Abstract
Caretakers and clinicians alike have long recognized that individuals with autism spectrum disorder (ASD) can have altered sensory processing, which can contribute to its core symptoms. However, the pathobiology of sensory alterations in ASD is poorly understood. Here we examined nocifensive behavior in ASD mouse models, the BTBR T+Itpr3tf/J (BTBR) and the fragile-X mental retardation-1 knockout (Fmr1-KO) mice. We also examined the effects of nicotine on nocifensive behavior given that nicotine, a nicotinic cholinergic receptor (nAChR) agonist that has antinociceptive effects and was shown to improve social deficits and decrease repetitive behaviors in BTBR mice. Compared to respective controls, both BTBR and Fmr1-KO had hyporesponsiveness to noxious thermal stimuli and electrical stimulation of C-sensory fibers, normal responsiveness to electrical stimulation of Aβ- and Aδ-fiber, and hyperresponsiveness to visceral pain after acetic acid intraperitoneal injection. In BTBR, nicotine at lower doses increased, whereas at higher doses, it decreased hotplate latency compared to vehicle. In a significantly different effect pattern, in control mice, nicotine had antinociceptive effects to noxious heat only at the high dose. Interestingly, these nocifensive behavior alterations and differential responses to nicotine antinociceptive effects in BTBR mice were associated with significant downregulation of α3, α4, α5, α7, β2, β3, and β4 nAChR subunits in several cerebral regions both, during embryonic development and adulthood. Taken together, these findings further implicate nAChRs in behaviors alterations in the BTBR model and lend support to the hypothesis that nAChRs may be a target for treatment of behavior deficits and sensory dysfunction in ASD.
Keywords: nicotine, nicotinic, autism, social behavior, nAChR, BTBR, nociception, nocifensive, pain
1. Introduction
Autism spectrum disorder (ASD) includes a group of neurodevelopmental disorders characterized by social communication and interaction deficits and repetitive, restricted patterns of behaviors, activities, or interests (American Psychiatric Association, 2013; Christensen et al., 2016; Constantino and Charman, 2015; Pedersen et al., 2012; Richards et al., 2015). Some of those restricted patterns of behavior or activities can be related to alterations in response to sensory input, as indicated in the diagnostic criteria for ASD in the Diagnostic and Statistical Manual of Mental Disorders – 5th edition (DSM-5) (American Psychiatric Association, 2013). Caretakers and clinicians alike have long recognized that individuals with ASD have altered responses to sounds, tastes, texture, smells, and/or apparent indifference to noxious stimuli (American Psychiatric Association, 2013; Ausderau et al., 2014a; Ausderau et al., 2014b; Ausderau et al., 2016; Patten et al., 2013). Studies using standardized scales to evaluate sensory phenotypes show that ASD individuals can display a spectrum of sensory response alterations with three predominant patterns. Hyperresponsiveness, a pattern in which ASD individuals show negative response to mild stimulation (Baranek et al., 2006; Ben-Sasson et al., 2009; Green et al., 2015). A second pattern is one of hyporesponsiveness, in which ASD patients show diminished or absent response to stimuli perceived as noxious or known to inflict pain (Ben-Sasson et al., 2009). A third pattern is that of sensory seeking behavior characterized by the pursuit of repeated exposure to given sensory stimuli. While the most prevalent pattern in ASD is that of hyporesponsiveness (Ben-Sasson et al., 2009), a large number of patients actually present a mixed pattern and simultaneously display hyper- and hyporesponsiveness to sensory stimuli(Baranek et al., 2006). Therefore, while it was only recently listed in the diagnostic criteria, sensory dysfunction in ASD individuals has been broadly described (Ausderau et al.,2014a; Ausderau et al., 2014b; Ausderau et al., 2016; Baum et al., 2015; Green et al., 2015; Patten et al., 2013; Tordjman et al., 2009).
While researchers have extensively reported on qualitative patterns of altered sensory processing in ASD, the neurobiological underpinnings of these alterations remain poorly understood. In a recent study using quantitative sensory testing, researchers showed that high-functioning adolescent with ASD have decreased thermal sensitivity (increased warmth and cold detection threshold) and that their thermal detection thresholds and heat pain thresholds correlated with their intelligence quotients (Duerden et al., 2015). In another study, high functioning ASD children were shown to have increased pain (lower pressure pain thresholds) and touch sensitivities (Von Frey monofilaments) compared to healthy children(Riquelme et al., 2016). Studies using questionnaires to evaluate behavioral responses to sensory stimuli show that in ASD individuals, higher levels of tactile hypo-responsiveness and increased tactile seeking behaviors significantly correlated with increased social impairment and repetitive behaviors (Baker et al., 2008; Foss-Feig et al., 2012; Hilton et al., 2010). Interestingly, others showed that sensory disturbances correlate with severity of ASD-related deficits in children, but not in adolescents or adults (Kern et al., 2007). However, it remains unknown whether these associations between social deficits and sensory dysfunctions are causative or are due to shared underlying pathobiology.
Studies in animal models of ASD have increased our understanding of altered behavior and pain sensitivity in ASD. For example, mice harboring mutations in the Mecp2 gene, a model of human Rett syndrome, a disorder which can be associated with ASD, display heat hyporesponsiveness at baseline and in the setting of inflammatory pain(Downs et al., 2010). In humans, mutations in the SHANK genes can be associated with ASD (Leblond et al., 2012; Leblond et al., 2014) and mice with null mutations of the Shank2 gene have social deficits (Schmeisser et al., 2012; Won et al., 2012) and hyporesponsiveness to mechanical and noxious heat stimuli as well as to inflammatory and neuropathic pain (Ko et al., 2016). Fragile X syndrome, a single gene neurodevelopmental disorder resulting from lack of expression of the mRNA binding protein-encoding the Fmr1 gene, is the leading known genetic cause of autism in humans. Mice with null mutations in the Fmr1 gene (Fmr1-KO), show social deficits (Spencer et al., 2011) and hyporesponsiveness to inflammatory and neuropathic pain (Price et al., 2007). Here, using different modalities of sensory stimulation, we investigated the nocifensive behavior in BTBR T+Itpr3tf/J (BTBR) mice, a well-studied model, which recapitulates core ASD phenotypes (Silverman et al., 2013; Silverman et al., 2015; Silverman et al., 2012; Silverman et al., 2010a) and in the Fmr1-KO mouse(Sorensen et al., 2015; The Dutch-Belgian Fragile, X Consortium., 2013). Additionally, we sought to determine whether nicotine, an agent that modulates behavior deficits would also modulate altered nociception in the BTBR model. As others and we have shown that the nicotinic cholinergic system modulates social deficits in the BTBR model (Karvat and Kimchi, 2014; Wang et al., 2015), we investigated whether nicotine, which is known to have analgesic effects, would also modulate nocifensive behavior in the BTBR mouse.
2. Material and methods
2.1. Animals
The investigational protocol was approved by the Children’s National Health System Institutional Animal Care and Use Committee and conducted in compliance with the Guide for the Care and Use of Laboratory Animals. Breeding pairs of BTBR T+Itpr3tf/J (BTBR) and C57BL/6J (B6, control strain for BTBR) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and bred in our animal facility. The BTBR strain has been extensively studied and is known to model the core symptoms of ASD (Brodkin, 2008; McFarlane et al., 2008; Price et al., 2007; Silverman et al., 2013; Silverman et al., 2015; Silverman et al., 2012; Silverman et al., 2010a; Wang et al., 2015). A balanced number of age-matched (8–22 weeks) male and female mice were included in BTBR and B6 studies. We also examined the nocifensive behavior in males FVB.129P2-Pde6b+ Tyrc-ch Fmr1tm1Cgr/J (Fmr1-KO) (The Dutch-Belgian Fragile, X Consortium., 2013) and FVB.129P2-Pde6b+ Tyrc-ch/AntJ (FVB, control strain for Fmr1-KO), both obtained from the Jackson Laboratory and bred in our animal facility. Male Fmr1-KO mice recapitulate features of human fragile X syndrome, a monogenic disorder associated with ASD, and display social deficits and hyperactivity (Sorensen et al., 2015). Mice of all strains were group housed (up to five/cage) on a 12h light cycle and had unrestricted access to food and water.
2.2. Nicotine trial
We examined the effect of chronic nicotine treatment on nocifensive behavior outcomes in B6 and BTBR mice. After baseline behavior outcome measurements, animals (age-matched male and female) were assigned to receive one of five doses [zero (vehicle), 50, 100, 200, or 400µg/mL, calculated as nicotine free-base] of (−) nicotine (hydrogen tartrate salt, Sigma-Aldrich, St. Louis, MO) in drinking water (Matta et al., 2007). Saccharin (2%, Sigma-Aldrich) was the vehicle for all nicotine solutions (Matta et al., 2007), which were prepared three times/week.
We also examined the effect of nicotine on visceral pain (writhing test, see below) in a different cohort of B6 and BTBR mice. In this cohort, we administered only two doses of nicotine (0 (vehicle) or 100µg/mL) and obtained behavior outcomes (number of writhes) only after treatment as to not repeat acetic acid injections.
2.3. Behavior assays
In order to minimize variability, each category of behavior assay was conducted by the same investigator between 9 AM and 2 PM and always included animals from control and mutant groups. During experiments examining nocifensive behavior, mutant mice and respective controls were examined during each session in order to control for the effect of time. Nocifensive behavior in response to “phasic pain” (Le Bars et al., 2001) was evaluated using thermal and electrical stimuli [hotplate latency, cold plate sensitivity, and current threshold (sensory fiber interrogation) in this order] and to “tonic pain” using the writhing test (Le Bars et al., 2001). Animals undergoing tail flick and the writhing test did not undergo any other nociception assay. Only one of testing paradigms was conducted per day and the investigator conducting quantitative sensory testing was unaware of the animals’ genotype and/or treatment.
2.3.1. Hotplate latency
In order to evaluate nocifensive response to noxious heat, mice were placed on a hotplate (Harvard Apparatus, Holliston, MA) and latency to display of pain-avoiding behaviors (jumping, stomping or repeated lifting or licking of hind or front paws) was measured(Le Bars et al., 2001). The hotplate temperature was chosen according to previous studies of ASD-like mouse models including Fmr1-KO and BTBR mice, which had been performed using a hotplate set at 55°C (Chadman et al., 2008; Ko et al., 2016; Silverman et al., 2010b; Veeraragavan et al., 2012). In order to avoid injury, animals were allowed to stay on the hotplate for a maximum of 30 seconds.
2.3.2. Tail-flick latency
Mice were gently held in a mouse holder and the middle third of the tail was placed over a radiant heat source (Ugo Basile, Varese, Italy) with infrared intensity set at 20%. Latency to tail flick (withdraw) away from the stimulus was measured to the nearest 0.1 s. Withdrawal of the tail stopped the stimulus, the latency to tail flick was recorded automatically, and the cut-off time was set at 15 s. The final tail flick latency was an average of three measurements obtained at least 1 min apart (Le Bars et al., 2001).
2.3.3. Cold Plate Sensitivity
We measured cold plate sensitivity using a Peltier cooler set at 2°C (Harvard Apparatus, Holliston, MA). Mice were observed on the cold plate for 5 minutes and their behavior -recorded. The time animals spent withdrawing from the cold surface (lifting front or hind paws, rubbing of front paws) was recorded with a stopwatch and the total time withdrawing from the cold plate was interpreted as cold plate sensitivity, i.e., the longer the time withdrawing from the cold plate, the higher the cold plate sensitivity (Choi et al., 1994).
2.3.4. Sensory nerve fiber interrogation using sine wave electrical stimulation
In order to evaluate specific somatosensory fibers in models of ASD, we used a sine-wave electrical stimulation paradigm at three frequencies: 5, 250 and 2000 Hz that preferentially stimulate C, Aδ, and Aβ fibers respectively as previously described (Finkel et al., 2012; Finkel et al., 2006; Koga et al., 2005; Spornick et al., 2011). Briefly, electrical stimuli generated by a neurostimulator (Neurotron, Inc, Baltimore, MD) were delivered to the tail of gently restrained mice. Stimuli at different frequencies (5, 250 and 2000 Hz) were delivered at increasing intensities, lasted one second and were set on a 50% duty cycle (each stimulus is followed by a one-second stimulus-free interval). Between stimulations at different frequencies, animals rested for one-minute. Vocalization was the nocifensive behavior outcome and its occurrence prompted termination of the stimulus. For each frequency, the electrical stimulus amperage that elicited audible vocalization or the maximum amperage delivered was defined as current threshold (Finkel et al., 2012; Finkel et al., 2006; Spornick et al., 2011). Current thresholds for each frequency were the average of five consecutive measurements obtained in response to 2000, 250, and 5 Hz sequentially. The current threshold unit of measurement is “unit” (U), which corresponds to 100 times the amperage that elicited audible vocalization.
2.3.5. Writhing test
Mutant mice and respective controls received a single intraperitoneal injection of 0.6% acetic acid diluted in phosphate buffered solution (10µl/g body weight). Animals were video-recorded and abdominal stretching/writhing activity was counted for 30 min following injections as described (Reichert et al., 2001). Mice undergoing the writhing test were euthanized upon completion of the test.
2.4. Nicotinic acetylcholine receptor subunits gene expression during adulthood and embryonic development
Prefrontal cortex, brain cortex, hippocampus, and cerebellum were dissected from adult naïve BTBR, B6, Fmr1-KO, and FVB mice. After anesthesia with isoflurane, animals were exsanguinated via cardiac puncture to avoid mRNA contamination during isolation. Pre-frontal cortex was obtained by slicing the first 2 mm of frontal cortex using a 1mm coronal mouse matrix (Roboz, Gaithersburg, MD), after carefully removing olfactory bulb. We isolated mRNA using a Trizol method with subsequent purification using the Qiagen mini-kit according to manufacturer’s instructions. We used a NanoDrop (Thermo Scientific, Wilmington DE) to quantify mRNA, and 1µg was used to synthesize cDNA (iScript Reverse Transcription Supermix for RT-qPCR, Bio-Rad). Relative gene expressions of nAChR subunits were quantified by realtime polymerase chain reaction using One-Step qRT-PCR Kit in the Abi Prism® 7900 (both from Applied Biosystems, Foster City, CA). Validated TaqMan hydrolysis probes (all from Thermo Scientific) to nAChR subunits (α2, α 3, α4, α5, α6, α7, β2, β3 and β4) were as follows: Mm00460630_m1 (Chrna2), Mm00520145_m1 (Chrna3), Mm00516561_m1 (Chrna4), Mm00616329_m1 (Chrna5), Mm00517529_m1 (Chrna6), Mm01312230_m1 (Chrna7), Mm00515323_m1 (Chrnb2), Mm00532602_m1 (Chrnb3), Mm00804952_m1 (Chrnb4)), and β-actin probe as the reference gene (Mouse ACTB ,#4352663, Applied Biosystems).
For analyses of nAChR expression during embryonic development, male and female BTBR or B6 mice were coupled overnight and separated early the following morning (counted as embryonic day 0.5 (E0.5) if pregnant). At E13, pregnant females (identified by visual inspection) were anesthetized and exsanguinated via cardiac puncture. The uterus was removed and each fetus dissected from its embryonic membranes. Because at this embryonic age it is not possible to recognize individual cerebral areas, all intracranial embryonic material was dissected after carefully removing brain meninges. For each pregnancy, embryonic brains from three to four fetuses were pooled and mRNA was isolated as described above. For each pregnancy, a single fetal pool was analyzed and for each strain, five pregnancies were assayed.
2.5. Statistical analysis
All statistical analyses were performed using STATA 12.0 (StataCorp., College Station, TX). We tested baseline nocifensive behavioral measurement differences using Kruskal-Wallis test or one way ANOVA, as appropriate. In the nicotine trial, we examined the effect of different nicotine doses on each nocifensive behavior outcome using a mixed-effect model. In each model, we introduced interaction terms between time and nicotine dose to assess the effect of treatment on change of outcome from baseline to post-treatment measurements compared to vehicle treatment. We separately examined whether there was a difference of treatment effect comparing BTBR and B6 mice strains by testing the interaction between strain and treatment effect. In all models, the effects of age, weight, or sex were adjusted for when appropriate. Model residuals were inspected and outliers removed for final analyses. P values <0.05 were considered statistically significant. RT-qPCR reactions were quantified and expression ratios (mutant vs. respective controls) were calculated using the Pfaffl method, REST2009 program from Qiagen(Pfaffl et al., 2002).
3. Results
3.1. Nocifensive behavior in BTBR mice
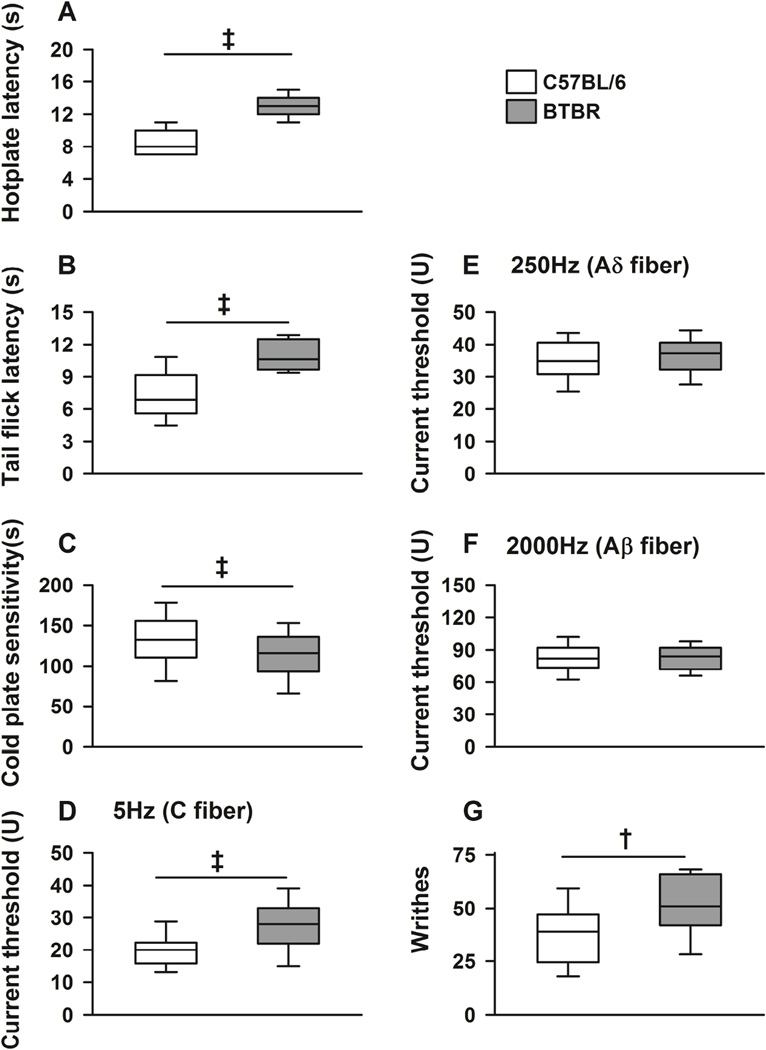
BTBR mice had increased tolerance to noxious heat stimuli as they had higher hotplate and tail flick response latencies (Figure 1A and 1B, χ2 (df) = 107.2 (1), p<0.001) compared to controls (B6) mice. In addition, BTBR mice had increased tolerance to noxious cold stimuli as they displayed lower sensitivity (less time withdrawing from the cold plate) to the cold plate compared to B6 animals (Figure 1C, χ2 (df) = 11.4 (1), p<0.001).
Figure 1. Nocifensive behavior phenotype in C57BL/6 (B6) and BTBR mice.
Box plots show median, interquartile range, and 5th and 95th percentiles for behavior outcomes in B6 (white) and BTBR (gray) mice. P values refer to comparisons of baseline measurement between strains. BTBR mice had higher hotplate (A, p<0.001) and tail flick response latencies (B, p<0.001) compared to controls (B6). C, BTBR mice had lower sensitivity (increased tolerance) to the cold plate (p<0.001) compared to B6 animals. D, BTBR had higher current threshold (increased tolerance) to electrical stimulation at frequencies (5Hz) that stimulate unmyelinated (C-fiber) sensory nerve fibers (p<0.001), but similar current thresholds in response to 250Hz (E, p=0.30) and 2000Hz (F, p=0.70) electrical stimulations, which stimulate Aδ and Aβ fibers respectively, compared to B6 animals. G, in a model of visceral pain (over 30 min after an intraperitoneal injection of 0.6% acetic acid), BTBR mice significantly more writhes compared to B6 animals (p=0.008). Baseline measurements were obtained before any nicotine treatment. Age-matched and balanced numbers of male and female mice were included. N ≥14 (male and female mice) per genotype for each outcome measurement. † indicates p≤0.01, and ‡ p≤0.001.
We then examined the nocifensive response to sine wave electrical stimulation in BTBR mice. Compared to B6 animals, BTBR had increased tolerance to electrical stimulation at the frequency that stimulate unmyelinated C-fibers (5Hz) as they had higher current threshold in response to 5Hz stimulation (Figure 1D, χ2 (df) = 32.2 (1), p<0.001). In contrast, BTBR mice had similar sensitivity to 250Hz and 2000Hz electrical stimulations, which recruit myelinated Aδ and Aβ fibers respectively, compared to B6 animals (Figure 1E and 1F).
We also examined nocifensive behavior in a model of visceral pain. Over 30 min after intraperitoneal injection of 0.6% acetic acid , BTBR mice showed hyperresponsiveness to visceral pain as they had significantly more writhes compared to B6 animals (Figure 1G, χ2 (df) = 6.41 (1), p=0.01).
3.2. Nocifensive behavior in a model of fragile X syndrome, a human disease associated with ASD
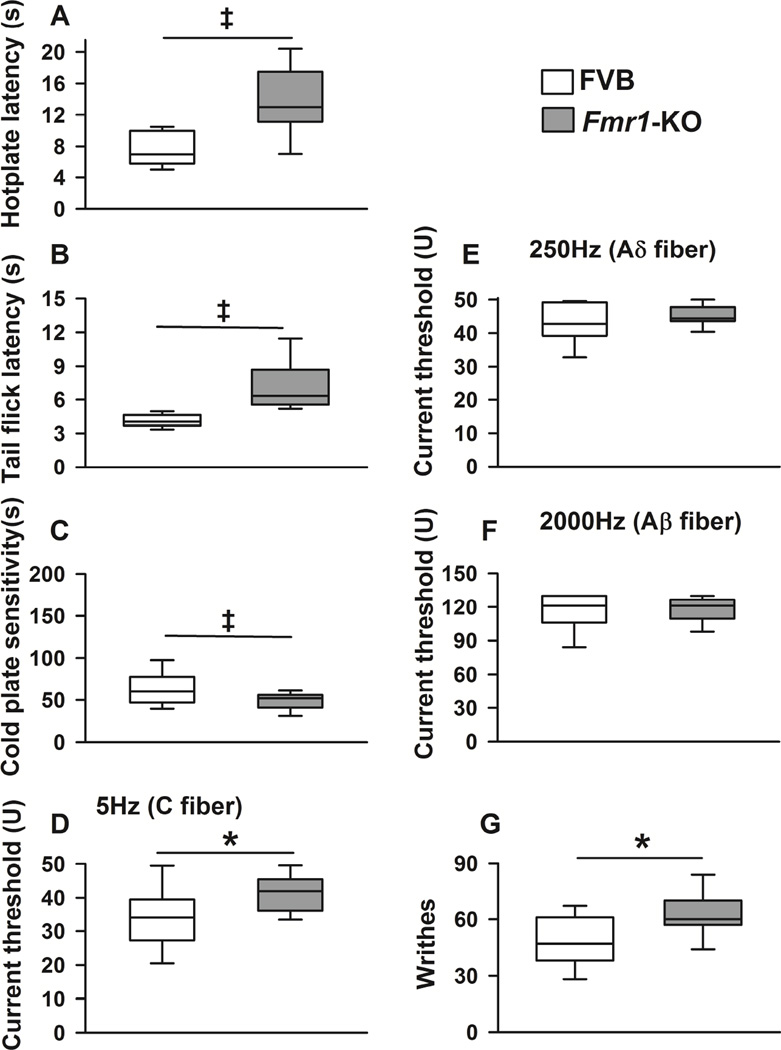
The Fmr1-KO, compared to its control mouse, displayed a pattern of nocifensive behavior similar to the BTBR mouse. Specifically, Fmr1-KO mice had increased tolerance to noxious heat as they had higher hotplate [H (df) = 19.66 (1), p<0.001] and tail flick [H (df) = 10.59 (1), p=0.001] latencies compared to FVB control mice (Figures 2A and 2B). Fmr1-KO mice also had increased tolerance to noxious cold stimuli as they displayed lower sensitivity to the cold plate compared to FVB animals (Figure 2C, F(1, 20) = 86.83, p<0.001).
Figure 2. Nocifensive behavior phenotype in FVB and Fmr1-KO mice.
Box plots show median, interquartile range, and 5th and 95th percentiles for behavior outcomes in FVB and Fmr1-KO mice. P values refer to comparisons of baseline measurement between strains. Fmr1-KO mice had increased tolerance to noxious heat as they had higher hotplate (A) and tail flick (B) latencies compared to FVB control mice (both p≤0.001). C, Fmr1-KO mice had increased tolerance to noxious cold stimuli as they displayed lower sensitivity to the cold plate compared to FVB animals (p=0.001). D, Fmr1-KO mice, compared to FVB animals, had higher current threshold in response to 5Hz stimulation (p=0.02). In contrast, compared to FVB mice, Fmr1-KO animals had similar sensitivity to 250Hz (E, p=0.19) and 2000Hz (F, p=0.85) electrical stimulation, which recruit myelinated Aδ and Aβ fibers respectively. G, Fmr1-KO mice also displayed significantly greater number of writhes after an intraperitoneal injection of 0.6% acetic acid compared to FVB mice (p=0.024). Age-matched males were investigated as only males have the behavior phenotypes of interest. N≥6 mice (males only) per group for each outcome measurement. indicates p<0.05, † indicates p≤0.01, and ‡ p≤0.001.
Regarding response to electrical stimulation, Fmr1-KO mice, compared to FVB animals, had higher current threshold in response to 5Hz stimulation (Figure 2D, F(1, 25) = 6.22, p=0.02), a pattern of hyporesponsiveness similar to that seen in BTBR mice. Further, compared to FVB mice, Fmr1-KO animals had similar sensitivity to 250Hz and 2000Hz (myelinated Aδ and Aβ fibers respectively) electrical stimulation (Figures 2E and 2F, p=0.19 and p=0.85 respectively).
Regarding nocifensive behavior in response to visceral pain, Fmr1-KO displayed significantly more writhes after intraperitoneal injection of 0.6% acetic acid compared to FVB mice (Figure 2G, χ2 (df) = 4.093 (1), p=0.04).
3.3. Differential effect of nicotine comparing B6 and BTBR mice
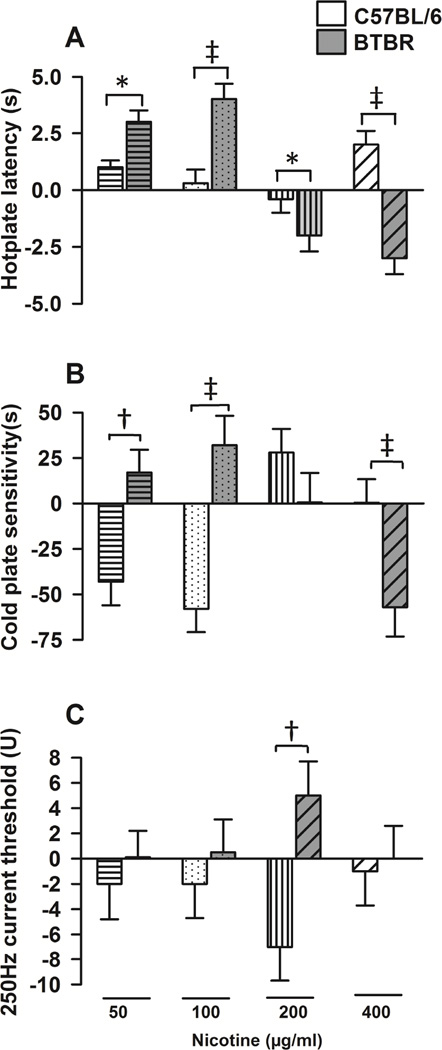
As others and we have shown that, the nicotinic cholinergic system modulates social deficits and repetitive behavior in BTBR mice and as nicotine is known to have analgesic effects, we examined its effects on nocifensive behavior in this model. We first asked whether nicotine treatment, compared with vehicle, had similar effects on nocifensive behavior comparing B6 and BTBR mice by examining time*treatment* strain interactions in the four-week nicotine trial. Figure 3 shows the comparisons of the effects of nicotine (compared with vehicle) on changes in behavior outcomes (post-treatment compared to baseline) in BTBR (gray bars) versus B6 (white bars) strains (time*treatment*strain interactions). We found that the effect of nicotine, compared with vehicle, indeed varied according to mouse strain. Specifically, compared with vehicle, four-week nicotine treatment at lower doses (50 and 100µg/ml) was associated with significantly greater increases on hotplate latency in BTBR compared to B6 mice (strain*time*treatment interaction z = −2.42, p=0.016 and z = −4.47, p<0.001 for 50 and 100µg/ml respectively, Figure 3A). At higher doses, compared with vehicle, nicotine also had a differential effect on nocifensive behavior comparing BTBR and B6 mice. At 200 and 400µg/ml nicotine led to significant decreases in hotplate latency in BTBR, whereas in B6 mice, it lead to no significant changes or increases in hotplate latency (strain*time*treatment interaction z = 2.05, p=0.040 and z = 6.12, p<0.001 for 200 and 400µg/ml respectively, Figure 3A). Concerning cold sensitivity, nicotine was also associated with different effect patterns in BTBR compared with B6 mice. Specifically, compared with vehicle, at lower doses (50 and 100µg/ml) nicotine led to significant increases in cold plate sensitivity in BTBR, whereas in B6 mice nicotine was associated with significant decreases in cold plate sensitivity (strain*time*treatment interaction z = −2.75, p=0.006 and z = −4.13, p<0.001 for 50 and 100µg/ml respectively, Figure 3B). In contrast, compared with vehicle, nicotine at 400µg/ml was associated with decreases in cold plate sensitivity in BTBR but with no significant changes in B6 mice (z = 3.26, p=0.001, Figure 3B). Regarding current thresholds, the effects of nicotine on 2000 and 5Hz were similar in BTBR and B6 mice. However in response to 250Hz stimulation, compared with vehicle, nicotine treatment at 200µg/ml was associated with slight increases in current thresholds in BTBR, but to slight decreases in B6, patterns of responses that were significantly different (z = −2.67, p=0.008, Figure 3C).
Figure 3. Differential effect of nicotine in C57BL/6 (B6) and BTBR mice.
Bars represent (means±SEM) nicotine effect compared with vehicle on behavior outcomes changes from baseline in B6 (white background bars) mice and BTBR (gray background bars). P values reflect refer to comparisons of the effect of nicotine vs. vehicle on changes from baseline between the two (BTBR vs. B6) strains (strain*time*treatment interactions). The nicotine doses indicated at the bottom of the figure apply to all panels. A, compared with vehicle, four-week nicotine treatment at lower doses (50 and 100µg/ml) was associated with significantly greater increases (from baseline) on hotplate latency in BTBR than in B6 mice (A, strain*time*treatment interaction p=0.016 and p<0.001 for 50 and 100µg/ml respectively). Compared with vehicle, at 200 and 400µg/ml nicotine led to significant decreases in hotplate latency in BTBR, whereas in B6 mice, it lead to no significant changes or increases in hotplate latency (A, strain*time*treatment interaction p=0.040 and p<0.001 for 200 and 400µg/ml respectively). B, concerning cold sensitivity, nicotine was also associated with a different pattern of effect in BTBR compared with B6. Compared with vehicle, at lower doses (50 and 100µg/ml) nicotine led to significant increases in BTBR, whereas in B6 mice nicotine was associated with significant decreases in cold plate sensitivity (B, strain*time*treatment interaction p=0.006 and p<0.001 for 50 and 100µg/ml respectively). Nicotine at 400µg/ml, compared with vehicle, was associated decreases in cold plate sensitivity in BTBR but with no significant changes in B6 mice (B, p=0.001). C, in response to 250Hz stimulation, compared with vehicle, nicotine treatment at 200µg/ml was associated with slight increases in current thresholds in BTBR, whereas in B6, to slight decreases in current thresholds in significantly different patterns (p=0.008). N≥10 (male and female) mice for each nicotine dose. *indicates p<0.05, † p≤0.01, and ‡ p≤0.001.
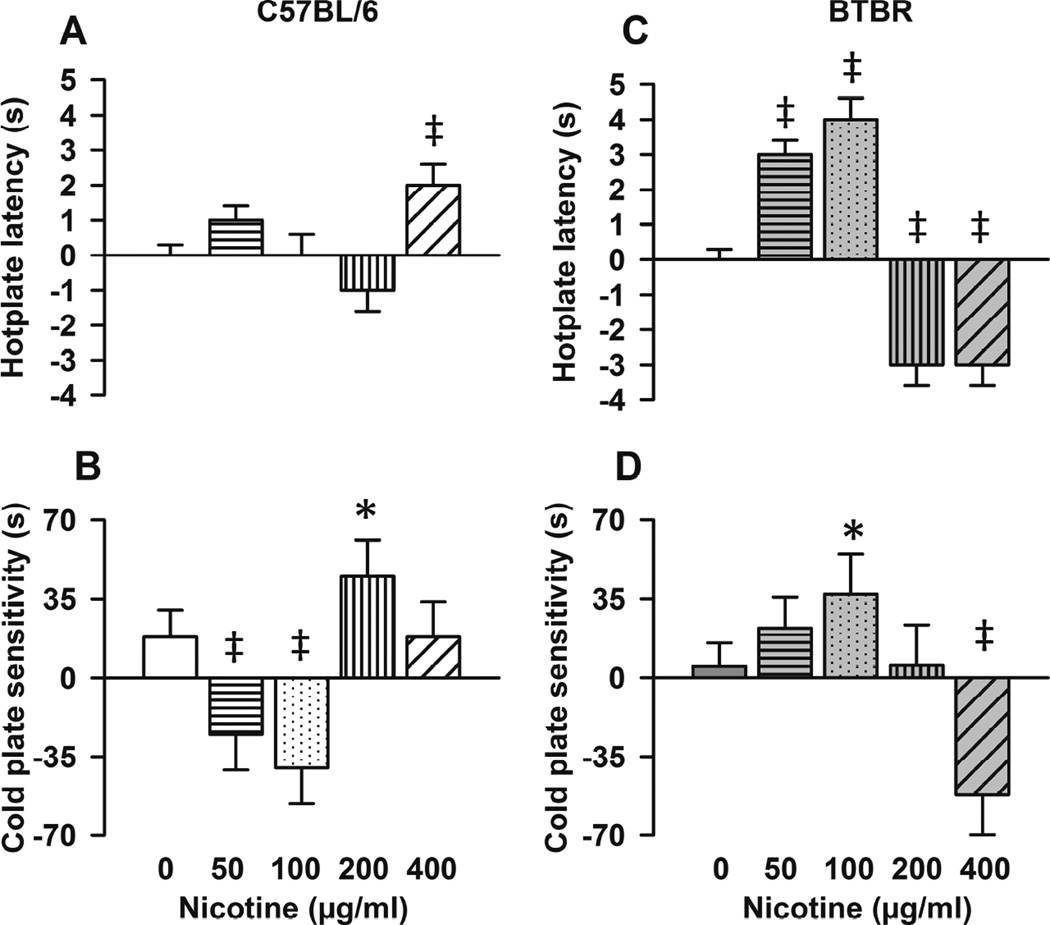
3.4. Effects of nicotine on nocifensive behavior in B6 (control) and BTBR mice
Figure 4 shows means±SEM changes (post-treatment vs. baseline) in behavior outcomes in B6 (4A and 4B) and in BTBR mice (4C and 4D) at each (0, 50, 100, 200, and 400µg/ml) nicotine dose (time*treatment interaction). In B6, nicotine only at the higher dose (400µg/ml) increased hotplate latency (Figure 4A, z = 3.18, p=0.001) compared with vehicle. Regarding cold sensitivity in B6 mice, at lower doses, nicotine decreased (time*treatment interaction at 50 µg/ml, z = −3.24, p= 0.001 and at 100 µg/ml, z = −3.48, p<0.001, Figure 4B), whereas at 200µg/ml, it increased (z = 2.12, p=0.034) cold plate sensitivity compared with vehicle. In addition in B6 mice, compared with vehicle, nicotine significantly decreased 2000Hz (χ2 (df) = 4.42 (1), p=0.036) and produced less of an increase in 250 Hz (χ2 (df) = 5.86 (1), p=0.016) and no changes in 5Hz current thresholds compared with vehicle (data not shown).
Figure 4. Effect of nicotine on nocifensive behavior in C57BL/6 (B6) and BTBR mice.
Bars represent (means±SEM) changes (post-treatment vs. baseline) in behavior outcomes in B6 (A and B) and in BTBR mice (C and D) at respective nicotine doses. P values reflect time (post nicotine vs. baseline) and treatment (vehicle vs. nicotine) interactions within indicated mouse strain. A, in B6 mice, nicotine only at the higher dose (400µg/ml) increased hotplate latency compared with vehicle (p=0.001). B, compared with vehicle, at lower doses (50µg/ml and 100µg/ml), nicotine decreased (B, time*treatment interaction, p≤0.001), whereas at 200µg/ml, it increased (p=0.034) sensitivity to cold plate in B6 mice. C, in BTBR mice, compared with vehicle, lower nicotine doses increased (time*treatment interaction, both p<0.001) and higher doses (200g/ml and 400µg/ml) decreased hotplate latency (both p=0.001, time*treatment interaction). D, compared with vehicle, at 100 µg/ml, nicotine increased (time*treatment interaction, p= 0.046), whereas at 400µg/ml, it decreased (p<0.001) sensitivity to cold plate in BTBR mice. N≥10 (male and female) per nicotine dose. *indicates p<0.05, † p≤0.01, and ‡ p≤0.001.
In BTBR mice, compared with vehicle, at lower doses, nicotine increased (50µg/ml, z = 6.69, and 100µg/ml, z = 7.19, both doses p<0.001, time*treatment interaction, Figure 4C) whereas, at higher doses ((200g/ml and 400µg/ml) nicotine decreased hotplate latency (both doses, z = −3.21, p=0.001, time*treatment interaction, Figure 4C). Regarding cold stimuli, compared with vehicle, at 100 µg/ml, nicotine increased (time*treatment interaction, z = 2.00, p= 0.046, Figure 4D), whereas at 400µg/ml, it decreased (z = −3.84, p<0.001, Figure 4D) cold sensitivity in BTBR mice. In BTBR mice, nicotine had no significant effect on electrical current thresholds in response to sinewave stimulation (data not shown).
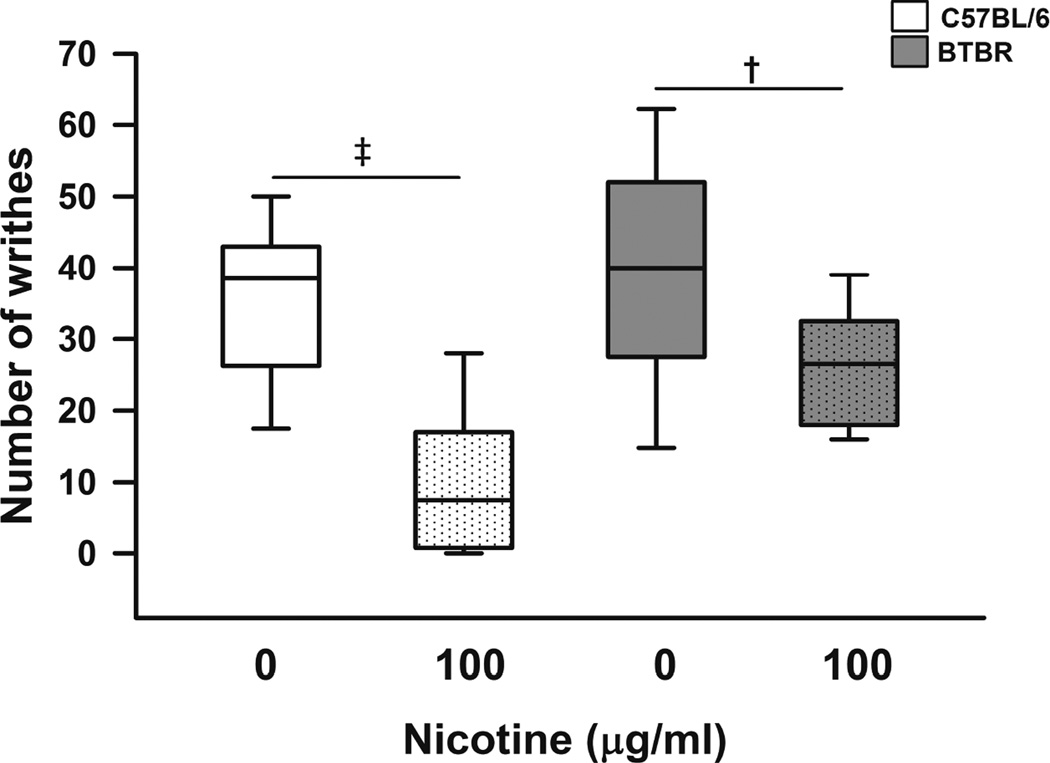
In a different cohort of animals, we examined the effect of nicotine on the writhing test and obtained behavior outcomes (number of writhes) only after treatment as not to repeat acetic acid injections. Overall, nicotine-treatment significantly decreased the number of writhes compared to vehicle (t=2.88, p=0.006, main effect of treatment). Specifically, nicotine-treated B6 and BTBR mice had significantly less writhes than saccharine-treated respective controls (p<0.001 for B6 and p=0.007 for BTBR) after intraperitoneal injection of acetic acid (Figure 5). Further, comparing the nicotine effect on visceral pain between the two strains (strain*treatment interaction), there was a trend (t=1.85, p=0.07) suggesting that nicotine was associated with greater decreases in writhes in B6 than in BTBR (Figure 5).
Figure 5. Effect of nicotine on visceral pain in C57BL/6 (B6) and BTBR mice.
Box plots show median, interquartile range, and 5th and 95th percentiles for number of writhes after acetic acid intraperitoneal injection in B6 (white) and BTBR (gray) mice. P values indicate comparisons between treatments (nicotine 0 or 100µg/ml) within respective strains. Nicotine-treated B6 and BTBR mice had significantly less writhes than saccharine-treated respective controls (p<0.001 for B6 and p=0.007 for BTBR). N=14 (age matched balanced number of male and female) mice per group. † indicates p≤0.01, and ‡ p≤0.001.
Water intake, mouse weight, and nicotine plasma levels with nicotine doses examined here, have been previously reported in B6 and BTBR mice (Wang et al., 2015). We chose not to evaluate the effects of nicotine on nocifensive behavior in Fmr1-KO mice because its effects (if any) on social behavioral deficits in the Fmr1-KO strain have not been characterized.
3.6. Expression of nicotinic acetylcholine receptor subunits in ASD mouse models
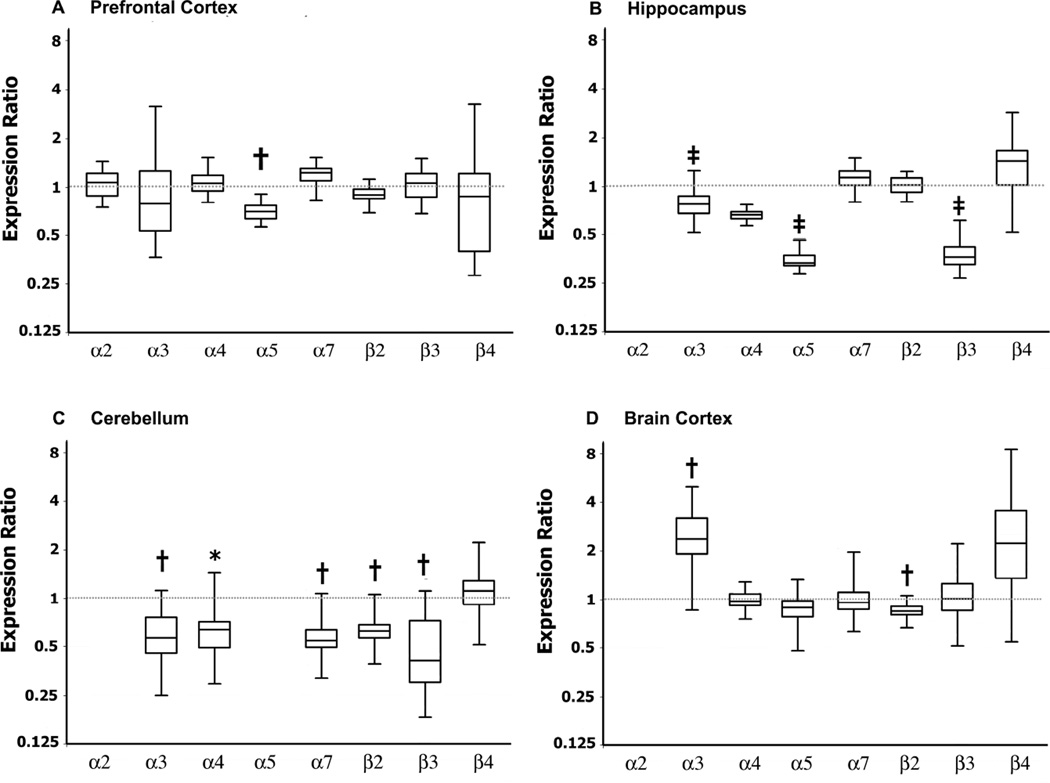
We then sought to determine whether there were alterations on expression levels of nAChR subunits (α2–7 and β2–4) in prefrontal cortex, hippocampus, cerebellum, and brain cortex in BTBR and Fmr1-KO mice compared to their respective controls. BTBR mice had significant downregulation of α5 (p=0.006, Figure 5A) nAChR receptor subunit in prefrontal cortex; α4 (p<0.0001), α5 (p=0.001), and β3 (p<0.0001) in hippocampus (Figure 5B); α3 (p=0.006), α4 (p=0.024), α5 (p=0.002), α7 (p=0.005), β2 (p<0.005), and β3 (p=0.01) in cerebellum (Figure 5C). In addition in brain cortex (excluding prefrontal cortex), BTBR mice had significant upregulation of α3 (p=0.004) and downregulation of β2 (p=0.004) nAChR subunits compared to B6 (Figure 5D). In all adult brain regions examined, α6 nAChR subunit mRNA expression was very low (data not shown).
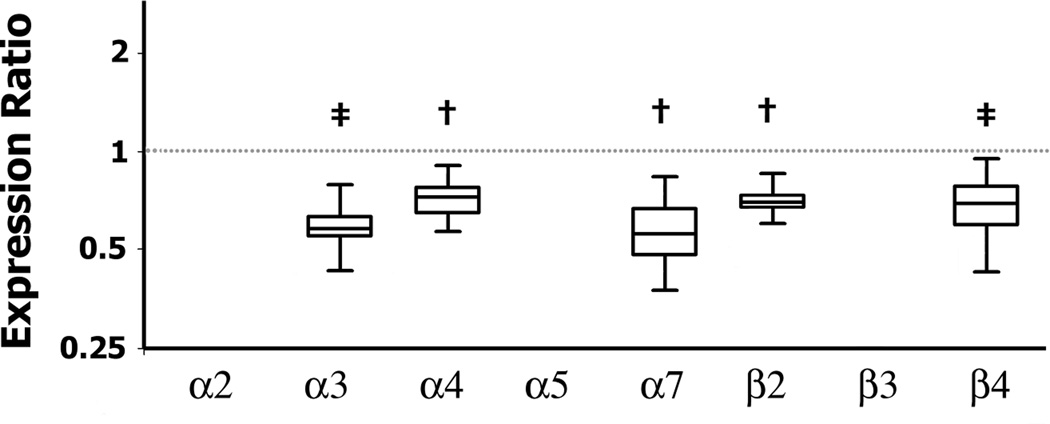
We then investigated whether in BTBR mice, these differences in brain nAChR subunits mRNA expression were present during embryonic development. We found that, embryonic (E13) BTBR brain had significantly reduced nAChR mRNA levels of α3 (p<0.0001); α4 (p=0.004), α7 (p=0.006), β2 (p=0.007), and β4 (p=0.001) compared to B6 fetuses (Figure 6). In fetal brains, α5, α6, and β3 nAChR subunit mRNA expression was very low at the time examined (data not shown).
Figure 6. Expression of nicotinic acetylcholine receptor (nAChR) subunits in adult BTBR mice.
Box plots show median and interquartile range of observations and whiskers represent minimum and maximum observations. P values refer to indicated gene expression ratios comparing BTBR and B6 (shown by the dotted line = 1). Compared to B6 mice, BTBR had significant downregulation of mRNA levels of α5 (A, p=0.006) nAChR subunit in prefrontal cortex; α4 (p<0.0001), α5 (p=0.001), and β3 (p<0.0001) in hippocampus (B); α3 (p=0.006), α4 (p=0.024), α5 (p=0.002), α7 (p=0.005), β2 (p<0.005), and β3 (p=0.01) in cerebellum (C). In addition in brain cortex, BTBR mice had significant upregulation of α3 (p=0.004) and downregulation of β2 (p=0.004) nAChR subunits compared to B6 mice (D). N= 6 mice per genotype. *indicates p<0.05, † p≤0.01, and ‡ p≤0.001,
Concerning Fmr1-KO mice, nAChR subunits mRNA levels prefrontal cortex and hippocampus were similar to FVB controls (data not shown). However, in cerebellum, Fmr1-KO mice had significantly decreased expression of α4 [0.625, (0.246, 1.141) 95% C.I, fold change and 95% confidence interval, p=0.032] and β4 [0.544, (0.277, 1.077) 95% C.I, p=0.023] compared to FVB controls.
4. Discussion
The results of the present study add to the existing literature showing that animal models carrying mutations known to occur in idiopathic and syndromic ASD have associated alterations in nociception and nocifensive behavior. Here we found that both, the BTBR and the Fmr1-KO mice on an FVB background had hyporesponsiveness to noxious hot and cold stimuli, and actually, hyperresponsiveness to visceral pain (acetic acid writhing test) compared to respective controls. We also found that nicotine, a prototypical nAChR agonist, which has been shown to modulate behavior deficits in BTBR mice, had differential effects in BTBR and mice with normal sociability. Taken together, these findings suggest that in ASD-like models, there are associated alterations in nocifensive behavior that can vary according to stimulation modality and that cholinergic agents that modulate social and repetitive behavior deficits can also modulate their altered nocifensive phenotype.
We found that compared with respective control strains, BTBR and Fmr1-KO mice displayed hyporesponsiveness to noxious thermal stimuli and electrical stimulation of C sensory fibers, similar responsiveness to electrical stimulation of Aβ and Aδ fibers, and hyperresponsiveness to visceral pain following intraperitoneal injections of acetic acid. Interestingly, these results mirror those findings in ASD individuals who display a mixed pattern (sensory hyper- and hyporesponsiveness) of nocifensive response (Baranek et al., 2006). We also found that the antinociceptive effects of nicotine in BTBR mice, were significantly different from the effects observed in control mice. In BTBR, at lower doses (50 and 100µg/ml), nicotine accentuated the hyporesponsiveness to the hotplate test but attenuated the hyporesponsiveness to the cold plate, an effect pattern that was significantly different from that observed in control B6 mice. At higher doses (200 and 400 µg/ml) nicotine decreased hotplate latency, normalizing responsiveness to thermal stimuli, but it decreased cold plate sensitivity, thus accentuating the hyporesponsiveness to noxious cold in BTBR mice. Therefore, these differential responses to the antinociceptive effects of nicotine raise the possibilities that the nicotinic cholinergic system could be altered in ASD and that nicotine, in addition to modulating social deficits and repetitive behaviors (Wang et al., 2015), also modulates the altered nocifensive phenotype in BTBR mice.
The findings that BTBR and Fmr1-KO mice have increased writhing after intraperitoneal injection of acetic acid, which models visceral pain, are relevant. While one must be circumspect about extrapolating findings in mice to humans, it is tempting to speculate that these findings in the models of ASD studied here recapitulate those in humans with ASD. In fact, a recent meta-analysis showed that children with ASD experience significant more gastrointestinal symptoms including more abdominal pain than those without ASD (McElhanon et al., 2014). Interestingly, nicotine modulated the nocifensive response in the ASD model reducing the number of writhes in a pattern suggestive that the effect of nicotine was lesser than in BTBR than in control mice. Therefore, these findings suggest that the models studied here are valuable for studies of increased visceral pain in ASD and that the nicotinic cholinergic system might have a role in modulating the nocifensive response to visceral pain in ASD models.
Others have shown that administration of nicotine acutely or chronically can yield nonlinear patterns of dose-effect relationships on animal behavior (Picciotto, 2003; Popke et al., 2000). For example, in rats, acute nicotine administration is associated with an U-shaped dose response in complex cognitive tasks that require accurate time perception while in tasks that evaluate response rates, nicotine yields a bell-shaped dose-effect relationship (Popke et al., 2000). We had previously reported that nicotine administered over four weeks at doses used in the present investigation, also yielded non-linear dose-effect relationships on social and repetitive behaviors in the BTBR and B6 mice (Wang et al., 2015). Thus, the findings of a nonlinear pharmacodynamic profile of chronic nicotine on nocifensive behavior in BTBR and B6 mice are in keeping with our previous results and those of others(Picciotto, 2003; Popke et al., 2000). One could only speculate that these non-linear patterns of nicotine dose-response curves reflect differences in molecular and/or genetic factors leading to different response patterns in BTBR and B6 mice. Alternatively, one could argue that nicotine at different doses activates and/or inhibits different neuronal circuits in the BTBR and B6 brain, thus leading to a non-liner dose-response.
Interestingly, the nocifensive behaviors alterations and the differential responses to nicotine in BTBR mice were associated with significant downregulation of the expression of several nAChR subunits (α3, α4, α7, β2, and β4) during embryonic development (E13) and adulthood. That altered nAChR subunit expression impacts animal behavior, synapse formation, and neuronal architecture has been shown in investigations of mice with null-mutations of specific nAChR subunits (Bailey et al., 2010; Role and Berg, 1996). For example, mice with α5 nAChR subunit null mutations have impaired attentional performance (Bailey et al., 2010), a deficit that is associated with lack of α5-dependent developmental peak in nicotinic signaling early in post-natal life and alterations in dendritic morphology in prefrontal cortex(Bailey et al., 2012). Mice with β2 nAChR subunit null mutations have behavior inflexibility shown by absence of adaptive behaviors and impaired capacity to interrupt ongoing behaviors (Avale et al., 2011; Granon et al., 2003). Interestingly, β2 null mutants also have impaired spatial learning at older age, which is coupled with neocortical hypertrophy, hippocampal pyramidal neuronal loss, astrogliosis, and microgliosis (Zoli et al., 1999). Therefore, taken together, our results and those of others raise the hypothesis that alterations in nAChRs subunits expression might play a role not only on social deficits and repetitive behavior, but also in the associated nocifensive behavior alterations in ASD-like mice.
That nAChRs subunits have a role on nocifensive behavior and on the antinociceptive effects of nicotine has also been previously shown (Bagdas et al., 2015; Damaj et al., 1999; Freitas et al., 2015; Saika et al., 2015; Wieskopf et al., 2015; Xanthos et al., 2015; Yalcin et al., 2011). In studies using nAChR agonists and antagonists as well as mice with nAChR subunits null mutations, researchers have shown that nAChRs containing α3, α4, α5, α6, α7, β2, and β4 subunits are involved in modulating nocifensive behaviors in response to acute noxious thermal stimuli (Damaj et al., 2007; Damaj et al., 1999; Marubio et al., 1999) and in response to inflammatory and neuropathic processes (AlSharari et al., 2012; Bagdas et al., 2015; Damaj et al., 1999; Freitas et al., 2015; Saika et al., 2015; Wieskopf et al., 2015; Xanthos et al., 2015; Yalcin et al., 2011). Others have also shown that mice with null mutations of α4, α5, or β2 have decreased response to the analgesic effects of nicotine, but intact response to the antinociceptive effects of morphine (Damaj et al., 2007; Marubio et al., 1999). Here we showed that while nicotine had an effect both in B6 (control) and BTBR mice, the effect of nicotine was significantly different in BTBR mice compared to the control strain. Further, these differential effects were observed at different nicotine doses and in response to different stimulation modalities. Therefore, future studies will test the hypotheses that 1) downregulation of nAChR subunits might have contributed to the altered nocifensive behavior phenotype in BTBR mice and 2) different nAChR subtypes modulate the differential responses to nicotine in BTBR compared to B6 mice.
While the mechanisms of altered nocifensive response in BTBR and Fmr1-KO mice are incompletely understood, this study supports the use of these two models for investigations of the pathobiology of altered sensory responsiveness associated with ASD. While these two models have entirely unrelated and different genetic abnormalities and backgrounds, it is noteworthy that BTBR and Fmr1-KO mice displayed similar nocifensive behavior phenotypes. One emerging principle in ASD is that circuit-level alterations could possibility explain the phenomenon that similar ASD behavior phenotypes could originate from syndromes associated with significant genetic disparities. In fact, researchers have hypothesized that imbalances in excitatory and inhibitory circuits could possibly contribute to the core behavior deficits in ASD (Zikopoulos and Barbas, 2013) and a few studies in BTBR (Han et al., 2014) and Fmr1-KO mice (Martin et al., 2014; Olmos-Serrano et al., 2011) support this hypothesis. For example, in BTBR, treatment with negative allosteric modulators of metabotropic glutamate receptor 5, which modulates excitatory neurotransmission, improves social deficits and reduces repetitive behavior (Silverman et al., 2012; Silverman et al., 2010a). Researchers have shown that BTBR mice have reduced GABAergic tone and treatment with agents that modulate excitatory/inhibitory balance, such as positive allosteric modulators of postsynaptic GABAA receptors and GABAB agonists or histone deacetylase inhibitors, improves social deficits and repetitive behaviors in these animals (Han et al., 2014; Kratsman et al., 2016; Silverman et al., 2015). One could then speculate that imbalances between excitatory and inhibitory circuits could also contribute to alteration in nocifensive behaviors displayed by BTBR and Fmr1-KO mice. Further, as researchers have shown that the antinociceptive effects of nicotine are altered in the setting of decreased GABAergic signaling (Varani et al., 2012), it is also conceivable that imbalances in excitatory/inhibitory signaling in BTBR, could at least in part have contributed to the differential effects of nicotine comparing BTBR and control mice. In turn, these findings in BTBR mice are compatible with the hypothesis that, chronic nicotine, possibly by its interaction with the GABAergic system, modulates nocifensive response as well as social deficits and repetitive behavior.
We note some limitations of this study. Some might argue that the nociception evaluation modalities examined here are reflexive in nature and therefore incompletely evaluate the influence of the effect of cholinergic agents on behavior domains that are relevant to pain perception and sensitivity such as anxiety, fear, and memory (Vierck and Yezierski, 2015). In fact, researchers have shown that changes in cholinergic tone can yield discrepant results in reflexive and operant escape evaluations of nociception (Vierck et al., 2016). Therefore, it is important that future studies determine the effect of nicotine on operant escape from nociceptive thermal stimulation in BTBR and control mice to further our understanding of the role of the cholinergic system on altered nociception in animal models of ASD. In addition, some might argue that by not correcting for multiple comparisons, the alpha error rate in the analysis of the mRNA levels data could have been inflated. We posit that by using robust and powerful tools for the analysis of behavior data (mixed-effects model (Liu et al., 2010; Wainwright et al., 2007)) and mathematical approaches based on a pair wise fixed reallocation randomization test to analyze the mRNA data (Pfaffl, 2001) added rigor to the analytical approach and minimized inflation of the alpha error rate.
Nevertheless, the results of the present study support the hypotheses that BTBR and Fmr1-KO mice model the nocifensive behavioral responses described in ASD. Therefore, they could be valuable for studies of the pathobiology of the altered sensory responses in ASD. The findings of altered expression of nAChR subunits both, during development and adulthood in ASD-like mice, support the hypothesis that altered nicotinic signaling might have a role in the pathobiology of ASD. Additionally, this study lends further support to the hypothesis that nAChR-directed interventions have the potential to, not only modulate social behavior deficits, but also to modulate the observed altered nocifensive response in autistic-like mice. Lastly, the present results can inform future studies seeking to determine what specific nAChR subtypes are involved in the modulation of nocifensive response in ASD.
Figure 7. Expression of nicotinic acetylcholine receptor (nAChR) subunits in BTBR mice during embryonic development.
Box plots show median and interquartile range of observations and whiskers represent minimum and maximum observations. P values refer to indicated gene expression ratios of fetal brain comparing BTBR and B6 (shown by the dotted line = 1). Fetal brains were collected from pregnant B6 and BTBR mice on E13. In BTBR fetal brains there were significant downregulation of α3 (p<0.0001), α4 (p=0.004), α7 (p=0.006), β2 (p=0.007), and β4 (p=0.001) nAChR mRNA levels compared to B6 fetuses. N= 5 pooled pregnancies per genotype. * indicates p<0.05, † p≤0.01, and ‡ p≤0.001.
Highlights.
Sensory alteration in autism spectrum disorder is associated with disease severity
Autism-like mouse models (BTBR) display altered nocifensive behaviors
BTBR have significant alterations in the expression of nicotinic receptor subunits
Nicotine modulates behavior deficits and decreases repetitive behavior in BTBR
Nicotine also modulates nociception alterations in BTBR mice
Acknowledgments
The authors are grateful to Drs. Joshua Corbin and Richard Levy for providing the Fmr1 mutant mice.
Funding: The work was supported by a grant from the Sheikh Zayed Institute for Pediatric Surgical Innovation, Children’s Research Institute, Children’s National Health System and by a National Institutes of Health Intellectual & Developmental Disabilities Research Center Grant (grant number P30HD040677)
Abbreviations
- ASD
Autism spectrum disorder
- BTBR
BTBR T+Itpr3tf/J
- FVB
FVB.129P2-Pde6b+ Tyrc-ch/AntJ, control strain for Fmr1-KO
- B6
C57BL/6J, control strain for BTBR
- Fmr1-KO
FVB.129P2-Pde6b+ Tyrc-ch Fmr1tm1Cgr/J
- nAChR
nicotinic acetylcholine receptor
- RT-qPCR
real-time reverse transcription polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article
Nicotine (PubChem CID: 89594); Saccharin (PubChem CID: 656582)
Disclosures
All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest and have no conflict of interest to disclose
Authorship
Conceived and designed the experiments: Li Wang, Luis E.F. Almeida, and Zenaide M.N.Quezado.
Performed the experiments: Li Wang, Luis E.F. Almeida, Margaret Nettleton, Alfia Khaibullina, Sarah Albani, Sayuri Kamimura, and Zenaide Quezado
Analyzed the data: Mehdi Nouraie, Alfia Khaibullina, and Zenaide M.N. Quezado.
Wrote the paper: Li Wang, Luis E.F. Almeida, and Zenaide M.N. Quezado.
Reviewed the manuscript: Li Wang, Luis E.F. Almeida, Margaret Nettleton, Alfia Khaibullina, Sarah Albani, Sayuri Kamimura, Mehdi Nouraie, and Zenaide M.N. Quezado.
References
- AlSharari SD, Carroll FI, McIntosh JM, Damaj MI. The Antinociceptive Effects of Nicotinic Partial Agonists Varenicline and Sazetidine-A in Murine Acute and Tonic Pain Models. Journal of Pharmacology and Experimental Therapeutics. 2012;342:742–749. doi: 10.1124/jpet.112.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Ausderau K, Sideris J, Furlong M, Little LM, Bulluck J, Baranek GT. National survey of sensory features in children with ASD: factor structure of the sensory experience questionnaire (3.0) J Autism Dev Disord. 2014a;44:915–925. doi: 10.1007/s10803-013-1945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausderau KK, Furlong M, Sideris J, Bulluck J, Little LM, Watson LR, Boyd BA, Belger A, Dickie VA, Baranek GT. Sensory subtypes in children with autism spectrum disorder: latent profile transition analysis using a national survey of sensory features. J Child Psychol Psychiatry. 2014b;55:935–944. doi: 10.1111/jcpp.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausderau KK, Sideris J, Little LM, Furlong M, Bulluck JC, Baranek GT. Sensory subtypes and associated outcomes in children with autism spectrum disorders. Autism Res. 2016 doi: 10.1002/aur.1626. [DOI] [PubMed] [Google Scholar]
- Avale ME, Chabout J, Pons S, Serreau P, De Chaumont F, Olivo-Marin JC, Bourgeois JP, Maskos U, Changeux JP, Granon S. Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J. 2011;25:2145–2155. doi: 10.1096/fj.10-178558. [DOI] [PubMed] [Google Scholar]
- Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. Biochem Pharmacol. 2015;97:590–600. doi: 10.1016/j.bcp.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CDC, Alves NC, Nashmi R, De Biasi M, Lambe EK. Nicotinic α5 Subunits Drive Developmental Changes in the Activation and Morphology of Prefrontal Cortex Layer VI Neurons. Biological Psychiatry. 2012;71:120–128. doi: 10.1016/j.biopsych.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AEZ, Lane A, Angley MT, Young RL. The Relationship Between Sensory Processing Patterns and Behavioural Responsiveness in Autistic Disorder: A Pilot Study. Journal of Autism and Developmental Disorders. 2008;38:867–875. doi: 10.1007/s10803-007-0459-0. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry. 2006;47:591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baum SH, Stevenson RA, Wallace MT. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Progress in Neurobiology. 2015;134:140–160. doi: 10.1016/j.pneurobio.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. J Autism Dev Disord. 2009;39:1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Brodkin ES. Social behavior phenotypes in fragile X syndrome, autism, and the Fmr1 knockout mouse: theoretical comment on McNaughton et al. (2008) Behav Neurosci. 2008;122:483–489. doi: 10.1037/0735-7044.122.2.483. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KV, Bilder D, Charles J, Constantino JN, Daniels J, Durkin MS, Fitzgerald RT, Kurzius-Spencer M, Lee LC, Pettygrove S, Robinson C, Schulz E, Wells C, Wingate MS, Zahorodny W, Yeargin-Allsopp M. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Charman T. Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)00151-9. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, Collins AC, Martin BR. Genetic Approaches Identify Differential Roles for α4β2* Nicotinic Receptors in Acute Models of Antinociception in Mice. Journal of Pharmacology and Experimental Therapeutics. 2007;321:1161–1169. doi: 10.1124/jpet.106.112649. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Aceto MD, Martin BR. Antinociceptive and Pharmacological Effects of Metanicotine, a Selective Nicotinic Agonist. Journal of Pharmacology and Experimental Therapeutics. 1999;291:390–398. [PubMed] [Google Scholar]
- Downs J, Geranton SM, Bebbington A, Jacoby P, Bahi-Buisson N, Ravine D, Leonard H. Linking MECP2 and pain sensitivity: the example of Rett syndrome. Am J Med Genet A. 2010;152A:1197–1205. doi: 10.1002/ajmg.a.33314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Taylor MJ, Lee M, McGrath PA, Davis KD, Roberts SW. Decreased sensitivity to thermal stimuli in adolescents with autism spectrum disorder: relation to symptomatology and cognitive ability. J Pain. 2015;16:463–471. doi: 10.1016/j.jpain.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Finkel J, Guptill V, Khaibullina A, Spornick N, Vasconcelos O, Liewehr DJ, Steinberg SM, Quezado ZM. The three isoforms of nitric oxide synthase distinctively affect mouse nocifensive behavior. Nitric Oxide. 2012;26:81–88. doi: 10.1016/j.niox.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel JC, Besch VG, Hergen A, Kakareka J, Pohida T, Melzer JM, Koziol D, Wesley R, Quezado ZM. Effects of aging on current vocalization threshold in mice measured by a novel nociception assay. Anesthesiology. 2006;105:360–369. doi: 10.1097/00000542-200608000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Heacock JL, Cascio CJ. Tactile responsiveness patterns and their association with core features in autism spectrum disorders. Research in Autism Spectrum Disorders. 2012;6:337–344. doi: 10.1016/j.rasd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas KC, Carroll FI, Negus SS. Effects of Nicotinic Acetylcholine Receptor Agonists in Assays of Acute Pain-Stimulated and Pain-Depressed Behaviors in Rats. Journal of Pharmacology and Experimental Therapeutics. 2015;355:341–350. doi: 10.1124/jpet.115.226803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granon S, Faure P, Changeux JP. Executive and social behaviors under nicotinic receptor regulation. Proc Natl Acad Sci U S A. 2003;100:9596–9601. doi: 10.1073/pnas.1533498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M. Neurobiology of Sensory Overresponsivity in Youth With Autism Spectrum Disorders. JAMA Psychiatry. 2015;72:778–786. doi: 10.1001/jamapsychiatry.2015.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Tai C, Jones CJ, Scheuer T, Catterall WA. Enhancement of inhibitory neurotransmission by GABAA receptors having alpha2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 2014;81:1282–1289. doi: 10.1016/j.neuron.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton CL, Harper JD, Kueker RH, Lang AR, Abbacchi AM, Todorov A, LaVesser PD. Sensory Responsiveness as a Predictor of Social Severity in Children with High Functioning Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2010;40:937–945. doi: 10.1007/s10803-010-0944-8. [DOI] [PubMed] [Google Scholar]
- Karvat G, Kimchi T. Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology. 2014;39:831–840. doi: 10.1038/npp.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Grannemann BD, Garver CR, Johnson DG, Andrews AA, Savla JS, Mehta JA, Schroeder JL. Sensory correlations in autism. Autism. 2007;11:123–134. doi: 10.1177/1362361307075702. [DOI] [PubMed] [Google Scholar]
- Ko H-G, Oh S-B, Zhuo M, Kaang B-K. Reduced acute nociception and chronic pain in Shank2−/− mice. Molecular Pain. 2016:12. doi: 10.1177/1744806916647056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1:13. doi: 10.1186/1744-8069-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratsman N, Getselter D, Elliott E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology. 2016;102:136–145. doi: 10.1016/j.neuropharm.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Leblond CS, Heinrich J, Delorme R, Proepper C, Betancur C, Huguet G, Konyukh M, Chaste P, Ey E, Rastam M, Anckarsater H, Nygren G, Gillberg IC, Melke J, Toro R, Regnault B, Fauchereau F, Mercati O, Lemiere N, Skuse D, Poot M, Holt R, Monaco AP, Jarvela I, Kantojarvi K, Vanhala R, Curran S, Collier DA, Bolton P, Chiocchetti A, Klauck SM, Poustka F, Freitag CM, Waltes R, Kopp M, Duketis E, Bacchelli E, Minopoli F, Ruta L, Battaglia A, Mazzone L, Maestrini E, Sequeira AF, Oliveira B, Vicente A, Oliveira G, Pinto D, Scherer SW, Zelenika D, Delepine M, Lathrop M, Bonneau D, Guinchat V, Devillard F, Assouline B, Mouren MC, Leboyer M, Gillberg C, Boeckers TM, Bourgeron T. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012;8:e1002521. doi: 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, Giuliano F, Stordeur C, Depienne C, Mouzat K, Pinto D, Howe J, Lemière N, Durand CM, Guibert J, Ey E, Toro R, Peyre H, Mathieu A, Amsellem F, Rastam M, Gillberg IC, Rappold GA, Holt R, Monaco AP, Maestrini E, Galan P, Heron D, Jacquette A, Afenjar A, Rastetter A, Brice A, Devillard F, Assouline B, Laffargue F, Lespinasse J, Chiesa J, Rivier F, Bonneau D, Regnault B, Zelenika D, Delepine M, Lathrop M, Sanlaville D, Schluth-Bolard C, Edery P, Perrin L, Tabet AC, Schmeisser MJ, Boeckers TM, Coleman M, Sato D, Szatmari P, Scherer SW, Rouleau GA, Betancur C, Leboyer M, Gillberg C, Delorme R, Bourgeron T. Meta-analysis of <italic>SHANK</italic> Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments. PLoS Genet. 2014;10:e1004580. doi: 10.1371/journal.pgen.1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Cripe TP, Kim MO. Statistical issues in longitudinal data analysis for treatment efficacy studies in the biomedical sciences. Mol Ther. 2010;18:1724–1730. doi: 10.1038/mt.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BS, Corbin JG, Huntsman MM. Deficient tonic GABAergic conductance and synaptic balance in the fragile X syndrome amygdala. J Neurophysiol. 2014;112:890–902. doi: 10.1152/jn.00597.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubio LM, Arroyo-Jimenez MdM, Cordero-Erausquin M, Lena C, Novere NL, d’Exaerde AdK, Huchet M, Damaj MI, Changeux J-P. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133:872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Corbin JG, Burns MP. The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev Neurosci. 2011;33:395–403. doi: 10.1159/000332884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten E, Ausderau KK, Watson LR, Baranek GT. Sensory Response Patterns in Nonverbal Children with ASD. Autism Res Treat. 2013;2013:436286. doi: 10.1155/2013/436286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen A, Pettygrove S, Meaney FJ, Mancilla K, Gotschall K, Kessler DB, Grebe TA, Cunniff C. Prevalence of autism spectrum disorders in Hispanic and non-Hispanic white children. Pediatrics. 2012;129:e629–e635. doi: 10.1542/peds.2011-1145. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR. Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Popke EJ, Mayorga AJ, Fogle CM, Paule MG. Effects of acute nicotine on several operant behaviors in rats. Pharmacol Biochem Behav. 2000;65:247–254. doi: 10.1016/s0091-3057(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert JA, Daughters RS, Rivard R, Simone DA. Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain. 2001;89:221–227. doi: 10.1016/s0304-3959(00)00365-1. [DOI] [PubMed] [Google Scholar]
- Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909–916. doi: 10.1016/S2215-0366(15)00376-4. [DOI] [PubMed] [Google Scholar]
- Riquelme I, Hatem SM, Montoya P. Abnormal Pressure Pain, Touch Sensitivity, Proprioception, and Manual Dexterity in Children with Autism Spectrum Disorders. Neural Plast. 2016;2016:1723401. doi: 10.1155/2016/1723401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic Receptors in the Development and Modulation of CNS Synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Saika F, Kiguchi N, Kobayashi Y, Kishioka S. Peripheral alpha4beta2 nicotinic acetylcholine receptor signalling attenuates tactile allodynia and thermal hyperalgesia after nerve injury in mice. Acta Physiologica. 2015;213:462–471. doi: 10.1111/apha.12437. [DOI] [PubMed] [Google Scholar]
- Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, Janssen A-L, Udvardi PT, Shiban E, Spilker C, Balschun D, Skryabin BV, Dieck St, Smalla K-H, Montag D, Leblond CS, Faure P, Torquet N, Le Sourd A-M, Toro R, Grabrucker AM, Shoichet SA, Schmitz D, Kreutz MR, Bourgeron T, Gundelfinger ED, Boeckers TM. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Oliver CF, Karras MN, Gastrell PT, Crawley JN. AMPAKINE enhancement of social interaction in the BTBR mouse model of autism. Neuropharmacology. 2013;64:268–282. doi: 10.1016/j.neuropharm.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, Crawley JN. GABA Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med. 2012;4:131ra151. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010a;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010b;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen EM, Bertelsen F, Weikop P, Skovborg MM, Banke T, Drasbek KR, Scheel-Kruger J. Hyperactivity and lack of social discrimination in the adolescent Fmr1 knockout mouse. Behav Pharmacol. 2015;26:733–740. doi: 10.1097/FBP.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Research. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spornick N, Guptill V, Koziol D, Wesley R, Finkel J, Quezado ZM. Mouse current vocalization threshold measured with a neurospecific nociception assay: the effect of sex, morphine, and isoflurane. J Neurosci Methods. 2011;201:390–398. doi: 10.1016/j.jneumeth.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Dutch-Belgian Fragile X Consortium, Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen A, Oostra BA, Reyniers E, De Boule K, D’Hooge R, Cras P, van Velzen D, Nagels G, Martin J-J, De Deyn PP, Darby JK, Willems PJ. Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 2013;78:23–33. [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Botbol M, Brailly-Tabard S, Perez-Diaz F, Graignic R, Carlier M, Schmit G, Rolland AC, Bonnot O, Trabado S, Roubertoux P, Bronsard G. Pain reactivity and plasma beta-endorphin in children and adolescents with autistic disorder. PLoS One. 2009;4:e5289. doi: 10.1371/journal.pone.0005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani AP, Moutinho LM, Bettler B, Balerio GN. Acute behavioural responses to nicotine and nicotine withdrawal syndrome are modified in GABAB1 knockout mice. Neuropharmacology. 2012;63:863–872. doi: 10.1016/j.neuropharm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Veeraragavan S, Graham D, Bui N, Yuva-Paylor LA, Wess J, Paylor R. Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of fragile X syndrome (FXS) Behav Brain Res. 2012;228:1–8. doi: 10.1016/j.bbr.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Yezierski RP. Comparison of operant escape and reflex tests of nociceptive sensitivity. Neurosci Biobehav Rev. 2015;51:223–242. doi: 10.1016/j.neubiorev.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Yezierski RP, Wiley RG. Pain sensitivity following loss of cholinergic basal forebrain (CBF) neurons in the rat. Neuroscience. 2016;319:23–34. doi: 10.1016/j.neuroscience.2016.01.038. [DOI] [PubMed] [Google Scholar]
- Wainwright PE, Leatherdale ST, Dubin JA. Advantages of mixed effects models over traditional ANOVA models in developmental studies: a worked example in a mouse model of fetal alcohol syndrome. Dev Psychobiol. 2007;49:664–674. doi: 10.1002/dev.20245. [DOI] [PubMed] [Google Scholar]
- Wang L, Almeida LE, Spornick NA, Kenyon N, Kamimura S, Khaibullina A, Nouraie M, Quezado ZM. Modulation of social deficits and repetitive behaviors in a mouse model of autism: the role of the nicotinic cholinergic system. Psychopharmacology (Berl) 2015;232:4303–4316. doi: 10.1007/s00213-015-4058-z. [DOI] [PubMed] [Google Scholar]
- Wieskopf JS, Mathur J, Limapichat W, Post MR, Al-Qazzaz M, Sorge RE, Martin LJ, Zaykin DV, Smith SB, Freitas K, Austin J-S, Dai F, Zhang J, Marcovitz J, Tuttle AH, Slepian PM, Clarke S, Drenan RM, Janes J, Al Sharari S, Segall SK, Aasvang EK, Lai W, Bittner R, Richards CI, Slade GD, Kehlet H, Walker J, Maskos U, Changeux J-P, Devor M, Maixner W, Diatchenko L, Belfer I, Dougherty DA, Su AI, Lummis SCR, Imad Damaj M, Lester HA, Patapoutian A, Mogil JS. The nicotinic α6 subunit gene determines variability in chronic pain sensitivity via cross-inhibition of P2×2/3 receptors. Science Translational Medicine. 2015;7:287ra272–287ra272. doi: 10.1126/scitranslmed.3009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, Lee H-R, Gee HY, Mah W, Kim J-I, Lee J, Ha S, Chung C, Jung ES, Cho YS, Park S-G, Lee J-S, Lee K, Kim D, Bae YC, Kaang B-K, Lee MG, Kim E. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- Xanthos DN, Beiersdorf JW, Thrun A, Ianosi B, Orr-Urtreger A, Huck S, Scholze P. Role of α5-containing nicotinic receptors in neuropathic pain and response to nicotine. Neuropharmacology. 2015;95:37–49. doi: 10.1016/j.neuropharm.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Charlet A, Cordero-Erausquin M, Tessier L-H, Picciotto MR, Schlichter R, Poisbeau P, Freund-Mercier M-J, Barrot M. Nociceptive thresholds are controlled through spinal β2-subunit-containing nicotinic acetylcholine receptors. Pain. 2011;152:2131–2137. doi: 10.1016/j.pain.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci. 2013;7:609. doi: 10.3389/fnhum.2013.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Picciotto MR, Ferrari R, Cocchi D, Changeux JP. Increased neurodegeneration during ageing in mice lacking high-affinity nicotine receptors. The EMBO Journal. 1999;18:1235–1244. doi: 10.1093/emboj/18.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]