined that the intracellular levels of Und-PP and Und-P are quite similar between E. coli and S. aureus (73). It is remarkable that the basal levels of these C55-isoprenoid pools have remained relatively unchanged between the two organisms, given that the two have such different amounts of PG (reviewed in reference 74) and that the two organisms diverged some 2 billion years ago (75). Why have the relative amounts of this lipid carrier remained unchanged in these very different organisms? One possibility is that an overabundance of membrane-associated polyisoprenols disrupts the architecture of the typical phospholipid bilayer (reviewed in reference 76). If so, then the conserved levels of bacterial Und-P may represent an evolutionary compromise that balances glycan biosynthesis with biophysical realities. This pressure to maintain a constant pool of Und-P might play an important role in determining the number of Und-P-dependent pathways that can be accommodated in any one organism. Because PG is essential, this structure may be the foundation around which all other Und-P-consuming pathways must be organized and delimited.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Weiss for plasmid pDSW361. We thank Andrea Harris of the UAMS Flow Cytometry Core Facility for help with flow cytometry and analysis and Allen Gies of the UAMS DNA Sequencing Core Facility for help with DNA sequencing.
Research reported in this publication was supported by National Institute of General Medical Sciences award number GM061019.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00550-16.
REFERENCES
- 1.Tytgat HL, Lebeer S. 2014. The sweet tooth of bacteria: common themes in bacterial glycoconjugates. Microbiol Mol Biol Rev 78:372–417. doi: 10.1128/MMBR.00007-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Ghachi M, Bouhss A, Blanot D, Mengin-Lecreulx D. 2004. The bacA gene of Escherichia coli encodes an undecaprenyl pyrophosphate phosphatase activity. J Biol Chem 279:30106–30113. doi: 10.1074/jbc.M401701200. [DOI] [PubMed] [Google Scholar]
- 3.Touz ET, Mengin-Lecreulx D. September 2008, posting date. Undecaprenyl phosphate synthesis. EcoSal Plus 2008 doi: 10.1128/ecosalplus.4.7.1.7. [DOI] [PubMed] [Google Scholar]
- 4.Kaur D, Brennan PJ, Crick DC. 2004. Decaprenyl diphosphate synthesis in Mycobacterium tuberculosis. J Bacteriol 186:7564–7570. doi: 10.1128/JB.186.22.7564-7570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grover S, Alderwick LJ, Mishra AK, Krumbach K, Marienhagen J, Eggeling L, Bhatt A, Besra GS. 2014. Benzothiazinones mediate killing of Corynebacterineae by blocking decaprenyl phosphate recycling involved in cell wall biosynthesis. J Biol Chem 289:6177–6187. doi: 10.1074/jbc.M113.522623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii K, Sagami H, Ogura K. 1986. A novel prenyltransferase from Paracoccus denitrificans. Biochem J 233:773–777. doi: 10.1042/bj2330773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higashi Y, Strominger JL, Sweeley CC. 1967. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A 57:1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umbreit JN, Strominger JL. 1972. Isolation of the lipid intermediate in peptidoglycan biosynthesis from Escherichia coli. J Bacteriol 112:1306–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkinson RJ, Hussey H, Baddiley J. 1971. Shared lipid phosphate carrier in the biosynthesis of teichoic acid and peptidoglycan. Nat New Biol 229:57–59. doi: 10.1038/newbio229057a0. [DOI] [PubMed] [Google Scholar]
- 10.Rick PD, Hubbard GL, Kitaoka M, Nagaki H, Kinoshita T, Dowd S, Simplaceanu V, Ho C. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8:557–567. doi: 10.1093/glycob/8.6.557. [DOI] [PubMed] [Google Scholar]
- 11.Wright A, Dankert M, Fennessey P, Robbins PW. 1967. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A 57:1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troy FA, Frerman FE, Heath EC. 1971. The biosynthesis of capsular polysaccharide in Aerobacter aerogenes. J Biol Chem 246:118–133. [PubMed] [Google Scholar]
- 13.Schmid J, Sieber V, Rehm B. 2015. Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 6:496. doi: 10.3389/fmicb.2015.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissborn AC, Rumley MK, Kennedy EP. 1991. Biosynthesis of membrane-derived oligosaccharides. Membrane-bound glucosyltransferase system from Escherichia coli requires polyprenyl phosphate. J Biol Chem 266:8062–8067. [PubMed] [Google Scholar]
- 15.Guan S, Bastin DA, Verma NK. 1999. Functional analysis of the O antigen glucosylation gene cluster of Shigella flexneri bacteriophage SfX. Microbiology 145(Pt 5):1263–1273. doi: 10.1099/13500872-145-5-1263. [DOI] [PubMed] [Google Scholar]
- 16.Trent MS, Ribeiro AA, Doerrler WT, Lin S, Cotter RJ, Raetz CR. 2001. Accumulation of a polyisoprene-linked amino sugar in polymyxin-resistant Salmonella typhimurium and Escherichia coli: structural characterization and transfer to lipid A in the periplasm. J Biol Chem 276:43132–43144. doi: 10.1074/jbc.M106962200. [DOI] [PubMed] [Google Scholar]
- 17.Feldman MF, Wacker M, Hernandez M, Hitchen PG, Marolda CL, Kowarik M, Morris HR, Dell A, Valvano MA, Aebi M. 2005. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc Natl Acad Sci U S A 102:3016–3021. doi: 10.1073/pnas.0500044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier-Dieter U, Starman R, Barr K, Mayer H, Rick PD. 1990. Biosynthesis of enterobacterial common antigen in Escherichia coli. Biochemical characterization of Tn10 insertion mutants defective in enterobacterial common antigen synthesis. J Biol Chem 265:13490–13497. [PubMed] [Google Scholar]
- 19.Samuel G, Reeves P. 2003. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr Res 338:2503–2519. doi: 10.1016/j.carres.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Whitfield C. 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 21.Brown S, Santa Maria JP Jr, Walker S. 2013. Wall teichoic acids of gram-positive bacteria. Annu Rev Microbiol 67:313–336. doi: 10.1146/annurev-micro-092412-155620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Typas A, Banzhaf M, Gross CA, Vollmer W. 2012. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol 10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanyal S, Menon AK. 2009. Flipping lipids: why an' what's the reason for? ACS Chem Biol 4:895–909. doi: 10.1021/cb900163d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatar LD, Marolda CL, Polischuk AN, van Leeuwen D, Valvano MA. 2007. An Escherichia coli undecaprenyl-pyrophosphate phosphatase implicated in undecaprenyl phosphate recycling. Microbiology 153:2518–2529. doi: 10.1099/mic.0.2007/006312-0. [DOI] [PubMed] [Google Scholar]
- 25.Yuasa R, Levinthal M, Nikaido H. 1969. Biosynthesis of cell wall lipopolysaccharide in mutants of Salmonella. V. A mutant of Salmonella typhimurium defective in the synthesis of cytidine diphosphoabequose. J Bacteriol 100:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glucksmann MA, Reuber TL, Walker GC. 1993. Genes needed for the modification, polymerization, export, and processing of succinoglycan by Rhizobium meliloti: a model for succinoglycan biosynthesis. J Bacteriol 175:7045–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhavsar AP, Beveridge TJ, Brown ED. 2001. Precise deletion of tagD and controlled depletion of its product, glycerol 3-phosphate cytidylyltransferase, leads to irregular morphology and lysis of Bacillus subtilis grown at physiological temperature. J Bacteriol 183:6688–6693. doi: 10.1128/JB.183.22.6688-6693.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rick PD, Barr K, Sankaran K, Kajimura J, Rush JS, Waechter CJ. 2003. Evidence that the wzxE gene of Escherichia coli K-12 encodes a protein involved in the transbilayer movement of a trisaccharide-lipid intermediate in the assembly of enterobacterial common antigen. J Biol Chem 278:16534–16542. doi: 10.1074/jbc.M301750200. [DOI] [PubMed] [Google Scholar]
- 29.Farha MA, Czarny TL, Myers CL, Worrall LJ, French S, Conrady DG, Wang Y, Oldfield E, Strynadka NC, Brown ED. 2015. Antagonism screen for inhibitors of bacterial cell wall biogenesis uncovers an inhibitor of undecaprenyl diphosphate synthase. Proc Natl Acad Sci U S A 112:11048–11053. doi: 10.1073/pnas.1511751112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jorgenson MA, Kannan S, Laubacher ME, Young KD. 2016. Dead-end intermediates in the enterobacterial common antigen pathway induce morphological defects in Escherichia coli by competing for undecaprenyl phosphate. Mol Microbiol 100:1–14. doi: 10.1111/mmi.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rendueles O, Beloin C, Latour-Lambert P, Ghigo JM. 2014. A new biofilm-associated colicin with increased efficiency against biofilm bacteria. ISME J 8:1275–1288. doi: 10.1038/ismej.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- 34.Weiss DS, Chen JC, Ghigo JM, Boyd D, Beckwith J. 1999. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J Bacteriol 181:508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Rudd KE. 2013. EcoGene 3.0. Nucleic Acids Res 41:D613–D624. doi: 10.1093/nar/gks1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega DE, Young KD. 2014. Accumulation of periplasmic enterobactin impairs the growth and morphology of Escherichia coli tolC mutants. Mol Microbiol 91:508–521. doi: 10.1111/mmi.12473. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Reeves PR. 1994. Escherichia coli K12 regains its O antigen. Microbiology 140(Pt 1):49–57. [DOI] [PubMed] [Google Scholar]
- 38.Burrows LL, Lam JS. 1999. Effect of wzx (rfbX) mutations on A-band and B-band lipopolysaccharide biosynthesis in Pseudomonas aeruginosa O5. J Bacteriol 181:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawamura T, Kimura M, Yamamori S, Ito E. 1978. Enzymatic formation of uridine diphosphate N-acetyl-d-mannosamine. J Biol Chem 253:3595–3601. [PubMed] [Google Scholar]
- 40.Sala RF, Morgan PM, Tanner ME. 1996. Enzymatic formation and release of a stable glycal intermediate: the mechanism of the reaction catalyzed by UDP-N-acetylglucosamine 2-epimerase. J Am Chem Soc 118:3033–3034. doi: 10.1021/ja960266z. [DOI] [Google Scholar]
- 41.Meier-Dieter U, Barr K, Starman R, Hatch L, Rick PD. 1992. Nucleotide sequence of the Escherichia coli rfe gene involved in the synthesis of enterobacterial common antigen. Molecular cloning of the rfe-rff gene cluster. J Biol Chem 267:746–753. [PubMed] [Google Scholar]
- 42.Stevenson G, Neal B, Liu D, Hobbs M, Packer NH, Batley M, Redmond JW, Lindquist L, Reeves P. 1994. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J Bacteriol 176:4144–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenutz R, Weintraub A, Widmalm G. 2006. The structures of Escherichia coli O-polysaccharide antigens. FEMS Microbiol Rev 30:382–403. doi: 10.1111/j.1574-6976.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- 44.Marquardt JL, Siegele DA, Kolter R, Walsh CT. 1992. Cloning and sequencing of Escherichia coli murZ and purification of its product, a UDP-N-acetylglucosamine enolpyruvyl transferase. J Bacteriol 174:5748–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizyed S, Oddone A, Byczynski B, Hughes DW, Berti PJ. 2005. UDP-N-acetylmuramic acid (UDP-MurNAc) is a potent inhibitor of MurA (enolpyruvyl-UDP-GlcNAc synthase). Biochemistry 44:4011–4017. doi: 10.1021/bi047704w. [DOI] [PubMed] [Google Scholar]
- 46.Whitfield C, Trent MS. 2014. Biosynthesis and export of bacterial lipopolysaccharides. Annu Rev Biochem 83:99–128. doi: 10.1146/annurev-biochem-060713-035600. [DOI] [PubMed] [Google Scholar]
- 47.Kadrmas JL, Raetz CR. 1998. Enzymatic synthesis of lipopolysaccharide in Escherichia coli. Purification and properties of heptosyltransferase I. J Biol Chem 273:2799–2807. [DOI] [PubMed] [Google Scholar]
- 48.Gronow S, Brabetz W, Brade H. 2000. Comparative functional characterization in vitro of heptosyltransferase I (WaaC) and II (WaaF) from Escherichia coli. Eur J Biochem 267:6602–6611. doi: 10.1046/j.1432-1327.2000.01754.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu D, Cole RA, Reeves PR. 1996. An O-antigen processing function for Wzx (RfbX): a promising candidate for O-unit flippase. J Bacteriol 178:2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnaitman CA, Klena JD. 1993. Genetics of lipopolysaccharide biosynthesis in enteric bacteria. Microbiol Rev 57:655–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong Y, Cunneen MM, Reeves PR. 2012. The Wzx translocases for Salmonella enterica O-antigen processing have unexpected serotype specificity. Mol Microbiol 84:620–630. doi: 10.1111/j.1365-2958.2012.08048.x. [DOI] [PubMed] [Google Scholar]
- 52.Liu MA, Stent TL, Hong Y, Reeves PR. 2015. Inefficient translocation of a truncated O unit by a Salmonella Wzx affects both O-antigen production and cell growth. FEMS Microbiol Lett 362:fnv053. doi: 10.1093/femsle/fnv053. [DOI] [PubMed] [Google Scholar]
- 53.Clarke BR, Cuthbertson L, Whitfield C. 2004. Nonreducing terminal modifications determine the chain length of polymannose O antigens of Escherichia coli and couple chain termination to polymer export via an ATP-binding cassette transporter. J Biol Chem 279:35709–35718. doi: 10.1074/jbc.M404738200. [DOI] [PubMed] [Google Scholar]
- 54.Cuthbertson L, Powers J, Whitfield C. 2005. The C-terminal domain of the nucleotide-binding domain protein Wzt determines substrate specificity in the ATP-binding cassette transporter for the lipopolysaccharide O-antigens in Escherichia coli serotypes O8 and O9a. J Biol Chem 280:30310–30319. doi: 10.1074/jbc.M504371200. [DOI] [PubMed] [Google Scholar]
- 55.Cabeen MT, Murolo MA, Briegel A, Bui NK, Vollmer W, Ausmees N, Jensen GJ, Jacobs-Wagner C. 2010. Mutations in the lipopolysaccharide biosynthesis pathway interfere with crescentin-mediated cell curvature in Caulobacter crescentus. J Bacteriol 192:3368–3378. doi: 10.1128/JB.01371-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D'Elia MA, Pereira MP, Chung YS, Zhao W, Chau A, Kenney TJ, Sulavik MC, Black TA, Brown ED. 2006. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J Bacteriol 188:4183–4189. doi: 10.1128/JB.00197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D'Elia MA, Millar KE, Beveridge TJ, Brown ED. 2006. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J Bacteriol 188:8313–8316. doi: 10.1128/JB.01336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xayarath B, Yother J. 2007. Mutations blocking side chain assembly, polymerization, or transport of a Wzy-dependent Streptococcus pneumoniae capsule are lethal in the absence of suppressor mutations and can affect polymer transfer to the cell wall. J Bacteriol 189:3369–3381. doi: 10.1128/JB.01938-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brimacombe CA, Stevens A, Jun D, Mercer R, Lang AS, Beatty JT. 2013. Quorum-sensing regulation of a capsular polysaccharide receptor for the Rhodobacter capsulatus gene transfer agent (RcGTA). Mol Microbiol 87:802–817. doi: 10.1111/mmi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.James DB, Gupta K, Hauser JR, Yother J. 2013. Biochemical activities of Streptococcus pneumoniae serotype 2 capsular glycosyltransferases and significance of suppressor mutations affecting the initiating glycosyltransferase Cps2E. J Bacteriol 195:5469–5478. doi: 10.1128/JB.00715-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reuber TL, Walker GC. 1993. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74:269–280. doi: 10.1016/0092-8674(93)90418-P. [DOI] [PubMed] [Google Scholar]
- 62.Marolda CL, Tatar LD, Alaimo C, Aebi M, Valvano MA. 2006. Interplay of the Wzx translocase and the corresponding polymerase and chain length regulator proteins in the translocation and periplasmic assembly of lipopolysaccharide O antigen. J Bacteriol 188:5124–5135. doi: 10.1128/JB.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Danese PN, Oliver GR, Barr K, Bowman GD, Rick PD, Silhavy TJ. 1998. Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J Bacteriol 180:5875–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laubacher ME, Ades SE. 2008. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J Bacteriol 190:2065–2074. doi: 10.1128/JB.01740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans KL, Kannan S, Li G, de Pedro MA, Young KD. 2013. Eliminating a set of four penicillin binding proteins triggers the Rcs phosphorelay and Cpx stress responses in Escherichia coli. J Bacteriol 195:4415–4424. doi: 10.1128/JB.00596-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranjit DK, Young KD. 2016. Colanic acid intermediates prevent de novo shape recovery of Escherichia coli spheroplasts, calling into question biological roles previously attributed to colanic acid. J Bacteriol 198:1230–1240. doi: 10.1128/JB.01034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clementz T, Bednarski JJ, Raetz CR. 1996. Function of the htrB high temperature requirement gene of Escherichia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J Biol Chem 271:12095–12102. doi: 10.1074/jbc.271.20.12095. [DOI] [PubMed] [Google Scholar]
- 68.Karow M, Fayet O, Cegielska A, Ziegelhoffer T, Georgopoulos C. 1991. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33 degrees C in rich media. J Bacteriol 173:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braun M, Silhavy TJ. 2002. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol Microbiol 45:1289–1302. doi: 10.1046/j.1365-2958.2002.03091.x. [DOI] [PubMed] [Google Scholar]
- 70.Feldman MF, Marolda CL, Monteiro MA, Perry MB, Parodi AJ, Valvano MA. 1999. The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O antigen assembly is independent of the chemical structure of the O repeat. J Biol Chem 274:35129–35138. doi: 10.1074/jbc.274.49.35129. [DOI] [PubMed] [Google Scholar]
- 71.Marolda CL, Vicarioli J, Valvano MA. 2004. Wzx proteins involved in biosynthesis of O antigen function in association with the first sugar of the O-specific lipopolysaccharide subunit. Microbiology 150:4095–4105. doi: 10.1099/mic.0.27456-0. [DOI] [PubMed] [Google Scholar]
- 72.Osborn MJ, Tze-Yuen RY. 1968. Biosynthesis of bacterial lipopolysaccharide. VII. Enzymatic formation of the first intermediate in biosynthesis of the O-antigen of Salmonella typhimurium. J Biol Chem 243:5145–5152. [PubMed] [Google Scholar]
- 73.Barreteau H, Magnet S, El Ghachi M, Touze T, Arthur M, Mengin-Lecreulx D, Blanot D. 2009. Quantitative high-performance liquid chromatography analysis of the pool levels of undecaprenyl phosphate and its derivatives in bacterial membranes. J Chromatogr B Analyt Technol Biomed Life Sci 877:213–220. doi: 10.1016/j.jchromb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 74.Vollmer W, Blanot D, de Pedro MA. 2008. Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 75.Feng DF, Cho G, Doolittle RF. 1997. Determining divergence times with a protein clock: update and reevaluation. Proc Natl Acad Sci U S A 94:13028–13033. doi: 10.1073/pnas.94.24.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartley MD, Imperiali B. 2012. At the membrane frontier: a prospectus on the remarkable evolutionary conservation of polyprenols and polyprenyl-phosphates. Arch Biochem Biophys 517:83–97. doi: 10.1016/j.abb.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raetz CR, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.