Abstract
Objective
The primary objective of this pilot study is to determine and compare the residence time in the vagina of biomarkers of semen exposure for up to 15 days post exposure. The biomarkers are prostate-specific antigen (PSA), Y chromosome DNA, the sex determining region of the Y chromosome (SRY) and testis-specific protein Y-encoded 4 (TSPY4). The secondary objectives are to determine if biomarker concentrations differed between intercourse and inoculation groups, to establish whether the sampling frequency post exposure affected biomarker concentrations and decay profile and to determine if biomarker concentrations in vaginal swabs obtained by the participant at home were similar to swabs obtained by the nurse in the clinic.
Study design
We randomized healthy women to unprotected intercourse (n=17) versus vaginal inoculation with the male partner’s semen in the clinic (n=16). Women were then further randomized to have vaginal swabs obtained at either 7 or 4 time points after semen exposure, up to 15 days post exposure, either obtained at home by the participant or in the clinic by the research nurse.
Results
PSA and SRY were markers of recent semen exposure. TSPY4 was detectable in approximately 50% of participants at 15 days post exposure. Unprotected intercourse resulted in significantly higher concentrations of select biomarkers. Sampling frequency and home versus clinic sampling had no significant effect on biomarker concentrations.
Conclusions
Objective biomarkers of recent or distant semen exposure may have great utility for verifying protocol compliance in a variety of clinical trials.
Keywords: Semen, Biomarker, Y chromosome, Clinical trials
1. Introduction
Objective biomarkers of vaginal exposure to semen were developed for use in forensic medicine to provide evidence of sexual contact [1]. Ultimately they may be used as surrogates for risk of pregnancy and sexually transmitted infections (STIs) and alternative endpoints in contraceptive efficacy trials [2–9], the assessment of slippage/breakage of condoms [7,8,10–14], and as an objective biomarker to validate self-reports of condom use and/or sexual activity [15–23].
Prostate-specific antigen (PSA) is the best characterized marker of seminal fluid (reviewed in Ref. [24]). PSA is detectable in the vagina immediately post exposure until approximately 24–48 h post exposure [1,8,10,11,25,26]. Other objective biomarkers of sperm or male cell exposure are linked to Y chromosome DNA (YcDNA), present in Y-bearing spermatozoa, immature germ cells and nongerminal male cells (e.g., epithelial cells and leukocytes). Two genes on the Y chromosome that are of particular interest are the sex determining region of the Y chromosome (SRY) and the testis-specific protein Y-encoded 4 (TSPY4) gene [27–29]. These biomarkers were utilized in small clinical trials, from the cell pellet from cervicovaginal lavage specimens [17] and vaginal swabs [5,17,26,30].
For new biomarkers to be used in future contraceptive and other clinical trials, it is important to determine residence time in the vagina under various exposure and sampling conditions. Only a few published studies were structured this way, with measurement of PSA and YcDNA as endpoints [8,10,11,26], and none compared methodologies to assess these new semen biomarkers.
The primary objective of this study is to describe the decay of SRY and TSPY4 in the vagina, for up to 15 days, using two different polymerase chain reaction (PCR) platforms, multiplex PCR and quantitative PCR (qPCR). Utilizing exposure to semen through unprotected vaginal intercourse or inoculation with the partner’s semen in the clinic, we measured and categorized biomarker detection post exposure and determined whether sampling frequency affects the decay of the markers. Finally, we compared concentrations of semen biomarkers obtained from vaginal swabs collected at home versus swabs obtained from the same participant by a research nurse in the clinic.
2. Materials and methods
This is a single-blinded, randomized, outpatient trial conducted at the Johns Hopkins University (JHU) Bayview Medical Center (Baltimore, MD) and the CONRAD Clinical Research Center (CRC) at the Eastern Virginia Medical School (EVMS) in Norfolk, VA. The institutional review board (IRB) (Approval Number NA_00016471) at the JHU (for the Bayview site) and the Chesapeake IRB (Protocol #Pro0003114) for the CONRAD CRC site approved this protocol. We screened monogamous couples at low risk of acquiring STIs, including a healthy, normally menstruating woman, aged 18–50 years, who was not at risk for pregnancy due to surgical sterilization or who was willing to become pregnant, and her male sexual partner, aged 18–55 years.
Women and men were interviewed and consented separately. Men enrolled in the study had to be in good health and had to have screening semen sample parameters as follows: volume: ≥1.8 mL (or ≥1.0 mL with a total sperm count of ≥120 million), total sperm count: 100–400 million, sperm concentration: ≥35 M/mL, total sperm motility (progressive motility+nonprogressive motility): ≥40%, viability: ≥70% vital cells, round cells: ≤5 M/mL and leukocytes: ≤1 M/mL.
We used the random permuted blocks method to generate allocation sequences assigning the couples to one of four subgroups (A1, A2, B1 and B2) in a 1:1:1:1 ratio. The sequences were created by a randomization manager, not otherwise involved in the statistical analyses of this study using a verified program based on the random function RANUNI in the SAS(r) System (SAS Institute, Cary, NC). The randomization groups and interventions are outlined in Table 1.
Table 1.
Randomization Groups and Interventions in Semen Biomarkers Study
| Semen Exposure Group | |||
|---|---|---|---|
|
| |||
| A
|
B
|
||
| Vaginal Inoculation with 2 mL of Partner’s Semen in Clinic (n=16) |
Unprotected Intercourse (n=17) |
||
| A1 (7 Time Points) | A2 (4 Time Points) | B1 (7 Time Points) | B2 (4 Time Points) |
| (n=8) | (n=8) | (n=8) | (n=9) |
| Clinic Sampling=4 | Clinic Sampling=4 | Clinic Sampling=5 | Clinic Sampling=3 |
| Home Sampling=4 | Home Sampling=4 | Home Sampling=3 | Home Sampling=6 |
| A1 Substudy | A2 Substudy | B1 Substudy | B2 Substudy |
| (n=3) | (n=2) | (n=2) | (n=2) |
| Clinic Sampling=1 | Clinic Sampling=0 | Clinic Sampling=0 | Clinic Sampling=0 |
| Home Sampling=2 | Home Sampling=2 | Home Sampling=2 | Home Sampling=2 |
The A1 and B1 groups underwent vaginal sampling for biomarkers of semen exposure at 7 time points after inoculation or intercourse (6 h, 24 h, 48 h, 72 h, 7 days, 11 days and 15 days post exposure).
The A2 and B2 underwent vaginal sampling for biomarkers of semen exposure at 4 time points after inoculation or intercourse (6 h, 24 h, 7 days and 15 days post exposure).
Women were given the choice of having all the vaginal swabs collected in the clinic by a nurse (“in-clinic sampling”) or having some samples collected in the clinic and collecting some at home (“mixed sampling”). For those opting for “mixed sampling”, all samples taken at 6 and 48 h were collected by the participant at home; a nurse in the clinic collected the remaining samples. Participants were instructed to abstain from vaginal or receptive oral intercourse during the 15 days of follow-up.
Up to two women in each of the four groups (A1, A2, B1 and B2) were invited to participate in a substudy. These women were asked to go through the study visits a second time, in their same group assignment, using the type of sampling that they did not use during their first time through to test the hypothesis that samples collected by participants yielded results similar to those collected in the clinic by the nursing staff.
Participants’ swabs were immediately placed in 1 mL of cold phosphate buffered saline (PBS). Next, the solution was spun for 5 min at 14,000 rpm. We sent the supernatant to the Centers for Disease Control and Prevention for PSA measurements, as previously described [31]. The pellet was resuspended in 700 μL PBS, and half was used for SRY qPCR (labeled “YcDNA”) analysis, and the other half was used for SRY-TSPY4 multiplex PCR and TSPY4 qPCR analysis, both performed as previously described [32,33].
We developed the TSPY4 qPCR using the Universal Probe Library system in combination with the Lightcycler TaqMan Master kit for the Lightcycler 2.0 (Roche Diagnostics, Indianapolis, IN). The method detected that TSPY4 amplified from sperm DNA as low as 1 pg and, in semen samples with DNA, as low as 2 pg. We used both qPCR and multiplex PCR to assess both Y chromosome biomarkers to measure semen exposure. Although both assays detect the SRY region of the Y chromosome, we use the terms SRY for the multiplex system and YcDNA for the qPCR system, to maintain consistency with previously published data [26,32,33].
Statistical analyses were performed using SAS version 9.3 (Carey, NC). Descriptive statistics were expressed as mean, median and standard deviation. Independent group comparisons for normally distributed data were compared with parametric methods and for nonnormally distributed data with nonparametric methods, as appropriate. Paired comparisons were performed to compare the quantity of biomarker detected on vaginal swabs obtained at home and in the clinic, from the same woman. Repeated-measures analysis was used to compare in clinic versus at home sampling among participants enrolled in the substudy. Statistical significance was detected at a p value of <.05.
3. Results
We screened 71 women and enrolled 33 couples (Table 1). Two women from the EVMS site withdrew from the main study. Only one woman (randomized to the B2 group) reported a protocol violation (unprotected intercourse) during the 15 days of follow-up. Usable swabs from the main study include the following: baseline (n=33), 6 h (n=33), 24 h (n=33), 48 h (n=15), 72 h (n=16), 7 days (n=32), 11 days (n=15) and 15 days (n=31). Nine women from the main study continued to the substudy and eight women provided at least one sample (Table 1). Usable swabs available for analysis from the substudy population include the following: baseline (n=8), 6 h (n=8), 24 h (n=8), 48 h (n=4), 72 h (n=4), 7 days (n=7), 11 days (n=3) and 15 days (n=7).
There were no differences in demographic characteristics among participants randomized to inoculation (Group A) versus intercourse (Group B) (Table 2), nor were there differences in baseline semen parameters or in baseline vaginal levels of the four semen biomarkers based on randomization group (all p values >.05, data not shown).
Table 2.
Demographics of the Female and Male Participants
| Males
|
Females
|
|||||
|---|---|---|---|---|---|---|
| Inoculation (Groups A1 and A2) (n=16) (STDEV or Column %) | Intercourse (Groups B1 and B2) (n=17) (STDEV or Column %) | p | Inoculation (Groups A1 and A2) (n=16) (STDEV or Column %) | Intercourse (Groups B1 and B2) (n=17) (STDEV or Column %) | p | |
| Age (years) | ||||||
| Mean (SD) | 37.0 (7.8) | 36.5 (8.3) | 0.87 | 33.6 (7.7) | 34.7 (7.5) | .81 |
| Ethnicity | ||||||
| Hispanic/Latino | 1 (6.3) | 0 (0.0) | 0.48 | 3 (18.8) | 0 (0.0) | .10 |
| Not Hispanic/Not Latino | 15 (93.8) | 17 (100) | 13 (81.3) | 17 (100) | ||
| Race | ||||||
| Black or African American | 7 (43.8) | 12 (70.6) | 0.11 | 6 (37.5) | 9 (52.9) | .29 |
| White | 9 (56.3) | 5 (29.4) | 10 (62.5) | 7 (41.2) | ||
| More than one race | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.9) | ||
| Education (years) | ||||||
| Mean (SD) | 14.7 (2.7) | 12.5 (1.7) | 0.34 | 14.9 (3.2) | 13.8 (1.8) | .88 |
| Partner Status | ||||||
| Living with partner | 16 (100) | 14 (82.4) | 0.12 | 16 (100) | 14 (82.4) | .12 |
| Not living with partner | 0 (0.0) | 3 (17.6) | 0 (0.0) | 3 (17.6) | ||
The data support that the frequency of sampling post semen exposure (7 versus 4 samplings) had no effect on the mean vaginal concentrations of all semen biomarkers regardless of whether women were exposed via inoculation or unprotected intercourse (all p values >.05, data not shown). There was one exception to this. At the first sampling, 6 h, women randomized to A1 had significantly higher mean vaginal concentrations of TSPY4 (9862±1158 ng/mL) compared to women randomized to A2 (TSPY4=7213±2372 ng/mL) (p=.02). This significant difference at 6 h was not seen in the timed intercourse group comparison (B1 versus B2).
At almost all time points, intercourse resulted in a trend toward higher mean concentrations of semen biomarkers than vaginal inoculation. These differences were significantly higher (p values <.05) at 6 and 24 h post exposure for various semen biomarkers (Supplemental Table 1).
Comparing self-swabbing at home versus nurse collected swabs, we found no significant differences in the mean concentrations of any biomarker at any time point (all p values >.36, data not shown).
The residence time of the biomarkers, based on exposure groups, is detailed in Supplemental Table 1 and graphically in Fig. 1a–e. Using multiplex technology, SRY can be detected up to 48 h, while TSPY4 can be detected up to 7 days. qPCR technology extended the window of detection of YcDNA and TSPY4.
Fig. 1.
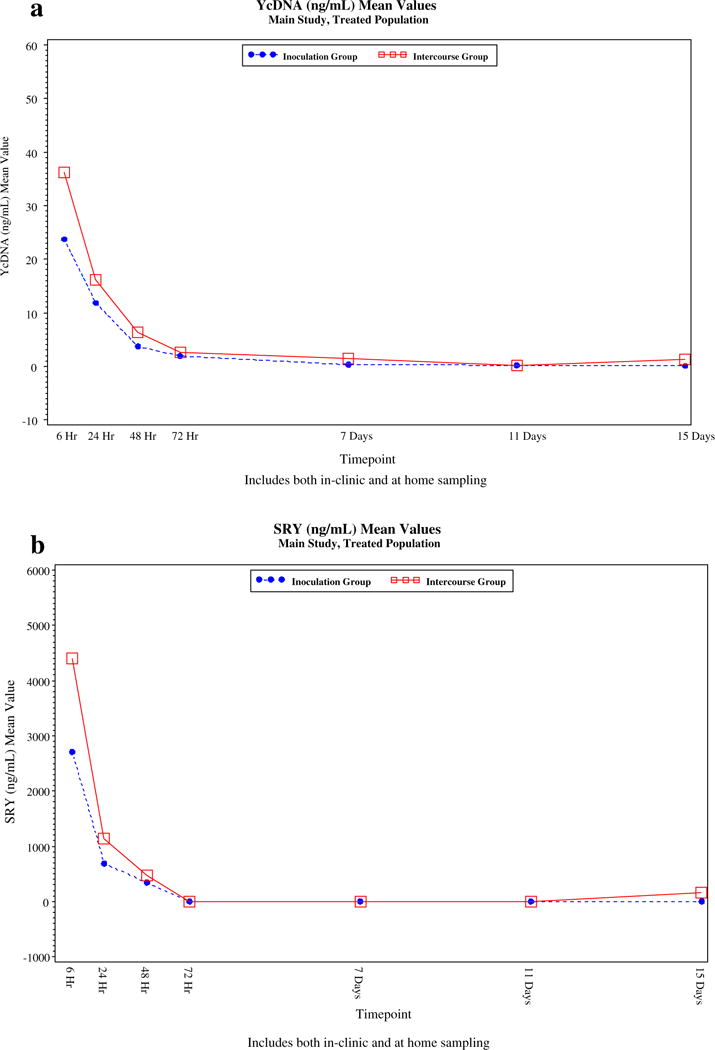
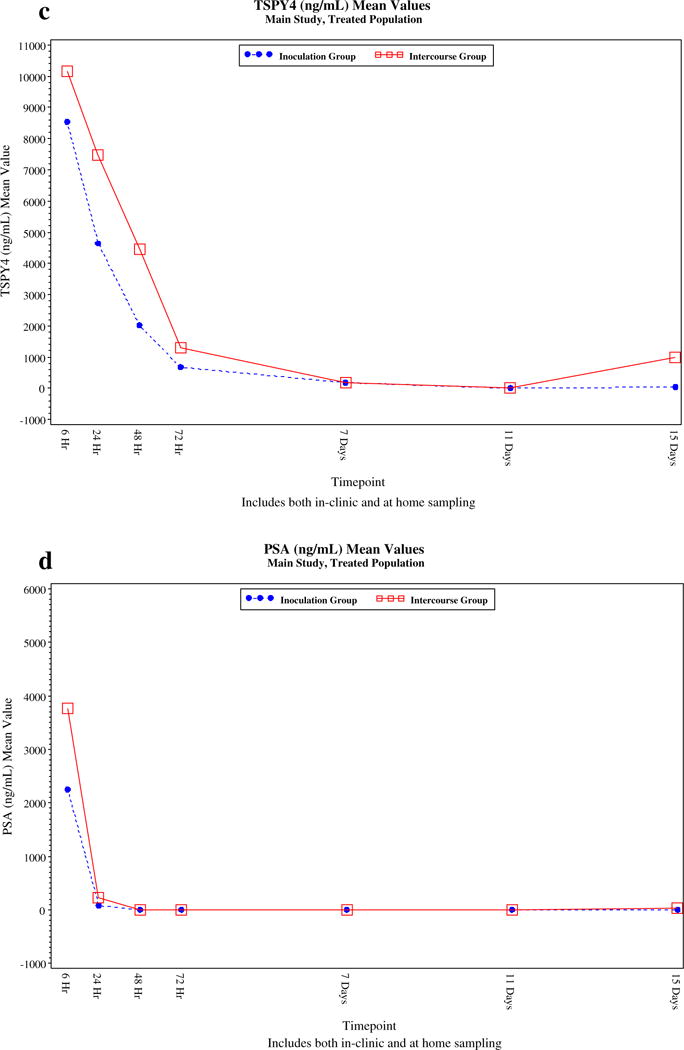
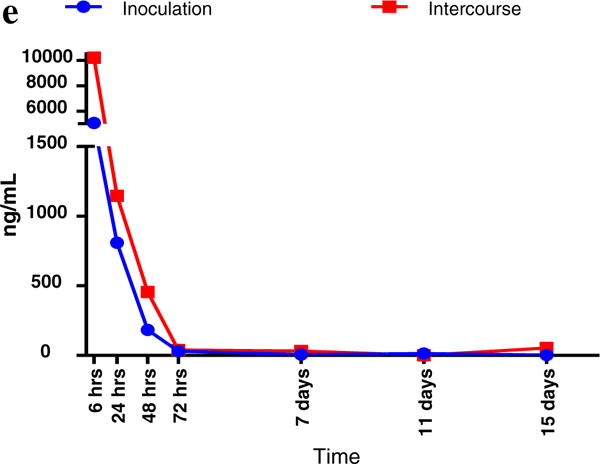
(a) Decay Curves for Semen Biomakers, Based on Vaginal Inoculation (Groups A1 and A2) versus Intercourse (Groups B1 and B2) YcDNA (SRY by qPCR). (b) Decay Curves for Semen Biomakers, Based on Vaginal Inoculation (Groups A1 and A2) versus Intercourse (Groups B1 and B2) SRY by Multiplex PCR. (c) Decay Curves for Semen Biomakers, Based on Vaginal Inoculation (Groups A1 and A2) versus Intercourse (Groups B1 and B2) TSPY4 by Multiplex PCR. (d) Decay Curves for Semen Biomakers, Based on Vaginal Inoculation (Groups A1 and A2) versus Intercourse (Groups B1 and B2) TSPY4 by Multiplex PCR. (e) Decay Curves for Semen Biomarkers, Based on Vaginal Inoculation (Groups A1 and A2) versus Intercourse (Groups B1 and B2) TSPY4 by qPCR (Mean Values, ng/mL), Main Study, Treated Population.
To determine the proportion of all participants (n=41), in the main (n=33) and substudies (n=8) with detectable biomarker concentrations at each time point, we considered YcDNA, SRY or TSPY4 concentrations of greater than 0.1 ng/mL and a PSA concentration of greater than 1.0 ng/mL as detectable, based on previous reports and the lowest limits of detection used in our assays [1,10,25,26]. This percentage is termed the actual detectable levels (Table 3). We then reexamined the data and used levels of PSA as an internal control, to detect presumed unreported protocol violations. Given the consistent data that PSA is present in the female genital tract for approximately 24–48 h after semen exposure [8,10,11,25,26], we assumed that once the PSA level was undetectable for an individual participant, subsequent PSA levels of 1 ng/mL or higher indicated new, recent semen exposure or unreported protocol violations. The adjustments were used to calculate the percent of detectable biomarkers at each visit, adjusted for presumed protocol violations.
Table 3.
Percent of Participants with Detectable Levels of Biomarkersa, Adjusted for Presumed Protocol Violations, All Main and Substudy Participants, at Each Time Point
| Variable | Proportion of Participants with Detectable Levels of Biomarkers in Main and Substudies (Actual Percent and Adjusted Percent for Presumed Protocol Violations, Using PSA as an Internal Control)
|
|
|---|---|---|
| Actual % Detected | Adjusted for Presumed Protocol Violations % Detected | |
| 6 h | ||
| YcDNA (SRY qPCR) | 97.6 | 97.6 |
| SRY (Multiplex) | 90.0 | 90.0 |
| TSPY4 (Multiplex) | 100 | 100 |
| TSPY4 (qPCR) | 100 | 100 |
| PSA | 100 | 100 |
| 24 h | ||
| YcDNA (SRY qPCR) | 95.2 | 95.2 |
| SRY (Multiplex) | 50.0 | 50.0 |
| TSPY4 (Multiplex) | 95.0 | 95.0 |
| TSPY4 (qPCR) | 100 | 100 |
| PSA | 69.0 | 69.0 |
| 48 h | ||
| YcDNA (SRY qPCR) | 89.5 | 89.5 |
| SRY (Multiplex) | 16.7 | 16.7 |
| TSPY4 (Multiplex) | 77.8 | 77.8 |
| TSPY4 (qPCR) | 88.9 | 88.9 |
| PSA | 16.7 | 16.7 |
| 72 h | ||
| YcDNA (SRY qPCR) | 80.0 | 80.0 |
| SRY (Multiplex) | 0 | 0 |
| TSPY4 (Multiplex) | 57.9 | 57.9 |
| TSPY4 (qPCR) | 78.9 | 78.9 |
| PSA | 10.0 | 10.0 |
| 7 days | ||
| YcDNA (SRY qPCR) | 38.5 | 38.5 |
| SRY (Multiplex) | 0 | 0 |
| TSPY4 (Multiplex) | 15.4 | 15.4 |
| TSPY4 (qPCR) | 64.1 | 64.1 |
| PSA | 0 | 0 |
| 11 days | ||
| YcDNA (SRY qPCR) | 16.7 | 17.6 |
| SRY (Multiplex) | 0 | 0 |
| TSPY4 (Multiplex) | 0 | 0 |
| TSPY4 (qPCR) | 50.0 | 47.1 |
| PSA | 5.5a | 0 |
| 15 days | ||
| YcDNA (SRY qPCR) | 18.4 | 16.1 |
| SRY (Multiplex) | 5.3 | 0 |
| TSPY4 (Multiplex) | 13.5 | 10.0 |
| TSPY4 (qPCR) | 50.0 | 48.4 |
| PSA | 18.4b | 0 |
One participant (out of 18) had an elevated PSA at 11 days after having undetectable PSA levels at 7 days and is therefore removed for the adjusted analysis for a suspected protocol violation.
Of 38 participants, 7 had an elevated PSA at 15 days after having undetectable PSA levels at 7 or 11 days and are therefore removed for the adjusted analysis for suspected protocol violations.
There were no adjustments made for presumed protocol violations for all time points up to 7 days because no woman had detectable levels of PSA (>1 ng/mL) after levels initially fell to zero. The internal control of PSA was applied to samples obtained at the 11 and 15 day sampling times. At 11 days, one participant had a vaginal PSA level of 9.3 ng/mL, after having a vaginal PSA levels of ranging from 0.02 to 0.11 ng/mL between 48 h and 7 days. At 15 days, 7 women out of 38 participants had elevated levels of PSA (range 1.16–384 ng/mL) after demonstrating undetectable PSA levels (<1 ng/mL) at day 7 and or day 11.
We found that the median vaginal concentration for all biomarkers was 0 ng/mL by 7 days post exposure. Table 3 demonstrates that SRY was a marker of recent intercourse (6–72 h), with the proportion of samples with detectable levels dropping to 0% by 72 h, while TSPY4 detection, by multiplex PCR, extended the window of detection up to 7 days post semen exposure in 15% of participants. qPCR increased the sensitivity and window of detection for both SRY (YcDNA) and TSPY4 (Table 3). TSPY4, when assessed by qPCR, was the most robust biomarker, detectable in approximately 47.1–50.0% of participants at 11 and 15 days post semen exposure.
4. Discussion
We confirmed that the multiplex technology enables us to combine a marker of recent semen exposure (SRY) with a marker of longer-term exposure (TSPY4). qPCR technology extended the window of detection beyond 7 days. TSPY4, measured with qPCR technology, was the most robust marker. No other study has measured these semen biomarkers, by both technologies, at defined time points, after exposure to semen by intercourse or vaginal inoculation.
Intercourse resulted in higher concentrations of YcDNA, SRY and TSPY4 at various time points for up to 7 days post exposure compared to vaginal inoculation. This is consistent with previous studies showing that higher volumes of inoculated semen (up to 1 mL) resulted in more consistent decay profiles of vaginal PSA and YcDNA levels [26]. We investigated the effect of frequency of genital sampling on biomarker concentrations because, in a previous study, frequent sampling was shown to decrease the sensitivity of detecting semen biomarkers among women exposed to very small volumes of semen (10 and 100 μL), which modeled volumes that might be encountered with a condom failure or preejaculate exposure [26]. We concluded that the number of sampling times after vaginal inoculation with 2 mL of semen or timed intercourse did not result in significant differences in the concentrations of semen biomarkers for up to 15 days post exposure. This finding has applications to the design of future clinical trials utilizing these biomarkers.
Our study confirms a previous study showing similar PSA levels among women sampled in the clinic versus providing swabs collected at home and extends these findings to newer semen biomarkers [8].
Finally, we found that the median concentration of all biomarkers was 0 ng/mL by 7 days post exposure, which suggests that participants in future clinical trials will likely need to provide at least weekly samples. The sensitivity of detecting markers post exposure is reduced as sampling is obtained greater than 7 days post exposure.
We confirmed that PSA [1,8,10,11,25,26] and SRY (assessed by multiplex PCR) were markers of recent semen exposure, with median concentrations of both markers falling to 0 ng/mL by 72 h post exposure. Multiplex PCR is beneficial because multiple genes may be assessed using a single aliquot of sample [32].
Our data support that SRY, assessed by multiplex PCR, extends the window of semen detection beyond that offered by the PSA measurement from 48 to 72 h. However, it is unknown whether SRY can be utilized in determining semen exposure from vasectomized men. Because PSA can be detected in semen from vasectomized men or men with low sperm counts [34] and can be assayed with a point-of-care test in the clinic, PSA will likely remain the semen biomarker of choice in detecting recent exposure to semen from this population.
In terms of assessing semen exposure beyond 72 h, qPCR technology improved the sensitivity of the biomarkers. Using qPCR to assess SRY (termed YcDNA) improves the sensitivity of detecting SRY past 7 days of exposure. TSPY4, assessed by multiplex PCR, was detectable in 15% of participants at 7 days post exposure and was virtually undetectable by 11 and 15 days post exposure. qPCR markedly improved the sensitivity of TSPY4, being the most robust marker, detectable in 47.1–50.0% of participants at 15 days post exposure.
The sensitivities and concentrations that we report in this study are consistent with other studies, utilizing inoculation or timed intercourse, assessing YcDNA [16,17,26,30]. The half-life of YcDNA is estimated to be 3.6 days [16,17,26] and a previous clinical study reported that YcDNA was absent by 15 days post exposure [17]. Strengths of this study include the ability to compare biomarker concentrations after timed intercourse versus vaginal inoculation, the number of sampling times post exposure, home versus in clinic sampling and the addition of SRY and TSPY4, assessed by both multiplex and qPCR technologies.
This pilot study has several limitations. We did not include an arm of the study without semen exposure, so we cannot calculate specificity. Although only one woman reported a protocol violation at her follow-up visit, PSA levels indicate that at least eight unreported protocol violations occurred. Gaps of 4 days or more existed between some of the samplings (72 h, 7 days, 11 days and 15 days). Since PSA disappears by 24–48 h post exposure, it is possible that there were additional unreported protocol violations. However, the mean and median levels of TSPY4 and SRY steadily declined after 11 days, supporting protocol adherence and our rationale for the adjusted analysis. Future studies, with more frequent (such as daily) sampling, are needed to further define the daily decay curves of these new biomarkers of semen exposure.
This was a pilot study to plot the decay of these biomarkers, based on exposure, sampling frequency and assessment method. Generalized mixed linear models controlling for within-subject correlation of measurements and potential between-subject variation in the rate of signal decay would likely have increased the statistical power of the comparisons and the precision of the estimates. However, the sample size was too small, with individual cell sizes ranging from 3 to 9 individuals. The small sample size often leads to failures of convergence and other modeling problems, as well as interpretational difficulties from the effect of a few influential observations.
In summary, there is an interest in developing biologic markers of semen exposure as reports of condom use and other sexual behaviors in clinical trials are fraught with recall, social desirability and reporting biases. Our data demonstrate that PSA and SRY via multiplex PCR are markers of recent (up to 72 h) semen exposure, while qPCR assessment of TSPY4 and SRY (YcDNA) are markers of semen exposure in the past 7–15 days. TSPY4 assessed by qPCR was the most sensitive long-term marker of semen exposure. It would be ideal to increase the sensitivity of detecting these biomarkers beyond 7 days, as women in clinical trials may be seen less frequently for follow-up. Our goal is to improve the sensitivity of the TSPY4 qPCR to detect semen exposure in nearly 100% of cases at 15 days. However, since menses, use of tampons or other intravaginal products, discharge and sex could all act to remove DNA, self-swabbing at home at weekly intervals may be the most feasible approach to detecting evidence of semen exposure. It is important to develop and characterize objective measures of product adherence and protocol compliance. Because women’s health trials often involve tracking intercourse or condom use, biomarkers of vaginal semen exposure will further the rational interpretation of data from these types of trials.
Supplementary Material
Footnotes
Funding: This work was supported by intramural CONRAD funds from the US Agency for International Development (Grants GPO-A-00-08-00005-00 and USAID APS OAA-A-14-00005) and an interagency agreement between CONRAD and the NICHD (#Y1-HD-0083). The findings and conclusions in this report are those of the authors do not necessarily reflect the official position of Centers for Disease Control and Prevention or CONRAD. Use of trade names is for identification only and does not imply endorsement by the US Department of Health and Human Services.
No authors report any conflicts of interest.
ClinicalTrials.gov Registration Number: NCT00984555.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.contraception.2016.05.012.
References
- 1.Graves HC, Sensabaugh GF, Blake ET. Postcoital detection of a male-specific semen protein. Application to the investigation of rape. N Engl J Med. 1985;312:338–43. doi: 10.1056/NEJM198502073120603. [DOI] [PubMed] [Google Scholar]
- 2.Mauck CK, Weaver MA, Schwartz JL, Walsh T, Joanis C. Critical next steps for female condom research–report from a workshop. Contraception. 2009;79:339–44. doi: 10.1016/j.contraception.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Mauck CK. Biomarkers of semen exposure. Sex Transm Dis. 2009;36:S81–3. doi: 10.1097/OLQ.0b013e318199413b. [DOI] [PubMed] [Google Scholar]
- 4.Mauck CK, Straten A. Using objective markers to assess participant behavior in HIV prevention trials of vaginal microbicides. J Acquir Immune Defic Syndr. 2008;49:64–9. doi: 10.1097/QAI.0b013e318183a917. [DOI] [PubMed] [Google Scholar]
- 5.Ghanem KG, Melendez JH, McNeil-Solis C, Giles JA, Yuenger J, Smith TD, et al. Condom use and vaginal Y-chromosome detection: the specificity of a potential biomarker. Sex Transm Dis. 2007;34:620–3. doi: 10.1097/01.olq.0000258318.99606.d9. [DOI] [PubMed] [Google Scholar]
- 6.Macaluso M, Lawson ML, Hortin G, Duerr A, Hammond KR, Blackwell R, et al. Efficacy of the female condom as a barrier to semen during intercourse. Am J Epidemiol. 2003;157:289–97. doi: 10.1093/aje/kwf212. [DOI] [PubMed] [Google Scholar]
- 7.Galvao LW, Oliveira LC, Diaz J, Kim DJ, Marchi N, van Dam J, et al. Effectiveness of female and male condoms in preventing exposure to semen during vaginal intercourse: a randomized trial. Contraception. 2005;71:130–6. doi: 10.1016/j.contraception.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Bahamondes L, Diaz J, Marchi NM, Castro S, Villarroel M, Macaluso M. Prostate-specific antigen in vaginal fluid after exposure to known amounts of semen and after condom use: comparison of self-collected and nurse-collected samples. Hum Reprod. 2008;23:2444–51. doi: 10.1093/humrep/den283. [DOI] [PubMed] [Google Scholar]
- 9.Walsh T, Warner L, Macaluso M, Frezieres R, Snead M, Wraxall B. Prostate-specific antigen as a biomarker of condom failure: comparison of three laboratory assays and self-reported condom use problems in a randomized trial of female condom performance. Contraception. 2012;86:55–61. doi: 10.1016/j.contraception.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macaluso M, Lawson L, Akers R, Valappil T, Hammond K, Blackwell R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59:195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 11.Lawson ML, Maculuso M, Bloom A, Hortin G, Hammond KR, Blackwell R. Objective markers of condom failure. Sex Transm Dis. 1998;25:427–32. doi: 10.1097/00007435-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Walsh TL, Frezieres RG, Peacock K, Nelson AL, Clark VA, Bernstein L, et al. Use of prostate-specific antigen (PSA) to measure semen exposure resulting from male condom failures: implications for contraceptive efficacy and the prevention of sexually transmitted disease. Contraception. 2003;67:139–50. doi: 10.1016/s0010-7824(02)00478-x. [DOI] [PubMed] [Google Scholar]
- 13.Walsh TL, Frezieres RG, Nelson AL, Wraxall BG, Clark VA. Evaluation of prostate-specific antigen as a quantifiable indicator of condom failure in clinical trials. Contraception. 1999;60:289–98. doi: 10.1016/s0010-7824(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 14.Walsh TL, Frezieres RG, Peacock K, Nelson AL, Clark VA, Bernstein L, et al. Effectiveness of the male latex condom: combined results for three popular condom brands used as controls in randomized clinical trials. Contraception. 2004;70:407–13. doi: 10.1016/j.contraception.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Pepin J, Fink GD, Khonde N, Sobela F, Deslandes S, Diakite S, et al. Improving second-generation surveillance: the biological measure of unprotected intercourse using prostate-specific antigen in vaginal secretions of West African women. J Acquir Immune Defic Syndr. 2006;42:490–3. doi: 10.1097/01.qai.0000222286.52084.9c. [DOI] [PubMed] [Google Scholar]
- 16.Jadack RA, Yuenger J, Ghanem KG, Zenilman J. Polymerase chain reaction detection of Y-chromosome sequences in vaginal fluid of women accessing a sexually transmitted disease clinic. Sex Transm Dis. 2006;33:22–5. doi: 10.1097/01.olq.0000194600.83825.81. [DOI] [PubMed] [Google Scholar]
- 17.Zenilman JM, Yuenger J, Galai N, Turner CF, Rogers SM. Polymerase chain reaction detection of Y chromosome sequences in vaginal fluid: preliminary studies of a potential biomarker for sexual behavior. Sex Transm Dis. 2005;32:90–4. doi: 10.1097/01.olq.0000149668.08740.91. [DOI] [PubMed] [Google Scholar]
- 18.Chomont N, Gresenguet G, Levy M, Hocini H, Becquart P, Matta M, et al. Detection of Y chromosome DNA as evidence of semen in cervicovaginal secretions of sexually active women. Clin Diagn Lab Immunol. 2001;8:955–8. doi: 10.1128/CDLI.8.5.955-958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aho J, Koushik A, Diakite SL, Loua KM, Nguyen VK, Rashed S. Biological validation of self-reported condom use among sex workers in Guinea. AIDS Behav. 2010;14:1287–93. doi: 10.1007/s10461-009-9602-6. [DOI] [PubMed] [Google Scholar]
- 20.Culhane JF, Nyirjesy P, McCollum K, Casabellata G, Di Santolo M, Cauci S. Evaluation of semen detection in vaginal secretions: comparison of four methods. Am J Reprod Immunol. 2008;60:274–81. doi: 10.1111/j.1600-0897.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 21.Gallo MF, Behets FM, Steiner MJ, Hobbs MM, Hoke TH, Van Damme K, et al. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sex Transm Dis. 2006;33:476–9. doi: 10.1097/01.olq.0000231960.92850.75. [DOI] [PubMed] [Google Scholar]
- 22.Gallo MF, Behets FM, Steiner MJ, Thomsen SC, Ombidi W, Luchters S, et al. Validity of self-reported ‘safe sex’ among female sex workers in Mombasa, Kenya–PSA analysis. Int J STD AIDS. 2007;18:33–8. doi: 10.1258/095646207779949899. [DOI] [PubMed] [Google Scholar]
- 23.Minnis AM, Steiner MJ, Gallo MF, Warner L, HObbs MM, van der Straten A, et al. Biomarker validation of reports of recent sexual activity: results of a randomized controlled study in Zimbabwe. Am J Epidemiol. 2009;170:918–24. doi: 10.1093/aje/kwp219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mauck CK, Doncel GF. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception. 2007;75:407–19. doi: 10.1016/j.contraception.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Kamenev L, Leclercq M, Francois-Gerard C. An enzyme immunoassay for prostate-specific p30 antigen detection in the postcoital vaginal tract. J Forensic Sci Soc. 1989;29:233–41. doi: 10.1016/s0015-7368(89)73257-6. [DOI] [PubMed] [Google Scholar]
- 26.Jamshidi R, Penman-Aguilar A, Wiener J, Gallo MF, Zenilman JM, Melendez JH, et al. Detection of two biological markers of intercourse: prostate-specific antigen and Y-chromosomal DNA. Contraception. 2013;88:749–57. doi: 10.1016/j.contraception.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel T, Schmidtke J. Structure and function of TSPY, the Y-chromosome gene coding for the “testis-specific protein”. Cytogenet Cell Genet. 1998;80:209–13. doi: 10.1159/000014982. [DOI] [PubMed] [Google Scholar]
- 28.Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–37. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- 29.Zimmermann B, Zhong XY, Holzgreve W, Hahn S. Real-time quantitative polymerase chain reaction measurement of male fetal DNA in maternal plasma. Methods Mol Med. 2007;132:43–9. doi: 10.1007/978-1-59745-298-4_5. [DOI] [PubMed] [Google Scholar]
- 30.Brotman RM, Melendez JH, Smith TD, Galai N, Zenilman JM. Effect of menses on clearance of Y-chromosome in vaginal fluid: implications for a biomarker of recent sexual activity. Sex Transm Dis. 2010;37:1–4. doi: 10.1097/OLQ.0b013e3181b5f15d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snead MC, Kourtis AP, Black CM, Mauck CK, Brown TM, Penman-Aguilar A, et al. Effect of topical vaginal products on the detection of prostate-specific antigen, a biomarker of semen exposure, using ABAcards. Contraception. 2013;88:382–6. doi: 10.1016/j.contraception.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacot TA, Zalenskaya I, Mauck C, Archer DF, Doncel GF. TSPY4 is a novel sperm-specific biomarker of semen exposure in human cervicovaginal fluids; potential use in HIV prevention and contraception studies. Contraception. 2013;88:387–95. doi: 10.1016/j.contraception.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Melendez JH, Giles JA, Yuenger JD, Smith TD, Ghanem KG, Reich K, et al. Detection and quantification of Y-chromosomal sequences by real-time PCR using the LightCycler system. Sex Transm Dis. 2007;34:617–9. doi: 10.1097/01.olq.0000258336.65285.31. [DOI] [PubMed] [Google Scholar]
- 34.Sensabaugh GF. Isolation and characterization of a semen-specific protein from human seminal plasma: a potential new marker for semen identification. J Forensic Sci. 1978;23:106–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


