Abstract
Objectives
Resting metabolic rate (RMR) reflects energetic costs of homeostasis and accounts for 60-75% of total energy expenditure (TEE). Lean mass and physical activity account for much RMR variability, but the impact of prolonged immune activation from infection on human RMR is unclear in naturalistic settings. We evaluate the effects of infection on mass-corrected RMR among Bolivian forager-horticulturalists, and assess whether RMR declines more slowly with age than in hygienic sedentary populations, as might be expected if older adults experience high pathogen burden.
Materials and Methods
RMR was measured by indirect calorimetry (Fitmate MED, Cosmed) in 1,300 adults aged 20-90 and TEE was measured using doubly labeled water (n= 40). Immune biomarkers, clinical diagnoses and anthropometrics were collected by the Tsimane Health and Life History Project.
Results
Tsimane have higher RMR and TEE than people in sedentary industrialized populations. Tsimane RMR is 18-47% (women) and 22-40% (men) higher than expected using six standard prediction equations. Tsimane mass-corrected TEE is similarly elevated compared to Westerners. Elevated leukocytes and helminths are associated with excess RMR in multivariate regressions, and jointly result in a predicted excess RMR of 10-15%. After age 40, RMR declines by 69 kcal/decade (p<0.0001). Controlling for lean mass and height accounts for 71% of age-related RMR decline, and adding indicators of infection minimally affects the age slope. The residual level of age-related decline from age 40 is 1.2% per decade.
Conclusion
High pathogen burden may lead to higher metabolic costs, which may be offset by smaller body mass or other energy-sparing mechanisms.
Keywords: resting metabolic rate (RMR), energetic expenditure, costs of infection, Tsimane, maintenance costs
Introduction
The size and allocation of an organism’s daily energy budget reflects its evolved strategies for growth, reproduction and maintenance, though trade-offs and integration of these functions across diverse environments in free-living organisms are poorly understood. In humans, roughly 60-75% of total energy expenditure (TEE, kcals/day) is spent on resting metabolic rate (RMR, kcals/day) (Manini 2010; Speakman and Selman 2003). RMR reflects the energetic costs of maintaining homeostasis. The sum of estimated RMR, activity energy expenditure (FAO/WHO), and dietary-induced thermogenesis is a common means of estimating TEE. However, these RMR and TEE estimations may be inappropriate in non-Western settings. Direct studies of TEE and RMR in humans and other species suggest that individuals adapt to increased activity levels via behavioral and physiological energy-sparing mechanisms, reducing RMR, other non-muscular activity, and TEE within narrow ranges (Dugas et al. 2011; Heini et al. 1991; Pontzer 2015). Further, standard RMR equations do not incorporate immune response and other maintenance costs that are commonly elevated outside of socioeconomically developed countries. The impact of prolonged immune activation from infection and enhanced baseline immunity on RMR in human populations facing high pathogenic burdens and energetic limitation is not currently known.
One set of vital metabolic challenges faced by all organisms including humans includes acute and chronic immune activation, and other maintenance functions that help tolerate or defend against pathogenic assault. Species such as primates with slow life histories specialize in energetically expensive specific defenses like cell-mediated and antibody-mediated immunity, rather than cheaper non-specific defenses like inflammation (Lee 2006). While maintenance of the immune system can be metabolically expensive, immune activation in particular is costly; a number of studies using avian and rodent models have quantified the energetic costs of infection or immune activation, while others have shown that energetic stress compromises certain immune function components (Frankenfield et al. 1994; Lochmiller and Deerenberg 2000; Nieman et al. 1990; Schmid-Hempel 2003; Zuk and Stoehr 2002). In humans, RMR increases by 8-14% in university students during an acute respiratory infection (Muehlenbein et al. 2010). RMR increases by 30% with sepsis (Carlsohn et al. 2011; Kreymann et al. 1993). Even vaccinations (e.g. typhoid fever) can raise RMR by 16% (Cooper et al. 1992). In humans and other species energetic costs of immune activation from infection or tissue injury may trigger “sickness behavior”, a broad coordinated adaptive response to promote energy conservation and reallocation (Hart 1988; Stieglitz et al. 2015b).
These estimates of acute immune response suggest that daily energy requirements vary depending on local pathogen burden, and all else equal may be higher in populations with chronically high pathogen load. Alternatively, such populations may adapt metabolically and/or behaviorally to chronic immune system activation and maintain TEE similar to that in other, less pathogenically burdened populations. Metabolic response to pathogens may also change with age, as maintenance becomes increasingly costly, and in response to other external pressures. Human metabolic adaptation and inherent energy allocation trade-offs may be less severe when more food is available (Pontzer et al. 2016). Similarly, the widely documented 1-2% per decade decline in adult RMR in industrialized populations (Elia et al. 2000; Fukagawa et al. 1990; Manini 2010; Van Pelt et al. 2001; Vaughan et al. 1991) may not generalize to pre-industrial populations with higher pathogen burden. Such population-level differences in metabolic adaptation and age-related decline could contribute to differences in obesity, chronic disease, and premature mortality (Fabbri et al. 2014; Ruggiero and Ferrucci 2006). To date, however, there have been few studies of human RMR in a free-living, energy-limited population with high rates of infectious morbidity. While RMR has been measured among Siberian Evenki, Keto and Yakut (Katzmarzyk et al. 1994; Snodgrass et al. 2006), Bolivian Aymara (Kashiwazaki et al. 1995; Kashiwazaki et al. 2009) and in low income countries (see Dugas et al. 2011), none of these studies have related RMR to indicators of infection or immune activation.
Here we estimate RMR among the Tsimane, a physically active population of forager-horticulturalists inhabiting a pathogen-rich environment in Amazonian Bolivia. We first assess the extent to which RMR measured by indirect calorimetry differs from RMR estimated from common prediction equations, and then test whether differences can be explained by a combination of environmental, anthropometric and health variables indicating infectious burden. We test whether indicators of infection are associated with higher RMR controlling for potential confounders, and then assess whether Tsimane RMR declines more slowly with age compared to hygienic, sedentary industrialized populations. We also measure TEE in a subset of our sample to examine the proportion of total daily energy requirement accounted for by RMR. By examining age-related changes in anthropometrics (e.g. fat and fat-free mass) and infection status, we test whether accounting for these conditions substantially attenuates the decline in Tsimane RMR with age.
Materials and Methods
Study Population
The Tsimane are forager-horticulturalists (population ~15,000) living in the Beni Department of the Bolivian Amazon, dispersed across 90+ villages ranging in size from 40-550 inhabitants. Many Tsimane are isolated from modern society and have not yet undergone an epidemiological and technological transition. Only two villages have any electricity (albeit intermittent), and there is no running water, sanitation, or waste management. Below we highlight relevant details about diet, physical activity and infection.
Tsimane diet remains largely traditional, with 66% of calories derived from cultivated staples (plantains, rice, manioc, corn), 17% from wild game, 7% from freshwater fish, and 6% from fruits and nuts. Estimated dietary contributions from carbohydrates, protein and fat are 72%, 14% and 14%, respectively (Martin et al. 2012). Less than 10% of calories come from market-derived foods. Obesity is rare in adulthood; Tsimane have 8-10 times lower levels of obesity than age- matched US peers (Gurven et al. 2009).
Tsimane are physically active throughout adulthood, spending roughly 5-6 hours/d in moderate activity; male and female estimated physical activity levels (PALs), using a combination of accelerometry and heart rate, are 2.15 and 1.85, respectively (Gurven et al. 2013). Tsimane living near the local town are not less active than Tsimane from other regions, perhaps because of the physical activity required for common wage labor options (e.g., logging, cash cropping). Men have higher PALs than women, although men’s activity exhibits more seasonal and age-related variation. Older Tsimane adults remain active but generally engage in less physically demanding activities with age because of greater infirmity. This is especially apparent for men, as their PAL declines by 10%-20% from the peak (achieved in the late 20s) to older adulthood (age 60+ years). Tsimane VO2max matches that of other subsistence populations, and is higher than estimates from industrialized populations. Their VO2max also declines more slowly than age-matched Canadians using a similar measurement method (Pisor et al. 2013). Tsimane also show reduced vascular aging (e.g. low rates of hypertension), perhaps due to their relatively high level of physical activity (Gurven et al. 2012a).
Tsimane are frequently diagnosed with an infection during annual clinical exams conducted by the Tsimane Health and Life History Project (THLHP) (respiratory: 20-30%, gastrointestinal: 10-30%, skin: 5%) (Gurven et al. 2012b). Elevated levels of white blood cells (WBCs) (>10,000 cells/mm3) are 10 times more prevalent among Tsimane than in the US. WBCs also decline with age among Tsimane, particularly lymphocytes and eosinophils, suggesting increasing maintenance costs because older adults are not less likely to experience infection than younger adults (Gurven et al. 2009). Systemic immunity shows many indications of chronic activation from infection with helminths, with 70% of Tsimane infected at any given time (Blackwell et al. 2011; Blackwell et al. 2013; Blackwell et al. 2015; Blackwell et al. 2016); coinfection is not uncommon (Blackwell et al. 2013; Martin et al. 2013). Serum immunoglobulins are two orders of magnitude higher than among US adults, especially for IgE (highly indicative of infection with helminths) (Blackwell et al. 2011). On average, 20% of WBCs are eosinophils, also indicative of intense parasitic infection, compared with the normal US reference range of <5%, with >90% of Tsimane adults in the clinically high range. Natural killer cells and B cell counts are approximately twice as high as typical US values (Blackwell et al. 2016). Tsimane also demonstrate higher levels of inflammation than those found in industrialized populations. C-reactive protein (CRP) is elevated in children, consistent with immune activation due to chronic exposure to acute infections, and increases with age (Gurven et al. 2009). Erythrocyte sedimentation rate (ESR) is also extremely high (Table 1), with mean levels of 27 mm/hour for males and 37 for females, compared with US reference ranges of <15 and <20, respectively.
Table 1.
Study sample description. Means ± SD shown for relevant variables.
| Female | Male | |||
|---|---|---|---|---|
|
|
||||
| Variable | 20-39 (n=236) | 40+ (n=415) | 20-39 (n=209) | 40+ (n=440) |
| RMR (kcals/day) | 1660 ± 320 | 1632 ± 327 | 1991 ± 341 | 1986 ± 359 |
| Age (years) | 31.2 ± 5.2 | 53.6 ± 11.4 | 31.9 ± 5 | 53 ± 10.5 |
| Weight (kg) | 57.5 ± 9.2 | 54.7 ± 9.5 | 62.4 ± 8 | 62.8 ± 8.8 |
| Height (cm) | 151.4 ± 6.3 | 150.4 ± 6.1 | 162.7 ± 6.4 | 161.5 ± 5.5 |
| FFM (kg) | 41.8 ± 4.6 | 39.8 ± 4.9 | 52.3 ± 6.1 | 50.4 ± 5.9 |
| Body Fat % | 26.4 ± 7.6 | 26.5 ± 7.1 | 16 ± 5.1 | 19.1 ± 6.2 |
| BMI (kg/ht2) | 25.1 ± 3.7 | 24.2 ± 4.1 | 23.6 ± 2.8 | 24 ± 2.9 |
| Hb (g/ml) | 12.5 ± 1.2 | 13 ± 1.2 | 14 ± 1.2 | 13.9 ± 1.4 |
| ESR (ml/mm) | 28.9 ± 11.9 | 27.1 ± 13.8 | 22.2 ± 11.5 | 21.1 ± 13.3 |
| WBC (×103 cells/uL) | 10.2 ± 2.7 | 9.4 ± 2.7 | 10.3 ± 3.1 | 9.5 ± 2.6 |
| Clinical diagnoses: | ||||
| Clincal helminths | 0.06 ± 0.24 | 0.16 ± 0.37 | 0.02 ± 0.15 | 0.16 ± 0.37 |
| Respiratory | 0.17 ± 0.38 | 0.18 ± 0.38 | 0.16 ± 0.36 | 0.09 ± 0.29 |
| Back pains | 0.23 ± 0.42 | 0.29 ± 0.46 | 0.39 ± 0.49 | 0.39 ± 0.49 |
| Gastrointestinal | 0.26 ± 0.44 | 0.23 ± 0.42 | 0.19 ± 0.4 | 0.16 ± 0.37 |
| All infection | 0.55 ± 0.76 | 0.55 ± 0.73 | 0.36 ± 0.57 | 0.49 ± 0.66 |
Participants
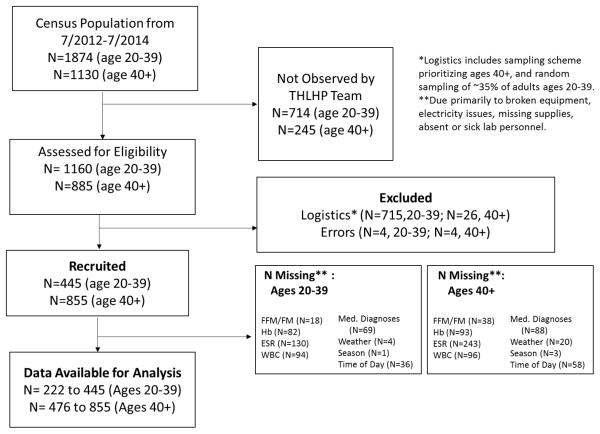
Study participants were adults aged 20+ years (mean±SD= 45.8±13.9, range: 20-90) across 46 villages visiting the THLHP mobile (within-village) health clinic for medical consultation by project physicians. This clinic was also composed of laboratory technicians trained to analyze biomarkers of infection, and bilingual (Spanish-Tsimane) research assistants conducting interviews. THLHP participation rates are ~85% of the sampled population (random sampling for ages 20-39 and near-complete sampling for ages 40+). Figure 1 provides a flowchart describing the sampling and participant recruitment. Data collection occurred from January 2012 through November 2014, including 445 adults age 20-39 (53% female) and 855 adults age 40+ (48% female). Tsimane total fertility rate is 9.1 births, breastfeeding duration is 19.2±7.3 months, and interbirth intervals are 30.7±10.6 months (McAllister et al. 2012; Veile et al. 2014); we thus estimate that 62% and 29% of women age 20-39 in our sample are breastfeeding or pregnant, respectively.
Figure 1.
Flowchart of participant recruitment and sample.
Resting metabolic rate (RMR)
RMR was measured using the Fitmate MED indirect calorimetry system (Cosmed, Italy). The seated participant relaxed while wearing an RMR mask during an initial habituation phase (~5 minutes), which was followed by 10 minutes of continuous data acquisition. The Fitmate employs a turbine flowmeter for measuring ventilation and a galvanic fuel cell oxygen sensor for analyzing the fraction of oxygen in expired gases. Sensors measure humidity, temperature, and barometric pressure for use in internal calculations. The Fitmate uses standard metabolic formulas to calculate oxygen uptake. Fitmate monitors oxygen uptake (VO2), ventilation (Ve), respiratory frequency (Rf), heart rate (HR) and fraction of O2 expired (FeO2). RMR (kcal/day) is estimated by a modified Weir equation: RMR = [5.675×VO2 + 1.593×VCO2 −21.7], where VO2 is the volume of oxygen in the breath (ml/min), and VCO2 is carbon dioxide output (ml/min) (Weir 1949). VCO2 is not measured directly but estimated assuming a fixed respiratory quotient (RQ) of 0.85, which has been shown to introduce little error in RMR estimation (Nieman et al. 2005; Nieman et al. 2003). The Fitmate is portable, easy to use, and has been validated against the Douglas bag system (Nieman et al. 2006), and it shows very high inter- and intra-day test-retest reliability for RMR measurement (Campbell et al. 2014).
Due to field conditions, a number of deviations from standard protocol were necessary. First, standard protocol requires 12 hours of fasting, which we could not guarantee, especially as measurements were taken throughout the day during THLHP surveillance (3.1% of RMR assessments began <8am, 61.4% between 8am-noon, 4.9% noon-2pm, 26.2% 2pm-5pm, 4.4% >5pm). 75.1% of participants reported having last eaten within 5 hours, 3.9% between 5-10 hours, and 20.9% 10+ hours prior to RMR testing. Time of day and time since the patient last ate were thus used as controls in all analyses. Second, temperature varied across days, and maintaining a temperature-controlled setting was not possible. Daily ambient temperature, humidity and precipitation were obtained from meteorological measures taken at the nearby San Borja airport (http://www.wunderground.com/history/airport/SLRY), and used as additional controls. Third, it was not possible to prevent physical activity during the 12 hours prior to RMR assessment. We also conservatively control for season (52.2% sampled in “dry” from May to August; 15.5% in “wet” from December to March; 32.3% in “other” during April and from September-November) because activity, pathogen burden, diet and climate can vary throughout the year.
Estimated RMR was based on six standard prediction equations devised for settings where direct or indirect calorimetry is unavailable: Oxford (Henry 2005), FAO (FAO/WHO/UNU 1985), Cunningham (Cunningham 1980), Harris-Benedict (Harris and Benedict 1918), Mifflin-St. Jeor (Mifflin et al. 1990), and Owen Weight (Owen et al. 1987; Owen et al. 1986) equations. These all use age, sex and anthropometric measures to estimate RMR, and a number of analyses have shown that different equations have varying degrees of accuracy depending on the age, ethnicity, physical fitness, body size and composition of the study sample (Frankenfield et al. 2005). Anthropometric measures include weight and height (except for Owen, which uses only weight) in all but the Cunningham equation, which instead uses fat-free mass; its reliance on fat-free mass has led some researchers to argue that Cunningham is more relevant for active populations than the other equations (Carlsohn et al. 2011; De Lorenzo et al. 1999). The Oxford equations were developed due to oversampling of Italians and undersampling of people from the tropics in formulation of the FAO equations, and tend to generate lower RMR estimates than the other equations (Henry 2005).
Total Energy Expenditure (TEE)
TEE (kcal/day) was measured in a subset (n=40, 44% male; mean±SD age: 48.6±14.2) using the doubly labeled water method (Speakman 1997). After providing a baseline urine sample, subjects ingested 114g (males) or 79g (females) of water enriched to 6% 2H2O and 10% H218O. Six urine samples were collected over the subsequent 12 days, and sent frozen to the Pontzer Lab (Hunter College, New York, USA) for determination of isotope concentrations (2H and 18O) via cavity ring down spectrometry (L2120i, Picarro Inc., Santa Clara CA, USA). Isotope dilution spaces and elimination rates were calculated via the slope-intercept method and used to calculate the mean rate of carbon dioxide production using equation 17.15 in Speakman (1997). Carbon dioxide production was converted to TEE using the modified Weir equation, assuming a respiratory quotient of 0.93, following dietary macronutrient estimates described in Martin et al. (2012). Isotope dilution was also used to determine fat-free mass for these subjects.
Physical activity was measured by accelerometry counts based on a three-day sample with an Actigraph GT3X accelerometer (Actigraph LLC, Pensacola, FL) in a subset of participants (n=28) in order to assess the relative impact of physical activity on TEE (see Gurven et al. 2013 for additional details).
Anthropometrics and biomarkers of infection
Height and weight were measured during medical exams using a Seca 213 portable stadiometer and Tanita scale (BF680). The scale also recorded body fat percentage by bioelectric impedance, which was used to calculate fat-free mass (FFM) and fat mass based on proprietary prediction equations. The TEE subsample permits a validation of the Tanita-based anthropometric measures. Correlations between Tanita-based and isotope dilution methods for FFM, fat mass and weight are 0.91, 0.74, and 0.91, respectively (all p’s<0.0001).
In-field blood analysis of fasting venous samples using the QBC Diagnostics dry hematology system (Drucker Diagnostics Inc., Port Matilda, PA) provided estimates of hemoglobin (Hb) and WBC. ESR was measured via the Westergren method (Westergren 1957). THLHP project physicians diagnosed illnesses and trauma presented by patients with the aid of bilingual Tsimane assistants. Diagnoses from the International Classification of Disease (ICD-10) are grouped into several categories, including respiratory ailments, back pain, and intestinal helminths. The latter category was based on having clinical symptoms of intestinal infection, and supplemented by laboratory confirmation from fecal samples analyzed by direct microscopy when possible (30.1% of cases) (Blackwell et al. 2013).
Ethics
Informed consent was obtained for all protocols from the Tsimane government that represents Tsimane interests and oversees research projects, from village officials for each participating village, and from all study participants. Consent procedures and protocols were approved by the University of California, Santa Barbara and University of New Mexico Institutional Review Boards.
Statistical Analyses
Multiple linear regressions of RMR and TEE were performed using SAS 9.3. Stepwise regressions using Akaike’s Information Criterion (AIC)-based stop criterion were used to determine best-fit models. Comparative analyses of RMR and TEE were performed by ordinary least squares (OLS) regression using 185 indirect calorimetry studies compiled by Dugas and colleagues (Dugas et al. 2011). To assess age changes in RMR, a series of regression models were conducted on adults age 40+, although some data were missing resulting in varying sample sizes (see Figure 1). Controlling for age and sex, cases with missing data had only slightly lower RMR (est=−42.6, p=0.065, β=−0.05). Nonetheless, analyses were run on several datasets created to insure that missing data did not skew results. These four datasets include: a) listwise deletion (n=471); b) raw data (n=471 to 855); c) imputed data using stochastic regression (n=855); and d) multiple imputations using Markov Chain Monte Carlo (MCMC) (n=855).
Results
Mean±SD RMR for men and women age 20+ is 1988±353 and 1642±325 kcals/day, respectively (Table 1). Men have higher RMR than women at all ages; RMR plateaus from ages 20-39, then declines with age thereafter (Figure 2). TEE for men and women is 3065±422 and 2186±366 kcals/day, respectively. The proportion of TEE that is RMR is higher in women (β=−0.33, p=0.05) and by age (β=0.31, p=0.07, n=32); predicted RMR/TEE from ages 20 to 80 is 63.7-78.6% for women and 56.3-71.1% for men.
Figure 2.
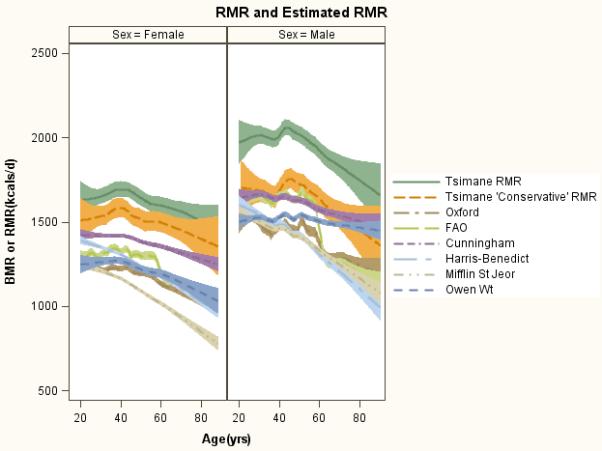
Measured and estimated RMR by age and sex. Estimations based on six prediction equations that have been widely applied to different populations. Tsimane ‘conservative’ RMR is a loess spline of adjusted raw data, using average regression cofficients of excess RMR across the six equations, correcting for season, time since last meal, time of day and precipitation. Mean±SD “conservative” RMR is 1678±354 for men and 1526±322 for women.
TEE and RMR
We examine whether RMR is associated with TEE, and whether the association is independent of FFM and physical activity. RMR is highly correlated with TEE (Pearson r=0.63; p<0.0001, df=36). Mean ± SD ratio of RMR/TEE is 0.71±0.12 for women (range: 0.56-0.90), and 0.64±0.10 for men (range: 0.49-0.85), well within the range observed in other populations. FFM alone accounts for 73% of the variance in TEE (p<0.0001, df=36, est=52.4±5.3). No other demographic or anthropometric variables (sex, age, height, fat mass, total body mass) are significantly associated with TEE in multiple regression including FFM (all other p’s>0.10). RMR remains positively associated with TEE after controlling for accelerometer-based estimates of calories expended per hour of physical activity (RMR: β=0.56, p<0.001; activity: β=0.37, p=0.019; Adj R2=0.57), but its magnitude and significance are reduced after controlling for FFM (RMR: β=0.18, p=0.150; FFM: β=0.70, p<0.0001; activity: β=0.19, p=0.076; Adj R2=0.81).
Do Tsimane have elevated RMR and TEE?
Tsimane measured RMR is 18-47% higher in women and 22-40% higher in men than BMR estimated using the six standard prediction equations (Supporting Information Table S1), including Oxford (Henry 2005), FAO (FAO/WHO/UNU 1985), Cunningham (Cunningham 1980), Harris-Benedict (Harris and Benedict 1918), Mifflin-St. Jeor (Mifflin et al. 1990), and Owen Weight (Owen et al. 1987; Owen et al. 1986) equations. Pearson correlations between measured and estimated RMR range from 0.32-0.41. Even the most accurate prediction (Cunningham) underestimates RMR by 253 kcals/day in women and 365 kcals/day in men (Supporting Information Table S1). RMR is only 55 (n=65) and 82 (n=77) kcals/day lower in women and men, respectively, after conservatively eliminating samples within 5 hours of eating, during mid-day and afternoon from noon-5pm and during dry season months; these lower RMR measures remain 14-42% higher (in women) and 17-35% higher (in men) than expected based on the five prediction equations.
Tsimane RMR is high relative to other studies where RMR is similarly measured by indirect calorimetry. Using energetic and anthropometric data from a recent meta-analysis, we compare Tsimane RMR with samples from “high” and “low” socioeconomic development as assessed by the Human Development Index (HDI) (Dugas et al. 2011). We find that Tsimane women’s RMR is higher than 11/11 of low or middle HDI samples, and 71/79 (90%) high HDI samples; men’s RMR is higher than 9/9 of low HDI samples, and 46/48 (96%) high HDI samples. Most strikingly, Tsimane RMR is higher by 482 kcals (p<0.0001) than RMR in 31 countries from 150 samples, after controlling for sex, mean age, body mass and physical activity (PAL) (generalized linear model, n=148, Adj R2=0.88) (Supporting Information Table S2, Figure 3).
Figure 3.
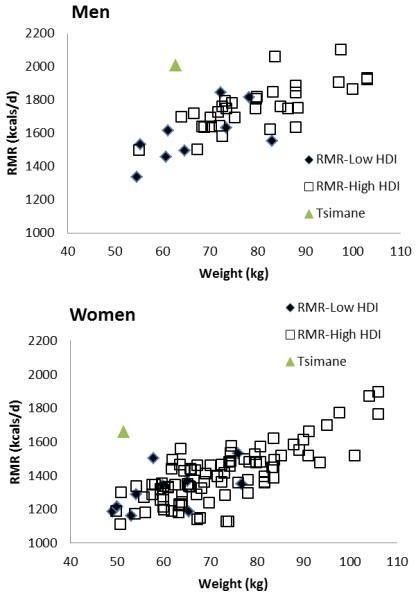
RMR in comparative perspective for adults. Comparative data from Dugas et al. 2011: Appendix. RMR is shown separately for developing (low or middle Human Development Index (HDI) populations, solid diamonds) and developed societies (high HDI, blue squares). Green triangle represents Tsimane.
Tsimane TEE is also high relative to other populations (Supporting Information Table S3). Tsimane TEE is 284 kcals/day higher relative to other populations (β=0.06, p=0.10) when controlling for weight, age and sex. When PAL is included in the models as an additional covariate, the Tsimane “excess” TEE reduces to 177 kcals/day (Supporting Information Table S3: Model 3). If RMR is added instead of PAL to the models, the Tsimane no longer appear different than other populations (p=0.88) (Supporting Information Table S3: Model 2).
Predictors of excess RMR
We first assess whether groups of variables summarizing anthropometric status, weather conditions at time of study, and medical diagnoses predict “excess” RMR, i.e. the deviation of measured RMR from its estimation based on the most conservative prediction equation (Cunningham), after controlling for age, sex, season, time of day, and time last eaten. Being taller, and having greater fat mass but lower weight are associated with having excess RMR (Table 2: Model 1). Lower daily average temperature, mean humidity, and precipitation are also associated with excess RMR (Table 2: Model 2). Lastly, excess RMR is higher among those with clinical symptoms of intestinal helminth infection, greater immune activation as indicated by elevated WBCs, high hemoglobin and back pains (Table 2: Model 3). In all models, men have greater excess RMR, and excess RMR is greatest in the dry season.
Table 2.
Predictors of “excess” RMR.
| Model 1: Anthropom (n=1155) |
Model 2: Weather (n=1139) |
Model 3: Medical (n=702) |
Model 4: Full (n=691) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Parameter | Est. | β | p | Est. | β | p | Est. | β | p | Est. | Β | p |
| Intercept | −697.1 | 0.00 | 0.0053 | 693.5 | 0.00 | <.0001 | −280.5 | 0.00 | 0.0508 | −927.9 | 0.00 | 0.0017 |
| Sex (1=male) | 188.1 | 0.29 | <.0001 | 115.6 | 0.18 | <.0001 | 108.9 | 0.17 | <.0001 | 178.3 | 0.28 | <.0001 |
| Age (yrs) | −1.2 | −0.05 | 0.0933 | |||||||||
| Time (<8:00 vs. 8-12pm) | 88.4 | 0.05 | 0.087 | 81.6 | 0.04 | 0.1241 | 62.9 | 0.03 | 0.3342 | |||
| [12-2pm vs. 8-12pm) | 60.2 | 0.04 | 0.1661 | 68.1 | 0.04 | 0.1306 | 123.3 | 0.09 | 0.0178 | |||
| [2-5pm vs. 8-12pm) | 63.3 | 0.09 | 0.0026 | 53.5 | 0.07 | 0.014 | 58.8 | 0.08 | 0.0311 | |||
| (>5pm vs. 8-12pm) | 39.0 | 0.02 | 0.4516 | −31.7 | −0.02 | 0.4914 | 40.5 | 0.03 | 0.5074 | |||
| Hrs ago ate (0-4 vs. 5-9) | 89.1 | 0.11 | 0.0954 | 134.3 | 0.17 | 0.0161 | 146.6 | 0.19 | 0.0257 | |||
| (10+ vs. 5-9) | 132.5 | 0.16 | 0.0179 | 115.9 | 0.14 | 0.0547 | 128.4 | 0.15 | 0.0598 | |||
| Season (Dry vs. wet) | 128.8 | 0.20 | <.0001 | 110.7 | 0.17 | 0.0002 | 136.7 | 0.21 | 0.0002 | 122.0 | 0.19 | 0.0007 |
| (Other vs. wet) | 76.9 | 0.11 | 0.0061 | 40.8 | 0.06 | 0.1721 | 77.2 | 0.12 | 0.0347 | 85.4 | 0.13 | 0.0162 |
| Fat-free mass (kg) | −8.7 | −0.20 | 0.0002 | |||||||||
| Fat mass (kg) | 22.7 | 0.42 | <.0001 | 12.7 | 0.24 | <.0001 | ||||||
| Height (cm) | 6.4 | 0.16 | 0.0002 | 6.5 | 0.17 | 0.0017 | ||||||
| Weight (kg) | −9.2 | −0.27 | <.0001 | . | ||||||||
| Mean Daily Temp (°C) | −5.3 | −0.11 | 0.0006 | |||||||||
| Mean Daily Humidity (%) | −2.1 | −0.07 | 0.0153 | |||||||||
| Daily Precipitation (mm) | −89.7 | −0.05 | 0.1123 | −144.2 | −0.08 | 0.0294 | ||||||
| Hemoglobin (mg/L) | 16.0 | 0.07 | 0.098 | |||||||||
| WBC (×103 cells/uL) | 8.8 | 0.08 | 0.0363 | 10.1 | 0.09 | 0.0142 | ||||||
| Helminths (1=yes) | 150.5 | 0.14 | 0.0002 | 131.3 | 0.13 | 0.0008 | ||||||
| Back pains (1=yes) | 47.5 | 0.07 | 0.0547 | 50.7 | 0.08 | 0.0347 | ||||||
|
| ||||||||||||
| Model Fit | Adj R2=0.1322 | Adj R2=0.1139 | Adj R2=0.1040 | Adj R2=0.1732 | ||||||||
Note: Excess is defined as measured RMR – estimated RMR from Cunningham prediction equation. Models consider variables on anthropometrics, weather, infection/ medical status, with age, sex, time since eaten, and time of day as controls. Each model employs stepwise selection method with AIC selection criterion. Additional variables in the models that were excluded from the stepwise selection include fat-free mass, fever, giardia (1=yes), respiratory ailment (1=yes), gastrointestinal ailment (1=yes). “est.” refers to unstandardized parameter estimate; β is the standardized regression coefficient
Stepwise regression with AIC stop criterion to yield a best-fit model starting with all variables, suggests that variables from all three macro-categories are associated with excess RMR (Table 2: Model 4; Supporting Information Table S4). Having recently eaten (std β=0.19-0.21), being male (β=0.11-0.29), taller (β=0.08-0.17), fatter (β=0.09-0.14) and older (β=0.07-0.19) have moderate to large effect sizes for excess RMR, but these are not consistently statistically significant in all best-fit models based on the five prediction equations (Supporting Information Table S4). We use average regression coefficients from the full models of sex-specific excess RMR to derive a “conservative” estimate of RMR by subtracting the effects of season, time of day, time since last meal and precipitation from an individual’s measured RMR, and then construct a loess smooth of “conservative” RMR in Figure 2. “Conservative” RMR is substantially lower than measured RMR, especially in men, where values overlap in late adulthood with predicted RMR based on the Cunningham equation.
Elevated WBCs (β=0.08-0.10), intestinal helminths (β=0.11-0.13) and greater back pain (β=0.07-0.09) were consistently associated with excess RMR across all six best-fit models. Based on the full regression models in Supporting Information Table S4, an adult diagnosed with helminths and marked WBC elevation (additional 3.0 × 106 cell/uL) can expect to have excess RMR of 143-168 kcals/day, depending on the prediction equation used. This amount reflects increases of 10-12% and 11-15% above predicted mean RMR in men and women, respectively, which are independent of other covariates. No interactions between anthropometric variables and WBC count, helminths or ESR are significant when added to the best fit models. The best fit models retained a number of the control variables: recent eating is associated with excess RMR of 140-160 kcals/day, dry season sampling with 105-129 kcals/day, and mid-day sampling with 117-136 excess RMR kcals/day. Despite the size and number of significant effects, our best-fit models explain only 9-19% of the variance in excess RMR, depending on which estimation equation is used as the baseline (Supporting Information Table S4).
Women’s lactation (proxied by having an infant <18 months) is associated with 223 kcals/d higher RMR (p=0.025) when controlling for age, time of day, last eaten, ambient temperature, precipitation, and season. However, the effect of lactational status is reduced to 170 kcals/d (p=0.067) in models that include anthropometric variables, and loses significance when additionally controlling for indicators of infection or immune activation (est=129, p=0.225). Because of this confounding with other variables, lactation did not appear in any of the best-fit models. Pregnancy was not significant in any of the models of RMR. Rather than being unimportant variables, we instead suspect that there is too little variation in pregnancy or lactation status among Tsimane women age 20-39 as 91% of women are either pregnant or breastfeeding. Unfortunately we do not have data on pregnancy trimester nor on breastfeeding intensity to obtain more fine-grained results.
RMR Decline with Age
RMR changes minimally from ages 20-39, but then declines starting by age 40 years (Figure 2). We test the extent to which this age decline diminishes after controlling for other time-varying factors that are associated with RMR. Supporting Information Figures S1 and S2 show the age profiles of anthropometric and biomedical variables for men and women. FFM, height, weight and WBC count decline with age in both sexes. Fat mass tends to decline after age 50 in women; fat mass and body fat percentage tend to increase with age in men. Serum hemoglobin tends to increase with age in women, but decreases with age in men. ESR shows a greater increase in men than women, though it remains higher in women overall.
We add covariates in a stepwise fashion to regression models of RMR on age for adults aged 40+ years and examine the change in the age slope (Table 3, Supporting Information Tables S6-S8). The baseline model controlling for sex shows a 69 kcal/decade decline in RMR (p<0.0001). Adding controls for ambient weather, time of day, and time since last eaten reduces the baseline age slope by 9%. It reduces further by 71% after considering FFM and height. Consideration of biomedical variables slightly improves overall model fit in Models 5-9 and reduces statistical significance of the age effect, but does not substantially alter its magnitude (range: 67-89% below baseline age effect). A stepwise regression model with AIC stop criterion using all the variables of Model 9 in Table 3 shows an age effect that is 68% below the baseline estimate. This level of decline is about 1.2% per decade from an initial 1,826 kcals/day average from ages 20-39. Overall, at least two-thirds of the decline in RMR with age is due to changes in other aspects of phenotypic condition indicating nutritional and health status. Results vary only minimally when not restricting the dataset to non-missing cases (Supporting Information Table S6) or when imputing missing data using two different methods (Supporting Information Tables S7 & S8).
TABLE 3.
Rate of RMR decline with age for adults age 40+.
| Model | Parameter estimate: AGE |
Std. error |
Pr > ∣t∣ | β | % Reduced from Model 1 |
% Decline per Decade |
Adj R2 |
Controlling for: |
|---|---|---|---|---|---|---|---|---|
| 1 | −6.85 | 1.05 | <.0001 | −0.194 | ------- | 3.7 | 0.246 | sex |
| 2 | −6.25 | 1.08 | <.0001 | −0.178 | 8.7 | 3.4 | 0.269 | + time since last ate, time of day, mean ambient temperature that day, mean precipitation that day |
| 3 | −2.30 | 1.12 | 0.0405 | −0.066 | 66.4 | 1.3 | 0.350 | + FFM |
| 4 | −1.96 | 1.12 | 0.0822 | −0.056 | 71.4 | 1.1 | 0.355 | + ht |
| 5 | −0.89 | 1.22 | 0.4674 | −0.025 | 87.0 | 0.5 | 0.353 | + Hb |
| 6 | −0.74 | 1.40 | 0.5977 | −0.021 | 89.2 | 0.4 | 0.351 | + ESR, WBC |
| 7 | −2.08 | 1.44 | 0.1486 | −0.060 | 69.6 | 1.1 | 0.367 | + helminth |
| 8 | −2.29 | 1.45 | 0.1151 | −0.066 | 66.6 | 1.3 | 0.368 | + fever |
| 9 | −2.28 | 1.45 | 0.1161 | −0.066 | 66.7 | 1.2 | 0.368 | + respiratory, giardia, back pains |
| step | −2.18 | 1.44 | 0.1311 | −0.063 | 68.1 | 1.2 | 0.367 | (+ sex, FFM, ht, Hb, WBC, helminths, respiratory infection, precipitation, time since last eaten) |
Effects of adding additional covariates to regression models on the rate of RMR decline with age. Model 1 is the baseline model controlling only for age. “Step” model is a stepwise regression with AIC stop criterion starting with all variables from Model 9.
Discussion
Tsimane RMR is much higher than predicted by standard equations that rely only on age and anthropometric measures. Standard equations are often poor predictors of RMR in select samples, such as professional athletes. For example, Harris-Benedict and Cunningham equations have grossly underestimated RMR in male heavyweight endurance athletes (Carlsohn et al. 2011). Prediction equations underestimated RMR in male rowers and canoeists by 133-202 kcals/day (Carlsohn et al. 2011). FAO equation overestimated RMR in a Vietnamese sample by 7-14% (Nhung et al. 2005). The closest predictions with the Cunningham equation still underestimated Tsimane RMR by over 250 kcals/day. However, no prediction equation can fit all individuals and situations (Wang et al. 2001). While FFM often accounts for the majority (~50-80%) of intra-population variation in daily RMR, a high level of intra-species variation in RMR not explained by differences in FFM, age and sex suggests the importance of other processes (Henry 2000; Weiss et al. 2012).
One source of additional variation in RMR that we isolated was the high burden of pathogens in a tropical environment. The high RMR is surprising given that RMR is expected to be lower in tropical climates with higher mean temperatures (Froehle 2008; Leonard et al. 2005). The costs of immune activation can be substantial: Tsimane adults with clinical symptoms of intestinal helminth infection have excess RMR of 116-138 kcals/d (Table S4). Elevated WBC counts are 10 times more prevalent among Tsimane than Americans; Tsimane WBCs are 2,600 cells/uL higher on average than US levels among adults age 18-49 (Blackwell et al. 2016), which in our model adds 23-28 excess RMR kcals/day. These findings build upon results from studies in Western populations showing RMR increases with infection (Muehlenbein et al. 2010). However, the role of infection on energy balance and maintenance costs in community-dwelling populations is still under-appreciated. It has been estimated that quiescent WBCs require approximately 382 kcals/day, whereas activated WBCs responding to an infection require an additional 36-118 kcals/day from glucose, glutamine, ketone bodies and fatty acid sources (Straub et al. 2010). Chronic inflammation due to chronic infection and/or repeated acute infections often induces an ‘energy appeal reaction’ (i.e. redirection of energy-rich fuels from stores to activated immune cells) to fuel sustained immune activation, resulting in elevated RMR via hypothalamic-pituitary-adrenal axis and sympathetic nervous system-directed activity.
With sustained immune activation, a number of co-morbid conditions can result from prolonged energetic allocation to immune defenses, including sickness behavior, cachexia, osteopenia, dyslipidemia and anemia (Straub et al. 2010). These energy allocation decisions are regulated by circadian rhythms of interacting neuroendocrine and immune systems, which help coordinate the storage and utilization of energy throughout the day (Straub et al. 2010). Sickness behavior is common with a pro-inflammatory state, and is associated with more sedentary behavior (Dantzer et al. 2008). Depressed affect has been associated with higher inflammatory cytokines and reduced physical activity in Tsimane (Stieglitz et al. 2015b). Consistent with these and other associations of prolonged immune investment, osteopenia (Stieglitz et al. 2015a), low HDL, LDL and total cholesterol (Gurven et al. 2009) and anemia are prevalent conditions among Tsimane. Despite their relatively active lifestyle and traditional diet, Tsimane bone mineral status, HDL, LDL and total cholesterol are substantially lower than among age-matched US peers. One hypothesis to be tested in future work is that these conditions may represent consequences of high RMR due to diversion of energy to maintain sustained immune responses.
High RMR in older age has been identified as an indication that greater energetic investment is needed to repair damage and maintain functional homeostasis. The expectation in “healthy aging” is that RMR should decline with age, due to lower FFM and physical activity, but also after considering the effects of changing body composition and fat composition (Luhrmann et al. 2010). Several organs decrease in mass at later ages, as do the metabolic rates of some tissues. These changes with age may be the result of tradeoffs meant to fuel other maintenance functions. If aging involves increasing costs of maintaining homeostasis, higher RMR should be associated with increasing multisystem dysregulation, physical frailty and cachexia-like muscle loss due to insufficient energy to meet the high metabolic needs of muscle homeostasis (Ruggiero and Ferrucci 2006; Straub et al. 2010). Some evidence is consistent with the idea that high RMR in older adults is associated with health deterioration. Older US adults from the Baltimore Longitudinal Study of Aging (BLSA) with no functional limitations or medical conditions had 109 kcals/day lower RMR than those suffering from chronic conditions and comorbidities (Schrack et al. 2014). Other studies show positive associations between RMR, morbidity and mortality (Ruggiero et al. 2008), leading some to label RMR a “candidate biomarker of global health status” (Ruggiero and Ferrucci 2006; Schrack et al. 2014). We found that RMR makes up a greater proportion of TEE with age and for women (whereas in the same limited sample, activity-based expenditure did not vary with age (p=0.73)), consistent with greater metabolic needs at later ages and lower relative energy available for other allocations. RMR declined with age among Tsimane over age 40 at a similar rate as in other populations, but most of this cross-sectional age effect was reduced after controlling for variation in anthropometric and health status. Additionally accounting for variation in hemoglobin levels eliminated the age effect altogether (Table 3). The lack of a robust age decline in RMR could suggest a higher level of frailty and morbidity among older Tsimane adults relative to other populations.
Study Limitations
Field conditions limited our ability to obtain RMR measures in a completely standardized manner that is temperature controlled with participants abstaining from food and activity for 12 hours. In the current study, we show that field conditions were responsible for some of the excess RMR as would be expected; time of sampling and recency of food consumption combined account for a maximum of up to 297 excess RMR kcals/day (Supporting Information Table S4). But on average, we conservatively estimate that these effects account for at most 26-46% of the excess RMR in women and 54-85% in men (Figure 2, Table S1). We also do not report estimates of diet-induced thermogenesis, which are likely to be greater in diets high in protein and carbohydrate, and lower in fat (Westerterp et al. 1999). Additionally, RMR is about ~70 kcals/day higher in a seated rather than supine testing position (Compher et al. 2006; Levine et al. 2000). Despite these limitations, “resting state” can be difficult to define, and minimal metabolism will be sensitive to many other factors, including menstrual cycle, wakefulness and nervousness (Ruggiero and Ferrucci 2006). Our data on breast feeding are limited, and the estimated cost of lactation reported here (223 kcal/day) is lower than previous estimates (Butte and King 2005). Similarly, our pregnancy status data are limited and show no significant increase in RMR while other studies in well-nourished populations report a 90-470 kcal/day increase in RMR depending on trimester (Butte et al. 1999). Under energy-limited conditions, BMR has been observed to slightly decrease or change only minimally during pregnancy as an energy-sparing strategy, along with lowering activity expenditure or increasing dietary intake (Jasienska 2009; Lawrence et al. 1987; Poppitt et al. 1994). Another possibility is that we were unable to find an effect because there was not a large enough sample of individuals who were not pregnant or lactating (only 9% of women aged 20-39 years). Additionally these women may have underlying pathologies impeding their reproductive state, and thus may not be comparable. Lastly, the cross-sectional design limits causal inference about age changes in RMR.
Conclusion
Tsimane RMR is high in comparison with other human populations, even after adjusting for body mass and other covariates. These results are confirmed by DLW analyses showing that high Tsimane RMR is consistent with their higher mass- and age-adjusted TEE. Tsimane TEE is similar to that of other populations when controlling for their higher RMR. Bolivian highland agropastoralists show similar TEE and lean body mass as Tsimane, but lower RMR and higher PAL (Kashiwazaki et al. 1995; Kashiwazaki et al. 2009). Our findings are consistent with TEE being constrained within a relatively narrow range (Pontzer 2015), whereas, given their physically active lifestyle, an additive, unconstrained model of energy expenditure would predict even higher Tsimane TEE than we document here. If greater immune surveillance and activation require higher resting energetic expenditure in a high pathogen tropical environment, we should expect reduced allocations towards other activities and physiological processes in populations like the Tsimane, including physical activity and cognitive performance (Ezeamama et al. 2005; Gurven et al. 2013; Trumble et al. 2014). It is noteworthy that energetic limitations do not appear to shunt energy away from reproductive effort, given the high fertility, short interbirth intervals, early menarche and intenstive breastfeeding patterns observed among Tsimane (McAllister et al. 2012; Veile et al. 2014). Future studies of the patterning and causes of RMR variation with age in different populations should help provide important insights about the changing maintenance costs affecting senescence.
Supplementary Material
Acknowledgments
We gratefully acknowledge the efforts of the Tsimane and Life History Project (THLHP) team. JS acknowledges support from the Agence Nationale de la Recherche (ANR) - Labex IAST.
Grants: NIH/NIA: R01AG024119, R56AG024119, P01AG022500
Footnotes
Disclosures: No conflicts of interest, financial or otherwise, are declared by the authors.
Authors Contributions: MG conceptualized the study and wrote the paper; MG and HK designed the study; HP, BT, JS, GY, AB revised the paper. HK and MG are co-directors of The Tsimane Health and Life History Project (THLHP), under which all data were collected. GY and DC trained field personnel and along with THLHP staff collected RMR and DLW data. HP analyzed DLW samples. BT supervised field lab operations. BB, AB, BT and JS organized the datasets, MG and BT conducted statistical analyses, and had final responsibility for content.
Literature Cited
- Blackwell AD, Gurven MD, Sugiyama LS, Madimenos FC, Liebert MA, Martin MA, Kaplan HS, Snodgrass JJ. Evidence for a Peak Shift in a Humoral Response to Helminths: Age Profiles of IgE in the Shuar of Ecuador, the Tsimane of Bolivia, and the U.S. NHANES. PLoS Negl Trop Dis. 2011;5(6):e1218. doi: 10.1371/journal.pntd.0001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Martin M, Kaplan H, Gurven M. Antagonism between two intestinal parasites in humans: the importance of co-infection for infection risk and recovery dynamics. Proceedings of the Royal Society B: Biological Sciences. 2013;280(1769) doi: 10.1098/rspb.2013.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, Martin M, Kaplan H, Gurven M. Helminth infection, fecundity, and age of first pregnancy in women. Science. 2015;350(6263):970–972. doi: 10.1126/science.aac7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Trumble BC, Maldonado Suarez I, Stieglitz J, Beheim BA, Snodgrass JJ, Kaplan H, Gurven M. Immune Function in Amazonian Horticulturalists. Annals of Human Biology. 2016 doi: 10.1080/03014460.2016.1189963. doi://10.1080/03014460.2016.1189963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EOB. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. The American Journal of Clinical Nutrition. 1999;69(2):299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutrition. 2005;8(7a):1010–1027. doi: 10.1079/phn2005793. [DOI] [PubMed] [Google Scholar]
- Campbell B, Zito G, Colquhoun R, Martinez N, St Louis C, Johnson M, Buchanan L, Lehn M, Smith Y, Cloer B. Inter-and intra-day test-retest reliability of the Cosmed Fitmate ProTM indirect calorimeter for resting metabolic rate. Journal of the International Society of Sports Nutrition. 2014;11(1):1–2. [Google Scholar]
- Carlsohn A, Scharhag-Rosenberger F, Cassel M, Mayer F. Resting metabolic rate in elite rowers and canoeists: difference between indirect calorimetry and prediction. Annals of Nutrition and Metabolism. 2011;58(3):239–244. doi: 10.1159/000330119. [DOI] [PubMed] [Google Scholar]
- Compher C, Frankenfield D, Keim N, Roth-Yousey L, Group EAW Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. Journal of the American Dietetic Association. 2006;106(6):881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Cooper A, Horan M, Little R, Rothwell N. Metabolic and febrile responses to typhoid vaccine in humans: effect of beta-adrenergic blockade. Journal of Applied Physiology. 1992;72(6):2322–2328. doi: 10.1152/jappl.1992.72.6.2322. [DOI] [PubMed] [Google Scholar]
- Cunningham JJ. A reanalysis of the factors influencing basal metabolic rate in normal adults. The American Journal of Clinical Nutrition. 1980;33(11):2372–2374. doi: 10.1093/ajcn/33.11.2372. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo A, Bertini I, Candeloro N, Piccinelli R. A new predictive equation to calculate resting metabolic rate in athletes. Journal of sports medicine and physical fitness. 1999;39(3):213. [PubMed] [Google Scholar]
- Dugas LR, Harders R, Merrill S, Ebersole K, Shoham DA, Rush EC, Assah FK, Forrester T, Durazo-Arvizu RA, Luke A. Energy expenditure in adults living in developing compared with industrialized countries: a meta-analysis of doubly labeled water studies. The American Journal of Clinical Nutrition. 2011;93(2):427–441. doi: 10.3945/ajcn.110.007278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia M, Ritz P, Stubbs R. Total energy expenditure in the elderly. European journal of clinical nutrition. 2000;54:S92–103. doi: 10.1038/sj.ejcn.1601030. [DOI] [PubMed] [Google Scholar]
- Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, Olveda RM, Kurtis JD, Mcgarvey ST. Helminth infection and cognitive impairment among Filipino children. The American journal of tropical medicine and hygiene. 2005;72(5):540–548. [PMC free article] [PubMed] [Google Scholar]
- Fabbri E, An Y, Schrack JA, Gonzalez-Freire M, Zoli M, Simonsick EM, Guralnik JM, Boyd CM, Studenski SA, Ferrucci L. Energy metabolism and the burden of multimorbidity in older adults: results from the Baltimore Longitudinal Study of Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2014 doi: 10.1093/gerona/glu209. glu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO/UNU . Protein and energy requirements. WHO; Geneva: 1985. [Google Scholar]
- Frankenfield D, Roth-Yousey L, Compher C, Group EAW Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. Journal of the American Dietetic Association. 2005;105(5):775–789. doi: 10.1016/j.jada.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Frankenfield DC, Wiles CE, III, Bagley S, Siegel JH. Relationships between resting and total energy expenditure in injured and septic patients. Critical care medicine. 1994;22(11):1796–1804. [PubMed] [Google Scholar]
- Froehle AW. Climate variables as predictors of basal metabolic rate: New equations. American Journal of Human Biology. 2008;20(5):510–529. doi: 10.1002/ajhb.20769. [DOI] [PubMed] [Google Scholar]
- Fukagawa NK, Bandini LG, Young JB. Effect of age on body composition and resting metabolic rate. American Journal of Physiology-Endocrinology and Metabolism. 1990;259(2):E233–E238. doi: 10.1152/ajpendo.1990.259.2.E233. [DOI] [PubMed] [Google Scholar]
- Gurven M, Blackwell AD, Rodríguez DE, Stieglitz J, Kaplan H. Does Blood Pressure Inevitably Rise With Age? Novelty and Significance Longitudinal Evidence Among Forager-Horticulturalists. Hypertension. 2012a;60(1):25–33. doi: 10.1161/HYPERTENSIONAHA.111.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Jaeggi AV, Kaplan H, Cummings D. Physical Activity and Modernization among Bolivian Amerindians. PLoS ONE. 2013;8(1):e55679. doi: 10.1371/journal.pone.0055679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Eid D, Vasunilashorn S, Kim J, Finch C, Crimmins E. Inflammation and infection do not promote arterial aging and cardiovascular disease among lean Tsimane forager-horticulturalists. PLoS ONE. 2009;4(8):e6590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurven M, Stieglitz J, Hooper PL, Gomes C, Kaplan H. From the womb to the tomb: The role of transfers in shaping the evolved human life history. Experimental Gerontology. 2012b;47:807–813. doi: 10.1016/j.exger.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Benedict FG. A biometric study of human basal metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1918;4(12):370. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Reviews. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Heini A, Schutz Y, Diaz E, Prentice AM, Whitehead RG, Jequier E. Free-living energy expenditure measured by two independent techniques in pregnant and nonpregnant Gambian women. American Journal of Physiology-Endocrinology and Metabolism. 1991;261(1):E9–E17. doi: 10.1152/ajpendo.1991.261.1.E9. [DOI] [PubMed] [Google Scholar]
- Henry C. Mechanisms of changes in basal metabolism during ageing. European journal of clinical nutrition. 2000;54:S77–91. doi: 10.1038/sj.ejcn.1601029. [DOI] [PubMed] [Google Scholar]
- Henry C. Basal metabolic rate studies in humans: measurement and development of new equations. Public Health Nutrition. 2005;8(7A):1133. doi: 10.1079/phn2005801. [DOI] [PubMed] [Google Scholar]
- Jasienska G. Reproduction and lifespan: Trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research. American Journal of Human Biology. 2009;21(4):524–532. doi: 10.1002/ajhb.20931. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki H, Dejima Y, Orias-Rivera J, Coward WA. Energy expenditure determined by the doubly labeled water method in Bolivian Aymara living in a high altitude agropastoral community. The American Journal of Clinical Nutrition. 1995;62(5):901–910. doi: 10.1093/ajcn/62.5.901. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki H, Uenishi K, Kobayashi T, Rivera JO, Coward WA, Wright A. Year-round high physical activity levels in agropastoralists of Bolivian Andes: Results from repeated measurements of DLW method in peak and slack seasons of agricultural activities. American Journal of Human Biology. 2009;21(3):337–345. doi: 10.1002/ajhb.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmarzyk PT, Leonard WR, Crawford MH. Resting metabolic rate and daily energy expenditure among two indigenous Siberian populations. American Journal of Human Biology. 1994;6:719–730. doi: 10.1002/ajhb.1310060606. [DOI] [PubMed] [Google Scholar]
- Kreymann G, Grosser S, Buggisch P, Gottschall C, Matthaei S, Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Critical care medicine. 1993;21(7):1012–1019. doi: 10.1097/00003246-199307000-00015. [DOI] [PubMed] [Google Scholar]
- Lawrence M, Coward W, Lawrence F, Cole T, Whitehead R. Energy requirements of pregnancy in the Gambia. The Lancet. 1987;330(8567):1072–1076. doi: 10.1016/s0140-6736(87)91492-9. [DOI] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integrative and Comparative Biology. 2006;46(6):1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Snodgrass JJ, Sorenson MV. Metabolic adaptations in indigenous Siberian populations. Annual Review of Anthropology. 2005;34:451–471. [Google Scholar]
- Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of nonexercise activity. The American Journal of Clinical Nutrition. 2000;72(6):1451–1454. doi: 10.1093/ajcn/72.6.1451. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88(1):87–98. [Google Scholar]
- Luhrmann P, Edelmann-Schafer B, Neuhauser-Berthold M. Changes in resting metabolic rate in an elderly German population: cross-sectional and longitudinal data. The journal of nutrition, health & aging. 2010;14(3):232–236. doi: 10.1007/s12603-010-0055-4. [DOI] [PubMed] [Google Scholar]
- Manini TM. Energy expenditure and aging. Ageing research reviews. 2010;9(1):1–11. doi: 10.1016/j.arr.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Blackwell AD, Gurven M, Kaplan H. Primates, Pathogens, and Evolution. Springer; 2013. Make new friends and keep the old? Parasite coinfection and comorbidity in Homo sapiens; pp. 363–387. [Google Scholar]
- Martin MA, Lassek WD, Gaulin SJ, Evans RW, Woo JG, Geraghty SR, Davidson BS, Morrow AL, Kaplan HS, Gurven MD. Fatty acid composition in the mature milk of Bolivian forager-horticulturalists: controlled comparisons with a US sample. Maternal & child nutrition. 2012;8(3):404–418. doi: 10.1111/j.1740-8709.2012.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister L, Gurven M, Kaplan H, Stieglitz J. Why do women have more children than they want? Understanding differences in women's ideal and actual family size in a natural fertility population. American Journal of Human Biology. 2012;24(6):786–799. doi: 10.1002/ajhb.22316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh Y. A new predictive equation for resting energy expenditure in healthy individuals. The American Journal of Clinical Nutrition. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Hirschtick JL, Bonner JZ, Swartz AM. Toward quantifying the usage costs of human immunity: altered metabolic rates and hormone levels during acute immune activation in men. American Journal of Human Biology. 2010;22(4):546–556. doi: 10.1002/ajhb.21045. [DOI] [PubMed] [Google Scholar]
- Nhung B, Khan N, Hop L, Lien D, Le D, Hien V, Kunii D, Sakai T, Nakamori M, Yamamoto S. FAO/WHO/UNU equations overestimate resting metabolic rate in Vietnamese adults. European journal of clinical nutrition. 2005;59(10):1099–1104. doi: 10.1038/sj.ejcn.1602199. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Austin MD, Benezra L, Pearce S, McInnis T, Unick J, Gross SJ. Validation of COSMED’s Fitmate™ in measuring oxygen consumption and estimating resting metabolic rate. Research in Sports Medicine. 2006;14(2):89–96. doi: 10.1080/15438620600651512. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Austin MD, Chilcote SM, Benezra L. Validation of a new handheld device for measuring resting metabolic rate and oxygen consumption in children. Int J Sport Nutr Exerc Metab. 2005;15(2):186–194. doi: 10.1123/ijsnem.15.2.186. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Johanssen LM, Lee J, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness. 1990;30(3):316–328. [PubMed] [Google Scholar]
- Nieman DC, Trone GA, Austin MD. A new handheld device for measuring resting metabolic rate and oxygen consumption. Journal of the American Dietetic Association. 2003;103(5):588–593. doi: 10.1053/jada.2003.50116. [DOI] [PubMed] [Google Scholar]
- Owen OE, Holup JL, D'Alessio DA, Craig ES, Polansky M, Smalley KJ, Kavle EC, Bushman MC, Owen LR, Mozzoli MA. A reappraisal of the caloric requirements of men. The American Journal of Clinical Nutrition. 1987;46(6):875–885. doi: 10.1093/ajcn/46.6.875. [DOI] [PubMed] [Google Scholar]
- Owen OE, Kavle E, Owen RS, Polansky M, Caprio S, Mozzoli MA, Kendrick ZV, Bushman M, Boden G. A reappraisal of caloric requirements in healthy women. The American Journal of Clinical Nutrition. 1986;44(1):1–19. doi: 10.1093/ajcn/44.1.1. [DOI] [PubMed] [Google Scholar]
- Pisor AC, Gurven M, Blackwell AD, Kaplan H, Yetish G. Patterns of senescence in human cardiovascular fitness: VO2max in subsistence and industrialized populations. American Journal of Human Biology. 2013;25(6):756–769. doi: 10.1002/ajhb.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer H. Constrained total energy expenditure and the evolutionary biology of energy balance. Exercise and Sport Sciences Review. 2015;43(3):110–116. doi: 10.1249/JES.0000000000000048. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Durazo-Arvizu R, Dugas L, Plange-Rhule J, Bovet P, Forrester TE, Lambert EV, Cooper RS, Schoeller DA, Luke A. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Current Biology. 2016;26(3):410–417. doi: 10.1016/j.cub.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppitt SD, Prentice AM, Goldberg GR, Whitehead RG. Energy-sparing strategies to protect human fetal growth. American Journal of Obstetrics and Gynecology. 1994;171(1):118–125. doi: 10.1016/s0002-9378(94)70087-7. [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Ferrucci L. The endeavor of high maintenance homeostasis: resting metabolic rate and the legacy of longevity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2006;61(5):466–473. doi: 10.1093/gerona/61.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero C, Metter EJ, Melenovsky V, Cherubini A, Najjar SS, Ble A, Senin U, Longo DL, Ferrucci L. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(7):698–706. doi: 10.1093/gerona/63.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Society of London B: Biological Sciences. 2003;270(1513):357–366. doi: 10.1098/rspb.2002.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. “IDEAL” aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. Journal of the American Geriatrics Society. 2014;62(4):667–672. doi: 10.1111/jgs.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JJ, Leonard WR, Tarskaia LA, Schoeller DA. Total energy expenditure in the Yakut (Sakha) of Siberia as measured by the doubly labeled water method. The American Journal of Clinical Nutrition. 2006;84(4):798–806. doi: 10.1093/ajcn/84.4.798. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Doubly Labelled Water: Theory & Practice. Chapman & Hall; London: 1997. [Google Scholar]
- Speakman JR, Selman C. Physical activity and resting metabolic rate. Proceedings of the Nutrition Society. 2003;62(03):621–634. doi: 10.1079/PNS2003282. [DOI] [PubMed] [Google Scholar]
- Stieglitz J, Beheim BA, Trumble BC, Madimenos FC, Kaplan H, Gurven M. Low mineral density of a weight-bearing bone among adult women in a high fertility population. American Journal of Physical Anthropology. 2015a;156(4):637–648. doi: 10.1002/ajpa.22681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieglitz J, Trumble BC, Thompson ME, Blackwell AD, Kaplan H, Gurven M. Depression as sickness behavior? A test of the host defense hypothesis in a high pathogen population. Brain, Behavior, and Immunity. 2015b;49:130–139. doi: 10.1016/j.bbi.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine–immune control in chronic inflammatory diseases. Journal of Internal Medicine. 2010;267(6):543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- Trumble BC, Stieglitz J, Thompson ME, Fuerstenberg E, Kaplan H, Gurven M. Testosterone and male cognitive performance in Tsimane forager-horticulturalists. American Journal of Human Biology. 2014 doi: 10.1002/ajhb.22665. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt RE, Dinneno FA, Seals DR, Jones PP. Age-related decline in RMR in physically active men: relation to exercise volume and energy intake. American Journal of Physiology-Endocrinology and Metabolism. 2001;281(3):E633–E639. doi: 10.1152/ajpendo.2001.281.3.E633. [DOI] [PubMed] [Google Scholar]
- Vaughan L, Zurlo F, Ravussin E. Aging and energy expenditure. The American Journal of Clinical Nutrition. 1991;53(4):821–825. doi: 10.1093/ajcn/53.4.821. [DOI] [PubMed] [Google Scholar]
- Veile A, Martin MA, McAllister L, Gurven M. Modernization and traditional breastfeeding patterns in the Bolivian Amazon. Social Science & Medicine. 2014;100:148–158. doi: 10.1016/j.socscimed.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Heshka S, Zhang K, Boozer CN, Heymsfield SB. Resting Energy Expenditure: Systematic Organization and Critique of Prediction Methods. Obesity Research. 2001;9(5):331–336. doi: 10.1038/oby.2001.42. [DOI] [PubMed] [Google Scholar]
- Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of physiology. 1949;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss CO, Cappola AR, Varadhan R, Fried LP. Resting Metabolic Rate in Old-Old Women with and without Frailty: Variability and Estimation of Energy Requirements. Journal of the American Geriatrics Society. 2012;60(9):1695–1700. doi: 10.1111/j.1532-5415.2012.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergren A. Diagnostic tests: the erythrocyte sedimentation rate range and limitations of the technique. Triangle; the Sandoz journal of medical science. 1957;3(1):20–25. [PubMed] [Google Scholar]
- Westerterp K, Wilson S, Rolland V. Diet induced thermogenesis measured over 24 h in a respiration chamber: effect of diet composition. International Journal of Obesity. 1999;23(3):287–292. doi: 10.1038/sj.ijo.0800810. [DOI] [PubMed] [Google Scholar]
- Zuk M, Stoehr AM. Immune defense and host life history. The American Naturalist. 2002;160(S4):S9–S22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.