Abstract
Clinical studies have indicated that circulating bile acid (BA) concentrations increase following bariatric surgery, especially following malabsorptive procedures such as Roux-en-Y gastric bypasses (RYGB). Moreover, total circulating BA concentrations in patients following RYGB are positively correlated with serum glucagon-like peptide-1 concentrations and inversely correlated with postprandial glucose concentrations. Overall, these data suggest that the increased circulating BA concentrations following bariatric surgery - independently of calorie restriction and body-weight loss - could contribute, at least in part, to improvements in insulin sensitivity, incretin hormone secretion, and postprandial glycemia, leading to the remission of type-2 diabetes (T2DM). In humans, the primary and secondary BA pool size is dependent on the rate of biosynthesis and the enterohepatic circulation of BAs, as well as on the gut microbiota, which play a crucial role in BA biotransformation. Moreover, BAs and gut microbiota are closely integrated and affect each other. Thus, the alterations in bile flow that result from anatomical changes caused by bariatric surgery and changes in gut microbiome may influence circulating BA concentrations and could subsequently contribute to T2DM remission following RYGB. Research data coming largely from animal and cell culture models suggest that BAs can contribute, via nuclear farnezoid X receptor (FXR) and membrane G-protein-receptor (TGR-5), to beneficial effects on glucose metabolism. It is therefore likely that FXR, TGR-5, and BAs play a similar role in glucose metabolism following bariatric surgery in humans. The objective of this review is to discuss in detail the results of published studies that show how bariatric surgery affects glucose metabolism and subsequently T2DM remission.
Keywords: Bariatric surgery, Type-2 diabetes, Bile acids, RXR, TGR-5, Gut microbiota, Roux-en-Y gastric bypasses
Core tip: Emerging evidence suggests that increased concentrations of circulating bile acids could, through their interaction with membrane (TGR-5) and nuclear (FXR) receptors, significantly contribute to improved glucose metabolism following bariatric surgery. This review presents information on the potential mechanism of bile acids on the remission of type-2 diabetes following bariatric surgery.
INTRODUCTION
The number of overweight and obese individuals increased worldwide by approximately 28% between 1980 and 2013[1]. Obesity is caused by many factors, such as lifestyle habits including physical activity, exposition to environmental factors, genetic and epigenetic factors, and hormones and gut microbiota, and is characterized by excessive fat accumulation and the development of several diseases, including type-2 diabetes (T2DM), which is a major public health problem at present[2-6]. However, the primary inducer of obesity and its associated disorders is inappropriate food intake, especially of food containing high amounts of fat and carbohydrates[7].
Bariatric surgery, which is currently the most effective treatment for obesity and its associated disorders, provides long-term control of T2DM in approximately 80% of patients, while the conventional therapy has never been as effective[8]. The supremacy of the bariatric procedure in T2DM therapy has been confirmed by independent randomized trials where it was compared to an intensive course of the conventional treatment[9,10]. Very recently, the “Joint Statement by International Diabetes Organizations” regarding the role of bariatric (metabolic) surgery in the Treatment Algorithm for T2DM was published[11]. The general conclusion of the statement is that bariatric (metabolic) surgery should be recommended for the treatment of patients with TMD2 and obesity. Moreover, in news published recently in the British Medical Journal[12], it is suggested that bariatric surgery “(…) is a highly cost effective therapy for patients with T2DM (…)”.
Roux-en-Y gastric bypass (RYGB), a commonly used bariatric procedure, creates a small pouch in the stomach and connects it to the proximal jejunum to form the Roux limb, which is anastomosed to the duodenal limb, forming a Y configuration (Figure 1B). The anatomical changes resulting from RYGB (namely the exclusion of a long section of the small intestine from the passage of food) encourage bile to reach the distal intestine in relatively high concentrations (because the excreted bile has not mixed with ingested food). These events are frequently associated with: (1) body mass loss and improvement in associated disorders, including T2DM; (2) modification of secretion and action of some gut hormones and peptides, including incretin hormones[13-15]; (3) an increase in circulating bile acid (BA) concentrations[14-16]; and (4) alterations in gut microbiota[14,17-20]. The alterations in gut microbiota, which play a crucial role in the conversion of primary BAs to secondary BAs, indirectly activate (or do not activate) nuclear and membrane receptors via modification of the structure of BAs, and may thus influence human glucose metabolism[21].
Figure 1.
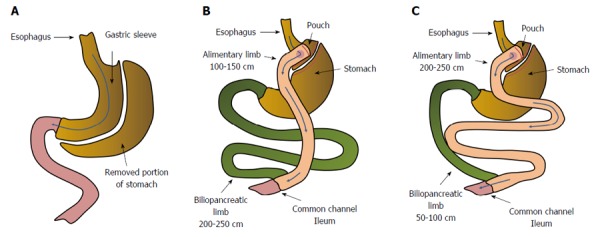
Anatomical changes in gastrointestinal tract resulting from: sleeve gastrectomy (A), Roux-en-Y gastric bypass - distal/scopinarized (B), Roux en Y gastric bypass - long limb (C).
It is generally believed that the improved glycemic control following RYGB, besides body weight loss and calorie restriction, can be the consequence of an increase in hepatic and peripheral insulin sensitivity, as well as of the increase in postprandial insulin secretion as a result of increased glucagon-like peptide-1 (GLP-1) secretion[13]. Some data suggest that body mass loss is an important determinant of T2DM remission one year following RYGB[22]. Other data indicate that changes in glycemic control following RYGB are mainly mediated by caloric restriction, but not by increased energy expenditure[15]. Since the improvement in glucose metabolism consequent on bariatric surgery is observed earlier than body mass loss, it is likely that the beneficial effect of RYGB (or other types of bariatric surgery) is at least in part independent of calorie restriction and body mass loss. So far, several mechanisms independent of body-mass loss for the improvement of glucose metabolism following bariatric surgery have been discussed[16]. However, the underlying mechanism of the action of bariatric surgery on glycemic control has not been fully elucidated.
Emerging evidence indicates that the increase in circulating bile acids and intestinal microbiota (via modification of the chemical structure and subsequently the biological activities of BAs) may play an important role in the improvement of glucose metabolism following bariatric surgery[16,23-27]. It should be emphasized that restrictive bariatric procedures, such as sleeve gastrectomy and gastric banding, which have no effect or less effect on circulating BA concentrations, display less influence on T2DM remission. On the other hand, after malabsorptive procedures such as RYGB, significant increases in circulating BA concentrations and a simultaneous improvement of glucose metabolism have frequently been observed[14,28-30]. Some authors have suggested that circulating BA concentrations increase following RYGB, independently of caloric restriction[31].
In summary, there are several clinical indications that an increase in gut, and subsequently in circulating, BA concentrations can occur following bariatric surgery (especially after malabsorptive procedures). In turn, BAs may play an important role in the improvement of glucose metabolism, via their binding to the nuclear or membrane receptors present in many organs, including the intestine, liver, pancreas, adipose tissue, and skeletal muscle[32-35]. The clinical observations were confirmed by animal studies indicating that: (1) RYGB contributes to increases in total circulating BAs in rats[36]; and (2) farnezoid X receptors (FXR) - one of the types of nuclear receptor activated by BAs - may be involved in improving glycemic control following bariatric surgery. For instance, in FXR knockout mice, the ability of bariatric surgery to improve glucose tolerance was significantly reduced[37]. Moreover, it has been shown that the administration of BAs to mice increases energy expenditure via the membrane G-protein - receptor (TGR-5) signaling pathway, preventing insulin resistance[38]. Moreover, the antidiabetic effect of agonists of TGR-5 other than BAs (for instance, oleanolic acid) in mice was also observed[39]. It is therefore likely that BAs and the nuclear and membrane receptors activated by BAs could play an important role in the regulation of glucose metabolism, and subsequently in T2DM remission in humans following bariatric surgery. However, caution needs to be taken when translating animal results to humans[36].
In recently published elegant review Penney et al[16] summarized the results regarding the role of BA and FXR and TGR-5 receptors in: (1) regulation of BA, lipid and energy metabolism; (2) glucose homeostasis; (3) incretin and other gut satiety hormones production; and (4) endoplasmic reticulum stress following bariatric surgery. The goal of this review is to focus on (1) BAs biosynthesis in liver; (2) BAs biotransformation in gut and enterohepatic circulation; and (3) the potential role of increased circulating BA concentrations and alterations of gut microbiota in improvement of glucose metabolism and subsequently in T2DM remission following bariatric surgery. In other words, this review offer a deeper understanding of the effect of bariatric surgery on T2DM remission.
EFFECT OF BARIATRIC SURGERY ON T2DM REMISSION: RESTRICTIVE PROCEDURES ARE LESS EFFECTIVE THAN MALABSORPTIVE PROCEDURES
As mentioned above, RYGB ameliorates most obesity related diseases, including T2DM[40]. As far as the remission of T2DM is concerned, biliopancreatic diversion with duodenal switch (BPD-DS)-a procedure where the longer intestinal limb is excluded from the alimentary passage - and transiting concentrated bile provided better results than RYGB, a procedure with markedly shorter biliary limbs (Figure 1)[41]. In turn, BPD-DS and RYGB are associated with a higher efficiency of T2DM remission than sleeve gastrectomy (SG), where no biliary exclusion is performed (Figure 1A)[42]. Several papers have indicated that improvements in insulin sensitivity were observed early after the surgery when the duodeno-jejunal exclusion is performed and cannot be only dependent on body mass loss[43-46]. Some authors have reported that the homeostatic model assessment of insulin resistance (HOMA-IR) had significantly improved even as soon as a few days after the gastric bypass[47-50]. Moreover, One Anastomosis Gastric Bypass (OAGB), where the concentrated bile transit is more than doubled in comparison to standard RYGB, provides greater improvement in HOMA-IR[47]. The reduction of the HOMA-IR value after SG is not as spectacular as observed following BPD-DS and RYGB, and is associated rather with the extreme caloric restriction resulting from the surgery[47].
The data suggest that the concentrated bile transit in the excluded jejunum, and the subsequently enhanced circulating concentrations of BAs, may play an important role in metabolic improvement following bariatric procedures. The mechanisms triggered by the altered digestive tract anatomy after the separation of the intestinal section from the alimentary passage are recognized as a key factor in foregut theory[51]. An overview of the anatomical changes in the gastrointestinal tract caused by various types of bariatric surgery is presented in Figure 1.
The effectiveness of gastric bypass in managing patients with type-1 diabetes mellitus or patients with low concentrations of C-peptide (below 1 ng/mL) has also been reported[52,53]. Significant reductions in exogenous insulin administration have been observed shortly following the operation in the studied patients.
Overall, the clinical data reviewed above support the idea that bariatric surgery, especially malabsorptive procedures, could be used in the treatment of both T1DM and T2DM.
ROLE OF THE LIVER IN PRIMARY BILE ACID BIOSYNTHESIS AND ENTEROHEPATIC BILE ACID CIRCULATION
In healthy subjects, cholic acid (CA) and chenodeoxycholic acid (CDCA) are the primary BAs synthesized from cholesterol mainly via the classic (or neutral) pathway in the liver endoplasmic reticulum. This pathway accounts for more than 90% of the total BA synthesis under physiological conditions[54]. Cholesterol in the presence of NADPH and O2 is converted to 7α-hydroxycholesterol by cytochrome P450 7α-hydroxylase (CYP7A1, encoded by CYP7A1), the rate limiting enzyme in both CA and CDCA biosynthesis (Figure 2). CYP7A1 is under negative feedback regulation by BAs, via BAs binding to FXR[55]. On the other hand, liver X receptor is involved in a feed-forward regulation of CYP7A1 dependent on oxysterol, which is formed from cholesterol (Figure 2). The 7α-hydroxycholesterol thus formed is then converted by 3β-hydroxy-∆5 -C27 steroid oxidoreductase (which isomerizes the ∆5 bond to the ∆4 position and oxidizes the 3β-OH to a 3-oxo group) to 4-cholesten-7α-ol-3-one, a precursor of both CA and CDCA (Figure 3A). In contrast to CDCA synthesis, CA requires the introduction of a hydroxyl group (OH group) in position 12α on the steroid skeleton. Therefore, 4-cholesten-7α-ol-3-one is hydroxylated to 4-cholesten-7α, 12α-diol-3-one by 12α hydroxylase (CYP8B1, encoded by CYP8B1). Recently, it has been found that BA-activated FXR increases the levels of MAFG (a product of the Mafg gene), which in turn inhibits CYP8B1 in mice[56]. Moreover, it has been shown that MAFG has no effect on CYP7A1. This suggests that MAFG may regulate the CDCA:CA ratio (and subsequently BA composition), and thus the hydrophobicity of BAs (by inhibiting CA synthesis without affecting CDCA synthesis), though not the BA pool size[56]. All the later steps in the formation of BAs, such as the conversion of 4-cholesten-7α-ol-3-one to chenodeoxycholyl-CoA or 4-cholesten-7α, 12α-diol-3-one to cholyl-CoA, are catalyzed by the same enzymes that are present in the hepatocytes (Figure 3B). Note that Figure 3B shows the pathway of cholyl-CoA biosynthesis. Biosynthesis of chenodeoxycholyl-CoA (which, unlike CA, does not contain a 12-OH group) takes place in the same manner and is catalyzed by the same liver enzymes (not shown). An alternative pathway (the acidic pathway) also begins in the hepatocytes with the mitochondrial 27-hydroxylation of cholesterol, catalyzed by CYP27A1 (encoded by CYP27A1) (not shown). Moreover, the mitochondrial 27-hydroxylation of cholesterol may additionally occur in extrahepatic tissue[57]. The conversion of cholesterol to BAs can also begin with 25-hydroxylation or 24-hydroxylation of cholesterol in extrahepatic tissue[58], though these pathway are probably responsible for less than 1% of the total production of BAs. 27-OH-cholesterol, 25-OH-cholesterol, and 24-OH-cholesterol are then hydroxylated by specific hydroxylases (CYP7B1 in the case of 27-OH-cholesterol, 25-OH-cholesterol or CYP39A1 or CYP7A1 in the case of 24-OH-cholesterol)[58,59]. Regardless of the initial hydroxylation of cholesterol, all the later steps in the biosynthesis of BAs occur in the liver, according to the scheme presented in detail in Figure 3B.
Figure 2.
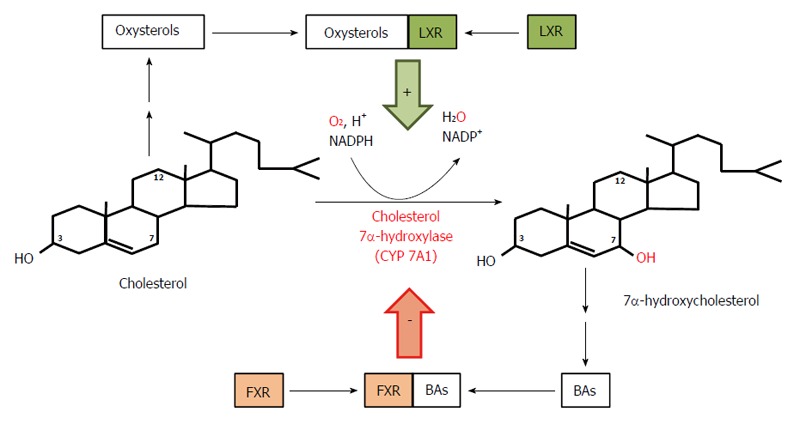
Course and regulation of reactions catalyzed by cholesterol 7α-hydroxylase - the rate limiting enzyme in bile acid biosynthesis. Bile acids (BAs) formed from 7α-hydroxycholesterol bind to farnezoid X receptor (FXR) and inhibit expression of the gene coding for 7α-hydroxylase, subsequently diminishing the rate of BA biosynthesis in liver. Oxysterols formed from cholesterol bind to liver X receptor (LXR) and stimulate expression of the gene coding for 7α-hydroxylase and the subsequent conversion of cholesterol to 7α-hydroxycholesterol and to BAs.
Figure 3.
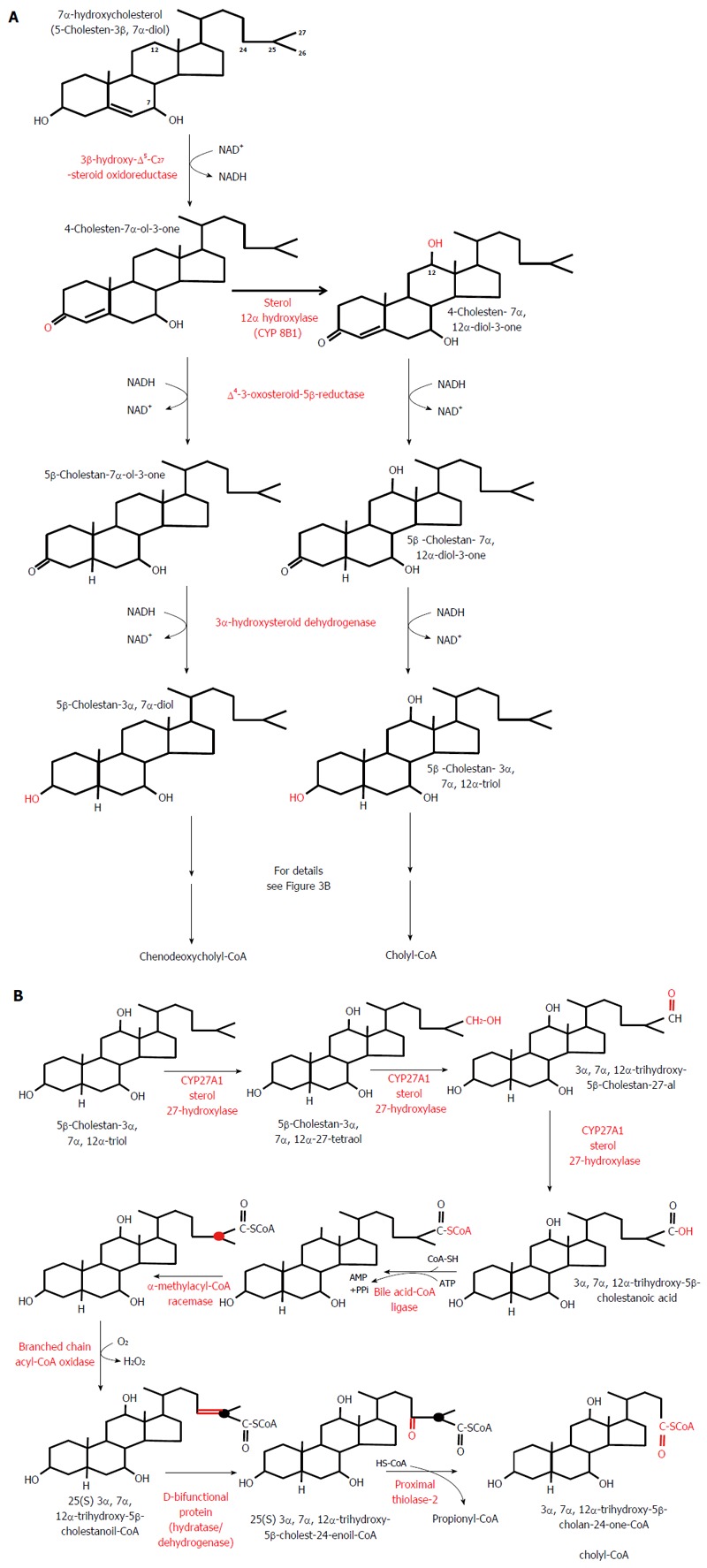
Classic pathway of bile acid biosynthesis in the liver. A: Conversion of 7α-hydroxycholesterol to 5β-cholestan-3α, 7α-diol - a precursor of chenodeoxycholyl-CoA - and of 7α-hydroxycholesterol to 5β-cholestan-3α, 7α,12α-triol - a precursor of cholyl-CoA. B: Conversion of 5β-cholestan-3α, 7α, 12α-triol to cholyl-CoA. Note that the conversion of 5β-cholestan-3α,7α-diol to chenodeoxycholyl-CoA biosynthesis takes place in the same manner and it is catalyzed by the same liver enzymes (not shown).
Most BAs, once formed, are immediately conjugated to glycine or taurine in liver peroxisomes, as illustrated in Figure 4. For example, Figure 4 shows the conversion of cholyl-CoA to glycocholic acid or taurocholic acid (TCA). The conversion of chenodeoxycholyl-CoA to glycochenodeoxycholic acid (GCDCA) or taurochenodeoxycholic acid (TCDCA) proceeds in the same manner (not shown). It is worth noting that conjugation changes the ability of BA to activate nuclear and membrane receptors. Primary BAs preferentially activate FXR, whereas TGR-5 is principally activated by secondary BAs[60].
Figure 4.
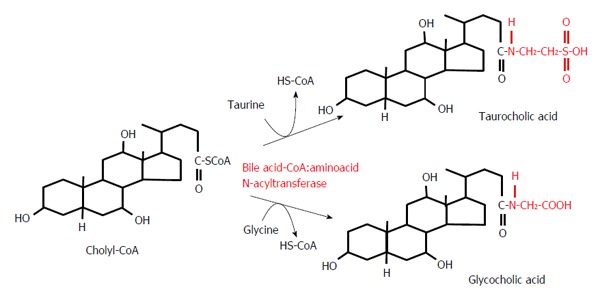
Conjugation reactions of cholyl-CoA with taurine or glycine.
BAs conjugated with taurine or glycine in the hepatocytes are excreted from the liver, chiefly via bile salts export proteins (BSEP) present at the canalicular membrane of the hepatocytes to canaliculi and then, via hepatic ducts, to the gallbladder, where the bile is concentrated during interdigestive periods before being released into the duodenum (Figure 5). Meals, especially those containing dietary fat, stimulate intestinal cells to secrete cholecystokinin, which in turn promotes the contraction of the gallbladder, facilitating the secretion of bile salts into the duodenum via the common bile duct (Figure 5). More than 95% of bile salts are reabsorbed by the intestinal cells (ileocytes) via apical sodium-dependent BA transporter (ASBT), also called ileal BA transporter (IBAT)[61], or through passive diffusion, mostly in the upper small intestine and colon[62,63] (Figure 5). In intestinal cells, BAs are transdiffused across the ileocytes or are bound to I-BABP (ileocyte BA-binding protein), also known as FABP6 (fatty-acid binding protein 6). The binding of BAs to I-BABP facilitates the transport of BAs across ileocytes. Interestingly, some BAs (such as TCA) increase I-BABP levels through activation of FXR and the subsequent stimulation of expression of the gene encoding I-BABP, suggesting that this protein plays an important role in regulation of enterohepatic BA circulation[64]. BA efflux from intestinal cells is mediated via organic solute transporters α and β (OSTα and OSTβ heterodimers) located in the basolateral membrane of intestinal cells[54] (Figure 5). The reabsorbed BAs enter portal circulation and are transported back to the liver (hepatocytes). Na+-taurocholate cotransport peptide and the organic anion transporting proteins, located in basolateral membrane of the hepatocyte, are responsible for sodium dependent uptake of conjugated and unconjugated BAs, respectively[33,54]. Figure 5 presents an overview of enterohepatic BA circulation. Some data suggest that the altered enterohepatic circulation of BAs could contribute to improved insulin sensitivity and cholesterol metabolism following biliopancreatic diversion[65].
Figure 5.
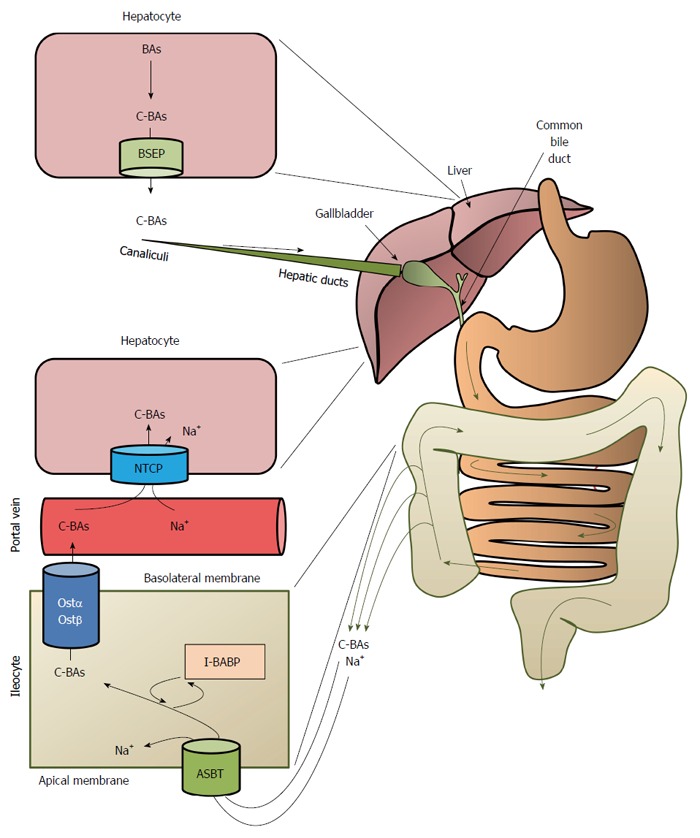
Overview of the enterohepatic circulation of bile acids. BAs: Bile acids; C-BAs: Conjugated bile acids; BSEP: Bile salt export proteins; ASBT: Bile acid transporter; I-BABP: Ileocyte bile-acid binding protein; OSTα/OSTβ: Organic solute transporters α/β; NTCP: Na+-taurocholate cotransport peptide.
After intestinal absorption and the return to the enterohepatic circulation, the conjugated and unconjugated BAs in the hepatocytes can undergo: (1) conversion to the corresponding CoA esters (CA to cholyl-CoA and CDCA to chenodeoxycholyl-CoA) by bile acid-CoA synthase (BACS; also called bile acid-CoA ligase, BACL) followed by reconjugation with glycine or taurine catalyzed by bile acid-CoA:amino acid N-acyltransferase, as presented in Figure 4 (only unconjugated BAs); (2) re-epimerization (conversion of the 3β-OH form to the 3α-OH form); (3) reduction (conversion of the O = form to the OH form); and (4) 7α-hydroxylation (in some species, though not in humans)[66]. It should be emphasized that the enterohepatic circulation of BAs is regulated by BA-activated FXR, which influences the levels of BSEP, ASBT, and IBBAP[67].
ROLE OF INTESTINAL MICROBIOTA IN SECONDARY BILE ACID PRODUCTION-INTERACTIONS BETWEEN BILE ACIDS AND INTESTINAL MICROBIOTA
Approximately 5% of bile salts (bile acids conjugated with glycine or taurine) escape enterohepatic circulation and undergo biotransformation to secondary BAs by intestinal microbiota enzymes. CA is converted to deoxycholic acid (DCA) and CDCA to litocholic (LCA)[68]. In healthy adults, the daily fecal loss of BAs amounts to 0.4-0.8 g, which is replenished by de novo BA biosynthesis (that is, conversion of cholesterol to BAs) in the liver, as described above and presented in Figures 2 and 3.
It is worth noting that the human intestine is colonized by about 100 trillion microbial cells[69], whose total biomass is approximately 1 kg[70]. The majority of gut microbiota are considered to be commensals, as they play important symbiotic roles, including: (1) the degradation of nondigested polysaccharides to short-chain fatty acids[71]; (2) the production of some vitamins (B and K)[72]; and (3) BA biotransformation. Six bacterial phyla-Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia-dominate in the gut of adult healthy human. Firmicutes and Bacteroidetes together can constitute over 90% of the microbiota, whereas the other phyla range between 2% and 10%[73]. The composition and activity of the gut microbiota is affected by energetic substrate availability and the physicochemical conditions in the alimentary tract. Substrates required by gut bacteria come from food ingested by host. The host also provides mucins, desquamated cells, and digestive enzymes, which can be used by gut bacteria, as well as BAs, which influence bacterial growth and are metabolized (undergo biotransformation) by bacteria. Gut microbial changes in response to any intervention-for example anatomical changes caused by bariatric surgery[19,36] or antibiotic treatment[74]-may also affect BA biotransformation. In other words, it may be supposed that changes in gut microbiota composition can influence the composition and concentrations of circulating BAs, which in turn can affect obesity and related disorders. However, it remains to be clarified further how bariatric surgery (and other treatments) modulates the gut microbiota towards a beneficial or harmful composition.
BA biotransformation occurs mainly in the large intestine, which is rich in bacteria (Table 1). Intestinal biotransformation is a very complex process involving several reactions, including: (1) removal of the glycine or taurine side chain, a process commonly known as the deconjugation of BAs; (2) 7α-dehydroxylation (removal of OH group at C-7α); (3) oxidation and epimerization of the C-3, C-7, or C-12 OH groups of BAs; (4) reduction of steroid skeleton (insertion of H into steroid structure); and (5) hydroxylation of BAs (insertion of an OH group)[68,75-78].
Table 1.
Abundance of bacterial species in various sections of the human intestine
| Intestine | Abundance of Bacteria | Bacteria |
| Small intestine | ||
| Duodenum | About 103 (bacteria/mL) | Lactobacillus1 |
| Streptococcus2 | ||
| Jejunum | About 104 (bacteria/mL) | Lactobacillus1 |
| Streptococcus2 | ||
| Staphylococcus | ||
| Veillonella2 | ||
| Ileum | 106-108 (bacteria/mL) | Enterobacteri1 |
| Enterococcus1 | ||
| Bacteroides12 | ||
| Clostridium12 | ||
| Lactobacillus1 | ||
| Veillonella | ||
| Large intestine | About 1011 (bacteria/g) | Bacteroides12 |
| Eubacterium2 | ||
| Bifidobacterium1 | ||
| Ruminococcus | ||
| Peptostreptococcus | ||
| Propionibacterium | ||
| Clostridium12 | ||
| Lactobacillus1 | ||
| Escherichia | ||
| Streptococcus2 | ||
| Methanobrevibacter |
Bacteria displaying bile salt hydrolase (BSH) activity;
Bacteria capable of catalyzing 7α-dehydroxylation. Based on data presented in[68].
Bile salt deconjugation is catalyzed by bile salt hydrolase (BSH) (Figure 6). This enzyme is present in various gut bacteria including: Clostridium, Bacteroides, Lactobacillus, Bifidobacterium, and Enterococcus (Table 1)[77]. In theory, deconjugation can begin in the small intestine, since bacteria displaying BSH activity are found there, though only in small numbers (Table 1). It is worth noting that deconjugated BAs are highly insoluble, toxic compounds and are excreted more rapidly than conjugated BAs[79].
Figure 6.
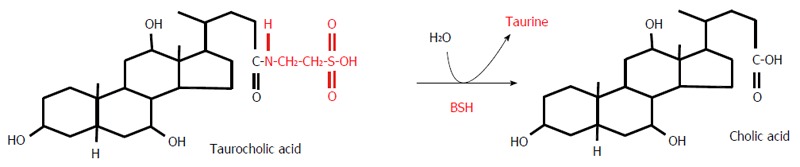
Deconjugation of taurocholic acid by bile salt hydrolase. BSH: Bile salt hydrolase.
The 7α-dehydroxylation contributes to the conversion of CA and CDCA to DCA and LCA, respectively. The formation of DCA and LCA - the secondary BAs that predominate in human feces (Table 2)-is considered the most quantitatively important biotransformation process of BAs[78]. This process is carried out by only a few strains of human intestinal bacteria, including Bacteroides, Clostridium, Streptococcus fecalis, and Veillonella[78]. Theoretically, dehydroxylation can take place also in the small intestine, since bacteria capable of performing such activities (dehydroxylation of CA) are found there (Table 1).
Table 2.
Composition of human biliary and fecal bile acids
| Bile acids | Biliary bile acids composition (% of total) | Fecal bile acids composition (% of total) |
| Cholic acid (CA) | 35% | 2% |
| Chenodeoxycholic acid (CDCA) | 35% | 2% |
| Deoxycholic acid (DCA) | 25% | 34% |
| Ursodeoxycholic acid (UDCA) | 2% | 2% |
| Lithocholic acid (LCA) | 1% | 29% |
| 12-oxo-Lithocholic acid (12-oxo-LCA) | - | 3% |
| Other | 2% | 28% |
Table shows percentage of both conjugated and unconjugated bile acids. Based on data presented in[68].
The oxidation and epimerization of the C-3, C-7, or C-12 hydroxy groups of BAs are catalyzed by α-hydroxysteroid dehydrogenases (α-HSDH) or β-HSDH, which are also present in gut bacteria.
It is worth noting that bile (especially the BAs it contains) significantly affects the survival and, subsequently the colonization, of some intestinal bacteria, especially in the small intestine[80]. Several mechanisms have been proposed for the direct antimicrobial action of BAs namely: (1) the interaction of BAs with bacterial membrane (where the BAs simply act as detergents); (2) acidification of bacterial cytoplasm; (3) bacterial DNA damage; and (4) alterations in bacterial proteins[66]. Studies with mice lacking FXR suggest that, besides direct antimicrobial action, BAs have indirect effects through FXR-induced antimicrobial peptide synthesis in the intestine[81]. Interestingly, Gram-positive bacteria are more sensitive to the deleterious effects of BAs than are Gram-negative bacteria[77]. It is worth noting that the gut bacteria benefit from metabolizing BAs. For instance, in catalyzing the deconjugation of BAs, gut bacteria can use glycine or taurine for their own metabolism[82]. Additionally, gut bacteria use BAs as sinks for the disposal of electrons liberated during bacterial fermentation[82].
The results discussed above indicate that BA pool size is controlled through 3 processes: (1) regulation of BA biosynthesis in the human liver; (2) enterohepatic circulation; and (3) the involvement of intestinal microbiota in the biotransformation of BAs[54]. As a result of their antimicrobial activity, BAs can affect the abundance and composition of gut microbiota[54,83,84]. This means that BAs and gut microbiota are closely integrated and affect each other: bile affects the growth and colonization of bacteria in the intestine, while bacteria contribute to the biotransformation of BAs. Theoretically, in pathological conditions, perturbations in the equilibrium between BA pool size and gut microbiota could occur. An intriguing example of such perturbation might be the state after bariatric surgery, when the gut contains more concentrated bile, which may affect the survival and subsequently the colonization of some gut bacteria, especially those involved in BA biotransformation and short-chain fatty acid production. As energetic substrates for colonocytes, short-chain fatty acids play an important role in maintaining intestinal barrier permeability[85].
EFFECT OF BARIATRIC SURGERY ON INTESTINE MICROBIOTA ABUNDANCE AND COMPOSITION
Gut microbiota is now considered an important factor affecting human health[86,87]. It is known that gut microbial composition is significantly altered in obese subjects, as compared to lean controls[88]. Using 16S rRNA to quantitatively identify bacteria, it has been shown that obesity is associated with a decrease in the relative abundance of Bacteroidetes vs Firmicutes[88-90]. Some data suggest that gut microbiota may play some role in the development of obesity[91-93] and obesity-related diseases[93,94], including T2DM[95,96]. In mice, gut microbiota composition changes significantly in response to dietary changes[97].
Several papers have pointed to significant changes in gut microbiota abundance and composition in humans and animal models as a possible beneficial effect of bariatric surgery[17,20,98-102]. Moreover, following bariatric surgery, an association has been reported between increased circulating BA concentrations and changes in gut microbial composition[19]. Altogether, the data discussed above suggest that changes in intestinal microbiota-possibly through the effects on BA pool size and primary:secondary BA ratio - may contribute to the beneficial effects of bariatric surgery on glucose metabolism[103,104]. However further studies are necessary to confirm this suggestion. Changes in gut microbiota following bariatric surgery were also seen to be closely linked to improvements in diabetic parameters and body mass loss[14,17-20].
It is worth noting that changes in gut microbiota following bariatric surgery are related to short-chain fatty acid production (mainly of butyrate and propionate)[19], which can influence glucose metabolism independently of BAs[95,105]. Moreover, it has been reported that changes in short-chain fatty acid biosynthesis by gut bacteria following RYGB may promote GLP-1 secretion[106]. It has recently been shown that the increased production of acetate by gut microbiota in rodents leads to activation of the parasympathetic nervous system, which in turn promotes insulin secretion[107]. Thus, it is not impossible that changes in gut microbiota following bariatric surgery, together with the subsequent changes in short-chain fatty acid production in the gut, could affect glucose metabolism independently or in combination with BAs. However, further studies are needed to confirm this supposition.
BILE ACIDS: LIGANDS FOR MEMBRANE AND NUCLEAR RECEPTORS INVOLVED IN THE REGULATION OF GLUCOSE METABOLISM
For many years, BAs were considered to be involved in (1) the digestion and absorption of dietary lipids (including fat-soluble vitamins); (2) cholesterol solubilization in gallbladder bile; (3) excretion of cholesterol (following cholesterol conversion to BAs) from the human body; and (4) regulation of survival, and subsequently colonization, of some gut bacteria. BAs circulating in the blood are now considered to be important regulatory molecules which, via binding to membrane or nuclear receptors, contribute to the homeostasis of carbohydrates and lipids. BAs also have effects on the immune system[108] and the apoptosis of colonic epithelial cells[109]. The main BAs detected in human serum are presented in Table 3[110].
Table 3.
Bile acids present in human serum. Based on data presented in[110]
| Primary bile acids | Secondary bile acids |
| Cholic acid (CA) | Lithocholic acid (LCA) |
| Chenodeoxycholic acid (CDCA) | Deoxycholic acid (DCA) |
| Glycocholic acid (GCA) | Ursodeoxycholic acid (UDCA) |
| Glycochenodeoxycholic acid (GCDCA) | Hyodeoxycholic acid (HDCA) |
| Taurocholic acid (TCA) | Glycolithocholic acid (GLCA) |
| Taurochenodeoxycholic acid (TCDCA) | Glycodeoxycholic acid (GDCA) |
| Glycoursodeoxycholic acid (GUDCA) | |
| Taurolithocholic acid (TLCA) | |
| Taurodeoxycholic acid (TDCA) | |
| Tauroursodeoxycholic acid (TUDCA) | |
| Taurohyodeoxycholic acid (THDCA) |
It is worth noting that ursodeoxycholic acid (UDCA) is also used as a drug for cholesterol gallstone dissolution therapy[111,112] and for treating patients with primary biliary cirrhosis[113]. It has recently been reported that the treatment of patients with UDCA significantly decreases gallstone formation following sleeve gastrectomy[114]. Moreover, the issue of using BA analogs as drugs in the treatment of NAFLD and NASH has also been discussed[115].
The known nuclear receptors activated by BAs (generally called bile acid activated receptors or BARs) are FXR (also known as NR1H4), vitamin D receptor (VDR, also known as NR1H1); constitutive androstane receptor (CAR, also known as NR1H3); and pregnane X receptor (PXR, also known as NR1H2). Although the role of PXR, CAR and VDR in the regulation of metabolism, including glucose metabolism, cannot be excluded[21,115-118], it seems that the activation of FXR by BAs (note that primary BA are preferential ligands of this receptor) plays an important role in the regulation of glucose metabolism[119]. CDCA, which activates FXR at EC50 in a concentration of approximately 10 μmol/L, appears to be the main physiological ligand for FXR. Other BAs could also activate FXR, but in significantly higher concentrations. The potency of BAs to activate FXR is as follow: CDCA > DCA > LCA > CA[64]. Through activation of FXR, BAs play a key role in liver and intestinal biosynthesis, enterohepatic transport, and homeostasis of BAs[64]. Accordingly, a deficiency in FXR may lead to cholestasis[120]. In the intestine, BAs bind to FXR and induce synthesis of fibroblast growth factor 19 (FGF-19) in humans or FGF 15 in rodents and secretion, which in turn circulates to the liver where it regulates BA biosynthesis and glucose metabolism[64]. In the liver, FGF 15/19 activates glycogen synthesis and inhibits gluconeogenesis via FGF receptor, which subsequently leads to a decrease in circulating glucose concentrations (Figure 7A)[64]. Thus, FGF 15/19 is sometimes referred to as a downstream metabolic effector of BAs. When insulin is an early-acting hormone released after a meal, FGF 15/19 is a late-acting hormone released in the fed state (with a half-life of about 30 min)[35]. By activating FXR, BAs also downregulate liver gluconeogenesis through inhibiting the gene expression of glucose 6-phosphatase, fructose 1,6-bisphosphatase, and phosphoenolpyruvate carboxykinase (PEPCK) (Figure 7B)[121]. However, some data suggest that PEPCK is upregulated by BAs via FXR[121]. In rodent hepatocytes, DCA-activated epidermal growth factor receptors ERB1/ERB2 and insulin receptor contribute to the activation of glycogen synthase through the PI3kinase/AKT/GSK3 signaling pathway[122]. Accordingly, the agonists of FXR decrease blood glucose concentrations[123]. Some authors suggest that BAs contribute to post-RYGB hypoglycemia, which sometimes occurs after bariatric surgery[124].
Figure 7.
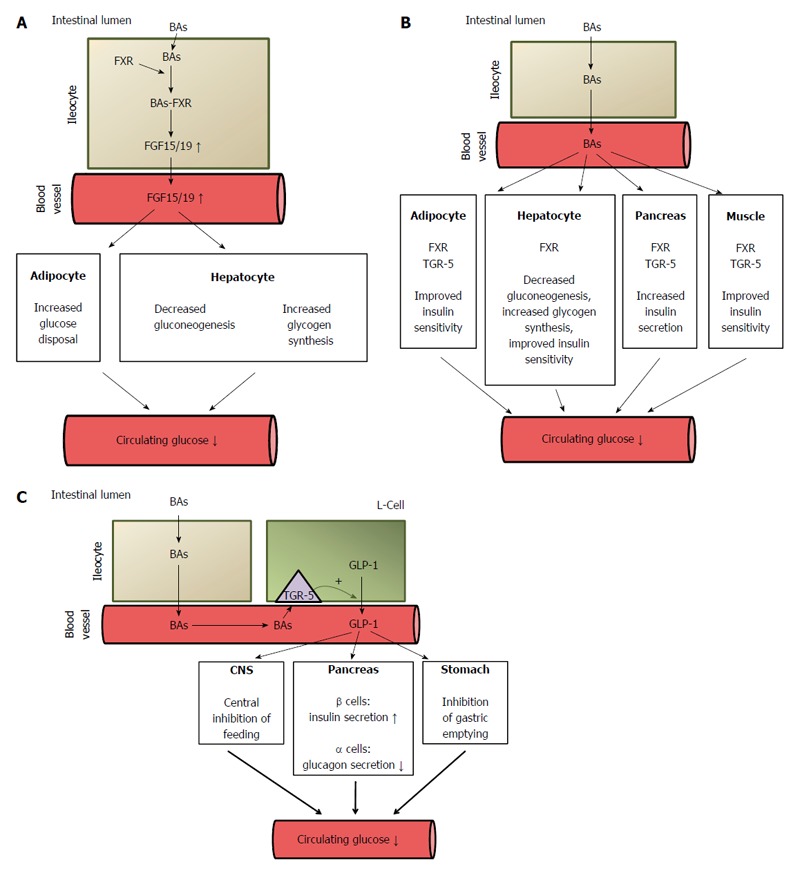
Potential mechanisms of bile acid mediated improvement of serum glucose concentration. A: Stimulatory effect of bile acids on FGF15/19 synthesis in intestinal cells (ileocytes). The activation of FXR in ileocytes by BAs leads to increased synthesis (via regulation of gene expression) and the release of fibroblast growth factor 15/19 (FGF 15/19) which, through the activation of the FGF-R present in hepatocyte and adipocyte membranes, regulates carbohydrate metabolism, leading to a decrease in circulating glucose concentrations. FGF 15/19 stimulates glycogen synthesis and inhibits gluconeogenesis in the liver and glucose disposal in adipose tissue. ↓: Decrease; ↑: Increase; B: Decreasing effect of BAs on circulating glucose concentration. BAs, by activating FXR, downregulate (via regulation of gene expression) liver gluconeogenesis and stimulate glycogen synthesis. BAs, by binding to FXR or to TGR-5 in pancreatic β-cells, stimulate insulin secretion. BAs, by binding to FXR or TGR-5 in adipose tissue and skeletal muscle, improve insulin sensitivity. ↓: Decrease; C: Potential mechanisms of BA-mediated decrease in circulating glucose concentrations after bariatric surgery caused by the increased release of GLP-1 by intestinal L-cells. ↓: Decrease; ↑: Increase. FXR: Farnezoid X receptor; FGR: Fibroblast growth factor; GLP: Glucagon-like peptide-1; BAs: Bile acids.
In mice, BAs bind to FXR in pancreatic β-cells and thus stimulate insulin secretion (Figure 7B)[125]. FXR knockout in mice leads to impaired glucose tolerance and insulin sensitivity[126]. The activation of FXR in the liver and gallbladder is associated with the regulation of BA synthesis and excretion, respectively.
Apart from nuclear receptors, BAs (secondary BAs are preferential ligands) can bind to membrane, G-protein coupled receptors such as TGR-5 (Takeda-G-protein-receptor, also known as G-protein-coupled bile acid receptor 1, GPBAR1), muscarine receptor (M3R), and sphingosine 1-phosphate receptor-2 (S1PR2), as well as epidermal growth factor receptor (EGFR), which belongs to the ErbB family of tyrosine kinase receptors[21,108,115,127,128]. The organ and tissue localizations of these nuclear and membrane receptors are presented in Figure 8.
Figure 8.
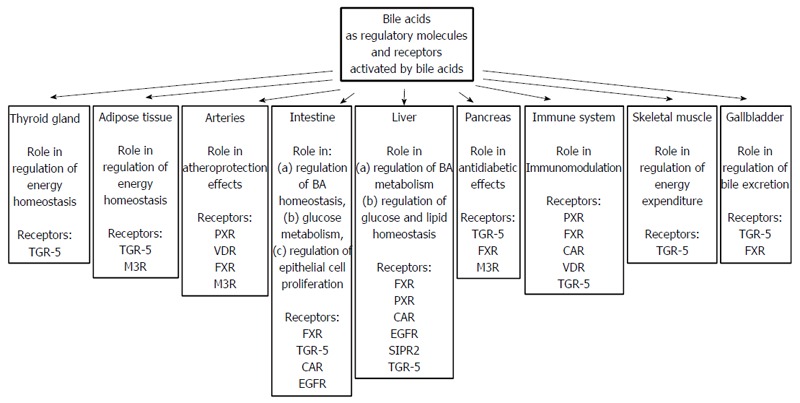
Bile acids as regulatory molecules and receptors activated by bile acids present in different organs.
As far as glucose metabolism is concerned, it seems that the activation of TGR-5 by BAs also plays an important role in the regulation of circulating glucose concentrations (Figure 7B)[123]. Taurolithocholic acid and LCA activate TGR-5 in nanomolar concentrations, which suggests that they are physiological ligands of this receptor. TGR5 is expressed in many organs including pancreatic β-cells, endocrine small intestine cells, the thyroid gland, the gallbladder, the liver, brown adipose tissue (BAT), cardiomyocytes, and macrophages[108,129,130]. Recent studies have shown that tauroursodeoxycholic acid (TUDCA) increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic cells and that TGR-5 is likely involved in this process (Figure 7B)[131]. Interestingly, this process is not associated with changes in glucose metabolism in pancreatic β-cells[131]. It may thus be supposed that the activation of both TGR-5 and FXR (discussed above) in pancreatic β-cells by BAs contributes to hyperinsulinemia developing late after RYGB[46]. Moreover, TGR-5 has been found in the brain, where it functions as a neurosteroid receptor[132]. Considering that TGR-5 is present in the brain and that BAs cross the blood-brain barrier, it may be supposed that activation of brain TGR-5 by BAs can also contribute to the beneficial effects of bariatric surgery.
The activation of TGR-5 in skeletal muscle cells (and BAT) leads to the induction of the cAMP-dependent signaling pathway, which results in increased energy expenditure (Figure 7B)[38,123,133]. The potency of BAs in activating TGR-5 is as follows: DCA>LCA>CDCA>CA[21]. In the intestine, the activation of TGR-5 by endocrine L cells (and by BAs[134]) leads to increased secretion of GLP-1 - an incretin hormone that increases the insulin secretion by pancreatic β-cells and inhibits the secretion of glucagon by pancreatic α-cells[123,133,135], thus affecting blood glucose concentration (Figure 7C). Moreover, BAs have been found to have a synergistic effect with glucose on the regulation of incretin hormone secretion[134]. In addition, gastric emptying and satiety mediated by GLP-1 could also contribute to its effect on circulating glucose concentration (Figure 7C)[136,137]. However, whether LCA activates TGR-5 under physiological conditions is not clear[55]; this may happen after bariatric surgery, when bile is concentrated and the subsequent circulating BA levels are elevated. An association has been found between circulating BAs and GLP-1 in patients who have undergone bariatric surgery[28,103,138]. Moreover, rectal administration of taurocholate resulted in an increase in circulating GLP-1, PYY, and insulin, but decreased glucose concentrations in obese T2DM volunteers[139]. It seems that TGR-5 also regulates BA pools, since these are significantly lower in TGR-5 (-/-) mice than in wild-type animals[115].
The activation of S1PR2 by BAs may also influence liver carbohydrate metabolism[127]. Recent studies suggest that blocking S1PR2 signaling could function as a novel therapeutic strategy for T2DM[140].
The activation of EGFR by BAs in the liver and intestine is associated with cell apoptosis[141] and cell proliferation[128], respectively. So far, the activation of muscarine receptors by BAs in context of the regulation of glucose homeostasis has not been reported.
Interestingly, BAs may exert many other biological functions via non-receptor-linked mechanisms involving the JNK1/2, ERK1/2, Akt1/2 signaling pathways, NO metabolism, and activation of cation channels[108]. Moreover, it has been shown that some BAs, such as UDCA or CA, are able to reduce endoplasmic reticulum (ER) stress[142,143]. Accordingly, significant reductions in ER stress have been observed following bariatric surgery on obese rats[144]. Thus, it is not impossible that the increase in circulating BAs following bariatric surgery may ameliorate ER stress, and subsequently improve glucose homeostasis.
Overall, the data discussed above suggest that by binding to membrane or nuclear receptors, and also through non-receptor mechanisms, BAs may influence glucose metabolism and subsequently contribute to the remission of T2DM following bariatric surgery when circulating BA concentrations are elevated.
EFFECT OF BARIATRIC SURGERY ON CIRCULATING BILE ACID CONCENTRATIONS AND DIABETES MARKERS: EVIDENCE THAT MALABSORPTIVE PROCEDURES ARE MORE EFFECTIVE THAN RESTRICTIVE PROCEDURES
Several papers have reported that circulating total BA concentrations significantly increase following bariatric surgery; however the results are inconsistent when BA composition is considered[14,16]. Moreover, the effect of bariatric surgery on serum BA concentration depends on the type of surgical procedure. In patients who have undergone gastric banding, no changes or decreases in serum BA concentrations were reported (Table 4)[28,138,145]. However, Nakatani et al[146] observed increased serum BAs one and three months after laparoscopic gastric banding (LAGB). Various effects of laparoscopic sleeve gastrectomy (LSG) on circulating BA concentrations have also been reported (Table 4). Steinert et al[147] found decreases in plasma BAs one week after LSG and increases three months and one year after the surgery. Belgaumkar et al[148] found decreases in primary BAs, and increases in secondary BAs six months after LSG. Increases in BA after LSG were documented by Nakatani et al[146] and Haluzíková et al[29].
Table 4.
Effects of various bariatric procedures on diabetic parameters and serum bile acid concentrations
| Patients | Type of bariatric procedure | Time interval from surgery to examination | Effect of surgery on diabetic parameters | Effect of surgery on serum bile acid concentrations | Ref. |
| Morbidly obese, n = 10 | LAGB | Various (after losing 20% of body weight) | Not presented | Decreased fasting BAs; no change in postprandial BAs | [28] |
| Morbidly obese, n = 6, preoperative BMI = 44 | Gastric banding | 42 d | Not presented | No change | [138] |
| Morbidly obese, n = 28, BMI = 46.0 | Gastric banding | 6-28 mo | Decreased serum glucose | Decreased primary BAs | [145] |
| Decreased serum insulin | No change in deoxycholic BAs | ||||
| Morbidly obese, n = 7, BMI = 43 | LSG | 1 wk, 3 mo, 1 yr | Decreased HOMA-IR | Decreased BAs after 1 wk | [147] |
| Increased BAs after 3 mo and 1 yr | |||||
| Morbidly obese, n =18, BMI = 60 | LSG | 6 mo | Decreased: fasting glucose , fasting insulin, HOMA-IR and HBA1c | No change in total BAs | [148] |
| Decreased primary BAs | |||||
| Increased secondary BAs | |||||
| Obese females, n = 17, BMI = 43 | LSG | 24 mo | Decreased: HbA1c, insulin, | Increased total BAs | [29] |
| HOMA-IR. | |||||
| Morbidly obese, n =15, BMI = 45 | LSG and LAGB | 1 and 3 mo | Decreased: HbA1c, insulin and HOMA-IR | Increased total, primary and secondary BAs | [146] |
| Morbidly obese, n = 8 | RYGB | Various (after losing 20% of body weight) | Not presented | Increased fasting and postprandial total and conjugated BAs | [28] |
| Morbidly obese, n = 9, preoperative BMI = 50 | RYGB | 2-4 yr | Lower fasting glucose and insulin | Higher total BA concentration | [103] |
| Morbidly obese, n = 37, nondiabetic, preoperative BMI = 48 | RYGB | About 200 d | No change in fasting serum glucose | No change in BAs after surgery | [150] |
| Morbidly obese, n =75, diabetic, preoperative BMI = 48 | RYGB | About 6 mo | Decreased fasting serum glucose and HbA1c | Increased total BAs | [150] |
| Severely obese women with T2DM, n = 13, preoperative BMI = 44 | RYGB | 1 mo and 2 yr | Decreased HOMA-IR | Reduced BAs after 1 mo | [32] |
| Increased BAs after 2 yr | |||||
| Obese patients, n = 63, BMI = 44 | RYGB | 15 mo | Decreased fasting glucose and HOMA-IR | Increased total BAs | [23] |
| Surgically obese, n = 5, BMI > 35 | RYGB | 1, 4, and 40 wk | Not presented | Increased conjugated BAs | [152] |
| No changed unconjugated BAs | |||||
| Obese females, n = 11, BMI = 44 | RYGB | 34 ± 16 mo | Increased postprandial insulin compared to controls | increased postprandial BAs comparing to controls | [149] |
| Morbidly obese, n = 30, BMI = 48 | RYGB | 8-13 mo | Decreased serum glucose and insulin concentration | Increased primary BAs, glycine BA, deoxycholic BA | [145] |
| Morbidly obese, n = 35, BMI = 48 | RYGB | 3 mo | Decreased HOMA-IR | Increased total BAs | [151] |
| Obese patients, n = 30, BMI = 46 | RYGB | 12 mo | Decreased fasting glucose and HOMA-IR | Increased total BAs, decreased taurine conjugated BAs | [153] |
| Morbidly obese, n = 7, BMI = 50 | LRYGB | 1 wk, 3 mo, 1 yr | Decreased HOMA-IR | Decreased BAs after 1 wk | [147] |
| Increased BAs after 3 mo and 1 yr | |||||
| Morbidly obese, n = 19, BMI = 43 | LRYGB and LSG/DJB | 1 and 3 mo | Decreased HBA1c, insulin, and HOMA-IR | Increased total, primary and secondary BA concentration | [146] |
| Morbidly obese, n = 12, preoperative BMI = 49 | Gastric bypass | 42 d | Not presented | Increased total BAs | [138] |
LSG: Laparoscopic sleeve gastrectomy; LAGB: Laparoscopic adjustable gastric banding; LSG/DJB: LSG with duodeno-jejunal bypass; RYGB: Roux-en-Y gastric bypass; LRYGB: laparoscopic RYGB.
Most studies have shown increases in circulating BA concentrations following RYGB (Table 4)[23,28,103,145,149-151]. However, there are some reports with differing results. For instance, Gerhard et al[150] found there to be no change in serum BAs in nondiabetic subjects, but serum BA levels increased in diabetic subjects after RYGB. Ahmad et al[152] reported increased levels of conjugated BAs, but no changes in unconjugated BAs following RYGB. Dutia et al[32] even found decreased serum BAs 1 mo after RYBG, whereas 2 years after surgery their levels were higher than before surgery. Simonen et al[153] found an increase in total BAs after RYGB, but taurine conjugated BAs had decreased. Nonetheless, most published data suggest that RYGB results in a postoperative increase of serum BAs in obese patients (Table 4). The increased levels of circulating BAs are probably due to the fact that BAs excreted into intestine have less time to mix with food prior to transit through the ileum, leaving more BAs free for reuptake and, probably more importantly, with the longer intestinal limb excluded from the alimentary passage and transited concentrated bile. Some studies have suggested that the BA concentrations were inversely correlated with 2 h post-meal serum glucose in patients after RYGB[103]. Others found a positive correlation between the increase in BA concentrations and fibroblast growth factor 19 (FGF-19), which regulates BA synthesis in the liver[150]. The study of Pournaras et al[138] also confirmed increases in BAs and FGF-19 4 and 42 d following gastric bypass. Interestingly, circulating levels of FGF-19 and BAs increased following RYGB, but not after pharmacological management in patients with T2DM[154]. Changes in serum BA levels were also positively correlated with FGF-19, incretin hormones, and peptide YY[32]. The increase in serum BA concentrations following RYGB was also confirmed in an obese diabetic rat model[155].
Overall, the majority of studies indicate that circulating BAs increase following RYGB (and other malabsorptive procedures) (Table 4). The increased circulating BA concentrations are usually associated with improvements in the glucose metabolism (Table 4), but are independent of caloric restriction[31]. Data regarding circulating BA concentrations and the associations between circulating BA concentrations and glucose metabolism following sleeve gastrectomy are inconsistent (Table 4). Based on the data in Table 4, it can be concluded that there is an association between circulating BA concentration and T2DM remission following malabsorptive procedures. However, more research is needed to confirm the connection between circulating BA concentration and the improvement in glucose metabolism following different bariatric surgery procedures.
POTENTIAL MECHANISM OF BILE ACID ACTION IN THE IMPROVEMENT OF GLUCOSE METABOLISM AFTER BARIATRIC SURGERY
Bariatric procedures involving the transport of concentrated bile by a section of small intestine excluded from the passage of food lead to increased absorption of BAs by ileocytes, and subsequent increases in BA blood concentrations[34]. In turn, BAs may interact with nuclear and membrane receptors-mainly FXR and TGR-5-in the intestines, liver, pancreas, adipose tissue, skeletal muscle cells, and other tissues, and improve glucose metabolism after bariatric surgery. Based on the data reported in the literature[23,32,103,146,147,150,151,153], we calculated the correlation between the mean circulating concentration of BAs and HOMA-IR or insulin concentrations in groups of patients before and after RYGB. Circulating total BA concentrations were inversely correlated with (1) HOMA-IR (R = -0.49, P < 0.05); and (2) insulin concentrations (R = -0.62, P < 0.01). This confirms, at least in part, the notion that elevated circulating concentrations of BAs could contribute to improved glucose metabolism in patients who have undergone bariatric surgery.
Recent studies on FXR knockout mice have indicated that FXR is necessary for improving glycemic control following bariatric surgery[19]. This strongly supports the idea that BAs could contribute as FXR ligands to improved glucose metabolism following bariatric surgery. The activation by BAs of FXR in ileocytes leads to the increased release of FGF 15/19, which regulates carbohydrate metabolism[32]. As mentioned above, FGF 15/19 stimulates glycogen synthesis and inhibits gluconeogenesis in the liver and glucose disposal in adipose tissue[34]. Moreover, the plasma levels of FGF 19 have been found to correlate inversely with HbA1c in diabetic patients[156]. The potential role of FGF 15/19 in improving glucose metabolism following bariatric surgery is supported by the coordinated increase in BAs and FGF 19 and the improvement of glucose metabolism in patients following bariatric surgery[32,138]. Another possible mechanism of the positive effect of elevated BAs following bariatric surgery is the direct activation of FXR in the liver, which leads to reduced gluconeogenesis[123]. In turn, the activation of FXR in muscles, the liver, and adipose tissue leads to improvements in insulin sensitivity[34,123]. The third potential mechanism of BA-mediated normalization of glycemia in bariatric patients is a through the stimulation of TGR-5 in L cells, which leads to the increased release of GLP-1[34]. This assumption is supported by improved incretin effect after RYGB[32] and the positive correlation seen between BAs and GLP-1 in patients who have undergone RYGB[28]. The potential mechanisms of BA-mediated improvement of glucose metabolism after bariatric surgery are presented in Figure 7. However, it should be noted that FXR activation in L cells leads to the opposite effect - inhibition of GLP-1 production[157]. This opposite effect of the BA induction of TGR-5 and FXR on GLP-1 release in L cells could possibly constitute some regulatory mechanism.
OTHER POTENTIAL MECHANISMS INVOLVED IN THE IMPROVEMENT IN GLUCOSE METABOLISM SUBSEQUENT UPON BARIATRIC SURGERY
Obesity is frequently associated with dyslipidemia, which includes hypertriglyceridemia, hypercholesterolemia, decreased HDL-cholesterol, and sometimes increased LDL-cholesterol concentrations in the blood[158]. It is generally accepted that hyperlipidemia plays an important role in the loss of glucose-stimulated insulin secretion in T2DM[159-161]. Bariatric surgery generally improves dyslipidemia[162]. Thus, it may be supposed that improvements in glucose metabolism following bariatric surgery could be a consequence of the reduction of circulating lipid concentrations[162]. A significant reduction in circulating triglyceride concentrations was observed from three months to four years after bariatric surgery[162]. In contrary, circulating non-estrified fatty acids (NEFA) concentrations, which also play an important role in the development of pancreatic β-cell dysfunctions and the subsequent progression of T2DM in obese subjects[159], significantly increased one month after bariatric surgery, but their concentrations normalized after three months[162]. Remission of T2DM has been shown to occur within a few days of bariatric surgery[163]. Thus, it is likely that the beneficial effects of bariatric surgery on glucose metabolism are independent of the circulating NEFA normalization, especially shortly after surgery. However, it is not impossible that normalization of circulating NEFA (or other lipid) concentrations may play some role in improving glucose metabolism a few months after bariatric surgery.
The improvement in glucose metabolism following bariatric surgery, based on the enhanced incretin effect, is commonly accepted[42,164]. However, the improvement in glucose metabolism following gastric bypass cannot be associated only with incretin hormones, because the incretin effect needs to be initiated through oral feeding, while patients cannot eat for the first few days after surgery, in order to avoid postoperative complications. Accordingly, the serum levels of GLP-1 (the main incretin hormone) is not markedly elevated in post-RYGB fasting patients, but is elevated after oral food intake[43,164]. Moreover, the improvement in glucose metabolism following bariatric surgery has also been observed under conditions in which the incretin system was inactivated[165].
Animal studies showed that bariatric surgery is associated with (1) increased production of GLP-1 in A. muciniphila[20,43]; and (2) modification of gut microbiota composition and lower intestinal and systematic dipeptidyl peptidase-4 (DPP-4) activity[166]. Thus, gut microbiota changes, leading to the elevated production of GLP-1 by intestinal endocrine cells (L cells) and decreased degradation of GLP-1 by DPP-4, may be another mechanism involved in the improvement of insulin resistance following bariatric surgery. However, further studies are needed to better understand the role of microbiota in incretin hormone modification after bariatric surgery.
Another potential mechanism for improving glucose metabolism following bariatric surgery may be associated with alterations in circulating adipokines. It has been shown that the concentrations of circulating proinflammatory adipokines (for instance, of leptin) increase, while those of anti-inflammatory adipokines (such as adiponectin) decrease in obesity and T2DM[167-169]. The dysregulation of adipokine secretion (especially leptin and adiponectin), together with other factors, such as lipid disturbance, could thus be a causal factor mediating insulin resistance and subsequently T2DM in obese patients. Bariatric surgery may overcome these disturbances. Accordingly, recently published data indicates an association between circulating adiponectin concentrations and the acute insulin response to intravenous glucose load in patients at one year and five years following bariatric surgery[170]. Thus, in the long term following bariatric surgery, the increase in circulating adiponectin concentration might play some role in T2DM remission, though it is unlikely that this would occur in the short term.
Overall, the data discussed in this review suggest that several factors could be involved in the remission of T2DM following bariatric surgery. It seems that the increase in circulating BA concentrations and alterations in gut microbiota play important role in this phenomenon.
CONCLUSION
Recent research indicates that the improvements in insulin sensitivity observed in patients who have undergone bariatric surgery, especially after gastric bypass procedures, are associated with elevated circulating BA concentration and changes in gut microbiota. Through the activation of FXR in enterocytes, the BAs increase the release of FGF 15/19, which in turn bind to FGFR, leading to (1) decreased gluconeogenesis and increased glycogen synthesis and glucose catabolism in the liver; and (2) improved insulin sensitivity and glucose disposal in adipose tissue. Moreover, BAs activate TGR-5 or FXR and thus lead to increases in (1) insulin secretion by pancreatic cells; (2) GLP-1 production in intestinal endocrine L-cells; (3) glucose catabolism in muscle and adipose tissue; and (4) insulin sensitivity with decreases of gluconeogenesis in the liver. Moreover, changes in gut microbiota following bariatric surgery also could be beneficial, as far as T2DM remission is concerned. Thus, increases in circulating BA concentrations and the interaction between BAs and gut microbiota (especially the role of microbiota in the biotransformation of BAs and the antimicrobial effects of BAs) following bariatric surgery would seem to be important factors leading to T2DM remission, besides body mass loss, calorie restriction, and changes in the concentrations of serum lipids and adipokines.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare no conflict of interest.
Peer-review started: July 14, 2016
First decision: August 8, 2016
Article in press: September 14, 2016
P- Reviewer: Bishu S, Hu S, Konishi T S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaska L, Mika A, Stepnowski P, Proczko M, Ratnicki-Sklucki K, Sledzinski T, Goyke E, Swierczynski J. The relationship between specific Fatty acids of serum lipids and serum high sensitivity C- reactive protein levels in morbidly obese women. Cell Physiol Biochem. 2014;34:1101–1108. doi: 10.1159/000366324. [DOI] [PubMed] [Google Scholar]
- 3.Sledzinski T, Sledzinski M, Smolenski RT, Swierczynski J. Increased serum nitric oxide concentration after bariatric surgery--a potential mechanism for cardiovascular benefit. Obes Surg. 2010;20:204–210. doi: 10.1007/s11695-009-0041-2. [DOI] [PubMed] [Google Scholar]
- 4.Swierczynski J, Sledzinski T, Slominska E, Smolenski R, Sledzinski Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes Surg. 2009;19:883–889. doi: 10.1007/s11695-008-9521-z. [DOI] [PubMed] [Google Scholar]
- 5.Swierczynski J, Sledzinski T. The Role of Adipokines and Gastrointestinal Tract Hormones in Obesity. In. Karcz K, Thomusch O, editors. Principles of metabolic surgery: Springer; 2012. [Google Scholar]
- 6.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 7.Rial SA, Karelis AD, Bergeron KF, Mounier C. Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals. Nutrients. 2016;8:pii: E281. doi: 10.3390/nu8050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutch DM, Clément K. Unraveling the genetics of human obesity. PLoS Genet. 2006;2:e188. doi: 10.1371/journal.pgen.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 10.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, Del Prato S, Ji L, Sadikot SM, Herman WH, et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care. 2016;39:861–877. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 12.Kmietowicz Z. Surgery for obese people with diabetes could save the NHS £100 000 a patient, finds study. BMJ. 2016;353:i3150. doi: 10.1136/bmj.i3150. [DOI] [PubMed] [Google Scholar]
- 13.Quercia I, Dutia R, Kotler DP, Belsley S, Laferrère B. Gastrointestinal changes after bariatric surgery. Diabetes Metab. 2014;40:87–94. doi: 10.1016/j.diabet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol. 2014;28:727–740. doi: 10.1016/j.bpg.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt JB, Pedersen SD, Gregersen NT, Vestergaard L, Nielsen MS, Ritz C, Madsbad S, Worm D, Hansen DL, Clausen TR, et al. Effects of RYGB on energy expenditure, appetite and glycaemic control: a randomized controlled clinical trial. Int J Obes (Lond) 2016;40:281–290. doi: 10.1038/ijo.2015.162. [DOI] [PubMed] [Google Scholar]
- 16.Penney NC, Kinross J, Newton RC, Purkayastha S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes (Lond) 2015;39:1565–1574. doi: 10.1038/ijo.2015.115. [DOI] [PubMed] [Google Scholar]
- 17.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med. 2015;21:702–714. doi: 10.1016/j.molmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Nosso G, Griffo E, Cotugno M, Saldalamacchia G, Lupoli R, Pacini G, Riccardi G, Angrisani L, Capaldo B. Comparative Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Glucose Homeostasis and Incretin Hormones in Obese Type 2 Diabetic Patients: A One-Year Prospective Study. Horm Metab Res. 2016;48:312–317. doi: 10.1055/s-0041-111505. [DOI] [PubMed] [Google Scholar]
- 23.Werling M, Vincent RP, Cross GF, Marschall HU, Fändriks L, Lönroth H, Taylor DR, Alaghband-Zadeh J, Olbers T, Le Roux CW. Enhanced fasting and post-prandial plasma bile acid responses after Roux-en-Y gastric bypass surgery. Scand J Gastroenterol. 2013;48:1257–1264. doi: 10.3109/00365521.2013.833647. [DOI] [PubMed] [Google Scholar]
- 24.Luo P, Yu H, Zhao X, Bao Y, Hong CS, Zhang P, Tu Y, Yin P, Gao P, Wei L, et al. Metabolomics Study of Roux-en-Y Gastric Bypass Surgery (RYGB) to Treat Type 2 Diabetes Patients Based on Ultraperformance Liquid Chromatography-Mass Spectrometry. J Proteome Res. 2016;15:1288–1299. doi: 10.1021/acs.jproteome.6b00022. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Ni Y, Bao Y, Zhang P, Zhao A, Chen T, Xie G, Tu Y, Zhang L, Su M, et al. Chenodeoxycholic Acid as a Potential Prognostic Marker for Roux-en-Y Gastric Bypass in Chinese Obese Patients. J Clin Endocrinol Metab. 2015;100:4222–4230. doi: 10.1210/jc.2015-2884. [DOI] [PubMed] [Google Scholar]
- 26.Cole AJ, Teigen LM, Jahansouz C, Earthman CP, Sibley SD. The Influence of Bariatric Surgery on Serum Bile Acids in Humans and Potential Metabolic and Hormonal Implications: a Systematic Review. Curr Obes Rep. 2015;4:441–450. doi: 10.1007/s13679-015-0171-x. [DOI] [PubMed] [Google Scholar]
- 27.Argyropoulos G. Bariatric surgery: prevalence, predictors, and mechanisms of diabetes remission. Curr Diab Rep. 2015;15:15. doi: 10.1007/s11892-015-0590-9. [DOI] [PubMed] [Google Scholar]
- 28.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–E712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haluzíková D, Lacinová Z, Kaválková P, Drápalová J, Křížová J, Bártlová M, Mráz M, Petr T, Vítek L, Kasalický M, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity (Silver Spring) 2013;21:1335–1342. doi: 10.1002/oby.20208. [DOI] [PubMed] [Google Scholar]
- 30.Dixon JB, le Roux CW, Rubino F, Zimmet P. Bariatric surgery for type 2 diabetes. Lancet. 2012;379:2300–2311. doi: 10.1016/S0140-6736(12)60401-2. [DOI] [PubMed] [Google Scholar]
- 31.Jahansouz C, Xu H, Hertzel AV, Serrot FJ, Kvalheim N, Cole A, Abraham A, Luthra G, Ewing K, Leslie DB, et al. Bile Acids Increase Independently From Hypocaloric Restriction After Bariatric Surgery. Ann Surg. 2015 doi: 10.1097/SLA.0000000000001552. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Dutia R, Embrey M, O’Brien CS, Haeusler RA, Agénor KK, Homel P, McGinty J, Vincent RP, Alaghband-Zadeh J, Staels B, et al. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes. Int J Obes (Lond) 2015;39:806–813. doi: 10.1038/ijo.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiorucci S, Cipriani S, Baldelli F, Mencarelli A. Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog Lipid Res. 2010;49:171–185. doi: 10.1016/j.plipres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Kuipers F, Groen AK. FXR: the key to benefits in bariatric surgery? Nat Med. 2014;20:337–338. doi: 10.1038/nm.3525. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, Yu L, Lin X, Cheng P, He L, Li X, Lu X, Tan Y, Yang H, Cai L, et al. Minireview: Roles of Fibroblast Growth Factors 19 and 21 in Metabolic Regulation and Chronic Diseases. Mol Endocrinol. 2015;29:1400–1413. doi: 10.1210/me.2015-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spinelli V, Lalloyer F, Baud G, Osto E, Kouach M, Daoudi M, Vallez E, Raverdy V, Goossens JF, Descat A, et al. Influence of Roux-en-Y gastric bypass on plasma bile acid profiles: a comparative study between rats, pigs and humans. Int J Obes (Lond) 2016;40:1260–1267. doi: 10.1038/ijo.2016.46. [DOI] [PubMed] [Google Scholar]
- 37.Raghow R. Ménage-à-trois of bariatric surgery, bile acids and the gut microbiome. World J Diabetes. 2015;6:367–370. doi: 10.4239/wjd.v6.i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 39.Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun. 2007;362:793–798. doi: 10.1016/j.bbrc.2007.06.130. [DOI] [PubMed] [Google Scholar]
- 40.Christou NV. Impact of obesity and bariatric surgery on survival. World J Surg. 2009;33:2022–2027. doi: 10.1007/s00268-009-0050-2. [DOI] [PubMed] [Google Scholar]
- 41.Clinical Guidelines - Recommendations of International Diabetes Federation, Chapter 06 Glucose Control Levels. Available from: https://www.idf.org/sites/default/files/IDF T2DM Guideline.pdf.
- 42.Cho YM. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metab J. 2014;38:406–415. doi: 10.4093/dmj.2014.38.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhee NA, Vilsbøll T, Knop FK. Current evidence for a role of GLP-1 in Roux-en-Y gastric bypass-induced remission of type 2 diabetes. Diabetes Obes Metab. 2012;14:291–298. doi: 10.1111/j.1463-1326.2011.01505.x. [DOI] [PubMed] [Google Scholar]
- 44.Hansen M, Sonne DP, Knop FK. Bile acid sequestrants: glucose-lowering mechanisms and efficacy in type 2 diabetes. Curr Diab Rep. 2014;14:482. doi: 10.1007/s11892-014-0482-4. [DOI] [PubMed] [Google Scholar]
- 45.Docherty NG, le Roux CW. Improvements in the metabolic milieu following Roux-en-Y gastric bypass and the arrest of diabetic kidney disease. Exp Physiol. 2014;99:1146–1153. doi: 10.1113/expphysiol.2014.078790. [DOI] [PubMed] [Google Scholar]
- 46.Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33 Suppl 1:S33–S40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- 47.Kaska Ł, Proczko M, Wiśniewski P, Stankiewicz M, Gill D, Śledziński Z. A prospective evaluation of the influence of three bariatric procedures on insulin resistance improvement. Should the extent of undiluted bile transit be considered a key postoperative factor altering glucose metabolism? Wideochir Inne Tech Maloinwazyjne. 2015;10:213–228. doi: 10.5114/wiitm.2015.52062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faria G, Preto J, da Costa EL, Guimarães JT, Calhau C, Taveira-Gomes A. Acute improvement in insulin resistance after laparoscopic Roux-en-Y gastric bypass: is 3 days enough to correct insulin metabolism? Obes Surg. 2013;23:103–110. doi: 10.1007/s11695-012-0803-0. [DOI] [PubMed] [Google Scholar]
- 49.Gómez-Abril S, Morillas-Ariño C, Ponce-Marco JL, Torres-Sánchez T, Delgado-Gomis F, Hernández-Mijares A, Rocha M. Short- and Long-Term Effects of Weight Loss on the Complement Component C3 After Laparoscopic Gastric Bypass in Obese Patients. Obes Surg. 2016 doi: 10.1007/s11695-016-2195-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 50.Severino A, Castagneto-Gissey L, Raffaelli M, Gastaldelli A, Capristo E, Iaconelli A, Guidone C, Callari C, Bellantone R, Mingrone G. Early effect of Roux-en-Y gastric bypass on insulin sensitivity and signaling. Surg Obes Relat Dis. 2016;12:42–47. doi: 10.1016/j.soard.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab. 2009;35:518–523. doi: 10.1016/S1262-3636(09)73459-7. [DOI] [PubMed] [Google Scholar]
- 52.Robert M, Belanger P, Hould FS, Marceau S, Tchernof A, Biertho L. Should metabolic surgery be offered in morbidly obese patients with type I diabetes? Surg Obes Relat Dis. 2015;11:798–805. doi: 10.1016/j.soard.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Garciacaballero M, Martínez-Moreno JM, Toval JA, Miralles F, Mínguez A, Osorio D, Mata JM, Reyes-Ortiz A. Improvement of C peptide zero BMI 24-34 diabetic patients after tailored one anastomosis gastric bypass (BAGUA) Nutr Hosp. 2013;28 Suppl 2:35–46. doi: 10.3305/nh.2013.28.sup2.6712. [DOI] [PubMed] [Google Scholar]
- 54.Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31:159–165. doi: 10.1097/MOG.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Aguiar Vallim TQ, Tarling EJ, Ahn H, Hagey LR, Romanoski CE, Lee RG, Graham MJ, Motohashi H, Yamamoto M, Edwards PA. MAFG is a transcriptional repressor of bile acid synthesis and metabolism. Cell Metab. 2015;21:298–310. doi: 10.1016/j.cmet.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norlin M, Wikvall K. Enzymes in the conversion of cholesterol into bile acids. Curr Mol Med. 2007;7:199–218. doi: 10.2174/156652407780059168. [DOI] [PubMed] [Google Scholar]
- 58.Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50 Suppl:S120–S125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norlin M, Toll A, Björkhem I, Wikvall K. 24-hydroxycholesterol is a substrate for hepatic cholesterol 7alpha-hydroxylase (CYP7A) J Lipid Res. 2000;41:1629–1639. [PubMed] [Google Scholar]
- 60.Sagar NM, Cree IA, Covington JA, Arasaradnam RP. The interplay of the gut microbiome, bile acids, and volatile organic compounds. Gastroenterol Res Pract. 2015;2015:398585. doi: 10.1155/2015/398585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camilleri M, Gores GJ. Therapeutic targeting of bile acids. Am J Physiol Gastrointest Liver Physiol. 2015;309:G209–G215. doi: 10.1152/ajpgi.00121.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 63.Grober J, Zaghini I, Fujii H, Jones SA, Kliewer SA, Willson TM, Ono T, Besnard P. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J Biol Chem. 1999;274:29749–29754. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- 64.Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol. 2013;368:17–29. doi: 10.1016/j.mce.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrannini E, Camastra S, Astiarraga B, Nannipieri M, Castro-Perez J, Xie D, Wang L, Chakravarthy M, Haeusler RA. Increased Bile Acid Synthesis and Deconjugation After Biliopancreatic Diversion. Diabetes. 2015;64:3377–3385. doi: 10.2337/db15-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085–1099. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 69.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Host-bacterial symbiosis in health and disease. Adv Immunol. 2010;107:243–274. doi: 10.1016/B978-0-12-381300-8.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allegretti JR, Kearney S, Li N, Bogart E, Bullock K, Gerber GK, Bry L, Clish CB, Alm E, Korzenik JR. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther. 2016;43:1142–1153. doi: 10.1111/apt.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hagey LR, Krasowski MD. Microbial biotransformations of bile acids as detected by electrospray mass spectrometry. Adv Nutr. 2013;4:29–35. doi: 10.3945/an.112.003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651. doi: 10.1016/j.femsre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Bortolini O, Medici A, Poli S. Biotransformations on steroid nucleus of bile acids. Steroids. 1997;62:564–577. doi: 10.1016/s0039-128x(97)00043-3. [DOI] [PubMed] [Google Scholar]
- 79.Chikai T, Nakao H, Uchida K. Deconjugation of bile acids by human intestinal bacteria implanted in germ-free rats. Lipids. 1987;22:669–671. doi: 10.1007/BF02533948. [DOI] [PubMed] [Google Scholar]
- 80.Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA, Ausina V, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551–557. doi: 10.1053/jhep.2003.50116. [DOI] [PubMed] [Google Scholar]
- 81.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA. 2006;103:3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Philipp B. Bacterial degradation of bile salts. Appl Microbiol Biotechnol. 2011;89:903–915. doi: 10.1007/s00253-010-2998-0. [DOI] [PubMed] [Google Scholar]
- 83.Merritt ME, Donaldson JR. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol. 2009;58:1533–1541. doi: 10.1099/jmm.0.014092-0. [DOI] [PubMed] [Google Scholar]
- 84.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 85.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hur KY, Lee MS. Gut Microbiota and Metabolic Disorders. Diabetes Metab J. 2015;39:198–203. doi: 10.4093/dmj.2015.39.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol. 2014;20:16452–16463. doi: 10.3748/wjg.v20.i44.16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr. 2011;31:15–31. doi: 10.1146/annurev-nutr-072610-145146. [DOI] [PubMed] [Google Scholar]
- 90.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 91.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caricilli AM, Saad MJ. Gut microbiota composition and its effects on obesity and insulin resistance. Curr Opin Clin Nutr Metab Care. 2014;17:312–318. doi: 10.1097/MCO.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 94.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 95.Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 96.Han JL, Lin HL. Intestinal microbiota and type 2 diabetes: from mechanism insights to therapeutic perspective. World J Gastroenterol. 2014;20:17737–17745. doi: 10.3748/wjg.v20.i47.17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST, Pan C, Civelek M, Rau CD, Bennett BJ, et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab. 2013;17:141–152. doi: 10.1016/j.cmet.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML, Xu A, Chavakis T, Bornstein AB, Ehrhart-Bornstein M, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13:514–522. doi: 10.1038/tpj.2012.43. [DOI] [PubMed] [Google Scholar]
- 99.Liou AP, Paziuk M, Luevano JM, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 101.Aron-Wisnewsky J, Doré J, Clement K. The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2012;9:590–598. doi: 10.1038/nrgastro.2012.161. [DOI] [PubMed] [Google Scholar]
- 102.Aron-Wisnewsky J, Clement K. The effects of gastrointestinal surgery on gut microbiota: potential contribution to improved insulin sensitivity. Curr Atheroscler Rep. 2014;16:454. doi: 10.1007/s11883-014-0454-9. [DOI] [PubMed] [Google Scholar]
- 103.Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring) 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring) 2014;22:390–400. doi: 10.1002/oby.20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 106.Kaji I, Karaki S, Kuwahara A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion. 2014;89:31–36. doi: 10.1159/000356211. [DOI] [PubMed] [Google Scholar]
- 107.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature. 2016;534:213–217. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vítek L, Haluzík M. The role of bile acids in metabolic regulation. J Endocrinol. 2016;228:R85–R96. doi: 10.1530/JOE-15-0469. [DOI] [PubMed] [Google Scholar]
- 109.Barrasa JI, Olmo N, Lizarbe MA, Turnay J. Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol In Vitro. 2013;27:964–977. doi: 10.1016/j.tiv.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 110.Scherer M, Gnewuch C, Schmitz G, Liebisch G. Rapid quantification of bile acids and their conjugates in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3920–3925. doi: 10.1016/j.jchromb.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 111.Yamamoto R, Tazuma S, Kanno K, Igarashi Y, Inui K, Ohara H, Tsuyuguchi T, Ryozawa S. Ursodeoxycholic acid after bile duct stone removal and risk factors for recurrence: a randomized trial. J Hepatobiliary Pancreat Sci. 2016;23:132–136. doi: 10.1002/jhbp.316. [DOI] [PubMed] [Google Scholar]
- 112.Stokes CS, Gluud LL, Casper M, Lammert F. Ursodeoxycholic acid and diets higher in fat prevent gallbladder stones during weight loss: a meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2014;12:1090–1100.e2; quiz e61. doi: 10.1016/j.cgh.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 113.Jackson H, Solaymani-Dodaran M, Card TR, Aithal GP, Logan R, West J. Influence of ursodeoxycholic acid on the mortality and malignancy associated with primary biliary cirrhosis: a population-based cohort study. Hepatology. 2007;46:1131–1137. doi: 10.1002/hep.21795. [DOI] [PubMed] [Google Scholar]
- 114.Adams LB, Chang C, Pope J, Kim Y, Liu P, Yates A. Randomized, Prospective Comparison of Ursodeoxycholic Acid for the Prevention of Gallstones after Sleeve Gastrectomy. Obes Surg. 2016;26:990–994. doi: 10.1007/s11695-015-1858-5. [DOI] [PubMed] [Google Scholar]
- 115.Yuan L, Bambha K. Bile acid receptors and nonalcoholic fatty liver disease. World J Hepatol. 2015;7:2811–2818. doi: 10.4254/wjh.v7.i28.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 117.Gao J, Xie W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol Sci. 2012;33:552–558. doi: 10.1016/j.tips.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao J, He J, Zhai Y, Wada T, Xie W. The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J Biol Chem. 2009;284:25984–25992. doi: 10.1074/jbc.M109.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jansen PL, Sturm E. Genetic cholestasis, causes and consequences for hepatobiliary transport. Liver Int. 2003;23:315–322. doi: 10.1034/j.1478-3231.2003.00856.x. [DOI] [PubMed] [Google Scholar]
- 121.Keitel V, Kubitz R, Häussinger D. Endocrine and paracrine role of bile acids. World J Gastroenterol. 2008;14:5620–5629. doi: 10.3748/wjg.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han SI, Studer E, Gupta S, Fang Y, Qiao L, Li W, Grant S, Hylemon PB, Dent P. Bile acids enhance the activity of the insulin receptor and glycogen synthase in primary rodent hepatocytes. Hepatology. 2004;39:456–463. doi: 10.1002/hep.20043. [DOI] [PubMed] [Google Scholar]
- 123.Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci. 2009;30:570–580. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 124.Rometo D, Korytkowski M. Perioperative Glycemic Management of Patients Undergoing Bariatric Surgery. Curr Diab Rep. 2016;16:23. doi: 10.1007/s11892-016-0718-6. [DOI] [PubMed] [Google Scholar]
- 125.Schittenhelm B, Wagner R, Kähny V, Peter A, Krippeit-Drews P, Düfer M, Drews G. Role of FXR in β-cells of lean and obese mice. Endocrinology. 2015;156:1263–1271. doi: 10.1210/en.2014-1751. [DOI] [PubMed] [Google Scholar]
- 126.Ding L, Pang S, Sun Y, Tian Y, Yu L, Dang N. Coordinated Actions of FXR and LXR in Metabolism: From Pathogenesis to Pharmacological Targets for Type 2 Diabetes. Int J Endocrinol. 2014;2014:751859. doi: 10.1155/2014/751859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids. 2014;86:62–68. doi: 10.1016/j.steroids.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dossa AY, Escobar O, Golden J, Frey MR, Ford HR, Gayer CP. Bile acids regulate intestinal cell proliferation by modulating EGFR and FXR signaling. Am J Physiol Gastrointest Liver Physiol. 2016;310:G81–G92. doi: 10.1152/ajpgi.00065.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 130.Vassileva G, Golovko A, Markowitz L, Abbondanzo SJ, Zeng M, Yang S, Hoos L, Tetzloff G, Levitan D, Murgolo NJ, et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398:423–430. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vettorazzi JF, Ribeiro RA, Borck PC, Branco RC, Soriano S, Merino B, Boschero AC, Nadal A, Quesada I, Carneiro EM. The bile acid TUDCA increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cells. Metabolism. 2016;65:54–63. doi: 10.1016/j.metabol.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 132.Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58:1794–1805. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- 133.Stepanov V, Stankov K, Mikov M. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J Recept Signal Transduct Res. 2013;33:213–223. doi: 10.3109/10799893.2013.802805. [DOI] [PubMed] [Google Scholar]
- 134.Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 136.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 137.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide 1(7-36) amide’s central inhibition of feeding and peripheral inhibition of drinking are abolished by neonatal monosodium glutamate treatment. Diabetes. 1998;47:530–537. doi: 10.2337/diabetes.47.4.530. [DOI] [PubMed] [Google Scholar]
- 138.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adrian TE, Gariballa S, Parekh KA, Thomas SA, Saadi H, Al Kaabi J, Nagelkerke N, Gedulin B, Young AA. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia. 2012;55:2343–2347. doi: 10.1007/s00125-012-2593-2. [DOI] [PubMed] [Google Scholar]
- 140.Kitada Y, Kajita K, Taguchi K, Mori I, Yamauchi M, Ikeda T, Kawashima M, Asano M, Kajita T, Ishizuka T, et al. Blockade of Sphingosine 1-Phosphate Receptor 2 Signaling Attenuates High-Fat Diet-Induced Adipocyte Hypertrophy and Systemic Glucose Intolerance in Mice. Endocrinology. 2016;157:1839–1851. doi: 10.1210/en.2015-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, Kukreja R, Valerie K, Nagarkatti P, El Deiry W, et al. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell. 2001;12:2629–2645. doi: 10.1091/mbc.12.9.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cummings BP, Bettaieb A, Graham JL, Kim J, Ma F, Shibata N, Stanhope KL, Giulivi C, Hansen F, Jelsing J, et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech. 2013;6:443–456. doi: 10.1242/dmm.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cao AL, Wang L, Chen X, Wang YM, Guo HJ, Chu S, Liu C, Zhang XM, Peng W. Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Invest. 2016;96:610–622. doi: 10.1038/labinvest.2016.44. [DOI] [PubMed] [Google Scholar]
- 144.Kohli R, Setchell KD, Kirby M, Myronovych A, Ryan KK, Ibrahim SH, Berger J, Smith K, Toure M, Woods SC, et al. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154:2341–2351. doi: 10.1210/en.2012-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63:891–902. doi: 10.1136/gutjnl-2013-305008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 147.Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, Beglinger C. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 2013;21:E660–E668. doi: 10.1002/oby.20522. [DOI] [PubMed] [Google Scholar]
- 148.Belgaumkar AP, Vincent RP, Carswell KA, Hughes RD, Alaghband-Zadeh J, Mitry RR, le Roux CW, Patel AG. Changes in Bile Acid Profile After Laparoscopic Sleeve Gastrectomy are Associated with Improvements in Metabolic Profile and Fatty Liver Disease. Obes Surg. 2016;26:1195–1202. doi: 10.1007/s11695-015-1878-1. [DOI] [PubMed] [Google Scholar]
- 149.De Giorgi S, Campos V, Egli L, Toepel U, Carrel G, Cariou B, Rainteau D, Schneiter P, Tappy L, Giusti V. Long-term effects of Roux-en-Y gastric bypass on postprandial plasma lipid and bile acids kinetics in female non diabetic subjects: A cross-sectional pilot study. Clin Nutr. 2015;34:911–917. doi: 10.1016/j.clnu.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 150.Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, Strodel WE, Still CD, Argyropoulos G. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36:1859–1864. doi: 10.2337/dc12-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J, Schaap FG. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29:48–51. doi: 10.1159/000324128. [DOI] [PubMed] [Google Scholar]
- 152.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013;37:1553–1559. doi: 10.1038/ijo.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Käkelä P, Pääkkönen M, Hallikainen M, Kolehmainen M, Uusitupa M, Moilanen L, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22:1473–1480. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sachdev S, Wang Q, Billington C, Connett J, Ahmed L, Inabnet W, Chua S, Ikramuddin S, Korner J. FGF 19 and Bile Acids Increase Following Roux-en-Y Gastric Bypass but Not After Medical Management in Patients with Type 2 Diabetes. Obes Surg. 2016;26:957–965. doi: 10.1007/s11695-015-1834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bhutta HY, Rajpal N, White W, Freudenberg JM, Liu Y, Way J, Rajpal D, Cooper DC, Young A, Tavakkoli A, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One. 2015;10:e0122273. doi: 10.1371/journal.pone.0122273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barutcuoglu B, Basol G, Cakir Y, Cetinkalp S, Parildar Z, Kabaroglu C, Ozmen D, Mutaf I, Bayindir O. Fibroblast growth factor-19 levels in type 2 diabetic patients with metabolic syndrome. Ann Clin Lab Sci. 2011;41:390–396. [PubMed] [Google Scholar]
- 157.Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, Perino A, Brighton CA, Sebti Y, Kluza J, et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629. doi: 10.1038/ncomms8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Howard BV, Ruotolo G, Robbins DC. Obesity and dyslipidemia. Endocrinol Metab Clin North Am. 2003;32:855–867. doi: 10.1016/s0889-8529(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 159.Janikiewicz J, Hanzelka K, Kozinski K, Kolczynska K, Dobrzyn A. Islet β-cell failure in type 2 diabetes--Within the network of toxic lipids. Biochem Biophys Res Commun. 2015;460:491–496. doi: 10.1016/j.bbrc.2015.03.153. [DOI] [PubMed] [Google Scholar]
- 160.Poitout V, Robertson RP. Minireview: Secondary beta-cell failure in type 2 diabetes--a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–342. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 161.Boucher A, Lu D, Burgess SC, Telemaque-Potts S, Jensen MV, Mulder H, Wang MY, Unger RH, Sherry AD, Newgard CB. Biochemical mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and reversal with a malate analogue. J Biol Chem. 2004;279:27263–27271. doi: 10.1074/jbc.M401167200. [DOI] [PubMed] [Google Scholar]
- 162.Carswell KA, Belgaumkar AP, Amiel SA, Patel AG. A Systematic Review and Meta-analysis of the Effect of Gastric Bypass Surgery on Plasma Lipid Levels. Obes Surg. 2016;26:843–855. doi: 10.1007/s11695-015-1829-x. [DOI] [PubMed] [Google Scholar]
- 163.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–481. doi: 10.1381/0960892053723402. [DOI] [PubMed] [Google Scholar]
- 164.Laferrère B. Do we really know why diabetes remits after gastric bypass surgery? Endocrine. 2011;40:162–167. doi: 10.1007/s12020-011-9514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gale EA. GLP-1-based therapies and the exocrine pancreas: more light, or just more heat? Diabetes. 2012;61:986–988. doi: 10.2337/db11-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Osto M, Abegg K, Bueter M, le Roux CW, Cani PD, Lutz TA. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav. 2013;119:92–96. doi: 10.1016/j.physbeh.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 167.Sledzinski T, Korczynska J, Hallmann A, Kaska L, Proczko-Markuszewska M, Stefaniak T, Sledzinski M, Swierczynski J. The increase of serum chemerin concentration is mainly associated with the increase of body mass index in obese, non-diabetic subjects. J Endocrinol Invest. 2013;36:428–434. doi: 10.3275/8770. [DOI] [PubMed] [Google Scholar]
- 168.Zabrocka L, Raczynska S, Goyke E, Sledzinski Z, Swierczynski J. BMI is the main determinant of the circulating leptin in women after vertical banded gastroplasty. Obes Res. 2004;12:505–512. doi: 10.1038/oby.2004.57. [DOI] [PubMed] [Google Scholar]
- 169.Matsuzawa Y. The role of fat topology in the risk of disease. Int J Obes (Lond) 2008;32 Suppl 7:S83–S92. doi: 10.1038/ijo.2008.243. [DOI] [PubMed] [Google Scholar]
- 170.Adami GF, Gradaschi R, Andraghetti G, Scopinaro N, Cordera R. Serum Leptin and Adiponectin Concentration in Type 2 Diabetes Patients in the Short and Long Term Following Biliopancreatic Diversion. Obes Surg. 2016;26:2442–2448. doi: 10.1007/s11695-016-2126-z. [DOI] [PubMed] [Google Scholar]


