Abstract
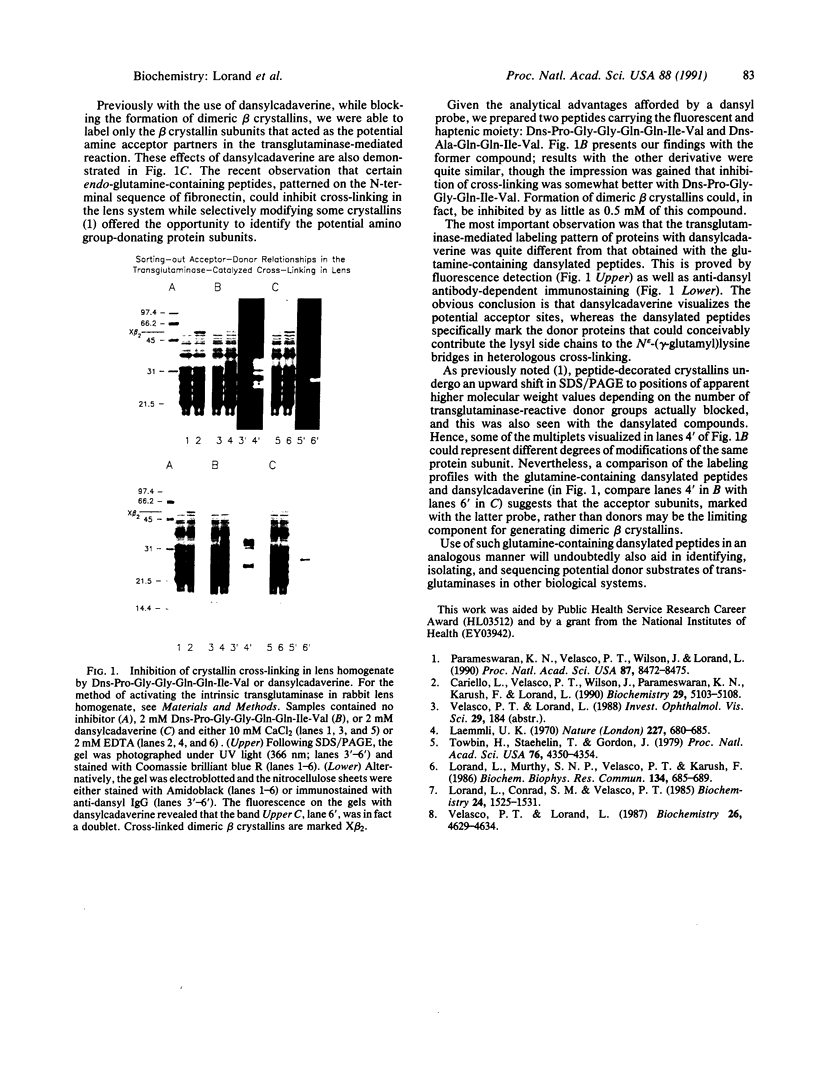
The dansyl-conjugated (Dns) peptides Dns-Pro-Gly-Gly-Gln-Gln-Ile-Val and Dns-Ala-Gln-Gln-Ile-Val, patterned on the N-terminal sequence of fibronectin, were synthesized and used for the transglutaminase (protein-glutamine:amine gamma-glutamyltransferase, EC 2.3.2.13)-directed selective blocking of lens proteins that otherwise might participate in donating lysyl side chains in forming N epsilon-(gamma-glutamyl)-lysine cross-linked oligomers and polymers. Labeling profiles with these peptides could be readily visualized by fluorescence as well as by immunoblotting with anti-dansyl antibody. The labeling patterns in rabbit lens homogenates were quite different with the dansylated peptides than those obtained with dansylcadaverine. Use of such glutamine-containing dansylated peptides should clearly aid in identifying, isolating, and sequencing potential donor substrates of transglutaminases in many biological systems.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cariello L., Velasco P. T., Wilson J., Parameswaran K. N., Karush F., Lorand L. Probing the transglutaminase-mediated, posttranslational modification of proteins during development. Biochemistry. 1990 May 29;29(21):5103–5108. doi: 10.1021/bi00473a015. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M., Velasco P. T. Formation of a 55 000-weight cross-linked beta crystallin dimer in the Ca2+-treated lens. A model for cataract. Biochemistry. 1985 Mar 12;24(6):1525–1531. doi: 10.1021/bi00327a035. [DOI] [PubMed] [Google Scholar]
- Lorand L., Murthy S. N., Velasco P. T., Karush F. Identification of transglutaminase substrates in inside-out vesicles from human erythrocytes: immunoblotting with anti-dansyl antibody. Biochem Biophys Res Commun. 1986 Jan 29;134(2):685–689. doi: 10.1016/s0006-291x(86)80474-0. [DOI] [PubMed] [Google Scholar]
- Parameswaran K. N., Velasco P. T., Wilson J., Lorand L. Labeling of epsilon-lysine crosslinking sites in proteins with peptide substrates of factor XIIIa and transglutaminase. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8472–8475. doi: 10.1073/pnas.87.21.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco P. T., Lorand L. Acceptor-donor relationships in the transglutaminase-mediated cross-linking of lens beta-crystallin subunits. Biochemistry. 1987 Jul 28;26(15):4629–4634. doi: 10.1021/bi00389a006. [DOI] [PubMed] [Google Scholar]